Abstract
Kv7 (KCNQ) potassium channel openers (enhancers) decrease neuropathic pain in experimental models. Here we show that C-fibers, and their associated small-diameter neurons in the dorsal root ganglia (both IB4- and TrkA-positive), expressed Kv7.5. In contrast, C-fibers did not express detectable levels of Kv7.2 or Kv7.3, which are instead localized to nodes of Ranvier and the cell bodies of large sensory neurons. These data suggest that Kv7.5 provides the primary M current in nociceptive neurons.
Indexing Terms: KCNQ, M-current, dorsal root ganglion, nociceptors, Kv7, Remak fibers
The Kv7 (KCNQ) family of K+ channels was originally characterized by their modulation by muscarinic agonists, giving rise to the name M-currents (Brown and Adams, 1980; Wang et al., 1998). In mammals, there are five Kv7 channels, Kv7.1–7.5; all can form homomeric channels, and Kv7.3 can form heteromeric channels with either Kv7.2 or Kv7.5 (Jentsch, 2000; Schroeder et al., 2000). All Kv7 channels are slowly activated by depolarization and play an important role in maintaining normal resting membrane potential. Dominant mutations of KCNQ2 or KCNQ3 cause benign familial neonatal convulsions, a form of generalized epilepsy confined to the neonatal period (Singh et al., 1998). In addition, at least one KCNQ2 mutation also causes neuromyotonia (Dedek et al., 2001), which is characterized by excessive excitability of distal motor axons.
We have previously shown that Kv7.2 and Kv7.3 channels are highly enriched in axon initial segments (AISs) and nodes of Ranvier (Devaux et al., 2004). Their localization is due to a consensus ankyrinG binding motif in the intracellular carboxy-terminus (Pan et al., 2006). This motif is present in all vertebrate voltage-gated Na+ channels (Nav1.1–1.9), Kv7.2, and Kv7.3, but not in Kv7.1, Kv7.4, or Kv7.5—a unique example of convergent evolution at the molecular level (Hill et al., 2008).
Kv7.5 mRNA has been detected in the small neurons of the dorsal root ganglia (DRG) (Passmore et al., 2003), but the localizations of the Kv7.5 protein in peripheral nerves and DRG are unknown. We show here that Kv7.5 is localized in the axons of the Remak bundles (unmyelinated axons and their associated Schwann cells), including their cutaneous branches, and is not detected at nodes of Ranvier. Furthermore, small-diameter DRG neurons, the origin of these unmyelinated afferents, express relatively more Kv7.5 than do large DRG neurons. Thus, Kv7.5 may be the relevant Kv7 channel expressed by C-fibers.
Materials and Methods
Animals and tissue sections
All procedures involving rodents were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Eight to 10-week-old adult Sprague-Dawley rats (n = 3) or C57BL/6 mice (n = 3) were anesthetized with ketamine/xylazine mix and killed by decapitation. Sciatic nerves, DRG (from L4-L6 spinal levels), and skin (both hairy and glabrous) were dissected and quickly embedded in OCT cooled in an acetone/dry ice slurry. The sciatic nerve fibers were teased apart with fine needles, mounted on SuperFrost Plus glass slides (Fisher Scientific, Pittsburgh, PA), dried overnight, and stored at −20°C. Ten-μm-thick cryostat sections were thaw-mounted onto Superfrost slides and stored at −20°C.
Axotomy was performed on anesthetized (60 mg/kg of ketamine, 7.5 mg/kg of xylazine) 30-day-old Sprague–Dawley rats (n = 3). The sciatic nerve was exposed at the sciatic notch and transected with iridectomy scissors and the skin incision was closed with wound clips. Four days after the surgery the animals were euthanized and the sciatic nerve segment distal to the transection site, as well as the corresponding contralateral sciatic nerve segment, were dissected and teased onto slides, and immunostained with antisera described below. For image recording, identical exposure times were used for both the transected and contralateral teased fibers.
Immunohistochemistry
Teased fibers and OCT sections were immersed in − 20°C acetone for 10 minutes, rinsed in Tris-buffer saline (TBS; pH 7.4), blocked at room temperature for 1 hour in TBS containing 5% fish skin gelatin and 0.5% Triton X-100, and incubated overnight at 4°C with various combinations of primary antibodies diluted in blocking solution. The slides were washed with TBS, incubated with the appropriate FITC-, TRITC-, and Cy5-conjugated donkey cross-affinity-purified secondary antibodies (Jackson ImmunoResearch, 1:200) at room temperature for 1 hour, washed with TBS, counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Invitrogen, La Jolla, CA), mounted with Vectashield (Vector Laboratories, Burlingame, CA), and examined by epifluorescence on a Leica DMR light microscope with a cooled Hama-matsu camera under the control of Openlab software (Improvision, Lexington, MA). When necessary, digital images were cropped and RGB histogram adjusted to fill entire tonal range using Photoshop (Adobe, San Jose, CA).
Antibody characterization
Please see Table 1 for a summary of all primary antibodies used. The KCNQ2N antiserum (Cooper et al., 2001) stained nodes and AISs of teased nerve fibers in an identical pattern as previously shown (Devaux et al., 2004; Pan et al., 2006). In addition, using the Lipofectamine 2000 kit (Invitrogen) with a method as previously described (Rasmussen et al., 2007), the KCNQ2N antiserum positively stained Hela cells that were transiently transfected with the cDNA encoding human Kv7.2 (kindly provided by Dr. Edward Cooper), but did not stain Hela cells transfected with human Kv7.5 cDNA (kindly provided by Dr. Thomas Jentsch). Finally, the nodal staining by the KCNQ2N antiserum was abolished in tissues of a Kv7.2 conditionally null mice, but was preserved in tissues of a wildtype littermate. The KCNQ3C antiserum also stained nodes in an identical pattern as previously described (Pan et al., 2006).
