Abstract
Psychosis involves dysregulation of response to stress, particularly to negative valence stimuli. Functional magnetic resonance imaging studies of psychosis have shown hyperactivity in hypothalamus, hippocampus, amygdala, and anterior cingulate cortex, and orbitofrontal and medial prefrontal cortices. Sex differences in these deficits may be associated with steroid hormone pathway abnormalities, i.e., dysregulation of the hypothalamic pituitary-adrenal and -gondal axes. We predicted abnormal steroid hormone levels in psychosis cases would be associated with hyperactivity in hypothalamus, amygdala, and hippocampus, and hypoactivity in prefrontal and anterior cingulate cortices in a sex-dependent way, with more severe deficits in men than women with psychosis. We studied 32 psychosis cases (50.0% women) and 39 controls (43.6% women) using a novel visual stress challenge while collecting blood throughout functional magnetic resonance imaging procedures. Males with psychosis showed hyperactivity across all hypothesized regions, including the hypothalamus and anterior cingulate cortex by family-wise corrected significance. Females showed hyperactivity in the hippocampus and amygdala and hypoactivity in orbital and medial prefrontal cortices, the latter by family-wise correction. Interaction of case status by sex was significant in the medial prefrontal cortex and, marginally so, in the left orbitofrontal cortex, with female cases (vs. healthy females and males) exhibiting the lowest activity. Male and female cases compared with their healthy counterparts were hypercortisolemic, which was associated with hyperactivity in prefrontal cortices in male cases and hypoactivity in female cases. This was further associated, respectively, with low bioavailable testosterone in male cases and low estradiol in female cases. Findings suggest disruptions in neural-hormone associations in response to stress are sex-dependent in psychosis, particularly in the prefrontal cortex.
Keywords: Schizophrenia, Sex differences, HPA axis, HPG axis, Stress response, Negative valence affect
1. Introduction
Schizophrenia has been associated with deficits in emotion recognition, discrimination (Heimberg et al., 1992; Schneider et al., 1995; Kohler et al., 2000; Streit et al., 2001), and experience (Berenbaum and Oltmanns, 1992; Schneider et al., 1995; Quirk et al., 1998; Epstein et al., 1999; Penn et al., 2000). These deficits, first recognized by Bleuler, were found in non-psychotic first-degree relatives (Docherty et al., 1994; Toomey et al., 1999), suggesting they represent vulnerability for schizophrenia (Phillips and Seidman, 2008; Phillips et al., 2011). Functional magnetic resonance imaging (fMRI) and positron emission tomography studies of emotional arousal in schizophrenia, particularly response to negatively-valenced stimuli or the so-called stress response, have consistently shown increased activation in hippocampus, amygdala and anterior cingulate cortex, coupled with decreased activation in prefrontal cortex (Wik and Wiesel, 1991; Epstein et al., 1999; Phillips et al., 1999; Crespo-Facorro et al., 2001; Taylor et al., 2002; Paradiso et al., 2003; Williams et al., 2004; Fernandez-Egea et al., 2010; Habel et al., 2010; Li et al., 2010), although this pattern was not consistent across all studies (Habel et al., 2010; Taylor et al., 2002). In fMRI studies, blood-oxygen-level-dependent (BOLD) signal changes in anterior cingulate cortex have been related to severity of delusions (Holt et al., 2011) and amygdala with affective symptoms (Strakowski et al., 2011), suggesting the need for analyses of traits as well as disorder per se to understand brain activity associated with the stress response.
Brain activity (BOLD) response to tasks of negative valence stimuli regardless of type of emotion have been associated with physiologic responses, such as autonomic arousal (Wik et al., 1991) and hypercortisolemia (Collip and Nicolson, 2011), underscoring their validity as defining the nature of a “stress response task”. These brain activity responses in schizophrenia were not explained by visual deficits (Phillips et al., 1999; Reske et al., 2009; Anticevic et al., 2010), medication (Schneider et al., 1998; Phillips et al., 1999; Streit et al., 2001), or cognition (Ursu et al., 2011). However, sex differences in brain activity to negative valence stimuli have been associated with steroid hormone fluctuations in healthy females and in schizophrenia.
Functional MRI studies have shown hyperarousal to negatively valenced stimuli in healthy women compared to men (Borod et al., 1993; George et al., 1996; Lang et al., 1998; Bradley et al., 2001; Cahill et al., 2001; Canli et al., 2002; Wager et al., 2003; Wrase et al., 2003; McClure et al., 2004; McRae et al., 2008; Domes et al., 2010). The magnitude of hyperarousal varied across the menstrual cycle in women, with attenuation of hyperactivity in response to stress during mid-cycle compared with early follicular (McManis et al., 2001; Wrase et al., 2003; McClure et al., 2004; Goldstein et al., 2005; Derntl et al., 2008; McRae et al., 2008; Andreano and Cahill, 2010; Goldstein et al., 2010b) and increased prefrontal and anterior cingulate cortices during the luteal phase, when progesterone was heightened (Ossewaarde et al., 2010; Wang et al., 2007). Menstrual cycle variation contributed to understanding sex differences in response to stress in that men resembled women in early follicular (Goldstein et al., 2010b), a pattern also seen in rodents (Figueiredo et al., 2013). Hyperactivity of hypothalamus in healthy men versus women was consistent across studies, controlled for menstrual cycle status and negatively correlated with estradiol levels (Goldstein et al., 2010b; Andreano and Cahill, 2010). Further, sex differences in laterality in this circuitry were demonstrated (Pardo et al., 1993; Cahill et al., 1996; George et al., 1996; Canli et al., 1999; Hamann et al., 1999; Damasio et al., 2000; Schneider et al., 2000; Cahill et al., 2001; Canli et al., 2002). Together, studies of sex differences in the healthy brain underscore the need to investigate sex differences in this circuitry systematically in psychoses, given the abundance of evidence demonstrating disrupted stress responses in these disorders.
Brain regions that respond to negatively valenced stimuli also regulate the hypothalamic-pituitary-adrenal (HPA) and HP-gonadal (HPG) systems, which are dysregulated in schizophrenia (Goldstein, 2006). Gonadal hormones, such as estradiol, modulate risk of psychotic illness across the lifespan (Walder et al., 2013). Likewise HPA dysregulation, at the adrenal, pituitary and central nervous system levels, contribute to the pathophysiology and etiology of schizophrenia (Holtzman et al., 2013; Koolschijn et al., 2008; Walker et al., 2010). Hippocampus, amygdala, hypothalamus, and anterior cingulate cortex are linked to endocrine function and neuroprotective and neurotoxic responses to reproductive steroid exposures (Herzog, 1989). Glucocorticoid receptors are located in the hippocampus, hypothalamus, prefrontal and anterior cingulate cortices, areas that are dense in sex steroid hormone receptors (Pacak et al., 1995; Koob, 1999). The hypothalamus, hippocampus and amygdala are involved in the regulation of HPA and HPG hormones, and anterior cingulate, medial, and dorsolateral prefrontal cortices influence autonomic and endocrine function (Price, 1999) integrating bodily states and goal-directed behavior. These brain regions are some of the most highly sexually dimorphic regions in the brain, demonstrating in vivo sex differences in brain volumes and brain activity in healthy populations (Filipek et al., 1994; Witelson et al., 1995; Giedd et al., 1996; Murphy et al., 1996; Paus et al., 1996; Passe et al., 1997; Rabinowicz et al., 1999; Nopoulos et al., 2000; Goldstein et al., 2001; Williams et al., 2005; Derntl et al., 2008; McRae et al., 2008; Domes et al., 2010; Mather et al., 2010), and schizophrenia (Gur et al., 1999; Frederikse et al., 2000; Goldstein et al., 2002; Goldstein et al., 2007; Mendrek, 2007).
We previously argued that there is shared pathophysiology between sex differences in stress response circuitry deficits and endocrine dysregulation in schizophrenia that originate during key fetal periods of sexual differentiation (Goldstein, 2006). Our hypotheses are based on the premise that normal sexual dimorphisms go awry in the development of schizophrenia (Goldstein et al., 2002), resulting in sex differences in adult stress response and neuroendocrine function. We hypothesize that sex differences in abnormalities in this circuitry are shared with other major psychoses, such as bipolar psychoses, whose etiologic origins begin in fetal development during this sensitive period. Thus, we predict participants with psychoses compared with healthy controls will demonstrate elevated BOLD signal in subcortical stress response circuitry regions and hypoactivity in cortical inhibitory regions. Furthermore, we expect the level of hyperactivity will be greater in men than women, and associated with elevated cortisol and low gonadal hormone deficits (low free androgens in men with psychoses; low estradiol in women with psychoses). Finally, although analyses are exploratory given our sample sizes, we predict shared sex-dependent stress response deficits in non-affective and affective psychoses.
2. Methods
2.1. Sample
Participants for this study were selected from adult offspring of a community sample of women who were originally recruited during their pregnancies 45 years ago, and have been followed by our team over the last 20 years, studies known as the New England Family Studies (NEFS) (Goldstein et al., 2013). In a series of case-control and high risk studies, we identified offspring participants (in their mid-forties) with psychoses. Expert diagnosticians (J.G., L.S. and J. Donatelli, Ph.D.) reviewed all information collected from systematic diagnostic interviews (First et al., 1996) and medical records, if available, to determine final best estimate diagnoses (Goldstein et al., 2010a; Seidman et al., 2013), resulting in 114 cases with DSM IV psychoses and 108 comparable controls (Goldstein et al., 2013).
