Abstract
Preterm premature rupture of fetal membranes (PPROM) is associated with infection and is one of the most common causes of preterm birth. Abnormalities of biglycan and decorin, two extracellular matrix proteoglycans, lead to preterm birth and abnormal fetal membrane morphology and abnormal signaling in the mouse model as well as being associated with inflammatory cascades. In humans, decorin dysregulation is associated with inflammation in PPROM. We investigated biglycan and decorin’s role in inflammation in fetal membranes using mouse models of intraperitoneal E. coli injections superimposed on biglycan and decorin deficiency. We assessed outcomes in vivo as well as in vitro using quantitative qPCR, Western blotting and ELISA techniques. Our results suggest that biglycan and decorin compensate for each other in the fetal membranes but lose the ability to do so in the setting of inflammation, leading to decreased latency to preterm birth. Furthermore, our findings suggest that biglycan and decorin play discrete roles in fetal membrane signaling pathways in inflammation, leading to changes in expression of MMP-8 and collagen α1VI, two components of the fetal membrane extracellular matrix that play a role in the pathophysiology of PPROM. In summary, these findings underline the importance of biglycan and decorin in fetal membranes as targets for the manipulation of fetal membrane extracellular matrix stability in the setting of inflammation.
Keywords: Preterm birth, MMP, collagen VI, PPROM, extracellular matrix
1. Introduction
Preterm birth is the leading cause of newborn morbidity and mortality in the United States (Murphy et al., 2012). About a third of preterm births are caused by preterm premature rupture of fetal membranes (PPROM) (Steer 2005). While no therapies for PPROM currently exist (Ananth and Vintzileos 2006) (Miyazaki et al. 2012) and the pathophysiology is unclear, infection as well as genetic susceptibility are thought to play a role (Parry and Strauss 1998; Shen et al. 2008).
We have shown that mice deficient in biglycan and decorin, two highly homologous extracellular matrix proteoglycans, deliver their pups prematurely and display fetal membrane abnormalities in morphology and signaling (Calmus et al. 2011; Wu et al. 2014). Biglycan and decorin are members of the small leucine-rich proteoglycan (SLRP) family (Iozzo 1999; Iozzo 2011; Iozzo and Murdoch 1996) and are the most abundant proteoglycans expressed in human fetal membranes (Gogiel et al. 2003; Meinert et al. 2001; Valiyaveettil et al. 2004). Biglycan and decorin are involved in a number of biological processes including tumor angiogenesis and fibrosis (Neill et al. 2013; Neill et al. 2012a; Neill et al. 2012b), cancer growth (Iozzo and Cohen 1993; Reed et al. 2005; Sofeu Feugaing et al. 2013), stem cell biology (Berendsen et al. 2011; Bi et al. 2005; Ichii et al. 2012), myogenesis (Brandan and Gutierrez 2013) and osteoarthritis and osteoporosis (Ameye et al. 2002; Ameye and Young 2002; Nikitovic et al. 2012). Furthermore, biglycan and decorin are involved in collagen fibrillogenesis and contribute to the mechanical properties of connective tissues (Chen et al. 2011; Reed and Iozzo 2002; Zhang et al. 2009). The absence of these proteoglycans leads to a decrease in connective tissue tensile strength (Corsi et al. 2002). Humans with abnormal post-translational processing of biglycan and decorin display a subtype of Ehlers-Danlos syndrome, a syndrome associated with a decrease in tensile strength of connective tissues as well as an increased risk of PPROM (Barabas 1966; Quentin et al. 1990). Furthermore, we have shown that decorin is dysregulated in human fetal membranes with PPROM (Horgan et al. 2014).
Beyond their structural roles, both biglycan and decorin have been implicated in a number of signaling pathways, including the TGF-β pathway. TGF-β signals via Smads (Liu et al. 1996), transcription factors that play a role in the modulation of the extracellular matrix. Both biglycan and decorin are regulated by TGF-β (Bassols and Massague 1988) and decorin modulates TGF-β activity via negative feedback mechanisms (Yamaguchi et al. 1990). Smad-2 and -3 regulate downstream gene expression of tissue inhibitors of matrix metalloproteinases (TIMPs) and collagens (Verrecchia et al. 2001), proteins that in turn modulate the mechanical stability of the fetal membrane extracellular matrix. Matrix metalloproteinases (MMPs) are linked to the pathogenesis of PPROM through the degradation of fetal membrane extracellular matrix proteoglycans and collagens (Ferrand et al. 2002). Furthermore, mutations in matrix metalloproteinase genes (Fujimoto et al. 2002) as well as collagen genes (Anum et al. 2009) have been identified as possible predisposing factors to PPROM. Additionally, proteoglycans interact with MMPs. For example, decorin induces the expression of MMP-1 (Huttenlocher et al. 1996). Importantly, we have shown that biglycan and decorin modulate the TGF-β-SMAD-MMP-TIMP-collagen pathway in fetal membranes in a gestational age dependent manner (Wu et al. 2014).
Substantial clinical data suggest that infection is a significant risk factor for PPROM (Torricelli et al. 2013). When exposed to an infectious insult, term human fetal membranes display increased MMP expression (Estrada-Gutierrez et al. 2010). We have shown a link between infection and decorin expression in human fetal membranes with PPROM (Horgan et al. 2014). Coupled with studies indicating that biglycan and decorin both play a role in inflammatory cascades (Babelova et al. 2009; Merline et al. 2011), these observations suggest that infection may significantly impact the biglycan and decorin-dependent signaling pathways that modulate fetal membrane mechanical properties.
However, while we know that mutations leading to abnormal processing of biglycan and decorin (Barabas 1966; Quentin et al. 1990) as well as infection (Torricelli et al. 2013) play a role in the pathogenesis of PPROM, the mechanism by which infection modulates the function of biglycan and decorin and their dependent signaling pathways in the fetal membranes is not known.
