Abstract
Background
Health care utilization peaks at the end-of-life (EOL) in patients with heart failure (HF). However, it is unclear what factors impact EOL utilization in patients with HF and if utilization has changed over time.
Methods and Results
Southeastern Minnesota residents with HF were prospectively enrolled into a longitudinal cohort study from 2003-2011. Patients who died before December 31, 2012 were included in the analysis. Information on hospitalizations and outpatient visits in the last year of life was obtained using administrative sources. Negative binomial regression was used to assess the association between patient characteristics and utilization. The 698 decedents (47.3% men, 53.4% preserved ejection fraction [EF]) experienced 1528 hospitalizations (median 2 per person, range 0-12, 37.6% due to cardiovascular causes) and 12,927 outpatient visits (median 14 per person, range 0-119) in their last year of life. Most patients (81.5%) were hospitalized at least once and 28.4% died in the hospital. Patients who were older and those with dementia had lower utilization. Patients who were married, resided in a skilled nursing facility, and had more comorbidities had higher utilization. Patients with preserved EF had higher rates of non-cardiovascular hospitalizations though other utilization was similar. Over time, rates of hospitalizations and outpatient visits decreased, while palliative care consults and enrollment in hospice increased.
Conclusions
While patient factors remain associated with differential health care utilization at the EOL, utilization declined over time and use of palliative care services increased. These results are encouraging given the high resource use in patients with HF.
Keywords: heart failure, skilled nursing facility, health care utilization, death, hospitalization, community
Each year in the United States, 275,000 patients die with heart failure (HF)1. In 2009, one in nine of all deaths listed HF as a contributing cause1. By 2030, the number of patients with HF is projected to increase by 25%, and the total annual costs of care to rise by 120% to $70 billion2. While the care of patients with HF is costly and burdensome throughout the disease trajectory3, utilization including hospitalizations peaks at the end-of-life (EOL)4, 5. The tremendous burden that EOL care in HF places on patients, caregivers, and the healthcare system has been well-recognized6, and there has been an impetus to improve EOL care through many mechanisms, including through the involvement of palliative care specialists in the management of patients with HF7.
However, in order to develop efficient, patient-centric care pathways, we must first gain a better understanding of the current distribution and predictors of hospitalizations and other health care utilization at the EOL, and identify whether utilization has changed over time. Recent work has suggested that hospitalization rates for HF have declined8, 9, but these data fail to capture what happens to patients living with HF as they develop advanced disease and approach EOL. In elderly Medicare beneficiaries from 2000-2007, 80% were hospitalized in the last six months of life, and costs to Medicare were higher in patients who were younger, had chronic obstructive pulmonary disease (COPD) and renal disease10. However, it is unknown how utilization may differ in patients with preserved compared with reduced ejection fraction (EF) and how it may be impacted by factors such as marital status and palliative care consultation services which cannot be captured using claims data. Furthermore, contemporary data are needed to delineate whether utilization has changed in recent years.
In order to fill these knowledge gaps, the primary aim of the study was to systematically examine the factors that predict hospitalizations and outpatient visits in the last year of life in community decedents with HF.
Methods
Study Design
This study was a cohort study conducted in southeastern Minnesota. This area is relatively isolated from other urban centers, and few providers, the largest of which is Mayo Clinic, deliver the vast majority of medical care to local residents. The Rochester Epidemiology Project11, a medical record linkage system, allows the indexing of medical care for residents, thus enabling the comprehensive capture of health-related events for the community's residents. The population in this area is representative of the state of Minnesota and the upper Midwest region of the U.S.12 Additionally, age- and sex-specific mortality rates were similar for Olmsted County (the largest county in southeastern Minnesota), the state of Minnesota and the entire U.S. Finally, broad disease trends are commensurate to national trends.13
Patient Population
Natural language processing of the electronic medical record text was used to identify potential patients with HF14. Following a clinical visit, documentation is transcribed and appears in the record within 24 hours, making prompt identification of patients with HF possible. The search was restricted to patients who were residents of Olmsted, Dodge, and Fillmore Counties in Minnesota and at least 20 years old. Experienced research nurses then reviewed the medical record to determine whether patients had active HF meeting Framingham criteria15. Patients were approached to participate in the study from September 2, 2003 through December 31, 2011, which included an echocardiogram and blood draw. Hospitalized patients were contacted in the hospital, and those in the outpatient setting were approached at their next clinic visit. All patients provided written authorization to participate, which was approved by the Mayo and Olmsted Medical Center Institutional Review Boards. Because we were interested in health care utilization in the last year of life, the analysis was restricted to participants who survived to hospital discharge (if hospitalized) at enrollment and subsequently died on or before December 31, 2012.