We recruited 32 participants (50% women) with psychoses and 39 healthy controls (~44% women) for this functional MRI (fMRI) study of sex differences in stress response circuitry and hormonal deficits in psychoses. Approximately 20% were non-New England Family Study subjects but were recruited using the same criteria and from the same community catchment area and were not different on any sociodemographic or clinical characteristic than the rest of the sample. “Psychoses” included so-called “non-affective psychoses” (schizophrenia, schizoaffective, depressed type and psychosis not otherwise specified) and “affective psychoses” (bipolar disorder with psychosis, schizoaffective disorder, bipolar type) (see Table 1), a categorization that has been previously validated in multiple studies (Faraone and Tsuang, 1985; Kendler et al., 1985; Goldstein et al., 2010a) and successfully applied by our group and others (Goldstein et al., 2010a). Healthy controls were adult offspring from the New England Family Study for whom parents and grandparents and parents’ and controls’ siblings were free of any known lifetime history of psychosis, bipolar, schizotypal, recurrent major depressive disorder, suicide attempts, or psychiatric hospitalizations, as described previously (Goldstein et al., 2010a). Human subjects and methods approval were at Harvard University, Brown University, Partners Healthcare system, and local psychiatric facilities. Written consent was obtained from all study participants, and subjects were compensated for their participation.
Table 1.
Demographic and clinical characteristics of participants with psychoses (PSY) and healthy controls (HC)
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Characteristic | Healthy control men (HC-M) (n=22) | PSY men (PSY-M) (n=16) | Healthy control women (HC-W) (n=17) | PSY women (PSY-W) (n=16) | ||||
|
| ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 45.32 | 3.00 | 43.06 | 5.37 | 45.00† | 2.06 | 40.69 | 6.26 |
|
| ||||||||
| Parental SESa | 6.62 | 1.51 | 5.36 | 1.83 | 4.85 | 1.24 | 6.45 | 1.59 |
| Education (years) | 14.45 | 2.65 | 13.33 | 3.09 | 13.91 | 1.73 | 13.78 | 2.15 |
| Estimated Full Scale IQb | 107.86 | 16.69 | 103.38 | 20.25 | 99.47 | 14.40 | 91.79 | 14.58 |
| Age at symptom onset (years) | -- | -- | 18.07 | 2.84 | -- | -- | 22.6 | 5.27 |
| Duration of illness (years) | -- | -- | 20.43 | 5.89 | -- | -- | 15.52 | 7.34 |
| Ethnicity (%Caucasian)c | 21/22 | 95% | 15/16 | 94% | 17/17 | 100% | 6/16 | 38% |
| Current psychotropic medicationd | -- | -- | 13/16 | 81% | -- | -- | 9/16 | 56% |
| Type of psychosise | -- | -- | 7 AP | 9 NP | -- | -- | 10 AP | 6 NP |
Significant mean difference in female participants: HC>PSY, t(31)=2.69, p<0.01.
Parental socioeconomic status (SES) was a composite index of family income, education, and occupation and ranged from 0.0 (low) to 9.5 (high).
Full Scale IQ estimated using the sum of age-scaled scores from the WAIS-R Vocabulary and Block Design subtests, and Sattler’s conversion table C-37 (Sattler, 1992) (p. 851). Data available on only 15 of 16 male PSY subjects and 15 of 16 female HC subjects.
One HC male and one PSY male were African American; Five PSY females were African American and two PSY females were Asian American.
Thirteen PSY men and nine PSY women were currently taking antipsychotic medications including aripiprazole, clozapine, olanzapine, paliperidone, perphenazine, quetiapine, risperidone, thiothixene, trifluoperazine and valproic acid.
AP = affective psychosis; NP = non-affective psychosis.
2.2. Sample description
Clinical and demographic characteristics are presented in Table 1. There were no significant differences between cases and healthy controls within sex, except for younger age of female cases compared with healthy women. (Given this, analyses controlled for age.) Of the 16 men with psychoses, 44% were classified affective, 56% non-affective. Of the 16 women with psychoses, ~62% were classified as affective, 38% non-affective. The male-female ratio of non-affective (specifically, schizophrenia) patients is typical for schizophrenia and reflects sex-dependent prevalence. Among subjects with psychoses, males reported younger ages of onset and longer illness durations than females, as previously demonstrated (Goldstein, 2006). The majority were Caucasian, with more minorities among cases than controls.
Given our previous work on sex differences in the healthy brain using this paradigm (Goldstein et al., 2005; Goldstein et al., 2010b), we recruited women during the late follicular/mid-cycle menstrual phase when sex differences in the healthy brain would be larger (co-occurring with higher estradiol and relatively low progesterone) than during early follicular timing. Here we present fMRI data in women (n=26) during mid-cycle timing, defined as 10–15 days from start of cycle. Seven women (three cases, four controls) did not have regular menstrual cycles due to conditions such as endometrial ablation or partial hysterectomy. However, average cycle length for women with and without psychoses was similar (controls: M = 30.26, SD = 3.46; cases: M = 29.21, SD = 2.95). There were no women in menopause assessed systematically by the standard, follicular stimulating hormone and estradiol level profile.
2.3. Clinical ratings
Mood and anxiety were assessed using the Profile of Mood States (POMS; McNair, 1992) and the Spielberger State-Trait Anxiety Inventory (STAI; Spielberger, 1983). The POMS rates the degree to which a number of affective adjectives apply to current mood state (0 – 4 scale). The STAI rates anxiety-related statements using a 1 – 4 scale, and differentiates “trait” from “state” anxiety. POMS and STAI were administered immediately pre- and post-scanning.
2.4. fMRI acquisition and paradigm
The fMRI studies were conducted using a Siemens Tim Trio 3T magnetic resonance scanner with a 12-channel head coil and the following parameters: 180 functional volumes were acquired using a spin echo, T2*-weighted sequence (repetition time=2000 ms; echo time=40 ms; field of view=200×200 mm; matrix=64×64; in-plane resolution=3.125 mm; slice thickness=5 mm; 23 contiguous slices aligned to the anterior commissure-posterior commissure plane). Our fMRI stress paradigm has been described previously and demonstrated reliability and validity in activating circuitry defining stress response to negative valence stimuli (Goldstein et al., 2005; Goldstein et al., 2010b). Briefly, participants viewed three stimulus blocks (6 images/block) for 30 s (1 image/5 s) ordered as: fixation, neutral, and negative. Subjects viewed this sequence four times during each of three runs (i.e., 72 images (24 images/condition/run), and pressed a button each time a new image appeared to ensure attention to images (see Fig. 1).
Figure 1. Illustrative Schemata of the fMRI Stress Response Task* and Timed Blood Draws.
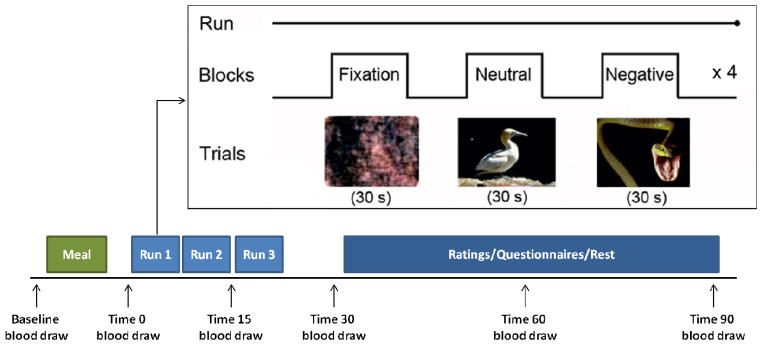
* fMRI task adapted from International Affective Picture System (Goldstein et al, 2005b; Lang et al, 2008; Goldstein et al, 2010b). Baseline blood draw was acquired at ~8am, fasting since midnight. A baseline in-scanner blood was acquired and then draws were timed to hormonal response to stress, i.e., pituitary (15, 30 min. post-stress challenge) and steroid hormones (60, 90 min. post-stress challenge).
Images were drawn from the International Affective Picture System (IAPS; Lang et al., 2008) according to affective valence and arousal (negative = unpleasant + high arousal, neutral = neutral + low arousal). This adapted set of images did not represent any particular IAPS-assessed emotion, but rather a set of images that evoked specifically quantified negative valence and high arousal levels, regardless of specific emotion and established in numerous population studies by the original Lang team (Lang et al., 1998). In numerous studies of ours, we demonstrated that this set of images evoked a stress response to negative imagery in the key brain regions of this circuitry that are associated with physiologic stress responses (e.g., Holsen et al., 2011; Holsen et al., 2013). Fixation images were based on Fourier transformations of each neutral image to create an image with the same physical properties of the original but without recognizable content. After fMRI, participants were shown two blocks of the negative and neutral images and provided subjective ratings of arousal using Self-Assessment Manikin (SAM; Bradley and Lang, 1994). Averaged scores from each block for negative and neutral images among cases and controls were similar (ts<1).
