Abstract
Background & Aims
Sustained JNK activation by saturated fatty acids plays a role in lipotoxicity and the pathogenesis of NASH. We have reported that the interaction of JNK with mitochondrial Sab leads to inhibition of respiration, increased ROS, cell death and hepatotoxicity. We tested whether this pathway underlies palmitic acid (PA)-induced lipotoxicity in hepatocytes.
Methods
Primary mouse hepatocytes from adeno-shlacZ or adeno-shSab treated mice and Huh7 cells were used.
Results
In PMH, PA dose dependently up to 1mM stimulated oxygen consumption rate (OCR) due to mitochondrial β-oxidation. At ≥ 1.5mM, PA gradually reduced OCR, followed by cell death. Inhibition of JNK, caspases or treatment with antioxidant butylated hydroxyanisole (BHA) protected PMH against cell death. Sab knockdown or a membrane permeable Sab blocking peptide prevented PA-induced mitochondrial impairment, but inhibited only the late phase of both JNK activation (beyond 4 hours) and cell death. PA increased P-PERK and downstream target CHOP in PMH but failed to activate the IRE-1α arm of the UPR. However, Sab silencing did not affect PA-induced PERK activation. Conversely, specific inhibition of PERK prevented JNK activation and cell death, indicating a major role upstream of JNK activation.
Conclusions
The effect of P-JNK on mitochondria plays a key role in PA-mediated lipotoxicity. The interplay of P-JNK with mitochondrial Sab leads to impaired respiration, ROS production, sustained JNK activation, and apoptosis.
Keywords: Palmitic acid, reactive oxygen species, apoptosis, mitochondria, hepatocytes
Introduction
Nonalcoholic steatohepatitis, mainly related to obesity and type II diabetes, is an important cause of cirrhosis in Western countries. This disease represents a progression from fatty liver to cirrhosis with hepatocellular death believed to be a pivotal factor in promoting inflammation and fibrosis [1-3]. The hepatocellular death is mainly induced by free fatty acids with saturated fatty acids such as palmitic acid being much more toxic than unsaturated fatty acids [4,5]. This phenomenon is referred to as lipotoxicity or lipoapoptosis [6-9]. The mechanism for palmitic acid induced lipotoxicity has been the subject of considerable investigation and has revealed a pivotal role for JNK in mediating the toxicity in hepatocytes [10-16]. The pathways for saturated fatty acid induced JNK activation have been extensively studied and evidence supports a role for Src dependent activation of the MAP3K, MLK3 [17-20]. Recently, autophagy-mediated degradation of KEAP-1 has been demonstrated to be upstream of JNK in palmitic acid induced apoptosis, possibly upstream of MLK3 [21]. The role of ER stress in activating ASK-1 has also been suggested [22] but recent evidence indicates that ER stress is somehow linked to MLK3 activation [11,20,23]. On the other hand, the effector cell death pathway which mediates the action of JNK in palmitic acid toxicity, has been linked to induction and activation of PUMA and Bim [13,21] pro-apoptotic Bcl2 family members which mediate mitochondrial permeabilzation. However, what determines the duration of sustained JNK activation required for toxicity is not fully understood.
Our laboratory has been investigating the mechanism of JNK mediated cell death in models of hepatotoxicity due to acetaminophen, TNF/galactosamine, and severe ER stress due to tunicamycin [24,25]. In all three models we have identified a key role for SH3BP5 (Sab), an outer membrane mitochondrial protein which is a binding target and substrate of JNK. When JNK phosphorylates Sab, mitochondrial respiration becomes impaired and ROS release is enhanced. This both sustains JNK activation, as ROS activate the MAPK pathways, and further impairs mitochondrial function. Thus, the interaction of JNK with mitochondria sustains JNK activation and ROS production which can promote MPT in APAP necrosis or MOMP via modulation of Bcl2 proteins in TNF and ER stress induced apoptosis. In all these models knockdown of Sab in vitro or vivo largely abrogates sustained JNK activation and thereby inhibits toxicity.
Since sustained JNK activation plays an important role in lipotoxicity, our goal in the present studies was to determine if palmitic acid induced JNK activation induces impaired mitochondrial function in a Sab dependent fashion and if this contributes to cell death.
Materials and methods
Animals and Reagents
Male C57BL/6NHsd mice (6–8 weeks of age) were obtained from Harlan Bioproducts for Science Inc. (Indianapolis, IN). Antisera to P-JNK, PERK, P-PERK, CHOP (Cell Signaling Technology), total JNK (JNK 1/2/3) (Santa Cruz Biotechnology), Gapdh and β-actin (Sigma Aldrich) and Sab (Proteintech, Abnova) were used. The P-JNK antiserum does not distinguish P-JNK 1 and 2. Palmitic acid, butylated hydroxyanisole (BHA), TUDCA, 4-PBA, tunicamycin, oligomycin, CCCP, rotenone, etomoxir, necrostatin-1 were from Sigma. JNK Inhibitor II (SP600125), PP2, Src inhibitor 1, PERK inhibitor 1 (GSK2606414) (EMD-Millipore) were dissolved as described by manufacturer. Organic solvent free palmitic acid-BSA (20mM stock) was prepared as follows. Equal volume of sodium palmitate (40mM) dissolved in 150mM NaCl at 70°C and 45% BSA (99% fat free, Roche) dissolved in 150mM NaCl at 37°C were mix gradually to generate palmitic acid-BSA stock (20mM palmitic acid/3.4mM BSA; ratio of 6:1) and stored in -80°C and stable for months. BSA control stock (3.4mM, 99% fat free) was prepared in 150mM NaCl at 37°C. Palmitic acid-BSA or BSA stock was thawed at 37°C and re-suspended thoroughly.
PMH isolation, culture, treatment and protein extraction
Primary mouse hepatocytes (PMH) from wild type or adenoviral shRNA (shlacZ or shSab) or ASO for JNK1 and 2 pretreated C57BL/6N mice were isolated and cultured as described previously (see supplementary methods) for adenoviral [24,26] and ASO preparation [27]. Briefly, 3 hours after plating of isolated hepatocytes in seeding medium (DMEM/F12 supplemented with HEPES, L-methionine, L-glutamine, penicillin, streptomycin, NaHCO3, insulin, hydrocortisol, FBS), medium was changed to serum free DMEM/F-12 culture medium and cells were rested overnight. Into the resting medium palmitic acid-BSA or relevant control reagents were added and incubated for 1, 4, 8, 16, 24 hours. At indicated times, hepatocytes were washed twice in DPBS and protein was extracted in RIPA lysis buffer supplemented with protease and phosphatase inhibitors. Whole cell lysate was centrifuged at 20,000Xg at 4°C for 15 min and supernatant was collected and stored in -80°C.