Data Collection
Potential Predictors of Health Care Utilization
Baseline characteristics at the time of study enrollment were collected from the medical record. Resting left ventricular EF was collected from transthoracic echocardiograms performed within 6 months prior to 2 months after study enrollment. Preserved EF was defined by an EF≥50%. The Charlson comorbidity index was used to assess comorbidity16. It is calculated based on the presence or absence of each of the following: myocardial infarction (MI), HF, peripheral vascular disease, cerebrovascular disease, dementia, COPD, connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, hemiplegia, moderate or severe renal disease, tumor (without metastasis vs. metastatic), leukemia, lymphoma, and acquired immunodeficiency syndrome. All patients had HF. Diabetes was defined by American Diabetes Association Criteria17 or use of diabetic medications. History of MI was defined using standard epidemiologic criteria18. Estimated glomerular filtration rate (eGFR) was calculated based on creatinine measured closest to the time of study enrollment using the Modification of Diet in Renal Disease equation19, and moderate/ severe renal disease was defined as an eGFR<60 mL/min per 1.73 m2. The remaining comorbidities were defined using physician diagnosis and billing codes. Body mass index was calculated using the last outpatient weight and the earliest adult height, and overweight/ obese was defined as body mass index≥25kg/m2. We determined whether a patient was cared for in a skilled nursing facility (SNF) in their last year of life using administrative billing codes and confirmed using manual chart review. The duration of stay in a SNF was not available. Patients were classified as “married” if they were married or living with a partner. Age at death was divided into quartiles (<75 years, 75-83 years, 84-90 years, ≥90 years). Year of death was categorized as 2003-06, 2007-09, and 2010-12. Hospice enrollment and palliative care consultations were identified using manual chart review.
Health Care Utilization
We examined health care utilization at the EOL, which was defined as hospitalizations and outpatient visits in the year prior to death or from the time from HF diagnosis until death if less than one year. The date of death was determined using death certificates filed in Olmsted County, obituary notices, and electronic files of death certificates obtained from the State of Minnesota Department of Vital and Health Statistics. Cause of death was obtained from death certificates; cardiovascular cause of death was defined by International Classification of Diseases 10th revision codes I01-I99. Information on hospitalizations and outpatient visits in Olmsted County was obtained from the Olmsted County Healthcare Expenditure and Utilization Database4, 20. Transfers between hospitals were considered a single hospitalization. The primary reason for admission was categorized as cardiovascular (ICD-9 codes 390-459) or non-cardiovascular (all other codes) based on principal ICD-9 code. Outpatient visits were categorized by provider type (cardiologist vs. non-cardiologist). As we were unable to identify provider type for some visits in the county after July 1, 2010, this analysis was restricted to patients that died prior to that date. Tests, imaging and outpatient procedures were not counted as outpatient visits and were not quantified.
Statistical Analysis
Baseline patient characteristics by SNF status were compared using t-tests for normally distributed continuous variables, Wilcoxon rank sum tests for non-normally distributed continuous variables, and chi square tests for categorical variables. Negative binomial regression was used to examine the relationship between patient baseline characteristics and the rate of hospitalizations and outpatient visits per person year of follow-up. Variables with no significant association with health care utilization in unadjusted analyses (malignancy, cerebrovascular disease, GFR) were not included in the multivariable models. All models were adjusted for incident vs. prevalent status at study enrollment. Patients were divided into quartiles by Charlson comorbidity index in the analysis. Predictors of cardiologist visits in the outpatient setting in the last year of life were analyzed using negative binomial regression. Given that the number of cardiology visits was fewer than other outcomes we examined, only variables that demonstrated a significant relationship with cardiologist visits on bivariate analysis were included in the multivariable model. A two-sided p value <0.05 was used as the level of significance for all analyses. Analyses were performed using Stata/SE Version 13.0 (College Station, Texas) and SAS version 9.3.
Results
In total, 1369 patients were enrolled in the study (Figure 1). Some patients were excluded from analysis as they lacked research authorization to examine utilization data or they died in the hospital at enrollment. Of the remaining 1243, 698 patients died prior to December 31, 2012, and are included in the analysis. There were only two patients who had an LVAD implanted before death, and they were retained in the analysis. The median (25th-75th percentile) follow-up time from HF diagnosis until death was 365 (237-365) days. Most decedents (n=486, 69.6%) had a full year of follow-up from HF diagnosis until death. The characteristics of the study population are shown in Table 1. Patients were elderly (mean age 82 years at death, range 36-105 years, 8% [n=56] <65 years old at death), 47.3% were men, and 53.4% had preserved EF. Approximately half (n=357, 52.7%) of patients died of a cardiovascular cause, which was more common in patients with reduced EF (57.4% vs. 48.9% in preserved EF, p=0.029). In the final year of life, 350 (50.7%) patients were cared for in a SNF. Patients residing in a SNF were older (85 vs. 79 years, p<0.001), more often female (63% vs. 43%, p<0.001), and more likely to have preserved EF (62% vs. 44%, p<0.001).
Figure 1.
