Abstract
Nicotine use is common among people with posttraumatic stress disorder (PTSD). Resilience, which is reflected in one's ability to cope with stress, has been shown to be associated with lower cigarette smoking and posttraumatic stress symptoms, but relationships among these three variables have not been examined. This study investigates the relationships of resilience and nicotine withdrawal with each other and in relation to PTSD symptoms. Participants were 118 cigarette smokers with PTSD seeking treatment for PTSD and nicotine use. Data were randomly cross-sectionally sampled from three time points: week 0, week 12, and week 27 of the study. Hierarchical multiple regression analyses revealed main effects of both resilience and nicotine withdrawal symptoms on PTSD severity, controlling for the sampled time point, negative affect, and expired carbon monoxide concentration. Consistent with prior research, PTSD severity was higher among individuals who were less resilient and for those who had greater nicotine withdrawal. There was an interaction between resilience and nicotine withdrawal on self-reported PTSD severity, such that greater resilience was associated with lower PTSD severity only among participants with low nicotine withdrawal symptoms. Among individuals with high nicotine withdrawal, PTSD severity was high, regardless of resilience level. These results suggest that resilience is a protective factor for PTSD severity for those with low levels of nicotine withdrawal, but at high levels of nicotine withdrawal, the protective function of resilience is mitigated.
Keywords: Posttraumatic stress disorder, resilience, smoking, addiction, trauma
Post-traumatic stress disorder (PTSD) is associated with significant burden, including greater days missed from work (Taylor et al., 2012), poorer health functioning (Asnaani et al., 2014), and greater healthcare utilization (Tuerk et al., 2013). Individuals with PTSD also have a significantly higher rate of smoking (approximately doubled) compared to those without PTSD (Feldner, Babson, & Zvolensky, 2007; Rasmusson et al., 2006). Further, higher rates of smoking and nicotine withdrawal have been identified as risk factors for the development of PTSD (Hawkins and Cougle, 2013; van der Velden et al., 2007; Feldner et al., 2007). That is, individuals who smoke are more likely to endorse clinically significant symptoms of PTSD (e.g. intrusions, avoidance behavior, and hostility) following a traumatic event than non-smokers (van der Velden et al., 2007; Beckham et al., 1996).
In addition, it has been found that smokers with PTSD are more likely to smoke heavily and report higher levels of nicotine dependence than those without the disorder (Feldner et al., 2007; Hapke et al., 2005). An additional source of concern is evidence for a greater difficulty quitting from smoking in daily smokers with PTSD, as compared to non-psychiatric smokers, specifically, greater number of failed lifetime quit attempts, more severe withdrawal quit problems (Marshall et al., 2008), and poorer outcomes in smoking cessation programs (Zvolensky et al., 2008; Beckham, Calhoun, Dennis, Wilson, & Dedert, 2013). Given these findings and the increased focus on developing smoking cessation treatments tailored for psychiatric populations (Ziedonis et al., 2008), there remains a recognized need to better understand the factors that affect the smoking-PTSD relationship (see for example: Richards et al., 2013).
Contrary to the exacerbation associated with smoking on PTSD severity, resilience is often regarded as a protective factor against the development of PTSD in trauma-exposed individuals. Resilience is conceptualized as a trait-like ability to effectively cope with stress and thrive despite hardship (Connor and Davidson, 2003). A commonly used of measure of resilience (the Connor-Davidson Resilience Scale, CD-RISC), assesses personal competence, tenacity, acceptance of change, ability to tolerate negative affect, finding strength in stress, and sense of purpose and control in life (Connor and Davidson, 2003). Higher resilience has been linked to lower risk of developing PTSD after experiencing trauma (Bensimon, 2012; Nishi et al., 2010), even after controlling for number of lifetime traumas (Wrenn et al., 2011). For example, in two matched cohorts of Austrian World War II survivors with and without PTSD, those with sub-threshold or threshold PTSD had significantly lower scores on the CD-RISC than those without PTSD (Tran et al., 2013).
Only a few studies, however, have examined how resilience may mitigate risk for PTSD. In a sample of adolescents, Fincham, Altes, Stein, and Seedat (2009) found that resilience moderated the relationship between childhood abuse/neglect and PTSD such that, in individuals with higher resilience, the association between childhood abuse/neglect and PTSD symptoms was significantly reduced. Similarly, in a sample of military combat veterans, resilience interacted with combat exposure such that those with higher resilience experienced lower levels of PTSD symptoms, regardless of the extent of combat exposure (Green et al., 2010). In addition, this study found that resilience was a particularly strong buffer against PTSD symptoms for those with the highest levels of combat exposure.
Resilience appears to play a similarly protective role against substance use. Higher resilience has been associated with less frequent smoking and lower nicotine dependence (Goldstein et al., 2013). In addition, higher resilience scores were associated with more days smoke-free in the month following a smoking cessation program (Pergadia, 2002). In a large sample of inner-city adults (n = 2,024), childhood abuse was associated with alcohol and illicit drug use in those with low resilience, but was unrelated to substance use in those high resilience (Wingo et al., 2014). Thus, resilience seems to function as a protective buffer between childhood trauma and both PTSD and substance use.