Isolation of mitochondria from PMH
PMH was rinsed and scrapped in 1ml of ice-cold homogenizing buffer (H-medium) with protease and phosphatase inhibitor cocktails, and collected into homogenizer in ice. Mitochondria from PMH 30×106 cells for each time point were pelleted by differential centrifugation as described before [24], and resuspended in RIPA lysis buffer.
Measurements of Respiration by Seahorse XF24 analyzer
PMH were plated in seeding medium for 5hrs in collagen coated 24 well XF24 cell culture microplates [25,28-30]. Cells were then rested overnight in serum free, phenol red free DMEM/F12 medium. The resting medium was then removed and cells were washed twice with DMEM running medium (XF assay modified DMEM supplemented with 2.5mM glucose, pH7.4) and incubated at 37°C without CO2 for 1hr to allow cells to pre-equilibrate with the assay medium. Palmitic acid or reagents of interest were diluted in running buffer, loaded into port-A, and injected into XF24 extracellular flux assay plate. Oligomycin, CCCP or rotenone (Sigma) diluted in DMEM running medium were loaded into port-B, port-C or port-D respectively. Final concentrations in XF24 cell culture microplates were 1μg/ml oligomycin, 20μM CCCP and 20μM rotenone. The sequence of measurements was as follow unless otherwise described. Basal level of oxygen consumption rate (OCR) was measured 3 times, and then port-A was injected and measured 7 times for 1, 4 or 6 hours. The medium was mixed every 10 minutes without data acquisition if intervals of OCR measurements were longer than 10 minutes. At the end of continuous real-time measurement of cellular OCR, port-B, port-C and port-D were injected sequentially and OCR was measured two times after each injection to determine mitochondrial or non-mitochondrial contribution of OCR. All measurements were normalized to average of 3 measurements of the basal (starting) level of cellular OCR of each well. Each sample was measured in 3-5 wells. Experiments were repeated 3-5 times with different cell preps. Mitochondrial oxidative-phosphorylation was determined by OCR before oligomycin injection minus OCR after oligomycin injection. Mitochondrial proton leak was determined by OCR after oligomycin injection minus OCR after rotenone injection. Non-mitochondrial OCR was determined by OCR after rotenone injection minus OCR of wells without cells. Mitochondrial reserve oxidative capacity was determined by OCR after CCCP injection minus OCR before oligomycin injection. Data was analyzed in groups of wells of each sample cell prep and statistical analysis was by ANOVA and t-test.
Biological Replicates and Statistical analysis
A minimum of three biological replicates were considered for all cell-based studies. Statistical analyses were performed using the Student's t test. p<0.05 was defined as statistically significant.
The methods for assessment of cell death, caspase 3 activation, immunoblotting, and peptide preparation are as we have previously reported [25,31] and details can be found in the supplementary material.
Results
Decreased mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity and cell death
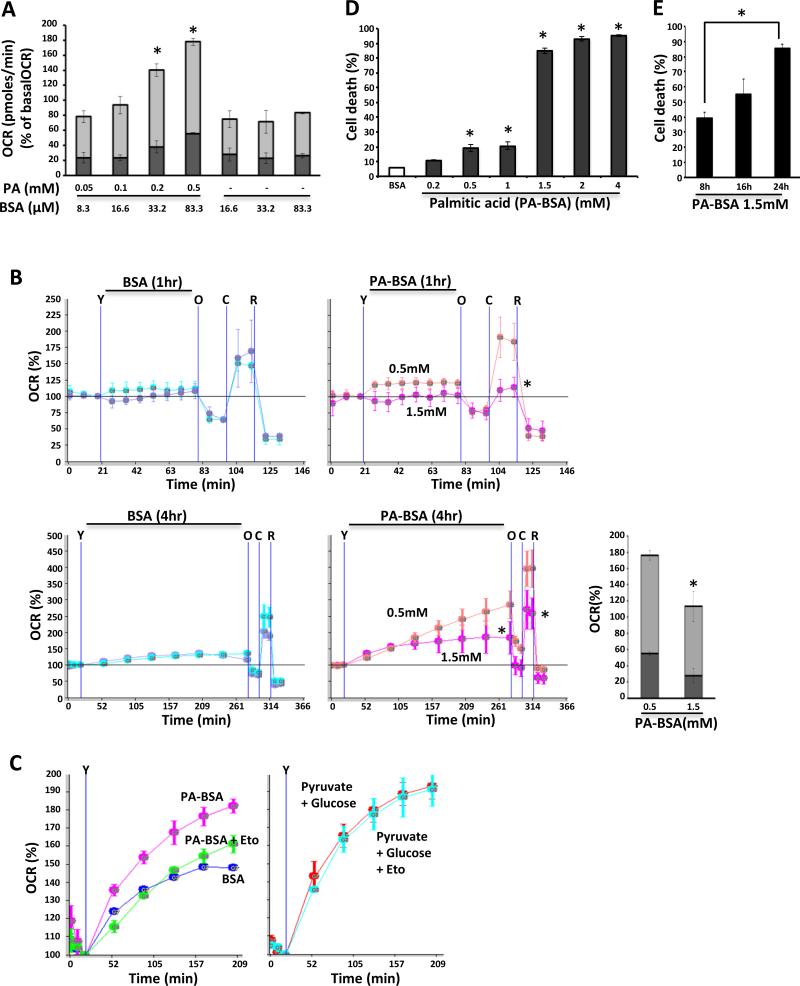
To determine the threshold of β-oxidation of primary mouse hepatocytes (PMH), we measured oxygen consumption induced by palmitic acid without supplementing mitochondrial respiratory substrates other than palmitic acid with low concentration glucose (2.5mM) using Seahorse analyzer (Fig.1A, B). Mitochondrial carnitine palmitoyltransferase (CPT)-1 inhibitor (Etomoxir) [32] prevented palmitic acid induced increased oxygen consumption rate (OCR), but did not prevent increased OCR in response to pyruvate 1mM with high concentration glucose (25mM) (Fig.1C). Thus, increased OCR represented palmitic acid induced mitochondrial β-oxidation. Cellular oxygen consumption of PMHs in response to palmitic acid steadily increased with dose up to 4 hour. At 4 hours after palmitic acid treatment, both ATP-producing (oligomycin inhibitable) and non-ATP producing (oligomycin uninhibitable proton leak) mitochondrial OCR increased dose dependently (180% of basal OCR with 0.5mM palmitic acid) (Fig.1 A). However, higher dose of palmitic acid (1.5mM) for 1 hour markedly decreased reserve capacity and by 4 hours, mitochondrial respiration (ATP-producing and proton leak) decreased 60% compared to 0.5mM (Fig.1B). Ultrapure fatty acid free bovine serum albumin alone (up to 1.7% BSA used to complex with palmitic acid 1.5mM) slightly (<20% of basal) increased cellular OCR, but proton leak change was not observed with different concentrations of BSA carrier control and OCR plateaued after 2 hours. At one hour after 1.5mM palmitic acid, mitochondrial oxidative phosphorylation slightly decreased and reserve capacity was significantly decreased compared to low dose 0.5mM palmitic acid. This indicated that activation of mitochondrial respiration in response to 0.5mM palmitic acid was not observed at the higher concentration of 1.5mM.
