Abstract
Environmental influences and insults by reproductive toxicant exposure can lead to impaired spermatogenesis or infertility. Understanding how toxicants disrupt spermatogenesis is critical for determining how environmental factors contribute to impaired fertility. While current animal models are available, understanding of the reproductive toxic effects on human fertility requires a more robust model system. We recently demonstrated that human pluripotent stem cells can differentiate into spermatogonial stem cells/spermatogonia, primary and secondary spermatocytes, and haploid spermatids; a model that mimics many aspects of human spermatogenesis. Here, using this model system, we examine the effects of 2-bromopropane (2-BP) and 1–2, Dibromo-3-chloropropane (DBCP) on in vitro human spermatogenesis. 2-BP and DBCP are non-endocrine disrupting toxicants that are known to impact male fertility. We show that acute treatment with either 2-BP or DBCP induces a reduction in germ cell viability through apoptosis. 2-BP and DBCP affect viability of different cell populations as 2-BP primarily reduces spermatocyte viability whereas DBCP exerts a much greater effect on spermatogonia. Acute treatment with 2-BP or DBCP also reduces the percentage of haploid spermatids. Both 2-BP and DBCP induce reactive oxygen species (ROS) formation leading to an oxidized cellular environment. Taken together, these results suggest that acute exposure with 2-BP or DBCP causes human germ cell death in vitro by inducing ROS formation. This system represents a unique platform for assessing human reproductive toxicity potential of various environmental toxicants in a rapid, efficient, and unbiased format.
Keywords: spermatogenesis, germ cell differentiation, reproductive toxicity, in vitro testing
Introduction
To date, most of the pioneering work in understanding the effects of environmental toxicants on male reproduction in pubertal and post-pubertal exposures has been done in mice and rats (for review,[1]) These rodent models, while very informative, possess challenges and limitations (for review, [1]) and typically focus on morphological/structural defects using histopathological studies to assess exposure-related damage. Because of the limitations of these models, sub-cellular effects of toxicant exposure are only now just being studied and are difficult to assess in the testis in vivo. Furthermore, these animal studies are laborious, time-consuming and expensive and may not replicate what happens in humans as rodent spermatogenesis is distinctly different from human spermatogenesis, with rodents possessing several spermatogonial amplifying events that humans do not have[2]. As such, human studies on the effects of toxicant exposure are largely relegated to clinical and epidemiological studies examining testis tissue defects or sperm quality. Thus for the case of human spermatogenesis, the underlying mechanisms by which environmental toxicants impair spermatogenesis are understudied and mainly extrapolated from work in rodents.
In 1996, the US Food Quality Protection Act ordered the EPA to implement a reproductive toxicant screening program to begin assessing the reproductive toxic potential for the large number of chemicals humans encounter[3]. The purpose of this Act was risk assessment for chemicals that potentially could contribute to human infertility[3]. To date, current models have been burdened by a lack of sensitivity leading to inconsistent or non-responses to toxicant exposures which in turn confound outcomes and lead to several false negative results. Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs), represent a unique system by which investigators can examine the effects of toxicants as these cells can be differentiated into any cell type in the adult organism, including germ cells[4–15]. Recently, we demonstrated that male human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs) are directly differentiated into adult-type spermatogonial stem cells/spermatogonia, pre-meiotic and post-meiotic spermatocytes, and post-meiotic spermatids[5]. Our work demonstrates the utility of hPSCs to be used to model several key aspects of human spermatogenesis in vitro and maintain critical windows of susceptibility necessary to evaluate the effects of reproductive toxicants on spermatogenic cell lineages. Likewise, this in vitro model system would permit determinations of sub-cellular mechanisms by which reproductive toxicants negatively impact human spermatogenesis and would allow investigators to examine whether mechanisms observed in rodents apply to human spermatogenic defects in a high throughput/high content approach.
1,2-dibromo-3-chloropropane (DBCP) is a banned nematicide that has been shown to cause male infertility[16–21]. In exposed workers, DBCP is gonadotoxic resulting in a loss of germ cells, including spermatogonia, causing oligo- or azoospermia, without affecting the somatic Leydig and Sertoli cells[16–21]. Clinical studies also indicate that DBCP interferes with meiosis as exposed male workers with observed sperm counts frequently generate aneuploid sperm[22]. Rodent studies on DBCP have been erratic as some models have shown gonadotoxicity, but others have not. Specifically, rats demonstrate less tolerance and higher lethality to lower doses of DBCP compared to their mouse counterparts[23]. Mouse models have indicated that DBCP does not induce spermatogonia cell death but instead blocks differentiation[24], a phenotype that to date has not been observed in human exposure cases. Likewise, mouse models indicate that Leydig cell loss plays a major role in DBCP-mediated infertility[25]. Thus a model that mimics human exposure phenotypes is needed to fully investigate the effects of DBCP on human spermatogenesis.
2-bromopropane (2-BP) was an alternative to ozone-depleting cleaning solvents and is also used in organic synthesis to add isopropyl groups to compounds[26]. Recently, the National Toxicology Program (NTP) concluded that there was sufficient evidence to suggest that 2-BP negatively impacts fertility in exposed males[27]. Occupational exposure to high levels of 2-BP has resulted in oligo- or azoospermia, and observations from clinical samples suggest that 2-BP reduces the number of premeiotic spermatocytes by affecting viability of both spermatogonia and spermatocytes by inducing germ cell apoptosis[28–33]. The effects of 2-BP exposure in humans appear to be on germ cell viability and not on somatic Leydig and Sertoli cell function or viability. Studies using mouse models indicated that 2-BP exposure in mice, like humans, shows a loss of spermatogonia and spermatocytes; but some studies implicate Leydig cell defects as the main factor behind 2-BP-mediated germ cell death in mice. This does not appear to be the case in humans. Even in the case of 2-BP, where rodent models mostly mimic human exposure phenotypes, a new model that simulates many aspects of human spermatogenesis would be of importance to examining the exact mechanism of how 2-BP interferes with human spermatogenesis, especially given that 2-BP is still used.
Here, we demonstrate that we can adapt our recently described human spermatogenic differentiation model to examine the effects of 2-BP and DBCP on in vitro human spermatogenesis. We demonstrate that our model mimics phenotypes observed in human exposure cases and that both 2-BP and DBCP directly affect germ cell viability in culture. Furthermore, we are able to utilize this system to examine one potential cellular mechanism by which 2-BP and DBCP affect germ cell survival. The work presented here also validates our model system as an appropriate platform for evaluating other environmental toxicants on human spermatogenic toxicity.