Table 1. List of Primary Antibodies Used.
| Name | Manufacturer | Dilution | Species | Type | Immunogen |
|---|---|---|---|---|---|
| KCNQ2N | Gift from Dr. Edward Cooper | 1:200 | Rabbit | Polyclonal | KCNQ2 N-terminus (residues 13-37) |
| KCNQ3C | Gift from Dr. Edward Cooper | 1:200 | Rabbit | Polyclonal | KCNQ3 C-terminus (residues 578-604) |
| KCNQ5 | Chemicon; AB5599 | 1:1000 | Rabbit | Polyclonal | KCNQ5 N-terminus (residues 12-93) |
| Kv7.5 | Chemicon; AB9792 | 1:200 | Rabbit | Polyclonal | KCNQ5 N-terminus (residues 89-103) |
| panNav | Sigma; clone K58/35 | 1:250 | Mouse | Monoclonal | CTEEQKKYYNAMKKLGSKK from the intracellular III-IV loop of Na+ channels |
| TrkA | R&D Systems; AF1056 | 1:100 | Goat | Polyclonal | Recombinant rat TrkA (Ala33 Pro418) |
| IB4 | Sigma; L2140 | 1:100 | Biotin-conjugated | N/A | Major affinity for terminal α-D-galactosyl residues of blood group B |
| GFAP | Gift from Dr. Virginia Lee | 1:200 | Rat | Monoclonal | Highly enriched glial filament proteins from bovine spinal cord |
The sequences of immunogen targeted by the KCNQ5 antiserum (Chemicon, Temecula, CA; AB5599) and the Kv7.5 antiserum (Chemicon, AB9792) were ascertained from both the manufacturer and from Caminos et al. (2007). Both antisera were able to positively label Hela cells transfected by the Kv7.5 cDNA but did not label Hela cells transfected by the Kv7.2 cDNA. In addition, peptide-blocking was performed, whereby slides of rat sciatic nerves and DRG were stained according to the above protocol, except that the AB5599 and the AB9792 Kv7.5 antisera (along with the panNav antibody) were preincubated with the purified peptide for the AB9792 antiserum (10 μg peptide per 1 μg of antibody in 100 μl for 12 hours at 37°C; the mixtures were then centrifuged for 15 minutes at 13,000 rpm and the supernatant was used in the primary antibody step); the blocking experiment was repeated three times and when images were taken with the microscope an identical exposure time was used for all blocking conditions. Preincubation with immunogen of AB9792 was able to diminish AB9792 staining of all tissues, but did not change AB5599's staining.
The panNav monoclonal antibody targets multiple voltage-gated Na+ channels, and has previously been shown to specifically stain the AISs and nodes of a wide range of nervous tissues (Rasband et al., 1999; Devaux et al., 2004; Pan et al., 2006); in our stainings it recognized the nodes of both rat and mouse sciatic nerves, as expected.
The TrkA antiserum has been shown by the manufacturer to be able to detect rat TrkA in both direct enzyme-linked immunosorbent assays (ELISAs) and western blots, with less than 1% crossreactivity with recombinant mouse TrkB and TrkC. It has been previously shown to label primarily small- and medium-sized rat DRG cells (Averill et al., 1995), and in our immunostaining of rat DRG sections the TrkA antiserum labeled primarily small- and medium-size neurons as well.
IB4 lectin is derived from Bandeiraea simplicifolia and binds to cells surface glycoprotein of GDNF-sensitive small-diameter DRG neurons (Kashiba et al., 2001). In our staining we used biotin-conjugated IB4 lectin and we observed that it preferentially labeled small-diameter DRG neurons, as shown previously (Ivanusic, 2009).
The glial fibrillary acidic protein (GFAP) antiserum recognizes a single band of 51 kDa on immunoblot of cyto-skeleton-enriched rat spinal cord lysate and has been shown to stain glial elements and astrocytes (Lee at al., 1984). When we stained rat sciatic nerve, the antibody labeled nonmyelinated Schwann cells, consistent with other studies using anti-GFAP antisera (Jessen et al., 1985, 1990; Cheng and Zochodne, 2002).
Kv7.5 quantification
DRG sections from three rats were triple-stained with Kv7.5 AB5599 antiserum, IB4 lectin, and TrkA antiserum, and imaged with a Leica Sp2 confocal microscope system. The embedding, sectioning, staining, and imaging were done in parallel. Cell areas and intensities of the three secondary antibodies specific to each of the primary antisera were measured using National Institutes of Health (NIH, Bethesda, MD) ImageJ. Cytoplasmic areas were calculated by subtracting the nucleus areas from the total cell areas; in order to measure intensities only near the center of cells, cells with nucleus area <80 μm2 or total cell area <255 μm2 were excluded in the final calculation. Cytoplasmic intensities were calculated by subtracting the nucleus intensities from the whole cell intensities. Data were collected in Microsoft Excel, and Student's t-tests were performed using SAS (SAS Institute, Cary, NC). For the comparison between IB4 and TrkA staining, a threshold of 20 was selected as the cutoff for clear positive staining.
To quantify mRNA level of Kv7.5, three samples of total RNA isolated from young adult rat sciatic nerve and DRG by CsCl2 gradient centrifugation (Chirgwin et al., 1979) was amplified and analyzed with the SuperScript One-Step reverse-transcription polymerase chain reaction (RT-PCR) with the Platinum Taq system (Invitrogen, 10928-042) according to manufacturer's protocol. NCBI Primer-BLAST using a known Rattus norvegicus mRNA sequence was used to design primer sets complementary to rat KV7.5 (forward, ACGTCACCACCTGCCTTGTTG; reverse, TGTAAGTTCAGTTCCTCTGTCGATCT) and myelin-associated glycoprotein (MAG; forward, AGCCACCGCCTTCAACCTGT; reverse, TGGCAAAGGCGACCACAGCA). To rule out genomic DNA contamination, two units of Platinum Taq DNA polymerase (Invitrogen, 10966-034) were used instead of the RT/Platinum Taq Mix in the reaction. The RT-PCR of the sciatic nerve total RNA sample was repeated three times.
Results
Kv7.5 is localized to Remak bundles
We immunostained unfixed teased fibers from rat sciatic nerves for Kv7.2 or Kv7.5, combined with a mouse monoclonal antibody against voltage-gated Na+ channels (panNav). As shown in Figure 1, nodes of Ranvier were strongly Kv7.2- and panNav-positive, as previously shown (Devaux et al., 2004; Pan et al., 2006). Remak bundles were panNav-positive, but were not detectably labeled by the Kv7.2 antiserum. The Kv7.5 antiserum (Chemicon, AB9792) produced the reciprocal result: nodes were not labeled, whereas Remak bundles were Kv7.5- (and pan-Nav)-positive. Another Kv7.5 antiserum produced the same staining pattern (Chemicon, AB5599; data not shown).
Figure 1.
