Abstract
miR-122, a completely conserved liver-specific miRNA in vertebrates, is essential for the maintenance of liver homeostasis. This 22 nucleotide RNA regulates diverse functions such as cholesterol, glucose and iron homeostasis, lipid metabolism and infection of hepatitis C virus (HCV) and of the parasitic protozoa, Leishmania donovani. It is the first miRNA that underwent successful clinical trials in HCV infected patients. In contrast, miR-122 expression is reduced in nonalcoholic steatohepatitis (NASH) patients, and in a subset of hepatocellular carcinoma (HCC) patients including hepatitis B virus (HBV) positive patients with highly invasive and metastatic cancer. Studies in mice genetically depleted of miR-122 have highlighted its critical role in liver biology. These mice progressively develop steatohepatitis, fibrosis and hepatocellular cancer, establishing it as a bona fide tumor suppressor. Additionally, delivery of miR-122 using a viral vector or liposomal nanoparticles resulted in liver tumor suppression in animal models. These results suggest miR-122 supplementation might be beneficial in NASH or HBV positive HCC patients. Furthermore, circulating miR-122 has emerged as a sensitive biomarker for liver injury. The ability of miR-122 to promote differentiation of embryonic and adult stem cells to hepatocyte-like cells in vitro suggests its potential role in driving the hepatic differentiation program. In this review, we will discuss the role of miR-122 in liver physiology and the deleterious consequences of its loss of function, its role as a sensitive biomarker for liver injury and therapeutic target. Development of novel technologies for targeted delivery of miR-122 to tumor cells and for direct monitoring of miR-122 in biological fluids is urgently needed for translating the basic research to the bedside. This review focuses on miR-122, the most abundant hepatic miRNA, in the context of liver health and diseases.
Keywords: Fibrosis, hepatitis, hepatocellular carcinoma, metabolism, microRNA-122, miR-122, miR-122 gene therapy, nanoparticles mediated miR-122 delivery
INTRODUCTION
miR-122 is a highly abundant liver-specific miRNA expressed in vertebrates [1–4]. Complete conservation of mature miR-122 in all vertebrates, reflects its critical role in the liver. Among other tissues, negligible expression of miR-122 was detected by RNA-sequencing only in the respiratory system both in humans and mice [3]. Curiously, this ~22 nucleotide miRNA plays critical roles in liver homeostasis by regulating expression of a large number of target mRNAs involved in diverse hepatic functions and also by suppressing nonhepatic genes. Its inhibition by pharmacological and genetic means results in dysregulation of systemic and hepatic lipid metabolism, iron homeostasis as well as differentiation of hepatocytes (reviewed in [5–8]). miR-122 is an important tumor suppressor and clinically, its hepatic [9] and circulating [10] levels are a prognostic marker in patients with HCC. Its hepatocyte-specific expression and its release from damaged hepatocytes early in liver injury in animal models [11] have made miR-122 an attractive biomarker for liver damage. Additionally, circulating miR-122 and HMGB1 levels have been identified as a sensitive and specific biomarker in patients with acetaminophen-induced liver injury [12]. Interestingly, all HCV serotypes take advantage of the abundant miR-122 in the liver to promote replication and translation of their genome (reviewed in [13]). In contrast, miR-122, down regulated in HBV positive HCC [9], appears to inhibit HBV replication in HCC cells in vitro [14].
MiR-122’s small size, liver-specific expression, and its multifarious functions have made miR-122 an enticing target for therapy both as mimic and anti-mir. In fact, a miR-122-specific inhibitor, miravirsen or SPC3649, is the first miRNA-targeted drug that has undergone successful phase II clinical trials to combat HCV infection [15]). However, miR-122’s essential role in maintaining hepatic phenotype and its loss of function causing hepatocarcinogenesis in mice [16, 17] suggest that the long-term depletion of miR-122 may have deleterious consequences in humans. Thus, HCV infected patients undergoing miravirsen therapy should be routinely monitored for liver function and cancer. In contrast, HBV infected HCC patients may benefit from miR-122 mimic therapy because it is down regulated in HBV positive tumors [9], and studies in different mouse models including orthotopic HCC xenografts have shown that miR-122 suppresses HCC growth and metastasis [18, 19]. Since it is down regulated in NASH patients at early stages [20, 21], these patients may benefit from miR-122 therapy to possibly restore liver function by reducing lipid accumulation, inflammation and fibrosis, as shown in the knockout mouse model [16, 17]. Our current studies highlight the fact that our mechanistic understanding of miR-122’s functions in liver health and disease is far from complete. In this review, we not only discuss biogenesis, regulation and functions of miR-122 in the mammalian liver, but also its role in various disease states, its emergence as an important therapeutic target, and different delivery methods for ectopic expression and depletion of this microRNA.
Discovery of miR-122
miR-122 was first discovered by cloning and sequencing of miRNAs from tissues in mice as a highly abundant liver-specific miRNA [2]. Subsequently, it has been shown that its expression starts after development of liver bud and attains maximal level after birth in mice [1]. Successful generation of germ-line knockout mice without overt developmental abnormalities affirms that miR-122 is dispensable for embryogenesis [16, 17]. However, expression of this developmentally regulated miRNA increases during late gestation and attains its maximal levels after birth in mice, implicating its important role in the fetal and adult liver. In situ hybridization in zebrafish detected miR-122 expression only in the liver [4] confirming it as a liver-specific miRNA. In the years to follow these early discoveries, many new findings on miR-122’s role in the liver would be uncovered.