2. Results
2.1. In the setting of inflammation, latency to preterm birth as well as live birth is decreased in the absence of biglycan and decorin
In order to assess the role that biglycan and decorin play in the maintenance of gestation to term in the setting of inflammation, we first examined the natural history of mouse gestation after the injection of E. coli intraperitoneally into pregnant mice at embryonic day 15. We found that the period between injection of live E. coli and preterm birth is significantly decreased in the Bgn+/−;Dcn−/− and Bgn−/−;Dcn+/− nulls compared to the wild-type E. coli injected mice as well as compared to the PBS injected Bgn+/−;Dcn−/− and Bgn−/−;Dcn+/− null mice (Figure 1A) (P=0.001). Furthermore, while the incidence of live pup birth is significantly decreased in the E. coli injected wild-type dams compared to the PBS injected wild-type dams, it decreased to 0 in the E. coli injected Bgn+/−;Dcn−/− and Bgn−/−;Dcn+/− mice (Figure 1B) (P=0.004). For comparison with the wild-type, we chose mice that have only one of four possible SLRP genes (two biglycan and two decorin). These mice are more affected than the biglycan or decorin single nulls but are less severely affected than the double nulls (Bgn−/−;Dcn−/−). The Bgn−/−;Dcn−/− mice delivered their pups extremely prematurely even without the additional environmental insult of an E. coli injection, and are thus not suitable for this experimental design. While there was no statistically significant difference in the latency between the E. coli exposed and the saline exposed wild-type mice, the standard deviation was larger in the E. coli exposed mice, demonstrating a range of latencies compared to the saline exposed mice from decreased latency to increased latency with dystocia.
Figure 1.
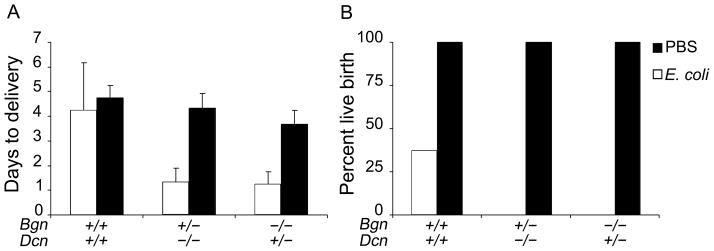
[A] Days to delivery are decreased in the Bgn+/−;Dcn−/− and Bgn−/−;Dcn+/− dams injected with E. coli compared to both the same genotype injected with PBS and the wild-type regardless of injection category (p=0.001 via ANOVA). Error bars = SD. [B] While the percentage of live pup births is decreased in the wild-type injected with E. coli compared to PBS injected wild-type dams, it is decreased to zero in the Bgn+/−;Dcn−/− and Bgn−/−;Dcn+/− dams injected with E. coli (p=0.004 via χ2). Gestational age embryonic day 15. n=3–8 per condition. Bgn=biglycan; Dcn=decorin.
2.2. Biglycan and decorin compensate for each other in fetal membrane transcription in the absence of inflammation
Next, we investigated whether biglycan and decorin would exhibit compensatory upregulation of gene expression in fetal membranes depending on exposure to the environmental insult of inflammation. We observed upregulation of biglycan transcript in decorin null fetal membranes in the absence of inflammation (PBS injected mice) (P=0.05), and similarly, upregulation of decorin transcript in biglycan null fetal membranes in the absence of inflammation (P=0.025)(Figure 2A and 2B).
Figure 2.
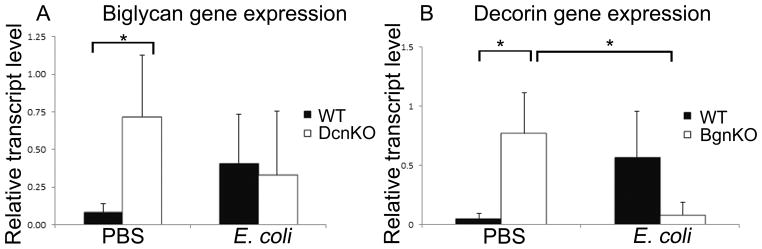
Biglycan and decorin compensate for each other in fetal membranes in the absence of inflammation, but not in the presence of inflammation. [A] Biglycan gene expression is increased in the PBS injected decorin null fetal membranes compared to wild-type (p=0.02); this compensatory increase does not occur in the setting of inflammation [B] Decorin gene expression is increased in the PBS-injected biglycan null fetal membranes compared to wild-type (p=0.006); this compensatory increase does not occur in the setting of inflammation, but decreased decorin gene expression occurs in the E. coli injected biglycan null fetal membranes compared to the PBS injected biglycan null fetal membranes (p=0.009). n=4 per genotype and condition. Error bars = SD. BgnKO=biglycan null; DcnKO=decorin null; WT=wild-type; PBS=phosphate buffered saline. Student’s t-test was performed for each set of data.
2.3. Biglycan and decorin compensatory transcription increase does not occur in inflammation, while decorin transcription is decreased in the E. coli injected mice compared to the PBS injected mice
Interestingly, the compensatory mechanisms between biglycan and decorin in the fetal membranes that are present in the absence of inflammation are modified by the presence of inflammation. In the E. coli injected fetal membranes, biglycan transcription remained unchanged in the decorin null samples compared to the wild-type samples, thus departing from the compensatory increase that it displayed in the absence of inflammation. Decorin transcription also displayed a lack of compensatory upregulation in the biglycan null in the setting of inflammation. Additionally, in the absence of biglycan, decorin transcription decreased in the E. coli injected mice compared to the PBS injected mice (P=0.01) (Figure 2A and 2B).
