Abstract
Introduction:
The aim of this study was to establish the efficacy of cryoablation for incidentally discovered small renal cell carcinomas in older patients with medical comorbidities.
Methods:
We carried out a retrospective chart analysis of outcomes of 70 patients treated by cryoablation. The inclusion criteria were age >56 years, medical comorbidities (Charlson class I–III), and suitability for cryoablation established by urologists and interventional radiologists. In total, 43 patients were male, 27 female, and the age range was 56 to 89. The lesions measured 1.5 to 4 cm; 29 were high-grade Fuhrman and 41 were low grade. All lesions were treated by 2 10-minute freezing cycles separated by an 8-minute thawing period. One to seven cryoprobes were inserted according to a preoperative, 3D computed tomography (CT)-based plan.
Results:
Results were assessed on follow-up CTs (at 8–9 months). Of the 70 patients, 68 were treated by cryoablations and surgical salvage procedures; these patients were free of disease for 23 to 72 months (mean 39). One patient experienced recurrence and the other was lost to follow-up. One or two cryoablations rendered 66 patients tumour-free and additional surgery rendered another 2 patients tumour-free. The location and configuration of the lesion affected outcomes. Of the 27 posterior lesions, there was 1 failure; of the postero-lateral lesions, there were 4 failures; of the anterior lesions, there were 5 lesions; finally of the 32 central or deep seated lesions, there were 9 failures. Implants with one and two cryoprobes had a high recurrence rate. Three major complications were managed by minor interventions. The mean hospitalization was 1.3 days and the procedure times were variable.
Conclusion:
Percutaneous cryoablation is recommended as a minimally invasive nephron-sparing treatment for amenable lesions in older patients with medical comorbidities.
Introduction
A reassessment of current treatments for renal cell carcinoma (RCC), consisting principally of segmental resection or radical nephrectomy, is affecting patient survival and quality of life. The new World Health Organization reclassification assigns an increasing number of suspect mass lesions to the benign group, thereby reducing the number of RCCs.1 Moreover, statistical analysis has shown intercurrent disease to be the prevalent cause of death in older patients with medical comorbidities rather than the RCC.2 These pertinent facts suggest an increased role for surveillance or minimally invasive treatment modalities in this patient group.3–6 Cryoablation is one option; it can achieve cancer-specific survival in 96% to 100% of patients.7–11 We have undertaken a retrospective analysis of results of cryoablation in 70 older patients with medical comorbidities.
Methods
In total, 70 patients treated by cryoablation for amenable RCC lesions between November 2005 and February 2011 were part of our retrospective study. The diagnosis of mass lesions with malignancy characteristics was established by contrast-enhanced multidetector computed tomography (CT) or magnetic resonance imaging. The institutional review board approval was waved and informed consent was obtained from all patients. Urologists and interventional radiologists assessed patients and offered surgical treatment modalities, cryoablation or surveillance with appropriate supervision for each patient. The inclusion criteria for cryoablation were RCCs <4 cm in size, age >56, and particularly coexistent medical comorbidities. Patient age ranged from 56 to 89 (mean 73.2) and 43 patients were male and 27 female. One or more comorbidities were present in all 70 patients. In total, 53 patients were class II Charlson comorbidity index, 5 patients were class III, and 12 were class I. Hypertension was present in 67 patients, diabetes in 21, congestive heart failure in 6, cardio-pulmonary disease in 11, obesity in 8, renal calculi in 3, pulmonary emboli in 1, emphysema in 1, prior cerebro-vascular accident in 2, and hepato-renal syndrome in 1.
All RCCs were clinical stage T1a, N0M0; 29 RCCs were high grade (Fuhrman) and 41 were low grade. Twelve RCCs were <2 cm in diameter, 35 were 2 to 3 cm, and 23 were 3 to 4 cm. Thirty-three RCCs were in the posterior and postero-lateral locations and exophytic (>50% of circumference projecting outside the renal capsule), 5 in the anterior location, and 32 in the central and deep location (Table 1).
Table 1.
