Abstract
Objective
To report the first cases of homozygous recessive mutations in NEFL, the gene that encodes the light subunit of neurofilaments (NFL).
Methods
Clinical and electrophysiologic data of all family members were evaluated, and a sural nerve biopsy from one affected child was examined by immunohistochemistry and electron microscopy. The ability of the mutant protein to form filaments was characterized in an established cell culture system.
Results
Four of five siblings developed a severe, progressive neuropathy beginning in early childhood. Serial nerve conduction studies showed progressively reduced amplitudes with age, and pronounced slowing at all ages. Visual evoked responses were slowed in three children, indicating that CNS axons were subclinically involved. All four affected children were homozygous for a nonsense mutation at glutamate 210 (E210X) in the NEFL gene; both parents were heterozygous carriers. A sural nerve biopsy from an affected patient at age 16 revealed markedly reduced numbers of myelinated axons; the remaining myelinated axons were small and lacked intermediate filaments. The E210X mutant protein did not form an intermediate filament network, and did not interfere with the filament formation by wild type human NFL in SW-13 vim- cells.
Interpretation
This is the first demonstration of a recessive NEFL mutation, which appears to cause a simple loss-of-function, resulting in a severe, early-onset axonal neuropathy with unique features. These results confirm that neurofilaments are the main determinant of axonal caliber and conduction velocity, and demonstrate for the first time that neurofilaments are required for the maintenance of myelinated PNS axons.
Keywords: neurofilament, Charcot-Marie-Tooth disease, CMT, myelin, axon, conduction velocity
Introduction
Neurofilaments are intermediate filaments and the main cytoskeleton component of vertebrate axons, and determine the axonal caliber 1, 2. They are heteropolymers 3, composed of heavy (NFH), medium (NFM), and light subunits (NFL). Knocking out the Nefl gene, which encodes NFL, eliminates neurofilaments in myelinated PNS, which fail to enlarge normally during development, and consequently have slowed conduction 4-6. Similarly, expressing a chimeric NFH-β-galactosidase fusion protein (that dimerizes and prevents the proper export of NFL, NHM, and NFH) also results in axonal hypotrophy related to an absence of axonal neurofilaments 7. The stoichiometry of neurofilament subunit expression is critical, as overexpressing any one subunit leads to diminished delivery of neurofilaments to axons 8-12, whereas coordinately expressing all three results in axonal hypertrophy 13.
Charcot-Marie-Tooth disease (CMT) is the eponym for uncomplicated hereditary neuropathies in humans, with more than 50 identified loci (http://www.molgen.ua.ac.be/CMTMutations). CMT is usually subdivided into demyelinating or axonal forms, in which demyelination or axonal loss appear to be the primary pathological events, respectively 14-16. Dominant mutations in NEFL cause clinically distinct forms of CMT, ranging from a severe neuropathy with an infantile onset, to a more moderate neuropathy with onset typically between 10 and 20 years 17-25. Herein we report the first recessive NEFL mutation (E210X), associated with a severe, early-onset neuropathy, characterized by slow conduction velocities, progressive axonal loss, and myelinated axons that lack intermediate filaments. In a cell model, E210X causes a simple loss-of-function. These data support the idea that neurofilaments are a key determinant of axonal caliber and conduction velocity, and demonstrate for the first time that neurofilaments are required for the maintenance of myelinated axons in PNS.
Methods
Patient data collection
The 16-year-old index patient (II-4 in Fig. 1) presented at age 2 years. He and his three affected siblings (II-1, II-3, II-5), one unaffected sibling (II-2), and parents underwent neurological and electrophysiological studies with standard techniques. Visual evoked responses (VERs) were elicited by pattern reversal stimulation to each eye using small and large check sizes. Blood samples were sent to Athena Diagnostics (Worcester, MA) for mutational analysis.
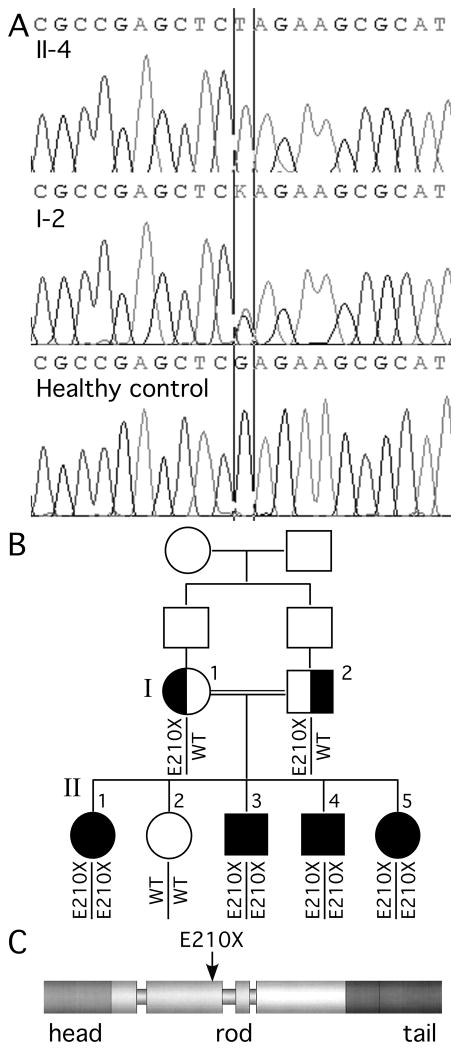
Fig. 1. Family pedigree and mutational analysis.
Panel A shows the electrophoretograms of the DNA sequence harboring the c.628G>T mutation for individuals II-4 and I-2 as well as a healthy control (the color versions are shown in Supplemental Fig. 1). In panel B, note that the unaffected sister (II-2) does not have the mutation, all affected children (II-1, II-3, II-4, and II-5) are homozygous, and both parents (I-1 and I-2) are heterozygous for the mutation. The siblings of the parents are not shown, and their parents are not available for genetic testing. Panel C shows the predicted effect of the E210X mutation on the domain structure of the human NFL protein.
Sural nerve biopsy
After obtaining informed consent, a sural nerve biopsy was taken from patient II-4. One cm of nerve was fixed in 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M phosphate buffer (PB, pH=7.4) for 20 h at 4°C, rinsed in PB, osmicated in 1% OsO4 (in 0.1 M PB) for 2 h, dehydrated in graded ethanols, and embedded in Embed-812 (Electron Microscopy Sciences). Thin sections (90 nm thick) were mounted on 2×1 mm single-slot, formvar-coated grids, stained with lead citrate and uranyl acetate, and photographed with a JOEL 1200 electron microscope. Axon density was assessed by manually counting myelinated axon inside the perineurium on low magnification electron micrographs with ImageJ. Another 1 cm segment was fixed in 4% paraformaldehyde in 0.1 M PB for 1 hour, and embedded in OCT. Ten micron thick sections were thaw-mounted, and immunostained with monoclonal antibodies against βIII tubulin (Sigma, dilution 1:50), NFL (Sigma, clone NR4, dilution 1:100), NFM (Sigma, dilution 1:200), NFH (Sigma, clone No142, dilution 1:400), as well as antisera against NFL-N terminus (Chemicon, dilution 1:500), NFL-C terminus (Santa Cruz; NFL C-15, dilution 1:300), NFM (from Dr. V. M.-Y. Lee dilution 1:300), NFH (Chemicon; dilution 1:300, or clone TA51 (from Dr. V. M.-Y. Lee), dilution 1:50), α-internexin (EnCor Biotechnology Inc. dilution 1:500), and peripherin (Chemicon, dilution 1:100). Digital images were obtained with a 60×, oil immersion objective on a FluoView FV1000 Olympus laser scanning confocal microscope, and were processed into figures using the ImageJ and Adobe Photoshop software.
Nefl-null mice
Adult Nefl-null mice 4 were perfused for electron microscopy (with 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M PB) or immunostaining (4% paraformaldehyde in 0.1 M PB for 1 h), as previously described 26. Frozen sections were immunostained as described above.
Construction of plasmids and site directed mutagenesis
The NEFLpCI plasmid containing human NEFL 27 (kindly provided by Drs. Liem and Perez-Olle) was amplified by PCR using oligonucleotide primers designed to include the open reading frame and incorporate a 5′ NheI site and a 3′ EcoRI site, using Platinum Pfx DNA polymerase (Invitrogen, Carlsbad, CA). The PCR product was digested, ligated into the pIRESpuro3 (Clontech, Palo Alto, CA) vector, which were used to transform DH5α competent cells. A large-scale plasmid preparation was made from a single colony (Qiagen, Valencia, CA), and the correct sequence (GenBank accession no. AY156690) was confirmed by direct sequencing. The E210X and Q333P mutations were introduced by PCR site-directed mutagenesis using the QuikChange kit (Stratagene, La Jolla, CA), with the following oligonucleotide primers (the underlined codon encodes the altered amino acid): E210X: 5′- ctcgcgccgagctctagaagcgcatcgac, 3′- gtcgatgcgcttctagagctcggcgcgag; Q333P: 5′- gcgctggagaagccgctgcaggagctg, 3′- cagctcctgcagcggcttctccagcgc. The resulting DNA was used to transform XL-1 Blue bacteria, and a large-scale plasmid preparation made from a single colony was sequenced at the Sequencing Core at the University of Pennsylvania to confirm the desired mutation.