Material and Methods
Human Embryonic Stem Cell Culture and Differentiation
NIH-approved WA01 (H1, WiCell, Madison, WI) male human embryonic stem cells were cultured and maintained in mTeSR1 (STEMCELL Technologies, Vancouver, Canada) on matrigel (Corning Life Sciences, Tewksbury, MA) as previously described[34]. Direct differentiation into spermatogenic lineages was performed as described[5] but with the following modification: human recombinant basic fibroblast growth factor (hbFGF) and glial-derived neurotrophic factor (GDNF) were from Peprotech (Rocky Hill, NJ). The same concentrations (1 ng/ml bFGF and 20 ng/ml GDNF) were used as previously described[5].
Cell Viability and Apoptosis Assays
Global cell viability was assessed by the Cell Toxicity Assay Kit (Abcam, Cambridge, MA) as per manufacturer’s instructions. This kit is a modified MTT assay. Absorbance values for calculating viability were determined using an Epoch Plate Reader (BioTek, Winooski, VT). % viability for each treatment was normalized to controls as per manufacturer’s instructions. Values shown represent average values from 3 separate trials. Statistical significance was evaluated using paired student’s t test, p < 0.01. Apoptosis and caspase activation assays were conducted using a flow cytometry-based approach. The Muse® Annexin V and Dead Cell Assay Kit and the Muse® Caspase 3/7 Assay Kit (both kits EMD Millipore, Billerica, MA) were used as per manufacturer’s instructions to prepare samples for flow cytometry. Samples were run on the Muse® benchtop flow cytometer (EMD Millipore, Billerica, MA). For each flow cytometry-based experiment, 10,000 events were analyzed.
High Content Imaging and Analyses
High content imaging of differentiated human embryonic stem cells was performed on the ThermoFisher Cellomics ArrayScan® VTI (Thermofisher, Waltham, MA). Quantitative analyses for average PLZF+ colony size and average intensity of PLZF staining per colony was determined using HCS Studio™ 2.0 Cell Analysis Software included with the ArrayScan® suite. 1000 events were analyzed per treatment condition (n = 1000). Statistical significance was evaluated using paired student’s t test, p < 0.01.
Cell Cycle and ROS Assays
Cell cycle assays were performed to examine the percentage of haploid cells in differentiating spermatogenic cultures. Cell cycle analyses were conducted using the Muse® Cell Cycle Assay Kit (EMD Millipore, Billerica, MA) as per manufacturer’s instructions. 10,000 events were analyzed for each treatment and the percentage of haploid cells calculated. Samples were run on the Muse® benchtop flow cytometer (EMD Millipore, Billerica, MA). Measurements of ROS were also conducted using a flow cytometry based approach. ROS generation was assessed using the Muse® Oxidative Stress Kit (EMD Millipore, Billerica, MA) as per manufacturer’s instructions. This kit determines the percentage of cells that are negative for ROS (healthy cells) and positive for ROS (cells exhibiting ROS). 10,000 events were analyzed for each treatment, and samples were run on the Muse® benchtop flow cytometer (EMD Millipore, Billerica, MA). % ROS+ was averaged from three separate experiments. Statistical significance was evaluated using paired student’s t test, p < 0.01.
Reagents
Other reagents used that are not listed above include: 2-bromopropane (2-BP), 1,2-dibromo-3-chloropropane (DBCP), and l-sulforaphane (all from Sigma-Aldrich, St. Louis, MO); PLZF antibody (R&D Systems, Minneapolis, MN); and HILI antibody (Bioss, Inc., Woburn, MA). Secondary antibodies for immunostaining were from LifeTechnologies (Carlsbad, CA). Hoechst for DNA staining was from Cell Signaling Technologies (Danvers, MA).
Results
2-BP and DBCP Induce Germ Cell Death in a Human in vitro Spermatogenic Model
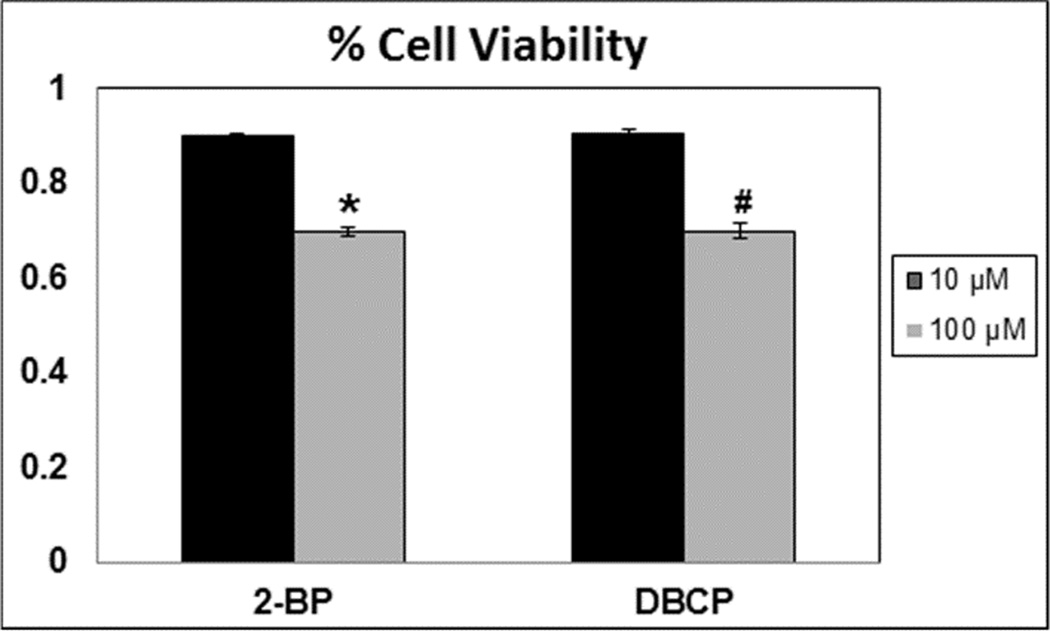
In the quest to satisfy the 1996 US Food Quality Protection Act ordering the implementation of a reproductive toxicant screening program, several models have been proposed (for review, [1]. However some of the proposed models have failed to recapitulate human reproductive toxicity as chemicals that normally induce germ cell death in humans failed to cause death in animal models (for review, [1]). Furthermore, whole testes analyses complicate sub-cellular examinations of defects caused by reproductive toxicants directly on spermatogenic cells. We recently developed an in vitro differentiation model using human ESCs and iPSCs that mimics many critical aspects of human spermatogenesis[5]. Specifically, we have previously shown that human pluripotent stem cells can be differentiated in vitro into adult-type stem and progenitor spermatonia. Secondly, differentiating stem cells give rise to cells that are phenotypically similar to premeiotic primary spermatocytes, post-meiotic secondary spermatocytes, and round spermatids[5]. Because this in vitro spermatogenic model more closely followed human spermatogenesis than rodent animal models, we sought to determine whether this model could be utilized for evaluate reproductive toxicity of various chemicals and examine underlying mechanisms of toxicity. As such, we first examined whether acute exposure to two known reproductive toxicants, 2-BP and DBCP, induces cell death. From clinical studies, 2-BP and DBCP have been shown to negatively affect human spermatogenesis by directly affecting germ cell viability rather than disrupt endocrine signaling from the testicular support cells[16–21, 28–33]. Human male embryonic stem cells (hESCs) were differentiated as described[5] for 10 days into advanced spermatogenic cells. This differentiation protocol reliably yields spermatogonial stem cells/spermatogonia, primary spermatocytes, post-meiotic secondary spermatocytes, and haploid spermatids. After 10 days differentiation, mixed germ cell cultures were treated 24 hr with either a low dose (10 µM) or high dose (100 µM) concentration of 2-BP or DBCP as indicated (Fig. 1). Higher concentrations of 2-BP or DBCP overnight significantly reduced cell viability, as assessed by a modified MTT assay (Cell Toxicity Assay Kit, Abcam) by approximately 30% (Fig. 1).