Fig. 1. Decreased β-oxidation in palmitic acid induced hepatic lipotoxicity.
(A) Palmitic acid (0.05-0.5mM) dose dependently activated mitochondrial OCR. Both mitochondrial oxidative phosphorylation OCR (light gray color) and proton leak OCR (dark gray color) were increased significantly compared to control BSA carrier; *P<0.05 versus BSA. (B) 1.5mM palmitic acid decreased mitochondrial reserve capacity at 1 hour and inhibited mitochondrial oxidative phosphorylation and proton leak and mitochondrial maximal OCR at 4 hours after treatment. The data are presented as means ± S.D of three - five wells of each sample for each experiment; *P<0.05, N = 5 experiments. (Y = injection of palmitic acid or BSA; O = injection of oligomycin; C = injection of CCCP; R = injection of rotenone). (C) Etomoxir (Eto) blocked increased OCR induced by palmitic acid (1mM) but not by pyruvate 1mM with glucose 25mM. Injection of compounds was indicated by “Y”. (D) PMH death increased with palmitic acid at and above 0.5mM, and 85% of cells died at 1.5mM for 24 hours; *P<0.05 versus BSA. (E) SYTOX green positive PMH cell death increased over time; [hexasterisk5]P<0.05. The data are presented as means ± S.D of five experiments;
Lipotoxicity of palmitic acid ultimately leads to cell death in different cell lines and isolated primary cells [4,12-16,19,32,33]. Slightly increased PMH cell death was seen at doses equal to or lower than 1mM palmitic acid. However, at higher dose (1.5mM or above), 85 – 95% PMH death was observed at 24 hours after treatment (Fig.1D). Cell death at 1.5mM PA increased with time from 40% at 8 hours to 55% at 16 hours to 85% of PMH at 24 hours (Fig.1E). Huh7 cells which have been widely used to study lipotoxicity [4,13] were more sensitive and death increased in a time and dose related fashion: 20% to 30% at 400μM from 16 to 24 hours and 55% to 80% at 800μM from 16 to 24 hours (Fig.S1). These studies indicated that PMH utilize palmitic acid as a bioenergetic substrate and higher doses of palmitic acid induce mitochondrial dysfunction and cell death.
Sab - JNK mediated impairment of mitochondrial respiration in palmitic acid induced lipotoxicity
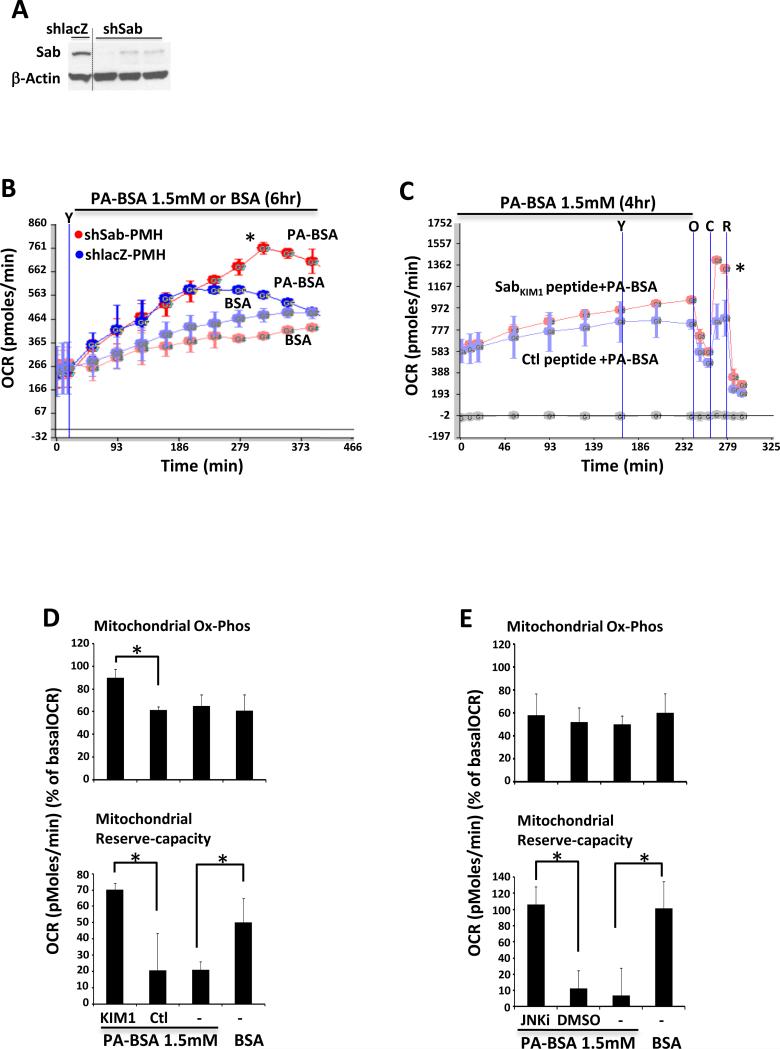
We have shown that tunicamycin induced ER stress inhibits mitochondrial OCR through a JNK-dependent effect mediated by mitochondrial Sab [25]. Although the precise mechanism for JNK activation in palmitic acid toxicity is more complex, we hypothesized that activated JNK in this context may be mediating the impaired respiration and contributing to cell death by interaction with mitochondrial Sab. To examine the role of Sab in palmitic acid inhibition of mitochondrial OCR we used Sab depleted PMH isolated from adenoviral shRNA treated Sab silenced mice (Fig.2A). OCR of PMH isolated from shlacZ control mice declined 4 hours after 1.5mM palmitic acid treatment. However, OCR of Sab depleted PMH continued to increase significantly (Fig.2B). Next, wild type PMHs were pretreated with cell membrane permeable SabKIM1 peptide or scrambled control peptide before and during 1.5mM palmitic acid treatment for 4 hours. SabKIM1 peptide maintained higher mitochondrial OCR and higher reserve capacity compared to the scrambled control peptide (Fig.2C). In addition, palmitic acid induced inhibition of mitochondrial reserve capacity was prevented and higher oxidative phosphorylation was seen at 1 hour after palmitic acid in the presence of SabKIM1 peptide (Fig.2D). To further examine the involvement of JNK activation, we treated wild type PMH with palmitic acid 1.5mM with or without JNK inhibitor. JNK inhibitor prevented palmitic acid 1.5mM inhibition of mitochondrial OCR (Fig.2E). Thus, JNK activation and mitochondrial Sab were required for palmitic acid induced inhibition of mitochondrial respiration.