What remains unclear is how resilience interacts with smoking withdrawal, and how this interaction may impact PTSD severity. As noted previously, there is considerable research showing that nicotine use exacerbates PTSD and vice versa (Beckham et al., 1996; Dedert et al., 2012; Feldner et al., 2008), and that resilience lowers risk for PTSD (Fincham et al., 2009; Green et al., 2010). However, no study to date has examined how these three variables intersect with one another. Due to the high prevalence of comorbidity between PTSD and nicotine use observed in clinical practice, the current study aimed to elucidate the relationships among PTSD, nicotine withdrawal, and resilience. We examined the main and interactive effects of resilience and nicotine withdrawal on total PTSD severity and PTSD symptom cluster severity (re-experiencing, avoidance, and hyperarousal) in a treatment-seeking sample of smokers with PTSD. We hypothesized that, consistent with prior research, resilience would have an inverse relationship with PTSD severity (Fincham et al., 2009), and that nicotine withdrawal would have a direct, proportionate relationship with PTSD (van der Velden et al., 2007). Similarly, given the evidence for a consistent positive influence of resilience on PTSD even with comorbid substance use (Pergadia, 2002; Wingo et al., 2014), we further hypothesized that individuals with high resilience would exhibit a weaker relationship between nicotine withdrawal and PTSD severity than those with low resilience, with resilience moderating the association between withdrawal and PTSD symptoms. Finally, we examined the effects of resilience and withdrawal on specific PTSD symptom clusters. Given the scarcity of data looking at this, the hypotheses for this last set of analyses was exploratory.
Material and Methods
Participants
Participants were 118 adult cigarette smokers with PTSD who were enrolled in an ongoing randomized clinical trial comparing the efficacy of varenicline and smoking cessation counseling with or without integrated prolonged exposure (PE) therapy for PTSD. Inclusion criteria for participants in the study included male and females (age ≥ 18) who were heavy cigarette smokers (≥10 cigarettes per day) and had a primary DSM-IV diagnosis of PTSD and a total score ≥20 on the PTSD Symptom Scale Interview (PSS-I; Foa et al., 1993). The exclusion criteria, designed to ensure safety, focused primarily on suicidal ideation or past suicide attempts, certain health conditions (specifically significant cardiovascular disease or uncontrolled hypertension in the past 6 months, and currently pregnant or nursing women), past or current psychosis, and continuing intimate relationship with a violent domestic partner. Individuals with a history of drug or alcohol abuse/dependence in the past 3 months or any unwillingness to not smoke marijuana during the first 13 weeks of the study were also excluded.
Participants were recruited through public advertising (e.g., ads in a free city newspaper and flyers) and direct referrals from health care providers. Of the 118 participants, 70 (59.3%) were men and 48 (40.7%) were women. Participants had a mean age of 41.9 years (SD = 10.3 years). The majority of participants (76.3%) were Black/African American, with the rest of the participants identifying as White (22.9%) or American Indian/Alaskan Native (0.9%). Eight participants (6.8%) identified as Hispanic or Latino. This study was conducted in compliance with the Declaration of Helsinki as revised in 2008 and received approval from the ethics boards at both sites at which the study was conducted. All participants received a full description of the study and then provided written informed consent.
Procedure
Data were collected from three time points over the course of the 27-week trial: week 0, week 12, and week 27. At each assessment, a masters- or doctoral-level clinician conducted an independent (blinded) evaluation of PTSD symptom severity. In addition, participants completed self-report questionnaires including measures of PTSD symptoms and resilience. Participants also met with a study nurse at each assessment point to complete a checklist of nicotine withdrawal symptoms.
Measures
Connor-Davidson Resilience Scale
The Connor-Davidson Resilience Scale (CD-RISC; Connor and Davidson, 2003) is a 25-item self-report questionnaire measuring resilience. Participants are asked to indicate how much they agree with items such as “I can deal with whatever comes my way” and “Even when things look hopeless, I don't give up.” Each item is rated on a 5-point Likert scale from 0 (not true at all) to 4 (true nearly all the time), with total scores ranging from 0 to 100. The CD-RISC was found by Connor and Davidson (2003) to have good internal consistency (Cronbach's alpha = .89), high test-retest reliability (intraclass correlation coefficient = .87), and good convergent validity. In the current sample, internal consistency for the CD-RISC was excellent (Cronbach's alpha = .97).
Posttraumatic Stress Diagnostic Scale
The Posttraumatic Stress Diagnostic Scale (PDS; Foa et al., 1997) is a self-report questionnaire assessing the severity of PTSD symptoms according to the DSM-IV. Participants are asked to rate their experience of 17 symptoms over the previous two weeks on 4-point Likert scales from 0 (not at all) to 3 (5 or more times a week/almost always), with total scores ranging from 0-51. The PDS includes the same three subscales as the PSS-I: re-experiencing (five items, range 0-15), avoidance (seven items, range 0-21), and hyperarousal (five items, range 0-15). The PDS has excellent internal consistency (Cronbach's alpha = .92) and good internal consistency for the three subscales (Cronbach's alphas: .78 for re-experiencing, .84 for avoidance, and .84 for hyperarousal; Foa et al., 1997). In addition, Foa and colleagues (1997) found that the PDS has high test-retest reliability (r = .83), as well as good convergent validity (82% accuracy, compared with the Structured Clinical Interview for DSM–III–R; Spitzer et al., 1990). In the present study, the PDS was found to have excellent internal consistency for the total score (Cronbach's alpha = .95), as well as good to excellent internal consistency for the three subscales (Cronbach's alphas = .90 for re-experiencing, .88 for avoidance, and .85 for hyperarousal).
PTSD Symptom Scale Interview
The PTSD Symptom Scale Interview (PSS-I; Foa et al., 1993) is a 17-item clinical interview that assesses the severity of PTSD symptoms according to the DSM-IV. Participants are asked about the nature and frequency of the symptoms that they have experienced over the previous 2 weeks, with responses coded on a 4-point scale from 0 (not at all) to 3 (5 or more times per week/very much). The interview yields a total score ranging from 0-51, as well as scores on three subscales: re-experiencing (five items, range 0-15), avoidance (seven items, range 0-21) and hyperarousal (five items, range 0-15). The PSS-I has good internal consistency (Cronbach's alpha = .85), and fair to good internal consistency for the three subscales (Cronbach's alpha = .69 for re-experiencing, .65 for avoidance, and .71 for hyperarousal; Foa et al., 1993). Foa and colleagues (1993) also found that the PSS-I has good test-retest reliability (r = .80) and excellent convergent validity (94% accuracy, compared with the Structured Clinical Interview for DSM–III–R; Spitzer et al., 1987). In the current sample, the PSS-I total score was found to have excellent internal consistency (Cronbach's alpha = .91), and the three subscales were found to have good internal consistency (Cronbach's alphas = .83 for re-experiencing, .83 for avoidance, and .74 for hyperarousal).