2.4. MMP-8 decreases in the wild-type and biglycan null fetal membranes in the setting of inflammation, while it remains unchanged in the decorin null
Next, we assessed the expression of the matrix metalloproteinases that play a significant role in fetal membrane rupture and PPROM. We found that inflammation leads to a decrease in MMP-8 expression in both the wild-type (P=0.03) and the biglycan null (P=0.05), but not in the decorin null, in which it remained unchanged irrespective of the presence of inflammation (Figure 3). MMP-9 expression was unchanged (data not shown).
Figure 3.
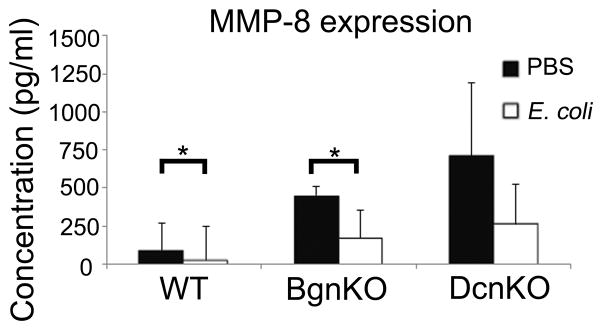
MMP-8 expression is decreased in fetal membranes of E. coli injected mice in the wild-type as well as the biglycan null mouse but not in the decorin null mouse. n=4 per genotype and condition (p=0.03 and 0.028). Error bars = SD. BgnKO=biglycan null; DcnKO=decorin null; WT=wild-type. Student’s t-test was performed for each set of data.
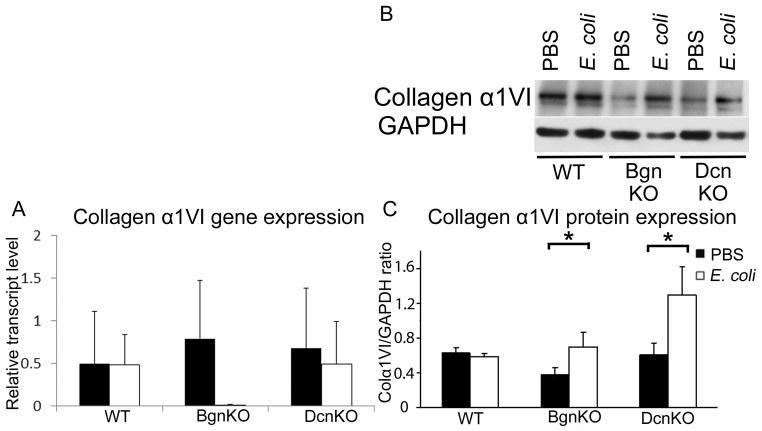
2.5. Collagen α1VI gene expression is unchanged in the setting of inflammation in the absence of biglycan or decorin, while collagen α1VI protein levels are increased
Given the role that collagens play in the maintenance of connective tissue mechanical strength and integrity, we next examined the biglycan- and decorin-dependent expression of collagen α1VI, a biglycan and decorin binding collagen, in inflammation. In the absence or presence of both biglycan and decorin, there was no change in collagen α1VI gene transcription levels (Figure 4A). However, in the absence of either biglycan (P=0.05) or decorin (P=0.03), collagen α1VI protein expression increased in the setting of inflammation compared to non-inflammation controls (Figure 4B and C).
Figure 4.
In the setting of inflammation, collagen α1VI protein expression is increased in the biglycan null and decorin null fetal membranes but not in the wild-type, while gene expression is unchanged. [A] Collagen α1VI gene expression is unchanged in the biglycan null and decorin null with or without inflammation compared to the wild-type. [B] Representative Western blots and [C] Summary data demonstrating increased collagen α1VI protein expression in the biglycan null and decorin null fetal membranes but not in the wild-type in the setting of inflammation (P=0.05 and 0.03). n=4 per genotype and condition. Error bars = SD. BgnKO=biglycan null; DcnKO=decorin null; WT=wild-type. Student’s t-test was performed for each set of data.
In summary, these results demonstrate that inflammation exacerbates the gestational phenotype of the biglycan/decorin null mouse and leads to abnormalities of transcriptional compensation between biglycan and decorin as well as leading to translational and transcriptional changes in downstream targets that play a role in fetal membrane stability.
3. Discussion
Biglycan and decorin are necessary for the maintenance of gestation to full term in a gene-dose dependent and compensatory manner (Calmus et al. 2011), while biglycan/decorin null fetal membranes display abnormal morphology (Wu et al. 2014). In this study, we show that biglycan and decorin play a role in the maintenance of gestation in the setting of inflammation as well as in the fetal membrane response to inflammation.
Others have shown that inflammation leads to preterm birth in the mouse model (Hirsch and Wang 2005; Reznikov et al. 1999) and is associated with PPROM in humans (Bendon et al. 1999). Our study has demonstrated that the superimposition of an environmental insult, in this case inflammation, on a genetic defect, leads to an exacerbation of the preterm birth phenotype compared to the preterm birth phenotype in the setting of an environmental insult without a genetic mutation. Furthermore, our study of the role of fetal membrane biglycan and decorin in the context of inflammation has demonstrated important mechanistic insight into the role of these proteoglycans in the pathophysiology of PPROM.