2.5. Novel hormonal sampling in imaging environment
Here we present a novel method for acquiring hormones in response to the visual stress challenge “in real time” during functional imaging. All subjects arrived at 7:30am for a fasting blood draw (overnight fasting after midnight). Blood samples were acquired throughout the scanning session, timed to hormonal responses based on pituitary (15–30 min post-visual stress challenge) and steroid hormones (60–90 min post-stress challenge) (see Fig. 1 for illustration of task and hormonal acquisition timing). When subjects arrived, nurses inserted a saline-lock intravenous (i.v.) line into the antecubital fossa of the non-dominant arm and baseline fasting blood was acquired. Participants then were given a small standardized breakfast and were administered questionnaires and then taken to the scanner.
Participant’s head was positioned at magnet’s isocenter, 5 min of pre-scan protocols were completed, and in-scanner baseline blood samples (Time 0) were acquired. Subsequent samples were drawn at 15 and 30 min in-scanner and 60 and 90 min out-of-scanner in a quiet room (approximately 30 cc of blood). Clotting time was allotted, samples spun to separate sera from blood cells and stored at −80 °C at the Brigham and Women’s Hospital-Harvard Partners Center for Genetics and Genomics. Harvard Clinical Translational Science Center laboratory analyzed hormones (estradiol, progesterone, testosterone, cortisol, sex hormone binding globulin (SHBG), and diepiandrostendione-sulfate (DHEAS)) in duplicate with commercial radioimmunoassay (RIA) kits [estradiol (sensitivity 20 pg/mL, intra-assay variation 12–21%), progesterone (sensitivity 0.08 ng/ml, intra-assay variation 6.11–11.19%), testosterone (sensitivity 10 ng/dl, intra-assay variation 4.22–7.08%), cortisol (sensitivity 0.04 μg/dl, intra-assay variation 4.4–6.7%), SHBG (sensitivity 0.33 nmol/l, intra-assay variation 4.5–4.8%), and DHEAS (sensitivity 2 μg/dl, intra-assay variation 1.6–8.3%): Access Immunoassay System, Beckman Coulter, Miami, FL]. DHEAS has anti-glucocorticoid action, and thus cortisol:DHEAS was used as a standard functional measure of hypercortisolemia, at 90 min post-visual stress challenge. The Free Androgen Index was calculated as the standard: [(Testosterone X 3.47)/SHBG].
2.6.1. fMRI data processing
Participants’ functional runs were pre-processed using Statistical Parametric Mapping-8 (Neuroimaging, 2008): motion realignment, normalization to Montreal Neurological Institute template, and spatial smoothing at 6-mm full width at half-maximum, which was then re-sampled to 3 mm isotropic. Statistical Parametric Mapping-8 analyzes voxels’ blood oxygen-level dependent (BOLD) time series applying a high-pass filter (180 s) to control for low-frequency scanner drift and modeling the time series with general linear models as a separate boxcar function convolved with a canonical hemodynamic-response basis function. Additionally, we added a regressor of no interest for each volume that created an artifactual change in global signal intensity. These volumes were identified using the artifact detection tool for Statistical Parametric Mapping-8 (Whitfield-Gabrieli, 2011), corresponding to participant movement between volumes >0.7 mm or a change in global signal intensity >3 standard deviations from the mean.
Masks were created excluding voxels outside the brain and including voxels in the brain regardless of signal intensity, to ensure that voxels in regions with high inter-participant variability in signal drop-out (e.g., orbitofrontal cortex) were not arbitrarily excluded. Linear contrasts of the effect of negative minus neutral images were used to create statistical parametric maps where significant voxels showed greater activation for negative than neutral stimuli for individuals and combined for group analyses.
2.6.2. Group statistical analyses
Independent sample t-tests were used to compare groups (within-sex; psychoses vs. healthy controls; non-affective versus affective psychoses) on the main contrast of interest (negative – neutral), treating participants as a random effect. Given specific hypotheses about stress circuitry and our previous work (Goldstein et al., 2005; Goldstein et al., 2010b), we used a priori regions of interest for small-volume correction of the results. Regions of interest were identified by manually segmenting the Montreal Neurologic Institute-152 brain template into hypothalamus, amygdala, anterior hippocampus, parahippocampal gyrus, orbital, medial prefrontal and anterior cingulate cortices, and periaqueductal gray and implemented as overlays on the Statistical Parametric Mapping-8 canonical brain using the Wake Forest University PickAtlas Region of Interest toolbox for Statistical Parametric Mapping (Maldjian et al., 2003). We applied a voxel-wise height threshold of p <0.05 (uncorrected for multiple comparisons), and a cluster was deemed significant if small-volume correction using anatomical regions of interest resulted in a peak-level family-wise error-corrected (FWE) p-value <0.05.
Following between-group analyses within Statistical Parametric Mapping-8, percent signal changes within a region of interest were extracted for each participant using the region-of-interest-extraction (REX) toolbox for Statistical Parametric Mapping-8 (Whitfield-Gabrieli, 2009). The percent signal change value represents the percent change in BOLD signal in the negative > neutral condition averaged across all voxels within an anatomical region of interest. This procedure extracts BOLD signal within an a priori region of interest using independently-derived anatomical coordinates (i.e., not based on results from between-group independent sample t-tests within Statistical Parametric Mapping-8). These percent signal change values were then exported to SAS (2001) for the following additional analyses.
First, percent signal change values were used to calculate an effect size difference (Cohen’s d) for regions of interest which met a threshold of p < .05 (uncorrected for multiple comparisons) between groups (e.g. Psychoses versus Healthy controls): d = 2t / √df, where t is the two-tailed independent samples t-test value for the between-group comparison of the percent signal change attributable to negative images (relative to neutral). For regions of interest demonstrating significant group differences (p < 0.05, FWE-corrected), steroid hormones were entered as covariates into mixed linear models to assess their impact on BOLD percent signal changes between-groups (e.g., Psychoses vs. Healthy Controls), within sex (given sex differences in gonadal hormones) and between sexes with regard to adrenal hormone responses. Hormone levels in the mixed models were natural log (ln) transformed due to significant skew |> 0.8| in order to normalize the distributions for analyses. Models within sex included estradiol and progesterone in women and free androgen index in men, and models shared across sex included cortisol:DHEAS levels (a standard measure of functional cortisolemia) 90 min post-stress challenge, controlled for baseline in-scanner values. We used 90-min values, controlled for in-scanner baseline, in order to assess in real time the physiologic hormonal responses to the stress challenge in tandem with the neural response. Finally, as a post-hoc exploratory analysis, regions of interest identified from the original Statistical Parametric Mapping-8 analyses above (within-sex, between-group results) which met statistical thresholding in one sex, but not the other, were analyzed using mixed linear models (SAS, 2001) to examine group (Psychoses, Healthy Controls) by sex interactions in percent signal change values in these regions of interest.
3. Results
3.1. Sex differences in stress circuitry
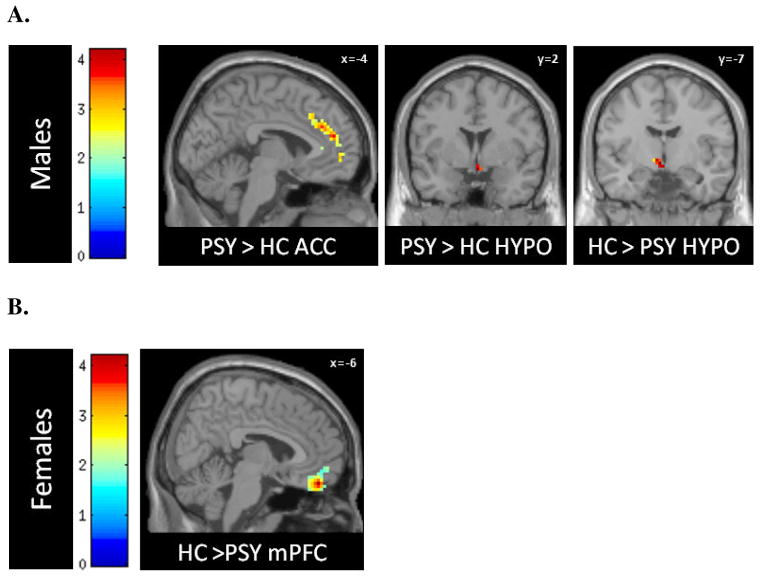
As seen in Table 2, compared with control males, males with psychoses showed significantly higher activity in most of the hypothesized stress response regions [right hypothalamus (z = 2.3; left anterior cingulate cortex (z = 3.08); medial prefrontal cortex (z = 1.73); bilateral orbiofrontal cortex (z = 2.05 [left] and z = 2.10 [right]; right parahippocampal gyrus (z = 2.12); and periaqueductal gray (z = 1.73)], with right hypothalamus and anterior cingulate cortex significant with FWE correction, p < 0.05. The only region showing significantly less activation in response to negative stimuli was left hypothalamus (FWE-corrected, p < 0.05, z = 2.48; see Table 2), but accompanied by significantly greater activation in right hypothalamus. Females with psychoses compared to healthy females showed hyperactivity in bilateral amygdala (z = 1.73 [left] and z = 1.71 [right]) and left anterior hippocampus (z = 1.95), and hypoactivity in medial prefrontal cortex (z = 2.91) and left orbitofrontal cortex (z = 1.84), with the former remaining significant at p < 0.05 after FWE-correction (see Table 2 and Fig. 2A,B).
Table 2.
