Abstract
Isoflavones (IFLs) are natural products to which humans have been traditionally exposed predominantly through soy foods; more recently humans are also exposed to them through soy protein addition to processed foods or through supplements. They are structurally similar to steroidal estrogens and can exert estrogenic or antiestrogenic effects depending on their concentrations and on the tissue considered. These properties qualify IFLs to be classified as phytoestrogens and are believed to account for many of the biological effects observed for soy and/or IFL exposure including benefits for bone and heart health or prevention of menopausal symptoms and certain types of cancer. In order to evaluate the function of IFLs, alone or when exposure happens through soy intake, pharmacokinetics and bioavailability are critical issues to be considered in epidemiologic and clinical research. For this purpose precise, accurate, robust, fast, and affordable techniques for IFL analyses are required.
Keywords: isoflavones, bioavailability, metabolism, analysis, soy, phytoestrogens
Introduction
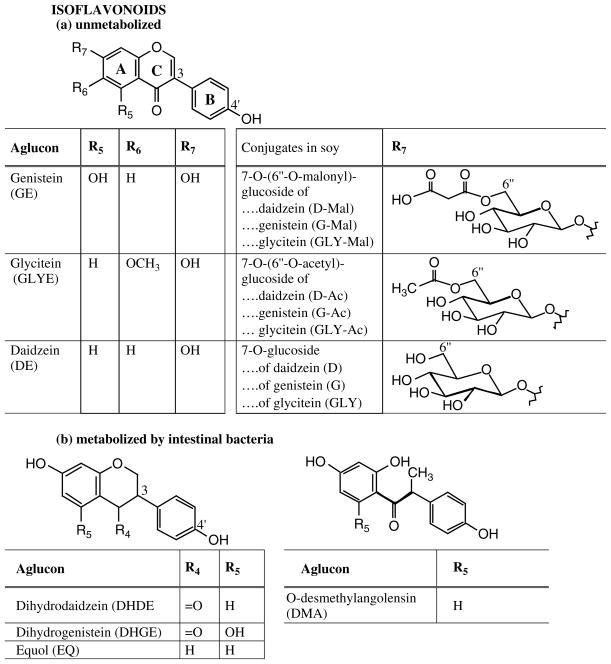
Epidemiologic and experimental studies suggest a preventive effect of soy or its associated isoflavones (IFLs) against chronic diseases including osteoporosis, cardiovascular disorders, menopausal symptoms, and particularly cancer (breast, prostate, colorectum, lung).[1 – 15] Although many of these effects are under debate, three recent epidemiologic studies show strong evidence that soy intake at young age prevents breast cancer later in life.[16 – 18] Animal studies had revealed this previously regarding genistein (GE), one of the major soy IFLs (Fig. 1).[19] Most of the evidence hints that the breast-cancer protective effect applies more to Asian populations, as confirmed by a recent meta-analysis.[20] This could be connected with the protective effect when exposure happens at young age because many Asians experience a soy-rich diet throughout life including childhood.
Figure 1.
Molecular structure of isoflavones, their conjugates in foods, and their metabolites formed by intestinal bacteria.
The structural similarity of IFLs to steroidal estrogens and the (anti)estrogenic effects of IFLs are reasons to classify IFLs as phytoestrogens and are the likely basis for many pharmacologic effects of soy intake.[21] Our recent findings on higher IFL bioavailability in children versus adults[22] and on cardioprotective effects independent from lipid profiles[23] strengthen the hypothesis that IFLs play an important role in the biological activity of soy exposure.[24]
The molecular structures of IFLs and estradiol (E2) superimpose to a high degree with the two hydroxyl groups (at C-3 and C-17 in E2 or C-7 and C-4′ in IFLs) at very similar locations in three-dimensional space (Fig. 2). This is substantiated by three-dimensional comparisons of the structures (Fig. 2). This could explain the potent binding to and, most importantly, transactivation of the estrogen receptor (ER), particularly the beta-subtype, by GE.[25] In order to increase the structural similarity a hydrogen bond of O C-4 to H-2′ in IFLs may be involved, mimicking ring B of E2 (Fig. 2). This is reinforced by the glyceollins which derive biogenetically from isoflavones by forming a pyran ring between C-4 to C-2′ and which possess notably strong antiestrogen and breast cancer-preventive effects.[26,27] Other structural features also play a role in this context because daidzein (DE) lacking the hydroxyl function at C-5 (Fig. 1) interacts with ER-beta much less.
Figure 2. Structural similarity of phytoestrogens to estrogens.
2D= two-dimensional, 3D= three-dimensional, *numbering according to isoflavones; 1 hydroxy groups on opposite sides of molecules
The evaluation of the health benefits of IFLs requires detailed knowledge of dietary IFL occurrence and thereby of the exposure to human populations. Consequently, a good understanding of the absorption, distribution, metabolism, elimination (ADME) and bioavailability of these phytoestrogens is needed, which demands robust, precise, accurate, fast and affordable analytical techniques applicable to a wide variety of biological and other matrices that our laboratory aimed to establish.
Occurrence
Soy and its products contain undoubtedly the highest IFL concentrations among foods;[10,28 – 31] therefore, but dependent on the population, dietary IFL exposure occurs mainly through intake of soy products. The latter contain typically a total of 0.01%–0.3% IFLs composed of glycosides of DE, GE, and glycitein (GLYE), roughly in a ratio of 1 : 1:0.1, while fermented products contain predominantly aglycons (Fig. 1).[10,28 – 31] Isoflavone concentrations in soy vary depending on a variety of environmental, genetic, harvesting and processing conditions.[32] In unprocessed soy foods 7-O-glucosides, namely daidzin (D), genistin (G), and glycitin (GLY), and 7-O-malonylglucosides, are the main native IFL conjugates while 7-O-acetylglycosides are formed by dry heating (Fig. 1);[33,34] aglycons occur at very low levels except in fermented soy foods.[31] Isoflavones occur in a variety of plants and are often used for chemotaxonomic purposes; they are particularly concentrated in the legumes (Fabaceae),[35] for example in kudzu (Pueraria lobata (Willd.) Ohwi) or red clover (Trifolium pratense L.) or of course soy (Glycine max (L.) Merr.). Isoflavone-rich plants other than soy are often not consumed in large amounts or are not considered food plants at all. A continuously updated database for IFL content in commonly consumed foods is available online.[36] Isoflavone supplements are increasingly popular particularly for ‘women’s health’ although their efficacy, in contrast to dietary exposure, is highly controversial,[37] their IFl levels are very variable and are little regulated in the USA,[38] and their doses are often at high levels that could lead to side effects.