Fig. 2. Sab - JNK mediated impairment of mitochondrial respiration.
(A,B) 10 days after Ad shlacZ or shSab treatment PMH were isolated. Efficient knockdown of Sab protein expression was observed (Western blot). BSA carrier control or palmitic acid 1.5mM were injected from port-A (indicated by “Y”) and cellular OCR was determined continuously for 6 hours; *P<0.05 shSab versus shlacZ. (C) Wild type PMH were exposed to scrambled control or SabKIM1 peptide (1μM) and palmitic acid 1.5mM just before loading the plate to Seahorse analyzer, and OCR was measured continuously for 4 hours and then mitochondrial respiration was determined by injection of oligomycin (O), CCCP (C), rotenone (R) from port-B, C, D. To maintain the peptide concentration, peptides (5μM) were injected from port-A (indicated by “Y”) at 2 hour of treatment; *P<0.05 SabKIM1 versus control (Ctl) peptide. (D) Decreased mitochondrial reserve capacity at 1 hour after treatment was prevented by SabKIM1 peptide (10μM); *P<0.05. (E) JNKi (20μM) prevented palmitic acid inhibition of mitochondrial reserve capacity at 1 hour; *P<0.05. The data are presented as means ± S.D of three - five wells of each sample for each experiment; N = 3 experiments.
Sab - JNK mediated lipotoxicity and cell death
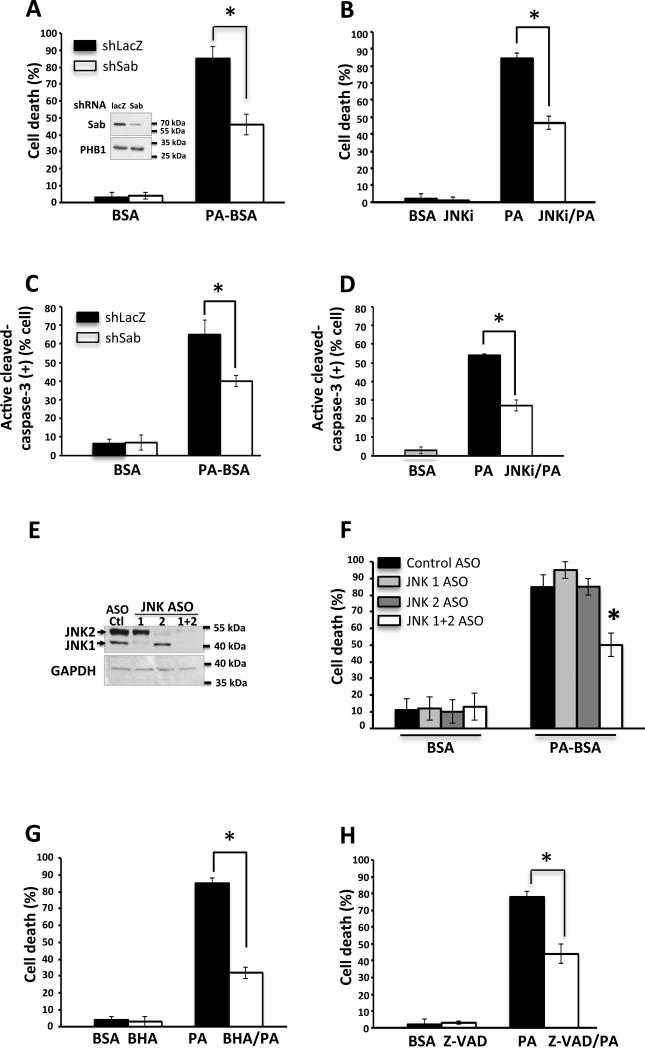
Palmitic acid induced apoptotic cell death has been reported in various cell lines [4,13,32,33]. To examine the palmitic acid induced cell death in PMH, mitochondrial Sab depleted PMH were treated with 1.5mM palmitic acid and cell death was measured at 16 hours and 24 hours. Depletion of Sab by adeno-shSab significantly prevented the palmitic acid induced cell death at 24 hours compared to adeno-shlacZ PMH death (Fig.3A). Similarly, inhibition of JNK activation by JNK inhibitor (SP600125) significantly decreased palmitic acid induced cell death at 24 hours compared to control in PMH (Fig.3B) and in Huh7 cells (not shown). In addition, 1.5mM palmitic acid induced caspase 3 activation in PMH was inhibited by Sab knockdown and by JNK inhibitor (Fig.3C, D). Since SP600125 is known to have off target effects [34], aside from potent inhibition of JNK, we confirmed that antisense knockdown of both JNK1 and 2 in hepatocytes isolated from mice treated in vivo [27] were protected against palmitic acid induced toxicity to a similar extent as SP600125. However, knockdown of JNK1 or 2 alone was not protective (Fig.3E, F). Cell death was inhibited by the antioxidant BHA (Fig.3G) and the pancaspase inhibitor Z-VAD-fmk (Fig.3H). Of note, necrostatin (15-60μM) did not protect (Fig.S3). This indicates that P-JNK and its mitochondrial target protein Sab are critical participants in palmitic acid induced apoptotic cell death and that the regulation of JNK activation is an important signaling event in this context. Since some residual cell death was observed with JNK inhibitor, BHA, Z-VAD-fmk, or PERK inhibitor (see below), we assessed if there were additive protective effects, but none were found, indicating that protection by all was though a common pathway involving JNK, oxidative stress and caspases.