Figure 1. Known reproductive toxicants decrease total germ cell viability in hESCs differentiated into spermatogenic cells.
Graphical representation showing that increasing concentrations of 2-BP and DBCP induce germ cell death in hESCs differentiated in in vitro spermatogenic conditions. Acute treatment with each reproductive toxicant was for 24 hours. Data shown represent average values +/ standard deviation from 3 separate experiments. * represents p < 0.01 statistical significance for 2-BP, # represents p < 0.01 for DBCP. % Viability was determined by normalizing values for 2-BP and DBCP to control values from modified MTT Assays.
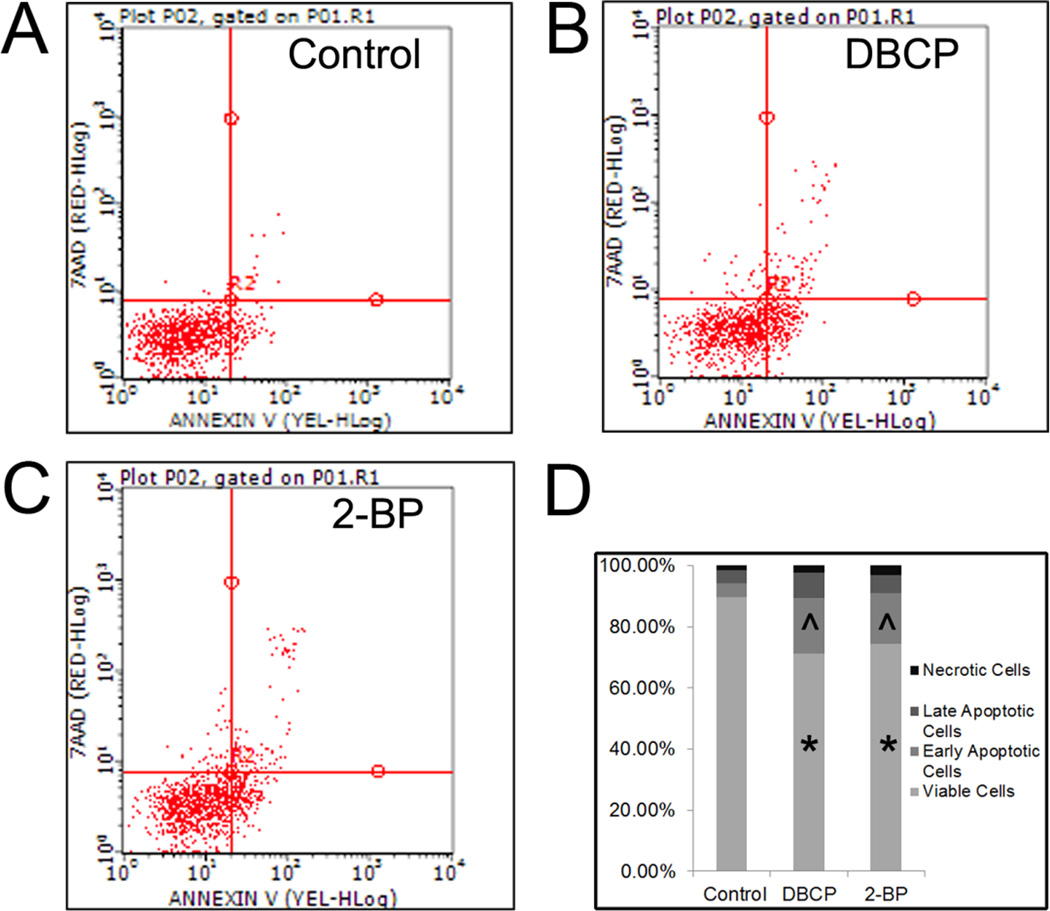
Because acute high exposure is known to cause infertility in workers exposed to either 2-BP or DBCP, we next examined whether acute, high dose exposure induced apoptosis in germ cells derived from hESCs in vitro. Using Annexin V flow cytometry analyses, we observed a significant increase in early apoptotic cells and a significant decrease in viable cells with 24 hr treatment with either 2-BP or DBCP (Fig. 2). Cell death from necrosis in our in vitro spermatogenic cultures was minimal as increased necrotic cell percentages in cultures treated with 2-BP or DBCP were not significant (Fig. 2D).
Figure 2. Annexin V analyses demonstrate that 2-BP and DBCP induce apoptosis in spermatogenic cells derived from hESCs.
Flow cytometry analyses indicating percent viable cells, percent early apoptotic cells, percent late apoptotic cells, and percent necrotic cells. Samples from three experiments for each condition were pooled and then 10,000 events were analyzed. (A) Vehicle control cells, (B) DBCP-treated cells, and (C) 2-BP-treated cells. Scatter plots for cell populations are shown. Lower left quadrant represents viable cells, lower right quadrant represents early apoptotic cells, upper right is late apoptotic, and upper left is necrotic. (D) Graphical summary of data collected in stacked graphs showing average percentages of cells in each population. * represents p < 0.01 statistical significance for decreased % of viable cells in DBCP and 2-BP treatments, ^ represents p < 0.01 statistical significance for increased % of early apoptotic cells in DBCP and 2-BP treatments.
