Abstract
Preadipocyte factor-1 (Pref-1) is made as a transmembrane protein containing EGF-repeats at the extracellular domain that can be cleaved to generate a biologically active soluble form. Pref-1 is found in islet β-cells and its level has been reported to increase in neonatal rat islets upon growth hormone treatment. We found here that Pref-1 can promote growth of pancreatic tumor derived AR42J cells. To examine Pref-1 function in pancreatic islets in vivo, we generated transgenic mouse lines overexpressing the Pref-1/hFc in islet β-cells using rat insulin II promoter (RIP). These transgenic mice exhibit an increase in islet mass with higher proportion of larger islets in pancreas compared to wild-type littermates. This is in contrast to pancreas from Pref-1 null mice that show higher proportion of smaller islets. Insulin expression and insulin secretion from pancreatic islets from RIP-Pref-1/hFc transgenic mice are increased also. Thus, RIP-Pref-1/hFc transgenic mice show normal glucose levels but with higher plasma insulin levels in both fasting and fed conditions. These mice show improved glucose tolerance. Taken together, we conclude Pref-1 as a positive regulator of islet β-cells and insulin production.
Keywords: Pref-1, Islet β-cells, transgenic mice, hyperinsulinemia, Dlk1, diabetes, obesity, insulin secretion
INTRODUCTION
Preadipocyte Factor-1 (Pref-1) is made as a transmembrane protein containing EGF-repeats at the extracellular domain, which is cleaved to generate soluble form. Our search for differentially-expressed genes that distinguish different stages of adipocyte development led to the identification of Pref-1, which is expressed in preadipocytes but not adipocytes [1–3]. In 3T3-L1 cells, constitutive expression of Pref-1 or treatment with soluble Pref-1 inhibits adipocyte differentiation, while knockdown of Pref-1 enhances adipocyte differentiation [4–6]. The inhibitory effect of Pref-1 on adipocyte differentiation is via activation of ERK/MAPK pathway [7]. By overexpression of Pref-1 in adipose tissue as well as by ablating Pref-1 gene, we demonstrated the critical role of Pref-1 in adipose development, as well as in bone development [4, 8–12].
During embryonic development, Pref-1 is expressed in a variety of tissues but its expression is progressively extinguished during development in most tissues, with the exception adipose precursors found in adipose tissue as well as certain neuroendocrine type of cells including islet β-cells [3,13,14]. It has been shown that the expression pattern of Pref-1 during pancreatic development is remarkably similar to that of PDX-1, a transcription factor that plays a crucial role in pancreatic development as well as maintenance of the β-cell phenotype [15–17]. During early pancreatic development, Pref-1 is expressed mostly in the ductal epithelial cells, but its expression gradually disappears with development, becoming primarily restricted to islet β-cells [13,14]. The expression levels for Pref-1 in islets of newborns are high but decrease drastically in adults. However, Pref-1 levels increase during pregnancy when β-cell hyperplasia occurs [18]. In vitro treatment of newborn pancreatic islets with prolactin/growth hormone increases Pref-1 levels accompanying the increase in proliferation of β–cells [18]. The expression pattern of Pref-1 and its regulation suggest a potential regulatory role of Pref-1 in islet β-cell growth.
To investigate whether Pref-1 functions as a mitogenic factor to affect islet β-cell function, we created transgenic mice expressing soluble Pref-1 fused to the human Fc using the rat insulin promoter. We report here that overexpression of Pref-1 in islet β-cells increases islet size, resulting in hyperinsulinemia and improvement of glucose tolerance in mice.
MATERIALS AND METHODS
Generation of RIP-Pref-1/hFc Transgenic Mice
The fusion gene fragment corresponding to the soluble form of Pref-1 and human constant region of immunoglobulin gamma was subcloned into the XbaI/Hind III site of a vector containing the RIP promoter (rat insulin II promoter, a generous gift from Dr. D. Hanahan. The 2.8-kb transgenic construct was excised by Bam HI and gel-purified using the QIAquick gel extraction kit (Qiagen). The construct was microinjected into single cell embryos of strain C57BL/6J X FVB and implanted into pseudo-pregnant female mice. At 3 weeks of age, a 0.5-cm portion of tail was removed from each mouse for DNA analysis. For PCR analysis of transgenic mice, primers specific for the 3' end of the Pref-1 cDNA (5'-CAC GAG CTG CCT GTT CAG CAG CC-3') and the 5' end of the human Fc cDNA sequence (5'-CTT GAC CTC AGG GTC TTC GTG-3') were used to amplify 254-bp fragments by the following thermocycling conditions: denaturation = 94 °C for 40 s, annealing = 55 °C for 60 s, and extension = 72 °C for 60 s for a total of 34 cycles, followed by 72 °C for 10 min. The transgene copy number was determined in different transgenic lines by Southern blot analysis using Pref-1 cDNA labeled by random priming with [α-32P] dCTP. For all experiments, age- and sex-matched nontransgenic wild-type littermates were used for comparison with the RIP-Pref-1/hFc transgenic mice. The detailed information on construction and generation of Pref-1 knockout mice has been described in our previous report (4). The studies were conducted with approval of the Animal Care and Use Committee at the University of California, Berkeley.