Fig. 3. Silencing of Sab or inhibition of JNK inhibited palmitic acid induced cell death.
(A) Cell death was inhibited by Sab knockdown, (B) JNK inhibitor refreshed 2 hourly, (C) Caspase 3 activation at 24 hours was inhibited by Sab knockdown and (D) JNK inhibitor, (E) Efficient knockdown of JNK protein expression was observed (Western blot), (F) Cell death (24 hours treatment of palmitic acid) with JNK 1 and/or 2 knockdown, (G) BHA, (H) Z-VAD-fmk (20μM). N = 3 experiments, *P<0.05.
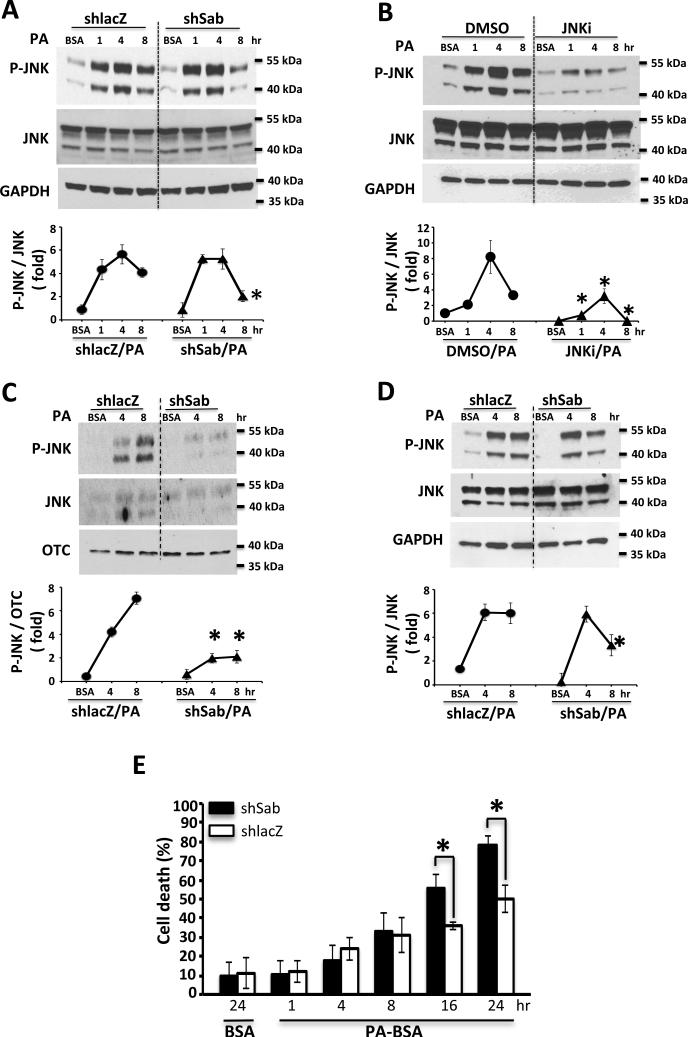
Accompanying palmitic acid induced impaired respiration and cell death, we verified that the toxic dose of palmitic acid induced sustained JNK activation in PMH (Fig.S2A). Interestingly, despite Sab knockdown markedly protecting against disturbed mitochondrial function and cell death, only the latter phase (8 hours) of JNK activation was inhibited but the earlier phase (1 and 4 hours) was not inhibited (Fig.4A). In contrast, as expected, the JNK inhibitor (Fig.4B) and JNK1 and 2 knockdown (Fig.S2B) markedly inhibited JNK throughout the time course (Fig.4C). In addition, the antioxidant BHA markedly inhibited both early and late JNK activation (Fig.S2C). We therefore examined the effect of Sab knockdown of P-JNK at 4 and 8 hours in total cell extracts and in mitochondria isolated from hepatocytes in the same experiments. While Sab knockdown markedly inhibited PA- induced P-JNK translocation to mitochondria at both time points, total cell P-JNK was only inhibited at the later time point (Fig.4D). Time course studies of PA-induced cell death indicated that Sab knockdown did not inhibit the cell death up to 8 hours but the continued increase in cell death at later times was inhibited after Sab knockdown (Fig.4E).
Fig. 4. Time course of JNK activation and effect of Sab knockdown, JNKi, and antioxidant.
(A) Knockdown of Sab did not affect JNK activation at 1 and 4 hours but decreased P-JNK at 8 hours. (B) JNK inhibitor as expected decreased palmitic acid induced sustained JNK activation. (C) Time course of JNK translocation to mitochondria following palmitic acid treatment. The graph represents P-JNK densitometry normalized to OTC. (D) Time course of JNK activation in whole cell lysate from same experiments as C. The graphs represent P-JNK densitometry normalized to JNK. N=3 experiment (E) Time course of cell death in shlacZ vs shSab. N=3 experiments,*P<0.05.