Relationship of tumour-free status attained after one cryoablation to Fuhrman grade and location of the mass
| Location | No. | Fuhrman grade | |
|---|---|---|---|
|
| |||
| High* | Low* | ||
| Posterior | 27 | 10 (1) | 17 (0) |
| Postero-lateral | 6 | 3 (3) | 3 (0) |
| Anterior | 5 | 4 (3) | 1 (1) |
| Central and deep | 32 | 12 (8) | 20 (1) |
| Total no. | 70 | 29 | 41 |
(n) number of failed cryoablations.
During the study period, 105 younger patients with suspicious renal masses were advised against thermal ablation by our urologists and interventional radiologists and offered laparoscopic or segmental resection. Of the other group of 68 patients, 42 were referred for laparoscopic and 26 for open cryoablation due to location and difficulty to access the lesions percutaneously.
Technique of cryoablation
In contrast to prior cryoablation studies, the positioning of cryoprobes was planned preoperatively based on axial, coronal, and volume-rendering images (General Electric high-speed, Milwaukee, WI; and Somatom 40 slice Siemens, Erlangen).5,7,12–17 The number of cryoprobes (2.4 and 3 mm, 4-cm freeze length, various shaft length, Endocare, Perc 24 system, Heathronics, Austin, TX) was determined by size, geometry, and morphology of the tumour to adequately cover the lesion with the resulting iceball. The probes were placed under CT guidance (contrast enhanced and 3D volume reconstruction) 1 to 1.5 cm apart, in a pattern akin to a radiation therapy implant, resulting in a freezing zone covering the lesion plus a 5-mm margin.5,7–11,15 Since cell-death is certain only within 3 mm of the iceballs margin (where a temperature of −20°C can be attained), a 5-mm safety margin is necessary.5,7–11,15–17 To attain this pattern, 1 probe was placed in 12, 2 probes in 16, 3 probes in 19, 4 probes in 15, 5 probes in 6, and 7 probes in 2 patients. The 7-probe-implant deployed the probes in 2 concentric rings, 3 and 4 respectively.
To prevent damage to adjacent structures (colon, duodenum, spleen, liver, pancreas and peritoneal lining) during the freezing cycle, we interposed a bolus of air, CO2, or saline.11,12,18,19 Under CT guidance, a catheter was introduced into the posterior para- or perirenal space using a 4-Fr micro-puncture set. We infused a 400 to 700 mL air, CO2 or saline until a 2-cm separation of critical structures and iceball were obtained (Fig. 1). Because of problems with conductivity, air was favoured over saline, though its rapid reabsorption may have required more frequent replenishing of the bolus.
Fig. 1a.
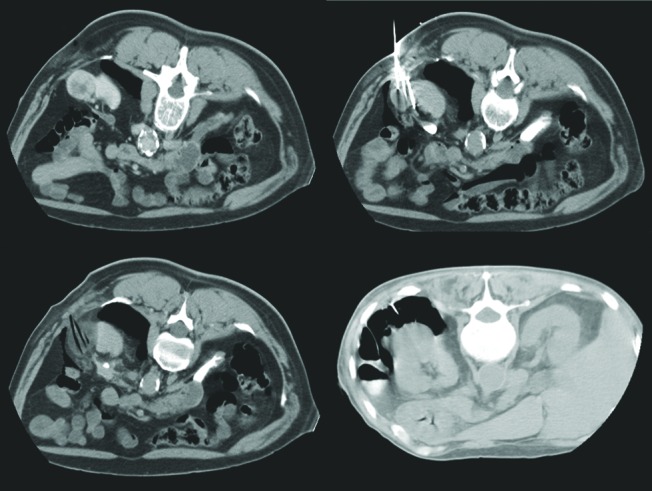
A computed tomography scan showing infusion of a 400 to 700 mL air, CO2 or saline until a 2-cm separation of critical structures and iceball were obtained.
Each mass was treated by two 10-minute freezing cycles, separated by an 8-minute thawing period. The double freeze-thaw cycle has been shown to increase liquefaction necrosis and hence improve efficacy.20,21 The interposed thawing causes cells to “burst” which is important to ensure cell death.5–11,20,21 During the freezing cycle, limited CTs are obtained every 3 to 4 minutes to affirm coverage of the lesion by the iceball.5,7,8,12,14,20 To identify possible “skip zones,” a contrast-enhanced CT was obtained after the second freeze cycle. In 5 patients the positions of 13 cryoprobes had to be adjusted or cryoprobes added to cover the “skip zone” by the iceball.