One hallmark of apoptosis is the activation of cell death machinery caspases (caspase-3, -7) which are activated in response to both intrinsic and extrinsic cues (for review,[35]). By flow cytometry, we examined caspase-3, -7 activation in our in vitro spermatogenic cultures. Both 2-BP and DBCP treatment elevated the percentage of early apoptotic cells, late apoptotic cells and dead cells compared to vehicle control treated spermatogenic cells (Supplemental Fig. 1). The percentage of total apoptotic and dead cells with 2-BP or DBCP was approximately 49% and 42% respectively compared to control at approximately 26% (Supplemental Fig. 1). Taken together with the MTT assay results and Annexin V results, these data suggest that 2-BP and DBCP induce in vitro germ cell death via apoptosis during acute high-dose exposures.
2-BP and DBCP Affect Viability of Distinct Populations of Spermatogenic Cells
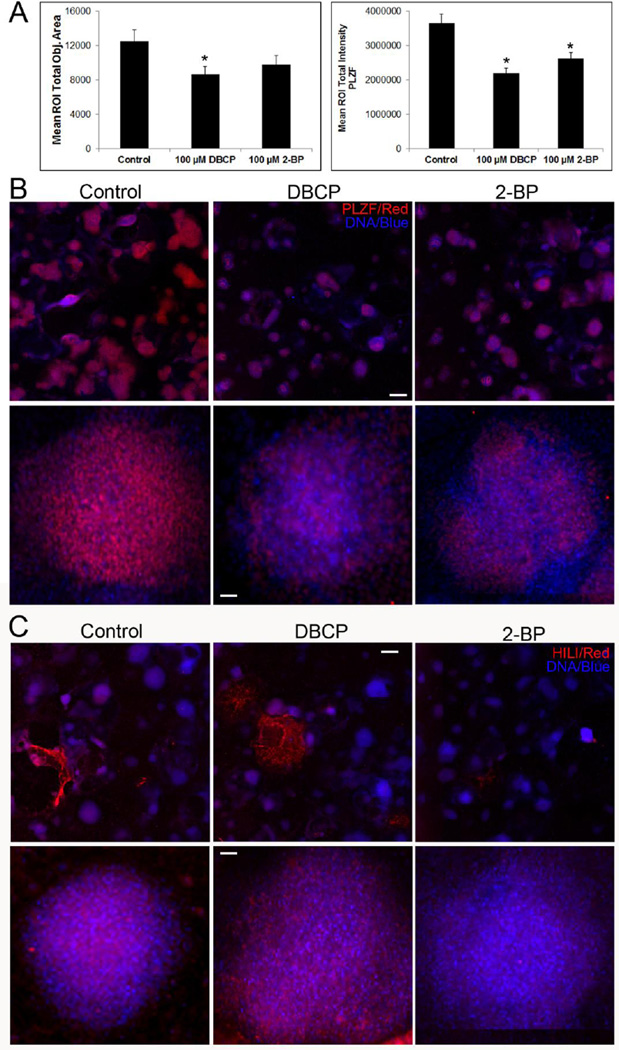
Once we observed that either 2-BP or DBCP induced germ cell death, we next sought to examine whether each toxicant affects a specific spermatogenic lineage. Results from epidemiological/clinical studies suggest that 2-BP and DBCP affect different spermatogenic cell lineages. Specifically, 2-BP depletes primary, pre-meiotic spermatocytes[28–33], whereas DBCP appears to target spermatogonial stem cells/spermatogonia[16–21]. To examine whether we observed a similar cell lineage-specific effect, we differentiated hESCs into spermatogenic lineages for 10 days, treated for 24 hr with 100 µM 2-BP or DBCP, and then analyzed for expression of the spermatogonial stem cell/spermatogonia maker PLZF (Fig. 3A and 3B) and the primary, pre-meiotic spermatocyte marker HILI (Fig. 3C). Using high content imaging, treatment with either 2-BP or DBCP reduced the number of PLZF+ colonies in differentiated hESCs (Fig. 3A, left panel and 3B, first row). Area measurements of PLZF+ colonies revealed that DBCP significantly reduced the area of PLZF+ colonies compared to vehicle control. 2-BP reduced colony area, but this reduction was not statistically significant (Fig. 3A, left panel). The overall intensity of PLZF staining, representing expression levels of PLZF, was significantly reduced by both DBCP and 2-BP (Fig. 3A, right panel). Closer examination of individual colonies showed a reduction in PLZF+ cells with both DBCP and 2-BP treatment (Fig. 3B, second row). In examination of HILI+ colonies and cells, DBCP showed no apparent reduction in HILI+ colonies compared to vehicle control treated cells, whereas, 2-BP reduced the number of HILI+ colonies, suggesting that 2-BP reduces the overall number of spermatocytes in culture (Fig. 3C, first row). Single colony examination showed no loss of HILI+ cells with DBCP treatment, but 2-BP treatment reduced the number of HILI+ cells (Fig. 3C, second row). These results suggest that DBCP and 2-BP affect viability of distinct populations of spermatogenic cells. Moreover, these results mimic phenotypic effects on spermatogenesis observed in human exposure cases.
Figure 3. DBCP and 2-BP reduce PLZF+ spermatogonia and HILI+ spermatocytes, respectively, in in vitro spermatogenic cultures.
(A) Graphical representation showing that 100 µM DBCP and 2-BP reduce overall colony size (left graph) and PLZF expression intensity (right graph) compared to controls. DBCP significantly reduces mean colony size area, whereas 2-BP and DBCP significantly reduce PLZF staining intensity. p < 0.01. (B) Representative 5× images obtained by the Cellomics ArrayScan VT1 of PLZF+ colonies used for analyses in (A). All images are taken under the same imaging conditions and parameters. Scale bar, 5000 µm. Second row depicts zoomed up images of representative individual colonies. Scale bar, 100 µm. (C) 2-BP reduces the number of HILI+ cells in culture. Representative images of HILI staining (red). Scale bar, 5000 µm. Second row shows zoomed representative images of individual colonies. Scale bar, 100 µm. Secondary-only control image is shown in Supplemental Figure 2.