Biogenesis and Processing of miR-122
The biogenesis of miR-122 follows the canonical pathway described in several excellent reviews [5, 6]. A few noteworthy properties are discussed here. Mir-122 gene is transcribed by RNA polymerase II (pol II) from a long non-coding RNA located on chromosome 18 in human and mouse generating ~4.5 kb pri-miR-122 [22], which is then processed to a 66nt pre-miR-122 in the nucleus and subsequently to mature miR-122 in the cytoplasm. In contrast to the mature miRNA, the pri-mir-122 sequence is poorly conserved in vertebrates, with no detected additional potential miRNAs, or conserved open reading frames within the primary transcript. Several liver-enriched transcription factors, e.g. C/EBPα, HNF1α, HNF3β, and HNF4α [9, 23] and HNF6 [24] were shown to activate Mir-122 gene expression in hepatic cell lines. Although the core promoter region of Mir-122 gene has been identified [23], the optimal promoter that drives its hepatocyte-specific transcription of this gene has not been fully elucidated. Recently, high-throughput sequencing of RNA polymerase II associated polyA+ and polyA- RNAs revealed that long intergenic noncoding (linc) RNAs harboring miRNAs, e.g. miR-122, belong to polyA-class (Nicholas Proudfoot, personal communication). Pre-miRNA stem-loops rather than polyadenylation signals serve as termination signals for such linc RNAs. Thus, discovery of miR-122 processed from a polII transcribed but non-polyadenylated linc RNA is conceptually novel.
One unique characteristic of the Mir-122 gene is the regulation of its expression by circadian rhythms by the transcriptional repressor, REV-ERBα [22]. While pri- and pre-miR-122 levels were shown to fluctuate by diurnal rhythm, levels of mature miR-122 remained constant, most likely due to its long half-life (>24hr). A cytoplasmic poly(A) polymerase, GLD2 that adds a single nontemplated adenosine to the 3′-end of miR-122 has shown to contribute to the stability of miR-122 [25]. Daily oscillation of the levels of some miR-122 targets e.g. Rell1, Smarcd1, Pparβ/δ suggests that a fraction of miR-122 pool, such as newly synthesized and/or distinct biochemically modified miR-122 might be associated with the miRISC (miRNA induced silencing complex), the functional entity that regulates expression of its targets in a circadian fashion. These findings suggest that miR-122 probably exhibits rhythmic functions despite its constant levels. However, regulation of miR-122 expression in the liver by physiological mediators such as the neuroendocrine system is still unknown.
miR-122’s Functions in Liver
It is not surprising that miR-122, perhaps the most abundant miRNA identified so far has important roles in regulating liver function and thereby maintaining systemic homeostasis. In fact, analysis of the genes that miR-122 normally represses showed that miR-122 plays a key role in preserving the adult liver’s differentiated state by suppressing nonhepatic genes [26]. Indeed, several studies have shown that ectopic miR-122 expression promotes differentiation of embryonic as well as adult stem cells of human and mouse origin to hepatocyte-like cells in vitro [27–29]. This suggests that miR-122 is involved in maintaining a hepatic gene expression signature and may potentially be used in combination with other factors in generating hepatocytes for therapeutic purpose in patients with liver failure. In fact, the first miR-122 target identified was the gene cationic amino acid transporter 1 (CAT-1) or Slc7a1, [1], which is expressed in many other adult tissue types and strongly expressed in fetal liver, yet under normal un-stressed circumstances, is repressed in adult hepatocytes [1, 30]. Similarly, Ndrg3 and Cutl1, targets of miR-122 are usually repressed in adult hepatocytes [26]. The functions of miR-122 in healthy liver cells may be as diverse as modulating circadian clock controlled gene expression through a deadenylase, Nocturnin [31], to involvement in maintaining systemic iron levels through the regulation of the expression of the hormone hepcidin by targeting the transcription factors hemochromatosis (Hfe) and hemojuvelin (Hjv) [32].
Among the most remarkable findings regarding miR-122’s roles in liver homeostasis are regulation of cholesterol and triglyceride metabolism and blood alkaline phosphatase level [17, 26, 33, 34]. Krutzfeldt et al made a seminal observation while testing the efficacy of systemic antimir delivery in depleting miRNAs in mice and their functional consequences [26]. miR-122 was silenced in vivo by injecting an antagomir, a cholesterol-conjugated 2′-O-methyl phosphorothioate-modified anti-sense oligonucleotide, that formed a duplex with hepatic miR-122 resulting in a 40% decrease in serum cholesterol levels compared to controls that correlated with down regulation of expression many genes involved in cholesterol biosynthesis and transport. Similar observations were reported by Esau et al by silencing miR-122 using a distinct antisense oligonucleotide (ASO), a 2′-O-methoxyethyl phosphorothioate ASO in normal mice and in mice that developed fatty liver upon feeding a high-fat diet (HFD) [33]. Reduced fat accumulation in mice fed HFD suggests ASO therapy for a short time may be effective in certain individuals with fatty liver disease. Subsequently, miR-122 was silenced in primates using locked nucleic acid (LNA) modified ASO, which resulted in a significant lowering of plasma cholesterol [35]. However, decreases in both potentially atherogenic LDL-cholesterol and cardio-protective HDL-cholesterol in rodents [16] and primates [35] suggest that implementing anti-miR-122 therapy may not be optimal for cardiovascular risk management in human patients.
miR-122 in Nonalcoholic Steatohepatitis and Autoimmune Primary Biliary Cirrhosis
miRNA array studies analyzing global miRNA gene expression profiles have identified miR-122 among the miR-NAs altered in NASH [20]. NASH occurs as an accumulation of inflammation-associated hepatic lipid, fibrosis and liver injury and may progress to cirrhosis and HCC [36]. Development of steatohepatitis in knockout mice that progresses to NASH indicates a causal role of miR-122 in NASH [17, 34]. Upregulation of expression of enzymes such as Agpat1, a conserved target of miR-122, Agpat3/9, Mogat1, Mogat2, Dgat1 and Ppap2a/2c involved in triglyceride biosynthesis and transport (Cidec, a miR-122 target) is likely to cause steatosis, whereas activation of Ccl2-Ccr2 axis plays a causal role in recruiting proinflammatory Cd11+Gr1+ cells in miR-122 depleted livers [34]. Chronic hepatitis in miR-122 knockout mice may lead to liver fibrosis due to increased synthesis and deposition of extracellular matrix by activated HSCs (hepatic stellate cells). Additionally, increased expression of profibrogenic transcription factor Klf6, a direct target of miR-122 [17], and Tgfβ [17, 34] may also play a causal role in hepatic fibrosis after miR-122 depletion. Based on these findings, it is likely that miR-122 is important for maintaining an environment that prevents the development of NASH. Recently, it has been shown that silencing of miR-122 is an early event in NASH development both in human patients and in STAM mice, a NASH-induced HCC model [21].