Uptake and metabolism
In order for IFL to be absorbed the native IFL-O-glucosides require hydrolysis followed by passive diffusion through the mucosa. Like steroids, reconjugation to glucuronides and sulphates occurs on the basolateral side of the enterocyte and/or in the liver.[39,40] Hydrolysis in the gut can happen either by lactate phloridzin hydrolase (LPH), an enzyme bound at the brush border membrane of enterocytes, or by the gut bacteria. Hydrolysis could possibly occur in the oral cavity[41] or in the stomach; however, this occurs in humans only to a small extent if at all.[39] Due to the absence of IFL-O-glucoside in the circulation[42] and the bile[43] transport of the intact glycoside through the apical membrane of the enterocyte via sodium-dependent glucose transporter 1 (SGLT1), as is the case for some flavonoids, does not take place. Whether the uptake can be interrupted by efflux of the aglycon from the enterocyte back into the lumen via multidrug resistance-associated protein 2 (MDRP2) is not known. To a minor extent further metabolism can take place in the liver to mono- or poly-hydroxylated or O-methylated isoflavonoids.[44,45] Enterohepatic recirculation of IFL glucuronides/sulfates directly from the liver to the lumen can also occur[46] followed by bacterial hydrolysis and reabsorption.
It is important to note that gut bacteria play a vital role because they not only hydrolyze IFL glycosides but also break down IFls to form the major known human metabolites dihydrodaidzein (DHDE), dihydrogenistein (DHGE), equol (EQ), and O-desmethylangolensin (DMA) or other minor metabolites including p-ethyl phenol from GE, and ultimately degrade IFLs to CO2.[47 – 49] The contribution of the gut flora in this context is highlighted by the extremely low levels of these metabolites in infants[50] and germ-free animals.[51] Urinary recovery of total IFLs in humans including all major metabolites is usually in the range of 10–40% of ingested dose, being significantly higher for DE than GE.[39,52,53] This combined with the minimal fecal IFL excretion[54] suggests bioconversion of IFLs to yet unknown metabolites (or CO2). In vitro experiments identified bacterial species responsible for the known conversions, particularly for that to EQ,[55 – 57] which, however, have not been confirmed in humans.
All metabolic studies after intake of soy or IFL preparations show great inter-individual variations of all ADME parameters for IFLs[53](reviewed in[58,59]). Many humans (30–60%) possess a gut flora that can produce equol (reviewed in[48,60]) occurring naturally as the optical S-isomer, which has higher biological effects than its other isomers.[61] Exact cutoffs for EQ production have only recently been suggested, based on a urinary EQ/DE ratio of 0.018,[62] but the assignment to an EQ excretor in the literature remains still somewhat arbitrary. A carbohydrate rich diet may increase EQ production while fat may reduce it.[63] Fiber was reported to diminish IFL uptake,[64] and oral antibiotics were shown to lead to a trend of decreased EQ and variable DMA production.[65] Vegetarians[62] and Asians,[66,67] who are usually habitual soy consumers, seem to have a greater ability to convert DE to EQ (ca 60%) than Western populations (ca 30%).[68] Whether men are more likely to produce EQ than women[62] remains to be confirmed in larger studies. In contrast, 80–90% of populations are DMA producers,[69] whereas all animals except pigs[70] are the opposite, namely extensive equol and small DMA producers. The health effects caused by the ability of the gut flora to produce DMA or EQ, which is more estrogenic, more present as ‘free’ aglycon (ca 50%), and less protein bound than its precursor DE’, are so far unclear and inconsistent.[30,48,62,71,72]
Metabolic studies by the current writers[73,74] and others[52,75 – 80] observed consistently a biphasic IFL appearance pattern in plasma and urine (Fig. 3) when humans consume soy or purified IFL preparations. Peak levels occur at 1–2 hours and again at 4–7 hours after intake. We investigated this in more detail by simultaneous soy challenge and drastic reduction of the intestinal microbiota through oral antibiotic therapy combined with mechanical bowel preparation.[39] We concluded that only approximately 10% of overall uptake occurs in the proximal small intestine, where probably LPH hydrolyzes IFLs to the bioavailable aglycon. The majority however, is absorbed after hydrolysis by the gut bacteria that start to occur in significant amounts in the more distal intestine. Interestingly this biphasic IFL appearance pattern persisted with intake of aglycons indicating a saturation limit regarding uptake in the proximal small intestine and possibly preferred gut locations where absorption can take place.
Figure 3.
Typical pattern of isoflavone appearance in urine and plasma over time after soy intake.
Bioavailability
Pharmacokinetic parameters from 16 studies summarized in Table 1 show greater bioavailability of GE versus DE and of glycosides versus aglycons (reviewed in [59]). Relative to their O-glycosides the aglycons DE and GE show a Cmax smaller by 43% and 37%, respectively, and a ca 17% shorter time to reach Cmax (tmax) while the elimination half lives (t1/2) are similar. Relative to DE GE has a 28% higher Cmax when dosed on a molar and 20% higher when dosed on a mg basis while tmax is 8% shorter and t1/2 23% longer. The comparisons for G vs. D show a similar trend (Cmax 16% and 9% higher, tmax 9% shorter, t1/2 36% longer). According to these parameters doses of 25 mg of DE and GE (aglucon equivalents) applied to subjects weighing 50 kg versus 75 kg would be expected to lead to peak plasma levels (Cmax) for DE of 980 versus 660 nM and GE of 1190 versus 790 nM; in contrast 25 mg aglucon equivalents of daidzin and genistin would lead to 1730 versus 1150 nM and 1890 versus 1260 nM, respectively. Relative to Cmax there will be residual IFL plasma levels of approximately 25%, 9%, and 4% 24 hours, 36 hours, and 48 hours after IFL intake, respectively. As expected and shown by us experimentally, IFL plasma accumulation over time was observed by dosing soy three times per day because the elimination was incomplete upon renewed exposure.[81] The correlation between dose and expected plasma levels in the circulation is almost linear with only a slight saturation effect.[82] However, the debate continues about whether fermented soy foods containing mostly IFL aglycons or unfermented soy foods containing mostly IFL O-glucosides result in better bioavailability. Aglycons as present in fermented soy products were found to result in higher IFL uptake by some[76,83 – 85] but not others;[39,82,86] some also found non-distinguishable differences.[87,88] In contrast, IFLs from liquid relative to solid foods were consistently found to be absorbed more quickly and more extensively[84] but the overall IFL exposure as measured by urine excretion or area under curve in plasma is often not significantly different.[39,78]
Table 1.
