Abstract
Background
Genomic research is challenging the tradition of informed consent. Genomic researchers in the United States, Canada, and parts of Europe are encouraged to use informed consent to address the prospect of disclosing individual research results (IRRs) to study participants. In the U.S., no national policy exists to direct this use of informed consent, and it is unclear how local Institutional Review Boards (IRBs) may want researchers to respond.
Objective and Methods
To explore publicly accessible IRB websites for guidance in this area, using summative content analysis.
Findings
Three types of research results were addressed in 45 informed consent templates and instructions from 20 IRBs based at centers conducting genomic research: 1) IRRs in general 2) incidental findings (IFs), and 3) a broad and unspecified category of, “significant new findings” (SNFs). IRRs were more frequently referenced than IFs or SNFs. Most documents stated that access to IRRs would not be an option for research participants. These nondisclosure statements were found to coexist in some documents with statements that SNFs would be disclosed to participants if related to their willingness to participate in research. The median readability of template language on IRRs, IFs, and SNFs was greater than a 9th grade level.
Conclusion
IRB guidance may downplay the possibility of IFs, and contain conflicting messages on IRR nondisclosure and SNF disclosure. IRBs may need to clarify why separate IRR and SNF language should appear in the same consent document. The extent of these issues, nationally and internationally, needs to be determined.
INTRODUCTION
Widespread interest and concern surrounds the prospect of disclosing to research participants the results of studies employing genome wide association, microarray, whole genome and exome sequencing, and other genomic technologies. Research results may be categorized as either general study results, which represent aggregate data usually published by the research team, or individual results, which are research findings relevant to particular participants.[1] This paper focuses on individual research results (IRRs) as well as incidental findings (IFs), which are research findings that were not the “direct object” of the study, [2] but that nonetheless may have potential health or reproductive importance for a research participant.[3–5] IFs have surfaced over the course of medical tests, surgeries, and medical imaging procedures for many years [6], and have more recently become an issue in genetic and genomic research.[7–9] A diverse terminology has been used to refer to IRRs and IFs, and consensus on usage has yet to emerge.[10]
A key possible goal of disclosing IRRs and IFs to research participants is to provide participants with information that can lead to timely preventative care or treatment.[11] In certain cases, this information can be life saving. However, IRR and IF disclosures can also be impractical and costly. They may be emotionally upsetting or falsely reassuring. They could lead to loss of privacy, confidentiality, and medical insurability, and possible social discrimination and stigmatization.[12, 13] In the research context, these outcomes could be considered potentially harmful to research participants, and therefore relevant to the “risk-benefit ratio” that is used to weigh the ethical acceptability of research.
The prospect of disclosing IRRs and IFs with associated risks and benefits has led to recommendations that informed consent processes should address this prospect, where applicable, so that people can factor it into their decisions about research participation. [7, 8] Yet, there is no broad consensus on whether or how informed consent processes should be used to these ends. There is an inherently limited amount of information that can be conveyed in a prospective consent process with respect to IFs. Participant disclosure preferences may also change over time and raise questions about sustaining the validity of an informed consent.[14] More fundamentally, it is not clear to what extent current federal informed consent regulations apply, or should apply, to the unique prospects of genomic research.
Researchers and research participants may be confused in cases where established informed consent language converges with newer ways of describing genetic and genomic research. For example, in the US “Common Rule” regulations governing informed consent, there is a requirement that, where appropriate, individuals be informed that “significant new findings developed during the course of the research which may relate to the subject’s willingness to continue participation will be provided to the subject”.[15] In other countries, including the UK and Canada, similar provisions are included for informed consent processes (for example, see http://www.rcn.org.uk/__data/assets/pdf_file/0010/78607/002267.pdf; http://www.hc-sc.gc.ca/sr-sr/advice-avis/reb-cer/applic-demande/_form_docs/e-eng.php).
