Abstract
Bisphenol A (BPA) is a synthetic chemical extensively used in many consumer products. It mimics estrogen activities and is related to developmental disorders and metabolic diseases. The current challenge of BPA detection is their low circulating levels at 0.1 ~10 ng/mL which is close to the detection limit of most of current analytical methods. In this report, we developed a simple, sensitive and accurate LCMS method after 1-methylimidazole-2-sulfonyl chloride derivatization. The method significantly improves sensitivity 5~9 fold over dansyl derivatization and approximately 100-fold without derivatization.
Keywords: Bisphenol A (BPA), ESI-LCMS, Orbitrap MS, 1-methylimidazole-2-sulfonyl derivatization
Introduction
Bisphenol A (BPA) is an industrial chemical present in many synthetic plastic materials, particular polycarbonates. It is the main intermediate in the synthesis of polycarbonate polymers and epoxy resins extensively used in many consumer products such as baby bottles, lining of food and beverage containers and dental fillings. Human exposure to BPA is prevalent, as it migrates from plastic products into food or water during the process of heating, allowing for the direct exposure to humans.[1,2] Exposure through medical supplies is also possible since BPA can leach into liquids from polycarbonate or PVC based medical containers or other devices. BPA was also shown to be absorbed by the skin leading to significant exposure via ink from cashier receipts. Once absorbed, BPA is glucuronidated and/or sulfated followed by elimination in urine and feces [3,4].
The biological activities of BPA have been extensively studied. It was reported that BPA exhibited endocrine disrupting properties and can cause reproductive and developmental defects in animals [5]. BPA is also associated with many medical disorders in humans such as cardiovascular diseases, diabetes, cancer, and liver enzyme abnormalities [6]. Better knowledge about its adverse health effects and recommended exposure thresholds was hindered by the lack of sufficient epidemiologic data. Consequently, monitoring human exposure to this prevalent environmental hazardous material gained increasing interests in recent years. Urine is the preferred matrix to measure environmental exposures since it integrates over long periods of exposure times and is non-invasive, however, it contains BPA levels in the sub-nanogram per milliliter range only which is difficult to measure.
The preferred analytical technique for BPA measurements in biological fluids is online- solid phase extraction (SPE) for the isolation and enrichment followed by liquid chromatography mass spectrometry (LCMS) in negative mode to monitor the negatively charged analytes [7]. The drawback of these methods is the low sensitivity with levels in healthy populations being measured near the limit of detection. Recently, BPA derivatization with dansyl chloride or ethyl chloroformate was reported to improve sensitivity during LCMS [8] or GCMS analysis [9]. An electrochemical bisphenol A sensor based on N-doped graphene sheets was proposed for BPA analysis with a detection limit of 5.0 × 10−9 mol/L [10] but whether this method works for urine is not known.
In this report, we intended to apply a new derivatization method to improve LCMS based sensitivity and overall analytical efficiency.
Experimental
Chemicals and reagents
Bisphenol A (BPA) and isotope-labeled BPA-13C12 used as internal standard were purchased from Cambridge Isotope Laboratories (Andover, MA) as 100 μg/mL in acetonitrile solutions. Dansy chloride (DSCl), 1-methylimidazole-2-sulfonyl chloride (ISCl), and sulfatase powder (from Helix pomatia, type H-1 >10,000 units/g) were purchased from Sigma-Aldrich (St. Louis, MO). β-glucuronidase (Escherichia coli-K12, solution in 50% glycerol, >140 U/mL) was obtained from Roche Applied Science (Indianapolis, IN). Standard reference material for BPA in urine (SRM 3673 ‘organic contaminants in non-smokers' urine’) was purchased from the National Institute of Standards and Technology (NIST, Gaithersburg, MD). LCMS grade water and formic acid were purchased from Sigma-Aldrich, and LCMS grade acetonitrile was purchased from Fisher Scientific (Fair Lawn, NJ).
Calibration standards
Calibration standards were prepared from the stock solution by serial dilution in acetonitrile. An eight point calibration curve was prepared covering a range from 0.1 ng/mL to 200 ng/mL. The working internal standard was prepared at 1 μg/mL in MeOH. 100 μL of calibration standard and 10 μL of internal standard (BPA-13C12, 1 μg/mL) were mixed, dried under nitrogen, and subjected to derivatization.
