Abstract
Neuroimaging studies have provided compelling evidence for abnormal hippocampal activity in schizophrenia. Most studies made inferences about baseline hippocampal activity using a single hemodynamic parameter (e.g., blood volume or blood flow). Here we studied several hemodynamic measures in the same cohort to test the hypothesis of increased hippocampal activity in schizophrenia. We used dynamic susceptibility contrast- (DSC-) magnetic resonance imaging to assess blood volume, blood flow, and mean transit time in the hippocampus of 15 patients with chronic schizophrenia and 15 healthy controls. Left and right hippocampal measurements were combined for absolute measures of cerebral blood volume (CBV), blood flow (CBF), and mean transit time (MTT). We found significantly increased hippocampal CBV, but normal CBF and MTT, in schizophrenia. The uncoupling of CBV and CBF could be due to several factors, including antipsychotic medication, loss of cerebral perfusion pressure, or angiogenesis. Further studies need to incorporate several complementary imaging modalities to better characterize hippocampal dysfunction in schizophrenia.
Keywords: Dynamic susceptibility contrast, DSC-MRI, Gadolinium, Mean transit time, CBV, CBF
1. Introduction
The hippocampus is abnormal in schizophrenia (Heckers and Konradi, 2010). Studies of the underlying cellular and molecular mechanisms have illustrated N-methyl-d-aspartate (NMDA) receptor hypofunction (Olney et al., 1999) and reduced gamma-aminobutyric acid-(GABA-) ergic interneuron density (Benes et al., 1998), especially in fast-spiking, parvalbumin-containing cells (Zhang and Reynolds, 2002; Konradi et al., 2011). These molecular changes may lead to excitation-inhibition imbalances (Lisman et al., 2008; Heckers and Konradi, 2014), which can be indirectly assessed through perfusion imaging methods. Initial positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies measured cerebral blood flow (CBF) or glucose metabolism (in the case of PET) or volume (CBV). These studies failed to achieve a general consensus: some reported increases in baseline hippocampal activity (Friston et al., 1992; Heckers et al., 1998; Malaspina et al., 2004), while others illustrated decreases (Buchsbaum et al., 1992; Tamminga et al., 1992; Nordahl et al., 1996) or no changes (Vita et al., 1995). Due to radiation exposure and poor spatial resolution, these methods are now used less frequently than comparable magnetic resonance imaging (MRI) methods (Small et al., 2011).
Recently implemented MRI-based methods require an endogenous (blood water) or exogenous (paramagnetic) contrast agent to generate CBV or CBF maps. Arterial spin labeling uses blood water magnetization as an endogenous contrast to analyze a single hemodynamic parameter, i.e., CBF, in the brain. This method has provided mixed results, finding increases (Pinkham et al., 2011), decreases (Scheef et al., 2010; Walther et al., 2011; Kindler et al., 2015), or no changes (Horn et al., 2009; Ota et al., 2014) in medial temporal lobe CBF in schizophrenia. More recently, contrast-enhanced, high resolution (submillimeter) steady-state imaging has been used to investigate CBV changes in schizophrenia. Though few in number, these initial studies support increased hippocampal Cornu Ammonis 1 (CA1) CBV (Schobel et al., 2009; Schobel et al., 2013; Talati et al., 2014a). Importantly, this method also characterizes a single hemodynamic parameter, i.e., CBV, to make inferences about basal metabolism. Dynamic susceptibility contrast- (DSC-) MRI may be able to resolve these mixed findings of hippocampal activity across different modalities due to its ability to assess several hemodynamic parameters, including CBV, CBF, and mean transit time (MTT) (Perkio et al., 2002).
Even though DSC-MRI has been used extensively to investigate metabolism and perfusion abnormalities in neurological disorders such as tumors and strokes, very few studies have implemented this technique to study psychiatric illnesses including schizophrenia (Renshaw et al., 1997). As recently reviewed by Théberge in 2008, only five articles have been published using this method in schizophrenia research (Théberge, 2008). Since the review, there have been two additional studies of bolus-tracking perfusion imaging with a paramagnetic agent in schizophrenia (Bellani et al., 2011; Peruzzo et al., 2011). Together, these studies illustrate several findings in schizophrenia: low and inverse hemispheric CBV as indirectly measured through contrast enhancement (CE) (Brambilla et al., 2007); increased CBV in the cerebellum (Loeber et al., 1999), caudate, and occipital cortex (Cohen et al., 1995); no alterations in CBV, CBF, or MTT in the cerebrum or cerebellum (Bellani et al., 2011); decreased frontal cortex CBV and CBF only when using a best predictor model containing clinical state, age, and length of illness (Peruzzo et al., 2011); variable time-to-peak (TTP) in the caudate (Fabene et al., 2007); and increased perfusion in the prefrontal cortex, temporal lobe, and posterior parietal cortices after dopamine receptor D1 agonist administration (Mu et al., 2007). Importantly, none of these studies used a region of interest-based analysis to report hemodynamic properties in the hippocampus in schizophrenia, even though functional abnormalities have been reported in this medial temporal lobe structure.
In this study, we used DSC-MRI to specifically study hippocampal perfusion properties (CBV, CBF, and MTT) in 15 patients with chronic schizophrenia and matched controls. Based on a previous report on this cohort using steady-state CBV imaging (Talati et al., 2014a), we hypothesized increased hippocampal perfusion, as measured by CBV and CBF.