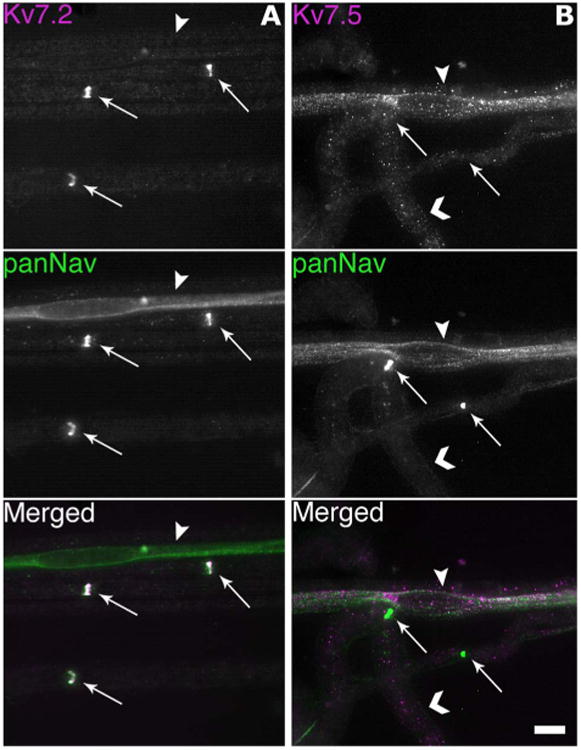
Remak bundles, but not nodes, express Kv7.5. These are digital images of unfixed teased fibers, double-labeled for either Kv7.2 or Kv7.5 (Chemicon, AB9792; magenta) and voltage-gated sodium channels (panNav; green). A: Note that Kv7.2 and panNav are colocalized at nodes (arrows), but Kv7.2 is not localized in a Remak bundle (arrowhead). B: Kv7.5 is not detected at nodes (arrows), but is expressed in a Remak bundle (arrowhead), and as a granular staining on myelinated axons (chevrons). Scale bar = 10 μm.
Because Kv7.5 can form heteromeric channels with Kv7.3 in vitro (Schroeder et al., 2000), we investigated whether Kv7.3 is also present in Remak bundles. The KCNQ3C antiserum we used does not label rat tissues well (due to the possibility that targeted residues of KCNQ3C is not conserved in rat; see Pan et al., 2006), so we immunostained unfixed mouse teased fibers with the Kv7.2, Kv7.3, or Kv7.5 (Chemicon, AB5599) antisera, combined with the panNav mouse monoclonal antibody. Similar to the above results, the Kv7.2 and Kv7.3 antisera labeled nodes but not the Remak bundles (Fig. 2), while the Kv7.5 antiserum produced the opposite result, labeling the Remak bundles but not the nodes. Therefore, Kv7.5 likely forms homomeric channels, but not heteromeric channels with Kv7.3, in Remak bundles. In a separate experiment, the Kv7.2 antiserum was used on a Kv7.2 conditionally null mouse, and the Kv7.2 staining of the nodes was abolished (with intact Kv7.3 nodal staining), thereby demonstrating the specificity of our Kv7.2 antiserum (unpubl. data; not shown).
Figure 2.
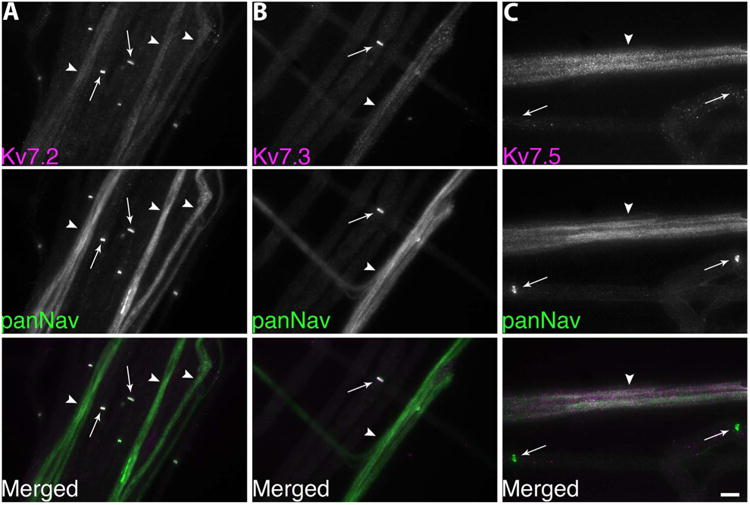
Remak bundles do not express Kv7.3. These are digital images of unfixed mouse teased nerves, double-labeled for either Kv7.2, Kv7.3, or Kv7.5 (AB5599; magenta) and voltage-gated sodium channels (panNav; green). A,B: Note that Kv7.2 and Kv7.3 are colocalized with panNav at nodes (arrows), but not in Remak bundles (arrowheads). C: The Kv7.5 antiserum labels Remak bundles (arrowheads) but not nodes (arrow). Scale bar = 10 μm.
The Chemicon AB9792 and AB5599 Kv7.5 antisera also produced granular staining of myelinated fibers (Fig. 1, chevrons). Specifically, much of the granular staining lies outside of the neurofilament-positive axons, but resides with the myelin sheaths (figures not shown). These two Kv7.5 antisera were raised against partially overlapping sequences of the intracellular N-terminus; this entire region shares little homology with Kv7.2. To ascertain their specificity, HeLa cells were transiently transfected to express either Kv7.2 or Kv7.5. Cells expressing Kv7.5, but not Kv7.2, were strongly labeled by both Kv7.5 antisera. Conversely, cells expressing Kv7.2, but not Kv7.5, were robustly labeled with the Kv7.2 antiserum but not by either Kv7.5 antisera (figures not shown).
To determine whether the Kv7.5 expression in the Remak bundles originates from axons or the surrounding Schwann cells, we immunostained teased fibers from the distal nerve stump of sciatic nerves 4 days posttransection. At this time, axons distal to the injury have degenerated, while their associated Schwann cells persist (Griffin and Thompson, 2008). Denervated Remak bundles were not panNav- or Kv7.5-positive, whereas Remak Schwann cells were GFAP-positive (Fig. 3). These results support the idea that Remak axons and not Remak Schwann cells express Kv7.5. While there exists the possibility that the disappearance of Kv7.5 staining is due to a loss of its expression from the Schwann cells rather than from the axons after nerve injury, we feel that this is a less likely explanation given the concurrent loss of panNav staining with the retention of robust GFAP staining, suggesting the loss of axons and the continued presence of its associated Schwann cells. In addition, as we will show, expression of Kv7.5 by the small-diameter DRG neurons also indicate that the loss of Kv7.5 staining is from the axons, since they originate from these DRG neurons. Finally, we performed a blocking experiment, utilizing the purified immunogen for the AB9792 antiserum. As shown in Figure 4, preincubation with the peptide eliminated the labeling of Remak bundles, and seemed to reduce granular staining of myelinated axons.
Figure 3.