Analysis of the E210X mutant
SW-13 vim- or SW-13 vim+ cells were grown in DMEM-F12 media supplemented with 5% FBS on 18 mm cover slips or in 100 mm plates to approximately 60% confluence, and transfected using FuGENE 6 reagent (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. After 2 days, the cells were immunostained or processed for immunoblot analysis. For immunostaining, cells were fixed in 4% paraformaldehyde at 4°C for 10 min, permeabilized in acetone at -20°C for 10 min, and blocked with 5% fish skin gelatin in PBS containing 0.1% Triton X for 1 hour at RT. Primary antibodies were added in the same blocking solution and the samples incubated overnight at 4°C. After washing in PBS, the appropriate TRITC-, FITC-, and Cy5-conjugated donkey antisera were added in the same blocking solution (1:200) and incubated at RT for 1 hour. Coverslips were mounted with Vectashield (Vector Laboratories Inc., Burlingame, CA) and samples were photographed under a Leica fluorescence microscope with a Hamamatsu digital camera C4742-95 connected to a G5 Mac computer, using the Openlab 2.2 software for deconvolution. For immunostaining, we used a mouse monoclonal antibody (Sigma, clone NR4; diluted 1:200) or a goat antiserum (Santa Cruz, NFL C-15; diluted 1:500) raised against C-terminal sequences of human NFL, a rabbit antiserum against a N-terminal sequence of NFL (Chemicon; diluted 1:1000), and a mouse monoclonal antibody against vimentin (Sigma, clone V9; dilution 1:200). For cells expressing both WT NFL and E210X mutant, we typically used the combination of the rabbit antiserum against the N-terminus of NFL (Chemicon) and a goat antiserum against the C-terminus of NFL (Santa Cruz).
For immunoblotting, cells were pelleted in cold Dulbecco's PBS (Life Technologies), lysed in ice-cold buffer (50 mM Tris, pH=7.0, 1% SDS, and 0.017mg/ml phenylmethylsulfonyl fluoride) containing Protease Inhibitor Cocktail (10 μl/ml, Sigma), followed by brief sonication on ice (Fisher Scientific). The protein concentration was determined (Bio-Rad Laboratories), and the lysate was incubated in loading buffer at 95°C for 4 min, separated on a 12% acrylamide 0.1% SDS gel, transferred to an Immobilon polyvinylidene fluoride membrane (Millipore) over 1 hour using a semidry transfer unit (Fisher Scientific). The blot was blocked (5% powdered skim milk and 0.5% Tween 20 in Tris-buffered saline) for 1 hour, and hybridized with a rabbit antiserum against the N-terminus of NFL (Chemicon; 1:10,000) or a goat antiserum against the C-terminus of NFL (Santa Cruz; 1:1000) overnight at 4°C. The membranes were washed, incubated with a donkey anti-rabbit antibody or an anti-goat antiserum coupled to peroxidase (Jackson ImmunoResearch 1:10,000) and visualized by enhanced chemiluminescence (ECL kit, Amersham, Piscataway, NJ) according to the manufacturer's protocols.
Results
Characterization of a family with a recessive E210X mutation
We identified a two-generational family from Palestine in which CMT segregated as an autosomal recessive trait (Fig. 1 and Table 1), affecting 4 of 5 children of consanguineous parents (first cousins). The index patient (II-4) presented at age 2 years with toe walking, and the family has subsequently been evaluated yearly in a neuromuscular clinic. All 4 affected individuals had hypotonia during infancy and early childhood, mildly delayed motor milestones, slowly progressive atrophy and weakness in distal muscles of the feet and hands, and bilateral pes cavus. At present, patient II-1 uses a walker and all others are ambulatory with bilateral molded ankle-foot orthoses. In addition, patient II-1 has moderate proximal leg weakness and a waddling gait; patient II-4 has mild to moderate proximal arm and shoulder girdle weakness. All have moderate sensory impairment in glove and stocking pattern. Their current CMT neuropathy scores 28 range from 16 to 27. None of them have ever had pathological reflexes, palpably enlarged nerves, ulcerated feet, cranial neuropathies, or clinically evident hearing loss. Although II-1 has learning difficulties, the other affected children do not, and three of them had normal MRIs of the brain. Both parents and the other sibling (II-2) are asymptomatic, with normal neurological examinations.
Table 1. Clinical features of patients with homozygous E210X mutations.
| Patients | II-1 | II-3 | II-4 | II-5 |
|---|---|---|---|---|
| Age of initial evaluation | 8 y | 2 y 8m | 2 y | 3 m |
| Age of onset (year) | ∼1.5 | ∼1.5 | ∼1.5 | ∼1.5 |
| Initial neuropathy symptoms | gait | gait | toe walking | delayed walking |
| Age (years/sex) | 23/F | 17/M | 16/M | 13/F |
| Motor*: arms: proximal | 4+ | 5 | 4+ | 5 |
| distal | 3 | 3 | 3 | 3 |
| legs: proximal | 4 | 5 | 4+ | 4 |
| distal | 0-1 | 3 | 3 | 1-2 |
| Sensory: pain/touch | normal | normal | normal | normal |
| position/vibration | ↓ at elb/kn | ↓ at wr/ank | ↓ at wr/ank | ↓ at elb/kn |
| Ankle jerks | absent | absent | absent | absent |
| Pes cavus | yes | no | yes | yes |
| Foot/ankle surgery | yes | no | yes | yes |
| Ambulation aids | walker | MAFO | MAFO | MAFO |
| CMT neuropathy score | 27 | 16 | 19 | 26 |
| MRI (brain) | normal | normal | normal | not done |
| VERs (P100 latency, L/R) | 116/126 ms | 132/129 ms | 123/124 ms | 108/122 ms |
MRC rating scale; elb/kn: elbows/knees; wr/ank: wrists/ankles; MAFO: molded ankle-foot arthosis; VERs: visual evoked responses; L/R: left/right
Nerve conduction studies were performed on all family members (including the asymptomatic parents and the asymptomatic sister), using surface electrodes (Table 2). Needle EMG was performed on all symptomatic patients. The sural and peroneal responses were normal in both parents and their asymptomatic child (II-2). In the affected children, the sural, ulnar, median and radial sensory responses were all absent, except for one ulnar response in patient II-3 at age 4 y and one radial response in patient II-5 at age 10; these had low amplitudes (4.5 and 17.0 μV, respectively) and slowed conduction velocities (14 and 9 m/s, respectively). The median and ulnar motor responses were severely slowed, ranging from 12 to 25 m/s, and had progressively smaller amplitudes with age. The peroneal and tibial responses were similarly slowed and progressively reduced with age, and became unobtainable in every affected child. EMG confirmed severe denervation in distal muscles. Visual evoked responses (VERs) were done in 2008, and were prolonged in three of four affected children (Table 1).
Table 2. Electrophysiologic data in initial and follow up studies.