2. Methods
2.1. Participants
Participants comprised 15 patients with schizophrenia or schizoaffective disorder (age range: 20-54 years) and 15 matched healthy controls (age range: 22-53 years), who provided informed consent in a manner approved by the Vanderbilt Institutional Board. Both groups were matched across several demographic variables, including age, race, and gender (Table 1). Subjects were recruited from the Vanderbilt Psychotic Disorders Program or the local community; they were paid for their participation. We used the Structured Clinical Interview for DSM-IV Axis I disorders [SCID (First, November 2002)] to establish all diagnoses and the Positive and Negative Syndrome Scale (Kay et al., 1987) to assess the clinical status of the patients. Most of the patients (12 of 15) were treated with antipsychotic medication, and their chlorpromazine-equivalent dosages (Gardner et al., 2010) are listed in Table 1. History of major neurological or medical illness was an exclusion criterion. Since nephrogenic systemic fibrosis is a rare adverse effect of gadolinium-containing contrast, we required a normal serum creatinine value for study eligibility and confirmed normal values again after study completion.
Table 1.
Subject demographics in individuals with schizophrenia and healthy controls
Groups are matched on age, gender, race, and subject and parental education. Values are reported as mean ± SD. The chlorpromazine (CPZ) equivalent doses could not be calculated for two subjects who were off medication at the time of the study and for one subject who was taking asenapine. PANSS denotes the Positive and Negative Syndrome Scale.
| Controls (n = 15) |
Schizophrenia (n = 15) |
Statistic | p-value | |
|---|---|---|---|---|
| Age (years) | 34.27 ± 9.06 | 36.20 ± 12.59 | t(25.43) = −0.483 | 0.63 |
| Males | 10 | 10 | Χ(1) = 0 | 1.00 |
| Race (W/B) | 11/4 | 10/5 | Χ(1) = 0.159 | 0.69 |
| Subject edu. (years) | 15.53 ± 2.77 | 14.20 ± 2.51 | t(28) = 1.38 | 0.18 |
| Avg. parental edu. (years) | 14.40 ± 1.91 | 14.19 ± 2.03 | t(28) = 0.30 | 0.77 |
| Duration of Illness (years) | 10.55 ± 8.86 | |||
| CPZ equivalent (mg/day) | 352.50 ± 160.35 | |||
| PANSS | Positive: 15.47 ± 7.10 | |||
| Negative: 17.47 ± 7.22 | ||||
| General: 28.07 ± 8.56 |
2.2. Dynamic susceptibility contrast imaging
Imaging was performed using a Phillips 3T Achieva scanner with an eight-channel SENSE head coil. T2*-weighted echo planar images were acquired perpendicular to the long axis of the hippocampus for accurate segmentation using the following sequence parameters: repetition time (TR) = 1600 ms, echo time (TE) = 54 ms, Field-of-View (FOV) = 240 × 240 mm2, spatial resolution: 1.5 mm3 isotropic, slices = 15, dynamics = 120. Fourteen dynamic scans were carried out before gadopentetate dimeglumine (Magnevist®, Bayer Schering Pharma, Germany, 0.1 mmol/kg) was injected at a constant rate of 5 ml/s through an 18G intravenous catheter in the antecubital vein via an MRI-compatible power injector (Medrad®, PA, USA). The bolus of contrast was immediately followed by a 40-ml saline flush at the same rate. The intravenous catheter was removed after the completion of the scan. To allow for accurate segmentation of the hippocampal formation (Moreno et al., 2007), high-resolution T1-weighted pre-contrast images were also acquired perpendicular to the long axis of the hippocampus using the following scan parameters: TR = 20 ms, TE = 3.41 ms, FOV = 256 × 256 mm2, slices = 15.
2.3. Analysis
2.3.1. CBV, CBF, and MTT calculations
AFNI (3dvolreg) (Cox, 1996) was used to correct for subject motion during the dynamic scans, and all images were registered to the first dynamic scan. First, voxel-wise signal contrast variations due to injection of gadolinium were converted to a concentration-time curve using Equation 1 (Ostergaard, 2004):
| (Eq. 1) |
where ΔR2* is the change in transverse relaxation rate (i.e., 1/T2*); TE is the echo time; S(t) is the signal intensity at a time t; and S0 is the baseline signal intensity at a voxel, calculated as the mean signal intensity at that voxel over the first 10 dynamic scans before contrast administration. Voxels located near the M1 segment of the middle cerebral artery were used to evaluate the arterial input function (AIF). Care was taken to place the voxels adjacent to the vessel to avoid susceptibility effects and signal cutoff as outlined by (van Osch et al., 2005). CBV, CBF, and MTT were calculated using singular value decomposition (SVD) with block circulant matrices (Ostergaard et al., 1996a; Ostergaard et al., 1996b). The threshold for the diagonal matrix generated using SVD was set to 0.15 to reduce oscillations of the derived tissue residue function. This threshold was chosen based on the high resolution and low signal-to-noise ratio of the images (SNR = 7.60±2.02). Regional CBF value was calculated as the peak value of the deconvolved tissue impulse response. Regional CBV was the ratio of the area under the tissue ΔR2* curve to the area under the AIF ΔR2* curve. Mean transit time (MTT) was calculated using Equation 2:
| (Eq. 2) |
Finally, a normal appearing white matter region of interest was chosen in the parietal region for each subject. Assuming a white matter CBF of 22 ml/100 g/min and a CBV of 2 ml/100 g (Ostergaard et al., 1996a), the regional values were scaled to obtain absolute CBF and CBV measures. Matlab (version 7.13.0.564, The MathWorks Inc, Natick, MA) was used to generate an in-house script to obtain the CBV, CBF, and MTT values.
2.3.2. Hippocampal segmentation
The T1-weighted pre-contrast image was used for blind, manual segmentation of the hippocampal formation by one rater (PT) and verified by another rater (SR). If there were any discrepancies, the region of interest (ROI) was drawn by both raters until a consensus was reached. Manual segmentation of each subject’s hippocampus generated 15 hippocampal ROIs for each hemisphere for a total of 30 hippocampal ROIs (15 left and 15 right). Hippocampal ROIs were down-sampled to reach the same spatial resolution of the T2*-weighted images.