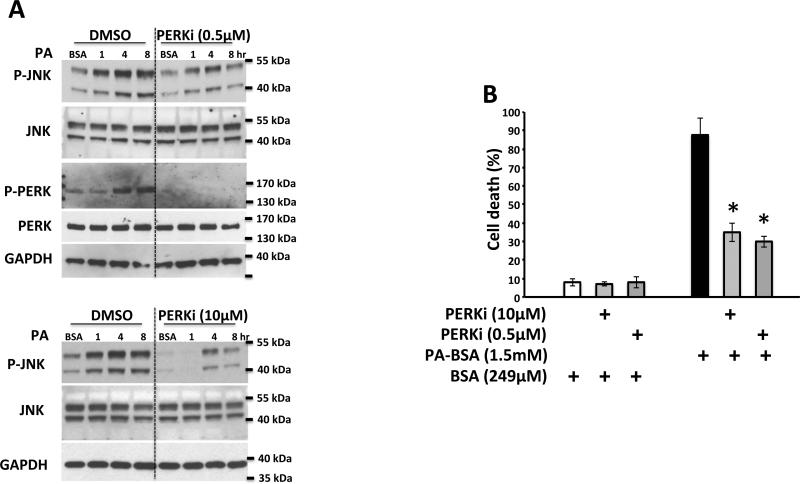
The finding of minimal effect of Sab knockdown on sustained JNK activation and cell death at early times, while inhibiting JNK activation and cell death at later time points, contrasts with the marked inhibitory effect of Sab knockdown on tunicamycin-induced sustained JNK activation, which we previously reported [25]. Therefore we explored the potential sources of Sab-independent sustained JNK activation. The Src inhibitor, PP2, inhibited JNK activation at 1 hour but not at 4, 6, 8 hours and had a modest protective effect on cell death induced by palmitic acid (Supplementary Fig.S4). In contrast, a highly specific PERK inhibitor blocked JNK activation at all-time points (Fig.5A) and protected against palmitic acid induced cell death (Fig.5B). Interestingly palmitic acid induced PERK activation was not inhibited by JNK inhibitor (Fig.S4A), BHA, (Fig.S5B), or by knockdown of JNK1 and 2 (Fig.S2) indicating PERK was upstream of JNK activation. PKR was also activated by palmitic acid but was not inhibited by the PERK inhibitor (Supplementary Fig.S5C). The IRE limb of ER stress was assessed by XBP1 splicing and there was no evidence of sXBP1 mRNA (not shown). Furthermore, neither tauroursodeoxycholic acid (500μg/ml) or 4-PBA (20mM) protected against JNK activation or cell death by palmitic acid (Supplementary Fig.S6 A-D) whereas TUDCA and PERK inhibitor protected against tunicamycin induced cell death and JNK activation (Supplementary Fig.S6E-H).
Fig. 5. Effect of and PERK inhibitor on PA induced JNK activation and cell death.
(A) Effect of PERK inhibitor (0.5 and 10μM) on time course P- JNK and P-PERK; (B) Effect of PERK inhibitor on PA-induced cell death at 24 hours. N=3 experiments,*P<0.05, versus DMSO vehicle.
Discussion
Saturated fatty acid induced apoptosis is of considerable potential importance in nonalcoholic steatohepatitis and may contribute to the progression to cirrhosis and liver cancer. The mechanism of this lipotoxicity has been linked to sustained JNK activation. However, despite considerable progress in elucidating the initiating pathways of JNK activation and cell death effector mechanisms induced by fatty acids, such as palmitic acid, little is known about the mechanisms that sustain prolonged JNK activation and the duration of activation required to promote cell death. We therefore decided to extend our studies on the mitochondrial targeted effects of JNK to the model of lipotoxicity to specifically test the hypothesis that the outer membrane JNK docking protein and substrate, Sab (SH3BP5), plays a key role. Sab is found exclusively in mitochondrial outer membrane. It contains C-terminal JNK docking sites facing the cytoplasm and is predicted to contain one transmembrane hydrophobic domain with N-terminal stretch facing the intermembrane space.
We previously demonstrated that sustained JNK activation and cell death in several models of hepatotoxicity, such as acetaminophen overdose, TNF/galactosamine, and tunicamycin-induced ER stress, depends on the expression of Sab [24,25]. We also demonstrated that the addition of P-JNK plus ATP, but not either alone, to isolated hepatic mitochondria induced an inhibition of respiration and enhanced ROS production [25]. Thus, JNK phosphorylation of Sab directly leads to an impairment of mitochondrial function and oxidative stress. In the case of acetaminophen, the reactive metabolite sufficiently impairs mitochondrial function to render mitochondria vulnerable to MPT due to enhanced ROS induced by JNK, while the high level of ROS and marked GSH depletion incapacitate apoptotic pathways; so the outcome is necrosis. In contrast, in most other contexts sustained JNK activation has been linked to the promotion of apoptosis by modulating pro- and anti- apoptotic Bcl2 family members leading to outer membrane permeablization, cytochrome c release, and effector caspase activation. Therefore, it seemed reasonable to hypothesize a similar mechanism for palmitic acid induced apoptosis.
When we treated PMH with palmitic acid, a dose dependent stimulation of β-oxidation- induced respiration was observed as expected [35]. However, above a threshold of palmitic acid, gradual impaired mitochondrial respiration and subsequent apoptosis were observed. These effects of palmitic acid were JNK dependent. Knockdown of Sab or the administration of a membrane permeant Sab inhibitory peptide prevented the mitochondrial impairment. Sab knockdown significantly inhibited PA-induced cell death and there was no additive effect of antioxidant or caspase inhibitor indicating a common pathway with Sab playing a pivotal role.
Our time course studies revealed that Sab and P-JNK translocation to mitochondria are dispensable for the early phase of JNK activation (up to 4 hours) and associated increased cell death (up to 8 hours). However, the continuation of sustained JNK and increasing cell death in the later phase are Sab dependent. In the later phase, prior Sab knockdown leads to a decline in P-JNK at 8 hours which precedes the inhibition of cell death seen at 16 hours. These findings mirror the JNK/Sab dependent impairment of mitochondrial function which begins in the early phase but worsens in the later phase prior to the increase in cell death. Thus, it appears that the effects of P-JNK on mitochondria to promote ROS production and cell death evolve over a much slower rate in this model than we have seen in other contexts [24,25,27]. The reasons for this latency in the current model remain uncertain but may be due to lower levels of JNK activation requiring longer time to induce adverse effects on mitochondria or the need to overcome a protective barrier.
One interesting finding was that the early phase of JNK activation was Src dependent, consistent with the findings of others [36], but as noted above, was not dependent on Sab. However, the later phase of JNK activation and ultimate cell death were Sab dependent. Another mechanism which has been implicated in JNK activation in lipotoxicity is ER stress [13, 37-40]. ER stress is known to activate ASK1 through the IRE-1 pathway [22, 41] but this has been shown to be dispensable in palmitic acid toxicity, and another mechanism for ER stress activation of JNK via MLK3 activation was suggested [19]. Although we found no evidence of activation of IRE-1 in PMH as reflected in absence of XBP-1 splicing or protection by chemical chaperone inhibition of ER stress, PERK was activated and a PERK inhibitor blocked JNK activation and cell death supporting a selective role for palmitic acid induced PERK activation upstream of JNK. Others have suggested that PERK and PKR can activate JNK [42-46]. Palmitic acid activated both kinases in PMH but the PERK inhibitor, which effectively inhibited PERK [47, 48], did not inhibit PKR. The mechanism for palmitic acid induced selective PERK activation and the MAPK signaling pathway which leads to JNK activation requires further study, but based on published work is likely to involve MLK3 [49-51]. Interestingly, ER stress in hematopoietic stem cells has recently been reported to selectively activate PERK and predispose to apoptosis [52]. Thus, selective PERK activation in response to ER stress is possible and is context dependent. Other potential mechanisms of palmitic acid toxicity, might also contribute upstream or downstream of JNK. These include NADPH oxidase [53-56] as a source of early phase BHA inhibitable oxidative stress [57,58], induction or posttranslational processing of Bcl2 family members, and lysosomal permeabilization [12]. However, further study of these mechanisms is beyond the current scope of this manuscript and should be addressed in the future.