Pharmacokinetic parameters of IFLs
| Cmaxa | rb | tmaxc | t1/2d | C24e | C36f | C48g | |
|---|---|---|---|---|---|---|---|
| (μM) | (hours) | (hours) | (rel. to Cmax) (%) | (rel. to Cmax) (%) | (rel. to Cmax) (%) | ||
| DE | 1.97 ± 0.63 | 0.93 | 6.2 ± 1.5 | 7.7 ± 3.3 | 20 | 7 | 2 |
| GE | 2.37 ± 0.41 | 0.71 | 5.7 ± 1.8 | 9.5 ± 3.2 | 26 | 11 | 5 |
| D* | 3.46 ± 0.75 | 0.85 | 7.5 ± 1.6 | 7.0 ± 1.6 | 20 | 6 | 2 |
| G* | 3.78 ± 1.00 | 0.85 | 6.8 ± 1.4 | 9.5 ± 1.7 | 29 | 12 | 5 |
Peak plasma levels in μM per mg IFL per kg BW.
correlation between dose and plasma levels by linear regression.
time to reach peak plasma levels.
time to reach peak plasma levels.
mean of 16 studies.[59]
% plasma level after 24 hours relative to peak plasma levels.
% plasma level after 36 hours relative to peak plasma levels.
% plasma level after 48 hours relative to peak plasma levels.
When dosed in aglycon units.
Intake compared to urine or plasma values
In several populations we found significant linear correlations of soy or IFL intake with urinary IFLs.[74,89] This was confirmed by others who found high correlations with either urinary IFLs,[90,91] plasma IFLs[1,92,93] or both.[66,94 – 97] The correlations were highest (up to r = 0.6) for recent intake data (food diaries) and weaker (approximately r = 0.2) or disappeared for long-term intake measures (food frequency questionnaires). This was expected considering the fast elimination pattern of IFLs. Very recent results from our collaborative work indicate that fasting morning serum IFL estimates will provide a poor index of long-term soy intake but that overnight urinary estimates perform much better.[98] Therefore, due to high reproducibility within subjects over time,[69,88] we and others recommend measuring IFLs in humans, preferably in overnight urine, as a reliable biomarker for soy intake.[99]
Apparent Bioavailability
We observed the same IFL appearance pattern in plasma and urine,[73,100] while others found high correlations between these matrices,[62,66,94 – 97,101] particularly when the timing of sample collection was considered accurately.[22,39,102] The correlation between IFL values in urine and blood are much improved by using area-under-curve (AUC) units for both matrices instead of urinary IFL excretion rate (UIER) for urine, a time-based unit, and IFL level for plasma, a volume-based unit.[22,39] This is largely due to the time domain being accurately considered by using the identical time intervals for the AUCs of both matrices. This is important to keep in mind because in living organisms IFL amounts in biological fluids change markedly over a given time period. It is vital to recognize that, at similar doses, more DE than GE appears in urine whereas the opposite is true for plasma. Therefore, the AUCplasma for DE is smaller than that for GE (accordingly the bioavailability of DE is smaller than that of GE) and the ratio AUCurine/AUCplasma much greater for DE than for GE, but most importantly, this ratio remains constant over any given time interval for each IFL.[39]
Advantages of using urine over plasma include its non-invasiveness (particularly important for research in children), its superiority at defining an EQ producer,[62] as well as the ability to collect a more concentrated matrix (particularly overnight urine) and large amounts, which leads to low quantitation limits. Collections can also be performed by participants themselves without medical supervision, in private, and, most importantly, can be accumulated over many hours (even days) reflecting exposures over much longer time periods compared to data from blood, which only reflects one given point in time per collection. This is confirmed as mentioned above by experimental findings of urinary estimates being a much better index of long-term soy intake than plasma values.[98] We suggest using UIERs as a reliable surrogate to determine circulating IFLs and thereby to assess IFL bioavailability. Since bioavailability is defined based on circulating levels, we propose using the term ‘apparent bioavailability’ when using urinary excretion data.
We recently found that the apparent IFL bioavailability in healthy children relative to healthy adults is ca 30–40% higher.[103] This is in excellent agreement with our previous reports on healthy infants,[102] school-aged children,[104] and (pre)pubertal girls.[105] These findings suggest a higher systemic IFL exposure in children versus adults at the same body weight (BW) adjusted soy dose. When considering that growing children eat much more per kg BW, the exposure in children is probably up to twofold higher relative to adults. This could result in children experiencing more benefits from the health effects of soy.[17,18] We believe that the IFL exposure after soy intake will stay far below levels that would give rise to concern regarding adverse effects.[106] Toxic activity is usually observed at much higher IFL levels and adverse effects have not been reported in populations with high soy intake.
Analysis
For accurate analyses, serious attention needs to be paid to calibration of analytical instrumentation. For this purpose we recommend preparing stock solutions of authentic standards by determining concentrations through absorbence readings followed by checking the purity via HPLC. This avoids problems with weighing inaccuracies, allows the repeated checking of existing stock solutions, and assures simple inter-laboratory comparability. While the determination of the molar extinction coefficient (ε) is critical it remains a reliable reference point even if it needs to be corrected because all final values can be adjusted linearly if ε value changes should become necessary. Table 2 shows that reported ε values for IFLs as a function of wavelength and solvent vary greatly which warrants an agreement on the true value to use for all analysts. For the sake of consistency we continue to use values reported by Ollis 1962[107] (daidzein, genistein) and Kelly et al. 1993[108] (glycitein) due to the lack of other published data when we started our IFL work in 1990.
Table 2.
Molar extinction coefficients (ε) of isoflavones as a function of wavelength and solvent
| Solvent | λ (nm) | ε (L/mol/cm) | Reference | |
|---|---|---|---|---|
| Daidzein | 96% EtOH | 250 | 20 893 | [107] |
| 100%EtOH | 262 | 24 739 | [128] | |
| n.a. | 249 | 31 563 | [129] | |
| MeOH | 248–249 | 27 100–27 200 | a* | |
| 80%MeOH | 250 | 27 542 | [130] | |
| Genistein | 96% EtOH | 263 | 37 154 | [107] |
| 100%EtOH | 262 | 35 842 | [128] | |
| n.a. | 263 | 35 323 | [129] | |
| EtOH | 263 | 35 000–38 400 | a* | |
| 96% EtOH | 262.5 | 37 291 | 0 | |
| n.a. | 261 | 33 113 | [131] | |
| 85% MeOH | 261 | 24 435 | [132] | |
| Glycitein | alcohol | 256 | 22 387 | [108] |
| n.a. | 256 | 25 388 | [129] | |
| Daidzin | n.a. | 249 | 26 830 | [129] |
| n.a. | n.a. | 23 749 | b* | |
| MeOH/Water | 250 | 28 561 | c | |
| Genistin | MeOH/Water | 262.5 | 39 129 | c |
| n.a. | 262.5 | 35 323 | [133] | |
| 85%aq EtOH | 262 | 39 000–40 000 | a* | |
| Glycitin | n.a. | 259 | 26 713 | [129] |
| Acetyldaidzin | n.a. | 256 | 29 007 | [129] |
| Acetylgenistin | n.a. | 261 | 38 946 | [129] |
| Acetylglycitin | n.a. | 260 | 29 595 | [129] |
| Malonyldaidzin | n.a. | 258 | 26 830 | [129] |
| Malonylgenistin | n.a. | 260 | 29 895 | [129] |
| Malonylglycitin | n.a. | 260 | 26 313 | [129] |
| Malonylglycitin | n.a. | 260 | 26 313 | [129] |
| Dihydrodaidzein | n.a. | 277 | 13 600 | d* |
| Dihydrogenistein | n.a. | 290 | 18 300 | d* |
| DMA | n.a. | 280 | 12 023 | d* |
| Equol | n.a. | 281 | 6761 | d* |
| Formononetin | n.a. | 256 | 29 512 | [107] |
| Biochanin-A | n.a. | 263 | 27 542 | [107] |
λ (nm) = wavelength of absorbance maximum in nanometer; EtOH = ethanol, MeOH = methanol; ε = molar extinction coefficient in L/mol/cm; DMA = O-desmethylangolensin; formononetin = 4′-O-methyl daidzein, Biochanin-A = 4′-O-methyl genistein;
Merck Index 10th edn; n.a. = not available;
private communications:
Sigma-Aldrich;
Nigel Botting 2004 (University of St Andrews, UK),
Purina/Nestle 2006,
Kristiina Wähälä 1998 (University of Helsinki, Finland).