| Patient | Age | SNAP (uV) | Motor NCV/DL (m/s) | CMAP (mV) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | M | U | R | M | U | P | T | M | U | P | T | ||
| II-5 | 4m | - | - | - | - | 12/6.1 | 18/6.3 | 13/4.5 | 11/8.1 | 3.8 | 2.6 | 0.5 | 0.8 |
| 10y | 0 | 0 | - | 17 | 18/13.5 | 20/9.8 | 1.8 | 2.4 | 0 | 0 | |||
| 13y | - | - | 0 | - | 14/10.9 | 21/6.9 | - | - | 1.2 | 1.8 | - | - | |
|
| |||||||||||||
| II-4 | 3y | 0 | - | 0 | - | 25/5.5 | - | 19/5.9 | 21/5.5 | 7.5 | - | 0.9 | 8.8 |
| 8y | - | - | 0 | 0 | 22/7.2 | 20/6.6 | 25/5.7 | 4.6 | 0.5 | 0 | 0.6 | ||
| 14y | 0 | - | - | 0 | 22/11.9 | 14/8.8 | 1.7 | 0.3 | 0 | 0 | |||
| 16y | - | - | 0 | - | 22/10.2 | 17/9.5 | - | - | 1.2 | 0.1 | - | - | |
|
| |||||||||||||
| II-3 | 4y | 0 | - | 4.5 | - | 21/6.6 | - | 10/10.6 | 18/6.9 | 3.1 | - | 0.4 | 7.2 |
| 15y | 0 | 0 | - | 0 | 19/10.9 | 21/7.3 | 1.8 | 2.2 | 0 | 0 | |||
| 17y | - | - | 0 | - | 24/9.3 | - | - | - | 2.1 | - | - | - | |
|
| |||||||||||||
| II-1 | 10y | - | 0 | - | - | 21/11.0 | 23/8.0 | 14/13 | 4.1 | 4.3 | 0 | 1.0 | |
| 20y | 0 | 0 | - | 0 | 20/13.6 | 19/6.8 | 1.5 | 1.9 | 0 | 0 | |||
| 23y | - | - | 0 | - | - | 23/6.2 | - | - | - | 1.0 | - | - | |
SNAP: sensory nerve action potential; CMAP: compound motor action potential; DL: distal motor latency; NCV: nerve conduction velocity; M: median; U: ulnar; P: peroneal; T: tibial; S: sciatic; R: radial; -: not done
DNA from all family members was analyzed at Athena Diagnostics. All exons encoding the open reading frame of the NEFL gene (Genbank no. NM_006158 for cDNA) were amplified by PCR, and both strands were sequenced. At position 628 in the cDNA (c.628), a homozygous G>T transversion was detected (c.628G>T), predicted to result in a glutamate>AMBER change at position 210 (E210X), in all four affected children. Both parents were heterozygous for this mutation, and their unaffected child did not harbor it (Fig. 1). Sequencing other genes that cause demyelinating forms of CMT (PMP22, GJB1/Cx32, MPZ, EGR2, PRX) and recessive axonal neuropathies (LMNA, GDAP1, and MFN2) in patients II-3 or II-1 did not show additional mutations, nor was the PMP22 gene duplicated or deleted. This is the first reported example of a homozygous NELF mutation associated with a severe, recessively inherited neuropathy.
Pathological findings in a sural nerve biopsy
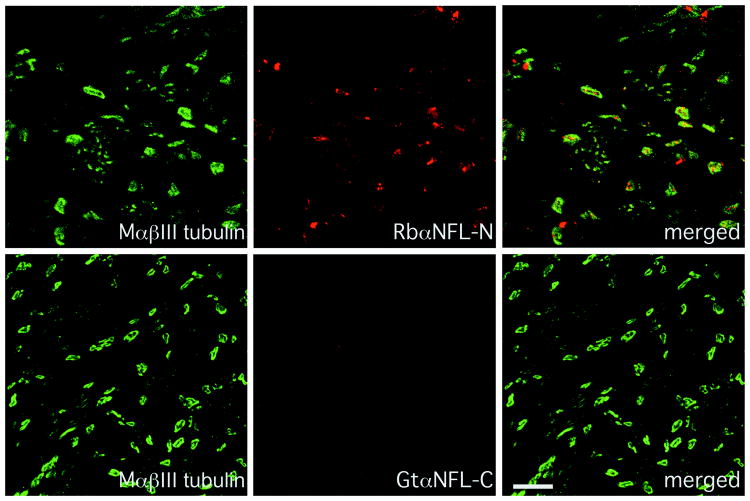
To provide more evidence that the recessive mutation was the cause of the neuropathy, we obtained a sural nerve biopsy from patient II-4 when he was 16 years old. To evaluate whether NFL was present, we immunostained sections of the biopsy with antisera against NFL. As shown in Fig. 2, an antiserum against the N-terminus labeled axons, as shown by the co-localization with βIII tubulin, an axon-specific tubulin 29, indicating that axons contained the E210X mutant protein. In contrast, an antiserum against the C-terminus of NFL (which was predicted to be absent in the E210X mutant protein), did not label the axons. Both antisera labeled axons in a biopsy from a patient with CMT4F (who had compound heterozygous PRX mutations; Supplemental Fig. 2), and neither labeled axons in the sciatic nerves (Supplemental Fig. 3) or spinal cord (data not shown) of Nefl-null mice, demonstrating their specificity.
Fig. 2. Altered NFL staining in a sural nerve biopsy.
These are confocal images of transverse sections from the sural nerve biopsy of patient II-4 at age 16 (who is homozygous for the E210X mutation), immunostained as indicated. Note the complete lack of staining with the goat antiserum against the C-terminus of NFL, whereas the rabbit antiserum against the N-terminus labels the axons, which are βIII tubulin-positive. Scale bar: 10 μm.
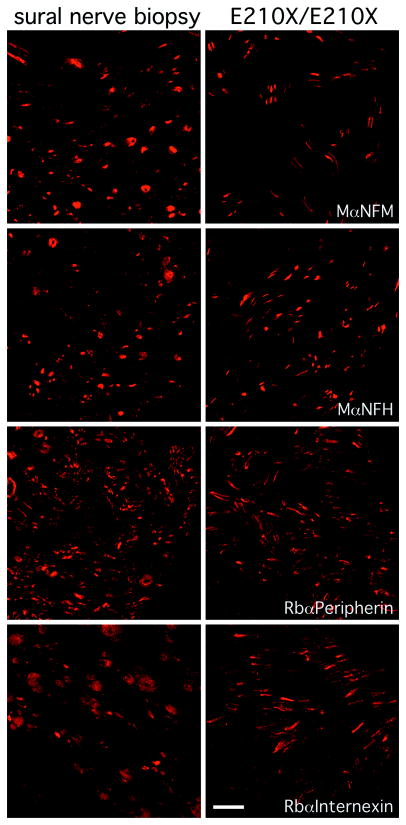
Both Nefl-null mouse and quiver quail (which have a recessive Q114stop mutation in Nefl 30) have low but detectable NFM- and NFH-immunoreactivity within axons even in the complete absence of NFL 4, 31. We confirmed this finding in the sciatic nerves (Supplemental Fig. 3) and spinal cord (data not shown) of Nefl-null mice. We then used these antibodies to label axons in the biopsy from the patient with the homozygous E210X mutation; a biopsy from a patient with an idiopathic neuropathy was used for comparison. As shown in Fig. 3, both NFM and NFH were present within the axons of both samples. Axons in both biopsies were also positive for both peripherin and α-internexin (Fig. 3), two intermediate proteins that are also expressed in axons and that can co-assemble with NFM and NFH to form filaments 3, 32. Thus, there was immunoreactivity for all five known intermediate filament proteins of axons.
Fig. 3. Intermediate filament subunits in a sural nerve biopsy.
These are confocal images of transverse sections of the sural nerve biopsy from patient II-4 at age 16 (right column), and an adult patient with an idiopathic neuropathy (left column), immunostained as indicated. The axons in both biopsies are immunoreactive for NFM, NFH, peripherin, and α-internexin. Scale bar: 10 μm.
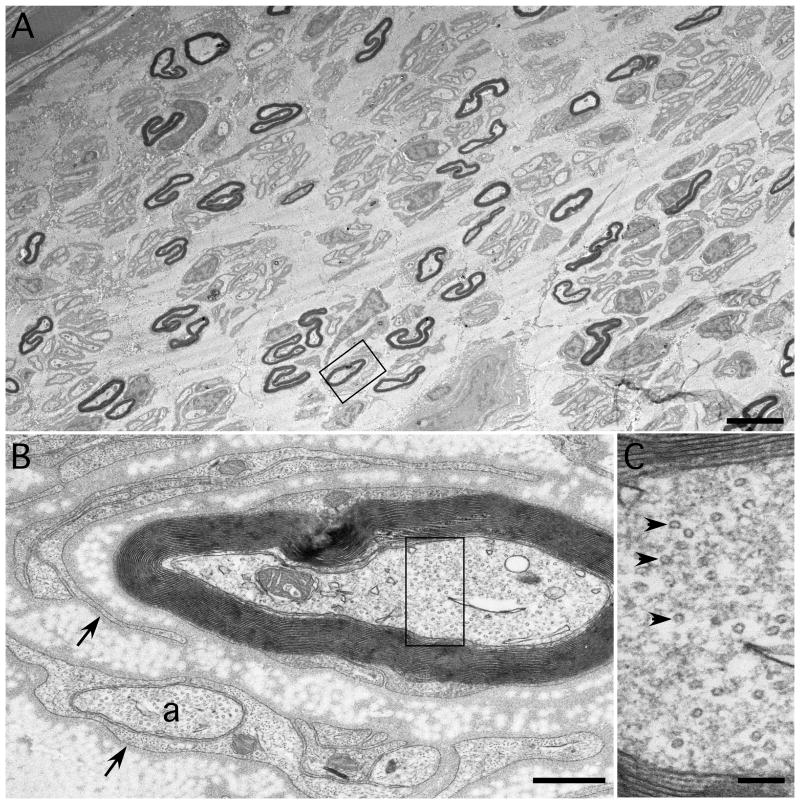
The homozygous E210X biopsy was also processed for electron microscopy. Transverse semi-thin sections (Supplemental Fig. 4) revealed that the number of myelinated axons was reduced: the density of myelinated axons was 1300/mm2, compared to ∼6000/ mm2 in normal controls 33. All myelinated axons were less than 3 microns in diameter, as has been described in Nefl-null mice 4, in which myelinated axons fail to enlarge during development. The myelinated axons themselves were often irregular in shape (Fig. 4). There were occasional clusters of regenerated axons, and some myelinated axons were partially surrounded by crescent of Schwann cell processes (Supplemental Fig. 5), but there was no evidence of actively degenerating axons (such as myelin debris). All fascicles were similarly affected. These results indicate that there is indolent but progressive axonal loss in patients with the homozygous E210X mutation.