Regions of activation comparing negative to neutral stimuli in healthy control and participants with psychoses: within sex by group contrasts
| Contrast | Men (n=38) | Women (n=32) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Region of interest (ROI) | x | y | z a | voxel | Z | p-valueb | FWE-corrected p-valuec | dd | x | y | z a | voxel | Z | p-valueb | FWE-corrected p-valuec | dd | |
| PSY > HC | |||||||||||||||||
|
| |||||||||||||||||
| L | Amygdala | −30 | −7 | −23 | 1 | 1.73 | 0.042 | 0.134 | 0.45 | ||||||||
| R | 27 | −4 | −20 | 1 | 1.71 | 0.044 | 0.139 | 0.37 | |||||||||
| R | Hypothalamus | 6 | 14 | −5 | 4 | 2.3 | 0.011 | 0.045 | 0.73 | ||||||||
| L | Anterior hippocampus | −33 | −19 | −17 | 5 | 1.95 | 0.025 | 0.09 | 0.44 | ||||||||
| R | Parahippocampal gyrus | 15 | −37 | −2 | 3 | 2.12 | 0.017 | 0.068 | 0.45 | ||||||||
| L | Anterior cingulate cortex | −6 | 47 | 19 | 18 | 3.08 | 0.001 | 0.026 | 0.29 | ||||||||
| L | Orbital frontal cortex | −42 | 32 | −14 | 6 | 2.05 | 0.02 | 0.213 | 0.39 | ||||||||
| R | 33 | 26 | −20 | 13 | 2.1 | 0.018 | 0.198 | 0.50 | |||||||||
| Medial prefrontal cortex | −3 | 56 | −8 | 12 | 2.05 | 0.02 | 0.212 | 0.51 | |||||||||
| Periaqueductal gray | 0 | −31 | −8 | 1 | 1.73 | 0.042 | 0.137 | 0.40 | |||||||||
|
| |||||||||||||||||
| HC > PSY | |||||||||||||||||
|
| |||||||||||||||||
| L | Hypothalamus | −6 | −7 | −5 | 8 | 2.48 | 0.007 | 0.031 | −0.73 | ||||||||
| L | Orbital frontal cortex | −45 | 26 | −14 | 2 | 1.84 | 0.033 | 0.272 | −0.44 | ||||||||
| Medial prefrontal cortex | −6 | 53 | −14 | 44 | 2.91 | 0.002 | 0.038 | −0.79 | |||||||||
Coordinates are presented in Montreal Neurologic Institute space.
Voxel-wise Z-score significance level p<0.05 uncorrected for multiple comparisons within a hypothesized region of interest; ROIs listed represent regions of significantly activated clusters within the a priori hypothesized ROI.
FWE rate (family-wise error rate) used for small volume correction: voxel-level significance level (FWE-corrected within the search volume of interest).
Effect sizes (d=Cohen’s d) based on average percent signal change values (beta weights averaged across an anatomical ROI) were obtained using the REX toolbox for Statistical Parametric Mapping-8.
FIGURE 2. Stress Response Circuitry Deficits in Psychoses in Male (A) and Female (B) Cases versus Healthy Controls.
A and B: Activations of hypothesized regions of interest were derived using the small volume correction tool in SPM8, restricted to anatomical borders defined by a manually segmented MNI brain. Peak voxel activations were significant at p<.05, FWE-corrected.
A) Male psychosis cases (PSY) showed significant hyperactivity compared to male controls in right hypothalamus (HYPO) and anterior cingulate cortex (ACC), and hypoactivity in left hypothalamus (HYPO).
B) Female cases showed hyperactivity in subcortical arousal regions, and hypoactivity in medial prefrontal cortex (mPFC) by FWE-correction and orbitofrontal cortex (not shown here, given trend-level significance).
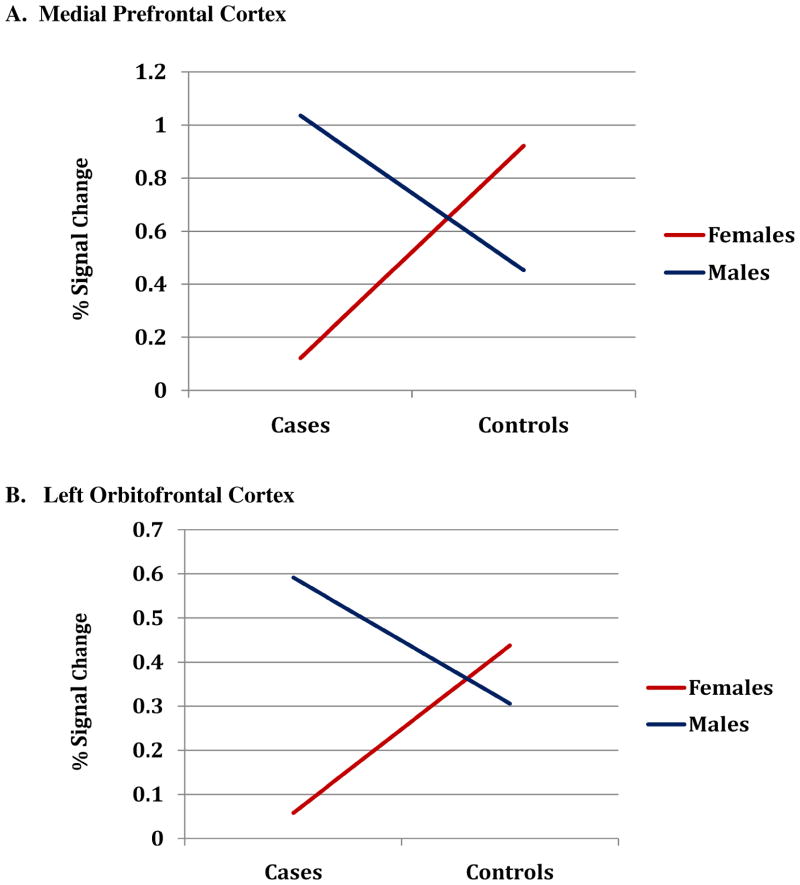
Important for demonstration of sex differences, there was a significant interaction of sex by case status in BOLD signal changes in medial prefrontal cortex (β=0.134, p=0.01) and marginally significant for left orbitofrontal cortex (β=0.07, p=0.09), with female cases exhibiting less activity than female controls and males (see Fig. 3).
Figure 3. Interaction of Case Status by Sex on BOLD signal intensity changes in Prefrontal Cortices (A) medial prefrontal cortex and (B) left orbital frontal cortex.
Interaction of sex by group on BOLD signal changes (negative > neutral stimuli) in response to stress were tested using mixed linear models (SAS 9.3, 2002–2010). Average percent BOLD-signal change (PSC) within an ROI was extracted for each subjects using ROI-extraction (REX) toolbox for SPM8 (Whitfield-Gabrieli, 2009). This value represents the average of PSC values across all voxels within an anatomical ROI. Interactions are illustrated (above) by sex-specific lines connecting mean PSC for cases and controls (mPFC: Females, Case (0.12) < Control (0.91); Males, Case (1.04) > Control (0.45). L_OFC: Females, Case (0.06) < Control (0.48); Males, Case (0.59) > Control (0.31). (a) mPFC (interaction: β = 0.13, P = 0.01), and (b) L_OFC (interaction: β = 0.07, P = 0.09).
3.2. Are steroid hormone deficits associated with sex differences in brain activity deficits?
Supplementary Table 1s is a descriptive table to present steroid hormone levels (untransformed) for cases and healthy controls by sex. In summary, at fasting baseline, males with psychoses had a lower free androgen index (M = 50.3 vs. M = 58.4, d = 0.43). Females with psychoses had lower estradiol levels than control females (M = 63.8 pg/ml vs. M = 91.0 pg/mL; d = 0.51) and higher progesterone (M = 4.2 ng/mL vs. M = 2.0 ng/mL; d = 0.49). Despite the medium effect sizes between cases and controls, mean differences (when log-transformed) were not significant (ps > 0.15), most likely given small sample sizes and substantial variability among the cases.
Regarding adrenal hormones, males and females with psychoses compared to healthy controls expressed higher levels of cortisol 90 minutes post-visual stress challenge: Males with psychoses vs. control males (M = 12.04 μg/dl vs. M = 9.95 μg/dl, d = 0.47); females with psychoses vs. control females (M = 10.78 μg/dl vs. M = 8.44 μg/dl, d = 0.52). In contrast, diepiandrostendione-sulfate (DHEA-S) was lower in males with psychoses vs. controls (M = 179.5 μg/dl vs. M = 220.96 μg/dl, d= 0.49), but higher in females with psychoses vs. controls (134.75 μg/dl vs. M = 108.40 μg/dL; d= −0.39). Cortisol: DHEAS ratio is typically used as a measure of functional cortisolemia.