We performed follow-up CTs to assess for residual or recurrent disease for the first 43 patients 2 to 3 months after the initial cryoablation. Enhancement in the rim of the treated lesion was considered evidence of residual disease. However based on recent reports and our own experience, we dismissed early enhancement as a reliable finding of residual tumour. Therefore, in the remaining 27 patients, we performed the first follow-up examination 8 to 9 months after the cryoablation to eliminate false positives of inflammatory neovascularity.22,23
Results
Of the 70 patients treated for RCC, 68 were treated by cryoablation and some surgical salvage procedures; they were free of disease 23 to 72 months (mean 39) after completion of treatment. One patient was lost to follow-up and one patient is alive with recurrent metastatic disease currently on chemotherapeutic management with sunitinib, a tyrosine kinase inhibitor.
At the 3-month follow-up of the first 43 patients, 28 patients achieved tumour-free status as established by enhanced CTs (Table 2a). Of these 43, 3 patients were retreated by segmental resections for a recurrence suspected on the basis of the 3-month follow-up CTs; they were tumour-free on histopathology of the resected segment (Table 2a). In total of the first group of 43, 31 were tumour-free after the first cryoablation. Likely the observed recurrence in 15 patients was due to inflammatory neovascularity that often perseveres up to 6 months after cryoablation.22,23 Perfusion CT may offer criteria to differentiate inflammatory from neoplastic neovascularity.24 After removing the 3 patients with false positives, we determined that after the second cryoablation the remaining 12 patients presumed to have residual or recurrent disease achieved tumour-free status (Table 2a) (Fig. 2).
Table 2a.
Outcome of cryoablation and salvage procedures
| Sequence of intervention | Procedures | Outcome | |||
|---|---|---|---|---|---|
|
| |||||
| Free of disease | Recurrence | False positive | Lost to follow-up | ||
| First group: At 3 months follow-up | 43 cryoablations | 28 | 15 | 3 | |
| Second group: At 9 months follow-up | 27 cryoablations | 21 | 5 | 1 | |
| Total no. | 70 | 49 | 30 | 3 | 1 |
Fig. 2.
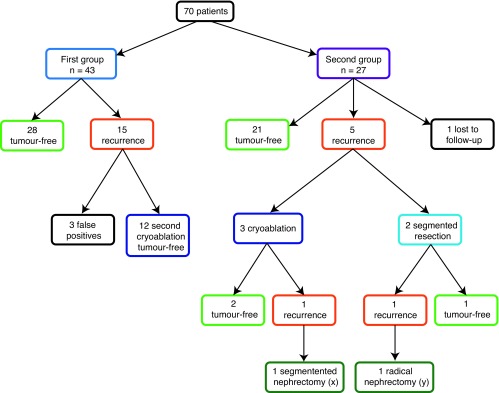
Patient outcomes.
In the second group of 27 patients, at the 9-month follow-up, 21 were tumour-free, 5 had recurrence and 1 patient was lost to follow-up (Table 2a). Of the 5 patients with recurrence, 3 had repeat cryoablations and 2 had segmental resections (Table 2b). Repeat follow-up CTs showed 1 recurrence after cryoablations and 1 recurrence after segmental resections (Table 2b). A radical nephrectomy rendered one of these patients tumour-free (follow-up 38 months) and a segmental resection failed to control the tumour in the other patient (Table 2b). This patient is now being followed with lung and brain metastasis under chemotherapy.
Table 2b.
Outcome of cryoablation and salvage procedures for the patients who recurred in the second group (n = 5)
| Sequence of intervention | Procedures | Free of disease | Recurrence | Lost to follow-up |
|---|---|---|---|---|
| First salvage intervention (n = 5) | 3 cryoablations | 2 | 1 (x) | |
| 2 segmental nephrectomies | 1 | 1 (y) | ||
| Second salvage intervention (n = 2) | 1 segmental nephrectomy (x) | 1 | ||
| 1 radical nephrectomy (y) | 1 |
In 65 patients the cryoprobes produced a satisfactory iceball covering the lesion. In 5 patients the position of 13 probes had to be adjusted to eliminate skip-zones.