Do these older provisions for disclosing significant new findings (SNFs) apply also to genetic or genomic IRRs and IFs? Some legal scholars believe so.[13] Yet, if this is the case, why require new language in informed consent documents concerning the disclosure of IRRs and IFs? Or, if new and different language is deemed necessary, why keep the more established language on SNFs? The proposal to reform key elements of the US Common Rule, including elements of informed consent, is in part driven by developments in genetic or genomic research.[16] Yet, the proposal does not currently address how converging categories of research findings such as IRRs, IFs, and SNFs should be distinguished in order to promote a clear and consistent informed consent message on the topic of results (non)disclosure.
In the absence of policy and regulations on how exactly informed consent processes should address the prospect of IRRs and IFs, the response of institutional review boards (IRBs) charged with protecting human research participants may be a critical indicator. In the U.S., IRBs not only review informed consent processes prior to study approval but are permitted to require that participants be given additional information, when in the IRB’s judgment this information would be protective of their rights or welfare.[13] This exploratory study sought to determine if and how IRBs in the U.S. are guiding genomic researchers with respect to informed consent and the prospect of IRRs, IFs, and, for reasons just discussed, SNFs as well.
METHODS
Data for this study were obtained from online, publicly available IRB documents, accessed between January 5, 2011 and April 21, 2011. A preliminary analysis of ten IRB websites was conducted to ascertain the feasibility of searching for and analyzing online informed consent guidance for content on IRRs, IFs, and SNFs. This analysis indicated that we could expect to locate two or more relevant documents per website approximately 10 pages each in length. Given project capacity, the decision was made to limit this exploratory study to material gathered from a total of 20 IRB websites.
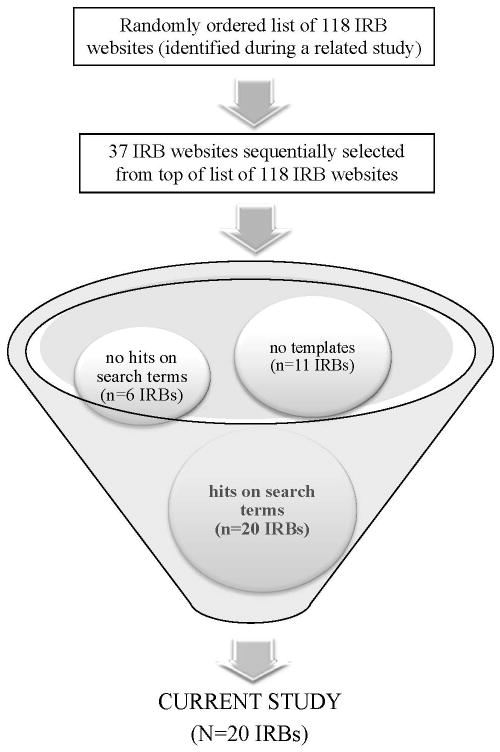
These 20 websites were located in the following way. First, we randomly ordered a list of 118 IRBs located at US institutions known to be conducting genome wide association studies (GWAS). GWAS are a first-generation genomic research study that emerged subsequent to the complete mapping of the human genome. They have led to the discovery of thousands of genetic variants contributing to variability in a range of common diseases including diabetes, cancer, and cardiovascular disease.[17] GWAS foreshadow the vast increases expected in research data through application of other genomic technologies and methods.
A list of 118 IRBs at GWAS-active centers was compiled through a systematic search of governmental and other resources (for further details see, Simon et al., in press).[14] IRBs needed to have publicly accessible websites in order to make the list. For the present study, IRBs were sequentially selected from the top of this randomly ordered list. A team member (who is also an IRB member) then visited the selected IRB’s website and explored its navigation panels for internal links to informed consent materials. If no materials were evident, the next IRB on the list of 118 IRBs was selected. If materials were found, these were electronically searched using a series of first-level search terms designed to determine if the materials contained any references likely to be relevant to the study (see Table 1). If materials yielded no “hits” on these first-level search terms, the IRB was replaced by the next IRB on the list of 118 IRBs. A total of 37 IRB websites needed to be searched before the desired sample of 20 IRB websites was reached. Figure 1 provides an illustrative overview of this sampling process.
Table 1.