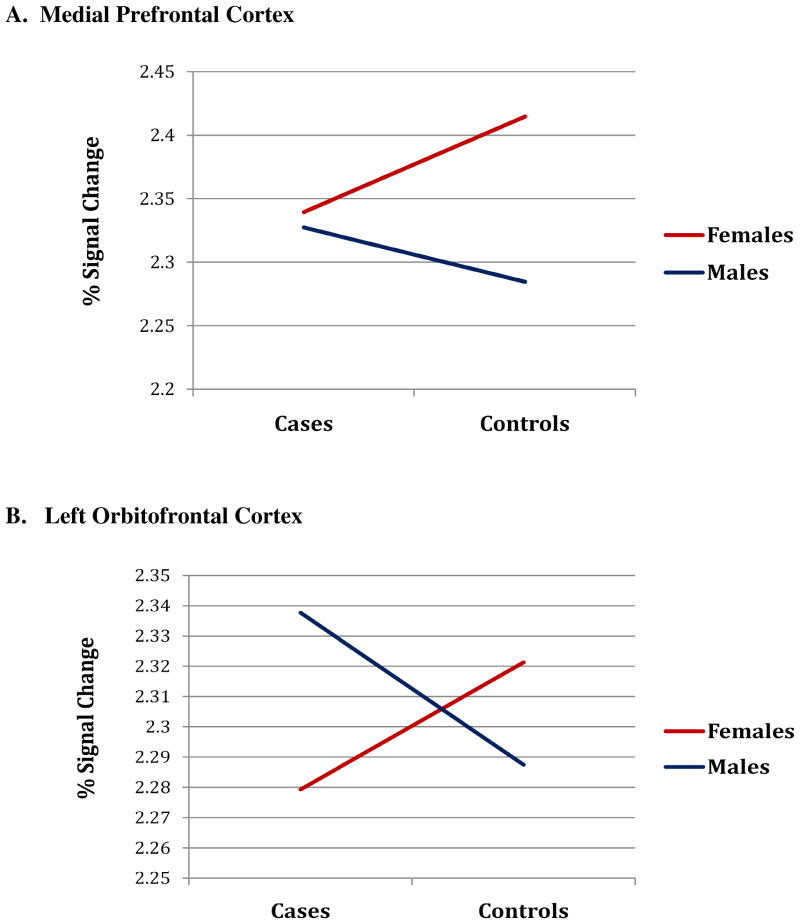
Hormonal abnormalities [in both sexes, cortisol:DHEAS response at 90 min post-stress challenge, controlled for in-scanner baseline level; in women, estradiol; in men, free androgen index] were entered into mixed models for regions showing significant BOLD changes in cases vs. controls by sex (i.e., medial prefrontal and left orbitofrontal cortices). As illustrated in Fig. 4A,B, high cortisol:DHEAS (i.e., hypercortisolemic) response to stress was associated with hyperactivity in prefrontal cortices in male cases and hypoactivity in prefrontal cortices in female cases compared with their healthy control counterparts. When cortisol:DHEAS was added to the model, it attenuated the beta estimates for the case-by-sex interaction by 72% in both medial prefrontal and left orbitofrontal cortices, thereby explaining much of the variance in the interaction with sex.
FIGURE 4. Interaction of Sex by Case Status on Signal Intensity Changes in Prefrontal Cortices at High Levels of Cortisol:DHEAS (i.e., hypercortisolemia).
Graphs represent mean percent signal change (natural log transformed BOLD) in the medial prefrontal cortex (A) and left orbitofrontal cortex (B) for subjects in the highest 75th percentile of the healthy control cortisol:DHEAS distribution at time 90. Hypercortisolemia in male cases was associated with higher medial prefontal and left orbitofrontal cortex signal changes compared with healthy controls and lower activity for female cases compared with healthy controls
Impact of low free androgen levels on hyperactivity in medial prefrontal cortex among the male cases versus controls was significant (β = 0.10, p<0.05), an effect that was, in part, accounted for by the high cortisol:DHEAS levels in male cases [i.e., in mixed model with androgens and cortisol:DHEAS, the beta for androgens was attenuated from β = 0.10 to β = 0.09, p=0.08]. Low estradiol was associated with hypercortisolemia in female cases with little correlation among the controls (Spearman’s r = −0.49 vs. −0.07, respectively). However, low estradiol did not account for variance in the impact on prefrontal cortex over and above hypercortisolemia in female cases vs. controls.
3.3. Does psychosis type matter?
Although we had less statistical power to test for differences by psychosis type, we conducted exploratory analyses of non-affective and affective psychoses cases and controls to provide initial insight into specificity of findings that could then be replicated in future studies (see Supplementary data, Table 2s). Briefly stated, in response to the mild stressful stimuli, males with non-affective versus affective psychoses were primarily similar except in right parahippocampal gyrus (p <0.05, z = 2.64). In contrast, females with non-affective compared with affective psychoses expressed significantly lower activity in the left hypothalamus (z = 3.28), right parahippocampal gyrus (z = 2.32), and periaqueductal gray (z = 2.24) and left anterior cingulate cortex (z = 3.21), all significant FWE-corrected (see Table and Fig. in 2s). Further, males versus females with non-affective psychoses showed significantly greater BOLD signal changes in left hypothalamus (z = 3.11) and right parahippocampal gyrus (z = 2.76) (p <0.05, FWE-corrected), with trend-level differences in left anterior cingulate cortex (p = 0.07, z = 2.80) and periaqueductal gray (p =0.07, z = 2.76). Males versus females with affective psychoses were similar. Thus, in response to a mild visual stress challenge, females compared to males with non-affective psychoses were significantly different (i.e., less BOLD response) than male and female healthy controls. Interaction tests were not significant, given the small sample sizes when separated by sex and psychosis type, thus replication is necessary. Hormonal abnormalities in association with brain activity deficits within males and females were similar regardless of psychosis type.
3.4. Potential confounds
Regarding psychotropic medications, there were three males and seven females who were unmedicated. While unmedicated males with psychoses had slightly elevated levels of brain activity compared to medicated males with psychoses, these values fell within one standard deviation of the male psychoses group mean (except for left amygdala and orbitofrontal cortex falling within 1.5 standard deviations). When medicated subjects were removed from the analysis, hyperactivity in case males compared with healthy control males was attenuated, but not eliminated. Hypoactivity in the left hypothalamus remained significant. For females, removing subjects on medications also attenuated (but did not eliminate) case-control differences, and hypoactivity in the medial prefrontal cortex remained significant. Thus, these findings demonstrated that, not surprisingly, medications affect brain activity in case men and women, but findings held in the unmedicated subjects. We had a small number of male cases off medications, and therefore our finding needs replication.
Regarding anxiety, again not surprisingly, cases were more anxious than control participants (see Table 3), measured by pre-scan state anxiety, post-scan state anxiety, and trait anxiety. However, change in state anxiety from pre- to post-scan did not vary by psychiatric status. Moreover, male and female cases and controls reported similar valence ratings (t (63) = −.38, n.s.) and arousal ratings of negative images (t (63) = 0.09, n.s.), and no significant interactions were present. Thus, state anxiety was not driving case-control differences in brain activity deficits by sex.
Table 3.
Mood and anxiety ratings in healthy control and participants with psychoses
| Men | Women | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Characteristic | Healthy control men (n=20) | PSY men (n=16) | Between-group comparisons | t | p | Healthy control women (n=17) | PSY women (n=16) | Between-group comparisons | t | p | ||||
|
| ||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||||
| Spielberger State-Trait Anxiety Inventory | ||||||||||||||
| Trait anxiety score | 32.50 | 6.46 | 41.75 | 11.73 | PSY>HC | 3.12 | .004 | 29.50 | 4.87 | 40.07 | 7.33 | PSY>HC | 4.76 | 0.00 |
| Pre-scan | PSY>HC | 3.19 | .004 | |||||||||||
| State anxiety score | 29.50 | 7.76 | 37.43 | 6.68 | 28.76 | 7.57 | 32.94 | 8.55 | ||||||
| Post-scan | 30.86 | 9.49 | 37.60 | 11.91 | 29.44 | 6.48 | 36.06 | 10.14 | PSY>HC | 2.62 | 0.04 | |||
| Change in state, pre- to post-scan | 1.65 | 6.64 | − 0.54 | 7.76 | 1.38 | 4.40 | 3.13 | 8.57 | ||||||
|
| ||||||||||||||
| IAPS stimuli ratingsa | ||||||||||||||
| Negative arousal | 5.05 | 2.33 | 4.79 | 2.14 | 4.47 | 2.10 | 4.68 | 2.56 | ||||||
| Negative valence | 7.35 | 1.05 | 7.29 | 1.55 | 7.47 | 1.42 | 7.79 | 1.59 | ||||||
STAI (Spielberger, 1983) rates anxiety-related statements using a 1 – 4 scale (“not at all” to “very much so”) with two subscales differentiating “trait” from “state” anxiety. After the fMRI scanning session, subjects rated a selection of the IAPS stimuli on valence and arousal using the Self-Assessment Manikin (SAM) (Bradley et al., 1994). No significant sex by case interaction effects.
4. Discussion
Compared with control males, males with psychoses expressed hyperactivity in most of the hypothesized stress response regions, demonstrating substantial effect sizes that were present regardless of psychosis type. In contrast, females with psychoses compared with healthy females showed hyperactivity in subcortical stress response regions and anterior cingulate cortex, and hypoactivity in orbital and medial prefrontal cortices, the latter of which were significantly different from males. We had adequate statistical power to test for sex differences in psychoses, and the sample presented here was generally representative of the population from which they were drawn, as shown in a recent publication (Goldstein et al., 2014a).
