Abstract
Several studies have shown that fasting plasma nitrite (NO2−) is an indicator of endothelial nitric oxide synthase (NOS) activity while plasma nitrate (NO3−) or the sum of NO2− and NO3− (NOx) do not reflect NOS function. Plasma NO2− can also be elevated through dietary NO3− where the NO3− is partially reduced to NO2− by oral bacteria and enters the plasma through the digestive system. NO3− is taken up from plasma by salivary glands and the cycle repeats itself. Thus, one may propose that salivary NO2− is an indicator of plasma NO2− and consequently of NO production. Many brands of nitric oxide (NO) saliva test strips have been developed that suggest that their product is indicative of circulatory NO availability. However, data supporting a relationship between salivary and plasma NO2− or NO bioavailability is lacking. Here we have measured basal salivary and plasma NO2− and NO3− to determine if any correlation exists between these in 13 adult volunteers. We found no significant correlation between basal salivary and plasma NO2−. Also no correlation exists between salivary NO3− and plasma NO2−. However, we did see a correlation between salivary NO3− and plasma NO3−, and between salivary NO2− and plasma NO3−. In a separate study, we compared the efficiency of salivary NO3− reduction with the efficacy of increasing plasma NO3− and NO2− after drinking beet juice, a high NO3−-containing beverage, in 10 adult volunteers. No significant correlation was observed between the ex vivo salivary reduction of NO3− to NO2− and plasma increases in NO3− or NO2−. These results suggest that measures of salivary NO3−, NO2− or NOx are not good indicators of endothelial function. In addition, the efficiency of saliva to reduce NO3− to NO2− ex-vivo does not demonstrate one’s ability to increase plasma NO2− following consumption of dietary NO3−.
Keywords: Nitrite, nitrate, nitric oxide bioavailability
1. Introduction
Determining a diagnostic marker of endogenous NO bioavailability has been a major topic of research, one which would have clinical implications for monitoring cardiovascular disease, metabolic syndrome, and other conditions [1; 2; 3; 4]. Endothelial dysfunction has been noted as a key event in early atherosclerosis. Due to defective synthesis, decreased levels of endothelium-derived NO characterize endothelial dysfunction [5]. In addition, increased scavenging of NO by oxygen radicals results in low NO bioavailability associated with endothelial dysfunction [6; 7]. Individuals with endothelial dysfunction show a decrease in flow-mediated dilation (FMD) and an increase of intima media thickness (IMT), both representative of atherosclerosis [8; 9; 10; 11]. Given the cost of procedures to measure FMD and IMT, it would be useful to establish a simple blood test to diagnose endothelial dysfunction or low NO bioavailability due to other conditions. The Kelm and Moncada labs have demonstrated that the majority of plasma NO2− is derived from constitutive NOS-activity [12; 13]. Observations have shown that upon regional nitric oxide synthase (NOS) inhibition in forearm circulation, vascular resistance increases linearly as plasma NO2− levels decrease, thereby establishing plasma NO2− as a potential measure of endothelial function [12].
In some of the same studies, plasma NO3− was ruled out as an indicator of NOS function. The Moncada lab showed that only 16% of isotopic L-arginine infused into circulation was represented in plasma NO3− levels versus 90% of plasma NO2− [13]. The Kelm laboratory demonstrated no significant change in plasma NO3− in mammals with inhibition of NOS activity [12]. These data are somewhat expected since plasma NO3− has many NOS-independent factors which can drastically change the basal levels such as dietary NO3− intake, denitrifying liver enzymes, and renal function [14; 15]. Interestingly, Hibbs and colleagues showed that inducible-nitric oxide synthase (iNOS) is one of the main contributors to circulating NO3− due to the increase of NO production after the addition of the cytokine-IL2 [16]. However, the general consensus is that, under most conditions, plasma NO3− does not reflect NOS function or NO bioavailability. In addition, there are even some investigators who suggest that plasma NO2− does not accurately reflect eNOS function and NO bioavailability [17].
In recent years, dietary NO3− has become a known contributor to the pool of bioavailable NO [18]. It is known that dietary NO3− is reduced in the oral cavity by tongue flora, specifically by Actinomyces and Veilonella species [19; 20]. Once ingested, NO2− is non-enzymatically reduced to NO in the gastric acidic milieu [21]. NO3− and remaining NO2− are rapidly absorbed in the small intestine. Plasma NO2− can then be reduced to NO by various mechanisms [18; 22]. Though most of the circulating NO3− is excreted in urine, approximately 25% is extracted by the salivary glands and recycled through the enterosalivary circulation [23]. Complementary to endogenous NO production, this cycle of dietary NO3− being converted to NO in physiology is referred to as the nitrate-nitrite-nitric oxide pathway [18].