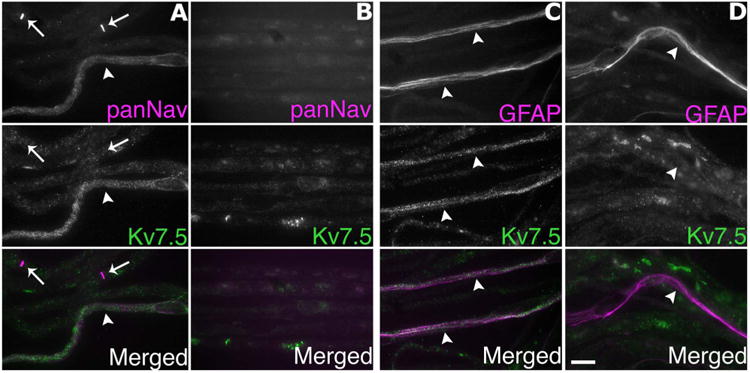
Axotomy abolishes Kv7.5 expression. These are digital images of unfixed rat teased fibers, taken from the nerve segment distal to the lesion (B,D) 4 days posttransection, or the corresponding segment of an unlesioned nerve (A,C). The fibers were double-labeled for Kv7.5 (Chemicon, AB9792; green) and either voltage-gated sodium channels (panNav; magenta) or glial fibrillary acidic protein (GFAP; magenta), labels Remak Schwann cells. Note that panNav-immunoreactivity is associated with nodes (arrows) and a Remak bundle (arrowhead), which is lost after axotomy (B), and that Kv7.5-immunoreactivity in Remak bundles (arrowheads) is also lost after axotomy, whereas GFAP-immunoreactivity persists. Scale bar = 20 μm.
Figure 4.
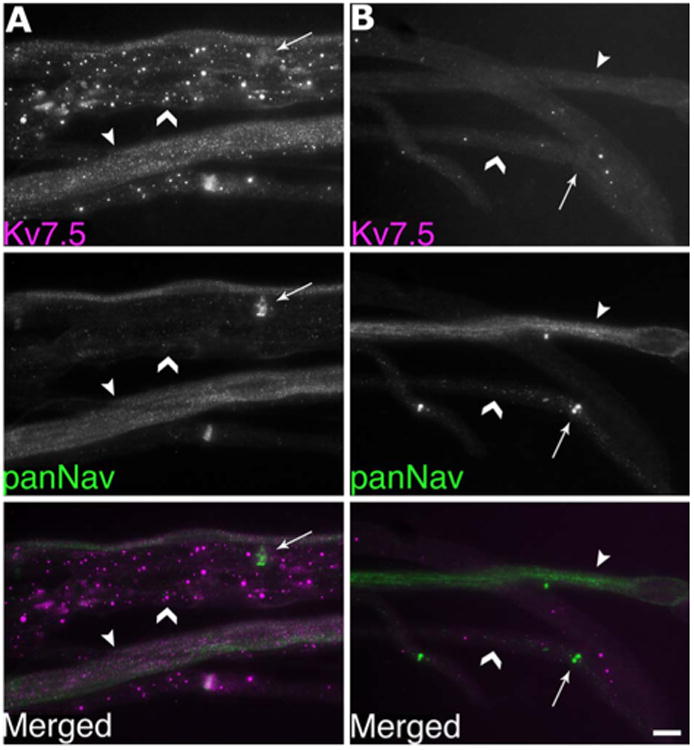
Cognate antigen reduces immunostaining of Remak bundles with Kv7.5 antiserum AB9792. These are images of unfixed teased fibers that were double-labeled for Kv7.5 (AB9792; magenta) and panNav (green), as indicated. The images were exposed for the same amount of time to facilitate direct comparison. Compared to teased fibers labeled with the AB9792 antiserum alone (A), preincubation with the control antigen (B) reduced the granular staining of myelinated axons (chevrons) and abolished the staining of Remak bundles (arrowheads), which are also labeled by the panNav antibody. Arrows indicate nodes. Scale bar = 10 μm.
We were suspicious that the granular staining of Kv7.5 represents background staining, as we are unaware of other antigens that are localized in this pattern. To investigate this, we performed RT-PCR on RNA samples prepared from rat sciatic nerve and DRG, with primer pairs for rat Kv7.5 and MAG (Fig. 5). Both the MAG and the Kv7.5 primers amplified the correctly sized products in both the sciatic nerve and the DRG RNA samples. In order to rule out the possibility of genomic DNA contamination in the RNA samples, we substituted DNA polymerase in place of the RT-PCR polymerase mix. In this case, the Kv7.5 primers did not amplify a product in either the sciatic nerve or the DRG sample, but it did amplify the expected size product from a DNA sample (result not shown). Thus, we cannot rule out the possibility that the granular staining of Kv7.5 of myelin sheaths is authentic.
Figure 5.
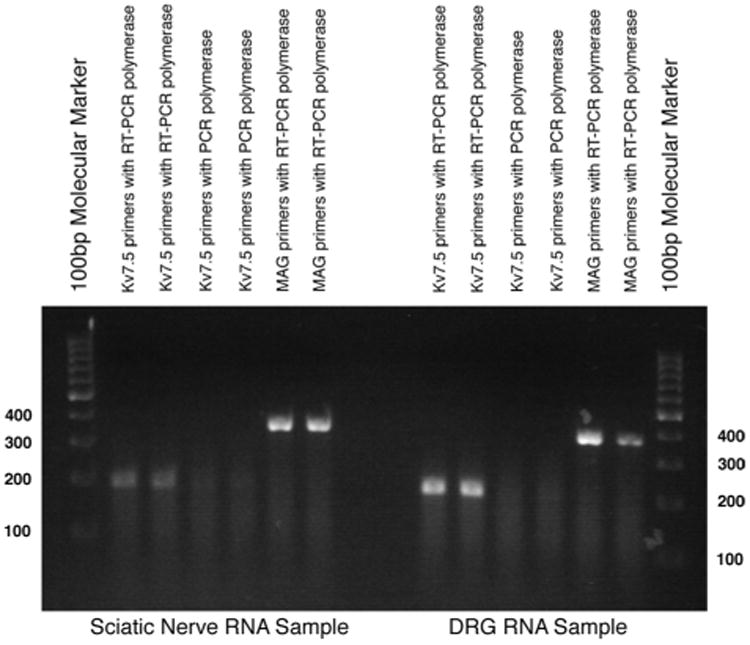
RT-PCR expression analysis of rat sciatic nerve and DRG RNA. Total RNA was isolated from adult rat tissues, duplicate samples were subjected to RT-PCR with the indicated primers and polymerases, and the reaction products were separated by gel electrophoresis. A ≈200 bp band, corresponding to the predicted size of the Kv7.5 mRNA sequence, was detected in both the DRG and the sciatic nerve RNA samples. When PCR polymerase is used instead of RT-PCR polymerase, the Kv7.5 primers did not amplify a similarly sized product from the RNA samples, indicating that they were not contaminated by genomic DNA. An ≈370 bp band, corresponding to the predicted size of MAG mRNA, was present in both RNA samples. Molecular markers are located in the first and last lanes (in 100 bps).