Structured Clinical Interview for DSM-IV
The SCID (SCID-IV; First & Gibbon, 2004) is a 60-minute, semi-structured interview that yields current and lifetime DSM-IV Axis I diagnoses for the major psychiatric disorders. The SCID was used to confirm diagnosis of post-traumatic stress disorder and to evaluate the presence of other Axis I and Axis II disorders to determine eligibility for the study. This interview is a widely used and reliable measure of psychopathology, with joint inter-rater reliability coefficients ranging from 0.60 to 0.83, depending on the disorder (Lobbestael, Leurgans, & Arntz, 2010).
Withdrawal Symptoms Checklist
The Withdrawal Symptoms Checklist – Weekly (WSC-W; Hughes et al., 1984) is a 21-item questionnaire assessing the presence and severity of 20 symptoms of nicotine withdrawal over the prior 7 days. The checklist includes items such as insomnia, nausea, and impatience, each rated on a 4-point scale from 0 (not present) to 3 (severe); total scores range from 0-60. The final question, rated dichotomously at baseline only, asks if these symptoms have caused significant distress or interference in the respondent's life. Hughes and colleagues (1984) found the measure to be reliable and valid: observers' ratings of subjects' symptoms were modestly consistent with subjects' self-reports at baseline (57-71% agreement, interrater correlation coefficients = 0.29-0.45) and more consistent during nicotine abstinence (67-91% agreement, interrater correlation coefficients = 0.33-0.60. In the same study, subjects who received a placebo instead of nicotine gum experienced the symptoms to a significantly greater degree (one-tailed t-tests, t (49) > 1.65, p < .05). In the current sample, the WSC-W was found to have good internal consistency (Cronbach's alpha = .86).
Statistical Analyses
Because data were collected as part of a treatment study, all participants had relatively high PTSD and smoking scores at week 0. In order to avoid restricting variability in our dependent variable (PTSD severity), data were cross-sampled from the week 0, week 12, and week 27 assessment points. Such a method allows for a true cross-sectional analysis of data from individuals with a greater range and combinations of PTSD and smoking severity, because using data from only one of the time-points (particularly the week 0 time-point) examines individuals who all have only a certain elevated severity level of PTSD and smoking behaviors (as required for entry into the study), and thus the subsequent analyses examining the relationships among the variables of interest are considerably more restrictive and less informative or generalizeable. Therefore, cases (and associated study variables collected at each time point) were randomly selected without replacement from each time point such that each participant was selected from one of the three assessment points. This means that each data point was unique (i.e. no person provided more than one data point, and all measures were taken from that same time-point for that participant). Such a method allowed us to examine associations among the variables of interest at various cross-sections of the sample, and therefore the stage of treatment would not affect the analyses.
Data were available for all 118 cases at week 0, 102 cases at week 12, and 102 cases at week 27. Therefore, data were selected from 43 cases at week 0, 38 cases at week 12, and 37 cases at week 27. Such a cross-sampling technique has been used before in other studies with trauma-exposed substance users (e.g. Gillihan et al., 2011). The independent variables consisted of scores on the WSC-W, CD-RISC and their interaction. The scores on the WSC-W and CD-RISC were mean-centered in order to make the different scales used in both measures more comparable and to more easily interpret their interaction term. In order to examine the effects of these variables on a more comprehensive measure of PTSD severity (total and symptom cluster scores), the outcome variables of self-reported PTSD (PDS) and clinician-assessed PTSD (PSS-I) were utilized as the dependent variables.
Data were analyzed using IBM SPSS Version 21.0. Bivariate correlations between all variables were conducted to measure their degree of association. Data were normally distributed, thus separate hierarchical linear multiple regressions were conducted to evaluate the contribution of resilience, nicotine withdrawal symptoms, and the interaction between the two to PTSD total symptom severity and the individual re-experiencing, avoidance, and hyperarousal symptom clusters. In the first block, assessment time point was entered as a covariate in order to account for any symptom reduction at later time points due to having completed PE. In the second block, resilience and withdrawal scores were entered in order to examine their main effects on PTSD severity. The final block included the interaction term between resilience and withdrawal symptoms to examine whether their interaction significantly contributed to PTSD symptoms. An assessment of the assumptions necessary for appropriate inclusion of all variables (i.e., absence of multicollinearity (VIF < 10), linearity, low incidence of outliers with standard residuals between -3.3 and 3.3, and homogeneity of variance) verified that none of these criteria were violated in any of the four regression models tested.
Results
Descriptive Characteristics of the Sample
Participants reported a mean of 25.2 years of smoking (SD = 11.4 years), indicating significant chronicity of nicotine use in the majority of the sample. In addition, 78.4% of the sample had made at least one prior smoking quit attempt, with the average number of quit attempts among these individuals being 5.9 (SD = 6.2). In addition, the most frequent comorbid current diagnosis (aside from PTSD) was major depressive disorder (36.4%), with significant past diagnoses of alcohol use disorder (33.9%), and other substance use disorders (including cocaine, opiates, and marijuana; 43.2%).