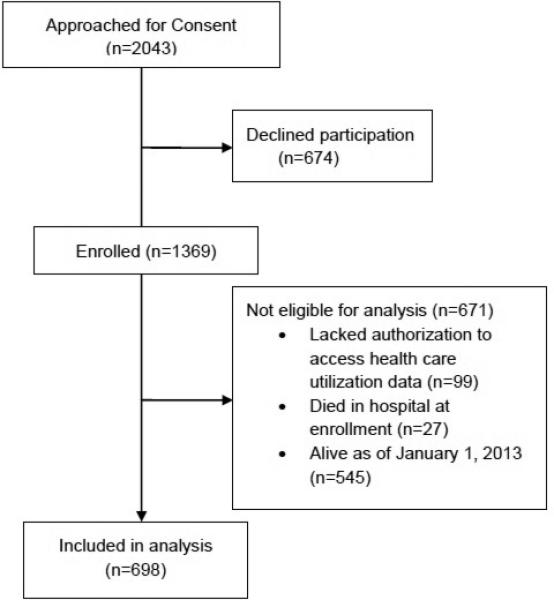
Study Population
Table 1.
Patient Characteristics
| Patient Characteristic | Missing | Overall (n=698) |
|---|---|---|
| Age at death, mean (SD), years | 0 | 82.2 (10.8) |
| Male | 0 | 330 (47.3) |
| Ejection fraction, mean(SD), % | 18 | 48.3 (16.2) |
| Preserved ejection fraction (≥50%) | 18 | 363 (53.4) |
| Comorbidities | ||
| Hypertension | 0 | 622 (89.1) |
| Diabetes mellitus | 0 | 262 (37.5) |
| COPD | 0 | 256 (36.7) |
| Prior myocardial infarction | 1 | 214 (30.4) |
| Malignancy | 0 | 257 (36.8) |
| Peripheral vascular disease | 0 | 240 (34.4) |
| Cerebrovascular disease | 0 | 249 (35.7) |
| Dementia | 0 | 50 (7.2) |
| eGFR<60 mL/min per 1.73 m2 | 0 | 526 (75.4) |
| Charlson comorbidity index | 1 | |
| 1-2 | 149 (21.4) | |
| 3-4 | 209 (30.0) | |
| 5-6 | 181 (26.0) | |
| >6 | 158 (22.7) | |
| Overweight/ obese (BMI≥25 kg/m2) | 0 | 417 (59.7) |
| Year of death | 0 | |
| 2003-2006 | 189 (27.1) | |
| 2007-2009 | 268 (38.4) | |
| 2010-2012 | 241 (34.5) | |
| Married/ Living with a Partner | 12 | 311 (45.3) |
| Resided in skilled nursing facility last year of | 7 | 350 (50.7) |
All numbers reported are N(%) unless otherwise noted. BMI= body mass index, COPD= chronic obstructive pulmonary disease, eGFR= estimated glomerular filtration rate
Health Care Utilization
At the EOL, 1528 hospitalizations (median 2 per person, range 0-12) and 12,927 outpatient visits (median 14 per person, range 0-119) occurred. Hospitalizations and days hospitalized increased in the months prior to death (Figure 2). In total, 569 (81.5%) patients were hospitalized at least once in their final year of life, and 198 (28.4%) died in the hospital. The median total days hospitalized in the last year was 9 (25th-75th percentile 3-21 range 0-160) per person. Of the hospitalizations, 575 (37.6%) were for cardiovascular causes, while the remainder were for non-cardiovascular causes. The most common reasons for hospitalization were HF (n=322, 21.1% of hospitalizations), pneumonia (n=80, 5.2% of hospitalizations), sepsis (n=66, 4.3% of hospitalizations), and arrhythmia (n=58, 3.8% of hospitalizations).
Figure 2. Number of Hospitalizations and Days Hospitalized in the Last Year of Life.
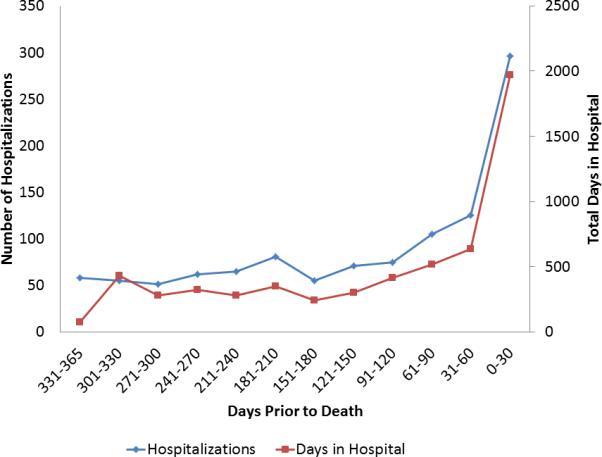
The number of hospitalizations and days hospitalized by days prior to death are shown for the 486 decedents with at least a full year of follow-up from heart failure diagnosis until death.