Search terms for identifying content relevant to study
| Search Term Levels | Search Terms | Objective |
|---|---|---|
| First level | “DNA” “gene(s)” “genetic(s)” “genome(s)” “genomic(s)” |
Identify informed consent templates or instructions with content relevant to genetics and/or genomics. |
| Second level 1. | “abnormal” “accidental” “CLIA” “clinical laboratory improvement amendments” “disclose” “discover” “disseminate” “incidental” “new finding” “new findings” “new information” “paternity” “pedigree” “results” “unanticipated” “unexpected” “unforeseen” “unintentional” “unrelated” “unusual” |
Identify content in informed consent template or instructions relevant to individual genetic or genomic research results, genetic or genomic incidental findings, and “significant new findings.” |
Second-level search terms were compiled through a review of terminology used in literature and conference meetings on return of results. Term stems as listed in the table as well as their variants (e.g. “disclose,” “disclosed,” and “disclosure”) were searched.
Figure 1.
Sampling process for current study
Content Analysis
A summative content analysis (SCA) was conducted to explore the nature and frequency of content on IRRs, IFs, and SNFs. Content analysis in general is a flexible method for analyzing text data, and SCA is an approach for understanding word and text usage, rather than meaning.[18] In this study, an SCA was conducted of downloaded IRB documents in order to identify and characterize the frequency and context of IRB guidance on IRRs, IFs, and SNFs.
A total of 45 informed consent templates and instructions contained relevant material (average = 2.3 documents per website, range = 1–4 per website). The average length of documents was 10 pages (range 1–27). Documents consisted mostly of informed consent templates (N=41, 91%) or separate instructions for investigators (N=4, 9%). Documents were downloaded to NVivo 8.0 (QSR International Pty Ltd, 2008), a qualitative software program that assists in data management. They were then searched with a more extensive list of second-level terms (see Table 1) designed to pinpoint content directly relevant to genetic or genomic research results.
Coding
Document content identified by our second-level search was located and independently read through and coded by two study coders. Coders read the immediate paragraph as well as the broader sectional context containing each second-level hit. Two coders independently categorized all content as “references” to IRRs, IFs, or SNFs. Both coders and the study PI met routinely to review codes for accuracy.
Readability Scores
The readability of informed consent templates is one approximate measure for the comprehensibility of informed consent documents.[19] We measured the grade level readability of select template language using the Flesch-Kincaid scale provided by Microsoft Word 2003. The scale assesses readability on the basis of the average number of syllables in a word and average number of words in a sentence. Scale measurements include a minimal grade level required to read and understand English text (range, 0 to 12). The scale is frequently used to assess the grade level readability of consent templates and documents, and has demonstrated reliability and validity.[19, 20] Because of significant variability in the volume and content of language on IRRs, IFs, and SNFs, we analyzed only language focused on the issue of whether or not results would be disclosed to research participants.
Promoting data confidentiality
We took steps to deidentify our study data since online search engines can link verbatim extracts to the original document source, or IRB. Several search engine tests we ran showed this was possible. To minimize this potential, we opted to paraphrase, edit, or create composite illustrative extracts. We re-ran online searches on every edited exemplar included in this report to check that extracts could not be reliably linked to their original online sources.
RESULTS
Website and document description
Twenty (54%) of 37 searched IRB websites met the study’s eligibility criteria. These 20 websites represented IRBs at 20 different U.S. institutions (15 degree granting, 5 non-degree granting) engaged in national and/or international GWAS, 7 (35%) of which were located in the Northeast, 7 (35%) in the Midwest, 3 (15%) in the West, and 3 (15%) in the South. A quarter (25%) of the 20 IRBs serve institutions ranked in the top 25 NIH grant-funded institutions for 2010 (http://report.nih.gov/award/organizations.cfm?ot=&fy=2010&state=US&ic=&fm=&orgid=&view=stateorg&sumcol=fun&sumdir=desc).