Small DRG neurons express Kv7.5
The largest and smallest axons in peripheral nerves originate from the largest and smallest neurons in DRG, respectively (Perry et al., 1991; Lozeron et al., 2004). To determine which neurons expressed Kv7.2 and Kv7.5, we immunostained unfixed sections of rat DRG (Fig. 6). The Kv7.2 antiserum predominantly labeled large-diameter neurons (which were largely panNav-negative), including their stem processes, which have many of the molecular features of nodes (data not shown). In contrast, the AB9792/Kv7.5 antiserum predominately labeled the smaller neurons, which were mostly panNav-positive. The other Kv7.5 antiserum (AB5599) gave similar results (data not shown). To quantify these results, we used NIH ImageJ to calculate the mean Kv7.5 (AB9792) immunofluorescence for individual DRG neurons imaged by confocal microscopy. As shown in Figure 7, for three different rats there was a negative correlation between cell size and Kv7.5 labeling, with the majority of the highly labeled cells (fluorescence intensity >20) having small diameters. Similar to the sciatic nerve, preincubation with the cognate peptide greatly diminished the staining of neurons by AB9792 (figure not shown).
Figure 6.
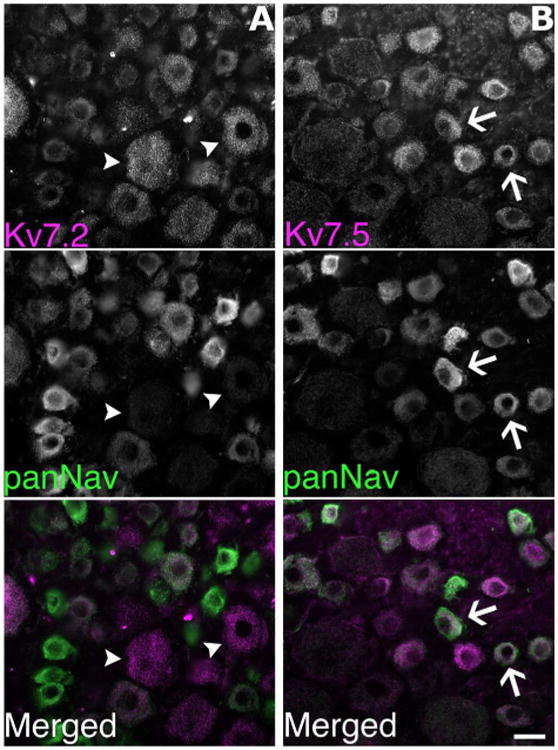
Kv7.5 is predominately expressed by the small DRG neurons. These are images of unfixed sections of rat lumbar DRG, double-labeled with rabbit antisera (magenta) against Kv7.2 or Kv7.5 (AB9792) and a panNav monoclonal antibody (green). Note that in (A) the Kv7.2 antiserum predominately labels large diameter neurons (two examples denoted with arrowheads), which are relatively unlabeled by the panNav antibody. In contrast, in (B) the Kv7.5 antiserum and the panNav antibody preferentially label small diameter neurons (two examples denoted with arrows). Scale bar = 20 μm.
Figure 7.
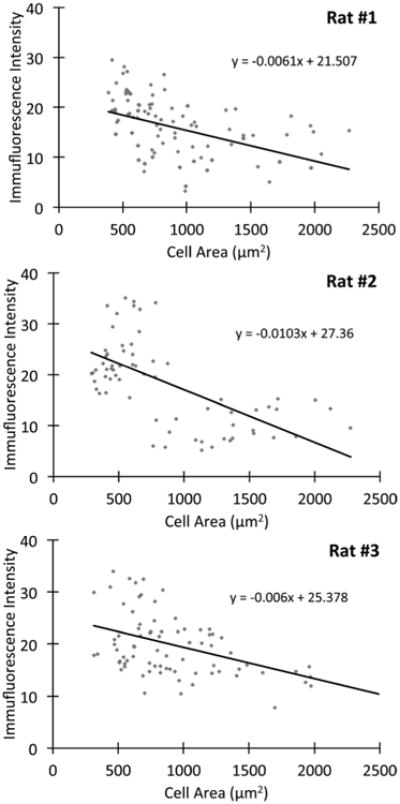
Smaller DRG neurons express relatively higher levels of Kv7.5. The level of Kv7.5/AB9792-immunoreactivity was measured in sections from lumbar DRG neurons from 3 adult rats. Note that Kv7.5-immunoreactivity is inversely related to the size of the DRG neurons, with the majority of the labeled cells (fluorescence intensity >20) being small (cell diameter less than 30 μm, or 701 μm2) (Rasband et al., 2001). The slope of the inverse relationship for each rat is statistically different as compared with the slope of no relationship (slope of 0), with a P-value of <0.05 (Student's t-test).
Small DRG neurons can be divided into either NGF-sensitive neurons that express TrkA (Averill et al., 1995) and the GDNF-sensitive neurons that express glycoprotein that can bind to IB4 (Kashiba et al., 2001), and because both of these neurons give off unmyelinated C fibers, both IB4 and TrkA serve as neurochemical markers of nociceptive neurons (Averill et al., 1995; Molliver et al., 1997; Kashiba et al., 2001; Priestley et al., 2002). To investigate this issue further, we triple-labeled sections of DRG from three individual rats for Kv7.5, IB4, and TrkA (Fig. 8). As expected, almost all of the small DRG neurons were either IB4- or TrkA-positive. Of the small neurons (diameter <30 μm), a similarly high proportion of IB4-positive and TrkA-positive were Kv7.5-positive neurons (Table 2). In contrast, all of the large-diameter, Kv7.2-positive neurons were IB4-negative, and a few were TrkA-positive. Taken together, these data show that Kv7.5 is mainly expressed by small-diameter neurons.
Figure 8.
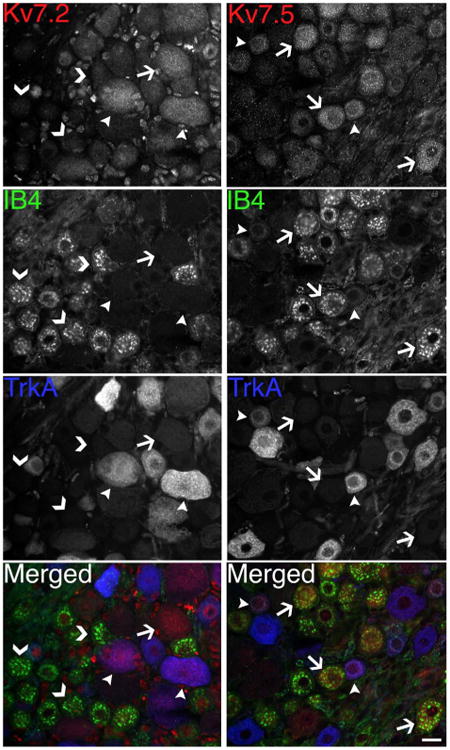
Kv7.5-positive DRG neurons are mostly IB4- or TrkA-positive. These are images of unfixed sections of rat DRG, triple-labeled as indicated for either Kv7.5 (AB9792; red) or Kv7.2 (red), as well as IB4 (green) and TrkA (blue). Note that most Kv7.5-positive neurons are IB4-positive (arrows); fewer are TrkA-positive (arrowheads). In contrast, none of the Kv7.2-positive neurons are IB4-positive (arrow), but some are TrkA-positive (arrowheads); all of the IB4-positive neurons are Kv7.2-negative (chevrons). Scale bar = 20 μm.