Our data indicate that biglycan and decorin compensate for each other in the fetal membranes by increasing transcription in a compensatory manner in the absence of inflammation. This compensatory upregulation of biglycan and decorin is similar to observations in multiple tissues including cornea, kidney, skin, bone and muscle (Ameye and Young 2002; Corsi et al. 2002; Zhang et al. 2009) and to our observations in the placenta as well as functionally in vivo during gestation (Calmus et al. 2011). We have not observed similar upregulation of biglycan protein expression in decorin-null fetal membranes and vice versa (Calmus et al. 2011). This may be secondary to protein compensation not being present in the fetal membranes or it may be because compensatory changes are present but not readily apparent with the techniques we utilized given the abundance of both SLRPs in fetal membranes. Interestingly, this ability to compensate for each other is disrupted by the presence of inflammation. On the other hand, when inflammation occurs in a wild-type genetic background, inflammation fails to evoke biglycan or decorin expression. Thus, it is likely that the underlying etiology for the decreased latency to preterm birth in the Bgn+/−;Dcn−/− and Bgn−/−;Dcn+/− mice is a result of a two hit hypothesis, in which a genetic predisposition is aggravated by an environmental factor. Specifically, genetic susceptibility is compensated for, but the compensation is then tempered by an environmental hit that deranges the compensation. This observation mirrors clinical observations in patients with Ehlers-Danlos syndrome, who display a significantly elevated incidence of preterm birth from PPROM compared to their unaffected siblings (Barabas 1966; Yen et al. 2006). The progeroid variant of Ehlers-Danlos syndrome displays a mutation leading to the secretion of abnormal, non-glycosylated biglycan and decorin (Quentin et al. 1990). Further support is lent to the concept of PPROM via connective tissue genetic abnormalities exacerbated by inflammation by the fact that women with a history of multiple PPROM have undiagnosed connective tissue anomalies similar to Ehlers-Danlos syndrome (Hermanns-Le et al. 2005).
One caveat to these observations is that the mice used for the in vivo E. coli injection experiments are heterozygous/homozygous and homozygous/heterozygous biglycan/decorin nulls (Bgn+/−;Dcn−/− and Bgn−/−;Dcn+/−). However, based on our prior data (Calmus et al. 2011), it is likely that our observations are a similar but tempered response compared to the homozygous/homozygous double nulls (Bgn−/−;Dcn−/−), which have too severe a phenotype to lend themselves to these types of experiments. At the same time, these observations are likely an exaggerated response compared to the single nulls (Bgn−/− or Dcn−/−), on which we have performed the in vitro component of this study.
When both biglycan and decorin are present in fetal membranes, inflammation leads to a decrease in MMP-8 protein expression, perhaps as a protective mechanism to counteract the membrane destructive/remodeling effects. Alternately, MMP-8 could be transcribed at the typical rate but secondary to inflammation there is higher turnover as it is being used to degrade the fetal membranes. In the absence of biglycan, MMP-8 protein expression is decreased in a manner similar to the wild-type. In the decorin null, on the other hand, MMP-8 protein expression is unchanged. Thus, the expected MMP-8 decrease in the presence of inflammation does not occur in the absence of decorin, but does occur in the absence of biglycan, indicating a role for decorin in the MMP-8 signaling pathway under inflammatory circumstances. Similarly, exogenous decorin gain-of-function experiments confirm that decorin regulates components of this pathway (Wu et al. 2014). These findings are similar to our previous findings, in which MMP-8 and MMP-9 gene and protein expression, as well as MMP-13 gene transcription increases in fetal membranes close to term (embryonic day 18) in the absence of decorin, but not in the absence of biglycan (Wu et al. 2014), suggesting that decorin is a membrane stabilizing factor. Furthermore, decorin suppresses the expression and activity of two MMPs associated with PPROM, MMP-2 and MMP-9 (Neill et al. 2012a). Also, decorin decreases prior to parturition as ozen in liquid nitrogen and stored at hile biglycan increases (Meinert et al. 2007). Interestingly, however, decorin induces the synthesis of a number of MMPs, including MMP-1, -2 and -14 (Schonherr et al. 2001) and during early gestation (embryonic day 12), the absence of decorin leads to a decrease in MMP-8 and -9 protein expression in fetal membranes. This suggests a role for decorin in extracellular matrix remodeling in early gestation as opposed to its membrane stabilizing role in late gestation and in inflammation.
Our data indicate that biglycan and decorin are both necessary for the maintenance of appropriate collagen α1VI levels in the setting of inflammation. Gene transcription of collagen α1VI is unchanged with inflammation or in the absence of biglycan or decorin. Protein expression, however, increases in the absence of biglycan or decorin in the setting of inflammation. One possible explanation for the increase in collagen α1VI in the absence of both biglycan and decorin is compensation for the structural role of the absent proteoglycans. In the setting of two hits, a genetic defect as well as inflammation, the increase in collagen α1VI expression may be a protective strategy to stabilize the fetal membranes. Decreased decorin in the kidney leads to increased accumulation of extracellular matrix with increased biglycan expression (Merline et al. 2009) and the absence of decorin leads to hepatic fibrosis (Baghy et al. 2011). Thus, alternately, the increase in collagen α1VI in the absence of these SLRPs may be a reflection of the anti-fibrotic role of these proteoglycans.
The diverse MMP-8 and collagen α1VI expression profiles in the absence of biglycan or decorin suggest that the two SLRPs signal via discrete pathways. Given that they compensate for each other, if they exerted their signaling via one common pathway, one would expect the MMP-8 and collagen α1VI levels to be unchanged between the wild-type and either null mouse. It is likely that compensation is not complete, coupled with some level of discrete functions. Biglycan and decorin play differential roles in mouse fetal membrane TGFβ signaling, leading to differential expression of the MMPs -8, -9 and -13, TIMPs -1, -2, -3 and -4, and collagen α1VI (Wu et al. 2014). Furthermore, the differences in expression and function may be related to the mid-pregnancy gestational age studied here, since biglycan and decorin play distinct signaling roles in a gestational age dependent manner (Wu et al. 2014).
Biglycan plays a role in inflammatory signaling as an inflammatory cascade activator via its role as a ligand for Toll-like receptors 2 and 4 (Babelova et al. 2009; Moreth et al. 2014; Zeng-Brouwers et al. 2014). Furthermore, decorin expression is evoked by septic inflammation, and decorin boosts inflammatory response in both sepsis and cancer (Merline et al. 2011). Thus, the role of biglycan and decorin in the maintenance of fetal membrane integrity in the setting of inflammation is likely to reflect complex interactions between their roles as connective tissue stabilizing proteins and their roles as extracellular matrix proinflammatory signaling molecules.