Sample preparation
100 μL of human urine was mixed with 50 μL of enzyme mixture (containing β-glucuronidase 2% v/v, and sulfatase 2 mg/mL; in 1M ammonium acetate, pH 6.5) and 10 μL of the internal standard (BPA-13C12, 1 μg/mL) and incubated at 37 °C for 90 min with horizontal rotation at 100 rpm. After the addition of 5 μL of glacial acetic acid for pH adjustment and extraction with 1 mL of methyl t-butyl ether (MtBE) by vortexting at 2000 rpm for 30 sec, the upper organic layer was transferred to an HPLC vial and dried under a nitrogen flow.
Derivatization of bisphenol A with dansyl chloride (DSCl)
Dried urine extracts or dried standards were mixed with 75 μL dansyl chloride (2 mg/mL in acetone) and 75 μL of sodium bicarbonate (100 mM, pH 9) in glass vials, then tightly capped and heated at 65°C for 15 min. The resulting mixture was transferred to HPLC inserts and 10 μL was injected for LCMS analysis.
Derivatization of BPA with 1-methylimidazole-2-sulfonyl chloride (ISCl)
Dry urine extracts or standards were treated as above except for replacing dansyl chloride with 75 μL ISCl (2 mg/mL in acetone).
LCMS analysis
The analysis was carried out on a model Accela ultra-HPLC system (Thermo Electron, Waltham, MA) including a model HTC Pal autosampler (Leap Technologies, Carrboro, NC) and a Q Exactive orbitrap mass spectrometer (Thermo Electron). 10 μL of the reaction mixture was injected onto a Hypersil Gold C18 column (50 x 2.1mm, 1.9 μm, Thermo, Waltham, MA) with a mobile phase consisting 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) at a flow rate of 800 μL/min. For the analysis of BPA-1-methyl imidazolesulfonyl (IS) derivatives, the following gradient was used (A/B, v/v): 70/30 to 35/65 from 0 to 5 min, then back to initial condition 70/30 and equilibrate for 3 min. The total run time was 8 min. The analysis of BPA-dansyl (DS) derivatives was carried out on an Agilent SB-C18 column (50 x 3.0 mm, 1.8 μm, Agilent, Santa Clara CA) using the same mobile phase at 800 μL/min flow rate with the following gradient: A/B (v/v) 60/40 to 10/90 from 0 to 10 min, keep at the same ratio for 1 min then back to initial condition and equilibrate for 5 min. The total run time was 16 min. Mass detection was carried out under positive electrospray ionization (ESI) mode with spray voltage of 4.5 kV, capillary temperature 350 °C, S-lens RF value 50, heater temperature 300 °C, in-source CID 5 eV. Nitrogen was used as sheath gas (pressure 45 units) and auxiliary gas (pressure 10 units). Two MS methods were applied: full scan was performed in a scan range of 150 to 1000 m/z, maximum injection time 100 ms, microscan 1, resolution 35000, and AGI target 1e6; targeted SIM (tSIM) scans the exact masses of BPA and BPA-13C12 (see table 1 for details), isolation width 1.5 mass units, maximum injection time 100 ms, microscan 1, resolution 35000, and AGI target 1e6.
Table 1.
Parameters of BPA- 1-methylimidazole-2-sulfonyl (IS) and dansyl (DS) adducts using orbitrap mass spectrometry in full scan (FS) and targeted SIM (tSIM) mode
| IS derivatization | DS derivatization | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| [M+H]+ | tR (min) | LOD (pg/mL) | [M+H]+ | tR (min) | LOD (pg/mL) | |||
|
|
||||||||
| tSIM | FS | tSIM | FS | |||||
| BPA | 517.12101 | 3.8 | 0.12 | 1.9 | 695.22441 | 11.6 | 1.1 | 10 |
| BPA-13C12 | 529.16126 | 3.8 | 707.26466 | 11.6 | ||||
LOD was defined as the concentration yielding a signal to noise ratio of three in standards
Method validation
Linearity was measured using an eight-point calibration curve by plotting the ratio (peak area of the analytes / peak area of the internal standard) versus analyte concentration. Precision was evaluated by repeated analysis of quality control (QC) urine samples. Two sets of internal urine pools, one high and the other low in BPA, were analyzed every day for four days, and the intra- and inter-day coefficient of variation (CV) was computed. Accuracy was evaluated by comparisons of our measured values versus defined concentrations of the standard reference material obtained from NIST (SRM 3673, urine).