Through this physiological pathway, it has been shown that dietary NO3− will increase plasma NO3− and NO2−. In addition, dietary NO3− has been shown to lower blood pressure with short and long term effects, be vasoprotective and reduce platelet aggregation, along with having acute effects on cerebral blood flow and an increase in exercise tolerance and performance [18; 24; 25; 26; 27; 28; 29; 30]. Daily dietary NO3− ingestion also improves endothelial function and vascular stiffness in hypercholesterolemia [31; 32; 33; 34].
As evidence suggests that basal plasma NO2− levels reflect NOS function and bioavailable NO, these measurements may have clinical utility. However, based on the nitrate-nitrite-NO cycle, one may also suggest that salivary NO2− could have the same utility, as recently pointed out [1]. Indeed, commercially available products exist that measure salivary NO2− and claim to report NO bioavailability. However, until now, no published studies have shown a positive correlation between basal plasma NO2− and salivary NO2− levels. Thus, in this work, we sought to investigate the basal levels of plasma and salivary NO2− and NO3−.
When studying increased plasma NO2− after a high NO3− load, Lundberg et al. observed attenuation after using an antibacterial mouthwash [35], suggesting saliva’s conversion from NO3− to NO2− greatly affects plasma NO2−. Consumption of high NO3−-containing food or drinks increases plasma NO3−, NO2−- and thus NOx. However, many studies have observed a significant variation with the increase in plasma levels among individuals [13; 24; 25; 28; 35]. It appears that some individuals are poor or non-responders with respect to dietary NO3− interventions as measured by increases in plasma NO2−[24; 27; 36; 37]. It would be useful to easily determine individuals’ efficacy at converting oral NO3− to plasma NO2−. In this study we hypothesized that saliva would reflect ability to convert NO3− to NO2− in the oral cavity and that this ability would correlate with an individual’s dietary NO3− to plasma NO2− conversion efficacy. Thus we conducted a second study where we examined both ex-vivo conversion of NO3− to NO2− in saliva as well as in vivo conversion of dietary NO3− to plasma NO2−.
2. Methods
2.1 Study Design
All human subjects use was approved by an internal review board following federal and institutional guidelines. For the basal levels study, 13 volunteers (8 male and 5 female) participated between the age of 18 and 80 years old. Volunteers reported to the lab at 9:00 am on the day of their participation. Individuals did not eat or drink within two hours of their participation. If the volunteers had eaten any food the morning of the sampling, they were told to avoid any high NO3− foods (ex. spinach, beets, lettuce, and other green leafy vegetables). In addition, volunteers did not use mouthwash but were permitted to brush their teeth. Upon arrival, blood was drawn from each volunteer from an antecubital vein and collected in a 4 mL lithium heparin vial. Simultaneously, volunteers expectorated 5 mL of saliva which was collected in a sterilized 50 mL Corning tube.
The beet juice study was ancillary to a larger study aimed at investigating potential additional benefits of beet juice combined with supervised exercise compared to supervised exercise alone. The larger study provided a great opportunity for the ancillary one discussed here to compare in vivo conversion of oral nitrate to plasma nitrite and ex vivo salivary conversion efficiency. 10 participants (5 male and 5 female) above the age of 55 were recruited. All recruits consumed a bottle of concentrated beet juice (Beet it Sport shot, 500 mg NO3−) a day for 6 weeks. On the first day of weeks 1, 3 and 6 participation, each recruit came in for sampling. As described for the study above, volunteers did not use mouthwash or eat any high NO3− foods but were permitted to brush their teeth. Blood was drawn before and 1 hour after beet juice consumption. Immediately before the blood draw, participants expectorated a 5 mL saliva sample into a 50 mL sterilized Corning tube. One plasma sample was excluded due to hemolysis during sample preparation. In addition, two anaerobic saliva samples were excluded due to their having dried out during the deoxygenation procedure.
2.2 Measurements
Since salivary NO strips claim to be indicative of physiological NO, we sought to determine what these strips actually test in saliva. Two brands of NO test strips, Nitric Oxide Test Strips (Berkeley Test) and Nitric Oxide Indicator Strips (Neogenis; Austin, TX), were placed in solutions of NaNO3− and NaNO2−. Since the strips have a colorimetric indicating tip, concentrations were varied in order to darken the strip with more reactant.