There are a few other caveats worthy of comment. We confirmed the protective effect of the JNK inhibitor by knocking down both JNK 1 and 2. However, neither alone showed protection indicating that both isoforms of JNK are interchangeable in our hepatocyte model system. In contrast, Singh et al. have shown that JNK1 versus 2 knockdown have distinct effects on steatohepatitis [59]. JNK2 knockdown increased steatohepatitis while JNK1 knockdown was protective. However, JNK knockdown was examined after 16 weeks of high fat diet with antisense given for the last 4 weeks. In addition, our findings on lack of effect of tauroursodeoxycholate on PA-induced P-JNK and cell death are somewhat discrepant with those of Cho et al [60]. However, their studies were conducted in Huh7-Ntcp cells at lower concentration of PA. Therefore, the apparent discrepancies between our findings and those mentioned are likely due to very different experimental models and conditions, which makes the findings difficult to compare.
In conclusion, we provide evidence that the lipotoxicity of palmitic acid in PMH involves the interplay of JNK with mitochondrial Sab which leads to mitochondrial dysfunction and triggers apoptosis. The upstream activation of JNK largely depends upon ER PERK activation and the downstream mitochondrial dysfunction, late sustained JNK activation, and apoptosis require effects of translocation of P-JNK to Sab on mitochondria which remain to be elucidated, but likely involve ROS production and effects of prolonged JNK activation on the Bcl2 family of regulators of the intrinsic pathway of apoptosis. Our findings point to the pivotal role of mitochondria in lipotoxicity [61-63].
Supplementary Material
Acknowledgments
Financial support
This work was supported by NIH grants RO1-DK067215 (NK) and RO1-AA014428 (NK), the USC Research Center for Liver Disease (P30-DK48522) Cellular and Tissue Imaging, Cell Separation and Culture, and Analytical/Metabolic/Instrumentation Cores and the Southern California Research Center for Alcoholic Liver and Pancreatic Disease (P30-AA11999).
Abbreviations
- ASK1
mitogen-activated protein kinase kinase kinase 5
- ATP
Adenosine 5′-triphosphate
- Bcl2
B-cell lymphoma 2
- BHA
butylated hydroxyanisole
- CCCP
Carbonyl cyanide 3-chlorophenylhydrazone
- CHOP
DNA-damage-inducible transcript 3
- ECAR
extracellular acidification rate
- ER
endoplasmic reticulum
- IRE1α
Inositol-requiring enzyme-1α
- JNK
c-Jun N-terminal kinase
- JNKi
JNK inhibitor SP600125
- MAP kinase
mitogen-activated protein kinase
- MOMP
mitochondrial outer membrane permeabilization
- MPT
membrane permeable transition
- NASH
nonalcoholic steatohepatitis
- O2•-
superoxide
- OCR
oxygen consumption rate
- Ox-Phos
oxidative phosphorylation
- OTC
ornithine carbamoyltransferase
- PA
palmitic acid
- PERK
eukaryotic translation initiation factor 2-alpha kinase
- PKR
protein kinase R
- PMH
primary mouse hepatocyte
- RCR
respiratory-control-ratio
- Reserve-capacity
reserve oxidative capacity
- ROS
reactive oxygen species
- Sab
SH3 homology associated BTK binding protein
- sXBP1
spliced X-box binding protein 1
- XBP1
X-box binding protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author's contributions
All the authors participated in the design and analysis of the work and writing of the manuscript. SW and TAT performed all the laboratory experiments. NK provided overall guidance.
Conflict of interest
The authors declare no conflict of interest.
Supplemental information
Supplemental information includes supplemental materials and methods, figure legends, figures and reference.
References
- 1.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24:830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 5.Nolan CJ, Larter CZ. Lipotoxicity: why do saturated fatty acids cause and monounsaturates protect against it? J Gastroenterol Hepatol. 2009;24:703–706. doi: 10.1111/j.1440-1746.2009.05823.x. [DOI] [PubMed] [Google Scholar]
- 6.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim SH, Kohli R, Gores GJ. Mechanisms of lipotoxicity in NAFLD and clinical implications. J Pediatr Gastroenterol Nutr. 2011;53:131–140. doi: 10.1097/MPG.0b013e31822578db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2013;52:165–174. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biden TJ, Boslem E, Chu KY, Sue N. Lipotoxic endoplasmic reticulum stress, cell failure, and type 2 diabetes mellitus. Trends Endocrinol Metab. 2014;25:389–398. doi: 10.1016/j.tem.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Solinas G, Naugler W, Galimi F, Lee MS, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci USA. 2006;103:16454–16459. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim SH, Gores GJ. Who pulls the trigger: JNK activation in liver lipotoxicity? J Hepatol. 2012;56:17–19. doi: 10.1016/j.jhep.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldstein AE, Werneburg NW, Li Z, Bronk SF, Gores GJ. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1339–1346. doi: 10.1152/ajpgi.00509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, et al. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52:586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockenfeller P, Ring J, Muschett V, Beranek A, Buettner S, Carmona-Gutierrez D, et al. Fatty acids trigger mitochondrion-dependent necrosis. Cell Cycle. 2010;9:2836–2842. doi: 10.4161/cc.9.14.12267. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim SH, Akazawa Y, Cazanave SC, Bronk SF, Elmi NA, Werneburg NW, et al. Glycogen synthase kinase-3 (GSK-3) inhibition attenuates hepatocyte lipoapoptosis. J Hepatol. 2011;54:765–772. doi: 10.1016/j.jhep.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakisaka K, Cazanave SC, Fingas CD, Guicciardi ME, Bronk SF, Werneburg NW, et al. Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G77–G84. doi: 10.1152/ajpgi.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaeschke A, Davis RJ. Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell. 2007;27:498–508. doi: 10.1016/j.molcel.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kant S, Swat W, Zhang S, Zhang ZY, Neel BG, Flavell RA, et al. TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway. Genes Dev. 2011;25:2069–2078. doi: 10.1101/gad.17224711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma M, Urano F, Jaeschke A. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol. 2012;56:192–198. doi: 10.1016/j.jhep.