Results and discussion
BPA is present in urine at very low levels, often below 1 ng/mL, in healthy populations and therefore poses great challenges for the accurate quantitation. Traditionally, BPA analysis required extensive sample preparation procedures to enrich BPA in order to be accurately detected. GCMS was previously reported for the analysis of BPA, but this requires large amount of starting material, extensive cleanup procedures and a derivatization step to yield a volatile product that can be ionized. In contrast, LCMS is more advantageous because the sample preparation steps are much less complicated and turn-around is much faster.
On-line SPE coupled to HPLC followed by (−)APCI MS was reported for the analysis of urinary BPA with a limit of detection of 0.1~0.4 ng/mL [7]. However, this method requires the injection of large amount of urine samples, thus leads to frequent change of online-SPE cartridges. Moreover, repeated injection of large amount of urine will decrease MS sensitivity and cause high CVs. Therefore, this method is less favorable for epidemiology studies that require thousands of samples to be analyzed with short turnaround times. Herein, our goal is to develop a sensitive, fast, and efficient method to detect BPA with a small volume of biological samples.
Dansylation has been widely used as an effective derivatizing reagent for modifying phenolic compounds because the sulfonyl chloride moiety readily and quantitative reacts with phenols [11] and the resulting dansyl (DS) adduct exhibits enhanced sensitivity for fluorescence analysis [12] and also for (+)ESI-LCMS detection. The latter reaches limits of detection of 5 pg/mL due to the high proton-affinity of the dansyl tag [8]. During our most recent studies to improve the LCMS sensitivity of phenolic steroids, we found a structurally similar sulfonyl compound that exhibit better sensitivity over DS [13]. In the current study, we translated this experience to BPA analyses by tagging the new 1-methylimidazolyl-sulfonyl moiety to BPA and compare that product to the DS derivative.
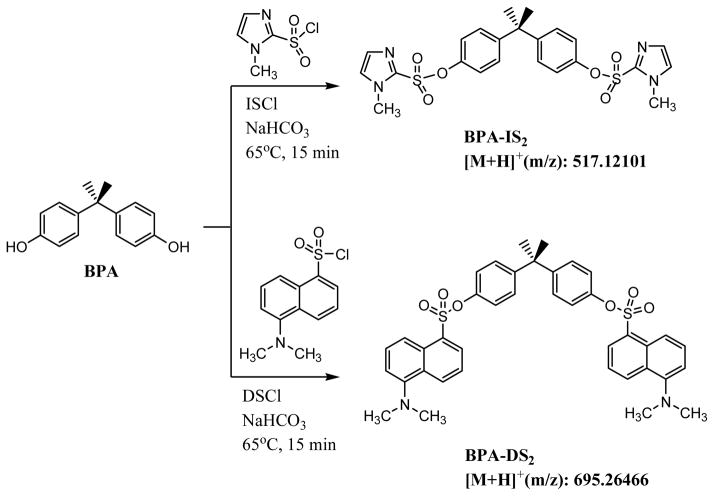
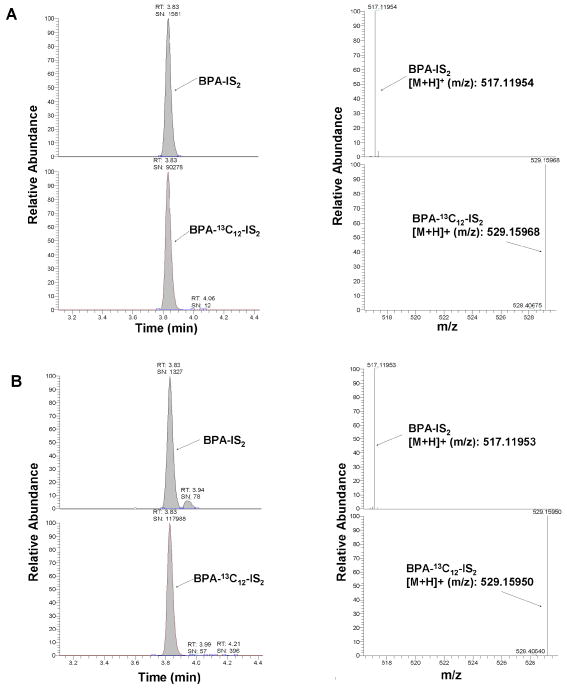
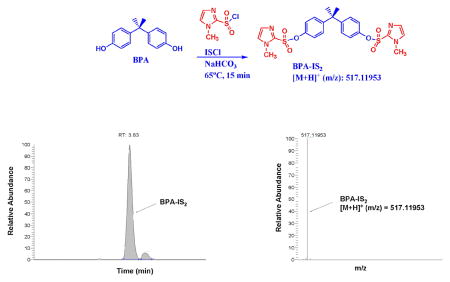
Similar to dansyl chloride, 1-methylimidazole-2-sulfonyl chloride (ISCl) contains a sulfonyl chloride functional group that underwent rapid and quantitative reaction with phenolic alcohols. The derivatization reaction of extracted urine with ISCl was carried out in a similar fashion as DSCl in the presence of aqueous sodium bicarbonate to afford quantitatively a double tagged BPA product (scheme 1). We optimized the reaction by variations of bases, solvents, reaction times, and temperature ranges. An important factor that we found to affect derivatization is the quality of the sulfonyl chloride reagent. By keeping dansyl chloride and 1-methylimidazole