Fig. 4. Ultrastructure of a sural nerve biopsy.
These are electron micrographs taken from a transverse section of a sural nerve biopsy from patient II-4 at age 16. In A, note that the reduced density of myelinated axons, all of which are small and many of which are irregularly shaped. The rectangle is enlarged in panel B, which shows a myelinated axon that is partially surrounded by Schwann cell processes (arrows), one of which encloses an unmyelinated axon (a). The rectangle is enlarged in panel C, which shows that the axon contains abundant microtubules (arrowheads), but no intermediate filaments. Scale bars: A, 5 μm; B, 0.5 μm; C, 0.1 μm.
To determine whether intermediate filaments were present in axons, we examined individual axons at high magnification. Myelinated axons had microtubules, but intermediate filaments were not present (Fig. 4C and Supplemental Fig. 5). Unmyelinated axons had microtubules and rare intermediate filaments (Supplemental Fig. 6). Schwann cells (especially those associated with unmyelinated axons or “denervated” Schwann cells) also had bundles of intermediate filaments. The absence of intermediate filaments in myelinated axons indicates that neither the truncated NFL mutant protein, nor the other intermediate filaments found in axons (peripherin and α-internexin) form intermediate filaments with NFM and NFH in myelinated PNS axons. In contrast, the non-myelinating and myelinating Schwann cells had intermediate filament proteins, likely glial fibrillary acid protein (GFAP) and vimentin 34, 35.
The E210X mutant is self-assembly incompetent
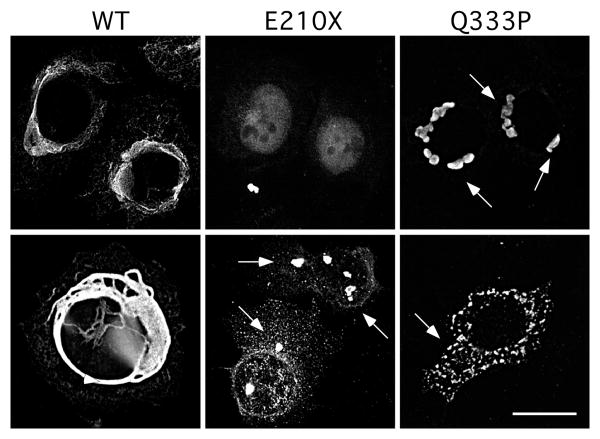
Expressing wild type (WT) human NEFL in SW-13 vim- cells, which lack intermediate filament proteins 36, results in a network of intermediate filaments comprised of NFL. The mutant NFL proteins encoded by dominant NEFL mutations, in contrast, do not form intermediate filaments 27, 37, 38. To determine whether the E210X mutation can form a filamentous network, we transfected SW-13 vim- cells to express the E210X mutant, and immuno-labeled them with an antiserum against the N-terminus of NFL. To exclude the possible reversion of the SW-13 vim- cells to the SW-13 vim+ cells, the cells were co-labeled with a mouse monoclonal antibody against vimentin, a non-neuronal intermediate filament. As shown in Fig. 5, the E210X mutant did not form filaments, but was typically found in a diffuse pattern, largely in the nucleus, with occasional cytoplasmic puncta in cells with higher levels of expression. As expected 27, 37-39, cells tranfected to express WT NFL formed bundles of filaments, whereas cells expressing the dominant mutant Q333P formed cytoplasmic aggregates. None of the cells examined expressed vimentin. Cells transfected with an “empty vector” had no NFL-immunoreactivity (data not shown). This experiment was repeated at least 6 times with similar results.
Fig. 5. The E210X mutant does not form filaments.
These are deconvolved images of SW-13 vim- cells, transiently transfected to express WT human NFL (WT), E210X, or Q333P, immunostained with a rabbit antiserum against the N-terminus of NFL and a mouse monoclonal antibody against vimentin (not shown), and counterstained with DAPI (not shown). Note that WT NFL forms a filamentous network, Q333P forms cytoplasmic aggregates (arrowheads), and E210X forms faint, diffuse puncta and occasional larger aggregrates (arrowheads). Scale bar: 20 μm.
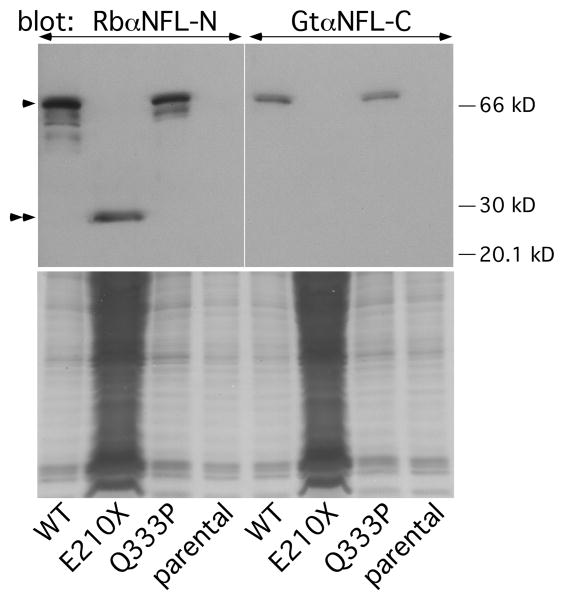
We corroborated these results by immoblotting lysates of transfected cells, using antisera against the N- or C-termini of NFL. As shown in Fig. 6, the N-terminal antiserum recognized a ∼30-kDa band in cells expressing E210X. This band was not found in lysates from cells expressing either WT NFL or Q333P; these lysates contained the expected ∼66 kDa band. Note that the E210X sample contains ∼30 times more protein relative to the lysates from cells expressing WT NFL or Q333P. The C-terminal antibody, moreover, detected the ∼66 kDa band in lysates from cells expressing either WT NFL or Q333P, but did not detect any bands in cells expressing E210X. Taken together, these results demonstrate that the E210X mutant does not accumulate properly in transfected cells, likely due to its inability to form filaments and/or enhanced degradation.
Fig. 6. Detection of the E210X mutant protein by immunoblotting.
The upper panel shows immunoblots of lysates from parental SW-13 vim- cells or cells transiently transfected to express WT human NFL (WT), E210X, or Q333P, probed with antisera against the N-terminus (left panel) or C-terminus (right panel) of NFL. Each lane contains 60 μg of lysate, except for the E210X sample, which was deliberately overloaded (∼1800 μg) to show the fainter NFL band (double arrowheads) of the truncated E210X mutant. The positions of the 20, 30, and 66 kDa size markers are shown, and full-length NFL protein is indicated by the single arrowhead. The lower panels are images of the Coomassie-stained gels after transfer.
The E210X mutant does not have dominant effects
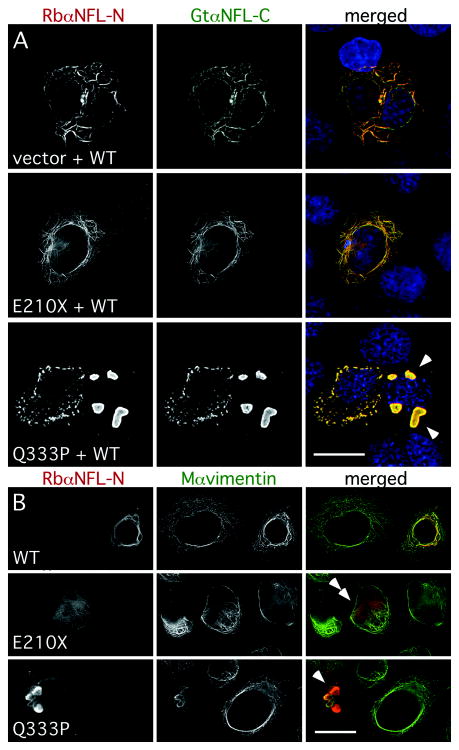
Six different dominant NFL mutants have been demonstrated to have a dominant effect on the ability of WT NFL to form filaments 27, 37, 38. Because the E210X mutation appears to be recessive, it may cause only a simple loss-of-function, and thus not disrupt the ability of WT NFL to form filaments. To investigate this possibility, we co-expressed WT NFL in the SW-13 vim- cells with the E210X mutant, the dominant mutant Q333P, or the “empty vector” (that was used to express the mutants). The cells were triple-labeled with a goat antiserum against the C-terminus (that does not detect E210X, Supplemental Fig. 7), a rabbit antiserum against the N-terminus (that detects E210X, Supplemental Fig. 7), and a mouse monoclonal antibody against vimentin. As shown in Fig. 7A, cells co-expressing WT NFL and E210X mutant formed filaments comparably to cells co-expressing WT NFL and an empty vector – mainly short, thin filaments, but to a lesser degree, extensive filamentous networks (all without vimentin; data not shown). In contrast, cells co-expressing WT NFL and Q333P largely formed intracellular aggregates of variable size as previously reported 38.