Cell Culture
AR42J cells were cultured in Ham’s F12K medium supplemented with 20% fetal bovine serum, 100 U/mL penicillin, and 0.1 mg/mL streptomycin. AR42J cells were suspended at 5×104 cells/ml of Ham’s F12K medium containing 20% FCS; 1 ml was used to seed each well of a 24 well plate. After 24 hours, conditioned media was added at 1 and 7% of final concentration to triplicate wells. Cells were assessed by the MTS-based cell titer assay (Promega). Briefly, cells were cultured in conditioned media for 48 and 72 hours and 200 µl of reagent containing MTS was added and the cells were further incubated at 37 °C for an hour. One hundred µl of media were transferred into 96-well plate and O.D. was determined at 490 nm.
Transfection of Pref-1/hFc into COS cells
Pref-1/hFc fusion gene cloned into pcDNA3.1 expression vectors were transiently transfected into COS cells using DEAE-Dextran in DMEM with 10% Serum Plus (JRH Biosciences) as described [10]. For control, pcDNA3.1 expression vector without insert was used for COS cell transfection. Twenty-four hours after transfection, the media were changed to DMEM supplemented with 10% fetal bovine serum (FBS) (Life Technologies). The conditioned media were collected 72 hours after transfection, centrifuged at 500 × g for 5 min, and stored at 4°C for less than one week before use.
RNA isolation and RT-PCR
Total RNA from pancreas was prepared using TriZOL reagent (Life Technologies). Total cellular RNA from pancreas of 3 week-old mice was reverse transcribed with SuperScript II (Gibco BRL). cDNA was amplified with Pref-1 primers (Forward; 5'-GCCATCGTCTTTCTCAACAAGTG-3', Reverse; 5'-GTAAGCATAGGCTTCACTCGATTC -3'), β-Actin primers (Forward; 5'-TCCTATGTGGGTGACGAGGC-3', Reverse; 5'-CATGGCTGGGGTGTTGAAGG-3'),
Histological Analysis
Pancreatic tissues from RIP-Pref-1/hFc and wild-type littermate mice (n = 5; 8–10 weeks old) were fixed for overnight in paraformaldehyde or Bouin’s solution, sectioned (6 µm) and stained with hematoxylin and eosin, at least 10 slides per mouse. Images of pancreas sections were captured and the islet area was measured using NIH image software. For each pancreas, every sixteenth section was investigated, and all detectable islets measured.
Immunohistochemistry
Double immunofluorescence staining of pancreas sections from 30-day old mice were performed sequentially by using primary antibodies of guinea pig anti-human insulin (1:200, Dako) and rabbit anti-bovine glucagon (1:200, Zymed) antibodies. The conjugated secondary antibodies used for immunofluorescence were Texas red-conjugated donkey anti-guinea pig IgG, FITC- or Texas red-conjugated donkey anti-rabbit IgG, and Texas red-conjugated donkey anti-human Fc IgG (1:100, Jackson).
Plasma Insulin and Lipid Metabolite Levels
Insulin levels for overnight fasted or non-fasted mice were measured by a Linco Rat RIA kit. Plasma triglyceride and cholesterol levels were determined using Triglyceride (INT) 10 and Infinity Cholesterol reagents (Sigma), respectively. Plasma free fatty acid levels were determined with NEFAC (Wako).
Glucose and Insulin Tolerance Tests
Glucose and insulin tolerance tests were performed on 10–12 weeks old transgenic mice and their wild-type littermates. For glucose tolerance test, D-glucose (2 mg/g of body weight) was injected intraperitoneally into overnight fasted mice. Tail-blood samples were taken at time 0, 30, 60, 90 and 120 min after injection for glucose measurement. Blood glucose levels were monitored by Accu-Chek glucometer (Roche). Insulin tolerance tests were performed on mice following a 5-hour fast. Animals were injected intraperitoneally with 0.75 U/kg body weight of insulin (Eli Lilly). Blood glucose levels were monitored at time 0, 30, 60, and 120 min after insulin injection.
Pancreatic Islet Isolation and Measurement of Insulin
Pancreatic islets were isolated using collagenase and counted and incubated in buffer containing 0.2% BSA, 20 mM HEPES [pH 7.2], 114 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.16 mM MgSO4, and 2.5 mM CaCl2 with 30mM Glucose for 60 min. Insulin release was measured using ELISA.
Statistical Analysis
Data are expressed as the means ± SEMs. The statistical differences in mean values between transgenic and wild-type littermates were assessed by Student's t test.