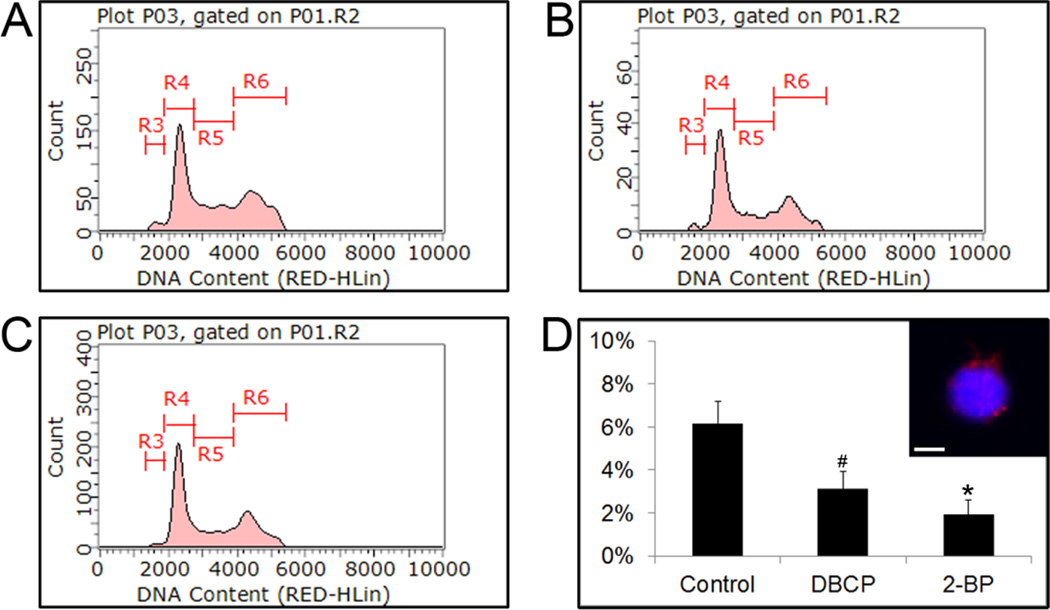
2-BP or DBCP Treatment Depletes Haploid Spermatids in in vitro Spermatogenic Cultures
In addition to observing germ cell death of spermatogonial stem cells/spermatogonia and primary spermatocytes, we next sought to examine whether acute treatment with either 2-BP or DBCP affected viability of haploid spermatids. In our differentiation protocol, we typically observed 5–6% haploid cell production with greater than 90% of these haploid spermatids representing round spermatids[5]. As above, we differentiated hESCs into spermatogenic lineages for 10 days and then treated 24 hr with either 100 µM 2-BP or DBCP. Acute treatment with either 2-BP or DBCP reduced the percentage of haploid cells (Fig. 4). Because the treatment was 24 hr, we conclude that the loss of haploid spermatids is most likely due to cell death and not necessarily a disruption in meiosis.
Figure 4. Acute treatment with reproductive toxicants 2-BP and DBCP reduce the number of haploid spermatids in culture following hESC in vitro spermatogenic differentiation.
Flow cytometry analyses of cell cycle profiles following acute 24 hr treatment with vehicle control (A), 100 µM DBCP (B), or 100 µM 2-BP (C). 10,000 events for each condition were examined. Representative profiles shown. R4, R5, and R6 populations on flow cytometry analyses correspond to G0/G1, S, and G2 phases respectively. (D) Graph of average percent haploid cell population (R3 population from plots) values with standard deviation is shown. * represents p < 0.01 statistical significance of decreased % haploid cells in DBCP and 2-BP treatments. Inset shows representative image of haploid sorted cells (R3 population) from our control group stained with protamine 1, a known spermatid marker. Scale bar, 5 µm.
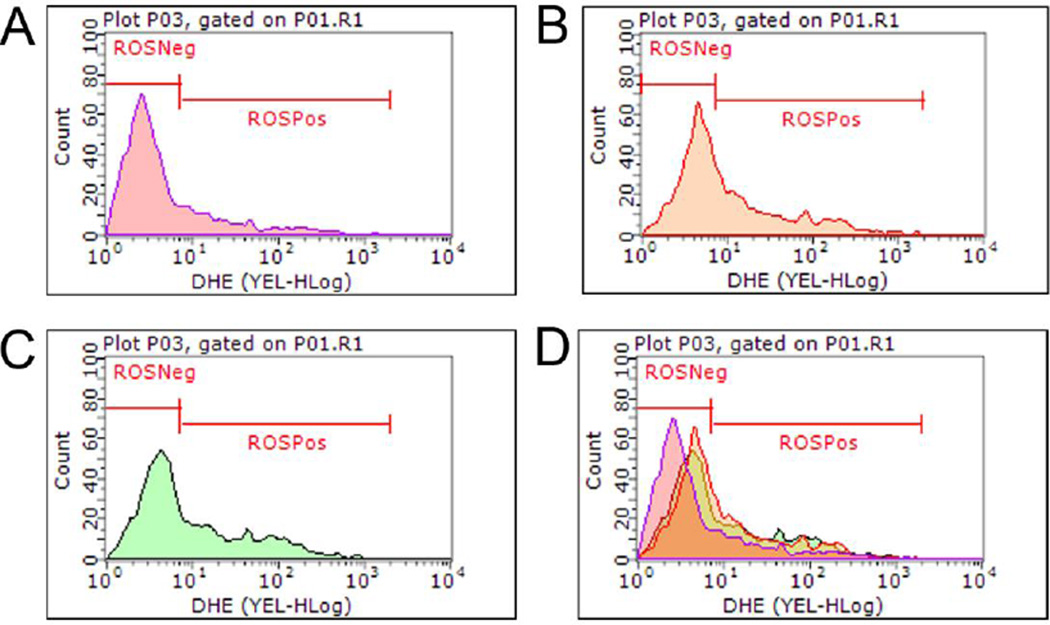
2-BP and DBCP Elevate Reactive Oyxgen Species Levels to Induce Cell Death
In our system, we demonstrated that both 2-BP and DBCP induce germ cell death and target certain populations of spermatogenic cells. Previous reports have indicated that the mammalian testis is particularly sensitive to the deleterious effects of ROS[36], so we next examined whether 2-BP or DBCP generated ROS as a potential mechanism in causing germ cell death. Using a flow cytometry-based approach, we analyzed ROS (+) and (−) cells by examining dihydroethidium (DHE) 24 hr after treatment with either 100 µM 2-BP or DBCP in spermatogenic cultures derived by differentiating hESCs for 10 days as described above. Both 2-BP (Fig. 5C) and DBCP (Fig. 5B) increased the number of ROS (+) cells, as indicated by elevated levels of DHE, in culture compared to vehicle control (Fig. 5A and Supplemental Fig. 2). Additionally, both 2-BP and DBCP treatment shifted the principal cell peak towards the ROS (+) population compared to vehicle control (Fig. 5D). These results suggest that both 2-BP and DBCP elevate ROS in spermatogenic cells derived from hESCs. To further confirm that both 2-BP and DBCP elevated ROS and induced oxidative stress, we performed Live-cell 2',7'-dichlorodihydrofluorescein diacetate (DCF) imaging of cells 15 minutes after treatment with either 100 µM 2-BP or 100 µM DBCP. DCF fluorescence was observed in cells treated with either 2-BP or DBCP in comparable levels to hydrogen peroxide (H2O2) treatment (Supplemental Fig. 3A). In response to oxidative stress, cells activate the NRF2 antioxidant response pathway in which NRF2 is released from oxidized KEAP1 and translocates to the nucleus to activate gene expression of antioxidants and detoxifying enzymes[37]. Both 2-BP and DBCP treatment caused a translocation of NRF2 into the nucleus (Supplemental Fig. 3B) further indicating that both toxicants induce oxidative stress.