Primary biliary sclerosis (PBC) is an autoimmune disease that causes damage and cirrhosis of intrahepatic bile ducts leading to liver failure [37]. miR-122 was among three miRNAs significantly down regulated in end-stage PBC suggesting that deregulation of miR-122 may be involved in mediating intrahepatic bile duct injury. High serum ALP (alkaline phosphatase) and GGT (gamma-glutamyl transferase) levels in miR-122 knockout mice supports this notion [16].
Role of miR-122 in Liver Tumor Suppression
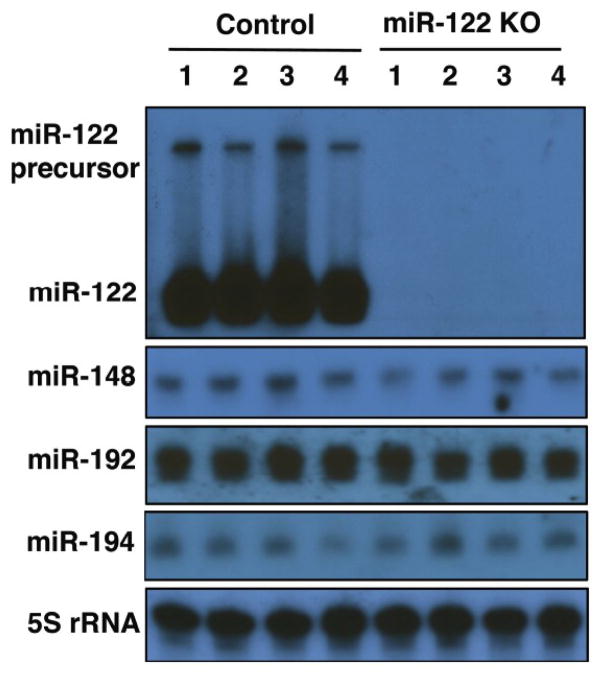
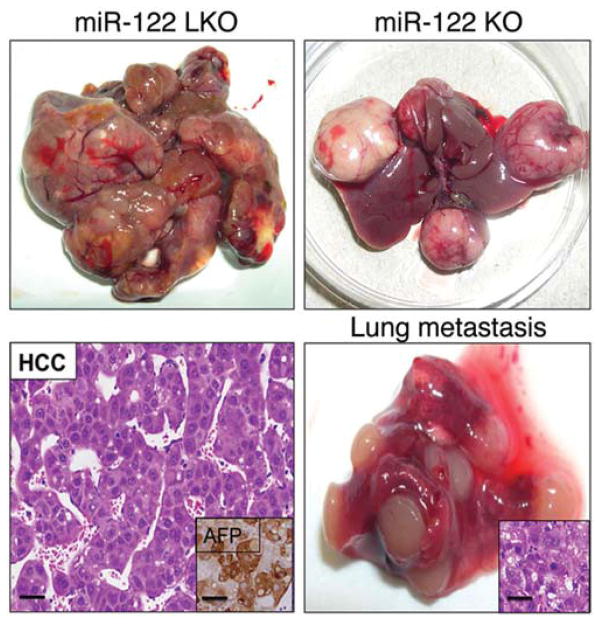
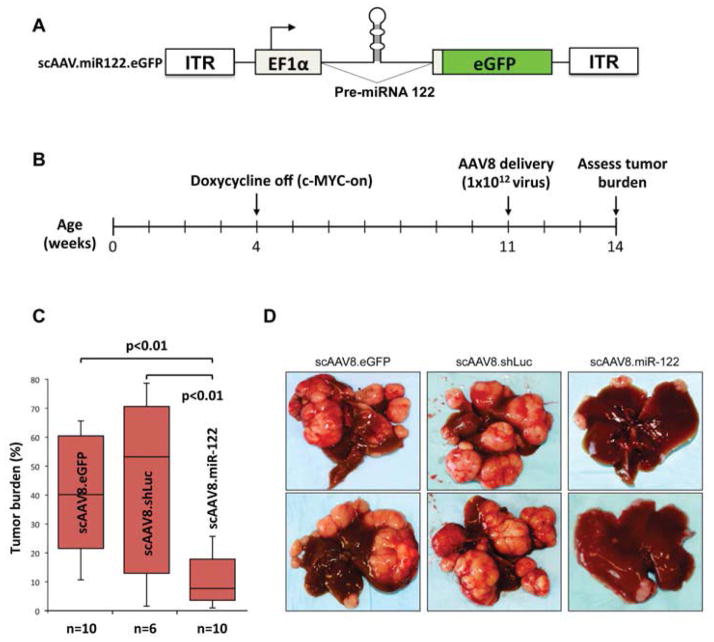
Studies from our lab initially demonstrated reduced level of miR-122 in primary HCCs of rodent and human origin compared to the matching benign livers [38], suggested its potential role as a tumor suppressor. Presently, it is well established that miR-122 is a bona fide tumor suppressor. The strongest evidence for miR-122’s tumor suppressor role comes from the fact that mice in which miR-122 was deleted systemically or only in the hepatocytes developed spontaneous HCC after one year of life [17, 34]. These mice progressively developed steatohepatitis, fibrosis, and HCC. Northern blot analysis confirmed loss of miR-122 in the knockout mice (Fig. 1). Fig. (2) illustrates the liver and metastatic lung tumors formed in these mice. Furthermore, depletion of miR-122 in liver predisposed mice to diethylnitrosamine, a potent liver carcinogen, induced biliary cyst formation at the pre-neoplastic stage, and facilitated HCC development by 8 months [34]. Activation of some fetal genes e.g. Igf2 and several oncogenes e.g. c-Myc, Iqgap1, Mapre1 is likely to play a causal role in tumorigenesis in these mice [16]. Interestingly, miR-122 and c-Myc exhibit reciprocal regulation in HCC [39]; miR-122 suppresses c-Myc transcription by targeting E2f1, a transcriptional activator, and Tfdp2, a co-activator, whereas c-Myc suppresses Mir-122 expression directly by binding to its promoter region and indirectly by downregulating several liver enriched transcription factors [39]. Indeed, miR-122 level is ablated in c-Myc - induced liver tumors, and miR-122 supplementation using a viral vector resulted in dramatic tumor regression (Fig. 3).