Similar measuring techniques were used for both basal and beet juice studies. Blood was taken from an antecubital vein and collected in a 4 mL Lithium heparin vial. The tubes were immediately centrifuged at 11,500 g for 2 min. The supernatant plasma was removed and immediately frozen on dry ice in aliquots of ~0.4 mL of plasma and stored in a −80 °C freezer. Plasma NO3− and NO2− were determined and labeled “basal levels.” For the beet juice studies, plasma NO2− levels were determined in the before- and 1 hr after-beet juice consumption timepoints. The difference was reported as the Δ plasma NO2−.
Plasma NO3− and NO2− levels were measured using an HPLC-based Eicom NOx Analyzer, model ENO-20 according to instructions of the manufacturer. For all measurements, standard curves were obtained and used for quantitative measurements.
For the basal levels study, each tube of saliva was centrifuged at 11,500 g for 5 minutes to exclude a large pellet of bacteria. The supernatant salivary matrix was removed and immediately frozen on dry ice in aliquots of ~1.0 mL of saliva until time of analysis. Freezing saliva is a method tested and employed by our lab which maintains the integrity of the sample after exclusion of the bacterial pellet[38; 39]. Before instrumental analysis, saliva was thawed and mixed 1:1 by volume with methanol for deproteination (a method tested which maintains NO3− and NO2− but allows for a cleaner sample to eliminate possible syringe clogging of the HPLC system of the NOx analyzer). Salivary NO3− and NO2− were determined and labeled “basal levels.”
For the beet juice study, each tube of saliva (5 mL) was immediately split into two samples, one designated for aerobic testing and the other for anaerobic testing. Both samples were placed in a round bottom flask and left to incubate in a 37 °C water bath. The anaerobic sample was capped and flushed with argon for 1 hr to deoxygenate and induce anaerobic activity. After 1 hr of incubation, 10 mM NaNO3− was added to each sample to mimic a high NO3− beverage. Starting with a 0 min time point, the NO2− produced was detected at 10 minute increments up to 90 minutes. The salivary rate (k) of NO2− production was calculated and reported in μM/min.
Salivary NO3− and NO2− levels were measured using chemiluminescence-based Nitric Oxide Analyzers (Sievers, Inc.) according to instructions of the manufacturer. For all measurements, standard curves were obtained and used for quantitative measurements.
2.3 Statistical Analysis
For the basal levels study, salivary NO3− and NO2− levels are reported as the mean value of three injections. Plasma NO3− and NO2− are reported as the sole value measured. For the beet juice study, the aerobic and anaerobic salivary rate of NO2− production was plotted against the change in plasma NO2−. A linear trendline was added to each plot to show relative correlation. Spearman correlation coefficients (r) were calculated to measure the linear correlation between the two variables plotted. An r value less than −0.5 or greater than 0.5 was considered significant. In addition, two-tailed p-values were calculated. A p-value less than 0.05 was considered significant.
3. Results
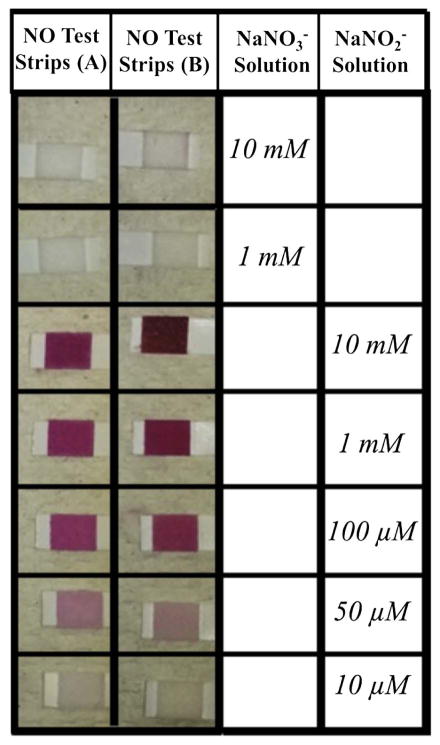
We first investigated sensitivity of oral commercial test strips to NO2− and NO3−. The salivary NO test strips reacted with NaNO2− in solution, but not NaNO3− (Fig 1). There was no colorimetric change when the strips were submerged in NaNO3− solution of 1 and 10 mM concentrations. When the strips were submerged in NaNO2−, a very apparent colorimetric change was seen. No detectable reaction was observed using the low concentration of 10 μM NaNO2. A light pink color was apparent at a concentration of 50 μM and the color darkened with increasing concentrations up to 10 mM NaNO2−.