References to IRRs, IFs, and SNFs
All references to IRRs, IFs, and SNFs were found in IRB documents that were explicitly genetic or genomic in focus (e.g., templates entitled, “Informed consent template for genetic tests/research studies”) or that contained components or instructions directed at genetic or genomic research (e.g., templates for clinical trials generally, with a section on genetic testing in research). References were distributed across the 20 IRB websites as follows:
References to IRRs
These were found in 39/45 (87%) documents and on all 20 (100%) websites. References to IRRs were typically framed as results about “you” or “the research participant,” thus distinguishing them from aggregate results. These IRRs were described as having “possible” or “potential” “importance,” “relevance,” or “significance” to the “health” or “wellbeing” of research participants, and, in the case of some documents, to their family. Out of 39 documents containing references to IRRs, almost half (N=19; 49%) included statements indicating that IRR disclosure was not an option, when compared to those indicating that disclosure was an option (N=10; 26%). Typical language indicating that IRR disclosure was an option included, “Even though the researchers are not looking to find or treat a medical problem, you will be contacted if we find a genetic test result that is important for you to know about.” Typical IRR nondisclosure language included, “You will not be provided with any results of the genetic tests you will be asked to undergo if you participate in this research.” Some IRB documents suggested that such nondisclosure statements could be accompanied by explanations that research was generally “not intended” for purposes of providing diagnostic information or clinical care, or that results might have “unknown value” and be “unreliable” at the current time. Ten (25%) documents provided researchers with the option of selecting either disclosure or nondisclosure language for their study, as was deemed appropriate.
References to IFs
These were found in 10 (22%) documents distributed over 6 (30%) websites, and typically referenced the possibility of uncovering unspecified types of IFs, for example, “Genetic information about you or your health that is unrelated to this study may be found through the tests we will do.” Three of the ten documents (30%) included statements indicating that IF disclosure was an option, for example: “You will be contacted if the researchers find any unusual or unexpected genetic test results in this research.” Six (60%) documents contained statements allowing researchers to select IF disclosure or nondisclosure language. Misattributed paternity was mentioned in five (11%) of the documents as a possible IF, for example, “Genetic tests may produce other information unrelated to this study, where the test may show that a parent is not the biological parent. This information will be kept confidential.”
References to SNFs
These were found in 26 (58%) IRB documents distributed over 15 (75%) websites and typically included statements such as, “you will be provided with any significant new findings or information discovered over the course of this research, which may affect your willingness to participate or continue participating in the study.” All (100%) SNF references indicated that disclosure would take place, assuming the findings were significant and could affect participant willingness to participate in the study. Only one SNF reference offered an explanation for when it would be appropriate to include this statement in a consent form, in this case, when research was “long term” and “interim information” was likely to surface over the course of the research.
Convergence of IRR and SNF language
Eleven (24%) of the 45 documents were informed consent templates that contained both IRR nondisclosure and SNF disclosure language. Typically, these two language sets appeared on different pages or in different sections of the template. For example, a template would state on one page that SNFs would be provided to participants if related to their willingness to participate or continue participating in the research, and on another that IRRs would not be disclosed. All but one of these templates (i.e., a tissue banking consent template) were general informed consent templates for clinical trials that included a genetic or genomic component.
Managing disclosure
Guidance on how IRR or IF disclosures would be managed was evident in nearly half (46%) of the documents relevant to studies with a disclosure option. IRBs provided language for addressing, or instructed researchers to address, who would be responsible for disclosing an IRR or IF, whether disclosures would be written, telephonic, or in person, and whether follow-up support services such as genetic counseling would be provided, and, if so, at whose expense (i.e., the institution’s, research study’s, or research participant’s).