Fig. (1).
Northern blot analysis of hepatic miRNAs in the wild type and miR-122 knockout livers (from reference [16]).
Fig. (2).
miR-122 liver specific (LKO) and germ-line (KO) mice develop spontaneous HCC and metastatic lung cancer after 12 months. Representative photographs are shown (from reference [16]).
Fig. (3).
A. Schematic representation of the recombinant scAAV harboring miR-122 minigene that generates miR-122. B. Timing of AAV8-miR-122 delivery after c-myc induction by doxycycline withdrawal) in Tet-O-Myc;LAP-tTA mice. C. Representative images of livers from AAV8, sh-luc-AAV8 and miR-122-AAV8 delivered mice (from reference [16]).
In vitro analyses with HCC and hepatic cell lines and ex vivo studies using immunocompromised athymic nude mice have been of major importance in understanding how miR-122 affects cell function, migration, invasiveness, response to therapeutics, and in identifying targets (reviewed in refs [5–8]). Of clinical importance, we were first to report that miR-122 was down regulated in human HCCs [38], and subsequently others showed that reduced levels of miR-122 correlated with clinical properties that lead to a poor prognosis [9,19]. It was found that just by ectopic expression of miR-122, these cells lose their tumorigenic properties, specifically growth, replication and survival properties, as well as angio-genesis, and migration and invasion properties. Thus, miR-122 over expressing cells revert back to an epithelial cell phenotype and had a significantly reduced ability to form tumors when transplanted into nude mice. Ectopic expression of miR-122 dramatically suppressed angiogenic properties of HUVEC cells in vitro, implicating it can be used as for anti-angiogenic therapy [18]. Additionally, ectopic miR-122 expression reduced clonogenic survival of these cells by the drug Sorafenib, a multi-kinase inhibitor and only approved drug in patients with advanced HCC [17]. Ectopic miR-122 expression induced apoptosis of HCC cells by the drug Doxorubicin, a DNA intercalating agent, which correlated with silencing of cyclin G1, a miR-122 target [40]. Therefore, combination of miR-122 restoration and chemotherapy may serve to impede tumor development in HCC patients.
The down regulation of miR-122 appears to be important in the onset and progression of tumorigenesis. We found that, in rats fed a hepatocarcinogenic folic acid, methionine, and choline-deficient (FMD) diet, miR-122 was down regulated both in pre-neoplastic nodules and in tumors [38]. Even so, we also found that in tumor-bearing mice produced using a distinct hepatocarcinogenesis diet model that results in nonalcoholic steatohepatitis (NASH)-induced HCC, miR-122 was down regulated at early stages when pre-neoplastic nodules are formed [40].
miR-122’s Roles in Viral (HCV, HBV) and Protozoan Infection
miR-122 has an atypical regulatory relationship with the HCV genome. HCV is a virus with a single stranded RNA genome, and like Hepatitis B Virus (HBV), can establish a chronic infection in its host that may lead to liver cirrhosis and subsequent development of HCC [41]. Unlike HBV, there is no effective vaccine to protect against HCV infection. Jopling et al made a seminal discovery demonstrating that a host microRNA, miR-122 facilitates replication of infectious virus in hepatic cells [42]. Santaris Pharma promptly took this key finding to the clinic by developing miravirsen, an anti-miR-122 drug that showed promising results in a phase 2a trial in chronic HCV type 1 infected patients [15]. The drug-treated patients showed prolonged dose-dependent abolishment or reduction in viremia without evidence of viral resistance, drug related toxicity, or escape mutations in the miR-122 cognate sites in the HCV RNA. The results of these trials may pave the way for an expansion of miRNA-based therapy, but long-term effects of miR-122 suppression in HCV infected patients with compromised liver function need to be determined and carefully managed. Interestingly, hepatic miR-122 level is significantly reduced in HBV infected patients compared to healthy controls, and negatively correlated with viral load and necro-inflammation in the liver [43]. Furthermore, contrary to HCV, miR-122 inhibited the HBV replication in hepatic cells in culture [44, 45].
Interestingly, the ENCODE project identified two SNPs, rs4309483 and rs4503880, in the upstream regulatory region of human Mir-122 gene in Han Chinese. This study showed that CC to AA SNP of rs4309483 may regulate the expression of miR-122 conferring protection from chronic HBV infection but increasing the risk for HCC in HBV carriers. However, it remains to be established how CC to AA SNP modulates pri-miR-122 expression.
One important recent finding is the protective role of miR-122 against the parasitic protozoa Leishmania that causes visceral leishmanisis, a deadly disease [46]. During leishmania infection, hepatocytes uptake exosomes containing the protozoal membrane metalloprotease GP63 released from infected mononuclear phagocytes. GP63 released from these exosomes then causes proteolytic inactivation of Dicer1, thus blocking processing of hepatic pre-miRNAs including pre-miR-122 to mature miRNAs. Thus, loss of hepatic miR-122 is likely to cause increased parasite burden in patients with visceral leishmanisis.