Traditionally, IFL analysis from foods or plants was performed by HPLC[109] or capillary zone electrophoresis[110,111] with UV or electrochemical detection[100] while GC/MS was usually applied for the determination of IFLs and their metabolites in human biological fluids including urine[108,112] plasma[113] and feces.[114,115] HPLC with photo diode array (PDA) detection was introduced in 1994 to measure these analytes in human urine.[53,116] Compared to GC/MS HPLC methods require fewer steps for sample preparation and analysis and demand less technician time and less expensive instrumentation. Higher sensitivity and selectivity for HPLC analyses can be achieved by detection with mass spectrometry (MS), which has gained extreme attention due to ease of use through electrospray ionization.[117 – 119] For food IFL analyses we favor PDA monitoring over fluorescence or electrochemical detection (ECD) due to its much better diagnostic value and over MS due its more affordable operation and extreme robustness except when levels become very low.[120] Although ECD results in higher sensitivity than PDA it is much less selective and can lead to false positive results.[100] We applied HPLC/PDA for the analysis of IFLs from foods,[28,31,121] urine,[53,122] plasma,[100] breast milk,[123] and nipple aspirate fluid.[124] In all projects that applied MS detection we could confirm our earlier findings that were based on PDA monitoring.[2,125]
Food analyses of IFLs were improved very recently in our laboratory by the application of ultra-HPLC technology, which reduced run times of 45 min to less than 9 min, a more than fivefold gain, while reducing solvent consumption and waste generation by a factor of more than eight. In brief, homogenized material was extracted with 80% aqueous methanol (v/v) containing 20 ppm flavone as internal standard by sonication (10 min) and stirring (2 hours) at room temperature yielding isoflavone conjugates and originally present aglycons. After centrifugation a clear aliquot was diluted 1 : 1 with 0.2 M acetate buffer (pH5) and 5 μL were injected into a ultra HPLC system (model Accela, Thermo, San Jose, CA) using a Hypersil Gold C18 (100 × 2.1 mm; 1.9 μm) reversed-phase column (ThermoFisher). Elution was performed at a flow rate of 0.5 mL/min (9600 PSI) with the following linear gradient: A = 0.1% formic acid in 50/50 methanol/acetonitrile (v/v), B = 0.1% formic acid in water; A/B (v/v): from 22/78 to 35/65 in 6 min, then to 50/50 in 0.2 min, hold at the same ratio for 0.8 min, then change to 90/10 in 0.01 min, and hold at the same ratio for 1 min to eliminate hydrophobic components, then change back to the initial conditions in 0.01 min and hold for 1 min for equilibration. Analytes were scanned between 200 and 400 nm by diode-array detection for identification purposes and quantitated at 260 nm after adjustment of internal standard recovery. Further improvements of this method could include increasing the flow rate, applying a steeper gradient, or heating the column but this was not warranted for our needs.
Isoflavone analyses from biological matrices including tissue are routinely analyzed in our laboratory by HPLC with electrospray ionization (negative mode) and has been improved by applying high-resolution tandem mass spectrometry (model TSQ Ultra, Thermo, San Jose, CA).[124,126,127] We frequently also include mammalian lignans (enterodiol, enterolactone), another class of phytoestrogens.
13C3-Labeled daidzein, equol, and enterolactone (University of St Andrews, UK), and 2H4 labeled genistein (University of Helsinki, Finland) are added as internal standards to each homogenized specimen, and hydrolysis is performed with β-glucuronidase and arylsulfatase (Roche Applied Sciences, Indianapolis, IN) followed by phase separation with methyl tertiary butyl ether with the assistance of a robotic extraction system (model Versa100, Aurora Biomed, Vancouver, Canada).[100] The ether fractions are dried under nitrogen at room temperature and redissolved in a 1 : 1 mixture of methanol:0.01% aq. formic acid. After injection of 25 μL, the extract is separated on a Gemini C18 (150 × 2.0 mm; 5 μm) reversed phase column coupled to a Gemini C18 (4.0 × 2.0 mm; 5 μm) direct-connect guard column (Phenomenex, Torrance, CA) using a linear gradient at 0.2 mL/min of MeOH/MeCN/Water = 15/15/70 to 30/30/40 in 2.5 min, to 40/40/20 in 5.5 min and back to 15/15/70 in 0.1 min for equilibration before subsequent injections; 0.5% aq. ammonia at 40 μL/ min is added post-column as dopant.
The general MS conditions are as follows: source, ESI; ion polarity, negative; spray voltage, 3000 V; sheath and auxiliary and Ion sweep gas, nitrogen; sheath gas pressure, 35 arbitrary units; auxiliary gas pressure, 10 arbitrary units; ion sweep gas pressure, 4 arbitrary units; ion transfer capillary temperature, 280 °C; scan type, high resolution selected reaction monitoring; collision gas, argon; collision gas pressure, 1.0 mTorr, source CID 10V; scan width, 0.002u; scan time, 0.02–0.08 s; Q1 peak width was set at 0.1–0.3 u full width at half maximum (FWHM) and Q3 peak width at 0.70 u FWHM. Mass spectrometric monitoring is started 4.0 minutes after sample injection by multiple reaction monitoring as previously[124] using transitions (only ions quantitated on are listed, collision energies applied in brackets) for daidzein from m/z 253.0 to m/z 223.0 (35eV), 208.0 (33eV), and 132.0 (40eV); for 13C3-daidzein from m/z 256.0 to m/z 226.0 (31eV), 211.0 (31eV), and 182.0 (15eV); for genistein from m/z 269.1 to m/z 159.1 (30eV), 133.0 (33eV), and 132.0 (43eV); for 2H4-genistein from m/z 273.0 to m/z 228.0 (44eV), 184.0 (44eV), and 137.0 (42eV); for equol from m/z 241.1 to m/z 135.0 (20eV), 121.0 (16eV), and 119.0 (20eV); for 13C3-equol from m/z 244.1 to m/z 120.0 (25eV); for DMA from m/z 257.1 to m/z 135.9 (25eV), m/z 109.0 (20eV), and m/z 108.0 (28eV); for 13C3-DMA from m/z 260.1 to m/z 109.0 (35eV); for enterolactone from m/z 297.0 to m/z 253.0 (22eV), 189.2 (39eV), and 121.0 (39eV); for 13C3-enterolactone from m/z 300.0 to m/z 255.2 (22eV), 191.0 (39eV), and 121.9 (39eV); and for enterodiol from m/z 301.0 to m/z 283.0 (27eV) and 253.0 (27eV). Limits of quantitation (LOQ) for all analytes were 1 nM for daidzein and genistein, 2 nM for equol, and 5 nM for enterolactone; between-day coefficients of variation ranged 4–18% for all analytes, while intra-day variation was half or less of that.
