Abstract
Introduction:
Data comparing the incidence of ureteroenteric strictures for Bricker and Wallace anastomoses are limited. This study compares both anastomotic techniques in terms of ureteroenteric stricture rates after radical cystectomy and ileal conduit urinary diversion.
Methods:
Electronic databases (Medline, EMBASE, and Cochrane database) were searched for studies comparing Bricker and Wallace ureteroeneteric anastomoses for ileal conduit urinary diversion after radical cystectomy. Meta-analyses were performed using the random effects method. The primary outcome measure was to determine differences in postoperative ureteroenteric stricture rates for both surgical techniques. Four studies describing 658 patients met the inclusion criteria. The total number of ureters used for ureteroeneteric anastomoses was 1217 (545 in the Bricker group and 672 in the Wallace group).
Results:
There were no significant differences in age (p = 0.472), gender (p = 0.897), duration of follow-up (p = 0.168), and duration to stricture development between groups (p = 0.439). The overall stricture rate was 29 of 1217 (2.4%); 16 of 545 ureters (2.9%) in the Bricker group and 13 of 672 ureters (1.9%) in the Wallace group. The Bricker anastomosis was not associated with a significantly higher overall stricture rate compared to the Wallace ureteroenteric anastomosis (odds ratio: 1.393, 95% confidence interval: 0.441–4.394, p = 0.572).
Conclusion:
Accepting limitations in the available data, we found no significant difference in the incidence of ureteroenteric stricture for Bricker and Wallace anastomoses.
Introduction
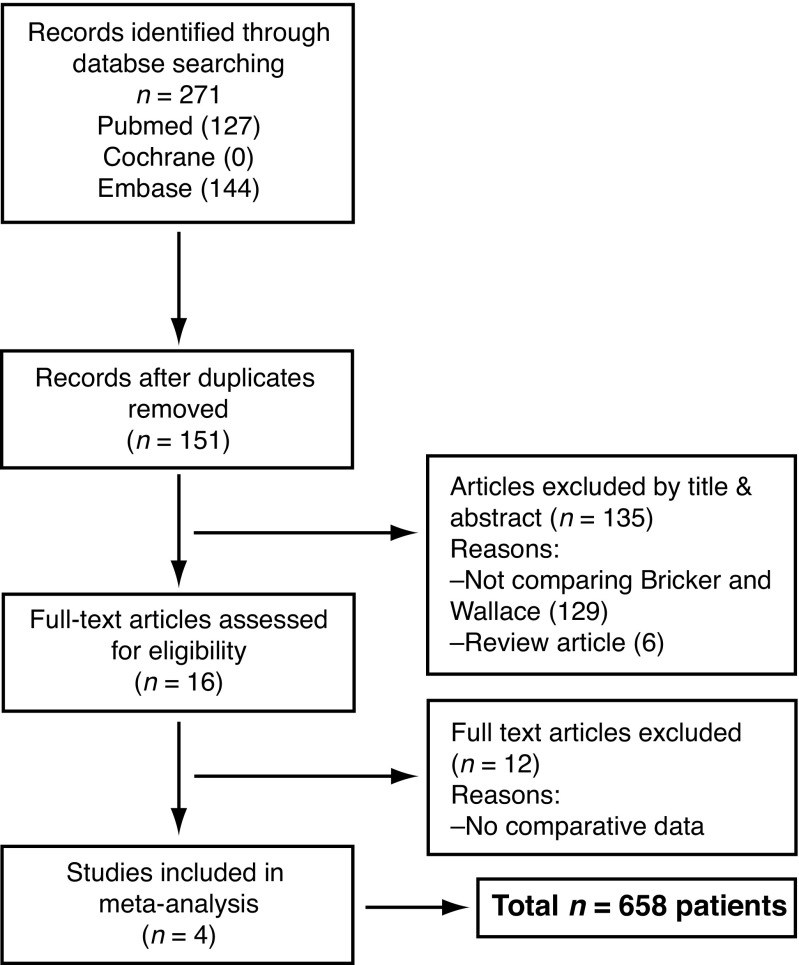
During the early and mid-twentieth century, North American urologists had a strong tradition of managing invasive bladder cancer with radical surgery due to relatively low postoperative morbidity (10%) and mortality rates (12%).1 At present, despite the popularity of continent urinary diversion and neobladder reconstruction, radical cystectomy and ileal conduit urinary diversion remain the most commonly performed curative surgical treatment option for patients with invasive bladder cancer. The two most common types of ureteroenteric anastomosis during the procedure are the refluxing Bricker and Wallace techniques. The Bricker technique, initially described in the early 1950s, involves spatulating and anastomosing each ureter to the serosa of the bowel segment separately (Fig. 1).2
Fig. 1.