Predictors of Health Care Utilization
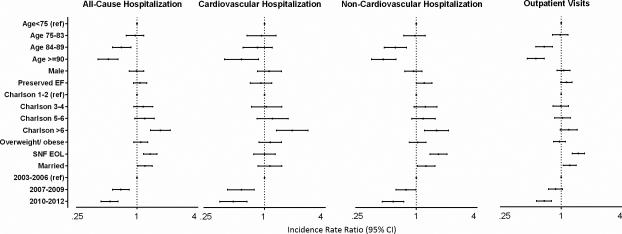
Several factors were associated with differential EOL utilization; the incidence rate ratios (IRR) predicting hospitalizations and outpatient visits are shown in Table 2 (unadjusted results) and Figure 3 (multivariable results). Lower utilization was seen in patients who were older. Patients who had a greater comorbidity burden, lived in a SNF in the final year of life, and were married had higher utilization.
Table 2.
Unadjusted Predictors of Hospitalizations and Outpatient Visits
| All-cause hospitalizations | Cardiovascular Hospitalizations | Non-cardiovascular hospitalizations | Outpatient Visits | |
|---|---|---|---|---|
| Male | 1.08 (0.92-1.27) | 1.29 (1.02-1.64)* | 0.97 (0.80-1.18) | 1.09 (0.95-1.26) |
| Age at Death (years) | ||||
| <75 | 1 | 1 | 1 | 1 |
| 75-83 | 1.05 (0.85-1.31) | 0.98 (0.71-1.37) | 1.10 (0.85-1.42) | 1.04 (0.86-1.25) |
| 84-89 | 0.73 (0.59-0.91) | 0.83 (0.59-1.16) | 0.68 (0.52-0.89) | 0.75 (0.62-0.91) |
| 90 and older | 0.53 (0.42-0.67)‡ | 0.53 (0.37-0.76)‡ | 0.53 (0.40-0.71)‡ | 0.63 (0.53-0.78)‡ |
| Married | 1.29 (1.10-1.52)† | 1.30 (1.03-1.65)* | 1.29 (1.06-1.56)* | 1.27 (1.11-1.45)† |
| Preserved ejection fraction | 1.08 (0.92-1.27) | 0.84 (0.66-1.07) | 1.25 (1.03-1.52)* | 1.09 (0.95-1.24) |
| Lived in a Skilled Nursing Facility | 1.22 (1.04-1.43)* | 0.88 (0.70-1.12) | 1.49 (1.24-1.81)‡ | 1.25 (1.09-1.42)† |
| Charlson comorbidity quartiles | ||||
| 1-2 | 1 | 1 | 1 | 1 |
| 3-4 | 1.24 (0.99-1.56) | 1.08 (0.77-1.52) | 1.35 (1.02-1.78) | 1.04 (0.86-1.26) |
| 5-6 | 1.36 (1.08-1.72) | 1.34 (0.95-1.91) | 1.36 (1.02-1.81) | 1.15 (0.95-1.39) |
| >6 | 1.98 (1.56-2.50)‡ | 2.07 (1.45-2.95)‡ | 1.89 (1.42-2.52)‡ | 1.33 (1.09-1.63)† |
| Individual Comorbidities | ||||
| Diabetes mellitus | 1.22 (1.04-1.43)* | 1.21 (0.95-1.54) | 1.23 (1.01-1.49)* | 1.15 (1.00-1.32) |
| COPD | 1.33 (1.13-1.57)‡ | 1.27 (0.99-1.63) | 1.34 (1.10-1.64)† | 1.11 (0.97-1.27) |
| Peripheral vascular disease | 1.38 (1.17-1.63)‡ | 1.80 (1.41-2.30)‡ | 1.15 (0.94-1.41) | 1.09 (0.95-1.25) |
| Cerebrovascular disease | 1.03 (0.87-1.21) | 1.12 (0.87-1.43) | 0.98 (0.80-1.19) | 1.05 (0.92-1.21) |
| eGFR<60 mL/min per 1.73m2 | 1.00 (0.83-1.20) | 0.96 (0.73-1.26) | 1.02 (0.82-1.28) | 1.07 (0.92-1.25) |
| Malignancy | 1.09 (0.93-1.29) | 0.98 (0.77-1.26) | 1.16 (0.95-1.42) | 1.07 (0.93-1.22) |
| Dementia | 0.55 (0.38-0.80)† | 0.42 (0.22-0.78)† | 0.63 (0.41-0.98)* | 0.77 (0.59-1.01) |
| Prior myocardial infarction | 1.12 (0.95-1.34) | 1.32 (1.02-1.70)* | 1.00 (0.81-1.24) | 1.03 (0.89-1.19) |
| Overweight or obese | 1.15 (0.98-1.36) | 1.21 (0.95-1.55) | 1.12 (0.92-1.37) | 1.01 (0.88-1.15) |
| Year of death | ||||
| 2003-2006 | 1 | 1 | 1 | 1 |
| 2007-2009 | 0.71 (0.58-0.87) | 0.62 (0.46-0.84) | 0.78 (0.61-1.00) | 0.91 (0.77-1.07) |
| 2010-2012 | 0.52 (0.42-0.64)‡ | 0.51 (0.37-0.69)‡ | 0.54 (0.42-0.70)‡ | 0.67 (0.56-0.79)‡ |
p<0.05
p<0.01
p<0.001.