Table 2. High Proportion of Small, IB4- and TrkA-Positive Neurons Are Kv7.5-Positive.
| IB4-positive only | TrkA-positive only | Total neurons | |
|---|---|---|---|
| Rat #1 | 12/17 (70.6%) | 5/6 (83.3%) | 17/23 (73.9%) |
| Rat #2 | 17/21 (81.0%) | 9/9 (100%) | 23/30 (76.7%) |
| Rat #3 | 17/20 (85.0%) | 8/10 (80%) | 25/30 (83.3%) |
The cell soma size as well as the fluorescence intensity of IB4, TrkA, and Kv7.5 was measured for ∼ 100 DRG neurons from 3 rats. For each rat we counted the number of small neurons (diameter < 30 μm) that were either IB4- or TrkA-positive (fluorescence intensity >20), and also determined whether they were Kv7.5-positive. For each rat the fraction of Kv7.5-positive small neurons to the total number small DRG neurons is shown. A similarly high proportion of IB4-positive and TrkA-positive small neurons are Kv7.5-positive.
Peripheral axons of cutaneous nociceptors express Kv7.5
Because Kv7 channel enhancers have local effects in the skin (Passmore et al., 2003), we wished to determine whether cutaneous afferents express Kv7.5. We immunostained unfixed sections of glabrous and hairy skin from rats for Kv7.2 or Kv7.5 and panNav. As shown in Figure 9, the panNav-positive unmyelinated axons were predominately Kv7.5-positive, and almost all were Kv7.2-negative. Preincubation of Kv7.5 antiserum with its cognate antigen greatly diminished the labeling of unmyelinated axons (figure not shown).
Figure 9.
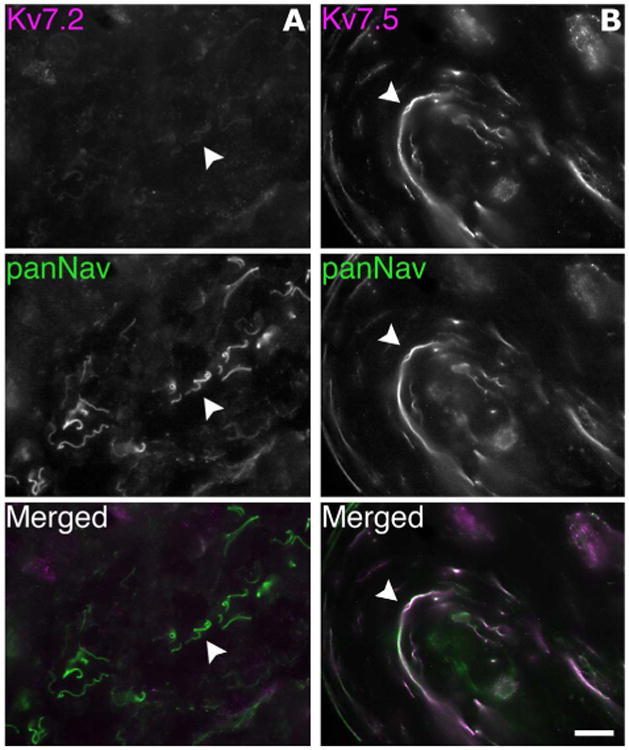
Cutaneous afferents express Kv7.5. These are digital images of unfixed section of hairy skin, double-labeled for Kv7.2 (magenta), Kv7.5 (Chemicon, AB9792; magenta), and voltage-gated sodium channels (panNav; green). A: The unmyelinated axons are labeled by the panNav antibody but not by the Kv7.2 antiserum (one example is denoted with an arrowhead). B: The unmyelinated axons are labeled by both the Kv7.5 antiserum and the panNav antibody (arrowhead). Scale bar = 20 μm.
Discussion
In this present study, we found that C-fibers, including their cutaneous branches, express Kv7.5 and not Kv7.2 or Kv7.3. Small-diameter DRG neurons also preferentially express Kv7.5 along with either IB4 or TrkA. Thus, Kv7.5 is the predominant Kv7 channel expressed by nociceptors, from their cell bodies to their terminals. In contrast to Kv7.2 and Kv7.3, Kv7.5 expression was not found at nodes of Ranvier. Although Kv7.5 subunits can form heteromeric channels with the Kv7.3 subunits (Schroeder et al., 2000), Kv7.3 was not colocalized with Kv7.5 in C-fibers, suggesting that the Kv7.5 primarily forms homomeric channels in C-fibers.
Although a previous study found that cultured DRG neurons express Kv7.2, Kv7.3, and/or Kv7.5 (Passmore et al., 2003), the authors did not emphasize that Kv7.5 is mainly localized to small-diameter neurons as we report here. However, they did show that the M-current was the dominant subthreshold sustained current in small sensory neurons, and that many were capsaicin-sensitive, confirming that they are nociceptors (Passmore et al., 2003). This observation is in agreement with our finding that the Kv7.5-positive small-diameter DRG neurons are also positive for either IB4 or TrkA, both neurochemical markers of nociceptors. Interestingly, several studies of nerve function suggest that C-fibers and their terminals are refractory to the M-channel blocker XE991 (Lang et al., 2008; Roza and Lopez-Garcia, 2008), while A-fibers are sensitive to inhibition (Schwarz et al., 2006; Brown and Passmore, 2009). Since the IC50 for XE991 inhibition is much higher for Kv7.5 (50–70 μM; Schroeder et al., 2000) than it is for Kv7.2/7.3 subunits (0.6 μM; Wang et al., 2000), the preferential expression of Kv7.5 by C-fibers may explain its insensitivity to XE991. Large neurons, in contrast, were IB4-negative and Kv7.2-positive, consistent with a previous study showing that IB4-negative neurons had a TEA-sensitive Kv current (with properties of slow kinetics and no inactivation) that was inhibited by 25 mM TEA (Vydyanathan et al., 2005), which should block Kv7.2 but not Kv7.5 (Hadley et al., 2000; Schroeder et al., 2000; Hadley et al., 2003). Our results contradict a recent report that Kv7.2-immunoreactivity is predominately localized to small sensory neurons and abundantly expressed in sciatic nerve axons (Rose et al., 2011). These findings are at odds with several reports that Kv7.2 is localized to nodes (Devaux et al., 2004; Pan et al., 2006; Schwarz et al., 2006) and our finding that large and not small sensory neurons are Kv7.2-positive. We have, furthermore, found that Kv7.2-immunoreactivity is lost in both nodes and large-diameter DRG neurons from a Kv7.2-conditional null mouse (unpubl. data), demonstrating the authenticity of our results. Taken together, different populations of sensory neurons express different levels of Kv7.2 and Kv7.5 (and likely Kv7.3); these channels likely affect their electrophysiological characteristics, akin to the differential expression of different voltage-gated Na channels in different kinds of sensory neurons (Waxman et al., 1999).