RESULTS
Effect of Pref-1 on growth of pancreatic tumor derived AR42J Cells
In developing fetal pancreas, Pref-1 is found mostly in ductal epithelial cells that are the precursors of both exocrine and endocrine cells [13]. We therefore, tested whether Pref-1 has influence on cell proliferation using AR42J cells in culture. The AR42J cells are derived from a chemically induced pancreatic acinar cell tumor but have the features of pluripotency of the common precursor cells of the pancreas. We expressed the full ectodomain of Pref-1 as a fusion protein to human Fc by transient transfection of the expression vector into COS cells. We previously demonstrated that the large soluble form is the biologically active Pref-1 and that the Fc fusion favors dimerization and makes the protein more stable [10]. As shown in Figure 3A, the Pref-1/hFc was detected by Westernblotting of the conditioned media from the Pref-1/hFc transfected COS cells. The AR42J cells were then cultured in media containing 10% fetal bovine serum and 7% of conditioned media. After 24, 48, and 72 h, cell proliferation was assessed by two methods: the tetrazolium bioreduction method (Promega) and actual counting of the cells. AR42J cell proliferation by tetrazolium method (Fig.1A) was increased by the addition of conditioned media containing Pref-1/hFc and the Pref-1 effects observed were dose-dependent. One percent of the final concentration of conditioned media could significantly increase the AR42J cell proliferation in comparison to the conditioned media from mock transfected COS cells, suggesting the mitogenic effect of Pref-1 on pancreatic precursor cells. Addition of 7 % of conditioned media increased AR42J cell proliferation at a higher magnitude than the addition of 1% conditioned media (Fig. 1A). Effect of Pref-1 on AR42J cell growth was estimated also by counting of cell numbers for 5 days after plating. Cell numbers were higher at each time point counted during growth in the presence of Pref-1, as shown in Figure 1B. However, exponential growth of cells could not be achieved in these experiments, probably because these cells usually grew in clusters and, as clusters enlarged, the growth rates attenuated. Regardless, the soluble Pref-1 clearly showed increase in AR42J cell growth.
Figure 3. Effect of Pref-1/hFc expression on Islet Size.
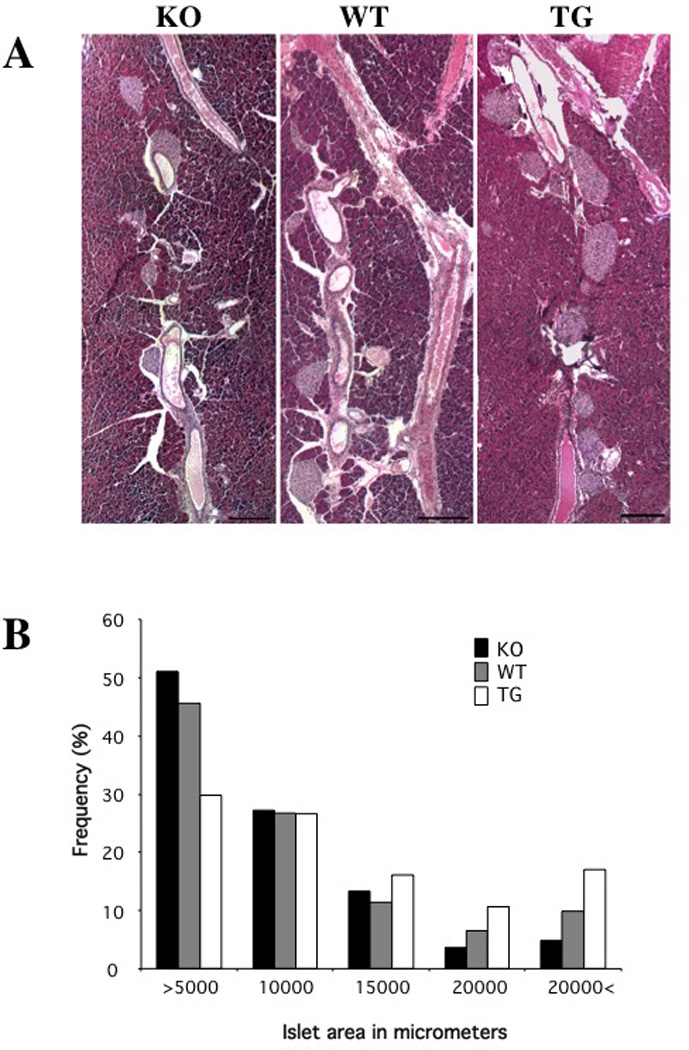
A: Hematoxylin and eosin staining of pancreas from Pref-1 homozygous knockout mice (left panel), wild-type control mice (middle panel), and RIP-Pref-1/hFc transgenic mice (right panel) at 8–10 weeks of age. Scale bar, 100 micron. B: Size distribution of islets. Data represent averages of five mice in each group, 10 slides per mice.
Figure 1. Effect of soluble Pref-1 on Proliferation of AR42J Cells.