Bricker ureteroenteric anastomosis. Ureters are spatulated and anastomosed independently to the ileal segment, (the ureteroileal anastomoses can be tunnelled). The proximal ileal loop is closed as indicated.
By 1962, ureteral urinary diversion with the Bricker ureteroileal anastomosis had been performed in over 300 patients for benign and malignant urological conditions with low complication rates.1 During the same time period, urologists in the United Kingdom and Europe adopted an alternative approach and irradiation (with radon, radium and open X-ray) was the standard of care for invasive bladder.3 A combined approach with irradiation and surgery was described in the early 1960s and popularized by Wallace and Hendry in the early 1970s and Blandy in the 1980s.4–6
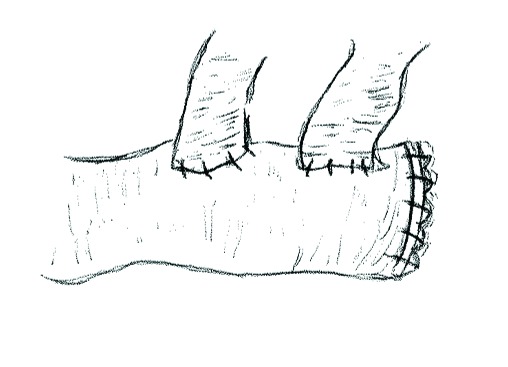
In the Wallace 1 surgical technique (Fig. 2, part A), described in 1966, both ureters are spatulated to the same length. Their medial walls are anastomosed together and the free edges of the newly conjoined ureters are then anastomosed to the proximal end of an open bowel segment.7 In the Wallace 2 technique (1969) or head-to-tail anastomosis (Fig. 2, part B), blood supply is protected by suturing the apex of one ureter to the end of the other.8 The posterior medial walls are sutured together, and then the ends and lateral walls are sutured to the bowel segment.
Fig. 2.
Wallace ureterouretero and ureteroenteric anastomoses. A. Wallace 1 technique: Ureters are spatulated to the same length and medial walls anastomosed in line. The free edges of the newly conjoined ureters are then anastomosed to the proximal end ileal loop. B. Wallace 2 technique (‘head-to-tail anastomosis’): After spatulation, the distal ureteric margin is anastomosed to apex of spatulated contralateral ureter. The free edges of the newly conjoined ureters are again anastomosed to the proximal end ileal loop.
Selecting one anastomotic technique over another is generally based on surgeon preference. A perceived disadvantage with the Bricker technique is the increased potential for stricture.9 It has been suggested that the Wallace technique is associated with an increased risk of bilateral ureteral obstruction at the site of the anastomosis secondary to poor surgical technique, tumour recurrence or bilateral calculi.10 Although both techniques have been established for well over 50 years, comparative data on the complication rates from both techniques remain sparse. The objective of the present study was to perform a meta-analysis of all studies that have compared ureteroenteric stricture rates between both surgical techniques.
Methods
Literature search and study selection
A systematic search of Medline and Embase was performed for all published studies comparing Bricker and Wallace ureteroeneteric anastomosis in patients undergoing urinary diversion by using the following in the search algorithm: (Bricker AND/ OR Wallace) AND (cystectomy) OR (urinary diversion). The Cochrane Central Register of Controlled Trials was also searched for articles that investigated complications for Bricker and Wallace ureteroeneteric anastamoses. Study selection was performed in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). The title and abstract of citations and full texts of potentially eligible trials were obtained. There were no language restrictions.