In summary, our findings suggest that biglycan and decorin compensate for each other in the fetal membranes but lose their ability to do so in the setting of inflammation, leading to decreased latency to preterm birth, and thus preterm birth earlier than inflammation alone would have led to. Furthermore, our findings suggest that biglycan and decorin play discrete roles in fetal membrane signaling pathways leading to changes in expression of MMP-8 and collagen α1VI, two components of the fetal membrane extracellular matrix that play a role in the pathophysiology of PPROM.
4. Materials and Methods
4.1. Mouse husbandry and genotyping
Wild-type mice (C3H) were purchased from Jackson Laboratories (Bar Harbor, ME). A Bgn−/− breeding pair (C3H) (generated by Marian Young (Xu et al. 1998)) was a gift from Justin Fallon. A Dcn+/− breeding pair (C57BL/6) was mated to the birth of homozygous null pups, which were then bred to establish the Dcn−/− colony. A Bgn−/− female was crossed with a Dcn−/− male to establish double heterozygous breeding pairs (Bgn+/−;Dcn+/− and Bgn−/0;Dcn+/−). These pairs were mated to breed Bgn+/−;Dcn−/− and Bgn−/−;Dcn+/− heterozygous-homozygous null pups. Breeding pairs were set up for mating at 5–7 weeks of age. Plugs were checked each morning, and the day of the plug was defined as embryonic day 0. Dams that were sacrificed at embryonic day 15 for tissue harvesting were sacrificed between 4 and 5 hours after E. coli or PBS injection. Approval was obtained from the Lifespan Institutional Animal Care and Use Committee. For genotyping, genomic DNA was extracted from each 3 mm tail biopsy sample using the High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany). Polymerase chain reaction (PCR) was performed using the Taq DNA Polymerase kit (New England Biolabs, Ipswich, MA) and the PTC-200 thermal cycler. The PCR product was run on a 1.8% w/v agarose gel to visualize the following diagnostic bands. The decorin PCR produced bands of 161 bp for the wild-type allele and 238 bp for the null allele. The biglycan PCR produced bands of 212 bp for the wild-type allele and 310 bp for the null allele.
4.2. Bacteria preparation
Previously frozen E. coli was inoculated on an agar plate overnight. The next morning one colony from the plate was incubated overnight in 10mL in Luria-Bertani (LB) broth (MP Biomedicals, Santa Ana, CA). The next morning, the culture was diluted 1:50 in fresh LB broth and incubated until an optical density of 0.5–0.8 was detected at 600nm, indicating the bacteria was in log phase growth. A more accurate bacterial concentration was determined post hoc by the plating of 106 serial dilutions in triplicate. Colony counts were performed on these plates the next morning. Colony forming units ranged from 3×106 to 1.4×107 CFU/ml.
4.3. Injection preparation
300mcl of the previously mentioned log phase bacterial broth was concentrated by centrifugation with the addition of 700mcl clean LB broth. The resulting pellet was washed with 1ml of sterile, room temperature phosphate buffered saline (PBS) and was concentrated again via centrifugation. The PBS was decanted and the pellet was re-suspended in 0.5ml of sterile, room temperature PBS. The resulting suspensions were drawn into 1ml tuberculin syringes and injected intraperitoneally into pregnant embryonic day 15 mice of the following genotypes: Bgn−/−;Dcn+/−, Bgn+/−;Dcn−/− and wild-type (Bgn+/+;Dcn+/+). Vehicle controls of 0.5ml sterile, room temperature PBS were also used. In order to assess preterm delivery rates, the mice were observed twice daily while health status and preterm deliveries were recorded. Health status was determined using an adaptation of the Mouse Sepsis Index scale (Biswas et al. 2002). The scale ranges from 5 for a normal healthy clinical status to 0 for death. Four is defined as slight illness with ruffled fur and lethargy, 3 is moderate illness with severe lethargy, ruffled fur and hunched back, 2 is severe illness with all signs of 3 plus exudative accumulation around the eyes, and 1 is a moribund state. Mice of both null genotypes injected with E. coli did not differ significantly from wild-type E. coli injected mice in their illness index. Nulls of both genotypes injected with E. coli had a mean score of 3.75 (SD 0.58) and wild-type mice injected with E. coli a mean score of 3.87 (SD 0.73) (P= 0.7).
Mice that were designated for tissue harvest were sacrificed 4 to 5 hours after E. coli or PBS injection on embryonic day 15.
4.4. Western blotting
Fetal membranes from each genotype and condition were dissected at embryonic day 15 between 4 and 5 hours after E. coli or PBS injection and frozen at −80°C Tissue samples were cut into small pieces and placed in 1.0 ml T-PER tissue protein extraction buffer (Pierce, Rockford, IL) with one tablet of proteinase inhibitor cocktail per 10 ml buffer (Roche, Basel, Switzerland). Tissue samples were then homogenized for 3×10s in an ice bath and kept on ice for 30 min. The homogenate was centrifuged at 10,000 × g at 4 °C for 8 min. The supernatant was collected and stored at −80°C until use. Total protein content was determined using the BCA assay (Pierce, Rockford, IL). 30 μg of total protein was loaded on duplicate 10% SDS-polyacrylamide gels and subsequently blotted to a polyvinylidene difluoride membrane. Following blocking in 5% milk in PBS-T buffer (PBS with 0.2% Tween 20) for 30 min, each blot was incubated overnight at 4°C with primary antibody. The blot was then washed with PBS-T buffer briefly and incubated with secondary antibody for 40 min. The Super-Signal West Pico chemiluminescent substrate kit (Pierce, Rockford, IL) was used prior to development of the blot membrane. The bands were compared with protein markers of known molecular size run in parallel on the same SDS-polyacrylamide gel (Bio-Rad, Hercules, CA). The membrane was stripped using Restore Western Blot stripping buffer (Thermo Scientific, Waltham, MA) for 10 min and blocked with 5% milk PBS-T for 20 min, then re-probed with anti-GAPDH antibody at a 1:800 dilution (Santa Cruz Biotech, Santa Cruz, CA) as an internal standard. Immunoblots were digitally scanned and densitometrically analyzed using Gel-Pro Analyzer (Media Cybernetics, Bethesda, MDRelative optical density (specific protein band to GAPDH) was normalized to the wild-type with PBS injection band. The experiment was repeated with three individual sets of samples.