2.3.3. Statistics
CBF, CBV, and MTT values across all posterior hippocampal slices were averaged for each hemisphere for each subject. This method produced the following six values for each subject: left and right hippocampal CBV, left and right hippocampal CBF, and left and right hippocampal MTT.
Because there was no a priori hypothesis about hemispheric lateralization, we performed an exploratory analysis testing for hemisphere effects. A repeated-measures analysis of variance (ANOVA) with hemisphere as a within-subject factor and group as a between-subject factor illustrated no main effect of hemisphere and no hemisphere by diagnosis interaction for CBV (F(1,28) = 1.83, p = 0.19; F(1,28) = 0.62, p = 0.44, respectively), CBF (F(1,28) = 2.17, p = 0.15; F(1,28)=0.11, p = 0.74, respectively), and MTT (F(1,28) = 0.76, p = 0.39; F(1,28) = 0.56, p = 0.46, respectively). Therefore, the primary analysis for each hemodynamic parameter (CBV, CBF, and MTT) was a between-group independent samples t-test after collapsing across hemisphere (average left/right). Because groups were well matched, age, gender, and race were not included as covariates in the analysis. Correlation analyses are reported using Pearson’s correlation. Statistical analyses were performed using The Statistical Package for Social Sciences software (SPSS version 20, IBM Corporation, Armonk, NY: http://www.spss.com).
3. Results
Fig. 1 depicts the ΔR2* changes before, during, and after gadolinium-contrast administration. Injection with a gadolinium bolus causes predominant R2* shortening, resulting in a loss of signal. As the contrast agent recirculates, the bolus diffuses and equilibrates with the blood in the microvasculature, allowing the tissue R2* to recover to nearly its pre-contrast value (shown in Fig. 2).
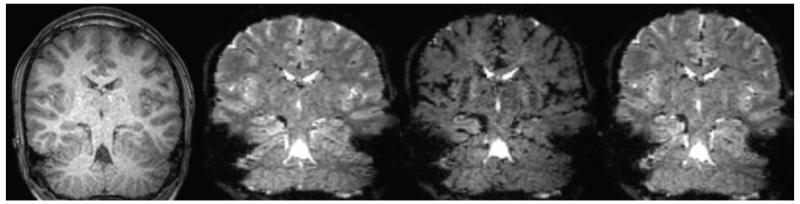
Fig. 1.
T1- and T2*-weighted dynamic scans in radiological orientation for a representative control subject with contrast-enhanced imaging.
A: T1-weighted structural image. B: T2*-weighted dynamic scan before contrast administration. C: Loss of signal intensity on T2*-weighted images during contrast administration. D: Recovery of signal intensity after contrast administration.
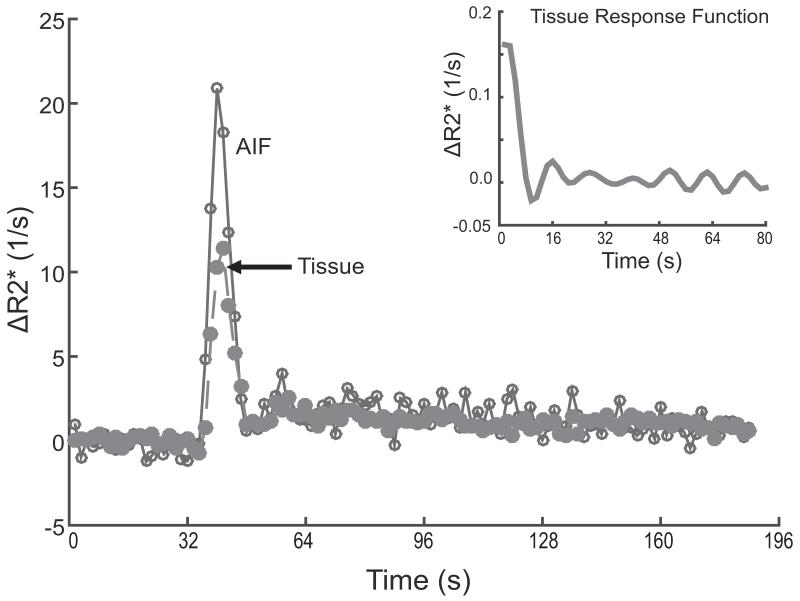
Fig. 2.
R2* signal recovery after gadolinium bolus passage in two different brain regions of a control subject
After a gadolinium bolus, R2* signal changes are evident across 120 dynamic scans (192 s) in select voxels near the M1 segment of the middle cerebral artery for the arterial input function (AIF, open circles connected by a solid line) and gray matter tissue (filled circles connected by a dashed line). The figure inset indicates the tissue response function obtained after deconvolution.
3.1. Hemodynamic imaging
Whole brain CBV-, CBF-, and MTT-weighted maps were generated for each subject (see Fig. 3) to extract hippocampal CBV, CBF, and MTT values. For healthy controls, hippocampal CBV (units = ml blood/100 g parenchyma) ranged from 2.69 to 5.32. Patients had a wider hippocampal CBV range (2.80 to 8.48). Hippocampal CBF (units = ml blood/100 g parenchyma per min) ranged from 27.75 to 76.71 in healthy controls and from 35.39 to 84.65 in patients. Finally, healthy control hippocampal MTT (units = seconds) ranged from 3.28 to 7.30 and patient hippocampal MTT ranged from 4.09 to 8.14. These values are similar to those previously published for medial temporal lobe CBF (Li et al., 2013) and CBV (Schobel et al., 2009; Talati et al., 2014b) and comparable to contrast-enhanced steady-state CBV values in the same cohort (Talati et al., 2014a).
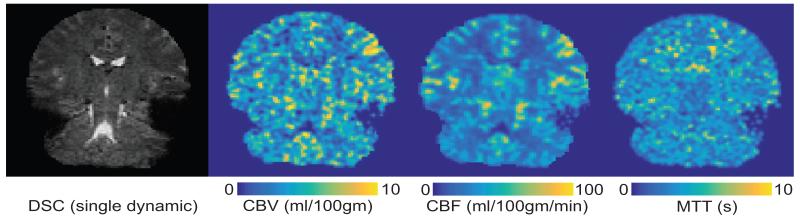
Fig. 3.