Fig. 1.
Sensitivity of test strips to NO2− and NO3−. Nitric Oxide Saliva Test Strips, Neogenis (A) and Berkeley (B), were dipped in a NaNO3− or NaNO2− solution. New unused test strips have a white tip where saliva is intended to be placed. Reaction with the strip caused a light pink to deep red colorimetric change.
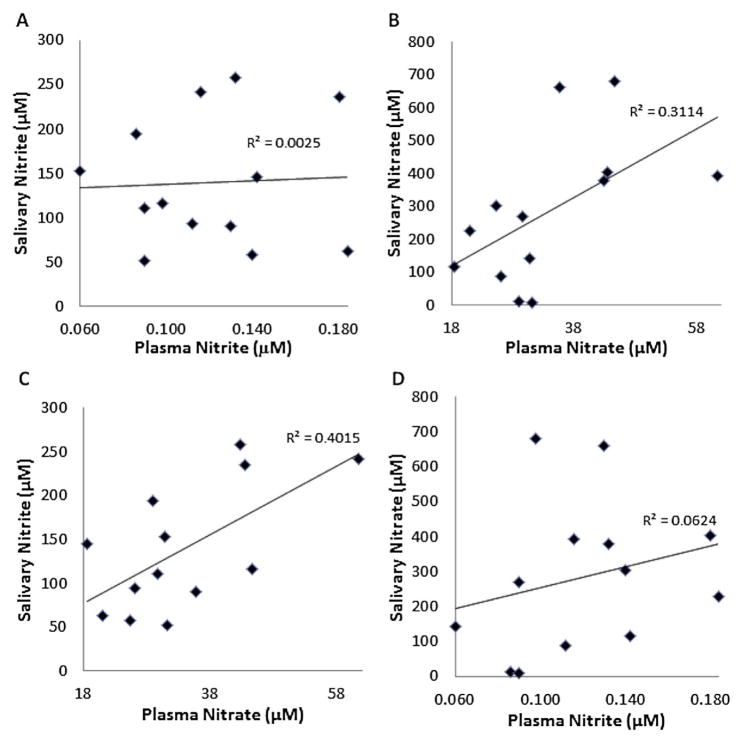
As these test strips appear to measure salivary NO2−, then, assuming plasma NO2− reflects NO bioavailability, the strips would measure NO bioavailability as long as salivary NO2− correlates with plasma NO2−. Thus, we compared basal salivary NO2− to plasma NO2−. We found that salivary NO2− in volunteers ranged from 51 to 257 μM while salivary NO3− ranged from 9 to 681 μM. Plasma NO2− in volunteers ranged from 60 to 184 nM while plasma NO3− ranged from 19 to 61 μM. Importantly, plasma NO2− did not significantly correlate with salivary NO2− (Fig 2A, p = 0.87). Plasma NO3− significantly correlated with salivary NO3− (Fig 2B, p = 0.05). Plasma NO3− significantly correlated with salivary NO2− (Fig 2C, p = 0.02). Basal plasma NO2− did not correlate with basal salivary NO3− (Fig 2D, p = 0.41).
Fig. 2.
Examination of basal salivary vs plasma levels of NO2− and NO3− (A) Basal plasma NO2− and salivary NO2− levels, p=0.87. (B) Plasma NO3− and salivary NO3−, p=0.048. (C) Plasma NO3− and salivary NO2−, p=0.020. (D) Plasma NO2− and salivary NO3− levels, p=0.41.
For the second study we investigated whether ex-vivo conversion of NO3− to NO2− in saliva correlates with in vivo conversion of dietary NO3− to plasma NO2−. Changes in plasma NO2− ranged from −270 to 740 nM. The average increase in plasma NO2− was 150 nM. The rates of salivary NO2− production ranged from 0 to 201 μM/min in aerobic saliva and 0 to 402 μM/min in anaerobic saliva. There was no correlation between the rate of aerobic salivary NO2− production and the change in plasma NO2− (Fig 3A). The trendline R2 value was 0.0240. The calculated r and p-values were 0.02 and 0.23, respectively. In addition, there was no correlation for the rate of anaerobic salivary NO2− production versus the change in plasma NO2− (Fig 3B). The trendline R2 value was 0.0003. The calculated r and p-values were 0.08 and 0.76, respectively. Each plot includes all of the week 1, 3 and 6 time points for each individual. It is worth noting that there was no correlation when comparing only week 1, 3 and 6 time points individually, aerobic or anaerobic salivary reduction of NO3− (data not shown).