Fig. 7. The E210X mutant does not affect the ability of WT NFL or vimentin to form a network.
(A) These are deconvolved images of SW-13 vim- cells, transiently transfected to express WT human NFL (WT) plus “empty vector” (vector), E210X, or Q333P, then immunostained with a rabbit antiserum against the N-terminus of NFL (red), a goat antiserum against the C-terminus of NFL (green), a mouse monoclonal antibody against vimentin (not shown), and counterstained with DAPI (blue). Note the filamentous network of NFL staining in cells expressing WT NFL plus vector or E210X, whereas cells expressing both WT NFL and Q333P form cytoplasmic aggregates (arrowheads). Scale bar: 20 μm. (B) These are deconvolved images of SW-13 vim+ cells, transiently transfected to express WT human NFL (WT), E210X, or Q333P, then immunostained with a rabbit antiserum against the N-terminus of NFL (red) and a mouse monoclonal antibody against vimentin (green). Note that vimentin and NFL staining are co-localized in cells expressing WT NFL (forming a network) or Q333P (which collapses the network and forms cytoplasmic aggregates; arrowheads), but not in cells expressing E210X, which is found in small puncta/diffuse staining that did not disrupt vimentin assembly (double arrowheads). Scale bar: 20 μm.
To determine whether the E210X mutant affects the assembly of a vimentin network, we transfected the NFL constructs into the SW-13 vim+ cells, which express vimentin. The cells were labeled with the antiserum against the N-terminus of NFL and a mouse monoclonal antibody against vimentin. As shown in Fig. 7B, WT NFL and vimentin were co-localized, indicating that NFL was incorporated into the endogenous vimentin network. Consistent with a previous report 38, the Q333P mutant was also incorporated into the vimentin network, but often caused it to “collapse” into large aggregates. In contrast, cells expressing the E210X mutant had the same diffuse pattern of NFL staining as inb Vim- cells; this did not co-localize with the vimentin staining or affect the endogenous vimentin network. Thus, at least in these assays, the E210X mutant does not have a dominant effect.
Discussion
A homozygous NEFL E210X mutation causes a severe, early-onset neuropathy
Siblings with a homozygous E210X mutation develop a severe neuropathy that clinically begins in infancy. The neuropathy is characterized by moderate-to-severe slowing of conduction velocities and progressive axonal loss, resulting in moderate to severe impairment according to the CMT neuropathy score 28. In some ways, these children resemble those with Déjérine-Sottas neuropathy (hereditary motor and sensory neuropathy type III or CMT3 are alternative designations), who have delayed motor development before 3 years of age, sometimes extending to infancy 40-42. Motor abilities may improve during the first decade, but this may be followed by progressive weakness to the point that many affected individuals use wheelchairs. NCVs are typically very slow (<10 m/s), with marked temporal dispersion but no conduction block 43. Nerves are often enlarged, and biopsies often reveal prominent “onion bulbs” and a complete absence of fibers containing normal/thick myelin sheaths; in most cases, axons have inappropriately thin myelin sheaths for the axonal caliber and/or are segmentally demyelinated. Historically, Déjérine-Sottas neuropathy was thought to be recessively inherited, but new dominant mutations in MPZ and PMP22 are the commonest causes 44 (R. Ouvrier, personal communication); dominant mutations of EGR2, and recessive mutations of MPZ, PMP22, PRX, EGR2, and FIG4, are rarer causes 14-16.
The children we describe approximate the clinical description of Déjérine-Sottas neuropathy 45 (see discussion by Plante-Bordeneuve and Said 46), but their nerve conductions are faster (∼20 m/s) and their nerve biopsy findings are distinct. Bearing these distinctions in mind, we find the term “severe, early-onset axonal neuropathy” (SEOAN) 47 is a more fitting name for their neuropathy, but we emphasize that homozygous NEFL mutations should also be considered in evaluation of children with Déjérine-Sottas neuropathy. In OMIM (http://www.ncbi.nlm.nih.gov/entrez/), which unfortunately labels recessively inherited axonal neuropathies with the confusing term “CMT2B”, our patients should be called CMT2B5. Recessive mutations of LMNA cause CMT2B1, recessive mutations in an unknown gene cause CMT2B2, and CMT2B3 and CMT2B4 should be reserved for recessive mutations in GDAP1 48 and MFN2 47 that cause an axonal neuropathy. If it were possible to avoid the confusion caused by the fact that dominant MFN2 mutations also cause SEOAN, then we would propose using the term SEOAN in place of CMT2B.
The slowed nerve conduction velocities observed in this family are problematic but interesting. According to the conventional criterion that is used to separated demyelinating from axonal neuropathies, 38 m/s, their neuropathy would be classified as “demyelinating” 49. Unlike most hereditary demyelinating neuropathies, however, the gene that is mutated - NEFL - is not prominently expressed by myelinating Schwann cells 50. Although a role for NFL in myelinating Schwann cells cannot be formally excluded, the histological analysis of the biopsy indicates that the absence of large, myelinated axons is the likely explanation for the slowed conduction. The prior analysis of the Nefl-null mouse and quiver quail strongly supports this interpretation, as in both cases, myelinated axons lack intermediate filaments and fail to develop their proper diameters during development, presumably because the E210X mutant does not assemble with NFM and NFH. As predicted 51, 52, the reduced axonal caliber results in markedly slowed nerve conduction velocities well into the “demyelinating range” 4, 53-56. Because their nerve conduction velocities were highly slowed even in early childhood (Table 2), it is likely that myelinated axons do not develop their normal calibers, although we cannot exclude the possibility that axonal atrophy contributes to the smaller axonal diameters.
The biology of recessive and dominant NEFL mutants
The E210X mutation has not been found in more than 11,000 individuals analyzed at Athena Diagnostics (Dr. S.D. Batish, personal communication). Although the genetics in our family indicated a recessive inheritance of the E210X mutation, we do not know the prevalence of this mutation in the Palestinian population. To demonstrate that this mutation was pathogenic, we expressed it in transfected cells. The E210X mutant was expressed at a much lower level than WT NFL or Q333P. Given the structure of the NEFL gene 57, nonsense-mediated decay likely increases the degradation of the E210X mutant mRNA in vivo 58. In keeping with this expectation, the mRNA level of NFL (but not those of NFM or NFH) is drastically reduced in quiver quail (affected animals are homozygous for a nonsense mutation, Q114X 30). The low level of E210X protein in transfected cells, however, likely reflects its inability to form filaments and/or enhanced degradation; it is independent of nonsense-mediated decay because cDNAs were used.
Of the nearly 20 dominant NEFL mutations, nearly all are in exon 1 (which encodes amino acids 1-349; all the head domain and most of the rod domain), and nearly all are missense mutations (amino acid substitutions). Unlike our patients with homozygous E210X mutations, affected individuals have axonal neurofilaments, which appeared to accumulate in abnormally large masses in some (P22S, 22; L286P, 23) but not all cases (E90K, 19; E396K 23). In transfected cells, dominant NFL mutants fail to form filaments and typically have dominant effects on co-expressed WT NFL. In transfected neurons, at least some dominant mutants appear to alter the axonal transport of neurofilaments 27, 37, 38, 59 60.
The corresponding analysis suggests that the E210X mutant protein causes simple loss-of-function. In transfected cells, the E210X mutant did not form filaments and did not co-assemble with WT NFL. These finding differ from the prior work that showed C-terminal deletions of mouse NFL interfere with the ability of co-expressed WT mouse NFL or vimentin to assemble filaments 61. There are two plausible explanations for this difference: mouse and human NFL behave differently because they are not identical, and the E210X was shorter than the ones analyzed by of Gill et al. (1990); L336X was their shortest deletion. Our results also differ from the effects of the T21frameshift mutation, which was identified as a heterozygous mutation in an isolated patient with a late-onset axonal neuropathy 62. Like the E210X mutant, this mutant did not assemble into filaments, but had a subtle effect on the assembly of WT NFL that we did not detect with the E210 mutant. If the T21frameshift mutation causes simple loss-of-function, then haplotype insufficiency of NEFL may be a cause of late-onset axonal neuropathy. Thus, it will be important to determine whether the heterozygous parents of our children develop an axonal neuropathy as they age.