Hemodynamic maps for a representative control subject in radiological orientation
A: T2*-weighted dynamic susceptibility contrast (DSC) dynamic scan. B-D: cerebral blood volume-(CBV-), cerebral blood flow-(CBF-), and mean transit time- (MTT-)weighted maps, respectively. Color bar denotes values for each hemodynamic map, with lighter colors corresponding to larger values.
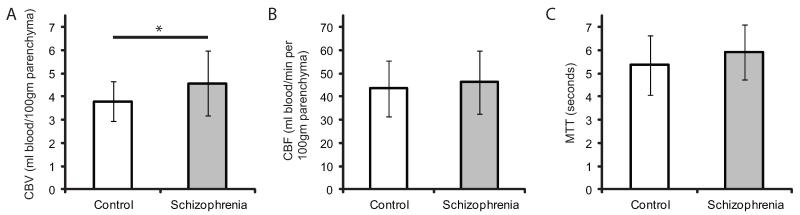
We first tested the hypothesis of increased hippocampal metabolism, as measured by CBV and CBF, in schizophrenia. Hippocampal CBV (mean ± SD) was increased in patients with schizophrenia compared with healthy controls (4.57 ± 1.41 vs. 3.79 ± 0.86, one-tailed t-test, t(28) = 1.83, p = 0.039, Cohen’s d = 0.67, Fig. 4A), when averaged across hemispheres. However, there were no group differences in CBF (one-tailed t-test, t(28) = −0.63, p = 0.28, d = 0.21, Fig. 4B). We then investigated whether hippocampal MTT was altered in schizophrenia and found no differences (two-tailed t-test, t(28) = −1.23, p = 0.22, d = 0.46), as illustrated in Fig. 4C.
Fig. 4.
Increased hippocampal blood volume, but not flow, in schizophrenia
Hemodynamic parameters (mean ± SD) for healthy controls and patients with schizophrenia generated from dynamic susceptibility contrast-(DSC)-MRI for the hippocampus. These values are averaged across hemisphere and slice along the long axis of the hippocampus. Fig. 4A illustrates increased hippocampal cerebral blood volume (CBV) in schizophrenia (one-tailed t-test, t(28) = 1.831, p = 0.039). Fig. 4B illustrates no group differences in hippocampal cerebral blood flow (CBF, one-tailed t-test, t(28) = −0.629, p = 0.28), and Fig. 4C illustrates no difference in hippocampal mean transit time (MTT) in schizophrenia (two-tailed t-test, t(28) = −1.230, p = 0.22). *p < 0.05.
We explored whether increased CBV in schizophrenia correlated with duration of illness, dose of antipsychotic medication, or psychopathology as assessed by the Positive and Negative Syndrome Scale (PANSS). Hippocampal CBV did not correlate with duration of illness (r = −0.28, p = 0.31), positive symptoms (r = −0.13, p = 0.65), negative symptoms (r = −0.43, p = 0.11), or general psychopathology (r = 0.001, p = 1.00) in the patient group. Three patients’ chlorpromazine (CPZ) equivalent dosages could not be calculated because there was either no chlorpromazine equivalent dose (n=1) or they were not taking any medication at the time of the study (n=2). For the remaining 12 patients, antipsychotic medication dose did not correlate with hippocampal CBV (r = 0.43, p = 0.16).
4. Discussion
This is the first study to use DSC-MRI to study hippocampal perfusion in schizophrenia. We report increased hippocampal CBV in chronic schizophrenia, similar to our previous study using steady-state imaging in the same cohort (Talati et al., 2014a). However, we did not find increased hippocampal CBF or MTT in schizophrenia.
Because of the well-established relationship between CBV and CBF (Grubb et al., 1974), many studies report CBV or CBF changes as a marker of basal activity. Here we report a novel finding in schizophrenia: increased hippocampal blood volume in the context of normal hippocampal blood flow. Our finding of increased hippocampal CBV is consistent with other gadolinium-enhanced studies (Schobel et al., 2009; Schobel et al., 2013). We did not observe increased hippocampal CBF using DSC-MRI, similar to what has been reported using arterial spin labeling (Horn et al., 2009; Ota et al., 2014) and nuclear medicine techniques (Vita et al., 1995). Even with the large variance in our dataset, the calculated effect sizes are larger for CBV (d = 0.67) than CBF (d = 0.21), suggesting that CBF changes may be more difficult to detect.
There might be several reasons for the finding of increased CBV in the context of normal CBF. First, previous studies have shown that antipsychotic medications do not affect hippocampal CBV (Schobel et al., 2009; Schobel et al., 2013; Talati et al., 2014a) but that they lower CBF in many brain regions (Miller et al., 1997; Novak et al., 2005; Ertugrul et al., 2009), including the hippocampus (Medoff et al., 2001; Lahti et al., 2003). Second, hippocampal excitation-inhibition imbalances (Lisman et al., 2008; Heckers and Konradi, 2014) in the prodromal or early stage of psychosis may lead to increased CBF to meet greater hippocampal metabolic demand. Increased CBF demands are mediated by increases in CBV via complex myogenic, chemical, neuronal, or metabolic autoregulatory mechanisms. Such CBV-CBF coupling occurs only when cerebral perfusion pressure remains unchanged. Therefore, a lack of CBF increase is likely due to a reduction in the cerebral perfusion pressure (Heilbrun et al., 1972; Chan et al., 1992). Finally, instead of microvascular dilation, increased CBV can be secondary to angiogenesis (Swain et al., 2003), which could be assessed via hemodynamic response imaging (i.e., carbogen challenge (95% O2+5% CO2) that vasodilates all functional blood vessels and a hypercapnic challenge (95% air+5% CO2) that only vasodilates mature blood vessels (Ben Bashat et al., 2012).