miR-122 Therapeutics for Liver Disease
miR-122 is a unique microRNA that has therapeutic potential both as an anti-mir in combating HCV infection and as a mimic against various liver diseases including NASH, fibrosis, cirrhosis and HCC. Table 1 summarizes the important findings regarding the development of these techniques in the context of liver-targeted delivery of miR-122. Significant interest has been generated surrounding the development of miRNA-based therapeutics, as expression of miR-NAs is deregulated in almost all types of liver disease, including cancer [7, 8, 47]. Many novel technologies are being developed to express or deplete specific miRNAs in the diseased cell types or organs (reviewed in [48, 49]). Their relatively small size and their ability to regulate a large number of cellular targets involved in multiple cellular pathways have made miRNAs attractive targets for therapy. Furthermore, the ability of certain miRNA mimics or antimirs to modulate the sensitivity of cancer cells to chemotherapeutic drugs suggests their potential application in combination therapy [18, 50, 51]. The technology to block miRNA expression is better developed than that to restore expression because chemically modified single-stranded oligonucleotides with high affinity binding to specific miRNA and with stability in biological fluids have been developed, however their delivery to specific cell types or tissues is still a major challenge. In fact, antagomirs (cholesterol conjugated chemically modified anti-mir oligonucleotides) [26] and LNA-anti-mirs (locked nucleic acid modified anti-sense oligonucleotides) [35] were effectively delivered systemically and subcutaneously, respectively, in the absence of a vehicle. In contrast, the delivery of a miRNA mimic is more challenging because they need to be double-stranded or contain a stem-loop structure of pre-miRNA, rendering them more prone to RNase degradation while in circulation. Thus, miRNA mimic oligonucleotides require vehicles for delivery in vivo to resist degradation. Furthermore, unlike anti-mirs, miRNA mimics could not be extensively modified as modifications might inhibit dissociation of guide and passenger strands hindering their loading on the miRISC complex, which is essential for suppressing their targets. Various vehicles or chemical modifications were designed and tested in cultured cells, experimental animal models, and recently in human patients. There are some excellent reviews on miRNA-based therapeutics [48, 49]. Here, we will focus on different delivery methods established for miR-122 mimics and antimirs.
Table 1.
Summary of selected studies on the in vivo delivery of miR-122 mimics, inhibitors or gene.
| Delivery Media | Physiological/Therapeutic Effect | Animal Model | Methods of Administration | Ref. |
|---|---|---|---|---|
| 2′-O-Me, phosphorothioate, 3′-cholesterol conjugated antimir | Hypocholestrolemia | Chow-fed mice | Intravenous | [26] |
| 2′-MOE, phosphorothioate antimir | Hypocholestrolemia and hypothriglyceridemia | High fat diet-fed obese mice | Intraperitoneal | [33] |
| LNA:DNA mixmer phos-phorothioate modified antimir (Miravirsen) | Hypocholestrolemia | African green monkeys | Intravenous/Intraperitoneal | [35] |
| Miravirsen | Iron homeostasis | Chow-fed mice | Intraperitoneal | [32] |
| Miravirsen | Reduced viral load | HCV infected Chimpanzees | Intravenous | [60] |
| Miravirsen | Undetectable or reduced viremia, no dose related toxicity | HCV (type 1) infected humans | Subcutaneous | [15] |
| rAAV9 expressing miR-122 TuD RNA | Hypocholesterolemia | Chow-fed mice | Intravenous | [58] |
| miR mimic/LNP-DP1 (cationic lipid nanoparticle) | Targeting miRNA to tumor cells | DEN-induced HCC in mice | Intravenous | [55] |
| rAAV8 expressing miR-122 | Dramatic reduction in liver tumor burden | Myc-induced liver tumor (Hepatoblastoma) | Intravenous | [16] |
Viral Delivery of the miR-122 Gene to the Liver and Tumors
Both viral and nonviral vehicles have been developed for the delivery of miR-122. Among different viral vectors, the AAV8 serotype showed the most promising results for gene therapy in the liver as it has a strong tropism for liver and can be administered intravenously [52]. The risk of insertional mutagenesis is minimal because most AAVs do not integrate into the host genome. The advantage of viral delivery is that a single administration of recombinant viral particles generates sustained, robust expression of a specific miRNA in post-mitotic cells [16, 53]. However, in replicating cells, the expression of the transgene drops with time due to the loss of replication-defective episomal viral DNA. The self-complementary AAV (scAAV) harboring a human Mir-122 mini-gene encoding a pri-miRNA fragment downstream of a strong pol II promoter, EF1a [16] was generated (Fig. 3A) that restored liver miR-122 level in the knockout mice comparable to the wild type level (data not shown). The advantage of scAAV is that the synthesis of the necessary second strand is independent of host cell DNA replication machinery [52]. This recombinant AAV8 dramatically inhibited tumor development in a c-Myc-induced liver cancer model, which exhibits silencing of miR-122 (Fig. 3B–D) [16]. However, one limitation of this method is that the AAV8 could not transduce larger tumors.
Non-Viral Delivery of miR-122 to Liver Cancer
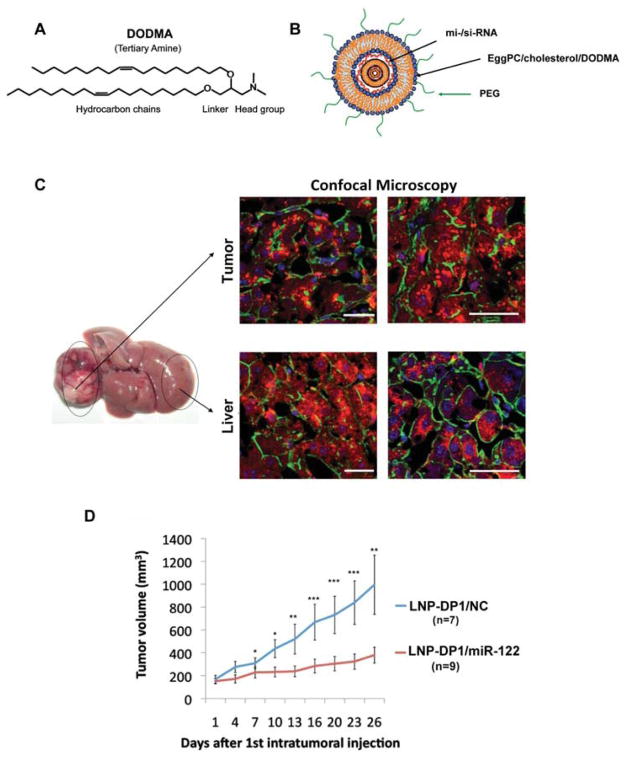
Although AAV8 mediated delivery generated high levels of miR-122 in the liver, non-viral systems are still considered safer because AAV8 can elicit several adverse effects, such as activation of the immune response, mutagenesis of the host genome due to occasional integration of the viral DNA and possible liver toxicity if delivered at high titer [54]. As an alternative approach, we developed LNP-DP1, a cationic lipid (DODMA) - based nanoparticle (Fig. 4A), for delivering miR-122 mimic (from Dharmacon) to HCCs developed in mice treated with diethylnitrosamine (DEN), a chemical carcinogen [55]. The advantage of the positively charged liposomal nanoparticles (LNPs) is that they can easily encapsulate negatively charged miRNAs forming lipo plexes (Fig. 4B) that stabilize miRNAs in blood circulation, facilitate uptake of anionic miRNAs through the negatively charged cell membranes and promote the release of encapsulated miRNAs from an endosome to the cytoplasm of target cells.