4.5. Antibodies
The following antibodies were used: rabbit polyclonal anti-COLα1VI (sc-20649, dilution 1:800) and mouse monoclonal anti-GAPDH (sc-137179, dilution 1:1000) (Santa Cruz Biotech, Santa Cruz, CA). Secondary antibody labeling was performed with anti-mouse and anti-rabbit IgG horseradish peroxidase conjugate (Cell Signaling, Danvers, MA).
4.6. ELISA
Wild-type, Bgn−/− and Dcn−/− dams injected with PBS vehicle as wells as dams of each genotype injected with E. coli were dissected at embryonic day 15 between 4 and 5 hours after E. coli or PBS injection. Fetal membranes were collected and flash frozen in liquid nitrogen and isopentane and stored at −80°C. Four whole fetal membranes from each condition were used. Each fetal membrane sample was homogenized in 1mL of protein lysate buffer (2.5ml Triton X and one tablet of Roche Complete EDTA-free Protease Inhibitor filled to 50ml volume), flash frozen in liquid nitrogen and stored at −80°C until used. Commercially available ELISA kits (MMP-8 from Anaspec, Fremont, CA; MMP-9 from Abnova, Taipei City, Taiwan) were run in triplicate according to the manufacturer’s instructions. Plates were read on a Bio-Rad Laboratories Microplate Reader at 450nm.
4.7. RNA, cDNA preparation and quantitative PCR (qPCR)
Fetal membranes from wild-type, Bgn−/− and Dcn−/− dams injected with PBS vehicle as wells as dams of each genotype injected with E. coli were dissected at embryonic day 15 between 4 and 5 hours after E. coli or PBS injection in 0.1mol l−1 phosphate-buffered saline, pH 7.4, snap-frozen in liquid nitrogen and stored at −80°C. RNA extraction, genomic DNA removal and conversion to cDNA was performed as described previously (Wu et al. 2014). qPCR reactions were performed on the 7500 Fast Real-Time PCR System thermocycler (Applied Biosystems, Foster City, CA) using the SYBR-Green method (Invitrogen, Carlsbad, CA). Primers were designed using Primer-Blast primer design software (National Library of Medicine, Bethesda, MD). GAPDH was used as a normalizer. Melting point analysis of the product was performed to ensure the absence of alternative products or primer dimers. Data analysis was performed via the comparative Ct method with a validation experiment. A standard sample of RNA pooled from samples of wild-type as well as biglycan and decorin null fetal membranes was used in each qPCR experiment as a calibrator whose relative transcript level was defined as 1. qPCR analysis was performed in triplicate. n=3–4 dams per genotype and condition.
4.8 Primer sequences
qPCR
GAPDH forward: CTCACAATTTCCATCCCAGAC, reverse: TTTTTGGGTGCAGCGAAC; collagen α1VI forward: CTGGTGAAGGAGAACTATGCAG, reverse: GTCTAGCAGGATGGTGATGTC; biglycan forward: ATTGCCCTACCCAGAACTTGAC, reverse: GCAGAGTATGAACCCTTTCCTG; decorin forward: TTCCTACTCGGCTGTGAGTC, reverse: AAGTTGAATGGCAGAACGC.
Genotyping
Biglycan wild-type allele forward TGATGAGGAGGCTTCAGGTT, reverse GCAGTGTGGTGTCAGGTGAG; biglycan null allele forward TGTGGCTACTCACCTTGCTG, reverse GCCAGAGGCCACTTGTGTAG; decorin allele forward CCTTCTGGCACAAGTCTCTTGG, decorin wild-type allele reverse TCGAAGATGACACTGGCATCGG; decorin null allele reverse TGGATGTGGAATGTGTGCGAG.
All primers were purchased from Invitrogen (Carlsbad, CA).
4.9 Statistical analysis
Analysis of differences in levels of gene and protein expression between genotypes was performed using the Student’s t-test or, in the case of non-normally distributed data, the nonparametric Mann-Whitney Rank Sum test. ANOVA and χ2 analysis were performed for the in vivo experiments. Where appropriate, Bonferroni correction was performed for multiple comparisons using the Student’s t-test. Analysis was performed using SigmaPlot 11.0 (San Jose, CA).
Acknowledgments
Grant support: National Institute of Child Health and Human Development grant 1K08HD054676 (to BEL), an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM12345 (to BEL) and National Institutes of Health grants R01 CA39481 and R01 CA47282 (to RVI).
Abbreviations
- PPROM
preterm premature rupture of fetal membranes
- SLRP
small leucine-rich proteoglycan
- Bgn−/−
biglycan null
- Dcn−/−
decorin null
- TGFβ
transforming growth factor β
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitor of matrix metalloproteases
Footnotes
The authors have no conflicts of interest.