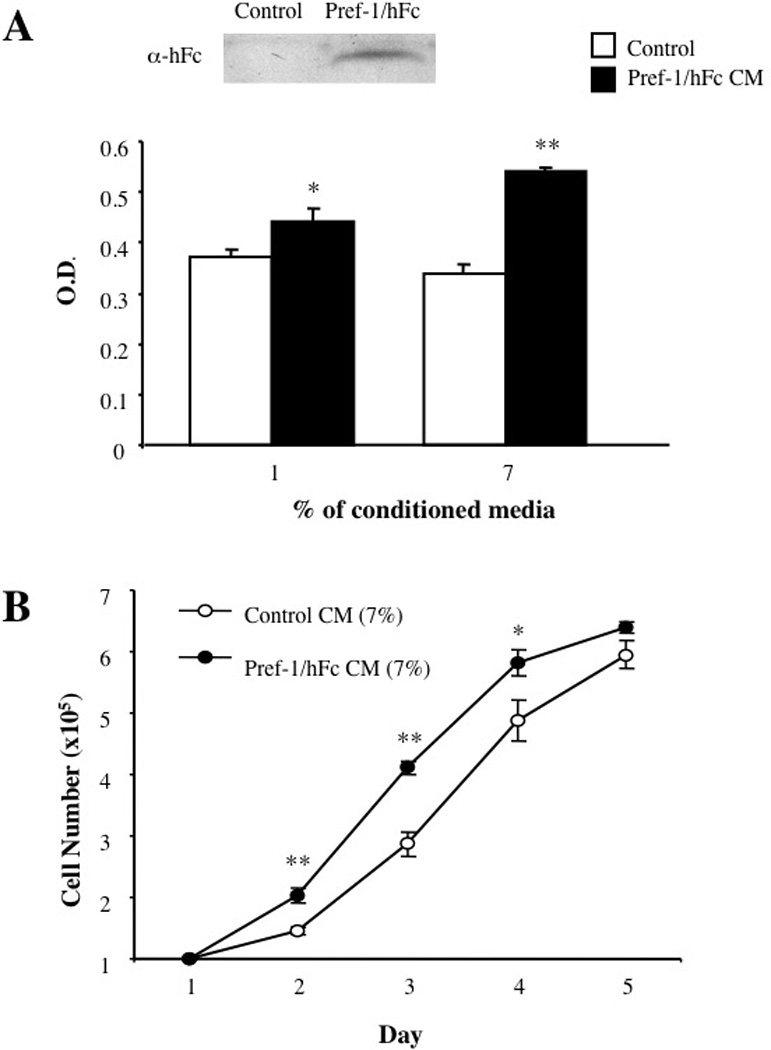
AR42J cells were cultured in media supplemented with either 1 or 7% conditioned media obtained from COS cells transfected with either Pref-1/hFc expression vector or empty vector. A. Conditioned media collected from control, vector transected and Pref-1/hFc transfected COS cells were subjected to western blot analysis using hFc antibodies. Cell proliferation was measured using MTS assay after 48 hours exposure to conditioned media. Cell proliferation is expressed as absorbance of formazan at 490 nm. Data represent the means ± SEMs for at least three experiments (each in triplicate). B: The cell numbers for AR42J cells maintained in the presence of conditioned media from Pref-1/hFC expression vector transfected COS cells or media from cells transfected with empty vector were counted at indicated time points. * < 0.05 and **, p < 0.01
Expression of Pref-1-hFc in Islet β-cells in Transgenic Mice and its Effect on Islet Morphology
To demonstrate the role of Pref-1 in islet β-cells in vivo, we generated transgenic mice overexpressing the Pref-1/hFc fusion protein, using the rat insulin promoter (RIP) (Fig. 2A). The RIP-Pref-1/hFc fusion gene was microinjected into mouse embryos, and four founder mice (C57BL/6–FAB genetic background) were identified. Two lines (tg-m and tg-h) that showed higher levels of transgene expression in the pancreas as detected by RT-PCR (Fig. 2B left panel) were selected for breeding and further analysis. Expression of transgene was also examined by Western blotting using anti-Fc antibodies (Fig. 2B right panel). There were no differences in bodyweights between wild type and RIP-Pref-1/hFc transgenic mice (data not shown). Blood parameters such as cholesterol and free fatty acid levels were normal, whereas blood triglyceride levels were somewhat lower in RIP-Pref-1/hFc transgenic mice (Suplementary Table 1).
Figure 2. RIP-Pref-1/hFc transgene Construct, Transgene Expression and Islet Morphology.
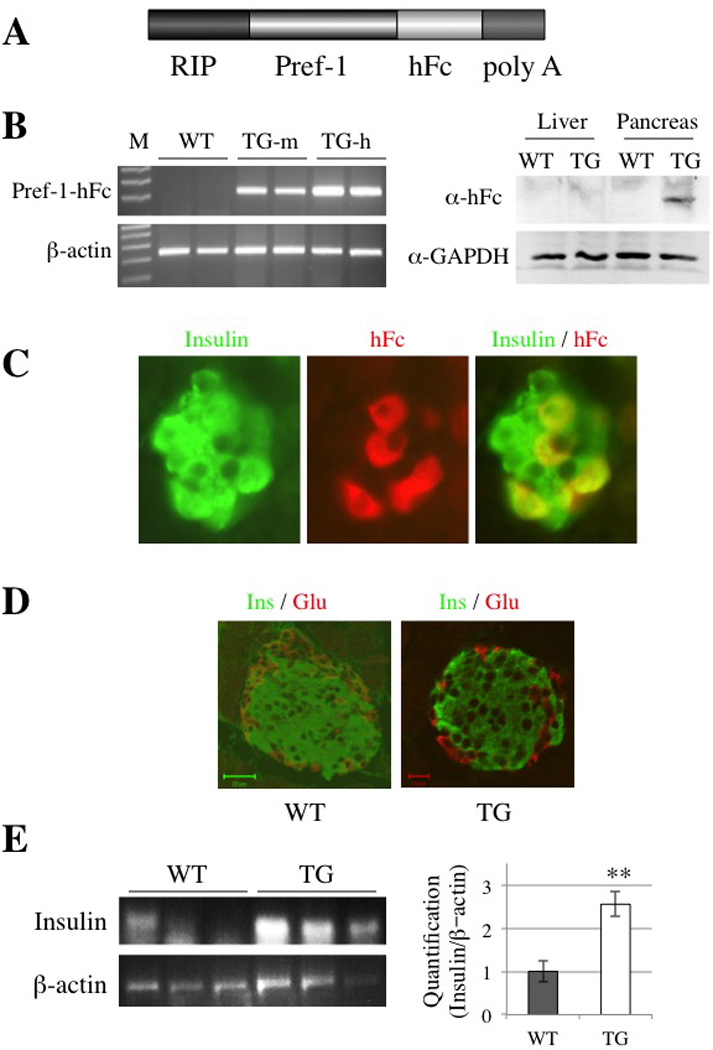
A: Schematic diagram of the RIP-Pref-1/hFc construct. RIP denotes the rat insulin II promoter, Pref-1/hFc represents the full ectodomain of Pref-1 fused in frame to the Fc portion of human immunoglobulin heavy chain gene, and Poly A denotes the bovine growth hormone polyadenylation sequence. B: Total pancreatic RNA from two transgenic mice of each lines and two wild-type littermates were analyzed by RT-PCR (left panel) to detect Pref-1/hFc transgene expression. β-actin was used as a control for RT-PCR. Based on transgene expression levels, Tg-m (mid expresser) and Tg-h (high expresser) lines were selected for further study (left panel). Immunoblotting of liver and pancreas using anti-hFc and anti-GAPDH antibodies (right panel). C: Immunohistochemical detection of Pref-1/hFc in the pancreas of transgenic mice. Sections of pancreas from RIP-Pref-1/hFc transgenic mice were stained for insulin (green) and hFc antibodies (red) and shown under high magnification (original magnification, ×400). Superimposed image is shown in the right panel. No signal for Pref-1/hFc transgene product was detected in pancreas from wild-type littermates (data not shown). D: Immunofluorescense staining of pancreas sections for insulin (Ins, green) and glucagon (Glu, red). WT; wild-type (bar 20 µm), TG; RIP-Pref-1/hFc transgenic mice (bar, 10 µm); Green scale bar, 20 micron and red scale bar, 10 micron. E: RT-PCR for insulin mRNA level from 3 different wild type and RIP-Pref-1/hFc transgenice pancreas (left pane), Quantification of the image were obtained using imageJ software (NIH), compare with the β-actin control.