A major goal in schizophrenia research is to link brain function with clinical features of the disease. Many perfusion imaging studies have quantified a single hemodynamic index (e.g., CBV or CBF) and correlated abnormal perfusion with psychopathology. For example, increased medial temporal lobe CBF has been associated with more severe general psychopathology (Friston et al., 1992) and positive symptoms (Liddle et al., 1992; Lahti et al., 2006), while increased hippocampal CBV has been associated with positive and negative symptoms (Schobel et al., 2009; for review, see Tregellas, 2014). Furthermore, increased CBF in the superior temporal gyrus predicted a reduction of auditory hallucinations after treatment with transcranial magnetic stimulation (Homan et al., 2012), suggesting that decreased perfusion in this region may predict which patients are likely to respond to this treatment. However, not all studies using CBV or CBF have supported correlations between brain function and positive/negative symptoms (Kawasaki et al., 1992; Talati et al., 2014a).
There are several assumptions that commonly underlie the DSC-MRI data analysis (Calamante et al., 2002). First, there is an assumption of linearity between tissue contrast agent concentration and changes in the R2* relaxation rate (Equation 1). This assumption has been validated (Boxerman et al., 1995; Simonsen et al., 1999) and likely holds true. Second, the arterial input function is presumed to represent the exact input to the tissue of interest (in this case, the hippocampus). The hippocampus has a differential blood supply, with the anterior region supplied by anterior hippocampal branch of the posterior cerebral artery (PCA) and anterior choroidal artery and the posterior region supplied by the middle and posterior hippocampal branches of the PCA (Huang and Okudera, 1997). In practice, however, the M1 segment of the MCA is used and has generated reliable CBF values when compared with PET studies (Zaro-Weber et al., 2012). Furthermore, we manually selected the AIF to avoid partial volume effects that can lead to under- or overestimation of the contrast agent concentration (van Osch et al., 2005). Other assumptions include constant capillary hematocrit and vascular permeability across brain regions and between subjects, which are not necessarily true.
The ideal imaging experiment to interrogate hippocampal hyperactivity would utilize a reliable, non-invasive imaging technique to longitudinally follow high-risk individuals who eventually convert to psychosis. Arterial spin labeling (ASL) is one such non-invasive method that has been shown to be reliable within and between scanning sessions (Parkes et al., 2004; Petersen et al., 2010; Xu et al., 2010; Gevers et al., 2011; Donahue et al., 2014). Even though ASL is gaining popularity due to increased signal-to-noise ratio at higher magnetic fields (e.g. 3 T) and ease of implementation, there are some drawbacks to this method: the reproducibility is quite variable in different brain regions (Wu et al., 2014), it has worse spatial and temporal resolution than DSC-MRI (Wintermark et al., 2005), the validity of the reported values varies depending on disease conditions (Tanaka et al., 2011; Zaharchuk et al., 2012; Choi et al., 2013; Nael et al., 2013; Jiang et al., 2014; White et al., 2014), and it is unclear how antipsychotic medication affects CBF values. Newer variants of arterial spin labeling acquire data over multiple post-labeling delays to assess several hemodynamic parameters (e.g. CBV and CBF) and have been shown to correlate with DSC-MRI (Wang et al., 2013). However, the spatial resolution still may lead to some challenges in interpreting CBF and CBV values in small structures like the hippocampus.
This study has a few limitations. The sample size is small but consistent with other CBV imaging studies of schizophrenia patients (Schobel et al., 2009; Talati et al., 2014a). Most of our patients were treated with antipsychotic medication, which can affect hippocampal hemodynamic parameters such as CBF (see above). Furthermore, this method does not provide enough spatial resolution for subfield-specific investigation. While the in-plane resolution is 1.5 mm isotropic, the actual in-plane resolution is closer to 2 mm when considering the point-spread function degradation due to the paramagnetic bolus passage (Wintermark et al., 2005). Therefore, subfield-specific testing (Small et al., 2011) was not feasible in this dataset.
In conclusion, we report increased hippocampal CBV, but not CBF, in chronic schizophrenia. Future studies need to incorporate several complementary imaging modalities to better characterize hippocampal dysfunction in schizophrenia.
Hippocampal activity is abnormal in schizophrenia
CBV or CBF is typically reported to indirectly assess hippocampal activity
We used DSC-MRI to assess several hippocampal hemodynamic parameters
Hippocampal CBV, but not CBF or MTT, is increased in schizophrenia
Complementary imaging methods are needed to best understand hippocampal dysfunction
Acknowledgements
The authors thank S. Kristan Armstrong for help with subject recruitment. The study was supported by the following NIH grants: R01 MH070560 awarded to S.H; R01 NS078828 supported S.R.; F30 MH102846 and T32 GM07347 provided support to P.T. The project described was supported by the National Center for Research Resources, Grant UR1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UR1 TR000445-06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Pratik Talati and Swati Rane both contributed equally to the data collection, analysis, and manuscript preparation. Jack Skinner contributed with data analysis and manuscript revisions. Stephan Heckers and John Gore helped with project inception, funding, data interpretation, and manuscript revisions.
Conflict of interest
The authors disclose no conflict of interest.