Figure 5. DHE labeling indicates that 2-BP or DBCP treatment induces ROS production following acute 24 hr treatment.
Flow cytometry-based analyses of DHE labeling. 10,000 events for each condition were analyzed, representative graphs are shown. (A) Vehicle control, (B) DBCP, and (C) 2-BP. (D) Merged flow cytometry graphs indicate that 2-BP and DBCP peaks shift towards the ROS+ population after 24 hr treatment with 100 µM of each indicated compound.
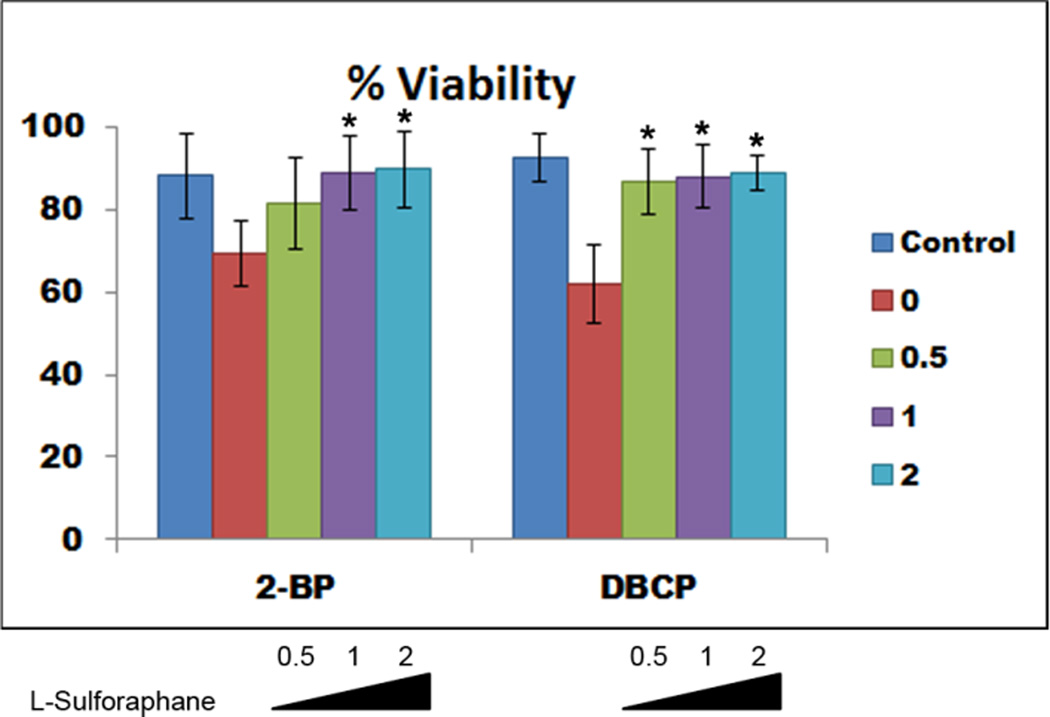
To further implicate oxidative stress as a mechanism for 2-BP- or DBCP-mediated germ cell death, we examined whether treatment with an antioxidant, l-sulforaphane, could mitigate the effects of 2-BP or DBCP. After 10 days post differentiation, germ cell cultures were treated overnight with vehicle control, 0.5 µM, 1.0 µM, or 2 µM l-sulforaphane prior to exposure to either 2-BP or DBCP. After pre-treatment with l-sulforaphane, germ cell cultures were re-fed with fresh differentiation medium containing vehicle control, 100 µM 2-BP, or 100 µM DBCP for 24 hours. Germ cell cultures were then analyzed for viability using Annexin V flow cytometry-based analyses. Pre-treatment with l-sulforaphane attenuated the reproductive toxicant-induced cell death and increased viability compared to cells not pre-treated with l-sulforaphane (Fig. 6). We did observe differential effects of l-sulforaphane pre-treatment as 1.0 µM significantly improved viability in 2-BP-treated cultures whereas 0.5 µM pre-treatment was sufficient to significantly rescue viability in DBCP-treated cultures (Fig. 6) These results taken together with the ROS generation data indicate that acute 2-BP or DBCP exposure results in oxidative stress that impacts viability of germ cells derived in vitro.
Figure 6. l-sulforaphane treatment rescues 2-BP- or DBCP-mediated cell death.
Graphical representation of flow cytometry data from 3 separate experiments showing that overnight treatment with l-sulforaphane blocked reproductive toxicant-mediated cell death. Data shown represent average % viable cells after 24 hours treatment with vehicle control or 100 µM 2-BP or DBCP as indicated. 10,000 events were analyzed for each condition. Indicated l-sulforaphane concentrations are shown. * represents p < 0.01 statistical significance of increased viability from 0 µM l-sulforaphane pre-treatment.
Discussion
Despite the fact that spermatogenesis occurs in a low oxygen environment, there are several different mechanisms that can cause oxidative stress[36, 38, 39]. Spermatogenic cells are particularly sensitive to oxidative damage, and oxidative stressors can negatively impact spermatogenesis[36, 38, 39]. Furthermore, oxidative stress has a profound effect on Leydig cell steroidogenesis, and defects in testosterone production can affect spermatogenesis[38]. To ensure proper propagation of the male germline, cells in the testis display a wide range of mechanisms to handle this oxidative stress[36, 38, 39]. Perturbations in this system can have catastrophic effects leading to male infertility. Thus reproductive toxicants that generate ROS are particularly damaging to male gametogenesis.