Correlational Analyses
Means and standard deviations for variables of interest among the cross-sectionally sampled data, as well as the strength and direction of bivariate correlations among them, are presented in Table 1. As shown in the table, both lower resilience and higher withdrawal symptoms were associated with higher total PTSD scores on both the PDS (resilience: r = -.31, p < .001; withdrawal: r = .55, p < .001) and the PSS-I (resilience: r = -.42, p < .001; withdrawal: r = .56, p < .001), as well as with higher scores on all three subscales for both PTSD measures. Withdrawal was most strongly correlated with hyperarousal symptoms (r = .55, p < .001, on both the PDS and PSS-I), while resilience had the strongest negative association with avoidance symptoms (PDS: r = -.33, p < .001; PSS-I: r = -.45, p < .001). Resilience and withdrawal scores were not significantly associated with each other (r = -.17, p = .067).
Table 1.
Descriptive statistics and zero order correlations (Pearson's r) among independent and dependent variables.
| Variable | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Withdrawal | 8.14 | 5.98 | - | ||||||||||
| 2. Resilience | 68.38 | 20.66 | -.17 | - | |||||||||
| 3. PDS | 21.47 | 14.13 | .55** | -.31** | - | ||||||||
| 4. PDS Reexp | 5.61 | 4.22 | .53** | -.21* | .90** | - | |||||||
| 5. PDS Avoid | 8.70 | 6.35 | .47** | -.33** | .94** | .75** | - | ||||||
| 6. PDS Hyp | 7.16 | 4.61 | .55** | -.29** | .94** | .81** | .83** | - | |||||
| 7. PSSI | 19.86 | 12.34 | .56** | -.42** | .74** | .66** | .69** | .71** | - | ||||
| 8. PSSI Reexp | 4.70 | 3.81 | .49** | -.23* | .66** | .68** | .58** | .61** | .83** | - | |||
| 9. PSSI Avoid | 7.74 | 5.71 | .47** | -.45** | .64** | .54** | .64** | .59** | .93** | .65** | - | ||
| 10. PSSI Hyp | 7.42 | 4.34 | .55** | -.41** | .68** | .57** | .61** | .71** | .90** | .64** | .75** | - | |
| 11. Exhaled CO | 14.35 | 5.80 | -.09 | .19* | -.06 | -.03 | -.05 | -.09 | -.05 | .03 | -.01 | -.14 | - |
| 12. Neg. Affect | 22.69 | 8.42 | .16 | -.22* | .27** | .23* | .28** | .23* | .22* | .15 | .24** | .18 | -.27** |
p < .01,
p < .001
Moderator Analysis
Overall PTSD severity
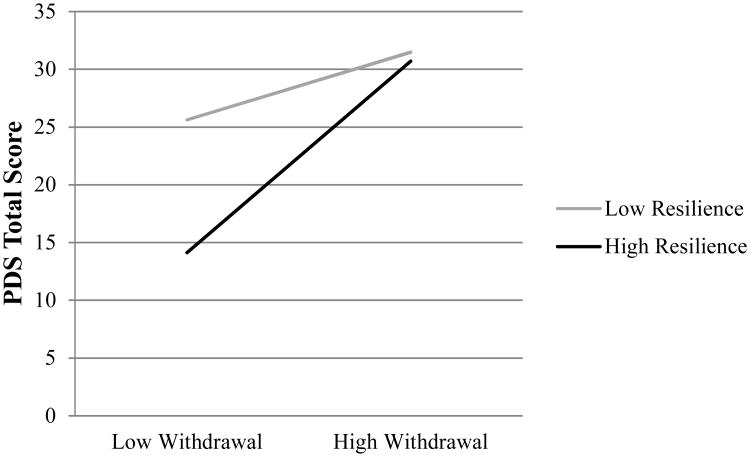
Analyses revealed that the model including resilience, withdrawal symptoms, and their interaction significantly explained 42% of the variance in total PTSD severity on the PDS (F(6, 109) = 13.36, p < .001) and 49% of the variance in total PTSD severity on the PSS-I (F(6, 109) = 17.49, p < .001). As shown in Tables 2 and 3, both resilience and withdrawal had significant main effects on PTSD severity such that higher resilience and lower withdrawal symptoms were each associated with lower total scores on both measures of PTSD. In addition, the interaction between withdrawal and resilience contributed a small but significant portion (3.2%) of the variance in total PDS scores (see Table 2). A graph of this interaction can be seen in Figure 1. For the PSS-I total score, no interactive effect was found for resilience and withdrawal (Table 3).
Table 2.
Hierarchical multiple regression analysis including resilience, withdrawal, and their interaction as contributors of PDS total and individual symptom cluster scores.
| DV | Variables | ΔR2 | β | Se | t | p | |
|---|---|---|---|---|---|---|---|
| PDS Total | 1. | Time Point | .191** | -.437 | .149 | -5.182 | .000 |
| 2. | Exhaled CO | .000 | -.032 | .207 | -.156 | .876 | |
| 3. | Neg. Affect | .053** | .402 | .143 | 2.802 | .006 | |
| 4. | Resilience | .148** | -.156 | .054 | -2.904 | .004 | |
| Withdrawal | .852 | .238 | 3.584 | .001 | |||
| 5. | Resil*Withdraw | .032* | .021 | .008 | 2.447 | .016 | |
|
| |||||||
| PDS Re-Experiencing | 1. | Time Point | .168** | -.217 | .045 | -4.799 | .000 |
| 2. | Exhaled CO | .000 | .010 | .063 | .159 | .874 | |
| 3. | Neg. Affect | .040* | .104 | .044 | 2.364 | .020 | |
| 4. | Resilience | .118** | -.027 | .017 | -1.571 | .119 | |
| Withdrawal | .275 | .075 | 3.685 | .000 | |||
| 5. | Resil*Withdraw | .036* | .007 | .003 | 2.478 | .015 | |
|
| |||||||
| PDS Avoidance | 1. | Time Point | .123** | -.279 | .070 | -4.000 | .000 |
| 2. | Exhaled CO | .000 | -.013 | .097 | -.129 | .897 | |
| 3. | Neg. Affect | .062** | .195 | .067 | 2.913 | .004 | |
| 4. | Resilience | .142** | -.080 | .026 | -3.136 | .002 | |
| Withdrawal | .328 | .113 | 2.913 | .004 | |||
| 5. | Resil*Withdraw | .025* | .008 | .004 | 2.060 | .042 | |
|
| |||||||
| PDS Hyperarousal | 1. | Time Point | .229** | -.277 | .048 | -5.826 | .000 |
| 2. | Exhaled CO | .001 | -.030 | .066 | -.453 | .652 | |
| 3. | Neg. Affect | .033** | .103 | .046 | 2.232 | .028 | |
| 4. | Resilience | .209** | -.050 | .018 | -2.829 | .006 | |
| Withdrawal | .249 | .078 | 3.205 | .002 | |||
| 5. | Resil*Withdraw | .023* | .006 | .003 | 2.082 | .040 | |
p < .05,
p < .01
Table 3.