References
- Bellani M, Peruzzo D, Isola M, Rambaldelli G, Perlini C, Baiano M, Cerini R, Andreone N, Barillari M, Mucelli RP, Balestrieri M, Tansella M, Bertoldo A, Brambilla P. Cerebellar and lobar blood flow in schizophrenia: a perfusion weighted imaging study. Psychiatry Research: Neuroimaging. 2011;193:46–52. doi: 10.1016/j.pscychresns.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, Artzi M, Ben Ami H, Aizenstein O, Blumenthal DT, Bokstein F, Corn BW, Ram Z, Kanner AA, Lifschitz-Mercer B, Solar I, Kolatt T, Palmon M, Edrei Y, Abramovitch R. Hemodynamic response imaging: a potential tool for the assessment of angiogenesis in brain tumors. PloS One. 2012;7:e49416. doi: 10.1371/journal.pone.0049416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biological Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magnetic Resonance in Medicine. 1995;34:555–566. doi: 10.1002/mrm.1910340412. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Cerini R, Fabene PF, Andreone N, Rambaldelli G, Farace P, Versace A, Perlini C, Pelizza L, Gasparini A, Gatti R, Bellani M, Dusi N, Barbui C, Nose M, Tournikioti K, Sbarbati A, Tansella M. Assessment of cerebral blood volume in schizophrenia: a magnetic resonance imaging study. Journal of Psychiatric Research. 2007;41:502–510. doi: 10.1016/j.jpsychires.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Haier RJ, Potkin SG, Nuechterlein K, Bracha HS, Katz M, Lohr J, Wu J, Lottenberg S, Jerabek PA, Trenary M, Tafalla R, Reynolds C, Bunney WE., Jr. Frontostriatal disorder of cerebral metabolism in never-medicated schizophrenics. Archives of General Psychiatry. 1992;49:935–942. doi: 10.1001/archpsyc.1992.01820120023005. [DOI] [PubMed] [Google Scholar]
- Calamante F, Gadian DG, Connelly A. Quantification of perfusion using bolus tracking magnetic resonance imaging in stroke: assumptions, limitations, and potential implications for clinical use. Stroke. 2002;33:1146–1151. doi: 10.1161/01.str.0000014208.05597.33. [DOI] [PubMed] [Google Scholar]
- Chan KH, Miller JD, Dearden NM, Andrews PJ, Midgley S. The effect of changes in cerebral perfusion pressure upon middle cerebral artery blood flow velocity and jugular bulb venous oxygen saturation after severe brain injury. Journal of Neurosurgery. 1992;77:55–61. doi: 10.3171/jns.1992.77.1.0055. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Kim HS, Jahng GH, Kim SJ, Suh DC. Pseudoprogression in patients with glioblastoma: added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiologica. 2013;54:448–454. doi: 10.1177/0284185112474916. [DOI] [PubMed] [Google Scholar]
- Cohen BM, Yurgelun-Todd D, English CD, Renshaw PF. Abnormalities of regional distribution of cerebral vasculature in schizophrenia detected by dynamic susceptibility contrast MRI. The American Journal of Psychiatry. 1995;152:1801–1803. doi: 10.1176/ajp.152.12.1801. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Faraco CC, Strother MK, Chappell MA, Rane S, Dethrage LM, Hendrikse J, Siero JC. Bolus arrival time and cerebral blood flow responses to hypercarbia. Journal of Cerebral Blood Flow and Metabolism. 2014;34:1243–1252. doi: 10.1038/jcbfm.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertugrul A, Volkan-Salanci B, Basar K, Karli Oguz K, Demir B, Ergun EL, Senturk S, Erbas B, Cila A, Ulug B. The effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: relationship with treatment response. Psychiatry Research: Neuroimaging. 2009;174:121–129. doi: 10.1016/j.pscychresns.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Fabene PF, Farace P, Brambilla P, Andreone N, Cerini R, Pelizza L, Versace A, Rambaldelli G, Birbaumer N, Tansella M, Sbarbati A. Three-dimensional MRI perfusion maps: a step beyond volumetric analysis in mental disorders. Journal of Anatomy. 2007;210:122–128. doi: 10.1111/j.1469-7580.2006.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer Robert L, Miriam Gibbon, Williams Janet B.W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/PSY SCREEN) Biometrics Research, New York State Psychiatric Institute; New York, NY: Nov, 2002. [Google Scholar]
- Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RS. The left medial temporal region and schizophrenia. A PET study. Brain. 1992;115(Pt 2):367–382. doi: 10.1093/brain/115.2.367. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. The American Journal of Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Gevers S, van Osch MJ, Bokkers RP, Kies DA, Teeuwisse WM, Majoie CB, Hendrikse J, Nederveen AJ. Intra- and multicenter reproducibility of pulsed, continuous and pseudo-continuous arterial spin labeling methods for measuring cerebral perfusion. Journal of Cerebral Blood Flow and Metabolism. 2011;31:1706–1715. doi: 10.1038/jcbfm.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb RL, Jr., Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Current Topics in Behavioral Neurosciences. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophrenia Research. 2014 doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neuroscience. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Heilbrun MP, Jorgensen PB, Boysen G. Relationships between cerebral perfusion pressure and regional cerebral blood flow in patients with severe neurological disorders. Stroke. 1972;3:181–195. doi: 10.1161/01.str.3.2.181. [DOI] [PubMed] [Google Scholar]
- Homan P, Kindler J, Hauf M, Hubl D, Dierks T. Cerebral blood flow identifies responders to transcranial magnetic stimulation in auditory verbal hallucinations. Translational Psychiatry. 2012;2:e189. doi: 10.1038/tp.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn H, Federspiel A, Wirth M, Muller TJ, Wiest R, Wang JJ, Strik W. Structural and metabolic changes in language areas linked to formal thought disorder. The British Journal of Psychiatry. 2009;194:130–138. doi: 10.1192/bjp.bp.107.045633. [DOI] [PubMed] [Google Scholar]