For Charlson comorbidity index quartiles, age at death, and year of death, p values are for trend across categories
COPD= chronic obstructive pulmonary disease, eGFR= estimated glomerular filtration rate
Figure 3. Predictors of Health Care Utilization in the Last Year of Life.
Multivariable models predicting total hospitalizations, cardiovascular and non-cardiovascular hospitalizations and outpatient visits are shown. All models are adjusted for incident vs. prevalent status at study enrollment.
Hospitalizations
The independent predictors of increased rate of hospitalization included younger age (IRR 0.52, 95% CI 0.41-0.65 for those age ≥90 years compared with <75 at death), residing in a SNF (IRR 1.35, 95% CI 1.16-1.58), being married (IRR 1.20, 95% CI 1.02-1.42), and more comorbidities (Charlson comorbidity index >6 vs 0-2: IRR 1.71, 95% CI 1.36-2.14, Figure 2). When individual comorbidities were included in the models rather than the Charlson comorbidity index, COPD (IRR 1.20, 95% CI 1.03-1.41) and peripheral vascular disease (IRR 1.29, 95% CI 1.10-1.51) were associated with increased hospitalizations, and dementia (IRR 0.64, 95% CI 0.45-0.91) with decreased hospitalizations (data not shown). While younger age and more comorbidities predicted increased rates of cardiovascular and non-cardiovascular hospitalizations (Figure 3), being cared for in a SNF at the EOL (IRR 1.66, 95% CI 1.37-2.01), being married (IRR 1.25, 95% CI 1.03-1.53), and having preserved EF (IRR 1.20, 95% CI 1.00-1.43) were only associated with increased non-cardiovascular hospitalizations. When examining individual comorbidities, peripheral vascular disease was associated with increased cardiovascular hospitalizations (IRR 1.66, 95% CI 1.29-2.14), and dementia with decreased cardiovascular hospitalizations (IRR 0.45, 95% CI 0.24-0.84). In contrast, only COPD was associated with increased non-cardiovascular hospitalizations (IRR 1.23, 95% CI 1.02-1.48). Sex, diabetes, renal dysfunction, malignancy, cerebrovascular disease, body mass index, and prior MI were not significant predictors of hospitalization rate.
While patients who were cared for at a SNF at the EOL had higher rates of hospitalizations, this excess risk was magnified in those who were younger (P value for interaction of age*SNF<0.001 for all-cause, cardiovascular and non-cardiovascular hospitalizations). For example, the IRR for hospitalization among those <75 years of age who were in a SNF compared to those not in a SNF at the EOL was 1.66 (95% CI 1.18-2.34) vs. 1.26 (95% CI 0.86-1.83) in the oldest SNF residents (aged 90 and older).
Outpatient Visits
Living in a SNF (IRR 1.48, 95% CI 1.29-1.70) and being married (IRR 1.22, 95% CI 1.05-1.41) were associated with increased outpatient visits, while older individuals had fewer outpatient visits (Figure 3). Neither number of comorbidities (Charlson comorbidity index, p for trend, 0.061) nor individual comorbidities were associated with differences in rates of outpatient visits. There was a trend toward more outpatient visits in patients with preserved EF (IRR 1.13, 95% CI 0.99-1.28).
We also evaluated the predictors of the combined endpoint of hospitalizations and outpatient visits at the EOL. The findings were similar to the predictors of hospitalizations, with higher rates of care utilization seen in patients who were younger at death, married, cared for in a SNF, had more comorbidities, and died in an earlier year (data not shown). There was a trend toward higher rates of hospitalization and outpatient visits in patients with preserved EF (IRR 1.13, 95% CI 0.99-1.28, p=0.065).
In total, 40.5% of patients (201 of 496 who died prior to July 1, 2010) had an outpatient visit with a cardiologist in the last year of life. Cardiologist visits comprised 9.9% of all outpatient visits. There was no change in the proportion of visits that were to cardiologists (rather than other types of providers) in the months preceding death. Cardiology visits occurred at a higher rate in patients who were younger, male, had reduced EF, were married, did not have dementia, and were not cared for in a SNF in the last year of life (p<0.05 for each on bivariate analysis). Adjusting for other factors, cardiologist visits in the last year of life were more common in patients who were married and lower in those who were older, had dementia, and were cared for in a SNF (Table 3).
Table 3.
Predictors of Cardiology Visits in the Last Year of Life
| *Patient Characteristic | Incidence Rate Ratio (95% CI ) | P value |
|---|---|---|
| Age at death (per 1 year increase) | 0.96 (0.94-0.98) | <0.001 |
| Male | 1.21 (0.81-1.82) | 0.35 |
| Preserved ejection fraction (≥50%) | 0.83 (0.57-1.22) | 0.34 |
| Dementia | 0.22 (0.09-0.54) | 0.001 |
| Married/ Living with a Partner | 2.43 (1.64-3.61) | <0.001 |
| Resided in skilled nursing facility Last Year of | 0.39 (0.26-0.57) | <0.001 |
Variables that were significantly (p<0.05) with cardiology visits in univariate analysis were included in the multivariable model.