Eligibility criteria
Studies with comparative data between Bricker and Wallace ureteroenteric anasatomoses were eligible for inclusion. Studies were included in the meta-analysis if comparable data on complications associated with both anastomotic techniques for urinary diversion were available. Studies without comparative data for both techniques were excluded. The primary end points of the study were differences in ureteroenteric stricture rates and their associated complications.
Data extraction and outcomes
The following information regarding each eligible trial was recorded: author names, journal, year of publication, study type, enrolment dates, length of follow-up, total number of patients, total number of ureters, patient demographics, history of radiotherapy, indication for ureteroenteric anastomosis, imaging modality for diagnosing ureteroenetric stricture, incidence of ureteroenteric stricture per renal unit (i.e., total stricture rate for all ureters), and mean duration to ureteroenteric stricture postoperatively.
Statistical analysis
Data was presented as a mean ± standard deviation where applicable. All pooled outcome measures were determined using a random-effects model as described by DerSimonian and Laird11 and the odds ratio (OR) was estimated with its variance and 95% confidence interval (CI). The random effects analysis weighted the natural logarithm of each study’s OR by the inverse of its variance plus an estimate of the between-study variance in the presence of between-study heterogeneity. As previously described,12 heterogeneity between ORs for the same outcome between different studies was assessed. This was through the use of the I2 inconsistency test and chi-square-based Cochran’s Q statistic test,13 in which p < 0.05 indicated significant heterogeneity. Analyses were conducted using Comprehensive Meta-analysis version 2 (Biostat Inc., Englewood, NJ) and Statsdirect version 2.5.6. (StatsDirect Ltd, Chesire, UK).
Results
Eligible studies
Four published studies containing comparative data on Bricker and Wallace ureteroenteric anastomoses were identified (Table 1).9,10,14,15 The initial search identified 271 articles and 16 full-text studies were assessed for eligibility; 12 of which were excluded (Fig. 3). These studies were excluded as they did not contain comparative data on Bricker and Wallace ureteroenteric anastomoses. All studies were published in English and were within the last 8 years. The spectrum of patients was reflective of modern clinical practice and all patients underwent urinary diversion after radical cystectomy for bladder cancer.
Table 1.
Summary of study characteristics
| First author, year | Country | Inclusion criteria | Total (N) | Available data (N) | Bricker | Wallace | Modality for diagnosing stricture | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| No. patients | No. ureters | No. patients | No. ureters | ||||||
|
| |||||||||
| Evangelidis et al., 200610 | USA | RC for bladder cancer | 237 | 198 | 86 | 162 | 112 | 224 | Ultrasound/CT abdopelvis, loopogram and renal scan |
| Desai et al., 201415 | USA | Robotic intracorporeal orthotopic neobladder during RC for bladder cancer | 136 | 132 | 46 | 92 | 86 | 172 | Not recorded |
| Kouba, et al., 20079 | USA | RC for bladder cancer | 186 | 186 | 94 | 187 | 92 | 184 | Surveillance imaging followed by diuretic renogram and by antegrade contrast study |
| Liu, et al., 201414 | China | RC for bladder cancer | 99 | 99 | 53 | 104 | 46 | 92 | Ultrasound followed by CT urogram |
| Total no. | 658 | 615 | 279 | 545 | 336 | 672 | |||
None of these studies were randomized controlled trials. RC: radical cystectomy; CT: computed tomography.
Fig. 3.
PRISMA diagram of studies identified in the systematic review and meta-analysis (PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses).
A total of 658 patients were included in these studies, and data were available for analysis from 615 patients: 279 in the Bricker arm and 336 in the Wallace arm (Table 1). The total number of ureters used for ureteroeneteric anastamoses was 1217: 545 in the Bricker arm and 672 in the Wallace arm. One trial was multicentre15 and all 4 trials were retrospective. Indications for withdrawals were described in detail for the 2 studies in which patients withdrew.10,15
Patient demographics
Patient demographics are detailed in Table 2. There were no significant differences between the Bricker and Wallace cohorts in terms of gender (n = 226 vs. n = 276, respectively, OR 0.975, 95% CI 0.665–1.429, p = 0.897), age (difference in means (DIM) 1.191 years, 95% CI: −2.057–4.440, p = 0.472), or duration of follow-up (DIM: 1.460 years, 95% CI: −0.616–3.535, p = 0.168). On random effects analysis, radiotherapy was not associated with a significantly higher overall stricture rate compared to patients who did not receive radiotherapy (Fig. 4, OR: 1.740, 95% CI: 0.614–4.931, p = 0.297).