sulfonyl chloride in desiccators we could maintain their reactivity. The obtained product was directly subjected to HPLC analysis using a 50 mm Hypersil Gold C18 column. We found that this column is more selective and faster than an Agilent SB C18 column. BPA-IS and interferences carrying the same mass were well-separated and BPA-IS eluted in less than 4 min with a total run time of 8 min (Figure 1). On the other hand, BPA-DS was best chromatographed on an Agilent SB C18 column and eluted at around 11 min with a total run time of 16 min. Since the IS tag is smaller than the DS tag (ca. 60% by MW) and its product less lipophilic, its elution is faster thereby requiring less solvent. MS analysis was carried out with orbitrap (+) ESI MS (model Q Exactive) because we found this instrument in most of our applications more sensitive compared to tandem MS [11]. The limit of detection (LOD) was determined for full scan (FS) and targeted SIM (tSIM) using a signal to noise ratio of 3. Table 1 shows the specific ion masses targeted in tSIM mode and the parameters used for quantitation of BPA, BPA-13C12-IS, and BPA-DS. Our results indicate that IS products can be measured more sensitively than DS products: in FS MS and tSIM mode, the LOD of BPA-IS were 1.9 pg/mL and 0.12 pg/mL, which is 5 times and 9 times lower than BPA-DS, respectively. The BPA-IS calibration curve is highly linear (R2 > 0.999) in the experimental concentration range of 0.1 ~ 200 ng/mL. Moreover, we found that in the lower calibration range of 0.1 ~ 10 ng/mL the IS curve (R2 > 0.99) showed a better linearity than the DS curve (R2 <0.98). Figure 1 showed typical chromatograms and mass spectra of the BPA-IS derivatives.
Scheme 1.
Derivatization reactions of bisphenol A (BPA) with 1-methylimidazole-2-sulfonyl chloride (ISCl) and dansyl chloride (DSCl).
Figure 1.
LC/MS chromatogram and spectrum of BPA-IS derivatives by (+ESI) tSIM from standards (A, 0.1 ng/ml) and a urine pool (B)
The IS method was validated for precision and accuracy using internal urine pools and a NIST urinary standard reference material (SRM 3673) under tSIM MS conditions (Table 2). Precision was evaluated using two sets of internal urine pools, one quality control (QC) at low (QC1) and one at high (QC2) BPA level, all analyzed in triplicates for four consecutive days. The low-level QC1 (mean level= 2.00 ng/mL) showed an intra-day CV of 7~13% (mean 11%) and an inter-day CV of 11%; the high-level QC2 (mean level= 9.34 ng/mL) showed better CVs, namely 2~7% (intra-day) and 4% (inter-day). SRM 3673 showed even better precision (1–4%). Accuracy of our IS method was evaluated using SRM 3673 which was found to have a BPA level of 2.00 ± 0.10 ng/mL. Therefore, high accuracy of our proposed method was proven with a deviation of only 4% from the certified concentration (1.92 ± 0.11 ng/mL)
Table 2.