Hierarchical multiple regression analysis including resilience, withdrawal, and their interaction as contributors of PSS-I total and individual symptom cluster scores.
| DV | Variables | ΔR2 | β | Se | t | p | |
|---|---|---|---|---|---|---|---|
| PSS-I Total | 1. | Time Point | .231** | -.743 | .127 | -5.849 | .000 |
| 2. | Exhaled CO | .000 | .020 | .177 | .115 | .909 | |
| 3. | Neg. Affect | .033* | .275 | .124 | 2.224 | .028 | |
| 4. | Resilience | .219** | -.224 | .043 | -5.153 | .000 | |
| Withdrawal | .632 | .192 | 3.296 | .001 | |||
| 5. | Resil*Withdraw | .008 | .009 | .007 | 1.300 | .196 | |
|
| |||||||
| PSS-I Re-Experiencing | 1. | Time Point | .173** | -.198 | .041 | -4.876 | .000 |
| 2. | Exhaled CO | .006 | .051 | .056 | .912 | .364 | |
| 3. | Neg. Affect | .017 | .062 | .040 | 1.557 | .122 | |
| 4. | Resilience | .107** | -.036 | .016 | -2.297 | .023 | |
| Withdrawal | .195 | .069 | 2.837 | .005 | |||
| 5. | Resil*Withdraw | .011 | .003 | .002 | 1.296 | .198 | |
|
| |||||||
| PSS-I Avoidance | 1. | Time Point | .147** | -.274 | .062 | -4.424 | .000 |
| 2. | Exhaled CO | .001 | .032 | .086 | .369 | .713 | |
| 3. | Neg. Affect | .048* | .155 | .060 | 2.589 | .011 | |
| 4. | Resilience | .218** | -.113 | .021 | -5.273 | .000 | |
| Withdrawal | .230 | .094 | 2.436 | .016 | |||
| 5. | Resil*Withdraw | .001 | .001 | .003 | .362 | .718 | |
|
| |||||||
| PSS-I Hyperarousal | 1. | Time Point | .248** | -.271 | .044 | -6.136 | .000 |
| 2. | Exhaled CO | .007 | -.063 | .061 | -1.030 | .305 | |
| 3. | Neg. Affect | .012 | .058 | .043 | 1.341 | .183 | |
| 4. | Resilience | .196** | -.075 | .016 | -4.810 | .000 | |
| Withdrawal | .207 | .069 | 3.020 | .003 | |||
| 5. | Resil*Withdraw | .016 | .005 | .002 | 1.840 | .068 | |
p < .05,
p < .01
Figure 1.
Moderation effect of resilience on the relationship between withdrawal and total PDS symptoms.
PTSD symptom clusters
Resilience, withdrawal, and their interaction explained 36% of the variance in re-experiencing symptoms (F(6, 109) = 10.31, p < .001) on the PDS and 31% of the variance in re-experiencing symptoms (F(6, 109) = 8.3, p < .001) on the PSS-I. There was a significant main effect of withdrawal on both PTSD measures: those with higher withdrawal symptoms had higher re-experiencing symptoms. There was also a main effect of resilience on the PSS-I, such that those with higher resilience had lower re-experiencing symptoms, although this main effect was not observed on the PDS (see Tables 2-3). However, withdrawal symptoms and resilience interacted to significantly contribute to re-experiencing symptoms on the PDS, in a pattern similar to the one observed with the total PDS score depicted in Figure 1.
A model including the same variables accounted for 35% of the variance in avoidance symptoms on the PDS (F(6, 109) = 9.87, p < .001) and 41% of the variance on the PSS-I (F(6, 109) = 12.9, p < .001). This model demonstrated significant main effects of resilience (such that those with higher resilience had lower avoidance symptoms) and withdrawal on both measures of PTSD, with a significant interaction effect on avoidance symptoms of the PDS only following the same patterns as observed in the total PDS score (see Tables 2-3).
Finally, the model examining hyperarousal symptoms accounted for 42% of the variance in these symptoms (F(6, 109) = 12.87, p < .001) on the PDS and 48% of the variance in these symptoms (F(6, 109) = 16.71, p < .001) on the PSS-I, showing significant main effects of resilience and withdrawal symptoms on hyperarousal symptoms in directions consistent with prior analyses on both measures of PTSD (see Tables 2-3). Again, there was a significant interaction effect between resilience and withdrawal on hyperarousal symptoms on the PDS only similar to the pattern depicted in Figure 1.
Contrary to the significant interactions found on the symptom clusters of the PDS, there were no significant interactive effects of resilience and withdrawal on any of the PSS-I symptom clusters.