Reemerging interest in soy and IFLs is due to recent findings on their cancer-preventing and other disease-preventing effects, particularly when exposure happens at young age and when intake persists throughout adulthood. Accurate, precise, and affordable analytical techniques will be the basis for future research aimed at defining the details and mechanisms of the IFL activities.
Figure 4.
Typical UHPLC trace of isoflavones from a soy protein extract. Detection at 260 nm using a Hypersil Gold C18 column (100 × 2.1 mm; 1.9 μm; Thermo, San Jose, CA) and a 0.5 mL/min flow rate (9600 PSI) of a linear gradient of 0.1% aq. formic acid versus methanol/acetonitrile(1 : 1). FL = flavone (internal standard), other details in Fig. 1 and in text. Concentrations of IFL peaks (μM): D = 17.7, GLY = 6.3, G = 26.5, D-Mal = 16.8, GLY - Mal = 3.6, D-Ac = 5.9, G-Mal = 26.7, DE = 3.4, GLYE = 1.7, G-Ac = 8.3, GE = 4.4.
Acknowledgments
Supported in part by NIH grants RR020890 and CA71789.
References
- 1.Wu AH, Yu MC, Tseng CC, Twaddle NC, Doerge DR. Carcinogenesis. 2004;25:77. doi: 10.1093/carcin/bgg189. [DOI] [PubMed] [Google Scholar]
- 2.Dai Q, Franke AA, Jin F, Shu XO, Hebert JR, Custer LJ, Cheng J, Gao YT, Zheng W. Cancer Epidemiol Biomarkers Prev. 2002;11:815. [PubMed] [Google Scholar]
- 3.Nagata Y, Sonoda T, Mori M, Miyanaga N, Okumura K, Goto K, Naito S, Fujimoto K, Hirao Y, Takahashi A, Tsukamoto T, Akaza H. J Nutr. 2007;137:1974. doi: 10.1093/jn/137.8.1974. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki M, Inoue M, Otani T, Sasazuki S, Kurahashi N, Miura T, Yamamoto S, Tsugane S. J Clin Oncol. 2008;26:1667. doi: 10.1200/JCO.2007.13.9964. [DOI] [PubMed] [Google Scholar]
- 5.Wu AH, Koh WP, Wang R, Lee HP, Yu MC. Br J Cancer. 2008;99:196. doi: 10.1038/sj.bjc.6604448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trock BJ, Hilakivi-Clarke L, Clarke R. J Natl Cancer Inst. 2006;98:459. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 7.Seow A, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Chia KS, Yu MC, Lee HP. Int J Cancer. 2002;97:365. doi: 10.1002/ijc.1615. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy A. Nurs Stand. 2005;19:44. [Google Scholar]
- 9.Setchell KD, Lydeking-Olsen E. Am J Clin Nutr. 2003;78:593S. doi: 10.1093/ajcn/78.3.593S. [DOI] [PubMed] [Google Scholar]
- 10.Adlercreutz H, Mazur W. Ann Med. 1997;29:95. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 11.Kurzer MS, Xu X. Ann Rev Nutr. 1997;17:353. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 12.Magee PJ, Rowland IR. Br J Nutr. 2004;91:513. doi: 10.1079/BJN20031075. [DOI] [PubMed] [Google Scholar]
- 13.Yan L, Spitznagel EL. Int J Cancer. 2005;20:667. doi: 10.1002/ijc.21266. [DOI] [PubMed] [Google Scholar]
- 14.Yan L, Spitznagel E. Int J Cancer Prev. 2004;1:281. [Google Scholar]
- 15.Duncan AM, Phipps WR, Kurzer MS. Best Pract Res Clin Endocrinol Metab. 2003;17:253. doi: 10.1016/s1521-690x(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 16.Thanos J, Cotterchio M, Boucher BA, Kreiger N, Thompson LU. Cancer Causes Control. 2006;17:1253. doi: 10.1007/s10552-006-0062-2. [DOI] [PubMed] [Google Scholar]
- 17.Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, Ruan Z, Gao YT, Zheng W. Cancer Epidemiol Biomarkers Prev. 2001;10:483. [PubMed] [Google Scholar]
- 18.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Carcinogenesis. 2002;23:1491. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 19.Lamartiniere CA. J Mammary Gland Biol Neoplasia. 2002;7:67. doi: 10.1023/a:1015722507237. [DOI] [PubMed] [Google Scholar]
- 20.Wu AH, Yu MC, Tseng CC, Pike MC. Br J Cancer. 2008;98:9. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotroneo MS, Wang J, Fritz WA, Eltoum IE, Lamartiniere CA. Carcinogenesis. 2002;23:1467. doi: 10.1093/carcin/23.9.1467. [DOI] [PubMed] [Google Scholar]
- 22.Franke A, Halm B, Ashburn L. Arch Biochem Biophys. 2008;476:161. doi: 10.1016/j.abb.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Walker S, Adams M, Franke A, Register T. Atherosclerosis. 2008;196:106. doi: 10.1016/j.atherosclerosis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiseman H. In: Flavonoids: Chemistry, Biochemistry, and Applications. Andersen O, Markham K, editors. Taylor & Francis Group, LLC; Boca Raton: 2006. p. 371. [Google Scholar]
- 25.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Endocrinology. 1997;138:863. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 26.Salvo VA, Boue SM, Fonseca JP, Elliott S, Corbitt C, Collins-Burow BM, Curiel TJ, Srivastav SK, Shih BY, Carter-Wientjes C, Wood CE, Erhardt PW, Beckman BS, McLachlan JA, Cleveland TE, Burow ME. Clin Cancer Res. 2006;12:7159. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- 27.Wood CE, Clarkson TB, Appt SE, Franke AA, Boue SM, Burow ME, McCoy T, Cline JM. Nutr Cancer. 2006;56:74. doi: 10.1207/s15327914nc5601_10. [DOI] [PubMed] [Google Scholar]
- 28.Umphress ST, Murphy SP, Franke AA, Custer LJ, Blitz CL. J Food Comp Anal. 2005;18:533. [Google Scholar]
- 29.Franke AA, Custer LJ, Cerna CM, Narala K. Proc Soc Exp Biol Med. 1995;208:18. doi: 10.3181/00379727-208-43826. [DOI] [PubMed] [Google Scholar]
- 30.Horn-Ross PL, Lee M, John EM, Koo J. Cancer Causes Control. 2000;11:299. doi: 10.1023/a:1008968003575. [DOI] [PubMed] [Google Scholar]
- 31.Franke AA, Hankin JH, Yu MC, Maskarinec G, Low SH, Custer LJ. J Agric Food Chem. 1999;47:977. doi: 10.1021/jf9808832. [DOI] [PubMed] [Google Scholar]
- 32.Tsukamoto C, Shimada S, Igita K, Kudou S, Kokubun M, Okubo K, Kitamura K. J Agric Food Chem. 1995;43:1184. [Google Scholar]
- 33.Barnes S, Kirk M, Coward L. J Agric Food Chem. 1994;42:2466. [Google Scholar]
- 34.Kudou S, Fluery Y, Welti D, Magnolato D, Uchida T, Kitamura K, Okubo M. Agric Biol Chem. 1991;55:2227. [PubMed] [Google Scholar]
- 35.Andersen O. In: Flavonoids: Chemistry, Biochemistry, and Applications. Andersen O, Markham K, editors. Taylor & Francis Group, LLC; Boca Raton: 2006. p. 1129. [Google Scholar]
- 36.U. US Department of Agriculture. [accesed 8 Dec.2008];2007 http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/isoflav/isoflav1-4.pdf.