- Huang YP, Okudera T. Arterial supply to the hippocampal formation. Neuroimaging Clinics of North America. 1997;7:31–50. [PubMed] [Google Scholar]
- Jiang J, Zhao L, Zhang Y, Zhang S, Yao Y, Qin Y, Wang CY, Zhu W. Comparative analysis of arterial spin labeling and dynamic susceptibility contrast perfusion imaging for quantitative perfusion measurements of brain tumors. International Journal of Clinical and Experimental Pathology. 2014;7:2790–2799. [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Maeda Y, Urata K, Yamaguchi N, Matsuda H, Hisada K, Takashima T. Regional cerebral blood flow in patients with schizophrenia. A preliminary report. European Archives of Psychiatry and Clinical Neuroscience. 1992;241:195–200. doi: 10.1007/BF02190252. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kindler J, Jann K, Homan P, Hauf M, Walther S, Strik W, Dierks T, Hubl D. Static and dynamic characteristics of cerebral blood flow during the resting state in schizophrenia. Schizophrenia Bulletin. 2015;41(1):163–170. doi: 10.1093/schbul/sbt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophrenia Research. 2011;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biological Psychiatry. 2003;53:601–608. doi: 10.1016/s0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31:221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- Li X, Sarkar SN, Purdy DE, Spence JS, Haley RW, Briggs RW. Anteroposterior perfusion heterogeneity in human hippocampus measured by arterial spin labeling MRI. NMR in Biomedicine. 2013;26:613–621. doi: 10.1002/nbm.2898. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, Frith CD, Frackowiak RS. Cerebral blood flow and mental processes in schizophrenia. Journal of the Royal Society of Medicine. 1992;85:224–227. doi: 10.1177/014107689208500415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in Neurosciences. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber RT, Sherwood AR, Renshaw PF, Cohen BM, Yurgelun-Todd DA. Differences in cerebellar blood volume in schizophrenia and bipolar disorder. Schizophrenia Research. 1999;37:81–89. doi: 10.1016/s0920-9964(98)00137-6. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Harkavy-Friedman J, Corcoran C, Mujica-Parodi L, Printz D, Gorman JM, Van Heertum R. Resting neural activity distinguishes subgroups of schizophrenia patients. Biological Psychiatry. 2004;56:931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Miller DD, Andreasen NC, O’Leary DS, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Effect of antipsychotics on regional cerebral blood flow measured with positron emission tomography. Neuropsychopharmacology. 1997;17:230–240. doi: 10.1016/S0893-133X(97)00042-0. [DOI] [PubMed] [Google Scholar]
- Moreno H, Wu WE, Lee T, Brickman A, Mayeux R, Brown TR, Small SA. Imaging the Abeta-related neurotoxicity of Alzheimer disease. Archives of Neurology. 2007;64:1467–1477. doi: 10.1001/archneur.64.10.1467. [DOI] [PubMed] [Google Scholar]
- Mu Q, Johnson K, Morgan PS, Grenesko EL, Molnar CE, Anderson B, Nahas Z, Kozel FA, Kose S, Knable M, Fernandes P, Nichols DE, Mailman RB, George MS. A single 20 mg dose of the full D1 dopamine agonist dihydrexidine (DAR-0100) increases prefrontal perfusion in schizophrenia. Schizophrenia Research. 2007;94:332–341. doi: 10.1016/j.schres.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Nael K, Meshksar A, Liebeskind DS, Wang DJ, Ellingson BM, Salamon N, Villablanca JP. Periprocedural arterial spin labeling and dynamic susceptibility contrast perfusion in detection of cerebral blood flow in patients with acute ischemic syndrome. Stroke. 2013;44:664–670. doi: 10.1161/STROKEAHA.112.672956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl TE, Kusubov N, Carter C, Salamat S, Cummings AM, O’Shora-Celaya L, Eberling J, Robertson L, Huesman RH, Jagust W, Budinger TF. Temporal lobe metabolic differences in medication-free outpatients with schizophrenia via the PET-600. Neuropsychopharmacology. 1996;15:541–554. doi: 10.1016/S0893-133X(96)00098-X. [DOI] [PubMed] [Google Scholar]
- Novak B, Milcinski M, Grmek M, Kocmur M. Early effects of treatment on regional cerebral blood flow in first episode schizophrenia patients evaluated with 99Tc-ECD-SPECT. Neuroendocrinology Letters. 2005;26:685–689. [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. Journal of Psychiatric Research. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Ostergaard L. Cerebral perfusion imaging by bolus tracking. Topics in Magnetic Resonance Imaging. 2004;15:3–9. doi: 10.1097/00002142-200402000-00002. [DOI] [PubMed] [Google Scholar]
- Ostergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: Experimental comparison and preliminary results. Magnetic Resonance in Medicine. 1996a;36:726–736. doi: 10.1002/mrm.1910360511. [DOI] [PubMed] [Google Scholar]
- Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magnetic Resonance in Medicine. 1996b;36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- Ota M, Ishikawa M, Sato N, Okazaki M, Maikusa N, Hori H, Hattori K, Teraishi T, Ito K, Kunugi H. Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophrenia Research. 2014;154:113–118. doi: 10.1016/j.schres.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magnetic Resonance in Medicine. 2004;51:736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Perkio J, Aronen HJ, Kangasmaki A, Liu Y, Karonen J, Savolainen S, Ostergaard L. Evaluation of four postprocessing methods for determination of cerebral blood volume and mean transit time by dynamic susceptibility contrast imaging. Magnetic Resonance in Medicine. 2002;47:973–981. doi: 10.1002/mrm.10126. [DOI] [PubMed] [Google Scholar]
- Peruzzo D, Rambaldelli G, Bertoldo A, Bellani M, Cerini R, Silvia M, Pozzi Mucelli R, Tansella M, Brambilla P. The impact of schizophrenia on frontal perfusion parameters: a DSC-MRI study. Journal of Neural Transmission. 2011;118:563–570. doi: 10.1007/s00702-010-0548-7. [DOI] [PubMed] [Google Scholar]