In this work, we demonstrate that using hESCs provides a novel in vitro spermatogenic model to assess how reproductive toxicants affect human spermatogenesis. Our results show that 2-BP and DBCP induce germ cell death similarly to that observed in men exposed to each toxicant. As predicted based on previous studies, each chemical affects different populations of germ cells, including spermatogonia (DBCP) and primary spermatocytes (2-BP), but both specifically affect germ cell viability. Rodent models often implicate the somatic support cells in germ cell viability, but our differentiation protocol lacks somatic support cells[5], and thus we are able to observe direct effects of each toxicant on germ cells specifically. Our results suggest that our in vitro spermatogenic model is ideal for examining the effects of various toxicants on male gametogenesis.
The concentrations of each toxicant used in this study represent an accidental occupational exposure. For DBCP, potential exposure routes include skin contact, ingestion, and inhalation[40]. While exposure in the US is limited, exposure through groundwater contamination in limited areas is possible[40]. However, DBCP is a listed chemical of concern at 8 hazardous waste sites[40], suggesting that acute occupational exposure is still possible. Workers exposed to DBCP can potentially inhale up to 97 µg per day and can potentially be exposed to 5 to 125,000 times that amount through contaminated soil via dermal contact[40]. Despite the fact that the risk of exposure to the general population is low, understanding the mechanism by which DBCP affects human spermatogenesis is still critical for individuals living near contaminated groundwater sites or working at DBCP-risk hazardous waste sites.
Likewise the dose of 2-BP utilized in this study reflects acute accidental exposure akin to the Korean electronics plant accident[28–33]. Workers were exposed to daily short-term doses up to 4,141 ppm[28–33] which roughly calculates to more than 100× the dose used in our study. 2-BP is still used today, and despite its recent classification by the NTP as a toxicant that negatively impacts gametogenesis, so exposure is possible. Understanding the mechanisms by which 2-BP impacts human spermatogenesis is critical given the wide use of 2-BP.
The work presented here represents an acute, short-term exposure, but our in vitro spermatogenesis model system would permit longer-term, lower dose studies to examine more subtle effects of each toxicant with prolonged exposures. Furthermore, our system can be utilized to examine the effects of known and unknown reproductive toxicants on human spermatogenic cells both in an acute, short-term format (24 hour) and in a longer-term setup (up to 10–20 days treatment). This system could be utilized to study the effects of known reproductive toxicants such as phthalates, dioxin, and endosulfan as well as predicted or unknown chemicals such as PFOS, HBCD, TDCPP and Aroclor. Because of the rapid differentiation protocol, and the ease of the system, we anticipate that our model, which mimics several aspects of human spermatogenesis, will serve as an ideal system going forward for examining the direct effects of known and unknown reproductive toxicants on human spermatogenic cells.
Supplementary Material
Highlights.
-
-
Utilization of a novel in vitro spermatogenic model for reproductive toxicity testing
-
-
Demonstration that two known reproductive toxicants, DBCP and 2-BP, can be studied in our differentiation protocol
-
-
Assessment of mechanisms by which known reproductive toxicants impact human spermatogenesis in vitro
-
-
Determination that DBCP and 2-BP affect spermatogenesis by inducing ROS generation to cause cell death
-
-
Recapitulation of clinical phenotypes due to exposure to either DBCP or 2-BP in vitro
Acknowledgments
This work is kindly supported by the National Institutes of Health, National Institute of Environmental Health Sciences P30ES019776 Human Exposome Research Center: Understanding Lifetime Exposures (HERCULES).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campion S, Catlin N, Heger N, McDonnell EV, Pacheco SE, Saffarini C, Sandrof MA, Boekelheide K. Male reprotoxicity and endocrine disruption. Exs. 2012;101:315–360. doi: 10.1007/978-3-7643-8340-4_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Human reproduction update. 2006;12:275–282. doi: 10.1093/humupd/dmk001. [DOI] [PubMed] [Google Scholar]
- 3.Tomerlin JR. The US Food Quality Protection Act--policy implications of variability and consumer risk. Food additives and contaminants. 2000;17:641–648. doi: 10.1080/026520300412573. [DOI] [PubMed] [Google Scholar]
- 4.Bucay N, Yebra M, Cirulli V, Afrikanova I, Kaido T, Hayek A, Montgomery AM. A novel approach for the derivation of putative primordial germ cells and sertoli cells from human embryonic stem cells. Stem Cells. 2009;27:68–77. doi: 10.1634/stemcells.2007-1018. [DOI] [PubMed] [Google Scholar]
- 5.Easley CAt, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE, Schatten GP. Direct Differentiation of Human Pluripotent Stem Cells into Haploid Spermatogenic Cells. Cell reports. 2012 doi: 10.1016/j.celrep.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eguizabal C, Montserrat N, Vassena R, Barragan M, Garreta E, Garcia-Quevedo L, Vidal F, Giorgetti A, Veiga A, Izpisua Belmonte JC. Complete meiosis from human induced pluripotent stem cells. Stem Cells. 2011;29:1186–1195. doi: 10.1002/stem.672. [DOI] [PubMed] [Google Scholar]
- 7.Fukunaga N, Teramura T, Onodera Y, Takehara T, Fukuda K, Hosoi Y. Leukemia inhibitory factor (LIF) enhances germ cell differentiation from primate embryonic stem cells. Cellular reprogramming. 2010;12:369–376. doi: 10.1089/cell.2009.0097. [DOI] [PubMed] [Google Scholar]