Fig. (4).
In vivo delivery of miR-122 mimic-loaded LNP-DP1 in DEN-induced HCCs developed in miR-122 KO mice (from reference [55]). A. The structure of the cationic lipid DODMA. B. The schematic representation of miRNA encapsulated by PEG modified LNP-DP1. C. Confocal microscopic imaging of Cy3-labeled siRNA formulated in LNP-DP1 in the benign liver and tumor tissues in a tumor-bearing miR-122 KO mouse. Four hours after intravenous administration of Cy3-labeled siRNA (2.5 mg/kg) tissues were processed for confocal microscopy. Red: Cy3-labeled siRNA; Green: cell outline (Phalloidin stained actin filament); Blue (DAPI): nucleus. Scale bars: 40μm. Red: Cy3-labeled siRNA; Yellow: collagen fibers; Blue: nucleus. Scale bars: 40μm. D. Growth curves of HCC xenograft tumors in nude mice intra-tumorally injected twice a week with LNP-DP1 loaded with NC miRNA (LNP-DP1/NC) or miR-122 (LNP-DP1/miR-122) at 10 μg per injection. P-value: *, P < .05; **, P < .01; ***, P < .001.
As a result, LNP-DP1 mediated Cy3 labeled si-/mi-RNA uptake was efficient not only in hepatocytes of the adjacent benign liver, but also in tumor cells (Fig. 4C). LNP-DP1 was highly effective in delivering miRNA to primary tumors developed using the DEN-induced HCC model in mice, which closely mimics human HCC, and was much more efficient than Invivofectamine (Life Technologies) in delivering si-/miRNA to tumor tissues [55]. LNP-DP1 formulated miR-122 was also effective in suppressing subcutaneous tumor growth in xenografts in immune-compromised mice (Fig. 4D) [55]. Systemic delivery of miR-122 in this model was not very effective due to the high deposition of extracellular matrix that impeded neovascularization in these tumors. Targeting of miR-122 to tumor cells might be further improved by tagging LNP-DP1 with specific targeting ligands, for example with SP94, a novel peptide that binds specifically to HCC cells [56] or with N-lactobionyl-dioleoyl phosphati-dylethanolamine (Lac-DOPE), a ligand for the asialoglyco-protein receptor (ASGR), which is expressed at a high levels in HCC cells [57]. Another goal may be to modify the zeta potential of LNP-DP1 so that it can form complexes to deliver small molecule drugs to HCC cells as part of a combination therapy with miR-122 mimic.
Delivery of miR-122 Inhibitors
The delivery of miRNA inhibitors depends heavily on the chemical modification of the inhibitors. miR-122 was one of the first miRNAs that could be effectively depleted in vivo by systemic delivery of chemically modified ASOs. As discussed in an earlier section, a miR-122 antagomir effectively depleted miR-122 in vivo [26]. However, a very high dose (80 mg/Kg) of antagomir was needed to inhibit miR-122 function in mouse livers. Several chemical modifications of the oligonucleotide have been developed to improve specificity, stability and delivery of anti-mirs to target cells/tissues (Table 1). Most of these modifications are at the 2′ position of the ribose ring as in 2′-fluoro (2′-F), 2′-O-methyl (2′-OMe), 2′-O-methyoxyethyl (2′-MOE), or 2′,4 -methylene′ (locked nucleic acid or LNA). These modifications serve to increase the binding affinity of an anti-mir to a specific miRNA, and increase its resistance to degradation by blood and tissue ribonucleases [48, 49]. Notably, recombinant AAV9 virus expressing miR-122 tough decoy (TuD) with two single stranded miR-122 binding sites flanked by two double-stranded stems, was highly specific and effective in long-term silencing of miR-122 in mice [58] (Table 1).
Miravirsen or SPC3649 is an LNA-, 2′-O-methyl- and phosphorothioate - modified 15 nucleotide (mer) LNA-DNA mixmer anti-miR-122 oligo, developed by Santaris Pharma. It effectively inhibited miR-122 function in vivo at a relatively low dose (2–10mg/kg) in the absence of a delivery system indicating high stability and specificity [15]. Miravirsen was the first miRNA - based therapeutic agent shown to be successful in clinical trials in HCV infected patients [15]. Subsequently, it was shown that an 8 mer LNA-anti-mir encompassing only the seed sequence (nucleotide 2–9) of miR-122 exhibited a much higher affinity compared to SPC3649 and demonstrated lower off-target effects [59], with a broad distribution throughout different organs after systemic delivery. The application of this LNA-antimir was first tested in chimpanzees chronically infected with HCV, which lowered viremia without causing generation of resistant mutants [60]. However, recent studies in knockout mice [16, 17] and human HCC patients [9, 19] have raised the concern that long-term inhibition of miR-122 might compromise liver function, lead to the development of NASH, or to metastatic HCC. Therefore, HCV infected patients undergoing Miravirsen therapy should be routinely monitored for liver functions and cancer development.