Table 2.
Comparison of patient demographics for Bricker and Wallace ureteroenteric anastomosis
| Variable | Bricker | Wallace | p value |
|---|---|---|---|
| Age (years) | 64 ± 3 | 63 ± 3 | 0.472 |
| No. male (%) | 212 (76) | 258 (77) | 0.897 |
| No. strictures/ ureter (%) | 16/545 (2.9) | 13/672 (1.9) | 0.572 |
| No. strictures/ patient (%) | 14/279 (5.7) | 13/336 (3.9) | 0.447 |
| Follow-up (months) | 27 ± 5 | 26 ± 5 | 0.168 |
| Duration to stricture (months) | 7 ± 5 | 8 ± 5 | 0.439 |
Fig. 4.
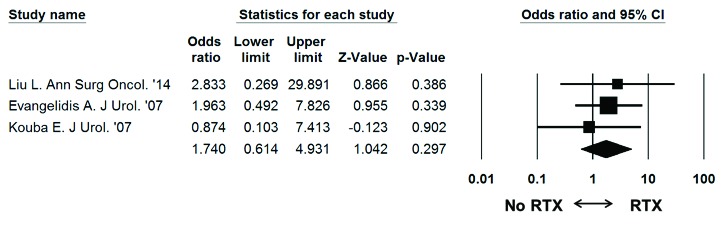
Meta-analysis of stricture rates in patients that received radiotherapy versus no radiotherapy in each study. Each study is shown by the point estimate of the odds ratio (OR; square proportional to the weight of each study) and 95% confidence intervals for the OR (extending lines); the combined OR and 95% confidence intervals by random-effects calculations are shown by diamonds. N = 483 (stricture radiotherapy: n = 5/70, stricture no radiotherapy: n = 18/413), p = 0.297; test for heterogeneity, Cochran Q = 0.6 (df: 2), p = 0.744; I2 = 0.0%.
Incidence of ureteroenteric stricture
All 4 studies included comparative data on the incidence of ureteroenteric stricture after Bricker and Wallace anastomoses (Table 2). Ureteral stricture developed in 29 of the 615 patients assessed (4.7%). The overall stricture rate for all ureters was 29 of 1217 (2.4%). In patients that underwent a Bricker anastamoses, ureteral stricture developed in 16 of 279 (5.7%). The overall stricture rate for all ureters in the Bricker group was 16 of 545 ureters (2.9%). Bilateral strictures developed in 2 patients in the Bricker group (0.7%). With the Wallace anastomoses, a ureteral stricture developed in 13 of 336 patients (3.9%). The overall stricture rate for all ureters in the Wallace group was 13 of 672 ureters (1.9%). Bilateral strictures developed in 3 patients in the Wallace group (0.9%).