Whether Kv7.5 functions in the conduction of normal C-fibers remains uncertain. Devaux et al. (2004) showed that retigabine (a Kv7-specific enhancer; Lerche et al., 2000; Brueggemann et al., 2007) mildly slows the conduction velocity of myelinated axons; they did not investigate C-fibers. In biopsied human sural nerves, retigabine increased the membrane threshold and modified the postspike recovery cycle of C-fibers (Lang et al., 2008), but these nerves were recovered from patients suffering from neuropathy or peripheral vascular disease. Roza and Lopez-Garcia (2008), however, reported that retigabine had no effect on C-fiber conduction in mouse saphenous nerve, but they only used one type of stimulus (a von Frey probe) and had a small sample size (five units). These results are not definitive in our opinion.
In contrast, Kv7 enhancers have substantial effects in rodent models of neuropathic/inflammatory pain. Kv7 enhancers (retigabine or flupirtine) reduce the excitability of injured axons to both mechanical and chemical stimuli (Passmore et al., 2003; Roza and Lopez-Garcia 2008), as well as the behavioral responses of rodents following nerve ligation or cutaneous injections of irritants (Blackburn-Munro et al., 2003; Passmore et al., 2003; Dost et al., 2004; Nielsen et al., 2004). Because these effects can be blocked by coadministrating Kv7 blockers, they most likely stem from enhanced Kv7 activity. Our finding that Kv7.5 is the main Kv7 subunit expressed by C-fibers focuses attention on the possible role of Kv7.5 in these models of pain, and the expression of Kv7.5 during the development of neuropathic pain warrants further investigation.
The ability of G-protein-coupled receptors (GPCRs) to modulate Kv7 channels gives rise to the possibility that GPCRs expressed by nociceptive axons modulate neuropathic/inflammatory pain. Local injection of inflammatory mediators that bind to GPCRs, such as bradykinin and certain proteases, can cause pain, presumably through the excitation and sensitization of the peripheral terminals of nociceptors (Linley et al., 2010). In small cultured DRG neurons, bradykinin (via the B2R receptor) increases intracellular Ca2+, which inhibits M-currents (Liu et al., 2010); in vitro study using CHO cells specifically shows intracellular Ca2+ only inhibits Kv7.2, Kv7.4, and Kv7.5 subunits (Gamper and Shapiro, 2005). Furthermore, injecting bradykinin into rat hindpaws produced nocifensive behavior that is reversed by retigabine, suggesting that bradykinin induces acute pain, at least in part, through its modulation of Kv7 channels (Liu et al., 2010). Similarly, ligands for protease-activated receptor 2 (PAR-2) inhibit M-current in small cultured DRG neurons by increasing intracellular Ca2+ (Linley et al., 2008). Injecting PAR-2 agonists into rat hindpaws produce nocifensive behavior similar to that produced by Kv7 blocker (XE991) injection, and the simultaneous injection of both compounds does not produce additive pain response, indicating that PAR-2 agonists act primarily through the Kv7 channels. Finally, beta-alanine, the ligand for MrgD receptors, inhibits the M-current in cultured IB4-positive sensory neurons (Crozier et al., 2007); the electrophysiological effects of beta-alanine are absent in Mrgprd-null sensory neurons, but the M-current was not measured (Rau et al., 2009). Taken together, the endogenous ligands of GPCRs may decrease the M-current of nociceptors, which we have reason to believe is largely generated by Kv7.5 channels, and lead to increased nociception.
Acknowledgments
We thank Drs. Edward Cooper, Virginia Lee, Thomas Jentsch, and Alvaro Villarroel for the cDNAs and antisera.
Grant sponsor: National Institutes of Health (NIH); Grant number: RO1NS43174.
Footnotes
The authors have no conflicts of interest.
Literature Cited
- Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–94. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn-Munro G, Jensen BS. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur J Pharmacol. 2003;460:109–116. doi: 10.1016/s0014-2999(02)02924-2. [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage sensitive K+ current in a vertebrate neuron. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Moran CJ, Barakat JA, Yeh JZ, Cribbs LL, Byron KL. Vasopressin stimulates action potential firing by protein kinase C-dependent inhibition of KCNQ5 in A7r5 rat aortic smooth muscle cells. Am J Physiol Heart Circ Physio. 2007;292:H1352–H1363. doi: 10.1152/ajpheart.00065.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminos E, Garcia-Pino E, Martinez-Galan JR, Juiz JM. The potassium channel KCNQ5/Kv7.5 is localized in synaptic endings of auditory brainstem nuclei of the rat. J Comp Neurol. 2007;503:363–378. doi: 10.1002/cne.21497. [DOI] [PubMed] [Google Scholar]
- Cheng C, Zochodne DW. In vivo proliferation, migration and phenotypic changes of Schwann cells in the presence of myelinated fibers. Neuroscience. 2002;115:321–329. doi: 10.1016/s0306-4522(02)00291-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin JM, Przbyla AE, MacDonald RJ, Rutter RJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci. 2001;21:9529–9540. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RA, Ajit SK, Kaftan EJ, Pausch MH. MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J Neurosci. 2007;27:4492–4496. doi: 10.1523/JNEUROSCI.4932-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek K, Kunath B, Kananura C, Reuner U, Jentsch TJ, Steinlein OK. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proc Natl Acad Sci U S A. 2001;98:12272–12277. doi: 10.1073/pnas.211431298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dost R, Rostock A, Rundfeldt C. The anti-hyperalgesic activity of retigabine is mediated by KCNQ potassium channel activation. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:382–390. doi: 10.1007/s00210-004-0881-1. [DOI] [PubMed] [Google Scholar]
- Gamper N, Li Y, Shapiro MS. Structural requirements for differential sensitivity of KCNQ K+ channels to modulation by Ca2+/calmodulin. Mol Biol Cell. 2005;16:3538–3551. doi: 10.1091/mbc.E04-09-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW, Thompson WJ. Biology and pathology of nonmyelinating Schwann cells. Glia. 2008;56:1518–1531. doi: 10.1002/glia.20778. [DOI] [PubMed] [Google Scholar]