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kant S, Barrett T, Vertii A, Noh YH, Jung DY, Kim JK, et al. Role of the mixed-lineage protein kinase pathway in the metabolic stress response to obesity. Cell Rep. 2013;4:681–688. doi: 10.1016/j.celrep.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cazanave SC, Wang X, Zhou H, Rahmani M, Grant S, Durrant DE, et al. Degradation of Keap1 activates BH3-only proteins Bim and PUMA during hepatocyte lipoapoptosis. Cell Death Differ. 2014;21:1303–1312. doi: 10.1038/cdd.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lizunov V, Chlanda P, Kraft M, Zimmerberg J. Long, saturated chains: tasty domains for kinases of insulin resistance. Dev Cell. 2011;21:604–606. doi: 10.1016/j.devcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Win S, Than TA, Fernandez-Checa JC, Kaplowitz N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014;5:e989. doi: 10.1038/cddis.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Than TA, Lou H, Ji C, Win S, Kaplowitz N. Role of cAMP-responsive element-binding protein (CREB)-regulated transcription coactivator 3 (CRTC3) in the initiation of mitochondrial biogenesis and stress response in liver cells. J Biol Chem. 2011;286:22047–22054. doi: 10.1074/jbc.M111.240481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krauss S, Zhang CY, Lowell BB. A significant portion of mitochondrial proton leak in intact thymocytes depends on expression of UCP2. Proc Natl Acad Sci U S A. 2002;99:118–122. doi: 10.1073/pnas.012410699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambers JW, Cherry L, Laughlin JD, Figuera-Losada M, Lograsso PV. Selective inhibition of mitochondrial JNK signaling achieved using peptide mimicry of the Sab kinase Interacting motif-1 (KIM1). ACS Chem Biol. 2011;6:808–818. doi: 10.1021/cb200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. 2011;1807:726–734. doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 33.Henique C, Mansouri A, Fumey G, Lenoir V, Girard J, Bouillaud F, et al. Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J Biol Chem. 2010;285:36818–36827. doi: 10.1074/jbc.M110.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartlett K, Eaton S. Mitochondrial β-oxidation. Eur J Biochem. 2004;271:462–469. doi: 10.1046/j.1432-1033.2003.03947.x. [DOI] [PubMed] [Google Scholar]
- 36.Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, et al. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147:173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Kitai Y, Ariyama H, Kono N, Oikawa D, Iwawaki T, Arai H. Membrane lipid saturation activates IRE1α without inducing clustering. Genes Cells. 2013;18:798–809. doi: 10.1111/gtc.12074. [DOI] [PubMed] [Google Scholar]
- 39.Ben Mosbah I, Alfany-Fernández I, Martel C, Zaouali MA, Bintanel-Morcillo M, Rimola A, et al. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell Death Dis. 2010;1:e52. doi: 10.1038/cddis.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–377. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y, Tian T, Huang T, Nakajima S, Saito Y, Takahashi S, et al. Subtilase cytotoxin activates MAP kinases through PERK and IRE1 branches of the unfolded protein response. Toxicol Sci. 2011;120:79–86. doi: 10.1093/toxsci/kfq368. [DOI] [PubMed] [Google Scholar]
- 43.Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19:1880–1891. doi: 10.1038/cdd.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peidis P, Papadakis AI, Muaddi H, Richard S, Koromilas AE. Doxorubicin bypasses the cytoprotective effects of eIF2α phosphorylation and promotes PKR-mediated cell death. Cell Death Differ. 2011;18:145–154. doi: 10.1038/cdd.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P, Langland JO, Jacobs BL, Samuel CE. Protein kinase PKR-dependent activation of mitogen-activated protein kinases occurs through mitochondrial adapter IPS-1 and is antagonized by vaccinia virus E3L. J Virol. 2009;83:5718–5725. doi: 10.1128/JVI.00224-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem. 2012;55:7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 48.Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73:1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- 49.Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, Davis RJ. Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol. 2005;25:3670–3681. doi: 10.1128/MCB.25.9.3670-3681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibrahim SH, Gores GJ, Hirsova P, Kirby M, Miles L, Jaeschke A, et al. Mixed lineage kinase 3 deficient mice are protected against the high fat high carbohydrate diet-induced steatohepatitis. Liver Int. 2014;34:427–437. doi: 10.1111/liv.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gadang V, Kohli R, Myronovych A, Hui DY, Perez-Tilve D, Jaeschke A. MLK3 promotes metabolic dysfunction induced by saturated fatty acid-enriched diet. Am J Physiol Endocrinol Metab. 2013;305:E549–556. doi: 10.1152/ajpendo.00197.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Galen P, Kreso A, Mbong N, Kent DG, Fitzmaurice T, Chambers JE, et al. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature. 2014;510:268–272. doi: 10.1038/nature13228. [DOI] [PubMed] [Google Scholar]
- 53.Lambertucci RH, Hirabara SM, Silveira Ldos R, Levada-Pires AC, Curi R, Pithon-Curi TC. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J Cell Physiol. 2008;216:796–804. doi: 10.1002/jcp.21463. [DOI] [PubMed] [Google Scholar]
- 54.Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191:1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 56.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 58.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho EJ, Yoon JH, Kwak MS, Jang ES, Lee JH, Yu SJ, et al. Tauroursodeoxycholic acid attenuates progression of steatohepatitis in mice fed a methionine-choline-deficient diet. Dig Dis Sci. 2014;59:1461–1474. doi: 10.1007/s10620-014-3217-0. [DOI] [PubMed] [Google Scholar]
- 61.Pérez-Carreras M, Del Hoyo P, Martín MA, Rubio JC, Martín A, Castellano G, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- 62.Begriche K, Massart J, Robin MA, Bonnet F, Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58:1497–1507. doi: 10.1002/hep.26226. [DOI] [PubMed] [Google Scholar]
- 63.Rolfe DF, Brand MD. The physiological significance of mitochondrial proton leak in animal cells and tissues. Biosci Rep. 1997;17:9–16. doi: 10.1023/a:1027327015957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.