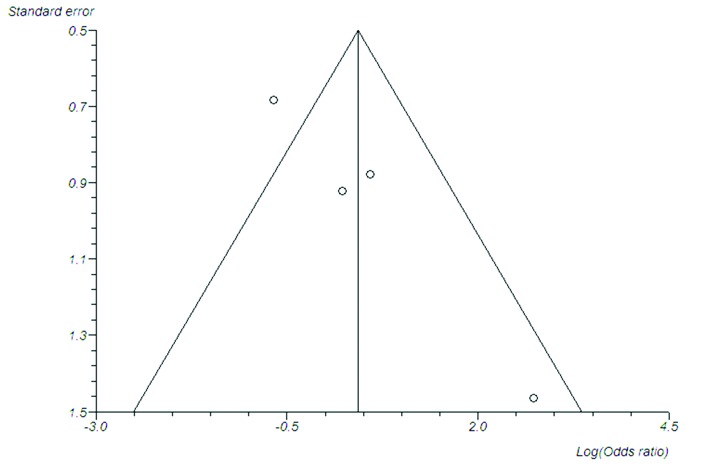
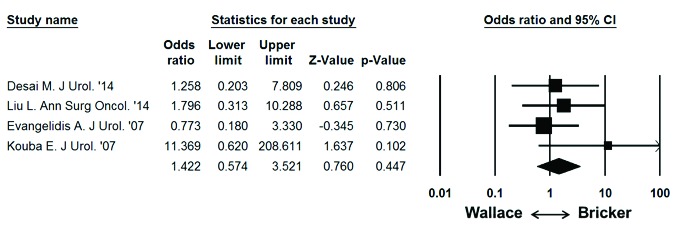
On random effects analysis of ureteroenteric stricture rate per ureter, the Bricker ureteroeneteric anastamosis was not associated with a significantly higher overall stricture rate compared to the Wallace ureteroenteric anastomosis (Fig. 5; 2.9% vs. 1.9%, OR: 1.393 95% CI: 0.441–4.394, p = 0.572). On random effects analysis of ureteroenteric stricture rate per patient, the Bricker ureteroeneteric anastomosis was not associated with a significantly higher overall stricture rate compared to the Wallace ureteroenteric anastomosis (Fig. 6; 5.7% vs. 3.9%, OR 1.422, 95% CI 0.574–3.521, p = 0.447). There were no significant differences between the Bricker and Wallace cohorts in time to stricture development (DIM: −2.343 years, 95% CI −8.275–3.589, p = 0.439). Analysis of the funnel plot (Fig. 7) demonstrated across trial publication bias.
Fig. 5.
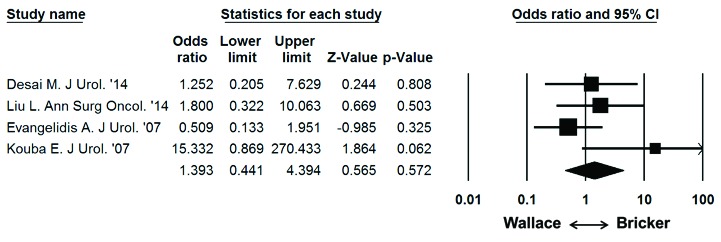
Meta-analysis of overall stricture rate per ureter and ureteroenteric anastomosis. Each study is shown by the point estimate of the odds ratio (OR; square proportional to the weight of each study) and 95% confidence intervals for the OR (extending lines); the combined OR and 95% confidence intervals by random-effects calculations are shown by diamonds. N = 1217, P = 0.572; test for heterogeneity, Cochran Q = 4.8 (df=3), p = 0.187; I2 = 37.6%.
Fig. 6.
Meta-analysis of overall stricture rate per patient and ureteroenteric anastomosis. Each study is shown by the point estimate of the odds ratio (OR; square proportional to the weight of each study) and 95% confidence intervals for the OR (extending lines); the combined Ors and 95% CIs by random-effects calculations are shown by diamonds. N = 615, P = 0.447; test for heterogeneity, Cochran Q = 2.7 (df = 3), p = 0.438; I2 = 0.0%.
Fig. 7.
Funnel plot to demonstrate publication bias (Begg-Mazumdar test [p = 0.330], Egger test [p = 0.023]).
A meta-analysis on the incidence of urinary tract calculi and tumour recurrence at the site of the ureteroenteric anastomosis was not performed as no patients developed disease recurrence or calculi during the follow-up period.
Discussion
Although Bricker and Wallace surgical techniques remain the two most common methods of ureteroenteric anastomosis for ileal conduit, there is little comparative data on their associated outcomes. Early studies have described complication rates relating to the ureteroenteric anastomosis ranging from 1.7% to 14% for both techniques.16–18 Limitations with earlier series are the absence of comparative data and the absence of a classification system for the type of ureteroenteric complication. In general, previous publications were observational without statistical comparisons postoperatively.19 To our knowledge, this is the first meta-analysis to compare ureteroenteric stricture rates between Bricker and Wallace techniques. Our main finding is that there was no significant difference in the incidence of ureteroenteric stricture after >2 years of follow-up.