We further found that differences across group (psychoses vs. healthy controls) and sex were differentially associated with steroid hormone abnormalities. Hypercortisolemia was present in male and female cases compared to their healthy counterparts, but had a differential effect on brain activity deficits in prefrontal cortex in males and females. Hypercortisolemia was associated with hyperactivity across stress response regions in men with psychoses, including prefrontal cortices. In contrast, hypercortisolemia was associated with hypoactivity in medial prefrontal (and orbitofrontal) cortices in females with psychoses, a difference that was not present among male and female controls. Not surprising, hypercortisolemia in cases was associated with low gonadal hormone expression regardless of sex (i.e, for male cases, low free androgen, and for female cases, low estradiol). The impact of low androgens on explaining hyperactivity in prefrontal cortex in male cases was only, in part, explained by hypercortisolemia, whereas the variance accounting for hypoactivity in prefrontal cortices in female cases was explained through its relationship to hypercortisolemia. These findings suggest adrenal and gonadal hormone abnormalities are associated with brain activity deficits in stress response regions but have differential effects on brain dependent on sex.
Neural-hormone deficits are not surprising given that stress response circuitry regions, such as anterior hypothalamus, amygdala, and hippocampus, are governed by the coordinated action of HPG and HPA axis hormones. They are regions dense in estrogen, progesterone, androgen, and glucocorticoid receptors (Kato et al., 1994; Donahue et al., 2000; Osterlund et al., 2000a; Osterlund et al., 2000b; Guerra-Araiza et al., 2002; McClellan et al., 2010; Zuloaga et al., 2011; Stratton et al., 2011). In fact, as evident in the cases in this study, HPA dysregulation, i.e., hypercortisolemia, had a significant impact on attenuating HPG response (i.e., lower gonadal hormone expression). There is a long history to the idea that HPA dysregulation is implicated in schizophrenia (Walker and Diforio, 1997), described as hypercortisolemic and hyperresponsive to stress (Breier et al., 1988; Walder et al., 2000), physiologic responses attributed to bipolar psychoses as well. Previous work, including our own, also demonstrated abnormalities in gonadal hormone levels (lower in cases) (Seeman and Lang, 1990; Häfner et al., 1991; Canuso et al., 2000; Kulkarni et al., 2001) and endocrine function (Beumont et al., 1974; Ghadirian et al., 1982; Sullivan and Lukoff, 1990; Reicher-Rossler et al., 1994). Findings in the study presented here extend earlier work demonstrating brain-hormone deficits may be shared across psychotic disorders.
Dysregulation of brain-hormone associations in women was also found in our recent study of HPG abnormalities in women with major depression compared with healthy controls, in a sample from the same New England Family Study population cohort (Jacobs et al., 2015). In that experimental within-woman design, 17β estradiol was significantly related to attenuation of BOLD activity in key subcortical stress response regions in healthy women, but no modulation by 17β estradiol in depressed women. In our previous study in these women with depression using the same fMRI paradigm as in the current study of psychoses, they were also hypercortisolemic, which related to hyperactivity across the stress response circuitry (Holsen et al., 2013). We suggested that abnormalities in the paraventricular hypothalamic nucleus may be one of the regions driving the steroid hormone deficits, given that in clinical, postmortem and preclinical studies, it has been implicated in depression (Bao et al., 2005; Tobet et al., 2013; Goldstein et al., 2014b). Abnormalities in this region in depression in women may be shared with psychoses in women.
In fact, we previously demonstrated structural abnormalities (increased volume) in the hypothalamus in schizophrenia, particularly in females in the area that included the paraventricular hypothalamic nucleus (Goldstein et al., 2007), which was also significantly associated with increased anxiety (Goldstein et al., 2007). The paraventricular hypothalamic nucleus (located in anterior hypothalamus) has the highest density of corticotropin releasing hormone in the brain and is the key brain region central to HPA-HPG axes function. Thus, abnormalities in this region could be mechanistically involved in a hyperactive subcortical stress response in psychoses for which, in females, there is less ability to inhibit or regulate by prefrontal cortex. Further, pituitary abnormalities in schizophrenia may also be present, given earlier work reporting pituitary volume abnormalities in first episode female schizophrenia (Pariante et al., 2004).
Together, our previous work on volumetric abnormalities in anterior hypothalamus coupled with findings here demonstrating hyperactivity in hypothalamus and other subcortical regions in response to stress and less ability to inhibit arousal, particularly by medial prefrontal cortex, underscore the importance of mechanistically understanding sex differences in neural-hormone deficit associations with psychoses compared with healthy controls. The studies suggest that sex differences in psychoses and disorders of mood/anxiety may share pathophysiology associated with mood dysregulation and anxiety, hypersensitivity to stress, and steroid hormone dysregulation, which may contribute to understanding some of the shared pathophysiology we found between non-affective and affective cases. In fact, previously, we also demonstrated that steroid hormone abnormalities were associated with sex-dependent deficits in arousal circuitry shared between stress circuitry and fear conditioning (Lebron-Milad et al., 2012; Lebron-Milad and Milad, 2012).
As discussed, a potential limitation of our study for investigating psychoses specificity is the small sample size for comparisons of non-affective and affective cases by sex. While our main comparisons of sex by psychoses case status had sufficient numbers, the more refined analyses of non-affective and affective cases by sex had low statistical power. Although results were exploratory and relegated to a Supplement, there were some significant brain activity differences within sex by psychosis type, corrected for multiple comparisons, potentially reflecting large effect sizes. Still, findings must be replicated. Further, the majority of male cases in this study were on medications and thus these findings must be replicated in a larger unmedicated sample.
The regulation of the stress response has been implicated in nearly every chronic disease, including major psychoses. We demonstrated here that an understanding of this in the brain necessitates a sex-dependent lens that implicates abnormalities in steroid hormone pathways. Shared significant case-control differences among men and women across psychoses were primarily in subcortical regions, with significant sex differences primarily in the cortical inhibitory control of arousal. Together, these regions are among those with the highest density of steroid hormone receptors in the brain underscoring the validity of the neural-hormone associations presented here. Our novel strategy for assessing brain-steroid hormone responses to stress in vivo in real time allowed us to refine our understanding of neural-hormone associations in ability to regulate the stress response. Our results also provide insights for the development of innovative sex-dependent therapeutics that implicate hormonal modulation or supplementation to psychotropic medication that may be relevant across psychoses.
Supplementary Material
Highlights.
Using fMRI, sex differences exist in stress circuitry deficits in psychoses
Male cases were hyperactive across subcortical and cortical stress circuitry
Female cases were hypoactive in prefrontal cortex
Brain activity deficits in medial prefrontal cortex were significant by sex
Neural-steroid hormone associations under stress are sex-dependent in psychosis
Acknowledgments
Funding
This study was supported by National Institute of Mental Health (NIMH) RO1 MH56956 (JMG, P.I.). In addition, original sample ascertainment was in part also supported by grants from the Stanley Medical Research Institute (SLB & JMG, LJS 1994–1997), NARSAD (LJS) and NIMH R01MH63951 (LJS, P.I) and with support from the Harvard Catalyst | Harvard Clinical and Translational Science Center (CTSC) (NIH Award UL1 RR025758).
The authors thank Monica Landi, M.S.W., June Wolf, Ph.D., and JoAnn Donatelli, Ph.D., for their contributions to clinical interviewing and diagnoses, respectively, Christiana Provencal, M.A., for her contribution to project management during the original sample acquisition. We also thank Stuart Tobet, Ph.D., and Robert Handa, Ph.D. (ORWH-NIMH P50 MH082679; JMG, ST, RH (PIs)), for their input on fetal disruptions of the sexual differentiation of brain development.
Footnotes
Disclosures
The authors have no financial interests or potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jill M. Goldstein, Email: jill_goldstein@hms.harvard.edu.
Katie Lancaster, Email: katie.lancaster@virginia.edu.
Julia M. Longenecker, Email: longenecker.julia@gmail.com.
Brandon Abbs, Email: brandon.abbs@gmail.com.
Laura M. Holsen, Email: lholsen@partners.org.
Sara Cherkerzian, Email: scherkerzian@partners.org.
Susan Whitfield-Gabrieli, Email: nmakris@partners.org.
Nicolas Makris, Email: swg@mit.edu.
Ming T. Tsuang, Email: mtsuang@ucsd.edu.
Stephen L. Buka, Email: stephen_buka@brown.edu.
Larry J. Seidman, Email: lseidman@bidmc.harvard.edu.
Anne Klibanski, Email: aklibanski@partners.org.