Fig. 3.
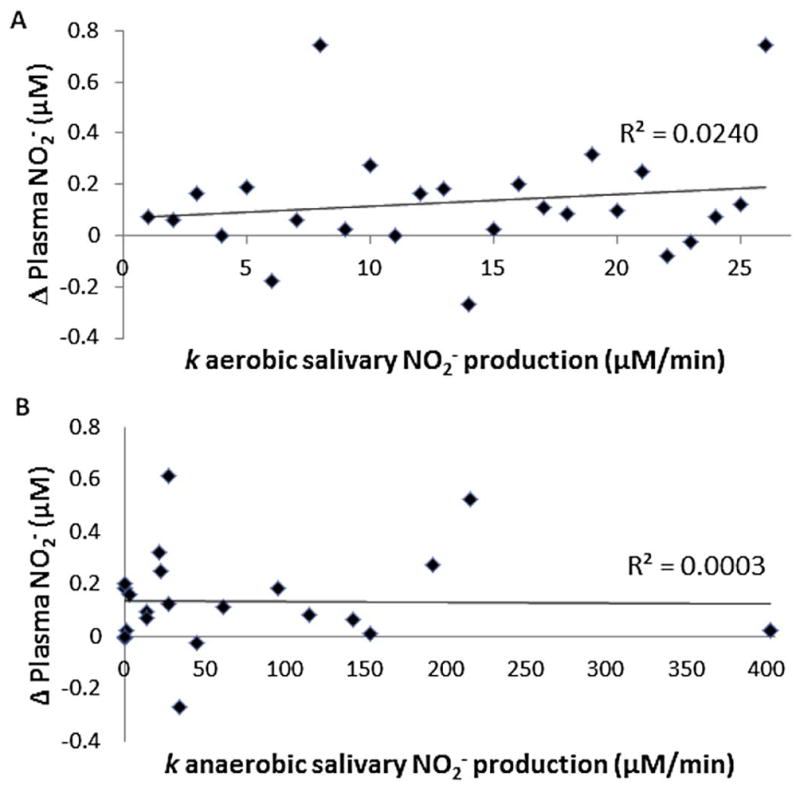
Comparison of ex vivo salivary conversion of NO3− to NO2− to in vivo conversion of oral NO3− to plasma NO2−. Patients (n = 10) were given 2.35 oz of concentrated beet juice (Beet-It) each day for six weeks. Blood was drawn from each patient before and 1 hr after (Δ Plasma NO2−) beet juice consumption at weeks 1, 3, and 6 (all 3 timepoints plotted for each individual). Saliva samples were expectorated early each morning (before breakfast or tooth brushing) at weeks 1, 3, and 6. Δ Plasma NO2− was plotted versus the rate of salivary NO2− production after 10 mM inorganic NO3− was added to (A) aerobic (p-value=0.23, corr=0.02) and (B) anaerobic saliva (p-value=0.76, corr=0.08). Neither condition shows a correlation.
4. Discussion
We have investigated potential methods to assess individuals’ basal NO bioavailability and a potential salivary-based method to assess the individuals’ ability to convert dietary NO3− to plasma NO2−. Our major findings were (i) Nitric Oxide Test Strips indicate a relative level of NO2− in saliva, (ii) basal plasma and salivary NO2− do not correlate, and (iii) ex vivo conversion of salivary NO3− to NO2− is not an indicator of in vivo dietary NO3− conversion to plasma NO2−. We also found that plasma NO3− correlated with both salivary NO3− and NO2−. As expected, there was no correlation between plasma NO2− and salivary NO3−.
As the Kelm lab has demonstrated, plasma NO2− may be indicative of endothelial function [8]. If salivary NO2− reflected plasma NO2−, it could be an easy way to measure endothelial function, as suggested recently [1]. However, we found no relation between basal and salivary NO2− (Figure 2A). These data suggest that salivary NO2− would not be a valid way to measure endothelial function and NO bioavailability. In addition, as we found that commercially available test strips measure salivary NO2− (Figure 1), those strips are not likely to accurately assess NO bioavailability. It should be noted that we used the test strips in a setting where liquid was applied to them. Future work may consider whether other employments, such as putting the strip directly on the tongue, give different results.