SUMMARY AND FUTURE DIRECTIONS
In 1993, when lin-4, the first microRNA, was discovered in the worm Caenorhabditis elegans [61], it was hard to conceive that in the years to come miRNAs would have a demonstrated involvement in crucial life processes and human disease states. miR-122 has a multitude of roles in metabolism, inflammation, viral/protozoan infection, and tumor suppression. Identification of miR-122’s targets and understanding the mechanisms by which specific targets modulate these functions may lead to new discoveries in liver biology and disease. The miR-122 knockout mouse is an ideal tool for furthering our knowledge of metabolism, liver fibrosis, tumorigenesis, and metastasis in vivo.
Modulating microRNAs in disease states, especially cancer, represents a promising frontier for pharmacotherapy that may be used in conjunction with classical chemotherapy to control consequences of deregulated miRNA levels within cells. Therapeutically targeting miR-122 may prove beneficial in patients with HBV/leishmania infection, in patients with HCV negative HCC, and even in preventing the development of NASH and cirrhosis, and pre-cancerous states in predisposed individuals. Similarly, development of technologies to directly quantify circulating miR-122, a specific biomarker for liver injury, will be a major breakthrough in diagnosis and prognosis of many liver diseases. However, many challenges remain in developing effective systems for the delivery of the miR-122 gene or mimics that can be taken to the clinic. The challenges include the cost of microRNA mimic and the delivery method. For delivery of miRNA using AAV vector, hurdles include variation in tissue tropism of the vector between humans and mice, efficiency of miRNA expression, immune response to viral proteins and dose-dependent toxicity of the vector in patients. Another major obstacle in implementing AAV8 mediated miRNA gene therapy is the loss of non-replicating episomal viral genome in rapidly proliferating cancer cells, causing rapid decline in recombinant miRNA gene expression (reviewed in [62]). In contrast, critical factors for in vivo liposome-mediated miRNA mimic delivery include nanoparticle size, charge, release from endosomes, and proper loading to the RISC complex to exert its function.. Furthermore, for proper loading to the RISC complex, it is essential that the miRNA mimic be double stranded. This makes mimic therapy much more expensive than anti-mir. For anti-mir delivery, minimizing off-target effect is crucial. Additionally, optimization of the delivery route such as intraportal or trans-arterial is also critical for successful miRNA therapy in HCC patients (reviewed in [49]).
Acknowledgments
This study was supported by grants (R01CA086978 and R01CA193244) from NIH and Pelotonia IDEA to K.G.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
Send Orders for Reprints to reprints@benthamscience.ae
References
- 1.Chang J, Nicolas E, Marks D, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1(2):106–13. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 2.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12(9):735–9. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 3.Landgraf P, Rusu M, Sheridan R, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–14. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wienholds E, Kloosterman WP, Miska E, et al. MicroRNA expression in zebrafish embryonic development. Science. 2005;309(5732):310–1. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 5.Filipowicz W, Grosshans H. The liver-specific microRNA miR-122: biology and therapeutic potential. Prog Drug Res. 2011;67:221–38. [PubMed] [Google Scholar]
- 6.Jopling C. Liver-specific microRNA-122: Biogenesis and function. RNA Biol. 2012;9(2):137–42. doi: 10.4161/rna.18827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu SH, Ghoshal K. MicroRNAs in Liver Health and Disease. Curr Pathobiol Rep. 2013;1(1):53–62. doi: 10.1007/s40139-012-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10(9):542–52. doi: 10.1038/nrgastro.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28(40):3526–36. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Wu C, Che X, et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50(2):136–42. doi: 10.1002/mc.20712. [DOI] [PubMed] [Google Scholar]
- 11.Ward J, Kanchagar C, Veksler-Lublinsky I, et al. Circulating microRNA profiles in human patients with acetaminophen hepatotoxicity or ischemic hepatitis. Proc Natl Acad Sci U S A. 2014;111(33):12169–74. doi: 10.1073/pnas.1412608111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antoine DJ, Dear JW, Lewis PS, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58(2):777–87. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jopling CL. Regulation of hepatitis C virus by microRNA-122. Biochem Soc Trans. 2008;36(Pt 6):1220–3. doi: 10.1042/BST0361220. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Xu Y, Hao J, Wang S, Li C, Meng S. MiR-122 in hepatic function and liver diseases. Protein Cell. 2012;3(5):364–71. doi: 10.1007/s13238-012-2036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 16.Hsu SH, Wang B, Kota J, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122(8):2871–83. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai WC, Hsu SD, Hsu CS, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122(8):2884–97. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai S, Nasser MW, Wang B, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284(46):32015–27. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai WC, Hsu PW, Lai TC, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49(5):1571–82. doi: 10.1002/hep.22806. [DOI] [PubMed] [Google Scholar]
- 20.Cheung O, Puri P, Eicken C, et al. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48(6):1810–20. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takaki Y, Saito Y, Takasugi A, et al. Silencing of microRNA-122 is an early event during hepatocarcinogenesis from nonalcoholic steatohepatitis. Cancer Sci. 2014;105(10):1254–60. doi: 10.1111/cas.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatfield D, Le Martelot G, Vejnar CE, et al. Integration of microRNA miR-122 in hepatic circadian gene expression. Genes Dev. 2009;23(11):1313–26. doi: 10.1101/gad.1781009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H, He JH, Xiao ZD, et al. Liver-enriched transcription factors regulate microRNA-122 that targets CUTL1 during liver development. Hepatology. 2010;52(4):1431–42. doi: 10.1002/hep.23818. [DOI] [PubMed] [Google Scholar]