Our results motivate a reconsideration of whether some putative polymorphisms of NEFL (http://www.molgen.ua.ac.be/CMTMutations) are recessive mutations. Whereas mutations that do not alter the amino acid sequences are almost certainly harmless polymorphisms, there are several putative polymorphisms that should alter the amino acid sequence of NFL. One of these, I213M, did not segregate with CMT in a family, but curiously did cause subtly altered neurofilament assembly in transfected cells 63, raising concerns about how to interpret the subtle perturbation caused by expressing the T21frameshift mutation (see above). Because individuals who are heterozygous for V76A, D468N, A498T, E525frameshift, and E527deleted are asymptomatic, these are probably not dominant mutations 17, 19, 64. Until individuals who are homozygous for these mutations have been identified, however, one cannot exclude the possibility that these are recessive mutations. This is feasible in the case of E527deleted, as 1% of Japanese patients are heterozygotes 65.
Neurofilaments are essential
The consequences of a homozygous recessive E210X mutation in humans underscore the unexpected importance of NFL in the biology of myelinated PNS axons. Unlike our patients, Nefl-null mice have no demonstrable axonal loss 4, 66, and quiver quail have minimal pathology 56, 67, 68. Provocative maneuvers, such as crushing peripheral nerves to provoke axonal regeneration, are required to discern differences between these mutant and WT animals 4, 69. If the E210X (human) and Q114X (quail) mutations result in loss-of-function only, as one would expect for the disrupted mouse Nefl gene, then this discrepancy likely relates to the much greater length of human nerves and/or the longer lifespan of humans.
Our study does not illuminate what essential roles neurofilaments serve in this regard, but altered axonal transport is an attractive possibility 37, 59, 70. In addition, the abnormal stoichiometry of neurofilaments due to the lack of NFL could also affect other intermediate filaments (such as α-internexin or peripherin), and contribute to the disease process 12. Finally, the finding that 3 of 4 affected children have prolonged VERs indicates that there is subclinical involvement of the myelinated axons in the CNS. The limited CNS involvement might owe to compensation by α-internexin, as has been demonstrated in Nefl-null mice 66.
Supplementary Material
Supplemental Fig. 1. Mutational analysis. These are the electrophoretograms of the DNA sequence harboring the c.628G>T mutation for individuals II-4 and I-2 as well as a normal control.
Supplemental Fig. 2. NFL staining in a sural nerve biopsy. These are confocal images of longitudinal sections of a sural nerve biopsy from a 10-year-old patient who has CMT4F, immunostained as indicated. Note the overlap of βIII tubulin-immunoreactivity with both the NFL-N- and the NFL-C-immunoreactivity. Scale bar: 10 μm.
Supplemental Fig. 3. NFM and NFH are present in Nefl-null peripheral axons. These are confocal images of transverse sections of sciatic nerves from adult WT (Nefl+/+) and Nefl-null (Nefl-/-) mice, immunostained as indicated. Each pair of images was exposed for the same amount of time. Compare to the Nefl+/+ nerve, the Nefl-/- nerve lacks NFL-N- and NFL-C-immunoreactivity, has fainter NFM- and NFH-immunoreactivity, and has smaller axons. Scale bar: 10 μm.
Supplemental Fig. 4. Light microscopy of the nerve biopsy. This is an image from a transverse section of one fascicle of the sural nerve biopsy of patient II-4 at age 16. Note the reduced density of myelinated fibers, which are all small in diameter. Scale bar: 10 μm.
Supplemental Fig. 5. Electron microscopy of a regenerated cluster. This is an electron micrograph from the sural nerve biopsy of patient II-4 at age 16. A myelinated axon is associated with Schwann processes (arrows), some of which enclose unmyelinated axons (a). n: Schwann cell nucleus. Scale bar: 0.5 μm.
Supplemental Fig. 6. Electron microscopy of a Remak bundle. This is an electron micrograph from the sural nerve biopsy of patient II-4 at age 16. Three unmyelinated axons (a) are surrounded by Schwann cell processes, in which bundles of intermediate filaments are circled. Scale bar: 0.1 μm.
Supplemental Fig. 7. Antisera against the N-terminus antiserum, but not the C-terminus, label the E210X mutant protein. These are deconvolved images of SW-13 vim- cells, transiently transfected to express WT human NFL (WT) or the E210X mutant, then immunostained as indicated. The RbαNFL-N (red), but not the GtαNFL-C (green), against the N- and C-termini of NFL, respectively, labels the E210X mutant. Scale bar: 20 μm.
Acknowledgments
We thank the family for their participation, Dr. W. Grover for referring the family, Dr. S.D. Batish (Athena Diagnostics) for performing the mutation analysis, Drs. R.K.H. Liem and R. Perez-Olle for the NFEL plasmid, Dr. R. Evans for the SW-13 cells, Dr. V.M.-Y. Lee for antibodies, Drs. J.-P. Julien and W.W. Schlaepfer for Nefl-null mice, S. Wong for technical assistance, and Drs. T. Ferguson, E. Lancaster, G. Nicholson, J. Orthmann-Murphy, R. Ouvrier, and W.W. Schlaepfer for discussions. This work was supported by the St. Christ Foundation as well as NIH grants KO8 DC005394 (to S.W.Y.) and RO1 NS43174 (to S.S.S.).
References
- 1.Friede RL, Samorajski T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat Rec. 1970;167:379–388. doi: 10.1002/ar.1091670402. [DOI] [PubMed] [Google Scholar]
- 2.Muma NA, Hoffman PN. Neurofilaments are intrinsic determinants of axonal caliber. Micron. 1993;24:677–683. [Google Scholar]
- 3.Lee MK, Xu Z, Wong PC, Cleveland DW. Neurofilaments are obligate heteropolymers in vivo. J Cell Biol. 1993;122:1337–1350. doi: 10.1083/jcb.122.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Q, Couillard-Despres S, Julien JP. Delayed maturation of regenerating myelinated axons in mice lacking neurofilaments. Exp Neurol. 1997;148:299–316. doi: 10.1006/exnr.1997.6654. [DOI] [PubMed] [Google Scholar]
- 5.Jacomy H, Zhu QZ, Couillard-Despres S, et al. Disrupting of type IV intermediate filament network in mice lacking the neurofilament medium and heavy subunits. J Neurochem. 1999;73:972–984. doi: 10.1046/j.1471-4159.1999.0730972.x. [DOI] [PubMed] [Google Scholar]
- 6.Elder GA, Friedrich VL, Bosco P, et al. Absence of the mid-sized neurofilament subunit decreases axonal calibers, levels of light neurofilament (NF-L), and neurofilament content. J Cell Biol. 1998;141:727–729. doi: 10.1083/jcb.141.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyer J, Peterson A. Neurofilament-deficient axons and perikaryla aggregates in viable transgenic mice expressing a neurofilament-b-galactosidase fusion protein. Neuron. 1994;12:389–405. doi: 10.1016/0896-6273(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 8.Cote F, Collard JF, Julien JP. Progressive neuronopathy in transgenic mice expressing the heavy neurofilament heavy chain - a mouse model of amyotrophic lateral sclerosis. Cell. 1993;73:35–46. doi: 10.1016/0092-8674(93)90158-m. [DOI] [PubMed] [Google Scholar]
- 9.Lee MK, Marzalek JR, Cleveland DW. A mutant neurofilament subunit causes massive, selective motor neuron death: implications for the pathogenesis of human motor neuron disease. Neuron. 1994;13:975–988. doi: 10.1016/0896-6273(94)90263-1. [DOI] [PubMed] [Google Scholar]
- 10.Collard J-F, Cote F, Julien J-P. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375:61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- 11.Ching GY, Chien C-L, Flores R, Liem RKH. Overexpression of a-internexin causes abnormal neurofilamentous accumulations and motor coordination deficits in transgenic mice. J Neurosci. 1999;19:2924–2986. doi: 10.1523/JNEUROSCI.19-08-02974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaulieu J-M, Jacomy H, Julien J-P. Formation of intermediate filament protein aggegates with disparate effects in two transgenic mouse models lacking the neurofilament light subunit. J Neurosci. 2000;20:5321–5328. doi: 10.1523/JNEUROSCI.20-14-05321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Marszalek JR, Lee MK, et al. Subunit composition of neurofilaments specifies axonal diameter. J Cell Biol. 1996;133:1061–1069. doi: 10.1083/jcb.133.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shy ME, Lupski JR, Chance PF, et al. Hereditary motor and sensory neuropathies: an overview of clinical, genetic, electrophysiologic, and pathologic features. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. 4th. Vol. 2. Philadelphia: Saunders; pp. 2005pp. 1623–1658. [Google Scholar]