Time Trends in Health Care Utilization
All utilization decreased over time. The proportion of patients who died in the hospital decreased over time from 32.8% in 2003-06 to 22.4% in 2010-2012 (p trend=0.019). The rate of hospitalizations was 46% less for patients who died from 2010-2012 and 31% less for those who died from 2007-2009 compared with 2003-2006 (IRR 0.54 and 0.69, respectively, p for trend <0.001, Figure 3). Similarly, the rate of outpatient visits was 32% less for those who died in 2010-2012 compared with 2003-2006. Trends were similar in patients with preserved and reduced EF.
Time Trends in Palliative and Hospice Care
In total, 242 (35.4%) patients enrolled in hospice a median (25th-75th percentile) of 21 (4-64) days prior to death. Use of hospice services increased over time from 28.7% in 2003-2006 to 42.2% in 2010-2012 (p <0.001, Figure 4). Palliative care consultations were obtained in 182 (26.5%) patients at a median (25th-75th percentile) of 27 (5-149) days prior to death, and also increased over time. There was no change in the proportion of patients residing in a SNF at the EOL (51.3% in 2003-06, 50.2% in 2007-2009, 50.6% in 2010-2012).
Figure 4.
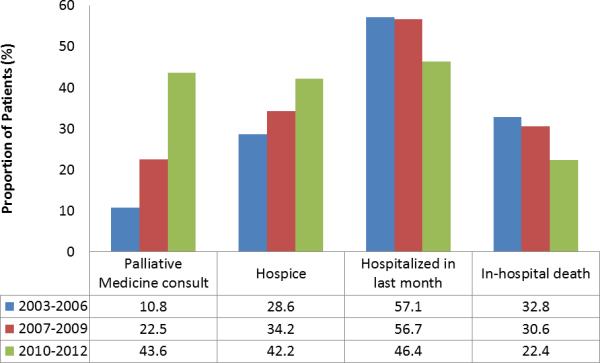
Secular Trends in Hospice Enrollment, Palliative Care Consultations, and Hospitalizations at the End-of-Life
Discussion
There were several important findings from this analysis of a community cohort of decedents with HF. First, patients who were older and had dementia had lower utilization at the EOL, while patients who were married, had more comorbidities, and were cared for at a SNF had higher utilization. Second we saw a dramatic decline in health care utilization at the EOL over time, concordant with increases in palliative care consultations and hospice enrollment. Third, health care utilization at EOL and time trends in utilization were similar in patients with preserved and reduced EF.
Impact of Patient Characteristics on Utilization
Most patients were hospitalized at least once in the last year of life, many were hospitalized multiple times and one-fourth spent more than three weeks hospitalized. While we expected to see lower utilization in those who were older, consistent with findings in Medicare beneficiaries10, we also saw lower utilization in those with dementia. In contrast, previous studies have suggested that elderly Medicare beneficiaries with newly diagnosed dementia are at higher risk for hospitalization than persons without dementia21. The lower utilization in patients with dementia at EOL seen herein may reflect a conscious choice to adopt a palliative approach to care given recognition of adverse prognosis associated with HF, and is worthy of additional analysis in future studies.
Conversely, utilization was higher in patients with more comorbidities. The comorbidity burden of patients with cardiovascular diseases such as HF has risen over time22, and portends adverse prognosis23. Herein, we found that utilization at the EOL rose with increasing comorbidity burden. Therefore, patients with HF and multimorbidity are high utilizers of services at the EOL, and care pathways that are not HF-specific, but rather encompass the growing population of patients with multimorbidity, need to be developed. While hospitalizations increased with greater comorbidity burden, we saw no difference in the rate of outpatient visits. Further data are needed to understand this pattern and to explore whether increased outpatient care could decrease use of emergency services.
We were surprised to find higher utilization in those who were married. Marriage has historically been considered protective against adverse outcomes in patients with cardiovascular disease24, 25, readmissions26 and hospitalizations for some conditions such as pneumonia27. However, we found that married individuals had higher rates of hospitalization and outpatient visits at the EOL. These findings require further exploration in future studies.
We also saw higher utilization in patients who were cared for at a SNF. One potential explanation is that SNF care may be more frequently utilized in patients with more complex disease or less psychosocial support, thereby predisposing them to readmissions and higher health care use. Similarly, Allen et al found a higher risk of 30-day readmission in patients with HF who were discharged to a SNF compared with those who were not28.
We found very few differences in EOL utilization in patients with preserved compared with reduced EF. While patients with preserved EF had a slightly higher risk of non-cardiovascular hospitalizations, there was no difference in overall risk of hospitalization, and the risk of outpatient visits was similar. This finding is in alignment with prior work demonstrating no difference in hospitalization risk4, 29 or 5-year costs30 in patients by EF.