Our study for basal levels was designed primarily to determine if physiological levels of salivary NO2− and plasma NO2− correlated. Without recently using mouthwash, consuming a high NO3− food or beverage, or medications, salivary and plasma NO2− in volunteers should reflect normal physiological levels. We observed no correlation between basal salivary and plasma nitrite levels. Interestingly, plasma NO3− correlated with both salivary NO3− and NO2− (Fig 2B & 2C). As noted by the Kelm laboratory, plasma NO3− is influenced by many NOS-independent factors such as dietary NO3− intake, saliva formation, and bacterial synthesis in the bowel [12]. The positive correlations between plasma NO3− and salivary NO2− and NO3− could be understood in terms of the nitrate-nitrite-NO cycle [18]. Salivary NO3− is converted to salivary NO2− by oral bacteria and thus, these levels correlate with each other [18; 19; 20; 22; 23]. NO3− enters the plasma and is taken back up into saliva [18; 22; 23]. Thus salivary and plasma levels of NO3− are linked. However, under basal conditions, plasma NO2− levels are largely due to eNOS function [40] and thus do not correlate with salivary NO3− or NO2−. Indeed, we did not see a correlation between plasma and salivary NO2− or NO3−.
To further rationalize the lack of a correlation between plasma and salivary NO2− levels, it is important to consider nitrogen oxide chemistry occurring during consumption and in the gut. As suggested by work looking at nitrogen oxide production in the oral cavity, it is likely that salivary NO2− is converted to further reduced nitrogen oxides such as NO, nitrous oxide, ammonia, and/or nitrogen gas [41; 42] and also reactive nitrogen oxide species [43] by bacteria which make up the human oral microbiome [44]. Additionally, the acidic milieu of the gastric lumen will likely affect the levels of plasma NO2− as evident in research showing increased NO levels after swallowing NO2− containing saliva [21; 22]. Thus, variations in factors that influence gastric pH and nitrogen oxide absorption as well as those that influence chemistry in the oral cavity could lead to additional variations in plasma NO2− that are not reflected in salivary NO2−.
The beet juice study was designed for exploring a correlation between in-vivo conversion of dietary NO3− to plasma NO2− and the efficacy of ex-vivo salivary NO3− conversion. Several labs have shown that dietary NO3− increases plasma NO2− substantially [23; 24; 25; 26]. However, the efficacy of this conversion varies dramatically among individuals, perhaps due to their microflora [13; 24; 25; 28; 35]. It would be useful to be able to easily assess individuals’ ability to convert dietary NO3− to plasma NO2−. We hypothesized that the rate of ex-vivo salivary NO3− to NO2− conversion would serve this purpose. However, we saw no correlation between the in vivo efficacy of converting dietary NO3− to plasma NO2− and the ex-vivo conversion rate. This lack of correlation may be due to the bacteria in the saliva samples we collected not being representative of the oral bacteria that are mainly responsible for NO3− to NO2− conversion in vivo. These bacteria are thought to form biofilms[19; 20] so that saliva may not necessarily reflect their number, distribution, or reflect optimum conditions for NO3− reduction.
Possible limitations to this study should be acknowledged. For the basal and beet juice studies, the sample sizes were 13 and 10 respectively. A larger sample size might show stronger correlations. For both studies we also used ex vivo salivary samples. As mentioned we used expectorated saliva to assess salivary NO3− to NO2− conversion. Tongue scraping or swabbing or NO3− to NO2− conversion in the oral cavity itself (gargling with NO3− and then expectorating) might show better correlations with in vivo dietary NO3− to plasma NO2− conversion. Plasma samples in the beet juice study were taken before and 1-hr after beet juice consumption. We have previously seen that plasma NO2− levels are close to maximum one hour after consumption [24]. However, others [25; 26] have found that plasma NO2− does not reach a semi-steady maximum value until three hours after consumption so that may have been a better choice for sampling. It’s possible that several time points (such as at 1, 2 and 3 hours after consumption) are needed to obtain the maximum plasma NOx levels after a high NO3− food or beverage for all individuals.
5. Conclusions
Our results argue against use of salivary NO2− as a marker for NO bioavailability. In addition, the efficacy of ex-vivo NO3− to NO2− conversion cannot be used to assess individuals’ ability to convert dietary NO3− to plasma NO2−.