- 15.Wrabetz L, Feltri ML, Kleopa KA, Scherer SS. Inherited neuropathies: clinical, genetic, and biological features. In: Lazzarini RA, editor. Myelin Biology and Disorders. Vol. 2. San Diego: Elsevier Academic Press; 2004. pp. 905–951. [Google Scholar]
- 16.Lupski JR, Garcia CA. Charcot-Marie-Tooth peripheral neuropathies and related disorders. In: Scriver CR, Beaudet AL, Sly WS, et al., editors. The Metabolic & Molecular Basis of Inherited Disease. Eighth. New York: McGraw-Hill; 2001. pp. 5759–5788. [Google Scholar]
- 17.Yoshihara T, Yamamoto M, Hattori N, et al. Identification of novel sequence variants in the neurofilament-light gene in a Japanese population: analysis of Charcot-Marie-Tooth disease patients and normal individuals. J Peripher Nerv Syst. 2002;7:221–224. doi: 10.1046/j.1529-8027.2002.02028.x. [DOI] [PubMed] [Google Scholar]
- 18.De Jonghe P, Mersivanova I, Nelis E, et al. Further evidence that neurofilament light chain gene mutations can cause Charcot-Marie-Tooth disease type 2E. Ann Neurol. 2001;49:245–249. doi: 10.1002/1531-8249(20010201)49:2<245::aid-ana45>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Jordanova A, De Jonghe P, Boerkoel CF, et al. Mutations in the neurofilament light chain gene (NEFL) cause early onset severe Charcot-Marie-Tooth disease. Brain. 2003;126:590–597. doi: 10.1093/brain/awg059. [DOI] [PubMed] [Google Scholar]
- 20.Georgiou D-M, Zidar J, Korosec M, et al. A novel NF-L mutation Pro22Ser is associated with CMT2 in a large Slovenian family. Neurogenetics. 2002;4:93–96. doi: 10.1007/s10048-002-0138-4. [DOI] [PubMed] [Google Scholar]
- 21.Lus G, Nelis E, Jordanova A, et al. Charcot-Marie-Tooth disease with giant axons - A clinicopathological and genetic entity. Neurology. 2003;61:988–990. doi: 10.1212/wnl.61.7.988. [DOI] [PubMed] [Google Scholar]
- 22.Fabrizi GM, Cavallaro T, Angiari C, et al. Giant axon and neurofilament accumulation in Charcot-Marie-Tooth disease type 2E. Neurology. 2004;62:1429–1431. doi: 10.1212/01.wnl.0000120664.07186.3c. [DOI] [PubMed] [Google Scholar]
- 23.Fabrizi GM, Cavallaro T, Angiari C, et al. Charcot-Marie-Tooth disease type 2E, a disorder of the cytoskeleton. Brain. 2007;130:394–403. doi: 10.1093/brain/awl284. [DOI] [PubMed] [Google Scholar]
- 24.Mersiyanova IV, Perepelov AV, Polyakov AV, et al. A new variant of Charcot-Marie-Tooth disease type 2 is probably the result of a mutation in the neurofilament-light gene. Amer J Hum Genet. 2000;67:37–46. doi: 10.1086/302962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miltenberger-Miltenyi G, Janecke AR, Wanschitz JV, et al. Clinical and electrophysiological features in Charcot-Marie-Tooth disease with mutations in the NEFL gene. Arch Neurol. 2007;64:966–970. doi: 10.1001/archneur.64.7.966. [DOI] [PubMed] [Google Scholar]
- 26.Scherer SS, Xu Y-T, Nelles E, et al. Connexin32-null mice develop a demyelinating peripheral neuropathy. Glia. 1998;24:8–20. doi: 10.1002/(sici)1098-1136(199809)24:1<8::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Olle R, Jones ST, Liem RKH. Phenotypic analysis of neurofilament light gene mutations linked to Charcot-Marie-Tooth disease in cell culture models. Hum Mol Genet. 2004;13:2207–2220. doi: 10.1093/hmg/ddh236. [DOI] [PubMed] [Google Scholar]
- 28.Shy ME, Blake J, Krajewski K, et al. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology. 2005;64:1209–1214. doi: 10.1212/01.WNL.0000156517.00615.A3. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee A, Roach MC, Trcka P, Luduena RF. Increased microtubule assembly in bovine brain tubulin lacking the type III isotype of b-tubulin. J Biol Chem. 1990;265:1784–1499. [PubMed] [Google Scholar]
- 30.Ohara O, Gahara Y, Miyake T, et al. Neurofilament deficiency in quail caused by nonsense mutation in neurofilament-L gene. J Cell Biol. 1993;121:387–395. doi: 10.1083/jcb.121.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamasaki H, Bennett GS, Itakura C, Mizutani M. Defective expression of neurofilament protein subunits in hereditary hypotrophic axonopathy of quail. Lab Invest. 1992;66:734–743. [PubMed] [Google Scholar]
- 32.Hirokawa N, Glicksman MA, Willard MB. Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton. J Cell Biol. 1984;98:1523–1536. doi: 10.1083/jcb.98.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malik RA, Tesfaye S, Newrick PG, et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia. 2005;48:578–585. doi: 10.1007/s00125-004-1663-5. [DOI] [PubMed] [Google Scholar]
- 34.Fields KL, Yen SH. A subset of Schwann cells in peripheral nerves contain a 50 KD protein antigenically related to astrocyte intermediate filaments. J Neuroimmunol. 1985;8:311–330. doi: 10.1016/s0165-5728(85)80070-9. [DOI] [PubMed] [Google Scholar]
- 35.Neuberger TJ, Cornbrooks CJ. Transient modulation of Schwann cell antigens after peripheral nerve transection of subsequent regeneration. J Neurocytol. 1989;18:695–710. doi: 10.1007/BF01187088. [DOI] [PubMed] [Google Scholar]
- 36.Sarria AJ, Nordeen SK, Evans RM. Regulated expression of vimentin cDNA in cells in the presence and absence of a preexisting vimentin filament network. J Cell Biol. 1990;111:553–565. doi: 10.1083/jcb.111.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownlees J, Ackerley S, Grierson AJ, et al. Charcot-Marie-Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum Mol Genet. 2002;11:2837–2844. doi: 10.1093/hmg/11.23.2837. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Olle R, Leung CL, Liem RKH. Effects of Charcot-Marie-Tooth-linked mutations of the neurofilament light subunit on intermediate filament formation. J Cell Sci. 2002;115:4937–4946. doi: 10.1242/jcs.00148. [DOI] [PubMed] [Google Scholar]
- 39.Carter J, Gragerov A, Konvicka K, et al. Neurofilament (NF) assembly; divergent characteristics of human and rodent NF-L subunits. J Biol Chem. 1998;273:5105–5108. doi: 10.1074/jbc.273.9.5101. [DOI] [PubMed] [Google Scholar]
- 40.Plante-Bordeneuve V, Parman Y, Guiochon-Mantel A, et al. The range of chronic demyelinating neuropathy of infancy: a clinico-pathological and genetic study of 15 unrelated cases. J Neurol. 2001;248:795–803. doi: 10.1007/s004150170096. [DOI] [PubMed] [Google Scholar]
- 41.Gabreëls-Festen A. Dejerine-Sottas syndrome grown to maturity: overview of genetic and morphological heterogeneity and follow-up of 25 patients. J Anat. 2002;200:341–356. doi: 10.1046/j.1469-7580.2002.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouvrier RA, McLeod JG, Pollard JD. Peripheral Neuropathy in Childhood. 2nd. London: MacKeith Press; 1999. p. 335. [Google Scholar]
- 43.Benstead T, Kuntz N, Miller R, Daube J. The elecrophysiologic profile of Déjérine-Sottas disease (HMSN III) Muscle Nerve. 1990;13:586–592. doi: 10.1002/mus.880130705. [DOI] [PubMed] [Google Scholar]
- 44.Nelis E, Haites N, Van Broeckhoven C. Mutations in the peripheral myelin genes and associated genes in inherited peripheral neuropathies. Hum Mutat. 1999;13:11–28. doi: 10.1002/(SICI)1098-1004(1999)13:1<11::AID-HUMU2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 45.Dejerine J, Sottas J. Sur la nevrite interstitielle, hypertrophique et progressive de l'enfance. C R Soc Biol. 1893;45:63–96. [Google Scholar]
- 46.Plante-Bordeneuve V, Said G. Dejerine-Sottas disease and hereditary demyelinating neuropathy of infancy. Muscle Nerve. 2002;26:608–621. doi: 10.1002/mus.10197. [DOI] [PubMed] [Google Scholar]
- 47.Nicholson GA, Magdelaine C, Zhu D, et al. Severe early-onset axonal neuropathy with homozygous and compound heterozygous MFN2 mutations. Neurology. 2008;70:1678–1681. doi: 10.1212/01.wnl.0000311275.89032.22. [DOI] [PubMed] [Google Scholar]