Potential risks and benefits of disclosure
Guidance that explicitly addressed the potential for participants to benefit from the disclosure of genetic or genomic results was evident in 2% of relevant documents. This guidance included instructions or model language specifying that “receiving results may reduce uncertainty” about a genetic condition or help a participant “plan for the future.” Guidance on the potential risks of results disclosure was evident in 18% of relevant documents. These included reference to the risk of discovering “information about yourself or your family that you do not want to know” and the potential for nonpaternity findings or genetic disorders not yet manifested. “Anxiety,” “stress,” “interpersonal tension,” “family problems,” and “low self-esteem” were listed as possible outcomes of IRR or IF disclosure. Researchers were instructed to acknowledge the potential for IRR or IF disclosure to lead to “accidental” breaches of privacy and confidentiality and, resultantly, to pose a risk to “employability,” “insurability,” “immigration,” “paternity suits,” or “social reputation.” Ten (22%) of the 45 documents contained language on the Genetic Information Discrimination Act (GINA), a US federal law that generally makes it illegal for health insurance companies, group health plans, and most employers to discriminate against people based on their genetic information.
One IRB encouraged researchers to address both the potential risks of knowing and not knowing about the results of genetic tests taken during the course of a research study. In a consent form template drawn up specifically for studies involving genetic testing, the template states that there may be “risks” in not knowing about test results and that if participants opted not to be contacted with results, they may “miss out” on the benefit of detecting an increased risk of developing a “treatable problem.”
Contact preferences
Guidance on how researchers should provide participants with the option of indicating whether or not they wish to be contacted with news of an IRR or IF was provided in one third (32%) of relevant documents. This guidance typically stated, “If one of our genetic tests for this research revealed something about your health that may be important for you to know, may we contact you?” One informed consent template asked participants whether it was acceptable for the researchers to also share IRR or IFs with: 1. the individual’s doctor 2. a spouse or family member, and/or 3. some other person or persons. Individuals were asked to check each of these options, depending on their decision.
Readability of model language
Selected language on IRRs, IFs, and SNFs was analyzed for grade-level readability using the Flesch-Kincaid scale (see Methods). IRR content for 34 informed consent templates had a median grade-level readability score of 9.6 (range = 4.0–22.0). SNF content for 25 templates had a median score of 11.3 (range = 5.6–22.1), and IF content for seven templates had a score of 11.3 (range = 9.9–12.4).
DISCUSSION
State of IRB guidance
This exploratory study was conducted with a sample of IRBs based at US institutions engaged in GWAS. Study findings suggest that IRB guidance may be lacking on informed consent and IRRs and IFs in particular. Many experts as well as members of the public believe that researchers have an ethical obligation to return actionable IRRs.[7, 8] However, this view is not shared by everyone, nor is it feasible in the case of many large-scale genomic research efforts to enact this ethical obligation.[21]
Based on our study findings, many IRBs emphasize nondisclosure of IRRs and IFs. Some suggest that informed consent documents can rationalize nondisclosure by claiming that research is not intended to confer clinical benefits on participants. In our opinion, this would not be an appropriate claim to make in an informed consent document because it is currently highly contested. It has been countered, for example, by Beskow and Burke in their use of an ancillary care framework to argue that researchers have a duty to “rescue” study participants if genetic information is discovered that meets actionable criteria.[9] IRBs are on more solid ground by suggesting that concerns about IRR and IF validity and accuracy can help explain the decision not to disclose results as these are, indeed, widely shared concerns.[5, 8]
Converging discourses
The tradition of informed consent is governed in the U.S. and elsewhere by regulations largely developed prior to the advent of genomic research. Problems can ensue where informed consent language crafted to meet these established regulations converge with more recently crafted institutional-level language on IRRs and IFs. The broad, unspecified category of SNFs is standard language for informed consent processes, yet it is unclear how encompassing this category is or should be. The US Office for Human Research Protections has clarified only that, in its view, SNFs are a “type of new risk information”.[22] IRBs construe SNFs as new side effects of experimental drugs, changes in the frequency of side effects, major changes in study design, and changes in standard of care, such that subjects may be placed at more risk if participation in the study continues (see, for example, http://www.usc.edu/admin/oprs/private/docs/oprs/pnp/SNIF_may2011.pdf; http://www.slu.edu/Documents/provost/irb/Re-Consenting_Subjects.doc). Yet, given the unspecified nature of SNFs, they could be interpreted—including by individuals asked to read a consent document—to potentially include IRRs and IFs. It is unclear whether such an inclusive interpretation reflects the original intent of the existing regulations on SNFs. In consent documents stating that IRRs or IFs will not be returned to research participants, the inclusion of SNF disclosure language may come across as confusing and contradictory. Efforts to reform the US Common Rule include the proposal to reduce standard “boilerplate” informed consent language that does little to genuinely inform participants.[23] This proposal may need to also address the potential for confusion and contradiction where established SNF language appears alongside newer language on genetic and genomic IRRs.