Interpreting Significant Interactions
To better understand the nature of the interaction effects associated with PDS total and subscale scores, specific values for each variable were substituted into the regression equations for each analysis, a post-hoc technique for significant interactions as outlined in detail by Holmbeck (2002) and utilized in other studies examining such interaction effects (Feldner et al., 2007; Gillihan et al., 2011). Briefly, two conditional moderating variables of resilience (high and low) were computed using the original mean-centered value of resilience +/- 1 SD. Subsequently, interaction terms of each of these conditional resilience variables with withdrawal were calculated. These interaction terms were then entered simultaneously into a post-hoc regression analysis with the conditional moderating resilience variable and withdrawal, in two separate models for high and low resilience. As shown in Figure 1, there was a steeper slope when resilience scores were high, indicating a stronger relationship between resilience and overall PTSD symptoms as measured by the PDS in individuals with low levels of withdrawal symptoms. Similarly, there were more negative associations between resilience and re-experiencing, avoidance, and hyperarousal symptoms as measured by the PDS in individuals with low withdrawal, following the same pattern illustrated in Figure 1. Significance testing of withdrawal symptoms (range of t-values: 3.85-4.72, all p-values < .01) in each regression model (Holmbeck, 2002) verified that high levels of resilience moderated the association between withdrawal symptoms and PTSD total, re-experiencing, avoidance, and hyperarousal symptoms in individuals with lower scores of withdrawal, but at higher scores of withdrawal, the influence of resilience on lowering PTSD scores was no longer significant.
Discussion
This study examined the interrelationships between resilience (a protective factor for the development of PTSD), nicotine withdrawal (a risk factor for PTSD), and PTSD symptom severity. As hypothesized, resilience was associated with lower self-reported and clinician-rated PTSD severity whereas nicotine withdrawal was associated with higher self-reported and clinician-rated PTSD severity, even after adjusting for the variance accounted for by assessment time-point, expired CO levels (which indicate current smoking status), and state negative affectivity. These main effects were evident for PTSD total symptom severity and generally for each PTSD symptom cluster (with the exception of a non-significant finding of resilience on self-reported re-experiencing symptoms). These findings are consistent with previous studies showing a negative relationship between resilience and PTSD (Bensimon, 2012; Wrenn et al., 2011) and with studies showing a positive relationship between nicotine withdrawal and PTSD (Beckham et al., 1996; Dedert et al., 2012; Feldner et al., 2008). The results of the current study support the utility in assessing for protective and risk factors in the treatment of smokers with PTSD, in order to more comprehensively understand how PTSD severity may be maintained or influenced by additional trait-like and state-like symptoms.
Contrary to our second hypothesis, however, the protective role of resilience against PTSD severity was only true for individuals with low levels of nicotine withdrawal when examining self-reported PTSD. Therefore, resilience served as a moderator between nicotine withdrawal and PTSD severity, but when individuals were experiencing higher levels of nicotine withdrawal, resilience no longer moderated the relationship between withdrawal and PTSD symptoms, suggesting a stronger direct influence of nicotine withdrawal on PTSD severity. This pattern of effects of resilience on the relationship between nicotine withdrawal and PTSD symptoms was observed for total, re-experiencing, avoidance, and hyperarousal symptoms of self-reported PTSD (although not in clinician-rated PTSD). The overpowering effect of nicotine withdrawal over resilience, and the similar pattern of interactive effects between these two variables on PTSD symptoms, combined and divided into clusters, has several important implications.
First, while recent research has focused on the protective nature of resilience and related constructs (e.g., social support and posttraumatic growth) on PTSD severity, the current results indicate that this relationship is impacted by the severity of nicotine withdrawal symptoms. Indeed, our results hint at a more nuanced buffering effect of resilience against severity of PTSD, and highlight the importance of nicotine withdrawal symptoms in particular (which have not been previously examined) as a risk factor in PTSD. Second, the interaction effects of resilience and nicotine withdrawal symptoms for each of the three cluster symptoms of PTSD deserve further investigation. In particular, is the overpowering effect of nicotine withdrawal over resilience for hyperarousal due to the overlap between hyperarousal symptoms and symptoms of nicotine withdrawal (e.g. irritability, insomnia)? The specificity of the observed interaction effects between resilience and withdrawal to re-experiencing symptoms is less clear, particularly since there was no main effect of resilience on self-reported re-experiencing symptoms. The significant interaction between avoidance symptoms and nicotine withdrawal should be interpreted with caution, because this subscale includes items that have now been pulled out into a new cluster (cognition/emotion) in DSM-5, and therefore do not particularly represent pure avoidance symptoms. Overall, there is currently scant data in the literature to provide explanations for the observed interaction effects of resilience and withdrawal on specific PTSD symptom clusters; therefore this area needs to be examined in greater detail. Finally, it is important to note that while resilience and nicotine withdrawal explained a significant portion of the variance in PTSD symptoms, the significant interactions between resilience and nicotine withdrawal observed explained considerably smaller portions of the variance (around 2-4% in each model). Thus, the clinical significance of these observed interactions is tentative.
This study has several notable strengths. First, the current findings give preliminary insight into how two important risk and protective factors for PTSD influence one another to impact PTSD symptoms. Second, the significant findings were observed in a clinical sample meeting criteria for both PTSD and nicotine dependence, as compared to the typically analogue samples studied previously (Bensimon, 2012; Fincham et al., 2009). In addition, the study relied on psychometrically sound, well-validated measures of all the constructs examined (PSS-I, PDS, CD-RISC, and WSC-W), which allowed for an adequate assessment of each of these factors (Connor and Davidson, 2003; Foa et al., 1997; Foa et al., 1993; Hughes et al., 1984). However, as noted above, the significant interaction effects emerged only when the self-reported PTSD measure (the PDS) was used, but no significant interactions were observed with the clinician-administered measure of PTSD (the PSS-I). Given the high degree of correlation in the current study and previously demonstrated high convergence between these two measures of PTSD, such a lack of finding with the PSS-I was puzzling. Previous research in this field has typically relied on self-reported symptoms of resilience, nicotine use, and PTSD, and therefore the findings with our self-reported measure of PTSD are consistent with these. Given this difference in findings between self-reported and clinician-reported symptoms (which most other studies have not used), it is important that future studies utilize a clinician-administered measure of PTSD, in order to put the current, novel findings with such a measure in context, and to clarify how robust the significant interactive effects found on the PDS are.