Validation of the IS method with urine samples by tSIM MS
| Concentration found (ng/mL) | Precision (CV) | Accuracy | ||
|---|---|---|---|---|
| Intra-day (n=3) | Inter-day (n=4) | - | ||
| QC1 (low) | 2.00± 0.22 | 7~13% | 11% | - |
| QC2 (high) | 9.34± 0.45 | 2~7% | 4% | - |
| SRM3673 | 2.00± 0.10 | 1~4% | 5% | 104% |
QC1= low level internal urine pool; QC2= high level internal urine pool; SRM 3673= standard reference urine purchased from NIST certified for 1.92 ± 0.11 ng/mL BPA
4. Conclusions
In this report, we developed a sensitive and accurate LCMS method for the determination of BPA in urine after derivatization with 1-methylimidazole-2-sulfonyl (IS) chloride in a simple and quantitative reaction. The resulting BPA-IS adduct showed a LOD of 0.12 pg/mL by tSIM and was 9 times more sensitive than the BPA-dansyl derivative. The precision and accuracy of the method was validated using internal quality control and SRM urine samples from NIST. The IS method requires much less turn-around-time and may also be useful for improving MS sensitivity of other phenol-containing compounds.
References
- 1.Lim DS, Kwack SJ, Kim KB, Kim HS, Lee BM. Potential risk of bisphenol A migration from polycarbonate containers after heating, boiling, and microwaving. Journal of toxicology and environmental health Part A. 2009;72 (21–22):1285–1291. doi: 10.1080/15287390903212329. [DOI] [PubMed] [Google Scholar]
- 2.Cao XL, Dufresne G, Belisle S, Clement G, Falicki M, Beraldin F, Rulibikiye A. Levels of bisphenol A in canned liquid infant formula products in Canada and dietary intake estimates. Journal of agricultural and food chemistry. 2008;56 (17):7919–7924. doi: 10.1021/jf8008712. [DOI] [PubMed] [Google Scholar]
- 3.Zalko D, Jacques C, Duplan Hln, Bruel S, Perdu E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere. 2011;82 (3):424–430. doi: 10.1016/j.chemosphere.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 4.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24 (2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom SFS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24 (2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300 (11):1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 7.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Analytical chemistry. 2005;77 (16):5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 8.Fox SD, Falk RT, Veenstra TD, Issaq HJ. Quantitation of free and total bisphenol A in human urine using liquid chromatography-tandem mass spectrometry. Journal of separation science. 2011;34 (11):1268–1274. doi: 10.1002/jssc.201100087. [DOI] [PubMed] [Google Scholar]
- 9.Mudiam MK, Jain R, Dua VK, Singh AK, Sharma VP, Murthy RC. Application of ethyl chloroformate derivatization for solid-phase microextraction-gas chromatography-mass spectrometric determination of bisphenol-A in water and milk samples. Analytical and bioanalytical chemistry. 2011;401 (5):1695–1701. doi: 10.1007/s00216-011-5226-6. [DOI] [PubMed] [Google Scholar]
- 10.Fan H, Li Y, Wu D, Ma H, Mao K, Fan D, Du B, Li H, Wei Q. Electrochemical bisphenol A sensor based on N-doped graphene sheets. Analytica Chimica Acta. 2012;711 (0):24–28. doi: 10.1016/j.aca.2011.10.051. [DOI] [PubMed] [Google Scholar]
- 11.Franke AA, Custer LJ, Morimoto Y, Nordt FJ, Maskarinec G. Analysis of urinary estrogens, their oxidized metabolites, and other endogenous steroids by benchtop orbitrap LCMS versus traditional quadrupole GCMS. Analytical and bioanalytical chemistry. 2011;401 (4):1319–1330. doi: 10.1007/s00216-011-5164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naassner M, Mergler M, Wolf K, Schuphan I. Determination of the xenoestrogens 4-nonylphenol and bisphenol A by high-performance liquid chromatography and fluorescence detection after derivatisation with dansyl chloride. Journal of chromatography A. 2002;945 (1–2):133–138. doi: 10.1016/s0021-9673(01)01522-9. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Franke AA. Improved profiling of estrogen metabolites by orbitrap LC/MS. Steroids. 2014 doi: 10.1016/j.steroids.2014.12.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]