- 37.Sirtori CR. Drug Saf. 2001;24:665. doi: 10.2165/00002018-200124090-00003. [DOI] [PubMed] [Google Scholar]
- 38.Setchell KDR, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirshner AS, Cassidy A, Heubi JE. J Nutr. 2001;131:1362S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 39.Franke AA, Custer LJ, Hundahl SA. Nutr Cancer. 2004;50(2):141. doi: 10.1207/s15327914nc5002_3. [DOI] [PubMed] [Google Scholar]
- 40.Setchell KD. J Nutr. 2000;130:654S. doi: 10.1093/jn/130.3.654S. [DOI] [PubMed] [Google Scholar]
- 41.Walle T, Browning AM, Steed LL, Reed SG, Walle UK. J Nutr. 2005;135:48. doi: 10.1093/jn/135.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Setchell KD, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE. Am J Clin Nutr. 2002;76:447. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- 43.Prasain JK, Xu J, Kirk M, Smith Johnson M, Sfakianos J, Barnes S. J Nutr. 2006;136:2975. doi: 10.1093/jn/136.12.2975. [DOI] [PubMed] [Google Scholar]
- 44.Heinonen SM, Hoikkala A, Wahala K, Adlercreutz H. J Steroid Biochem Mol Biol. 2003;87:285. doi: 10.1016/j.jsbmb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Kulling SE, Honig DM, Metzler M. J Agric Food Chem. 2001;49:3024. doi: 10.1021/jf0012695. [DOI] [PubMed] [Google Scholar]
- 46.Sfakianos J, Coward L, Kirk M, Barnes S. J Nutr. 1997;127:1260. doi: 10.1093/jn/127.7.1260. [DOI] [PubMed] [Google Scholar]
- 47.Setchell KDR, Brown NM, Lydeking-Olsen E. J Nutr. 2002;132:3577. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 48.Atkinson C, Frankenfeld CL, Lampe JW. Exp Biol Med (Maywood) 2005;230:155. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- 49.Bannwart C, Adlercreutz H, Fotsis T, Wahala K, Hase T, Brunow G. Finn Chem Lett. 1984;4–5:120. [Google Scholar]
- 50.Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. The Lancet. 1997;350:23. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 51.Bowey E, Adlercreutz H, Rowland I. Food Chem Toxicol. 2003;41:631. doi: 10.1016/s0278-6915(02)00324-1. [DOI] [PubMed] [Google Scholar]
- 52.Setchell KDR, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, Wolfe BE, Brashear WT, Desai P, Oldfield MF, Botting NP, Cassidy A. Am J Clin Nutr. 2003;77:411. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- 53.Franke AA, Custer LJ. J Chromatogr B. 1994;662:47. doi: 10.1016/0378-4347(94)00390-4. [DOI] [PubMed] [Google Scholar]
- 54.Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S. J Nutr. 1995;125:2307. doi: 10.1093/jn/125.9.2307. [DOI] [PubMed] [Google Scholar]
- 55.Wang XL, Hur HG, Lee JH, Kim KT, Kim SI. Appl Environ Microbiol. 2005;71:214. doi: 10.1128/AEM.71.1.214-219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Decroos K, Vanhemmens S, Cattoir S, Boon N, Verstraete W. Arch Microbiol. 2005;183:45. doi: 10.1007/s00203-004-0747-4. [DOI] [PubMed] [Google Scholar]
- 57.Ueno T, Uchiyama S. Ann Nutr Metab. 2001;45 [Google Scholar]
- 58.Cassidy A. J AOAC Int. 2006;89:1182. [PubMed] [Google Scholar]
- 59.Nielsen IL, Williamson G. Nutr Cancer. 2007;57:1. doi: 10.1080/01635580701267677. [DOI] [PubMed] [Google Scholar]
- 60.Yuan JP, Wang JH, Liu X. Mol Nutr Food Res. 2007;51:765. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
- 61.Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE. Am J Clin Nutr. 2005;81:1072. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 62.Setchell KD, Cole SJ. J Nutr. 2006;136:2188. doi: 10.1093/jn/136.8.2188. [DOI] [PubMed] [Google Scholar]
- 63.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Nutr Cancer. 2000;36:27. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 64.Tew BY, Xu X, Wang HJ, Murphy PA, Hendrich S. J Nutr. 1996;126:871. doi: 10.1093/jn/126.4.871. [DOI] [PubMed] [Google Scholar]
- 65.Halm B, Franke AAFL-CIO, Ashburn LA, Hebshi SM, Wilkens LR. Nutrition and Cancer. 2008;60:14. doi: 10.1080/01635580701586747. [DOI] [PubMed] [Google Scholar]
- 66.Arai Y, Uehara M, Sato Y, Kimira M, Eboshida A, Adlercreutz H, Watanabe S. J Epidemiol. 2000;10:127. doi: 10.2188/jea.10.127. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe S, Yamauchi M, Sobue T, Takahashi T, Miura T, Arai Y, Mazur W, Wähälä K, Adlercreutz H. J Nutr. 1998;128:1710. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- 68.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Proc Soc Exp Biol Med. 1998;217:335. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 69.Frankenfeld CL, Atkinson C, Thomas WK, Gonzalez A, Jokela T, Wahala K, Schwartz SM, Li SS, Lampe JW. Br J Nutr. 2005;94:873. doi: 10.1079/bjn20051565. [DOI] [PubMed] [Google Scholar]
- 70.Gu L, House SE, Prior RL, Fang N, Ronis MJ, Clarkson TB, Wilson ME, Badger TM. J Nutr. 2006;136:1215. doi: 10.1093/jn/136.5.1215. [DOI] [PubMed] [Google Scholar]