Patient characteristics also had an impact on the type of provider seen at the EOL. Patients that were older, not married, had dementia, and were cared for in a SNF were less likely to see a cardiologist in the outpatient setting in the last year of life. This finding is in alignment with other data suggesting that older and more infirm patients are less likely to receive subspecialist care.31, 32
Changes in Utilization Over Time
From 2003 to 2012, we saw a marked decline in hospitalizations and outpatient visits at the EOL. Patients were less likely to die in the hospital and more often enrolled in hospice in recent years, which parallels national trends reported by the Centers for Disease Control33 and in Medicare beneficiaries10, 34. We also saw a dramatic increase in palliative care consultations. While no specific incentives or systems were in place to prompt these consultations, this may reflect increased recognition by clinicians of the importance of palliative care in patients with HF approaching the EOL. While we were not poised to directly examine whether increased use of palliative care consultations and hospice care led to decreased utilization, both have been shown to decrease hospitalizations and the odds of inhospital death35-36. While our data do not inform us whether these shifting patterns of care are in alignment with patient preferences and resulted in improved quality of life, it has been established that most Americans prefer to die at home37, 38 and hospice improves caregiver satisfaction with care39, 40. While we found that palliative care consultations increased markedly during the study period, other health care systems without access to this resource may not see the same temporal trends.
Limitations and Strengths
Several factors should be considered when interpreting these data. We do not have information on the duration patients resided in a SNF at the EOL, which would be of interest to examine in future studies. While commonly used in health services research, administrative data has limitations. For example, reliance upon billing codes to identify the primary reason for hospitalization may result in misclassification. Health care utilization outside of the county was not captured. However, comparison with Medicare data would suggest that only 5% of all hospitalizations for elderly residents of Olmsted County occur outside of the county. Participants in the study were, on average, younger than non-participants, which should be considered when interpreting study results. As we thoroughly investigated the association between multiple characteristics and outcomes, the possibility that multiple testing could contribute to finding an association that doesn't exist cannot be excluded. However, this study has several notable strengths. We captured hospitalizations and outpatient visits at the EOL in a well-characterized group of decedents with HF. We were able to capture data such as marital status and EF that are generally not available in large insurance claims databases. This cohort includes patients with a wide range of age, EF, and comorbidity burden, thus providing a comprehensive depiction of the range of those dying with HF in the community.
Clinical Implications
The population of patients with HF continues to evolve, and a growing number of patients have multimorbidity. Multiple comorbid conditions in addition to HF can make it challenging to provide comprehensive patient care throughout the HF trajectory, but also at the EOL. These data underscore the high utilization in this population, and highlight the need for EOL care pathways that encompass patients with a high comorbidity burden. Care directed only at the patient's HF may not decrease utilization. The concurrent trends of increased utilization of palliative and hospice services and decreased overall health care utilization at the EOL are notable. It will be critical to continue to identify appropriate triggers and barriers to palliative care involvement in the care of patients with advanced HF and to equip clinicians with the knowledge and tools that they need to practice primary palliative care themselves41.
Conclusions
Care at the EOL has changed over time in community patients with HF. We saw increased consultations with palliative care specialists, increased enrollment in hospice and decreased utilization at the EOL. While these are important changes that deserve acknowledgment, health care utilization at the EOL remains high in patients in certain patient subgroups, including those who have multimorbidity and those cared for in SNF, and further work is needed to define and achieve optimal EOL care in these populations.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by grants from the National Institutes of Health (R01 HL72435 [VL Roger] and K23 HL116643 [SM Dunlay]).
Footnotes
Disclosures
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: Heart disease and stroke statistics--2014 update. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunlay SM, Shah ND, Shi Q, Morlan B, VanHouten H, Long KH, Roger VL. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4:68–75. doi: 10.1161/CIRCOUTCOMES.110.957225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai AS, Stevenson LW. Rehospitalization for heart failure: Predict or prevent? Circulation. 2012;126:501–506. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 6.Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, Cook NR, Felker GM, Francis GS, Hauptman PJ, Havranek EP, Krumholz HM, Mancini D, Riegel B, Spertus JA. Decision making in advanced heart failure: A scientific statement from the american heart association. Circulation. 2012;125:1928–1952. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. Journal of the American College of Cardiology. 2013;61:1078–1088. doi: 10.1016/j.jacc.