Configuration, morphology, and geometry of the lesion greatly influenced the potential for a successful ablation. It was difficult to create an adequate iceball to cover small lesions with 1 or 2 cryoprobes.5–7,10,12,20,25–27 Hence the high failure rate of 38% in this group (8 of 21 patients). Conversely, when using 3 or more cryoprobes, the rate of failure dropped to 18% (9 of 49 patients) (Table 3).
Table 3.
Relationship of tumour-free status attained after cryoablation to the number of cryoprobes and high and low Fuhrman grade mass lesions
| Fuhrman grade | ||
|---|---|---|
|
| ||
| No. cryoprobes | High* | Low* |
| 1 | 4 (3) | 1 (1) |
| 2 | 5 (3) | 11 (1) |
| 3 | 10 (3) | 16 (0) |
| 4 | 5 (3) | 10 (0) |
| 5 | 4 (2) | 2 (0) |
| 7 | 1 (1) | 1 (0) |
| Total no. | 29 | 41 |
(n) number of failed cryoablations.
Location of the lesion was a major factor governing success or failure of the ablation procedure (p = 0.0001) (Table 4). Size had no significant impact on attaining tumour-free status (p = 0.3753). In 27 exophytic posterior lesions, we recorded only 1 failure, and this was in a lesion that was implanted with only 1 cryoprobe (Table 4). In anterior-located lesions we had 4 failures in 5 patients. However, again 1 patient had 1 cryprobe in the high-Fuhrman grade lesion. (Table 4). In the 32 lesions in the central and deep locations, we had 9 failures (28%). However, 4 failures occurred in patients in whom 1 or 2 cryoprobes had been used (Tables 4).
Table 4.
Relationship of tumour-free status attained after one cryoablation to cryoprobes deployed, size and location of mass lesion
| Location | |||||
|---|---|---|---|---|---|
|
| |||||
| No. cryoprobes | Size mass | Posterior* | Postero-lateral* | Anterior* | Central and deep* |
| 1 | <2 cm | 4 (1) | 2 (1) | 1 (1) | 5 (1) |
| 2 | 2–3 cm | 8 (0) | 2 (1) | 0 | 6 (3) |
| 3 | 2–3 cm | 7 (0) | 2 (1) | 2 (1) | 8 (2) |
| 4 | 3–4 cm | 3 (0) | 0 | 1 (1) | 11 (1) |
| 5 | 3–4 cm | 4 (0) | 0 | 1 (1) | 1 (1) |
| 7 | 3–4 cm | 1 (0) | 0 | 0 | 1 (1) |
| Total no. | 27 | 6 | 5 | 32 | |
(n) number of failed cryoablations.
Grade (Fuhrman) of tumour significantly influenced outcome and tumour-free survival (p = 0.0001). Of the 29 high-grade tumours, 14 (48%) were tumour-free; of the 41 low-grade tumours 39 were tumour-free.
In 11 patients, saline (n = 6) and air (n = 5) interpositions were performed to safeguard adjacent structures against freeze damage. We observed no damage to 4 colons, 2 duodenums, 3 livers, 1 spleen, 1 pancreas, and 1 ureteropelvic junction at risk.
We encountered 3 major and 5 minor complications (Clavian classification). In 1 patient, active post-ablation bleeding was treated first with blood transfusions and then embolization. In a second patient, a substantial perirenal and pararenal hematoma developed hours post-cryoablation, causing hypotension and mandating 2 blood transfusions. In a third patient, a urine leak developed the day following cryoablation of a central lesion abutting urothelium, which had not been protected by warm saline perfusion. Drainage by a double “J” catheter for 6 weeks resulted in closure of the dehiscence. Two minor hematomas resolved without sequellae as did 2 febrile reactions.