Highlights.
The correlation of basal plasma and salivary NOx are investigated
Ex-vivo salivary NO3− reduction is compared to in-vivo dietary NO3− to plasma NO2−
We report that basal salivary and plasma NO2− levels show no correlation.
Salivary NO3− to NO2− does not correlate with in vivo dietary NO3− to plasma NO2−
Acknowledgments
This work was supported by NIH grant HL058091 and by the Translational Science Center of Wake Forest University.
Abbreviations
- NOS
nitric oxide synthase
- FMD
flow-mediated dilation
- iNOS
inducible nitric oxide synthase
- IMT
intima media thickness
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bryan NS. The potential use of salivary nitrite as a marker of NO status in humans. Nitric Oxide. 2015;45:4–6. doi: 10.1016/j.niox.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Silver JH. Nitrite and Nitric Oxide as Potential Diagnostic Markers in Acute Vascular Diseases. Journal of Neurology and Neurophysiology. 2011;S1 [Google Scholar]
- 3.Bredt DS. Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free radical research. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 4.Clini E, Volterrani M, Pagani M, Bianchi L, Porta R, Gile’ LS, Giordano A, Ambrosino N. Endogenous nitric oxide in patients with chronic heart failure (CHF): relation to functional impairment and nitrate-containing therapies. International Journal of Cardiology. 2000;73:123–130. doi: 10.1016/s0167-5273(00)00211-4. [DOI] [PubMed] [Google Scholar]
- 5.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial Dysfunction, Oxidative Stress, and Risk of Cardiovascular Events in Patients With Coronary Artery Disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 6.Salisbury D, Bronas U. Reactive Oxygen and Nitrogen Species: Impact on Endothelial Dysfunction. Nursing Research. 2015;64:53–66. doi: 10.1097/NNR.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 7.Lu X, Guo X, Wassall CD, Kemple MD, Unthank JL, Kassab GS. Reactive oxygen species cause endothelial dysfunction in chronic flow overload. 2011 doi: 10.1152/japplphysiol.00786.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radical Biology and Medicine. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Mancini GBJ, Dahlöf B, Díez J. Surrogate Markers for Cardiovascular Disease: Structural Markers. Circulation. 2004;109:IV-22–IV-30. doi: 10.1161/01.CIR.0000133443.77237.2f. [DOI] [PubMed] [Google Scholar]
- 10.Nambi V, Pedroza C, Kao LS. Carotid intima-media thickness and cardiovascular events. The Lancet. 2012;379:2028–2030. doi: 10.1016/S0140-6736(12)60652-7. [DOI] [PubMed] [Google Scholar]
- 11.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid Intimal-Medial Thickness Is Related to Cardiovascular Risk Factors Measured From Childhood Through Middle Age: The Muscatine Study. Circulation. 2001;104:2815–2819. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 12.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Gödecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radical Biology and Medicine. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes PM, Leone AM, Francis PL, Struthers AD, Moncada S. The L-Arginine: Nitric Oxide Pathway Is the Major Source of Plasma Nitrite in Fasted Humans. Biochemical and Biophysical Research Communications. 1995;209:590–596. doi: 10.1006/bbrc.1995.1541. [DOI] [PubMed] [Google Scholar]
- 14.Tannenbaum SR, Witter JP, Gatley SJ, Balish E. Nitrate and Nitrite: Origin in Humans. Science. 1979;205:1332–1337. doi: 10.1126/science.205.4413.1335. [DOI] [PubMed] [Google Scholar]
- 15.Kelm M. Nitric oxide metabolism and breakdown. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1999;1411:273–289. doi: 10.1016/s0005-2728(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 16.Hibbs JB, Westenfelder C, Taintor R, Vavrin Z, Kablitz C, Baranowski RL, Ward JH, Menlove RL, McMurry MP, Kushner JP. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. Journal of Clinical Investigation. 1992;89:867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsikas D. Circulating and excretory nitrite and nitrate: Their value as measures of nitric oxide synthesis, bioavailability and activity is inherently limited. Nitric Oxide. 2015;45:1–3. doi: 10.1016/j.niox.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 19.Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, Torregrossa AC, Tribble G, Kaplan HB, Petrosino JF, Bryan NS. Metagenomic Analysis of Nitrate-Reducing Bacteria in the Oral Cavity: Implications for Nitric Oxide Homeostasis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. European Journal of Oral Sciences. 2005;113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 21.