The Wallace ureteroenteric anastomosis was popular in the United Kingdom during the 1970s as it was technically easier, quicker, and less likely to precipitate ureteroenteric stricture disease as demonstrated Clark and colleagues in which the incidence of ureteroenteric stricture was 2% in 101 patients that were followed up to 12 years.20 It has been suggested that a potential disadvantage of a conjoined ureteroenteric anastomosis in cases of cystectomy for cancer (i.e., Wallace) is that tumour recurrence in one ureter may affect both kidneys.10 This hypothesis has never been demonstrated in a clinical study or meta-analysis. In the present review, there was no evidence of tumour recurrence in either study group after a mean follow-up >2 years. Also the incidence of benign bilateral ureteroenteric stricture disease was almost identical in both groups. This finding suggests that significant risk factors (e.g., prostatic urethra involvement, CIS and recurrent tumour) for tumour recurrence are not a contraindication for performing the Wallace surgical technique. Another potential disadvantage associated with the Wallace technique is bilateral renal obstruction secondary to calculus/calculi at the ureteroenteric anastomosis.14 However, no patients in the Wallace arm of the review developed a urinary tract calculus during the follow-up period. In fact, one could argue that the Wallace technique is less likely to obstruct as the conjoined anastomosis is more patent than its Bricker counterpart at its proximal and distal ends.
Studies that suggest the Bricker anastomosis may be associated with a greater overall benign ureteroenteric stricture rate due to chronic ischaemia at the anastomotic site and/or excessive mobilisation of ureteral tissue lack statistical evidence to support this assumption.9,10 In the 1970s, Patil and colleagues demonstrated a ureteroenteric stricture rate of 3% in 37 patients that underwent Bricker ureteroenteric anastomosis.21 In the present review, no significant difference in the incidence of ureteroenteric stricture was observed between techniques. It is arguable that all benign ureteroenteric strictures may not have been captured as a longer time period (i.e., >2 years) may be required for their development. However, previous studies have demonstrated that benign ureteroenteric stricture disease typically manifests within the first 12 months of the diversion procedure.22
Our meta-analysis demonstrates no significant differences in patient demographics between groups. In addition, we note no significant difference in stricture rates in patients who received radiotherapy compared to patients who did not. Although previous data have suggested that radiotherapy is not a predictor for the development of ureteroenteric strictures,22 one study has demonstrated a trend for higher stricture rates in patients previously treated with radiotherapy as expected.10 A subgroup analysis of ureteroenteric stricture rates in irradiated Bricker and Wallace arms was not performed as this data were only recorded in one study.4
Our study has its limitations. Firstly, the number of studies included was small due to the paucity of comparative studies available on both techniques. No studies were randomized or prospective. Three studies involved open surgery and one was robotic. Due to these issues, there may have been an element of selection bias, as demonstrated by the evident publication bias (Fig. 7). A randomized trial however is unlikely to be performed in this setting as based on the current data, 7350 patients would be required to have an 80% chance of detecting, as significant at the 5% level, a decrease in the primary outcome measure (stricture) from 2.9% in the Bricker group to 1.9% in the Wallace group. A future, prospective multicentre observational study would be beneficial in definitively demonstrating these techniques as equivalent or either of them as particularly superior.
Conclusion
Findings from the present review demonstrate no statistically significant difference in the rates of ureteroenteric stricture disease for Bricker and Wallace anastomotic techniques. As this finding is independent of confounding variables, such as age gender, radiotherapy and length of follow-up, it appears that selection of either diversion method should be based on surgeon preference.