Risks and benefits
More IRB documents in our study contained a statement on the potential risks associated with IRR or IF disclosure, when compared to the potential benefits. This difference may reflect the emphasis frequently placed in informed consent documents on the potential risks of research participation as opposed to the potential benefits, which are frequently framed as other-oriented and societal. Greater emphasis may need to be placed in informed consent documents on the potential benefits of IRR and IF disclosures, since the primary reason IRRs or IFs warrant disclosure lies in their capacity to benefit individuals through arming them with actionable medical or health information. At the same time, participants need to adequately grasp the relationship of potential harms to benefits of disclosure. The primary benefit associated with IRR/IF disclosures is the insight gained into one’s risk for a specific condition, while a key possible harm of disclosure is the surprise, shock, or emotional distress that may follow from this insight. Such psychological and emotional responses may be highly complex and variable from case to case. Yet, this would be true also of the psychological or emotional harms posed by other nongenomic research studies, and need not necessitate an unnecessary level of detail and speculation in an informed consent document. Consent documents should also explain that IRR and IF disclosures carry an inherent risk of other people or entities accessing and linking IRR or IF information to the identity of the research participant. Few documents that we analyzed explicitly made this connection.
While many people profess a theoretical desire to receive research results, some people may prefer not to receive them for personal, social, religious, or cultural reasons. Alongside the important message that the consequences of not accessing clinically actionable IRRs or IFs may be serious, informed consent documents may need to acknowledge this entitlement to nondisclosure, where relevant and applicable. In sum, explanations of the potential risks and benefits of IRR and IF disclosures is emerging in IRB guidance. Whether these explanations are adequately framed and detailed for participants to truly appreciate the stakes involved, however, is unclear. In particular, efforts are needed to ensure that risks and benefits of disclosure are not treated as disconnected “either/or” possibilities. They need to be framed relationally, such that people understand the inherently interconnected nature of the potential risks and benefits of IRR/IF disclosure.
Readability
Mean grade-level readability of research result language exceeded the 8th grade reading level aspired to by many IRBs.[19] In a recent study of consent forms for US and international HIV network trials sponsored by the NIH, Kass et al.[20] found that median grade-level readability of actual study consent forms was 9.2, with international site forms having lower (i.e., better) readability scores than US forms. The challenge of presenting and explaining scientific concepts in informed consent processes is well known, and will likely be amplified in the case of consent for genetic and genomic research.[25] Further work is needed to determine how best to manage the challenge of presenting IRR and IF informed consent language that meets IRB readability standards and public capabilities.
STUDY LIMITATIONS
Informed consent templates and documents have been analyzed with growing frequency for evidence of the nature and variability of research protection policy and guidance.[19] Data for this study were obtained exclusively from Web sites. Although it is likely that online IRB materials accurately reflect local IRB practices, [19] we did not solicit additional materials such as actual consent forms or IRB application forms for investigators, which may have contained additional information. Researchers sometimes modify template language, with the result that the content and readability of the actual consent document may change. Because our sample of IRBs were all associated with institutions conducting GWAS, our findings may not be reflective of the guidance that IRBs may be providing at other institutions, including where research studies are being conducted using next-generation sequencing.
CONCLUSION
IRBs in the US not only review informed consent processes prior to study approval, but are permitted to require that participants be given additional information, when in the IRB’s judgment this information would be protective of their rights or welfare.. IRBs vary in their interpretations according to institutional commitments and regulations, applicable law, and standards of professional conduct and practice.. IRBs may have limited capacity and uncertain impact in their efforts to develop and integrate effective, adequately detailed IRR and IF language into informed consent processes when technologies, guidelines, and best practices in this area are still evolving.