In order to better document the Pref-1/hFc transgene expression in these transgenic mice, we performed immunohistochemical analysis of Pref-1/hFc fusion protein in pancreatic islets using hFc antibodies. No hFc staining was observed in islets from wild-type control mice (data not shown), whereas high levels of hFc signal were detected in the islets of transgenic mice. By judging hFc staining, transgene expression was colocalized with insulin, indicating Pref-1 expression in islet β-cells (Fig. 2C). To test whether overexpression of Pref-1 driven by the rat insulin promoter influences islet structure and histology, pancreas sections were immunostained with antibodies against insulin and glucagon and compared to those of wild-type littermates (Fig. 2D). While no striking gross changes in the islet architecture were detected, insulin positive ß-cells were surrounded by a rim of glucagon-positive α-cells in the RIP-Pref-1/hFc transgenic mice. We did not detect significant changes in islet density in pancreas of the Pref-1/hFc transgenic mice. However we observed stronger staining of insulin in transgenic islets. We tested insulin expression levels in pancreas by RT-PCR and performed quantification of the images from 3 different mice in each group. As shown in Figure 2E left and right panels, insulin mRNA levels were significant higher in Pref-1/hFc transgenic mice than in control littermates.
Expression of Pref-1/hFc in Islet β-cells Results in Increase of Islet Mass
We next carried out quantitative morphometric analysis of pancreas sections by measuring the islet surface area throughout the pancreas to determine if expression of the Pref1/hFc transgene had an effect on islet mass. We also employed Pref-1 homozygous knockout mice that we previously generated for comparison in this experiment. The percentages of islet area for varying diameters were estimated in wild-type, Pref-1/hFc transgenic and Pref-1 null mice (Fig. 3A and B). Compared to wild-type littermates, the pancreas from mice expressing the Pref1/hFc transgene showed a greater percentage of larger islets and a smaller proportion of very small islets. Conversely, Pref-1 null mice showed a decreased proportion of islets with larger diameters as compared to wild-type mice. Overall these data indicate that Pref-1 indeed promotes an increase in islet mass.
The Metabolic Effects of Islet β–cell Expression of Pref-1-hFc in Transgenic Mice
To determine whether the increase in β-cell mass would affect the glucose homeostasis, we measured blood glucose and insulin levels. Fasted and non-fasted glucose levels were not significantly different between wild-type and RIP-Pref-1/hFc transgenic mice (Fig. 4A right panel). However, blood insulin levels in both fasted and non-fasted states were about three-fold higher in RIP-Pref-1/hFc transgenic compared to those in wild-type mice (Fig. 4A middle panel). Thus, RIP-Pref-1/hFc transgenic mice were normoglycemic but hyperinsulinemic. The insulin/glucose ratio was significantly higher in the RIP-Pref-1/hFc transgenic mice than in normal wild-type mice at both the fasted and fed conditions (Fig. 4A right panel).