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-shapiro DB, Kozlov AV, Lancaster JR, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-addo GB, Schechter A, Shiva S, Tsuchiya K, Van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nature Chemical Biology. 2009;5:865–9. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radical Biology and Medicine. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Presley TD, Morgan AR, Bechtold E, Clodfelter W, Dove RW, Jennings JM, Kraft RA, Bruce King S, Laurienti PJ, Jack Rejeski W, Burdette JH, Kim-Shapiro DB, Miller GD. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24:34–42. doi: 10.1016/j.niox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb AJ, NP, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs A, Ahluwalia A. Acute Blood Pressure Lowering, Vasoprotective, and Antiplatelet Properties of Dietary Nitrate via Bioconversion to Nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapil V, Khambata R, Robertson A, Caulfield M, Ahluwalia A. Dietary Nitrate Provides Sustained Blood Pressure Lowering in Hypertensive Patients: A Randomized, Phase 2, Double-Blind, Placebo-Controlled Study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones A. Dietary Nitrate Supplementation and Exercise Performance. Sports Medicine. 2014;44:35–45. doi: 10.1007/s40279-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly J, Vanhatalo A, Bailey SJ, Wylie LJ, Tucker C, List S, Winyard PG, Jones AM. Dietary nitrate supplementation: effects on plasma nitrite and pulmonary O2 uptake dynamics during exercise in hypoxia and normoxia, American Journal of Physiology - Regulatory. Integrative and Comparative Physiology. 2014;307:R920–R930. doi: 10.1152/ajpregu.00068.2014. [DOI] [PubMed] [Google Scholar]
- 29.Larsen FJM, Ekblom BMDP, Sahlin KP, Lundberg JOMDP, Weitzberg EMDP. Effects of Dietary Nitrate on Blood Pressure in Healthy Volunteers. The New England Journal of Medicine. 2006;355:2792–3. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 30.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiologica. 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 31.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1281–8. doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- 33.Heiss C, Meyer C, Totzeck M, Hendgen-Cotta UB, Heinen Y, Luedike P, Keymel S, Ayoub N, Lundberg JO, Weitzberg E, Kelm M, Rassaf T. Dietary inorganic nitrate mobilizes circulating angiogenic cells. Free Radical Biology and Medicine. 2012;52:1767–1772. doi: 10.1016/j.freeradbiomed.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Mateos A, Hezel M, Aydin H, Kelm M, Lundberg JO, Weitzberg E, Spencer JPE, Heiss C. Interactions between cocoa flavanols and inorganic nitrate: Additive effects on endothelial function at achievable dietary amounts. Free Radical Biology and Medicine. 2015;80:121–128. doi: 10.1016/j.freeradbiomed.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Govoni M, Jansson EÅ, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. Journal of Applied Physiology. 2013;115:325–336. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 37.Wilkerson D, Hayward G, Bailey S, Vanhatalo A, Blackwell J, Jones A. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. European Journal of Applied Physiology. 2012;112:4127–4134. doi: 10.1007/s00421-012-2397-6. [DOI] [PubMed] [Google Scholar]
- 38.Salimetrics. Saliva Collection and Handling Advice. Salimetrics, LLC 3rd; 2013. [Google Scholar]
- 39.Veerman ECI, vandenKeybus PAM, Vissink A, Amerongen AVN. Human glandular salivas: Their separate collection and analysis. European Journal of Oral Sciences. 1996;104:346–352. doi: 10.1111/j.1600-0722.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 40.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–9. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zetterquist W, Pedroletti C, Lundberg JO, Alving K. Salivary contribution to exhaled nitric oxide. European Respiratory Journal. 1999;13:327–333. doi: 10.1034/j.1399-3003.1999.13b18.x. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber F, Stief P, Gieseke A, Heisterkamp IM, Verstraete W, de Beer D, Stoodley P. Denitrification in human dental plaque. BMC BIOLOGY. 2010;8:24–24. doi: 10.1186/1741-7007-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahama U, Hirota S, Oniki T. Production of nitric oxide-derived reactive nitrogen species in human oral cavity and their scavenging by salivary redox components. Free radical research. 2005;39:737–745. doi: 10.1080/10715760500043561. [DOI] [PubMed] [Google Scholar]
- 44.Dewhirst FE, Chen T, Izard J, Paster BJ, Anne CRT, Yu WH, Lakshmanan A, Wade WG. The Human Oral Microbiome. Journal of Bacteriology. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]