IRBs may benefit from multi-source input on the issue of integrating IRR and IF disclosure prospects into the tradition of informed consent. It may be critical that they receive assistance from expert consultants, national-level bodies, and public interest advocates before formalizing and routinizing local informed consent IRR and IF policy and guidance. IRBs also need recourse to empirical evidence on the kinds of IRR and IF information that people generally want and have the capacity to understand in an informed consent context. The degree to which newer IRB guidance on IRRs and IFs is compatible with established SNF language needs careful examination. These and related efforts may benefit in turn through international discussion and collaboration on best practices for appropriately informing prospective research participants about possible discovery and disclosure of individual-level genomic research results.
Table 2.
Distribution of IRR, IF, and SNF references by website
| IRB Websites | Individual Research Results (IRRs) | Incidental Findings (IFs) | Significant New Findings (SNFs) |
|---|---|---|---|
| 1 | ✓ | ✓ | |
| 2 | ✓ | ✓ | |
| 3 | ✓ | ✓ | |
| 4 | ✓ | ||
| 5 | ✓ | ||
| 6 | ✓ | ✓ | ✓ |
| 7 | ✓ | ✓ | |
| 8 | ✓ | ✓ | ✓ |
| 9 | ✓ | ✓ | |
| 10 | ✓ | ||
| 11 | ✓ | ✓ | |
| 12 | ✓ | ✓ | ✓ |
| 13 | ✓ | ✓ | |
| 14 | ✓ | ✓ | |
| 15 | ✓ | ✓ | |
| 16 | ✓ | ✓ | |
| 17 | ✓ | ✓ | |
| 18 | ✓ | ✓ | ✓ |
| 19 | ✓ | ✓ | |
| 20 | ✓ | ✓ |
Acknowledgments
The study was supported by an (ARRA) grant from the National Human Genome Institute of the National Institutes of Health (NIH) (RC1HG005786). Support was also provided by Grant Number TL1RR024981 (training support for DB) from the National Center for Research Resources (NCRR), a part of the NIH. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We are also grateful for the comments and suggestions of the anonymous reviewers of this manuscript.
References
- 1.Shalowitz DI, Miller FG. Disclosing individual results of clinical research: implications of respect for participants. 2005;294:737–740. doi: 10.1001/jama.294.6.737. [DOI] [PubMed] [Google Scholar]
- 2.Clayton EW. Incidental findings in genetics research using archived DNA. J Law Med Ethics. 2008;36:286–291. doi: 10.1111/j.1748-720X.2008.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office of Biorepositiories and Biospecimen Research (OBBR) [Accessed on: 2011 July 25];Workshop on release of research results to participants in biospecimen studies. workshop summary. http://biospecimens.cancer.gov/global/pdfs/NCI_Return_Research_Results_Summary_Final-508.pdf.
- 4.Office of Biorepositories and Biospecimen Research (OBBR) [Accessed on: 2011 June 14];NCI best practices for biospecimen resources [report] http://biospecimens.cancer.gov/global/pdfs/Revised%20NCI%20Best%20Practices_public%20comment.pdf.