Figure 4. The Metabolic Effects in RIP-Pref-1/hFc Transgenic Mice.
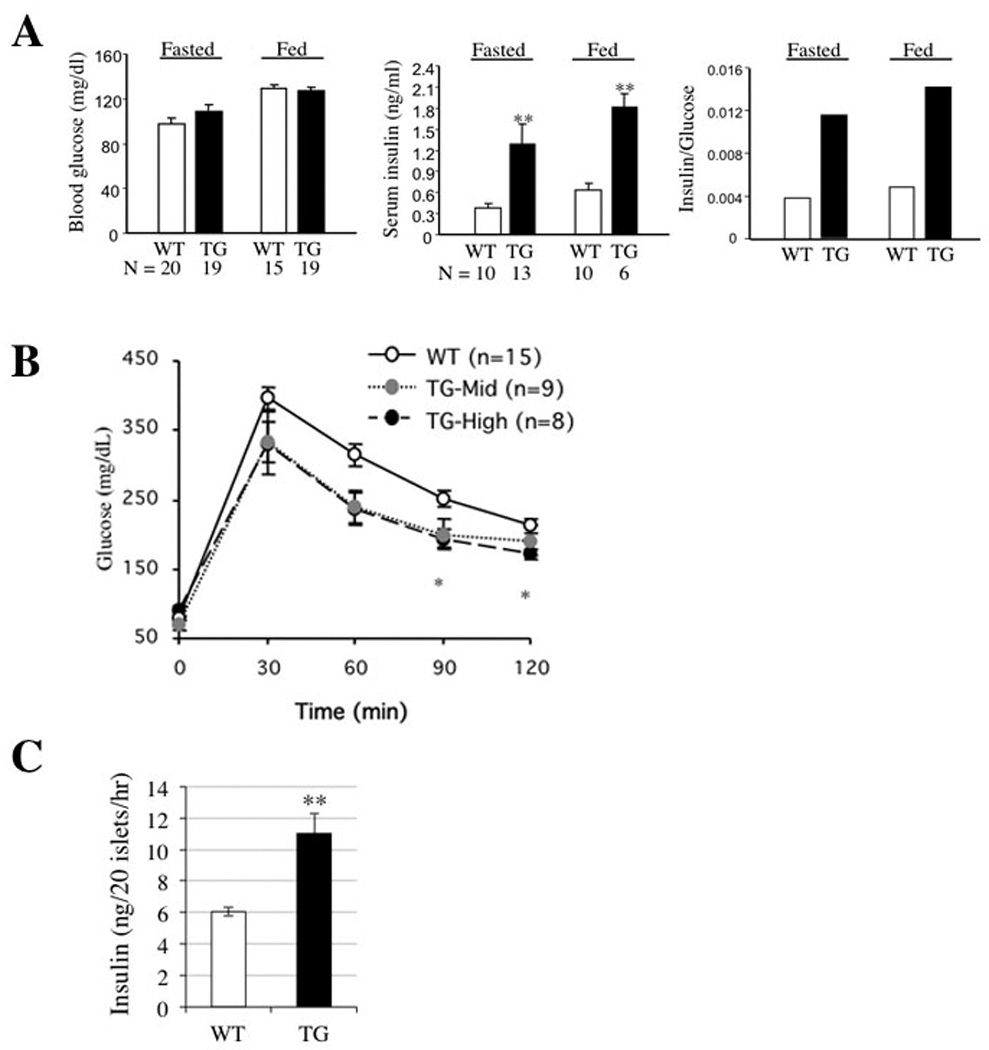
RIP-Pref-1/hFc transgenic mice and wild-type controls at 10–12 weeks of age were used. Blood glucose and insulin levels were measured after overnight fast and in fed states. A. Blood glucose levels (left panel), plasma insulin levels (middle panel), and the insulin/glucose ratio (right panel). Data represent means ± SEMs. B: Glucose tolerance tests were performed in the two lines of RIP-Pref-1/hFc transgenic mice and wild-type controls at 10–12 weeks of age. Tg-mid (mid expresser) and Tg-high (high expresser) lines. Data are presented as means ± SEMs * < 0.05 and ***, p < 0.01. C: Insulin secretion from isolated pancreas of wild-type and RIP-Pref-1/hFc transgenic mice. Experiments were repeated twice. Data represent means ± SEMs. **, < 0.01.