References
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53 (4):1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophrenia Bulletin. 2010;38 (3):608–621. doi: 10.1093/schbul/sbq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128 (Pt 6):1301–1313. doi: 10.1093/brain/awh448. [DOI] [PubMed] [Google Scholar]
- Beumont PJV, Gelder MG, Friesen HG, Harris GW, MacKinnon PCB, Mandelbrote BM, Wiles DH. The effects of phenothiazines on endocrine function: I. British Journal of Psychiatry. 1974;124:413–419. doi: 10.1192/bjp.124.5.413. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. Journal of Abnormal Psychology. 1992;101 (1):37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borod J, Martin C, Alpert M, Brozgold A, Welkowitz J. Perception of facial emotion in schizophrenic and right brain-damaged people. Journal of Nervous and Mental Disease. 1993;181:494–502. doi: 10.1097/00005053-199308000-00004. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cogispoti M, Sabatinelli D, Lang PJ. Emotion and motivation. II: Sex differences in picture processing. Emotion. 2001;1 (3):300–319. [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25 (1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Breier A, Wolkowitz OM, Doran AR, Bellar S, Pickar D. Neurobiological effects of lumbar puncture stress in psychiatric patients and healthy volunteers. Psychiatry Research. 1988;25:187–194. doi: 10.1016/0165-1781(88)90050-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory. 2001;75 (1):1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JDE. Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences of the United States of America. 2002;99 (16):10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Glover G, Gabrieli JDE. fMRI identifies a network of structures correlated with retention of positive and negative emotional memory. Psychobiology. 1999;4:411–452. [Google Scholar]
- Canuso CM, Goldstein JM, Green AI. Schizophrenia in women: the role of estrogen. Primary Psychiatry. 2000;7 (4):38–44. [Google Scholar]
- Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LLB, Hichwa RD. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA. 2001;286 (4):427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3 (10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Derntl B, Kryspin-Exner I, Fernbach E, Moser E, Habel U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Hormones and Behavior. 2008;53 (1):90–95. doi: 10.1016/j.yhbeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Sledge WH, Wexler BE. Affective reactivity of language in stable schizophrenic outpatients and their parents. Journal of Nervous and Mental Disease. 1994;182 (6):313–318. doi: 10.1097/00005053-199406000-00001. [DOI] [PubMed] [Google Scholar]
- Domes G, Schulze L, Bottger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC. The neural correlates of sex differences in emotional reactivity and emotion regulation. Human Brain Mapping. 2010;31 (5):758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue JE, Stopa EG, Chorsky RL, King JC, Schipper HM, Tobet SA, Blaustein JD, Reichlin S. Cells containing immunoreactive estrogen receptor-alpha in the human basal forebrain. Brain Research. 2000;856 (1–2):142–151. doi: 10.1016/s0006-8993(99)02413-0. [DOI] [PubMed] [Google Scholar]
- Epstein J, Stern E, Silbersweig D. Mesolimbic activity associated with psychosis in schizophrenia: symptom-specific PET studies. Annals of the New York Academy of Sciences. 1999;877:562–574. doi: 10.1111/j.1749-6632.1999.tb09289.x. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Tsuang MT. Quantitative models of the genetic transmission of schizophrenia. Psychological Bulletin. 1985;98:41–66. [PubMed] [Google Scholar]
- Fernandez-Egea E, Parellada E, Lomeña F, Falcon C, Pavia J, Mane A, Horga G, Bernardo M. 18-FDG PET study of amygdalar activity during facial emotion recognition in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2010;260 (1):69–76. doi: 10.1007/s00406-009-0020-6. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cerebral Cortex. 1994;4 (4):344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID -I/P, Version 2.0) American Psychiatric Press; Washington, DC: 1996. [Google Scholar]
- Frederikse ME, Lu A, Aylward E, Barta PE, Sharma T, Pearlson GD. Sex differences in inferior parietal lobule volume in schizophrenia. American Journal of Psychiatry. 2000;157 (3):422–427. doi: 10.1176/appi.ajp.157.3.422. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh P, Herscovitch P, Post RM. Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biological Psychiatry. 1996;40:859–871. doi: 10.1016/0006-3223(95)00572-2. [DOI] [PubMed] [Google Scholar]
- Ghadirian AM, Chouinard G, Annable L. Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. Journal of Nervous and Mental Disease. 1982;170:463–467. doi: 10.1097/00005053-198208000-00004. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Goldstein JM. Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Hormones and Behavior. 2006;50 (4):612–622. doi: 10.1016/j.yhbeh.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Buka SL, Seidman LJ, Tsuang MT. Specificity of familial transmission of schizophrenia psychosis spectrum and affective psychoses in the New England family study’s high-risk design. Archives of General Psychiatry. 2010a;67 (5):458–467. doi: 10.1001/archgenpsychiatry.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Cherkerzian S, Seidman LJ, Donatelli JA, Remington AG, Tsuang MT, Hornig M, Buka SL. Prenatal maternal immune activation disruption and sex-specific risk for psychoses. Psychological Medicine. 2014a;44 (15):3249–3261. doi: 10.1017/S0033291714000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Holsen LM, Tobet SA, Handa RJ. Fetal hormonal programming of the increased risk of major depressive disorder in women over the lifespan. Frontiers in Neuroscience. 2014b;8:247. doi: 10.3389/fnins.2014.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. Journal of Neuroscience. 2010b;30 (2):431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. Journal of Neuroscience. 2005;25 (40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11 (6):490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Makris N, Ahern T, O’Brien LM, Caviness VS, Jr, Kennedy DN, Faraone SV, Tsuang MT. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biological Psychiatry. 2007;61:935–945. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, Caviness VS, Jr, Faraone SV, Tsuang MT. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Archives of General Psychiatry. 2002;59 (2):154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I. Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Research Bulletin. 2002;59 (2):105–109. doi: 10.1016/s0361-9230(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. Journal of Neuroscience. 1999;19 (140):4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel U, Chechko N, Pauly K, Koch K, Backes V, Seiferth N, Shah NJ, Stöcker T, Schneider F, Kellermann T. Neural correlates of emotion recognition in schizophrenia. Schizophrenia Research. 2010;122 (1–3):113–123. doi: 10.1016/j.schres.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Häfner H, Behrens S, DeVry J. An animal model for the effects of estradiol on dopamine-mediated behavior: mplications for sex differences in schizophrenia. Psychiatry Research. 1991;38:125–134. doi: 10.1016/0165-1781(91)90038-q. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Heimberg C, Gur RE, Erwin RJ, Shtasel DL, Gur RC. Facial emotion discrimination: III. Behavioral findings in schizophrenia. Psychiatry Research. 1992;42:253–265. doi: 10.1016/0165-1781(92)90117-l. [DOI] [PubMed] [Google Scholar]
- Herzog AG. A hypothesis to integrate partial seizures of temporal lobe origin and reproductive endocrine disorders. Epilepsy Research. 1989;3:151–159. doi: 10.1016/0920-1211(89)90043-0. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Spaeth SB, Lee JH, Ogden LA, Klibanski A, Whitfield-Gabrieli S, Goldstein JM. Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. Journal of Affective Disorders. 2011;131:379–387. doi: 10.1016/j.jad.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Lancaster K, Klibanski A, Whitfield-Gabrieli S, Cherkerzian S, Buka S, Goldstein JM. HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience. 2013;250:733–742. doi: 10.1016/j.neuroscience.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Holsen LM, Lancaster K, Makris N, Whitfield-Gabrieli S, Remington A, Weiss B, Buka S, Klibanski A, Goldstein JM. 17β-Estradiol differentially regulates stress circuitry activity in healthy and depressed women. Neuropsychopharmacology. 2015;40 (3):566–576. doi: 10.1038/npp.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Yamada-Mouri N. Gene expression of progesterone receptor isoforms in the rat brain. Hormones and Behavior. 1994;28 (4):454–463. doi: 10.1006/hbeh.1994.1043. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gruenberg AM, Tsuang MT. Subtype stability in schizophrenia. American Journal of Psychiatry. 1985;142 (7):827–832. doi: 10.1176/ajp.142.7.827. [DOI] [PubMed] [Google Scholar]
- Kohler C, Bilker W, Hagendoorn M, Gur RE, Gur RC. Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biological Psychiatry. 2000;48:127–136. doi: 10.1016/s0006-3223(00)00847-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biological Psychiatry. 1999;46 (9):1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Riedel A, de Castella AR, Fitzgerald PB, Rolfe TJ, Taffe J, Burger H. Estrogen--a potential treatment for schizophrenia. Schizophrenia Research. 2001;48:137–144. doi: 10.1016/s0920-9964(00)00088-8. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International Affective Picture System (IAPS), Affective Ratings of Pictures and Instruction Manual. [Google Scholar]