- 24.Laudadio I, Manfroid I, Achouri Y, et al. A feedback loop between the liver-enriched transcription factor network and miR-122 controls hepatocyte differentiation. Gastroenterology. 2012;142(1):119–29. doi: 10.1053/j.gastro.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 25.D’Ambrogio A, Gu W, Udagawa T, Mello CC, Richter JD. Specific miRNA stabilization by Gld2-catalyzed monoadenylation. Cell Rep. 2012;2(6):1537–45. doi: 10.1016/j.celrep.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krutzfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438(7068):685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 27.Davoodian N, Lotfi AS, Soleimani M, Mowla SJ. MicroRNA-122 overexpression promotes hepatic differentiation of human adipose tissue-derived stem cells. J Cell Biochem. 2014;115(9):1582–93. doi: 10.1002/jcb.24822. [DOI] [PubMed] [Google Scholar]
- 28.Deng XG, Qiu RL, Wu YH, et al. Overexpression of miR-122 promotes the hepatic differentiation and maturation of mouse ESCs through a miR-122/FoxA1/HNF4a-positive feedback loop. Liver Int. 2014;34(2):281–95. doi: 10.1111/liv.12239. [DOI] [PubMed] [Google Scholar]
- 29.Doddapaneni R, Chawla YK, Das A, Kalra JK, Ghosh S, Chakraborti A. Overexpression of microRNA-122 enhances in vitro hepatic differentiation of fetal liver-derived stem/progenitor cells. J Cell Biochem. 2013;114(7):1575–83. doi: 10.1002/jcb.24499. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125(6):1111–24. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Kojima S, Gatfield D, Esau CC, Green CB. MicroRNA-122 modulates the rhythmic expression profile of the circadian deadenylase Nocturnin in mouse liver. PLoS One. 2010;5(6):e11264. doi: 10.1371/journal.pone.0011264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castoldi M, Vujic Spasic M, Altamura S, et al. The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice. J Clin Invest. 2011;121(4):1386–96. doi: 10.1172/JCI44883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esau C, Davis S, Murray SF, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3(2):87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Hsu SH, Wang B, Kutay H, et al. Hepatic loss of miR-122 predisposes mice to hepatobiliary cyst and hepatocellular carcinoma upon diethylnitrosamine exposure. Am J Pathol. 2013;183(6):1719–30. doi: 10.1016/j.ajpath.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elmen J, Lindow M, Schutz S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452(7189):896–9. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 36.Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8(1):35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 37.Padgett KA, Lan RY, Leung PC, et al. Primary biliary cirrhosis is associated with altered hepatic microRNA expression. J Autoimmun. 2009;32(3–4):246–53. doi: 10.1016/j.jaut.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kutay H, Bai S, Datta J, et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem. 2006;99(3):671–8. doi: 10.1002/jcb.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Yu B, Ren W, et al. Enhanced hepatic delivery of siRNA and microRNA using oleic acid based lipid nanoparticle formulations. J Control Release. 2013;172(3):690–8. doi: 10.1016/j.jconrel.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang B, Majumder S, Nuovo G, et al. Role of microRNA-155 at early stages of hepatocarcinogenesis induced by choline-deficient and amino acid-defined diet in C57BL/6 mice. Hepatology. 2009;50(4):1152–61. doi: 10.1002/hep.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436(7053):953–60. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- 42.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309(5740):1577–81. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Qiu L, Yan X, et al. Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G(1)-modulated P53 activity. Hepatology. 2012;55(3):730–41. doi: 10.1002/hep.24809. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Shen A, Rider PJ, et al. A liver-specific microRNA binds to a highly conserved RNA sequence of hepatitis B virus and negatively regulates viral gene expression and replication. FASEB J. 2011;25(12):4511–21. doi: 10.1096/fj.11-187781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan CG, Wang CM, Tian C, et al. miR-122 inhibits viral replication and cell proliferation in hepatitis B virus-related hepatocellular carcinoma and targets NDRG3. Oncol Rep. 2011;26(5):1281–6. doi: 10.3892/or.2011.1375. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh J, Bose M, Roy S, Bhattacharyya SN. Leishmania donovani targets Dicer1 to downregulate miR-122, lower serum cholesterol, and facilitate murine liver infection. Cell Host Microbe. 2013;13(3):277–88. doi: 10.1016/j.chom.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gambari R, Fabbri E, Borgatti M, et al. Targeting microRNAs involved in human diseases: a novel approach for modification of gene expression and drug development. Biochem Pharmacol. 2011;82(10):1416–29. doi: 10.1016/j.bcp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Broderick JA, Zamore PD. MicroRNA therapeutics. Gene Ther. 2011;18(12):1104–10. doi: 10.1038/gt.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13(8):622–38. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 50.Fornari F, Gramantieri L, Giovannini C, et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69(14):5761–7. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- 51.Miller TE, Ghoshal K, Ramaswamy B, et al. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283(44):29897–903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genetics. 2011;12(5):341–55. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 53.Kota J, Chivukula RR, O’Donnell KA, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–17. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–65. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu SH, Yu B, Wang X, et al. Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomedicine. 2013;9(8):1169–80. doi: 10.1016/j.nano.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lo A, Lin CT, Wu HC. Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol Cancer Ther. 2008;7(3):579–89. doi: 10.1158/1535-7163.MCT-07-2359. [DOI] [PubMed] [Google Scholar]
- 57.Zhang M, Zhou X, Wang B, et al. Lactosylated gramicidin-based lipid nanoparticles (Lac-GLN) for targeted delivery of anti-miR-155 to hepatocellular carcinoma. J Control Release. 2013;168(3):251–61. doi: 10.1016/j.jconrel.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie J, Ameres SL, Friedline R, et al. Long-term, efficient inhibition of microRNA function in mice using rAAV vectors. Nat Methods. 2012;9(4):403–9. doi: 10.1038/nmeth.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Obad S, dos Santos CO, Petri A, et al. Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet. 2011;43(4):371–8. doi: 10.1038/ng.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327(5962):198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee RC, Feinbaum RL, Ambros V. The C.elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 62.Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genetics. 2014;15(7):445–51. doi: 10.1038/nrg3742. [DOI] [PMC free article] [PubMed] [Google Scholar]