A major methodological limitation to this study was that measures of overall nicotine dependence such as the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al., 1991) were only administered at the beginning of the study, thus precluding the examination of how nicotine dependence interacts with resilience using the cross-sampling technique employed in the study. However, to this end, nicotine withdrawal has been a less examined, and yet highly relevant, construct of state-like nicotine abstinence (whether this abstinence is acute, overnight, or withdrawal associated with smoking cessation) to be studied within the context PTSD. This study therefore sheds light on an area within the field of comorbid smoking and PTSD that has been relatively neglected. In addition, this study did examine expired CO levels, a biochemical verification of smoking status which is superior to subjective self-report (Shiffman et al., 1997). Indeed, the results regarding the impact of nicotine withdrawal severity and resilience were still evident, even after controlling for smoking status/rate as measured by CO levels. Similarly, it is important to note that the effects of resilience and nicotine withdrawal were significant beyond the effects of general negative affectivity, which itself has been implicated in higher nicotine dependence and relapse among smokers with PTSD (Beckham et al., 2013). Still, given the paucity of studies examining these constructs together, the current findings should be replicated in various samples of smokers with PTSD.
Several important future directions for empirical examination emerge from the current study findings. It would be fruitful for investigators to examine how resilience moderates the relationship between nicotine withdrawal symptoms and PTSD over the course of treatment. Such investigation could reveal the mechanisms underlying PTSD reduction and smoking cessation via prolonged exposure therapy and other empirically supported treatments for PTSD. Are these changes mediated by a change in the relationships between and among resilience, nicotine withdrawal, and PTSD? In sum, the present results highlight the value of examining the impact of resilience relative to exacerbating variables such as nicotine withdrawal, and offer researchers and clinicians with a more nuanced and informed approach to interventions designed to target either of these factors in smokers with PTSD.
Highlights.
Cigarette smoking contributes to exacerbation and maintenance of PTSD symptoms.
Resilience implicated in pathology of nicotine withdrawal and PTSD separately.
Regressions examined main/interactive effects of resilience and withdrawal on PTSD.
Resilience and withdrawal both had significant main effects on PTSD severity.
Resilience moderated relationship between withdrawal & PTSD at low withdrawal only.
Acknowledgments
This work was funded by National Institute of Drug Abuse grant awarded to Dr. Edna B. Foa (R01-DA023507-01A1). The authors express their sincerest appreciation to the study nurse Patricia Imms, research assistants on this study Michelle Capozzoli, Janice Paton, Catherine Coogan and Elizabeth Alpert, and to the patients who were brave enough to share their struggles with our research team by participating in this study.
Please note that the study was supported by National Institute of Drug Abuse grant R01-DA023507-01A1, awarded to Dr. Edna B. Foa.
Role of the Funding Source: This work was funded by National Institute of Drug Abuse grant awarded to Dr. Edna B. Foa (R01-DA023507-01A1).
Footnotes
Conflicts of Interest: There are no conflicts of interest for Dr. Asnaani, Ms. Alpert, or Dr. McLean.
Contributors: Anu Asnaani, Ph.D., University of Pennsylvania, Dr. Asnaani contributed to the writing and the conceptualization of this manuscript, as well as the statistical analysis.
Elizabeth Alpert, B.A., University of Pennsylvania, Ms. Alpert contributed to the writing and the conceptualization of this manuscript, as well as the statistical analysis.
Carmen P. McLean, Ph.D., University of Pennsylvania, Dr. McLean contributed to the writing and the conceptualization of this manuscript.
Edna B. Foa, Ph.D., University of Pennsylvania, Dr. Foa contributed to the writing and the conceptualization of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asnaani A, Reddy MK, Shea MT. The impact of PSTD symptoms on physical and mental health functioning in returning veterans. J Anxiety Disord. 2014;28:310–7. doi: 10.1016/j.janxdis.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Lytle BL, Vrana SR, Hertzberg MA, Feldman ME, Shipley RH. Smoking withdrawal symptoms in response to a trauma-related stressor among Vietnam combat veterans with posttraumatic stress disorder. Addict Behav. 1996;21:93–101. doi: 10.1016/0306-4603(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Calhoun PS, Dennis MF, Wilson SM, Dedert EA. Predictors of lapse in first week of smoking abstinence in PTSD and non-PTSD smokers. Nicotine Tob Res. 2013;15:1122–29. doi: 10.1093/ntr/nts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon M. Elaboration on the association between trauma, PTSD and posttraumatic growth: The role of trait resilience. Pers Individ Dif. 2012;52:782–7. [Google Scholar]
- Connor KM, Davidson JRT. Development of a new resilience scale: The Connor-Davidson Resilience Scale (CD-RISC) Depress Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- Dedert EA, Calhoun PS, Harper LA, Dutton CE, McClernon FJ, Beckham JC. Smoking withdrawal in smokers with and without posttraumatic stress disorder. Nicotine Tob Res. 2012;14:372–6. doi: 10.1093/ntr/ntr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and post-traumatic stress: A critical review of the empirical literature. Clin Psychol Rev. 2007;27:14–45. doi: 10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Vujanovic AA, Gibson LE, Zvolensky MJ. Posttraumatic stress disorder and anxious and fearful reactivity to bodily arousal: A test of the mediating role of nicotine withdrawal severity among daily smokers in 12-hr nicotine deprivation. Exp Clin Psychopharmacol. 2008;16:144–55. doi: 10.1037/1064-1297.16.2.144. [DOI] [PubMed] [Google Scholar]