- 48.Cuesta A, Pedrola L, Sevilla T, et al. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat Genet. 2002;30:22–25. doi: 10.1038/ng798. [DOI] [PubMed] [Google Scholar]
- 49.Harding AE, Thomas PK. The clinical features of hereditary motor and sensory neuropathy types I and II. Brain. 1980;103:259–280. doi: 10.1093/brain/103.2.259. [DOI] [PubMed] [Google Scholar]
- 50.Fabrizi C, Kelly BM, Gillespie CS, et al. Transient expression of the neurofilament proteins NF-L and NF-M by Schwann cells is regulated by axonal contact. J Neurosci Res. 1997;50:291–299. doi: 10.1002/(SICI)1097-4547(19971015)50:2<291::AID-JNR17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 51.Arbuthnott EB, Boyd IA, Kalu KU. Ultrastructural dimensions of myelinated peripheral nerve fibres in the cat and their relation to conduction velocity. J Physiol. 1980;308:125–157. doi: 10.1113/jphysiol.1980.sp013465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffman PN, Cleveland CW, Griffin JW, et al. Neurofilament gene expression: A major determinant of axonal caliber. Proc Natl Acad Sci USA. 1987;84:3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kriz J, Zhu QZ, Julien JP, Padjen AL. Electrophysiological properties of axons in mice lacking neurofilament subunit genes: disparity between conduction velocity and axon diameter in absence of NF-H. Brain Res. 2000;885:32–44. doi: 10.1016/s0006-8993(00)02899-7. [DOI] [PubMed] [Google Scholar]
- 54.Elder GA, Friedrich VL, Lazzarini RA. Schwann cells and oligodendrocytes read distinct signals in establishing myelin sheath thickness. J Neurosci Res. 2001;65:493–499. doi: 10.1002/jnr.1179. [DOI] [PubMed] [Google Scholar]
- 55.Sakaguchi T, Okada M, Kitamura T, Kawasaki K. Reduced diameter and conduction velocity of myelinated fibers in the sciatic nerve of a neurofilament-deficient mutant quail. Neurosci Lett. 1993;153:65–68. doi: 10.1016/0304-3940(93)90078-y. [DOI] [PubMed] [Google Scholar]
- 56.Zhao JX, Ohnishi A, Itakura C, et al. Smaller number of large myelinated fibers and focal myelin thickening in mutant quails deficient in neurofilaments. Acta Neuropathol. 1993;86:242–248. doi: 10.1007/BF00304138. [DOI] [PubMed] [Google Scholar]
- 57.Julien J-P, Grosveld F, Yazdanbaksh K, et al. The structure of a human neurofilament gene (NF-L): a unique exon-intron organization in the intermediate filament gene family. Biochim Biophy Acta. 1987;909:10–20. doi: 10.1016/0167-4781(87)90041-8. [DOI] [PubMed] [Google Scholar]
- 58.Baker KE, Parker R. Nonsense-mediated mRNA decay: terminating erroneous gene expression. Curr Opin Cell Biol. 2004;16:293–299. doi: 10.1016/j.ceb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Perez-Olle R, Lopez-Toledano MA, Goryunov D, et al. Mutations in the neurofilament light gene linked to Charcot-Marie-Tooth disease cause defects in transport. J Neurochem. 2005;93:861–874. doi: 10.1111/j.1471-4159.2005.03095.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhai JB, Lin H, Julien JP, Schlaepfer WW. Disruption of neurofilament network with aggregation of light neurofilament protein: a common pathway leading to motor neuron degeneration due to Charcot-Marie-Tooth disease-linked mutations in NFL and HSPB1. Hum Mol Genet. 2007;16:3103–3116. doi: 10.1093/hmg/ddm272. [DOI] [PubMed] [Google Scholar]
- 61.Gill SR, Wong PC, Monteiro MJ, Cleveland DW. Assembly properties of dominant and recessive mutations in the small mouse neurofilament (NF-L) subunit. J Cell Biol. 1990;111:2005–2019. doi: 10.1083/jcb.111.5.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leung CL, Nagan N, Graham TH, Liem RKH. A novel duplication/insertion mutation of NEFL in a patient with Charcot-Marie-Tooth disease. Am J Med Genet A. 2006;140:1021–1025. doi: 10.1002/ajmg.a.31242. [DOI] [PubMed] [Google Scholar]
- 63.Kabzinska D, PerezOlle R, Goryunov D, et al. Is a novel I214M substitution in the NEFL gene a cause of Charcot-Marie-Tooth disease? Functional analysis using cell culture models J Peripher Nerv Syst. 2006;11:225–231. doi: 10.1111/j.1529-8027.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 64.Andrigo C, Boito C, Prandini P, et al. A novel out-of-frame mutation in the neurofilament light chain gene (NEFL) does not result in Charcot-Marie-Tooth disease type 2E. Neurogenetics. 2005;6:49–50. doi: 10.1007/s10048-004-0202-3. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto M, Yoshihara T, Hattori N, Sobue G. Glu528del in NEFL is a polymorphic variant rather than a disease-causing mutation for Charcot-Marie-Tooth disease in Japan. Neurogenetics. 2004;5:75–77. doi: 10.1007/s10048-003-0159-7. [DOI] [PubMed] [Google Scholar]
- 66.Levavasseur F, Zhu QZ, Julien J-P. No requirement of a-internexin for nervous system development and for radial growth of axons. Mol Brain Res. 1999;69:104–112. doi: 10.1016/s0169-328x(99)00104-7. [DOI] [PubMed] [Google Scholar]
- 67.Yamasaki H, Itakura C, Mizutani M. Hereditary hypotrophic axonopathy with neurofilament deficiency in a mutant strain of the Japanese quail. Acta Neuropathol. 1991;82:427–434. doi: 10.1007/BF00293376. [DOI] [PubMed] [Google Scholar]
- 68.Zhao JX, Ohnishi A, Itakura C, et al. Smaller axon and unaltered numbers of microtubules per axon in relation to number of myelin lamellae of myelinated fibers in the mutant quail deficient in neurofilaments. Acta Neuropathol. 1995;89:305–312. doi: 10.1007/BF00309623. [DOI] [PubMed] [Google Scholar]
- 69.Jiang XM, Zhao JX, Ohnishi A, et al. Regeneration of myelinated fiber after crush injury is retarded in sciatic nerves of mutant Japanese quails deficient in neurofilaments. Acta Neuropathol. 1996;92:467–472. doi: 10.1007/s004010050548. [DOI] [PubMed] [Google Scholar]
- 70.de Waegh SM, Lee VM-Y, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Mutational analysis. These are the electrophoretograms of the DNA sequence harboring the c.628G>T mutation for individuals II-4 and I-2 as well as a normal control.
Supplemental Fig. 2. NFL staining in a sural nerve biopsy. These are confocal images of longitudinal sections of a sural nerve biopsy from a 10-year-old patient who has CMT4F, immunostained as indicated. Note the overlap of βIII tubulin-immunoreactivity with both the NFL-N- and the NFL-C-immunoreactivity. Scale bar: 10 μm.
Supplemental Fig. 3. NFM and NFH are present in Nefl-null peripheral axons. These are confocal images of transverse sections of sciatic nerves from adult WT (Nefl+/+) and Nefl-null (Nefl-/-) mice, immunostained as indicated. Each pair of images was exposed for the same amount of time. Compare to the Nefl+/+ nerve, the Nefl-/- nerve lacks NFL-N- and NFL-C-immunoreactivity, has fainter NFM- and NFH-immunoreactivity, and has smaller axons. Scale bar: 10 μm.
Supplemental Fig. 4. Light microscopy of the nerve biopsy. This is an image from a transverse section of one fascicle of the sural nerve biopsy of patient II-4 at age 16. Note the reduced density of myelinated fibers, which are all small in diameter. Scale bar: 10 μm.
Supplemental Fig. 5. Electron microscopy of a regenerated cluster. This is an electron micrograph from the sural nerve biopsy of patient II-4 at age 16. A myelinated axon is associated with Schwann processes (arrows), some of which enclose unmyelinated axons (a). n: Schwann cell nucleus. Scale bar: 0.5 μm.
Supplemental Fig. 6. Electron microscopy of a Remak bundle. This is an electron micrograph from the sural nerve biopsy of patient II-4 at age 16. Three unmyelinated axons (a) are surrounded by Schwann cell processes, in which bundles of intermediate filaments are circled. Scale bar: 0.1 μm.
Supplemental Fig. 7. Antisera against the N-terminus antiserum, but not the C-terminus, label the E210X mutant protein. These are deconvolved images of SW-13 vim- cells, transiently transfected to express WT human NFL (WT) or the E210X mutant, then immunostained as indicated. The RbαNFL-N (red), but not the GtαNFL-C (green), against the N- and C-termini of NFL, respectively, labels the E210X mutant. Scale bar: 20 μm.