- Hadley JK, Noda M, Selyanko AA, Wood IC, Abogadie FC, Brown DA. Differential tetraethylammonium sensitivity of KCNQ1–4 potassium channels. Br J Pharmacol. 2000;129:412–415. doi: 10.1038/sj.bjp.0703086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley JK, Passmore GM, Tatulian L, Al-Qatari M, Ye F, Wickenden AD, Brown DA. Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and sub-unit composition of native ganglionic M channels deduced from block by tetraethylammonium. J Neurosci. 2003;23:5012–5019. doi: 10.1523/JNEUROSCI.23-12-05012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Nishino A, Nakajo K, Zhang G, Fineman JR, Selzer ME, Okamura Y, Cooper EC. Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet. 2008;4:e1000317. doi: 10.1371/journal.pgen.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanusic JJ. Size, neurochemistry, and segmental distribution of sensory neurons innervating the rat tibia. J Comp Neurol. 2009;517:276–283. doi: 10.1002/cne.22160. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Uchida Y, Senba E. Difference in binding by isolectin B4 to trkA and c-ret mRNA-expressing neurons in rat sensory ganglia. Brain Res Mol Brain Res. 2001;95:18–26. doi: 10.1016/s0169-328x(01)00224-8. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Morgan L, Brammer M, Mirsky R. Galactocerebroside is expressed by non-myelin-forming Schwann cells in situ. J Cell Biol. 1985;10:1135–1143. doi: 10.1083/jcb.101.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Morgan L, Stewart HJ, Mirsky R. Three markers of adult non-myelin-forming Schwann cells, 217c (Ran-1), A5E3 and GFAP: development and regulation by neuron-Schwann cell interactions. Development. 1990;109:91–103. doi: 10.1242/dev.109.1.91. [DOI] [PubMed] [Google Scholar]
- Lang PM, Fleckenstein J, Passmore GM, Brown DA, Grafe P. Retigabine reduces the excitability of unmyelinated peripheral human axons. Neuropharmacology. 2008;54:1271–1278. doi: 10.1016/j.neuropharm.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Lee VM, Page CD, Wu HL, Schlaepfer WW. Monoclonal antibodies to gel-excised glial filament protein and their reactivities with other intermediate filament proteins. J Neurochem. 1984;42:25–32. doi: 10.1111/j.1471-4159.1984.tb09692.x. [DOI] [PubMed] [Google Scholar]
- Lerche C, Scherer CR, Seebohm G, Derst C, Weii AD, Busch AE, Steinmeyer K. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J Biol Chem. 2000;275:22395–22400. doi: 10.1074/jbc.M002378200. [DOI] [PubMed] [Google Scholar]
- Linley JE, Rose K, Patil M, Robertson B, Akopian AN, Gamper N. Inhibition of M current in sensory neurons by exogenous proteases: a signaling pathway mediating inflammatory nociception. J Neurosci. 2008;28:11240–11249. doi: 10.1523/JNEUROSCI.2297-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley JE, Rose K, Ooi L, Gamper N. Understanding inflammatory pain: ion channels contributing to acute and chronic nociception. Eur J Physiol. 2010;459:657–669. doi: 10.1007/s00424-010-0784-6. [DOI] [PubMed] [Google Scholar]
- Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+activated Cl- channels. J Clin Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozeron P, Krarup C, Schmalbruch H. Regeneration of unmyelinated and myelinated sensory nerve fibres studied by a retrograde tracer method. J Neurosci Methods. 2004;138:225–232. doi: 10.1016/j.jneumeth.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Nielsen AN, Mathiesen C, Blackburn-Munro G. Pharmacological characterization of acid-induced muscle allodynia in rats. Eur J Pharmacol. 2004;487:93–103. doi: 10.1016/j.ejphar.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore GM, Selyanko AA, Mistry M, A-Qatari M, Marsh SJ, Mattews EA, Dickenson AH, Brown TA, Burbidge SA, Main M, Brown DA. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MJ, Lawson SN, Robertson J. Neurofilament immunoreactivity in populations of rat primary afferent neurons: a quantitative study of phosphorylated and non-phosphorylated subunits. J Neurocytol. 1991;20:746–58. doi: 10.1007/BF01187848. [DOI] [PubMed] [Google Scholar]
- Priestley JV, Michael GJ, Averill S, Liu M, Willmott N. Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can J Physiol Pharmacol. 2002;80:495–505. doi: 10.1139/y02-034. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Peles E, Trimmer JS, Levinson SR, Lux SE, Shrager P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J Neurosci. 1999;19:756–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:13373–13378. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen HB, Frokjaer-Jensen C, Jensen CS, Jensen HS, Jorgensen NK, Misonou H, Trimmer JS, Olesen SP, Schmitt N. Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J Cell Sci. 2007;120:953–963. doi: 10.1242/jcs.03396. [DOI] [PubMed] [Google Scholar]
- Rau KK, McIlwrath SL, Wang H, Lawson JJ, Jankowski MP, Zylka MJ, Anderson DJ, Koerber HR. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J Neurosci. 2009;29:8612–8619. doi: 10.1523/JNEUROSCI.1057-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K, Ooi L, Dalle C, Robertson B, Wood IC, Gamper N. Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain. 2011;152:742–754. doi: 10.1016/j.pain.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza C, Lopez-Garcia JA. Retigabine, the specific KCNQ channel opener, blocks ectopic discharges in axotomized sensory fibres. Pain. 2008;138:537–545. doi: 10.1016/j.pain.2008.01.031. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinreich F, Ku-Bisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, Kaji R, Bostock H. KCNQ channels mediates IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006;573:17–34. doi: 10.1113/jphysiol.2006.106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quattlebaum T, Murphy JV. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18:25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- Vydyanathan A, Wu ZZ, Chen SR, Pan HL. A-type voltage-gated K+ currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons. J Neurophysiol. 2005;93:3401–3409. doi: 10.1152/jn.01267.2004. [DOI] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Wang HS, Brown BS, McKinnon D, Cohen IS. Molecular basis for differential sensitivity of KCNQ and IKs channels to the cognitive enhancer XE991. Mol Pharmacol. 2000;57:1218–1223. [PubMed] [Google Scholar]
- Waxman SG, Cummins TR, Dib-Hajj S, Fjell J, Black JA. Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain. Muscle Nerve. 1999;22:1177–1187. doi: 10.1002/(sici)1097-4598(199909)22:9<1177::aid-mus3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]