- 5.Wolf SM. Introduction: The challenge of incidental findings. J Law Med Ethics. 2008;36:216–218. doi: 10.1111/j.1748-720X.2008.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illes J, Kirschen MP, Edwards E, et al. Ethics. Incidental findings in brain imaging research. Science. 2006;311:783–784. doi: 10.1126/science.1124665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bookman EB, Langehorne AA, Eckfeldt JH, et al. Reporting genetic results in research studies: Summary and recommendations of an NHLBI working group. Am J Med Genet A. 2006;140:1033–1040. doi: 10.1002/ajmg.a.31195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabsitz RR, McGuire A, Sharp RR, et al. Ethical and practical guidelines for reporting genetic research results to study participants: Updated guidelines from a National Heart, Lung, and Blood Institute working group. Circ Cardiovasc Genet. 2010;3:574–580. doi: 10.1161/CIRCGENETICS.110.958827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beskow LM, Burke W. Offering individual genetic research results: Context matters. Sci Transl Med. 2010;2:1–5. doi: 10.1126/scitranslmed.3000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoppers BM, Dam A. Return of results: towards a lexicon? J Law Med Ethics. 2011;39:577–582. doi: 10.1111/j.1748-720X.2011.00624.x. [DOI] [PubMed] [Google Scholar]
- 11.Roberts JS, Shalowitz DI, Christensen KD, et al. Returning individual research results: development of a cancer genetics education and risk communication protocol. J Empir Res Hum Res Ethics. 2010;5:17–30. doi: 10.1525/jer.2010.5.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dressler LG. Disclosure of research results from cancer genomic studies: state of the science. Clin Cancer Res. 2009;15:4270–4276. doi: 10.1158/1078-0432.CCR-08-3067. [DOI] [PubMed] [Google Scholar]
- 13.Wolf SM, Paradise J, Caga-anan C. The law of incidental findings in human subjects research: Establishing researchers’ duties. J Law Med Ethics. 2008;36:361–383. 214. doi: 10.1111/j.1748-720X.2008.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon C, Williams JK, Shinkunas L, et al. Informed consent and genomic incidental findings: IRB chair perspectives. J Empir Res Hum Res Ethics. 2011 doi: 10.1525/jer.2011.6.4.53. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services, Office for Human Research Protections. [Accessed on: 2011 December 15];Protection of Human Subjects, 45 C.F.R. § 46.116 (b) 2009 (5) http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html#46.116.
- 16.U.S. Department of Health and Human Services, Office for Human Research Protections. [Accessed on: 2011 December 15];Advanced Notice of Proposed Rule Making. 2011 http://www.hhs.gov/ohrp/humansubjects/index.html.
- 17.Johnson AD, Bhimavarapu A, Benjamin EJ, et al. CLIA-tested genetic variants on commercial SNP arrays: Potential for incidental findings in genome-wide association studies. Genet Med. 2010;12:355–363. doi: 10.1097/GIM.0b013e3181e1e2a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. 2005;15:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 19.Paasche-Orlow MK, Taylor HA, Brancati FL. Readability standards for informed-consent forms as compared with actual readability. N Engl J Med. 2003;348:721–726. doi: 10.1056/NEJMsa021212. [DOI] [PubMed] [Google Scholar]
- 20.Kass NE, Chaisson L, Taylor HA, et al. Length and Complexity of US and International HIV Consent Forms from Federal HIV Network Trials. J Gen Intern Med. 2011 Jul 6; doi: 10.1007/s11606-011-1778-6. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller FA, Christensen R, Giacomini M, et al. Duty to disclose what? Querying the putative obligation to return research results to participants. J Med Ethics. 2008;34:210–213. doi: 10.1136/jme.2006.020289. [DOI] [PubMed] [Google Scholar]
- 22.Office for Human Research Protections (OHRP) [Accessed on: 2011 September 20];Institutional review board review of protocol and informed consent changes [correspondence] http://www.hhs.gov/ohrp/policy/consent/nci200870929.html.
- 23.U.S. Department of Health and Human Services Office of the Secretary, Food and Drug Administration. Human subjects research protections: Enhancing protections for research subjects and reducing burden, delay, and ambiguity for investigators [proposed rules] [Accessed on: 2011 December 15];Federal Register. 2011 Jul 26;76:44512–44531. http://www.gpo.gov/fdsys/pkg/FR-2011-07-26/pdf/2011-18792.pdf. [Google Scholar]
- 24.Christensen KD, Roberts JS, Shalowitz DI, et al. Disclosing individual CDKN2A research results to melanoma survivors: Interest, impact, and demands on researchers. Cancer Epidemiol Biomarkers Prev. 2011;20:522–529. doi: 10.1158/1055-9965.EPI-10-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson GE. Is Informed Consent Broken? Am J Med Sci. 2011 Aug 3; doi: 10.1097/MAJ.0b013e31822a6c47. Published Online First. [DOI] [PubMed] [Google Scholar]