- Petersen ET, Mouridsen K, Golay X. The QUASAR reproducibility study, Part II: Results from a multi-center Arterial Spin Labeling test-retest study. NeuroImage. 2010;49:104–113. doi: 10.1016/j.neuroimage.2009.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham A, Loughead J, Ruparel K, Wu WC, Overton E, Gur R. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Research: Neuroimaging. 2011;194:64–72. doi: 10.1016/j.pscychresns.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw PF, Levin JM, Kaufman MJ, Ross MH, Lewis RF, Harris GJ. Dynamic susceptibility contrast magnetic resonance imaging in neuropsychiatry: present utility and future promise. European Radiology. 1997;7(Suppl 5):216–221. doi: 10.1007/pl00006895. [DOI] [PubMed] [Google Scholar]
- Scheef L, Manka C, Daamen M, Kuhn KU, Maier W, Schild HH, Jessen F. Resting-state perfusion in nonmedicated schizophrenic patients: a continuous arterial spin-labeling 3.0-T MR study. Radiology. 2010;256:253–260. doi: 10.1148/radiol.10091224. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of General Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen CZ, Ostergaard L, Vestergaard-Poulsen P, Rohl L, Bjornerud A, Gyldensted C. CBF and CBV measurements by USPIO bolus tracking: reproducibility and comparison with Gd-based values. Journal of Magnetic Resonance Imaging. 1999;9:342–347. doi: 10.1002/(sici)1522-2586(199902)9:2<342::aid-jmri29>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature Reviews: Neuroscience. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- Talati P, Rane S, Kose S, Blackford JU, Gore J, Donahue MJ, Heckers S. Increased hippocampal CA1 cerebral blood volume in schizophrenia. NeuroImage: Clinical. 2014a;5:359–364. doi: 10.1016/j.nicl.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati P, Rane S, Kose S, Gore J, Heckers S. Anterior-Posterior Cerebral Blood Volume Gradient in Human Subiculum. Hippocampus. 2014b;24:503–509. doi: 10.1002/hipo.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Archives of General Psychiatry. 1992;49:522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nagaoka T, Nair G, Ohno K, Duong TQ. Arterial spin labeling and dynamic susceptibility contrast CBF MRI in postischemic hyperperfusion, hypercapnia, and after mannitol injection. Journal of Cerebral Blood Flow and Metabolism. 2011;31:1403–1411. doi: 10.1038/jcbfm.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théberge J. Perfusion magnetic resonance imaging in psychiatry. Topics in Magnetic Resonance Imaging. 2008;19:111–130. doi: 10.1097/RMR.0b013e3181808140. [DOI] [PubMed] [Google Scholar]
- Tregellas JR. Neuroimaging biomarkers for early drug development in schizophrenia. Biological Psychiatry. 2014;76:111–119. doi: 10.1016/j.biopsych.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Osch MJ, van der Grond J, Bakker CJ. Partial volume effects on arterial input functions: shape and amplitude distortions and their correction. Journal of Magnetic Resonance Imaging. 2005;22:704–709. doi: 10.1002/jmri.20455. [DOI] [PubMed] [Google Scholar]
- Vita A, Bressi S, Perani D, Invernizzi G, Giobbio GM, Dieci M, Garbarini M, Del Sole A, Fazio F. High-resolution SPECT study of regional cerebral blood flow in drug-free and drug-naive schizophrenic patients. The American Journal of Psychiatry. 1995;152:876–882. doi: 10.1176/ajp.152.6.876. [DOI] [PubMed] [Google Scholar]
- Walther S, Federspiel A, Horn H, Razavi N, Wiest R, Dierks T, Strik W, Muller TJ. Resting state cerebral blood flow and objective motor activity reveal basal ganglia dysfunction in schizophrenia. Psychiatry Research: Neuroimaging. 2011;192:117–124. doi: 10.1016/j.pscychresns.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Wang DJ, Alger JR, Qiao JX, Gunther M, Pope WB, Saver JL, Salamon N, Liebeskind DS. Multi-delay multi-parametric arterial spin-labeled perfusion MRI in acute ischemic stroke - Comparison with dynamic susceptibility contrast enhanced perfusion imaging. NeuroImage: Clinical. 2013;3:1–7. doi: 10.1016/j.nicl.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CM, Pope WB, Zaw T, Qiao J, Naeini KM, Lai A, Nghiemphu PL, Wang JJ, Cloughesy TF, Ellingson BM. Regional and voxel-wise comparisons of blood flow measurements between dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) and arterial spin labeling (ASL) in brain tumors. Journal of Neuroimaging. 2014;24:23–30. doi: 10.1111/j.1552-6569.2012.00703.x. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza S, Soustiel JF, Nariai T, Zaharchuk G, Caille JM, Dousset V, Yonas H. Comparative overview of brain perfusion imaging techniques. Stroke. 2005;36:e83–99. doi: 10.1161/01.STR.0000177884.72657.8b. [DOI] [PubMed] [Google Scholar]
- Wu B, Lou X, Wu X, Ma L. Intra- and interscanner reliability and reproducibility of 3D whole-brain pseudo-continuous arterial spin-labeling MR perfusion at 3T. Journal of Magnetic Resonance Imaging. 2014;39:402–409. doi: 10.1002/jmri.24175. [DOI] [PubMed] [Google Scholar]
- Xu G, Rowley HA, Wu G, Alsop DC, Shankaranarayanan A, Dowling M, Christian BT, Oakes TR, Johnson SC. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer’s disease. NMR in Biomedicine. 2010;23:286–293. doi: 10.1002/nbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharchuk G, El Mogy IS, Fischbein NJ, Albers GW. Comparison of arterial spin labeling and bolus perfusion-weighted imaging for detecting mismatch in acute stroke. Stroke. 2012;43:1843–1848. doi: 10.1161/STROKEAHA.111.639773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaro-Weber O, Moeller-Hartmann W, Heiss WD, Sobesky J. Influence of the arterial input function on absolute and relative perfusion-weighted imaging penumbral flow detection: a validation with (1)(5)O-water positron emission tomography. Stroke. 2012;43:378–385. doi: 10.1161/STROKEAHA.111.635458. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophrenia Research. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]