To determine whether the increased islet mass by Pref-1 expression brings about changes in glucose tolerance, we measured plasma glucose concentrations after an intraperitoneal injection of glucose. As shown in Figure 4B, fasting blood glucose concentrations in RIP-Pref-1/hFc mice were comparable with those in wild-type mice. However, 30 and 60 min after an intraperitoneal injection of glucose (2 mg/g body weight), blood glucose levels in both lines of RIP-Pref-1/hFc mice were slightly lower than those in wild-type littermates. Significantly lower levels of blood glucose were detected in high expresser mice 90–120 min after the glucose injection, and mid-expresser mice also showed similar low levels although not statistically significant. These results suggest that the RIP-Pref-1/hFc mice have increased ability for glucose disposal. In this regard, we found by ITT that RIP-Pref-1/hFc mice had impairment of glucose disposal in response to insulin, confirming that improved glucose disposal observed by GTT was not due to changes in insulin responsiveness by peripheral tissue (data not shown). To examine whether insulin release was higher in transgenic mice, we employed isolated islets to stimulate insulin secretion by incubating them with 30 mM glucose for 1 hr. Indeed, compare to wild type islets, RIP-Pref-1/hFc transgenic islets showed significant higher insulin secretion, indicating RIP-Pref-1/hFc transgenic islets responded to glucose stimulation even better than wild type islets (Fig. 4C). These data suggest that improved glucose tolerance in RIP-Pref-1/hFc mice is due to the increase in islet mass and higher insulin secretion from islet β-cells.
DISCUSSION
Formation of new β–cells may occur either by the replication of islet β–cells or by neogenesis from pancreatic ductal precursor cells. During embryonic development, Pref-1 is found in most of the pancreatic parenchymal cells, but later during development Pref-1 level decreases and its expression becomes restricted to the β-cells of islet of Langerhans [13]. Therefore, Pref-1 can be detected in islets of fetal, neonatal, and pregnant rats [13,14]. Furthermore, growth hormone or prolactin-induced β-cell growth was shown to correlate with increased Pref-1 expression, suggesting Pref-1 as a downstream mediator of growth hormone/prolactin on β-cell proliferation that may occur during gestational diabetes [18]. Overall, these observations suggest a possible role of Pref-1 in β-cell growth. In the present studies we found that Pref-1 induces the proliferation of AR42J pancreatic cells and that expression of Pref-1 in the islet β-cells of transgenic mice induces increased islet size to bring about metabolic effects of hyperinsulinemia and improved glucose clearance. In contrast, ablation of Pref-1 gene in mice induces a significantly smaller size of islets compared to age-matched wild-type mice. The effects of Pref-1 on islet size as shown above in Pref-1 transgenic and Pref-1 knockout mice, and the proliferative effect of Pref-1 observed in in vitro cultured cells, support our hypothesis that Pref-1 acts as a positive effector for β-cell growth.
The hyperinsulinemia in the transgenic mice is observed both in fasting and in fed states, but the fasting plasma glucose levels did not differ significantly between transgenic and wild-type littermates. An inappropriate hyperinsulinemia in RIP-Pref-1/hFc transgenic mice was correlated with the observed increase in insulin mRNA levels in islets of these transgenic mice. In addition, hyperinsulinemia caused by chronic expression of Pref-1 in β-cells in transgenic mice correlated with increased islet size. Since we did not detect neither an increase in islet density in the pancreas nor gross structural changes in islets, we conclude that although proportion of insulin-positive cells in the islet was normal, the total number of β-cells would be higher in Pref-1/hFc transgenic mice and this would lead to hyperinsulinemia. We observed an increase in insulin secretion from islets isolated from RIP-Pref-1/hFc transgenic mice.
Pref-1 has been extensively studied for its anti-adipogenic function. Pref-1 has been reported to be an imprinted gene that is paternally expressed due to differential methylation [19, 20]. Our studies on Pref-1 knockout mice demonstrated that Pref-1 is indeed a paternally expressed and that Pref-1 is important in fetal growth and development [4]. Several reports on spatiotemporal expression of Pref-1 indicated that Pref-1 is expressed in most of the parenchymal cells of fetal pancreas [13, 14]. In the present studies, we analyzed the proliferative effects of pref-1 on a rat pancreatic tumoral cell line, AR42J. The results showed that pref-1 conditioned media significantly stimulated AR42J cell proliferation in a dose-dependent fashion. Mitogenic effect of Pref-1 has been observed in thymocytes in culture. Kaneta et al. reported that Pref-1 increased thymocyte proliferation and the addition of Pref-1 antibody inhibited cell proliferation [21]. Furthermore, Pref-1 was proposed to control thymocyte cellularity via increasing HES-1 expression. It may be that the mitogenic effect of Pref-1 is limited in certain cell types, since we did not observe increase in proliferation in 3T3-L1 preadipocytes in response to Pref-1 [7]. The disparate responsiveness to Pref-1 of the different cell types may be the result of the possible dual roles of Pref-1 signals in cell fate decision and proliferation. Although Pref-1 increased cell proliferation of pancreatic AR42J cells as in thymocytes, we did not observe changes in HES-1 expression in our system (data not shown). Understanding the molecular mechanism underlying Pref-1 action would require cloning of the putative Pref-1 receptor and elucidation of downstream signaling pathways.
In summary, our present studies show an important role of a soluble form of Pref-1 in pancreatic islet mass and insulin/glucose homeostasis. Although the mechanism underlying Pref-1 function needs further investigation, increase in islet and β–cell mass in our transgenic mice may be at least in part via increase in cell proliferation.
Supplementary Material
Highlights.
Pref-1 promotes growth of pancreatic tumor derived AR42J cells.
Overexpression of Pref-1 in islet β-cells results increase in islet mass with higher insulin secretion.