Of the total 70 patients in this study, 52 were discharged after 5 to 8 hours of observation, 9 patients after overnight admission to the short stay unit, 7 patients were admitted for 2 days, 1 patients was admitted for 3 days, and 1 patient was hospitalized for a total of 2 weeks.
Operating times varied widely depending on size, complexity of the lesion, and number of cryoprobes deployed as well as use of ancillary interventions, such as bolus interposition and retrograde ureteral perfusion with warming solutions, from 42 to 225 minutes (mean 98 minutes).
Discussion
The management of malignant renal masses has been significantly influenced and altered by two factors. The first influential factor is that despite the increase in suspect renal masses identified on abdominal CTs, the numbers assigned to the RCC group has declined reflecting the new reclassification criteria of the World Health Organization.1
A revision of indications for surgery is the second factor. While prompt surgical excision of renal malignancies had been the accepted standard of care, recent data have shown conclusively that an increase in size from 1.5 cm to 3 cm does not alter the rate of tumour-free survival.3,4,28–30 Moreover, recent reports have shown minimal metastatic progression during surveillance or follow-up after cryoablation, which allows delay of definitive surgery without affecting tumour-free survival.3,4,30 To further improve identification of recurrent tumour by imaging studies, the use of CT-guided biopsy has been advocated.31 Furthermore, recent statistical analysis has shown the cause of death in older patients with RCC and medical comorbidities to be more likely intercurrent disease than RCC.2 These newly emerged concepts make surveillance or management by minimally invasive techniques, such as cryoablation, a viable alternative to surgery, for older patients with medical comorbidities.3,4,30 While segmental or laparoscopic resection remains the gold standard for treating amenable RCCs, recent data show acceptable results6–12,14,25–27,32 (98% tumour-free survival for segmental resection and a 93% to 98.7% tumour-free survival for cryoablation.5–11,14,17,20,25–29,32–34
The efficacy of cryoablation treating RCCs in our patients is 97% (68 of 70) based on imaging follow-up criteria (lack of enhancement of ablated tissue), which is similar to that reported in the literature (93.3%–98.7%).7–9,12,14,22,23,25–27,32,34–36
Similar to reported experiences, we found that lesion location greatly affected the rate of success of cryoablation.5,25,26 For lesions in the anterior location, our rate of success was only 20%, for central or deep seated lesions 68%, and for posterior lesions 96%. Steriotactic percutaneous cryoablation may offer advantages for lesions in such locations.13 Conversely location of lesions did not affect tumour-free status attained by open or laparoscopic segmental resection, though it adversely affected the rate of complications.28,29,33,35 This raises the question of whether an anterior or deep location of a lesion should be an exclusion criterion for cryoablation, and whether surgical management should be recommended in these cases.16,25–29,32,33,36
Conclusion
We have found that hydro-displacement of critical organs and protection of urothelium against freeze-damage by perfusion with warm saline prevented complications in adjacent organs or urothelium injury in all but one of our patients. Based on our experience, percutaneous cryoablation is recommended as a minimally invasive nephron-sparing treatment for amenable lesions in older patients with medical comorbidities.