- 71.Frankenfeld CL, McTiernan A, Aiello EJ, Thomas WK, LaCroix K, Schramm J, Schwartz SM, Holt VL, Lampe JW. Cancer Epidemiol Biomarkers Prev. 2004;13:1156. [PubMed] [Google Scholar]
- 72.Frankenfeld CL, McTiernan A, Thomas WK, LaCroix K, McVarish L, Holt VL, Schwartz SM, Lampe JW. Maturitas. 2006;53:315. doi: 10.1016/j.maturitas.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 73.Fanti P, Sawaya PB, Custer LJ, Franke AA. J Am Soc Nephrol. 1999;40:382. doi: 10.1681/ASN.V104864. [DOI] [PubMed] [Google Scholar]
- 74.Franke AA, Yu MC, Maskarinec G, Fanti P, Zheng W, Custer LJ. Biochem Soc Trans. 1999;27:308. doi: 10.1042/bst0270308. [DOI] [PubMed] [Google Scholar]
- 75.King RA, Bursill DB. Am J Clin Nutr. 1998;67:867. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- 76.Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. J Nutr. 2006;136:2291. doi: 10.1093/jn/136.9.2291. [DOI] [PubMed] [Google Scholar]
- 77.Anupongsanugool E, Teekachunhatean S, Rojanasthien N, Pongsatha S, Sangdee C. BMC Clin Pharmacol. 2005;5:2. doi: 10.1186/1472-6904-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Pascual-Teresa S, Hallund J, Talbot D, Schroot J, Williams CM, Bugel S, Cassidy A. J Nutr Biochem. 2005;17:257. doi: 10.1016/j.jnutbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 79.Vergne S, Bennetau-Pelissero C, Lamothe V, Chantre P, Potier M, Asselineau J, Perez P, Durand M, Moore N, Sauvant P. Br J Nutr. 2008;99:333. doi: 10.1017/S0007114507803953. [DOI] [PubMed] [Google Scholar]
- 80.Zubik L, Meydani M. Am J Clin Nutr. 2003;77:1459. doi: 10.1093/ajcn/77.6.1459. [DOI] [PubMed] [Google Scholar]
- 81.Gardner C, Chatterjee L, Morris J, Franke AA. [accesed 8 Dec.2008];J Nutr Biochem. 2008 Jul 3; [epub]. http://www.ncbi.nlm.nih.gov/sites/entrez.
- 82.Setchell KD, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, Creutzinger V, Heubi JE. J Nutr. 2003;133:1027. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 83.Hutchins AM, Slavin JL, Lampe JW. J Am Diet Assoc. 1995;95:545. doi: 10.1016/S0002-8223(95)00149-2. [DOI] [PubMed] [Google Scholar]
- 84.Cassidy A, Brown JE, Hawdon A, Faughnan MS, King LJ, Millward J, Zimmer-Nechemias L, Wolfe B, Setchell KD. J Nutr. 2006;136:45. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]
- 85.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. J Nutr. 2000;130:1695. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 86.Tsangalis D, Wilcox G, Shah NP, Stojanovska L. Br J Nutr. 2005;93:867. doi: 10.1079/bjn20041299. [DOI] [PubMed] [Google Scholar]
- 87.Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, Offord EA. J Nutr. 2002;132:2587. doi: 10.1093/jn/132.9.2587. [DOI] [PubMed] [Google Scholar]
- 88.Maskarinec G, Watts K, Kagihara J, Hebshi SM, Franke AA. Br J Nutr. 2008:1. doi: 10.1017/S0007114508898686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jaceldo-Siegl K, Fraser GE, Chan J, Franke A, Sabate J. Am J Clin Nutr. 2008;87:1422. doi: 10.1093/ajcn/87.5.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atkinson C, Skor HE, Fitzgibbons ED, Scholes D, Chen C, Wahala K, Schwartz SM, Lampe JW. Cancer Epidemiol Biomarkers Prev. 2002;11:253. [PubMed] [Google Scholar]
- 91.Slavin JL, Karr SC, Hutchins AM, Lampe JW. Am J Clin Nutr. 1998;68:1492S. doi: 10.1093/ajcn/68.6.1492S. [DOI] [PubMed] [Google Scholar]
- 92.Heald CL, Ritchie MR, Bolton-Smith C, Morton MS, Alexander FE. Br J Nutr. 2007;98:388. doi: 10.1017/S0007114507700703. [DOI] [PubMed] [Google Scholar]
- 93.Frankenfeld CL, Patterson RE, Horner NK, Neuhouser ML, Skor HE, Kalhorn TF, Howald WN, Lampe JW. Am J Clin Nutr. 2003;77:674. doi: 10.1093/ajcn/77.3.674. [DOI] [PubMed] [Google Scholar]
- 94.Grace PB, Taylor JI, Low YL, Luben RN, Mulligan AA, Botting NP, Dowsett M, Welch AA, Khaw KT, Wareham NJ, Day NE, Bingham SA. Cancer Epidemiol Biomarkers Prev. 2004;13:698. [PubMed] [Google Scholar]
- 95.Ritchie MR, Morton MS, Deighton N, Blake A, Cummings JH. Br J Nutr. 2004;91:447. doi: 10.1079/BJN20031062. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto S, Sobue T, Sasaki S, Kobayashi M, Arai Y, Uehara M, Adlercreutz H, Watanabe S, Takahashi T, Iitoi Y, Iwase Y, Akabane M, Tsugane S. J Nutr. 2001;131:2741. doi: 10.1093/jn/131.10.2741. [DOI] [PubMed] [Google Scholar]
- 97.Low YL, Taylor JI, Grace PB, Mulligan AA, Welch AA, Scollen S, Dunning AM, Luben RN, Khaw KT, Day NE, Wareham NJ, Bingham SA. Nutr Cancer. 2006;56:31. doi: 10.1207/s15327914nc5601_5. [DOI] [PubMed] [Google Scholar]
- 98.Fraser GE, Franke AA, Jaceldo-Siegl K, Bennett H. J Nutr. 2008 in preparation. [Google Scholar]
- 99.Lampe JW. J Nutr. 2003;133(Suppl 3):956S. doi: 10.1093/jn/133.3.956S. [DOI] [PubMed] [Google Scholar]