Footnotes
Competing interests: The authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Butcher HR, Jr, Sugg WL, McA C, et al. Ileal conduit method of ureteral urinary diversion. Ann Surg. 1962;156:682–91. doi: 10.1097/00000658-196210000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bricker EM. Bladder substitution after pelvic evisceration. Surg Clin North Am. 1950;30:1511–21. doi: 10.1016/s0039-6109(16)33147-4. [DOI] [PubMed] [Google Scholar]
- 3.Riches EW, Jacobs A, Wallace DM, et al. Discussion on the treatment of carcinoma of the bladder. Proc R Soc Med. 1952;45:191–206. [PMC free article] [PubMed] [Google Scholar]
- 4.Hendry WF. Morbidity and mortality of radical cystectomy (1971–78 and 1978–85) J R Soc Med. 1986;79:395–400. doi: 10.1177/014107688607900706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace DM. Strategy of surgical management of bladder cancer. Proc R Soc Med. 1973;66:286–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Blandy JP, England HR, Evans SJ, et al. T3 bladder cancer--the case for salvage cystectomy. Br J Urol. 1980;52:506–10. doi: 10.1111/j.1464-410X.1980.tb03101.x. [DOI] [PubMed] [Google Scholar]
- 7.Wallace DM. Ureteric diversion using a conduit: a simplified technique. Br J Urol. 1966;38:522–7. doi: 10.1111/j.1464-410X.1966.tb09747.x. [DOI] [PubMed] [Google Scholar]
- 8.Wallace DM. Uretero-ileostomy. Br J Urol. 1970;42:529–34. doi: 10.1111/j.1464-410X.1970.tb04498.x. [DOI] [PubMed] [Google Scholar]
- 9.Kouba E, Sands M, Lentz A, et al. A comparison of the Bricker versus Wallace ureteroileal anastomosis in patients undergoing urinary diversion for bladder cancer. J Urol. 2007;178(3 Pt 1):945–8. doi: 10.1016/j.juro.2007.05.030. discussion 948–9. [DOI] [PubMed] [Google Scholar]
- 10.Evangelidis A, Lee EK, Karellas ME, et al. Evaluation of ureterointestinal anastomosis: Wallace vs Bricker. J Urol. 2006:1755–8. doi: 10.1016/S0022-5347(05)01020-7. discussion 1758. [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Dunne C, Burke JP, Morrow M, et al. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27:1615–20. doi: 10.1200/JCO.2008.17.5182. [DOI] [PubMed] [Google Scholar]
- 13.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Chen M, Li Y, et al. Technique selection of Bricker or Wallace ureteroileal anastomosis in ileal conduit urinary diversion: A strategy based on patient characteristics. Ann Surg Oncol. 2014;21:2808–12. doi: 10.1245/s10434-014-3591-z. [DOI] [PubMed] [Google Scholar]
- 15.Desai MM, Gill IS, de Castro, et al. Robotic intracorporeal orthotopic neobladder during radical cystectomy in 132 patients. J Urol. 2014;192:1734–40. doi: 10.1016/j.juro.2014.06.087. [DOI] [PubMed] [Google Scholar]
- 16.Knap MM, Lundbeck F, Overgaard J. Early and late treatment-related morbidity following radical cystectomy. Scand J Urol Nephrol. 2004;38:153–60. doi: 10.1080/00365590310020060. [DOI] [PubMed] [Google Scholar]
- 17.Farnham SB, Cookson MS. Surgical complications of urinary diversion. World J Urol. 2004;22:153–60. doi: 10.1007/s00345-004-0429-5. [DOI] [PubMed] [Google Scholar]
- 18.Pantuck AJ, Han KR, Perrotti M, et al. Ureteroenteric anastomosis in continent urinary diversion: Long-term results and complications of direct versus nonrefluxing techniques. J Urol. 2000;163:450–5. doi: 10.1016/S0022-5347(05)67898-6. [DOI] [PubMed] [Google Scholar]
- 19.Yang WJ, Cho KS, Rha KH, et al. Long-term effects of ileal conduit urinary diversion on upper urinary tract in bladder cancer. Urology. 2006;68:324–7. doi: 10.1016/j.urology.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Clark PB. End-to-end ureteroileal anastomosis for ileal conduits. Br J Urol. 1979;51:105–9. doi: 10.1111/j.1464-410X.1979.tb02841.x. [DOI] [PubMed] [Google Scholar]
- 21.Patil U, Glassberg KI, Waterhouse K. Ileal conduit surgery with a nippled ureteroileal anastomosis. Urology. 1976;7:594–7. doi: 10.1016/0090-4295(76)90083-2. [DOI] [PubMed] [Google Scholar]
- 22.Nieuwenhuijzen JA, de Vries RR, Bex A, et al. Urinary diversions after cystectomy: The association of clinical factors, complications and functional results of four different diversions. Eur Urol. 2008;53:834–42. doi: 10.1016/j.eururo.2007.09.008. [DOI] [PubMed] [Google Scholar]