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for medicare beneficiaries, 1998-2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unroe KT, Greiner MA, Hernandez AF, Whellan DJ, Kaul P, Schulman KA, Peterson ED, Curtis LH. Resource use in the last 6 months of life among medicare beneficiaries with heart failure, 2000-2007. Arch Intern Med. 2011;171:196–203. doi: 10.1001/archinternmed.2010.371. [DOI] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd. History of the rochester epidemiology project: Half a century of medical records linkage in a us population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: An illustration from the rochester epidemiology project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pakhomov SV, Buntrock J, Chute CG. Prospective recruitment of patients with congestive heart failure using an ad-hoc binary classifier. J Biomed Inform. 2005;38:145–153. doi: 10.1016/j.jbi.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 15.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 18.Roger VL, Killian J, Henkel M, Weston SA, Goraya TY, Yawn BP, Kottke TE, Frye RL, Jacobsen SJ. Coronary disease surveillance in olmsted county objectives and methodology. J Clin Epidemiol. 2002;55:593–601. doi: 10.1016/s0895-4356(02)00390-6. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Leibson CL, Katusic SK, Barbaresi WJ, Ransom J, O'Brien PC. Use and costs of medical care for children and adolescents with and without attention-deficit/hyperactivity disorder. JAMA. 2001;285:60–66. doi: 10.1001/jama.285.1.60. [DOI] [PubMed] [Google Scholar]
- 21.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–172. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the united states. J Am Coll Cardiol. 2013;61:1259–1267. doi: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glynn LG, Buckley B, Reddan D, Newell J, Hinde J, Dinneen SF, Murphy AW. Multimorbidity and risk among patients with established cardiovascular disease: A cohort study. Br J Gen Pract. 2008;58:488–494. doi: 10.3399/bjgp08X319459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandra V, Szklo M, Goldberg R, Tonascia J. The impact of marital status on survival after an acute myocardial infarction: A population-based study. Am J Epidemiol. 1983;117:320–325. doi: 10.1093/oxfordjournals.aje.a113544. [DOI] [PubMed] [Google Scholar]
- 25.King KB, Reis HT. Marriage and long-term survival after coronary artery bypass grafting. Health Psychol. 2012;31:55–62. doi: 10.1037/a0025061. [DOI] [PubMed] [Google Scholar]
- 26.Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71–77. doi: 10.3122/jabfm.2013.01.120107. [DOI] [PubMed] [Google Scholar]
- 27.Mor A, Ulrichsen SP, Svensson E, Berencsi K, Thomsen RW. Does marriage protect against hospitalization with pneumonia? A population-based case-control study. Clin Epidemiol. 2013;5:397–405. doi: 10.2147/CLEP.S50505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen LA, Hernandez AF, Peterson ED, Curtis LH, Dai D, Masoudi FA, Bhatt DL, Heidenreich PA, Fonarow GC. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4:293–300. doi: 10.1161/CIRCHEARTFAILURE.110.959171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Krumholz HM. Outcomes in heart failure patients with preserved ejection fraction: Mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41:1510–1518. doi: 10.1016/s0735-1097(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 30.Liao L, Jollis JG, Anstrom KJ, Whellan DJ, Kitzman DW, Aurigemma GP, Mark DB, Schulman KA, Gottdiener JS. Costs for heart failure with normal vs reduced ejection fraction. Arch Intern Med. 2006;166:112–118. doi: 10.1001/archinte.166.1.112. [DOI] [PubMed] [Google Scholar]
- 31.Foody JM, Rathore SS, Wang Y, Herrin J, Masoudi FA, Havranek EP, Radford MJ, Krumholz HM. Predictors of cardiologist care for older patients hospitalized for heart failure. Am Heart J. 2004;147:66–73. doi: 10.1016/j.ahj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 32.McBride D, Hardoon S, Walters K, Gilmour S, Raine R. Explaining variation in referral from primary to secondary care: Cohort study. BMJ. 2010;341:c6267. doi: 10.1136/bmj.c6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Health, United States, 2010: With special feature on death and dying. http://www.cdc.gov/nchs/data/hus/hus10.pdf. [PubMed]
- 34.Teno JM, Gozalo PL, Bynum JP, Leland NE, Miller SC, Morden NE, Scupp T, Goodman DC, Mor V. Change in end-of-life care for medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309:470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Back AL, Li YF, Sales AE. Impact of palliative care case management on resource use by patients dying of cancer at a veterans affairs medical center. J Palliat Med. 2005;8:26–35. doi: 10.1089/jpm.2005.8.26. [DOI] [PubMed] [Google Scholar]
- 36.Kelley AS, Deb P, Du Q, Aldridge Carlson MD, Morrison RS. Hospice enrollment saves money for medicare and improves care quality across a number of different lengths-of-stay. Health Aff. 2013;32:552–561. doi: 10.1377/hlthaff.2012.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: A qualitative systematic literature review of patient preferences. J Palliat Med. 2000;3:287–300. doi: 10.1089/jpm.2000.3.287. [DOI] [PubMed] [Google Scholar]
- 38. [july 16, 2014];End-of-life care. Dartmouth atlas of healthcare. Http://www.Dartmouthatlas.Org/keyissues/issue.Aspx?Con=2944.
- 39.Teno JM, Gozalo PL, Lee IC, Kuo S, Spence C, Connor SR, Casarett DJ. Does hospice improve quality of care for persons dying from dementia? J Am Geriatr Soc. 2011;59:1531–1536. doi: 10.1111/j.1532-5415.2011.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teno JM, Clarridge BR, Casey V, Welch LC, Wetle T, Shield R, Mor V. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291:88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 41.Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368:1173–1175. doi: 10.1056/NEJMp1215620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.