References
- Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. Faseb J. 2002;16(7):673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12(9):107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19(12):773–782. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- Anum EA, Hill LD, Pandya A, Strauss JF., 3rd Connective tissue and related disorders and preterm birth: clues to genes contributing to prematurity. Placenta. 2009;30(3):207–215. doi: 10.1016/j.placenta.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Grone HJ, Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284(36):24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghy K, Dezso K, Laszlo V, Fullar A, Peterfia B, Paku S, Nagy P, Schaff Z, Iozzo RV, Kovalszky I. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab Invest. 2011;91(3):439–451. doi: 10.1038/labinvest.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabas AP. Ehlers-Danlos syndrome: associated with prematurity and premature rupture of foetal membranes; possible increase in incidence. Br Med J. 1966;5515:682–684. doi: 10.1136/bmj.2.5515.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassols A, Massague J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988;263(6):3039–3045. [PubMed] [Google Scholar]
- Bendon RW, Faye-Petersen O, Pavlova Z, Qureshi F, Mercer B, Miodovnik M, Das AF, Meis PJ, Moawad AH, Iams JD, McNellis D. Fetal membrane histology in preterm premature rupture of membranes: comparison to controls, and between antibiotic and placebo treatment. The National Institute of Child Health and Human Development Maternal Fetal Medicine Units Network, Bethesda, MD, USA. Pediatr Dev Pathol. 1999;2(6):552–558. doi: 10.1007/s100249900161. [DOI] [PubMed] [Google Scholar]
- Berendsen AD, Fisher LW, Kilts TM, Owens RT, Robey PG, Gutkind JS, Young MF. Modulation of canonical Wnt signaling by the extracellular matrix component biglycan. Proc Natl Acad Sci U S A. 2011;108(41):17022–17027. doi: 10.1073/pnas.1110629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Stuelten CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG, Chen XD, Young MF. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280(34):30481–30489. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- Biswas B, Adhya S, Washart P, Paul B, Trostel AN, Powell B, Carlton R, Merril CR. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect Immun. 2002;70(1):204–210. doi: 10.1128/IAI.70.1.204-210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandan E, Gutierrez J. Role of skeletal muscle proteoglycans during myogenesis. Matrix Biol. 2013;32(6):289–297. doi: 10.1016/j.matbio.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Calmus ML, Macksoud EE, Tucker R, Iozzo RV, Lechner BE. A mouse model of spontaneous preterm birth based on the genetic ablation of biglycan and decorin. Reproduction. 2011;142(1):183–194. doi: 10.1530/REP-10-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sun M, Meng X, Iozzo RV, Kao WW, Birk DE. Pathophysiological mechanisms of autosomal dominant congenital stromal corneal dystrophy: C-terminal-truncated decorin results in abnormal matrix assembly and altered expression of small leucine-rich proteoglycans. Am J Pathol. 2011;179(5):2409–2419. doi: 10.1016/j.ajpath.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17(7):1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- Estrada-Gutierrez G, Gomez-Lopez N, Zaga-Clavellina V, Giono-Cerezo S, Espejel-Nunez A, Gonzalez-Jimenez MA, Espino y Sosa S, Olson DM, Vadillo-Ortega F. Interaction between pathogenic bacteria and intrauterine leukocytes triggers alternative molecular signaling cascades leading to labor in women. Infect Immun. 2010;78(11):4792–4799. doi: 10.1128/IAI.00522-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrand PE, Parry S, Sammel M, Macones GA, Kuivaniemi H, Romero R, Strauss JF., 3rd A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod. 2002;8(5):494–501. doi: 10.1093/molehr/8.5.494. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, Romero R, Strauss JF., 3rd A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002;277(8):6296–6302. doi: 10.1074/jbc.M107865200. [DOI] [PubMed] [Google Scholar]
- Gogiel T, Bankowski E, Jaworski S. Proteoglycans of Wharton’s jelly. Int J Biochem Cell Biol. 2003;35(10):1461–1469. doi: 10.1016/s1357-2725(03)00128-6. [DOI] [PubMed] [Google Scholar]
- Hermanns-Le T, Pierard G, Quatresooz P. Ehlers-Danlos-like dermal abnormalities in women with recurrent preterm premature rupture of fetal membranes. Am J Dermatopathol. 2005;27(5):407–410. doi: 10.1097/01.dad.0000175529.42615.42. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12(3):145–155. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Horgan CE, Roumimper H, Tucker R, Lechner BE. Altered Decorin and Smad Expression in Human Fetal Membranes in PPROM. Biol Reprod. 2014 doi: 10.1095/biolreprod.114.121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A, Werb Z, Tremble P, Huhtala P, Rosenberg L, Damsky CH. Decorin regulates collagenase gene expression in fibroblasts adhering to vitronectin. Matrix Biol. 1996;15(4):239–250. doi: 10.1016/s0945-053x(96)90115-8. [DOI] [PubMed] [Google Scholar]
- Ichii M, Frank MB, Iozzo RV, Kincade PW. The canonical Wnt pathway shapes niches supportive of hematopoietic stem/progenitor cells. Blood. 2012;119(7):1683–1692. doi: 10.1182/blood-2011-07-369199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274(27):18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Cohen I. Altered proteoglycan gene expression and the tumor stroma. Experientia. 1993;49(5):447–455. doi: 10.1007/BF01923588. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Goldoni S, Berendsen A, Young MF, editors. Small leucine-rich proteoglycans. Springer; 2011. pp. 197–231. [Google Scholar]
- Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10(5):598–614. [PubMed] [Google Scholar]
- Liu F, Hata A, Baker JC, Doody J, Carcamo J, Harland RM, Massague J. A human Mad protein acting as a BMP-regulated transcriptional activator. Nature. 1996;381(6583):620–623. doi: 10.1038/381620a0. [DOI] [PubMed] [Google Scholar]