Footnotes
Competing interests: The authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Scolarius TA, Serrano MF, Grubb RL, et al. Effect of reclassification on the incidence of benign and malignant renal tumors. J Urol. 2010;183:455–8. doi: 10.1016/j.juro.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 2.Miller DC, Ruterbush J, Colts JS, et al. Contemporary clinical epidemiology of renal cell carcinoma: Insight from a population based case-control study. J Urol. 2010;184:2254–8. doi: 10.1016/j.juro.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Poppel H, Jonian S. Is surveillance an option for the treatment of small renal masses? Eur Urol. 2007;52:1323–30. doi: 10.1016/j.eururo.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Rosales JC, Haramis G, Moreno J, et al. Active surveillance for renal cortical neoplasms. J Urol. 2010;183:1698–702. doi: 10.1016/j.juro.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Allen BC, Remer EM. Percutaneous cryoablation of renal tumors: Patient selection, technique and postprocedural imaging. Radiographics. 2010;30:900–7. doi: 10.1148/rg.304095134. [DOI] [PubMed] [Google Scholar]
- 6.Campbell SC, Novick AC, Belldegrun A, et al. Guidelines for management of clinical T1 renal mass. J Urol. 2009;182:1271–9. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Schmit GD, Atwell TD, Callstrom MR, et al. Percutaneous cryoablation of renal masses of >3cm: Efficacy and safety in treatment of 108 patients. J Endourol. 2010;24:1255–63. doi: 10.1089/end.2009.0328. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez R, Cizman Z, Hong K, et al. Prospective analysis of the safety and efficacy of percutaneous cryoablation for T1NxMx biopsy proven renal cell carcinoma. Cardiovasc Intervent Radiol. 2011;34:573–8. doi: 10.1007/s00270-010-9934-7. [DOI] [PubMed] [Google Scholar]
- 9.Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of small renal mass: A metaanalysis. Cancer. 2008;113:2671–80. doi: 10.1002/cncr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandi G, Wen CC, Hedican SP, et al. Cryoablation of small renal masses: Assessment of outcome at one institution. BJU Int. 2007;100:798–801. doi: 10.1111/j.1464-410X.2007.07158.x. [DOI] [PubMed] [Google Scholar]
- 11.Vricella GJ, Haaga JR, Adler AL, et al. Percutaneous cryoablation of renal masses: Impact of patient selection and treatment parameters on outcome. Urology. 2011;77:649–54. doi: 10.1016/j.urology.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Zagoria RJ, Traver MA, Werle DM, et al. Oncologic efficacy of CT guided percutaneous radio-frequency ablation of small renal cell carcinoma. AJR Am J Roentgenol. 2007;189:2129–34. doi: 10.2214/AJR.07.2258. [DOI] [PubMed] [Google Scholar]
- 13.Haber GP, Crouzet S, Remer EM, et al. Stereotactic percutaneous cryoablation for renal tumors: Initial clinical experience. J Urol. 2010;183:884–8. doi: 10.1016/j.juro.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Breda A, Anterasian C, Bellegrun A, et al. Management and outcome of tumor recurrence after focal ablation renal therapy. J Endourol. 2010;4:749–52. doi: 10.1089/end.2009.0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young JL, Kolla SB, Pick DL, et al. In vitro, ex vivo and in vivo isotherms for renal cryotherapy. J Urol. 2010;183:752–8. doi: 10.1016/j.juro.2009.09.072. [DOI] [PubMed] [Google Scholar]
- 16.Long CJ, Kutikov A, Canter DJ, et al. Percuteneous versus surgical cryoablation of the small renal mass: Is efficacy compromised? BJU Int. 2011;107:1376–80. doi: 10.1111/j.1464-410X.2010.09851.x. [DOI] [PubMed] [Google Scholar]
- 17.Strom KH, Derweesh I, Stroup SP, et al. Recurrence rates after percutaneous and laparoscopic renal cryoablation of small renal masses: Does the approach make a difference? J Endourol. 2011;25:371–5. doi: 10.1089/end.2010.0239. [DOI] [PubMed] [Google Scholar]
- 18.Allaf ME, Lang EK. Bowel separation before percutaneous cryoablation. J Urol. 2008;180:721–3. doi: 10.1016/j.juro.2008.04.099. [DOI] [PubMed] [Google Scholar]