- Fincham DS, Altes LK, Stein DJ, Seedat S. Posttraumatic stress disorder symptoms in adolescents: Risk factors versus resilience moderation. Compr Psychiatry. 2009;50:193–9. doi: 10.1016/j.comppsych.2008.09.001. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M. The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II) Hoboken, NJ: John Wiley & Sons, Inc; 2004. [Google Scholar]
- Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychol Assess. 1997;9:445–51. [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6:459–73. [Google Scholar]
- Gillihan SJ, Farris SG, Foa EB. The effect of anxiety sensitivity on alcohol consumption among individuals with comorbid alcohol dependence and posttraumatic stress disorder. Psychol Addict Behav. 2011;25:721–6. doi: 10.1037/a0023799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AL, Faulkner B, Wekerle C. The relationship among internal resilience, smoking, alcohol use, and depression symptoms in emerging adults transitioning out of child welfare. Child Abuse Negl. 2013;37:22–32. doi: 10.1016/j.chiabu.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Green KT, Calhoun PS, Dennis MF, Beckham JC. Exploration of the resilience construct in posttraumatic stress disorder sensitivity and functional correlates in military combats veterans who have served since September 11, 2001. J Clin Psychiatry. 2010;71:823–30. doi: 10.4088/JCP.09m05780blu. [DOI] [PubMed] [Google Scholar]
- Hapke U, Schumann A, Rumpf HJ, John U, Konerding U, Meyer C. Association of smoking and nicotine dependence with trauma and posttraumatic stress disorder in a general population sample. J Nerv Mental Dis. 2005;193:843–846. doi: 10.1097/01.nmd.0000188964.83476.e0. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Cougle JR. The effects of nicotine on intrusive memories in nonsmokers. Exp Clin Psychopharmacol. 2013;21:434–42. doi: 10.1037/a0033966. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. J Pediatr Psychol. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Pickens RW, Krahn D, Malin S, Luknic A. Effect of nicotine on the tobacco withdrawal syndrome. Psychopharmacology. 1984;83:82–7. doi: 10.1007/BF00427428. [DOI] [PubMed] [Google Scholar]
- Lobbestael J, Leurgans M, Arntz A. Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II) Clin Psychol Psychopath. 2010;18:75–79. doi: 10.1002/cpp.693. [DOI] [PubMed] [Google Scholar]
- Marshall EC, Zvolensky MJ, Vujanovic AA, Gibson LE, Gregor K, Bernstein A. Evaluation of smoking characteristics among community-recruited daily smokers with and without posttraumatic stress disorder and panic psychopathology. J Anx Disord. 2008;22:1214–1226. doi: 10.1016/j.janxdis.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi D, Matsuoka Y, Kim Y. Posttraumatic growth, posttraumatic stress disorder and resilience of motor vehicle accident survivors. Biopsychosoc Med. 2010;4 doi: 10.1186/1751-0759-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergadia ML. Personal resources and smoking cessation: Promoting assets and preventing liabilities. 2002 [Google Scholar]
- Rasmusson AM, Picciotto MR, Krishnan-Sarin S. Smoking as a complex but critical covariate in neurobiological studies of posttraumatic stress disorders: A review. J Psychopharmacol. 2006;20:693–707. doi: 10.1177/0269881106060193. [DOI] [PubMed] [Google Scholar]
- Richards CS, Cohen LM, Morrell HE, Watson NL, Low BE. Treating depressed and anxious smokers in smoking cessation programs. J Consult Clin Psychol. 2013;81:263–273. doi: 10.1037/a0027793. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: Illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol. 2006;74:276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M. Structured clinical interview for DSM-III-R (SCID) New York: Biometrics Research Department, New York State Psychiatric Institute; 1987. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured clinical interview for DSM-III-R-Patient ed, (with psychotic screen; SCID-P) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- Taylor BC, Hagel EM, Carlson KF, Cifu DX, Cutting A, Bidelspach DE, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq war veteran VA users. Med Care. 2012;50:342–6. doi: 10.1097/MLR.0b013e318245a558. [DOI] [PubMed] [Google Scholar]
- Tran US, Glück TM, Lueger-Schuster B. Influence of personal and environmental factors on mental health in a sample of Austrian survivors of World War II with regard to PTSD: Is it resilience? BMC Psychiatry. 2013;13 doi: 10.1186/1471-244X-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk PW, Wangelin B, Rauch SAM, Dismuke CE, Yoder M, Myrick H, et al. Health service utilization before and after evidence-based treatment for PTSD. Psychol Serv. 2013;10:401–9. doi: 10.1037/a0030549. [DOI] [PubMed] [Google Scholar]
- van der Velden PG, Grievink L, Olff M, Gersons BPR, Kleber RJ. Smoking as a risk factor for mental health disturbances after a disaster: A prospective comparative study. J Clin Psychiatry. 2007;68:87–92. doi: 10.4088/jcp.v68n0112. [DOI] [PubMed] [Google Scholar]
- Wingo AP, Ressler KJ, Bradley B. Resilience characteristics mitigate tendency for harmful alcohol and illicit drug use in adults with a history of childhood abuse: A cross-sectional study of 2024 inner-city men and women. J Psychiatr Res. 2014;51:93–9. doi: 10.1016/j.jpsychires.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn GL, Wingo AP, Moore R, Pelletier T, Gutman AR, Bradley B, et al. The effect of resilience on posttraumatic stress disorder in trauma-exposed inner-city primary care patients. J Natl Med Assoc. 2011;103:560–6. doi: 10.1016/s0027-9684(15)30381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National institute of mental health report. Nicotine Tob Res. 2008;10:1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Gibson LE, Vujanovic AA, Gregor K, Bernstein A, Kahler C, et al. Impact of posttraumatic stress disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine Tob Res. 2008;10:1415–1427. doi: 10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]