RIP-Pref-1/hFc transgenic mice show improved glucose tolerance.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grant DK 50828 to H. S. S. We thank Sarah De Val and Sunjoo Lee for technical help. We also thank Dr. D. Hanahan for the RIP promoter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Smas CM, Chen L, Sul HS. Cleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol Cell Biol. 1997;17:977–988. doi: 10.1128/mcb.17.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smas CM, Green D, Sul HS. Structural characterization and alternate splicing of the gene encoding the preadipocyte EGF-like protein pref-1. Biochemistry. 1994;33:9257–9265. doi: 10.1021/bi00197a029. [DOI] [PubMed] [Google Scholar]
- 3.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 4.Moon YS, Smas CM, Lee K, Villena JA, Kim KH, Yun EJ, Sul HS. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol. 2002;22:5585–5592. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Sul HS. Ectodomain shedding of preadipocyte factor 1 (Pref-1) by tumor necrosis factor alpha converting enzyme (TACE) and inhibition of adipocyte differentiation. Mol Cell Biol. 2006;26:5421–5435. doi: 10.1128/MCB.02437-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Zhao L, Smas C, Sul HS. Pref-1 interacts with fibronectin to inhibit adipocyte differentiation. Mol Cell Biol. 2010;30:3480–3492. doi: 10.1128/MCB.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KA, Kim JH, Wang Y, Sul HS. Pref-1 (preadipocyte factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Mol Cell Biol. 2007;27:2294–2308. doi: 10.1128/MCB.02207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudak CS, Gulyaeva O, Wang Y, Park SM, Lee L, Kang C, Sul HS. Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Rep. 2014;8:678–687. doi: 10.1016/j.celrep.2014.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee K, Villena JA, Moon YS, Kim KH, Lee S, Kang C, Sul HS. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1) J Clin Invest. 2003;111:453–461. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mei B, Zhao L, Chen L, Sul HS. Only the large soluble form of preadipocyte factor-1 (Pref-1), but not the small soluble and membrane forms, inhibits adipocyte differentiation: role of alternative splicing. Biochem J. 2002;364:137–144. doi: 10.1042/bj3640137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villena JA, Choi CS, Wang Y, Kim S, Hwang YJ, Kim YB, Cline G, Shulman GI, Sul HS. Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): a new model of partial lipodystrophy. Diabetes. 2008;57:3258–3266. doi: 10.2337/db07-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Sul HS. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab. 2009;9:287–302. doi: 10.1016/j.cmet.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tornehave D, Jansen P, Teisner B, Rasmussen HB, Chemnitz J, Moscoso G. Fetal antigen 1 (FA1) in the human pancreas: cell type expression, topological and quantitative variations during development. Anat Embryol (Berl) 1993;187:335–341. doi: 10.1007/BF00185891. [DOI] [PubMed] [Google Scholar]
- 14.Tornehave D, Jensen CH, Teisner B, Larsson LI. FA1 immunoreactivity in endocrine tumours and during development of the human fetal pancreas; negative correlation with glucagon expression. Histochem Cell Biol. 1996;106:535–542. doi: 10.1007/BF02473268. [DOI] [PubMed] [Google Scholar]
- 15.Peers B, Leonard J, Sharma S, Teitelman G, Montminy MR. Insulin expression in pancreatic islet cells relies on cooperative interactions between the helix loop helix factor E47 and the homeobox factor STF-1. Mol Endocrinol. 1994;8:1798–1806. doi: 10.1210/mend.8.12.7708065. [DOI] [PubMed] [Google Scholar]
- 16.Peshavaria M, Gamer L, Henderson E, Teitelman G, Wright CV, Stein R. XIHbox 8, an endoderm-specific Xenopus homeodomain protein, is closely related to a mammalian insulin gene transcription factor. Mol Endocrinol. 1994;8:806–816. doi: 10.1210/mend.8.6.7935494. [DOI] [PubMed] [Google Scholar]
- 17.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlsson C, Tornehave D, Lindberg K, Galante P, Billestrup N, Michelsen B, Larsson LI, Nielsen JH. Growth hormone and prolactin stimulate the expression of rat preadipocyte factor-1/delta-like protein in pancreatic islets: molecular cloning and expression pattern during development and growth of the endocrine pancreas. Endocrinology. 1997;138:3940–3948. doi: 10.1210/endo.138.9.5408. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt JV, Matteson PG, Jones BK, Guan XJ, Tilghman SM. The Dlk1 and Gtl2 genes are linked and reciprocally imprinted. Genes Dev. 2000;14:1997–2002. [PMC free article] [PubMed] [Google Scholar]
- 20.Takada S, Tevendale M, Baker J, Georgiades P, Campbell E, Freeman T, Johnson MH, Paulsen M, Ferguson-Smith AC. Delta-like and gtl2 are reciprocally expressed, differentially methylated linked imprinted genes on mouse chromosome 12. Curr Biol. 2000;10:1135–1138. doi: 10.1016/s0960-9822(00)00704-1. [DOI] [PubMed] [Google Scholar]
- 21.Kaneta M, Osawa M, Sudo K, Nakauchi H, Farr AG, Takahama Y. A role for pref-1 and HES-1 in thymocyte development. J Immunol. 2000;164:256–264. doi: 10.4049/jimmunol.164.1.256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