- 19.Bodily KD, Atwell TD, Mandrekar JN, et al. Hydrodisplacement in the percutaneous cryoablation of 50 renal tumors. AJR Am J Roentgenol. 2009;194:779–83. doi: 10.2214/AJR.08.1570. [DOI] [PubMed] [Google Scholar]
- 20.Littrup PJ, Ahmed A, Aoun MD, et al. CT guided percutaneous cryotherapy, of renal masses. J Vasc Intervent Radiol. 2007;18:383–92. doi: 10.1016/j.jvir.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Woolley ML, Schulsinger DA, Durand DB, et al. Effect of freezing parameters (freeze cycle and thaw process) on tissue destruction following renal cryoablation. J Endourol. 2002;16:519–22. doi: 10.1089/089277902760367494. [DOI] [PubMed] [Google Scholar]
- 22.Porter CA, IV, Woodrum DA, Callstrom MR, et al. MRI after technically successful cryoablation: Early contrast enhancement is a common finding. AJR Am J Roentgenol. 2010;194:790–3. doi: 10.2214/AJR.09.2518. [DOI] [PubMed] [Google Scholar]
- 23.Stein AJ, Mayes JM, Mouraview V, et al. Persistent contrast enhancement several months after laparoscopic cryoablation of the small renal mass may not indicate recurrent tumor. J Endourol. 2008;22:2433–9. doi: 10.1089/end.2008.0261. [DOI] [PubMed] [Google Scholar]
- 24.Squillaci E, Manenti G, Ciccio C, et al. Perfusion CT monitoring of cryoablated renal cell tumors. J Exp Clin Cancer Res. 2009;28:138–51. doi: 10.1186/1756-9966-28-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmit GD, Atwell TD, Leibovich BC, et al. Percutaneous cryoablation of anterior renal masses: Technique, efficacy and safety. AJR Am J Roentgenol. 2010;195:1418–22. doi: 10.2214/AJR.09.3530. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg HD, Kim CY, Tsivian M, et al. Percutaneous cyroablation of renal lesions with radiographic ice ball involvement of the renal sinus: analysis of collecting system complications. AJR Am J Roentgenol. 2011;196:935–9. doi: 10.2214/AJR.10.5182. [DOI] [PubMed] [Google Scholar]
- 27.Derweesh IH, Malcom JB, Diblasio CJ, et al. Single center comparison of laparoscopic cryoablation and CT guided percutaneous cryoablation for renal tumors. J Endourol. 2008;22:2461–7. doi: 10.1089/end.2008.0196. [DOI] [PubMed] [Google Scholar]
- 28.Russo P. Should elective partial nephrectomy be performed for renal cell carcinoma >4 cm in size? Nat Clin Pract Urol. 2008;5:482–3. doi: 10.1038/ncpuro1177. [DOI] [PubMed] [Google Scholar]
- 29.Miller DC, Hollingworth JM, Hafez KJ, et al. Partial nephrectomy for small renal masses: An emerging quality of care concern? J Urol. 2006;175:853–7. doi: 10.1016/S0022-5347(05)00422-2. [DOI] [PubMed] [Google Scholar]
- 30.Volpe A, Jewett MA. The natural history of small renal masses. Nat Clin Pract Urol. 2005;2:384–90. doi: 10.1038/ncpuro0254. [DOI] [PubMed] [Google Scholar]
- 31.Kramer BA, Whelan CM, Vestal JC, et al. RF. Increasing the number of biopsy cores before renal cryoablation increases the diagnostic yield. J Endourol. 2009;21:283–6. doi: 10.1089/end.2008.0347. [DOI] [PubMed] [Google Scholar]
- 32.Park SH, Kang SH, Ko YH, et al. Cryoablation for endophytic renal cell carcinoma: Intermediate-term oncologic efficacy and safety. Korean J Urol. 2010;51:518–24. doi: 10.4111/kju.2010.51.8.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Link RE, Bhayani SR, Allaf ME, et al. Exploring the learning curve, pathological outcomes and perioperative morbidity of laparoscopic partial nephrectomy performed for renal mass. J Urol. 2005;173:1890–4. doi: 10.1097/01.ju.0000154777.24753.1b. [DOI] [PubMed] [Google Scholar]
- 34.Atwell TD, Callstrom MR, Farrell MA, et al. Percutaneous renal cryoablation: Local control at mean 26 months followup. J Urol. 2010;184:1291–5. doi: 10.1016/j.juro.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Tsivian M, Chen V, Kim CY, et al. Complications of laparoscopic and percutaneous renal cryoablation in a single tertiary referral center. Eur Urol. 2010;58:142–7. doi: 10.1016/j.eururo.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 36.Sidana A, Aggarawal P, Feng Z, et al. Complications of renal cryoablation: A single center experience. J Urol. 2010;184:42–7. doi: 10.1016/j.juro.2010.03.013. [DOI] [PubMed] [Google Scholar]