- Lebron-Milad K, Abbs B, Milad MR, Linman C, Rougemount-Bücking A, Zeidan MA, Holt DJ, Goldstein JM. Sex differences in the neurobiology of fear conditioning and extinction: a preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biology of Mood and Anxiety Disorders. 2012;2 (1):1–10. doi: 10.1186/2045-5380-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad M. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biology of Mood & Anxiety Disorders. 2012;2 (1):3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chan RCK, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophrenia Bulletin. 2010;36 (5):1029–1039. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19 (3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mather M, Lighthall NR, Nga L, Gorlick MA. Sex differences in how stress affects brain activity during face viewing. Neuroreport. 2010;21 (14):933–937. doi: 10.1097/WNR.0b013e32833ddd92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan KM, Stratton MS, Tobet SA. Roles for gamma-aminobutyric acid in the development of the paraventricular nucleus of the hypothalamus. Journal of Comparative Neurology. 2010;518 (14):2710–2728. doi: 10.1002/cne.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, Charney DS, Ernst M, Pine DS. A developmental examination of gender differences in brain engagement during evaluation of threat. Biological Psychiatry. 2004;55 (11):1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- McManis MH, Bradley MM, Berg WK, Cuthbert BN, Lang PJ. Emotional reactivity in children: verbal, physiological, and behavioral responses to affective pictures. Psychophysiology. 2001;38:222–231. [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman L. Profile of Mood States. EdITS/Educational and Industrial Testing Service, Inc; San Diego: 1992. pp. 1–40. [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ. Gender differences in emotion regulation: an fmri study of cognitive reappraisal. Group Processes & Intergroup Relations. 2008;11 (2):143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A. Reversal of normal cerebral sexual dimorphism in schizophrenia: evidence and speculations. Medical Hypotheses. 2007;69 (4):896–902. doi: 10.1016/j.mehy.2007.01.064. [DOI] [PubMed] [Google Scholar]
- Murphy DGM, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, Rapoport SI. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Archives of General Psychiatry. 1996;53 (7):585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- Neuroimaging TWTCf. SPM: Statistical Parametric Mapping, version 8. London, UK: 2008. [Google Scholar]
- Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Research: Neuroimaging. 2000;98 (1):1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. Journal of Clinical Endocrinology and Metabolism. 2000a;85 (10):3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000b;95 (2):333–342. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Frontiers in Neuroendocrinology. 1995;16 (2):89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Andreasen NC, Crespo-Facorro B, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. American Journal of Psychiatry. 2003;160 (10):1775–1783. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- Pardo J, Pardo P, Raichle M. Neural correlates of self-induced dysphoria. American Journal of Psychiatry. 1993;150:713–719. doi: 10.1176/ajp.150.5.713. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Vassilopoulou K, Velakoulis D, Phillips L, Soulsby B, Wood SJ, Brewer W, Smith DJ, Dazzan P, Yung AR, Zervas IM, Christodoulou GN, Murray R, McGorry PD, Pantelis C. Pituitary volume in psychosis. British Journal of Psychiatry. 2004;185:5–10. doi: 10.1192/bjp.185.1.5. [DOI] [PubMed] [Google Scholar]
- Passe TJ, Rajagopalan P, Tupler LA, Byrum CE, MacFall JR, Krishnan KRR. Age and sex effects on brain morphology. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1997;21 (8):1231–1237. doi: 10.1016/s0278-5846(97)00160-7. [DOI] [PubMed] [Google Scholar]
- Paus T, Otaky N, Caramanos Z, MacDonald D, Zijdenbos A, D’Avirro D, Gutmans D, Holmes C, Tomaiuolo F, Evans AC. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: hemispheric asymmetries, gender differences and probability maps. Journal of Comparative Neurology. 1996;376 (4):664–673. doi: 10.1002/(SICI)1096-9861(19961223)376:4<664::AID-CNE12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Penn D, Combs D, Ritchie M, Francis J, Cassisi J, Morris S, Townsend M. Emotion recognition in schizophrenia: further investigation of generalized versus specific deficit models. Journal of Abnormal Psychology. 2000;109:512–516. [PubMed] [Google Scholar]
- Phillips LK, Giuliano AJ, Lee EH, Faraone SV, Tsuang MT, Seidman LJ. Emotion-cognition interaction in people at familial high risk for schizophrenia: the impact of sex differences. Journal of Abnomal Psychology. 2011;120 (4):993–998. doi: 10.1037/a0023542. [DOI] [PubMed] [Google Scholar]
- Phillips LK, Seidman LJ. Emotion processing in persons at risk for schizophrenia. Schizophrenia Bulletin. 2008;34 (5):888–903. doi: 10.1093/schbul/sbn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Williams L, Senior C, Bullmore ET, Brammer MJ, Andrew C, Williams SC, David AS. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Research: Neuroimaging. 1999;92 (1):11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Price JL. Prefrontal cortical networks related to visceral function and mood. Annals of the New York Academy of Sciences. 1999;877:383–396. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- Quirk SW, Strauss ME, Sloan DM. Emotional responses as a function of symptoms in schizophrenia. Schizophrenia Research. 1998;32:31–39. doi: 10.1016/s0920-9964(98)00039-5. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, Dean DE, Petetot JMC, de Courten-Myers GM. Gender differences in the human cerebral cortex: more neurons in males; more processes in females. Journal of Child Neurology. 1999;14 (2):98–107. doi: 10.1177/088307389901400207. [DOI] [PubMed] [Google Scholar]
- Reicher-Rossler A, Hafner H, Stumbaum M, Maurer K. Can estradiol modulate schizophrenic symptomatology? Schizophrenia Bulletin. 1994;20:203–214. doi: 10.1093/schbul/20.1.203. [DOI] [PubMed] [Google Scholar]
- Reske M, Habel U, Kellermann T, Backes V, Jon Shah N, von Wilmsdorff M, et al. Differential brain activation during facial emotion discrimination in first-episode schizophrenia. Journal of Psychiatric Research. 2009;43(6):592–599. doi: 10.1016/j.jpsychires.2008.10.012. [DOI] [PubMed] [Google Scholar]
- SAS Institute, Inc. SAS Software, Version 8. Author; Cary, NC: 2001. [Google Scholar]
- Sattler JM. Assessment of Children. 3. Jerome M. Sattler; San Diego: 1992. [Google Scholar]
- Schneider F, Gur RC, Gur RE, Shtasel DL. Emotional processing in schizophrenia: neurobehavioral probes in relation to psychopathology. Schizophrenia Research. 1995;17:67–75. doi: 10.1016/0920-9964(95)00031-g. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Kessler C, Salloum JB, Posse S. Gender differences in regional cerebral activity during sadness. Human Brain Mapping. 2000;9:226–238. doi: 10.1002/(SICI)1097-0193(200004)9:4<226::AID-HBM4>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, Salloum JB, Posse S, Grodd W, Müller-Gärtner HW. Differential amygdala activation in schizophrenia during sadness. Schizophrenia Research. 1998;34 (3):133–142. doi: 10.1016/s0920-9964(98)00085-1. [DOI] [PubMed] [Google Scholar]
- Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophrenia Bulletin. 1990;16:185–194. doi: 10.1093/schbul/16.2.185. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Cherkerzian S, Goldstein JM, Agnew-Blais J, Tsuang MT, Buka SL. Neuropsychological performance and family history in children at age 7 who develop adult schizophrenia or bipolar psychosis in the New England Family Studies. Psychological Medicine. 2013;43:119–131. doi: 10.1017/S0033291712000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Stratton MS, Budefeld T, Majdic G, Tobet S. Embryonic GABA-B receptor blockade alters adult hypothalamic structure and anxiety- and depression-like behaviors in mice [abstract]. Society of Neuroscience 41st Annual Meeting; Washington, DC. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M, Ionnides A, Sinnemann T, Wolwer W, Dammers J, Zilles K, Gaebel W. Disturbed facial affect regulation in patients with schizophrenia associated with hypoactivity in distributed brain regions: a magnetoencephalographic study. American Journal of Psychiatry. 2001;158 (9):1429–1436. doi: 10.1176/appi.ajp.158.9.1429. [DOI] [PubMed] [Google Scholar]
- Sullivan G, Lukoff D. Sexual side effects of antipsychotic medication: evaluation and interventions. Hospital and Community Psychiatry. 1990;41:1238–1241. doi: 10.1176/ps.41.11.1238. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Decker LR, Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophrenia Research. 2002;58 (2–3):159–172. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- Tobet S, Handa R, Goldstein JM. Sex-dependent pathophysiology as predictors of comorbidity of major depressive disorder and cardiovascular disease. Pflügers Archiv - European Journal of Physiology. 2013;465 (5):585–594. doi: 10.1007/s00424-013-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey R, Seidman LJ, Lyons MJ, Faraone SV, Tsuang MT. Poor perception of nonverbal social-emotional cues in relatives of schizophrenic patients. Schizophrenia Research. 1999;40:121–130. doi: 10.1016/s0920-9964(99)00036-5. [DOI] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Gard MG, Minzenberg MJ, Yoon JH, Ragland JD, Solomon M, Carter CS. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. American Journal of Psychiatry. 2011;168 (3):276–285. doi: 10.1176/appi.ajp.2010.09081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19 (3):513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Walder DJ, Walker EF, Lewine RJ. Cognitive functioning, cortisol release, and symptom severity in patients with schizophrenia. Biological Psychiatry. 2000;48 (12):1121–1132. doi: 10.1016/s0006-3223(00)01052-0. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychological Review. 1997;104 (4):667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S. REX Software; Cambridge, MA: 2009. [Google Scholar]
- Whitfield-Gabrieli S. Artifact Detection Tools (ART) Cambridge, MA: 2011. Release version 07/19/11. [Google Scholar]
- Wik G, Wiesel FA. Regional brain glucose metabolism: correlations to biochemical measures and anxiety in patients with schizophrenia. Psychiatry Research: Neuroimaging. 1991;40 (2):101–114. doi: 10.1016/0925-4927(91)90002-8. [DOI] [PubMed] [Google Scholar]
- Williams LM, Barton MJ, Kemp AH, Liddell BJ, Peduto A, Gordon E, Bryant RA. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. NeuroImage. 2005;28 (3):618–626. doi: 10.1016/j.neuroimage.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Harris AW, Liddell BB, Brammer MJ, Olivieri G, Skerrett D, Phillips ML, David AS, Peduto A, Gordon E. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. American Journal of Psychiatry. 2004;161 (3):480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. Journal of Neuroscience. 1995;15 (5 Pt 1):3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Klein S, Gruesser SM, Hermann D, Flor H, Mann K, Braus DF, Heinz A. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neuroscience Letters. 2003;348 (1):41–45. doi: 10.1016/s0304-3940(03)00565-2. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Carbone DL, Hiroi R, Chong DL, Handa RJ. Dexamethasone induces apoptosis in the developing rat amygdala in an age-, region-, and sex-specific manner. Neuroscience. 2011;199:535–547. doi: 10.1016/j.neuroscience.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.