- Meinert M, Eriksen GV, Petersen AC, Helmig RB, Laurent C, Uldbjerg N, Malmstrom A. Proteoglycans and hyaluronan in human fetal membranes. Am J Obstet Gynecol. 2001;184(4):679–685. doi: 10.1067/mob.2001.110294. [DOI] [PubMed] [Google Scholar]
- Meinert M, Malmstrom A, Tufvesson E, Westergren-Thorsson G, Petersen AC, Laurent C, Uldbjerg N, Eriksen GV. Labour induces increased concentrations of biglycan and hyaluronan in human fetal membranes. Placenta. 2007;28(5–6):482–486. doi: 10.1016/j.placenta.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Merline R, Lazaroski S, Babelova A, Tsalastra-Greul W, Pfeilschifter J, Schluter KD, Gunther A, Iozzo RV, Schaefer RM, Schaefer L. Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J Physiol Pharmacol. 2009;60(Suppl 4):5–13. [PMC free article] [PubMed] [Google Scholar]
- Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhao JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal. 2011;4(199):ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Furuhashi M, Yoshida K, Ishikawa K. Aggressive intervention of previable preterm premature rupture of membranes. Acta Obstet Gynecol Scand. 2012 doi: 10.1111/j.1600-0412.2012.01432.x. [DOI] [PubMed] [Google Scholar]
- Moreth K, Frey H, Hubo M, Zeng-Brouwers J, Nastase MV, Hsieh LT, Haceni R, Pfeilschifter J, Iozzo RV, Schaefer L. Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol. 2014 doi: 10.1016/j.matbio.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill T, Jones HR, Crane-Smith Z, Owens RT, Schaefer L, Iozzo RV. Decorin induces rapid secretion of thrombospondin-1 in basal breast carcinoma cells via inhibition of Ras homolog gene family, member A/Rho-associated coiled-coil containing protein kinase 1. FEBS J. 2013;280(10):2353–2368. doi: 10.1111/febs.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill T, Painter H, Buraschi S, Owens RT, Lisanti MP, Schaefer L, Iozzo RV. Decorin antagonizes the angiogenic network: concurrent inhibition of Met, hypoxia inducible factor 1alpha, vascular endothelial growth factor A, and induction of thrombospondin-1 and TIMP3. J Biol Chem. 2012a;287(8):5492–5506. doi: 10.1074/jbc.M111.283499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol. 2012b;181(2):380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitovic D, Aggelidakis J, Young MF, Iozzo RV, Karamanos NK, Tzanakakis GN. The biology of small leucine-rich proteoglycans in bone pathophysiology. J Biol Chem. 2012;287(41):33926–33933. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry S, Strauss JF., 3rd Premature rupture of the fetal membranes. N Engl J Med. 1998;338(10):663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- Quentin E, Gladen A, Roden L, Kresse H. A genetic defect in the biosynthesis of dermatan sulfate proteoglycan: galactosyltransferase I deficiency in fibroblasts from a patient with a progeroid syndrome. Proc Natl Acad Sci U S A. 1990;87(4):1342–1346. doi: 10.1073/pnas.87.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19(4–5):249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- Reed CC, Waterhouse A, Kirby S, Kay P, Owens RT, McQuillan DJ, Iozzo RV. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24(6):1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- Reznikov LL, Fantuzzi G, Selzman CH, Shames BD, Barton HA, Bell H, McGregor JA, Dinarello CA. Utilization of endoscopic inoculation in a mouse model of intrauterine infection-induced preterm birth: role of interleukin 1beta. Biol Reprod. 1999;60(5):1231–1238. doi: 10.1095/biolreprod60.5.1231. [DOI] [PubMed] [Google Scholar]
- Schonherr E, Schaefer L, O’Connell BC, Kresse H. Matrix metalloproteinase expression by endothelial cells in collagen lattices changes during co-culture with fibroblasts and upon induction of decorin expression. J Cell Physiol. 2001;187(1):37–47. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Shen TT, DeFranco EA, Stamilio DM, Chang JJ, Muglia LJ. A population-based study of race-specific risk for placental abruption. BMC Pregnancy Childbirth. 2008;8:43. doi: 10.1186/1471-2393-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofeu Feugaing DD, Gotte M, Viola M. More than matrix: the multifaceted role of decorin in cancer. Eur J Cell Biol. 2013;92(1):1–11. doi: 10.1016/j.ejcb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Steer P. The epidemiology of preterm labour. Bjog. 2005;112(Suppl 1):1–3. doi: 10.1111/j.1471-0528.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- Torricelli M, Conti N, Galeazzi LR, Di Renzo GC, Petraglia F. Epidemiology of early pre-term delivery: relationship with clinical and histopathological infective parameters. J Obstet Gynaecol. 2013;33(2):140–143. doi: 10.3109/01443615.2012.743980. [DOI] [PubMed] [Google Scholar]
- Valiyaveettil M, Achur RN, Muthusamy A, Gowda DC. Characterization of chondroitin sulfate and dermatan sulfate proteoglycans of extracellular matrices of human umbilical cord blood vessels and Wharton’s jelly. Glycoconj J. 2004;21(6):361–375. doi: 10.1023/B:GLYC.0000046276.77147.b2. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276(20):17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Horgan CE, Carr O, Owens RT, Iozzo RV, Lechner BE. Biglycan and decorin differentially regulate signaling in the fetal membranes. Matrix Biol. 2014;35:266–275. doi: 10.1016/j.matbio.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20(1):78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yen JL, Lin SP, Chen MR, Niu DM. Clinical features of Ehlers-Danlos syndrome. J Formos Med Assoc. 2006;105(6):475–480. doi: 10.1016/S0929-6646(09)60187-X. [DOI] [PubMed] [Google Scholar]
- Zeng-Brouwers J, Beckmann J, Nastase MV, Iozzo RV, Schaefer L. De novo expression of circulating biglycan evokes an innate inflammatory tissue response via MyD88/TRIF pathways. Matrix Biol. 2014;35:132–142. doi: 10.1016/j.matbio.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284(13):8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]