- 100.Franke AA, Custer LJ, Wang W, Shi SJ. Proc Soc Exp Biol Med. 1998;217:263. doi: 10.3181/00379727-217-44231. [DOI] [PubMed] [Google Scholar]
- 101.Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. J Nutr. 2004;134:1998. doi: 10.1093/jn/134.8.1998. [DOI] [PubMed] [Google Scholar]
- 102.Franke AA, Halm BM, Custer LJ, Tatsumura Y, Hebshi S. Am J Clin Nutr. 2006;84:406. doi: 10.1093/ajcn/84.1.406. [DOI] [PubMed] [Google Scholar]
- 103.Halm B, Ashburn L, Franke A. Br J Nutr. 2007;98:998. doi: 10.1017/S0007114507771866. [DOI] [PubMed] [Google Scholar]
- 104.Franke AA, Halm BM, Ashburn LA. Nutrition and Cancer. 2008;60:627. doi: 10.1080/01635580802065310. [DOI] [PubMed] [Google Scholar]
- 105.Maskarinec G, Morimoto Y, Novotny R, Nordt FJ, Stanczyk FZ, Franke AA. Nutr Cancer. 2005;52:22. doi: 10.1207/s15327914nc5201_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Munro IC, Harwood M, Hlywka JJ, Stephen AM, Doull J, Flamm WG, Adlercreutz H. Nutr Rev. 2003;61:1. doi: 10.1301/nr.2003.janr.1-33. [DOI] [PubMed] [Google Scholar]
- 107.Ollis WD. In: The Chemistry of Flavonoid Compounds. Geissman TA, editor. Macmillan; New York: 1962. p. 353. [Google Scholar]
- 108.Kelly GE, Nelson C, Waring MA, Joannou GE, Reeder AY. Clin Chim Acta. 1993;223:9. doi: 10.1016/0009-8981(93)90058-c. [DOI] [PubMed] [Google Scholar]
- 109.Wang G, Kuan SS, Francis OJ, Ware GM, Carman AS, Agric J. Food Chem. 1990;38:185. [Google Scholar]
- 110.Aussenac T, Lacombe S, Dayde J. Am J Clin Nutr. 1998;68:1480S. doi: 10.1093/ajcn/68.6.1480S. [DOI] [PubMed] [Google Scholar]
- 111.Mellenthin O, Galensa R. J Agric Food Chem. 1999;47:594. doi: 10.1021/jf980749h. [DOI] [PubMed] [Google Scholar]
- 112.Adlercreutz H, Fotsis T, Bannwart C, Wahala K, Brunow G, Hase T. Clin Chim Acta. 1991;199:263. doi: 10.1016/0009-8981(91)90120-2. [DOI] [PubMed] [Google Scholar]
- 113.Adlercreutz H, Fotsis T, Lampe J, Wahala T, Makela T, Brunow G, Hase T. Scand J Clin Lab Invest. 1993;53:5. doi: 10.3109/00365519309090693. [DOI] [PubMed] [Google Scholar]
- 114.Kurzer MS, Lampe JW, Martini MC, Adlercreutz H. Cancer Epidem Biomarkers & Prevention. 1995;4:353. [PubMed] [Google Scholar]
- 115.Adlercreutz H, Fotsis T, Kurzer MS, Wähälä K, Mäkelä T, Hase T. Anal Biochem. 1995;225:101. doi: 10.1006/abio.1995.1114. [DOI] [PubMed] [Google Scholar]
- 116.Xu X, Wang HJ, Murphy PA, Cook L, Hendrich S. J Nutr. 1994;124:825. doi: 10.1093/jn/124.6.825. [DOI] [PubMed] [Google Scholar]
- 117.Twaddle NC, Churchwell MI, Doerge DR. J Chromatogr B. 2002;777:139. doi: 10.1016/s1570-0232(02)00275-1. [DOI] [PubMed] [Google Scholar]
- 118.Barnes S, Coward L, Kirk M, Sfakianos J. Proc Soc Exp Biol Med. 1998;217:254. doi: 10.3181/00379727-217-44230. [DOI] [PubMed] [Google Scholar]
- 119.Franke AA, Murphy SP, Le Marchand L, Zheng W, Custer L. J Nutr. 2002;132:592S. [Google Scholar]
- 120.Franke AA, Custer LJ, Arakaki C, Murphy S. J Food Comp Anal. 2004;17:1. [Google Scholar]
- 121.Franke AA, Custer LJ, Cerna CM, Narala KK. J Agric Food Chem. 1994;42:1905. [Google Scholar]
- 122.Zheng W, Dai Q, Custer LJ, Shu XO, Wen WQ, Jin F, Franke AA. Cancer Epidemiol Biomarkers Prev. 1999;8:35. [PubMed] [Google Scholar]
- 123.Franke AA, Custer LJ, Tanaka Y. Am J Clin Nutr. 1998;68:1466S. doi: 10.1093/ajcn/68.6.1466S. [DOI] [PubMed] [Google Scholar]
- 124.Maskarinec G, Hebshi S, Custer L, Franke A. Eur J Cancer Prevention. 2008;17:67. doi: 10.1097/CEJ.0b013e3281108101. [DOI] [PubMed] [Google Scholar]
- 125.Franke AA, Halm BM, Custer LJ, Tatsumura Y, Hebshi S. Am J Clin Nutr. 2006;84:406. doi: 10.1093/ajcn/84.1.406. [DOI] [PubMed] [Google Scholar]
- 126.Blair RM, Appt SE, Franke AA, Clarkson TB. J Nutr. 2003;133:2262. doi: 10.1093/jn/133.7.2262. [DOI] [PubMed] [Google Scholar]
- 127.Franke AA, Custer LJ, Wilkens LR, Le Marchand L, Nomura AN, Goodman MT, Kolonel LN. J Chromatogr B. 2002;777:43. doi: 10.1016/s1570-0232(02)00216-7. [DOI] [PubMed] [Google Scholar]
- 128.Wiseman H, Casey K, Clarke DB, Barnes KA, Bowey E, Agric J. Food Chem. 2002;50:1404. doi: 10.1021/jf011243t. [DOI] [PubMed] [Google Scholar]
- 129.Murphy PA, Barua K, Hauck CC. J Chromatogr B. 2002;777:129. doi: 10.1016/s1570-0232(02)00342-2. [DOI] [PubMed] [Google Scholar]
- 130.Walz E. Justus Liebigs Ann Chem. 1931;489:118. [Google Scholar]
- 131.Williams CA, Harborne JB. In: Methods in Plant Biochemistry. Dey PM, Harborne JB, editors. Academic Press; London: 1989. p. 421. [Google Scholar]
- 132.Walter ED. J Am Oil Chem Soc. 1941;63:3273. [Google Scholar]
- 133.Hendrich S, Murphy SP. In: Handbook of Neutraceuticals and Functional Foods. Wildman REC, editor. CRC Press; Boca Raton: 2001. p. 55. [Google Scholar]