- 8.Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 9.Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panula S, Medrano JV, Kee K, Bergstrom R, Nguyen HN, Byers B, Wilson KD, Wu JC, Simon C, Hovatta O, Reijo Pera RA. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Human molecular genetics. 2011;20:752–762. doi: 10.1093/hmg/ddq520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park TS, Galic Z, Conway AE, Lindgren A, van Handel BJ, Magnusson M, Richter L, Teitell MA, Mikkola HK, Lowry WE, Plath K, Clark AT. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells. 2009;27:783–795. doi: 10.1002/stem.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teramura T, Takehara T, Kawata N, Fujinami N, Mitani T, Takenoshita M, Matsumoto K, Saeki K, Iritani A, Sagawa N, Hosoi Y. Primate embryonic stem cells proceed to early gametogenesis in vitro. Cloning and stem cells. 2007;9:144–156. doi: 10.1089/clo.2006.0070. [DOI] [PubMed] [Google Scholar]
- 13.Tilgner K, Atkinson SP, Golebiewska A, Stojkovic M, Lako M, Armstrong L. Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells. 2008;26:3075–3085. doi: 10.1634/stemcells.2008-0289. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi K, Hasegawa K, Chuma S, Nakatsuji N, Suemori H. In vitro germ cell differentiation from cynomolgus monkey embryonic stem cells. PloS one. 2009;4:e5338. doi: 10.1371/journal.pone.0005338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West FD, Mumaw JL, Gallegos-Cardenas A, Young A, Stice SL. Human haploid cells differentiated from meiotic competent clonal germ cell lines that originated from embryonic stem cells. Stem cells and development. 2011;20:1079–1088. doi: 10.1089/scd.2010.0255. [DOI] [PubMed] [Google Scholar]
- 16.Amann RP, Berndtson WE. Assessment of procedures for screening agents for effects on male reproduction: effects of dibromochloropropane (DBCP) on the rat. Fundamental and applied toxicology : official journal of the Society of Toxicology. 1986;7:244–255. doi: 10.1016/0272-0590(86)90154-5. [DOI] [PubMed] [Google Scholar]
- 17.Bjorge C, Wiger R, Holme JA, Brunborg G, Andersen R, Dybing E, Soderlund EJ. In vitro toxicity of 1,2-dibromo-3-chloropropane (DBCP) in different testicular cell types from rats. Reprod Toxicol. 1995;9:461–473. doi: 10.1016/0890-6238(95)00038-c. [DOI] [PubMed] [Google Scholar]
- 18.Holme JA, Soderlund EJ, Brunborg G, Lag M, Nelson SD, Dybing E. DNA damage and cell death induced by 1,2-dibromo-3-chloropropane (DBCP) and structural analogs in monolayer culture of rat hepatocytes: 3-aminobenzamide inhibits the toxicity of DBCP. Cell biology and toxicology. 1991;7:413–432. doi: 10.1007/BF00124075. [DOI] [PubMed] [Google Scholar]
- 19.Potashnik G, Porath A. Dibromochloropropane (DBCP): a 17-year reassessment of testicular function and reproductive performance. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 1995;37:1287–1292. doi: 10.1097/00043764-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Slutsky M, Levin JL, Levy BS. Azoospermia and oligospermia among a large cohort of DBCP applicators in 12 countries. International journal of occupational and environmental health. 1999;5:116–122. doi: 10.1179/oeh.1999.5.2.116. [DOI] [PubMed] [Google Scholar]
- 21.Whorton D, Milby TH, Krauss RM, Stubbs HA. Testicular function in DBCP exposed pesticide workers. Journal of occupational medicine. : official publication of the Industrial Medical Association. 1979;21:161–166. [PubMed] [Google Scholar]
- 22.Kapp RW, Jr, Picciano DJ, Jacobson CB. Y-chromosomal nondisjunction in dibromochloropropane-exposed workmen. Mutation research. 1979;64:47–51. doi: 10.1016/0165-1161(79)90136-5. [DOI] [PubMed] [Google Scholar]
- 23.Teramoto S, Saito R, Aoyama H, Shirasu Y. Dominant lethal mutation induced in male rats by 1,2-dibromo-3-chloropropane (DBCP) Mutation research. 1980;77:71–78. doi: 10.1016/0165-1218(80)90122-6. [DOI] [PubMed] [Google Scholar]
- 24.Meistrich ML, Wilson G, Shuttlesworth GA, Porter KL. Dibromochloropropane inhibits spermatogonial development in rats. Reprod Toxicol. 2003;17:263–271. doi: 10.1016/s0890-6238(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 25.Kelce WR, Raisbeck MF, Ganjam VK. Gonadotoxic effects of 2-hexanone and 1,2-dibromo-3-chloropropane on the enzymatic activity of rat testicular 17 alpha-hydroxylase/C17,20-lyase. Toxicology letters. 1990;52:331–338. doi: 10.1016/0378-4274(90)90043-l. [DOI] [PubMed] [Google Scholar]
- 26.NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of 2-Bromopropane (2-BP) Ntp Cerhr Mon. 2003:i-III11. [PubMed] [Google Scholar]
- 27.Boekelheide K, Darney SP, Daston GP, David RM, Luderer U, Olshan AF, Sanderson WT, Willhite CC, Woskie S. NTP-CERHR Expert Panel Report on the reproductive and developmental toxicity of 2-bromopropane. Reprod Toxicol. 2004;18:189–217. doi: 10.1016/j.reprotox.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Ichihara G, Ding X, Yu X, Wu X, Kamijima M, Peng S, Jiang X, Takeuchi Y. Occupational health survey on workers exposed to 2-bromopropane at low concentrations. American journal of industrial medicine. 1999;35:523–531. doi: 10.1002/(sici)1097-0274(199905)35:5<523::aid-ajim10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, Jung K, Hwang T, Jung G, Kim H, Park J, Kim J, Park D, Park S, Choi K, Moon Y. Hematopoietic and reproductive hazards of Korean electronic workers exposed to solvents containing 2-bromopropane. Scandinavian journal of work, environment & health. 1996;22:387–391. doi: 10.5271/sjweh.159. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y, Park J, Moon Y. Hematopoietic and reproductive toxicity of 2-bromopropane, a recently introduced substitute for chlorofluorocarbons. Toxicology letters. 1999;108:309–313. doi: 10.1016/s0378-4274(99)00103-4. [DOI] [PubMed] [Google Scholar]
- 31.Son HY, Kim YB, Kang BH, Cho SW, Ha CS, Roh JK. Effects of 2-bromopropane on spermatogenesis in the Sprague-Dawley rat. Reprod Toxicol. 1999;13:179–187. doi: 10.1016/s0890-6238(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, Faqi AS, Yang J, Ding X, Jiang X, Chahoud I. Male reproductive toxicity and beta-luteinizing hormone gene expression in sexually mature and immature rats exposed to 2-bromopropane. Human & experimental toxicology. 1999;18:683–690. doi: 10.1191/096032799678839536. [DOI] [PubMed] [Google Scholar]
- 33.Yu IJ, Chung YH, Lim CH, Maeng SH, Lee JY, Kim HY, Lee SJ, Kim CH, Kim TG, Park JS, Moon YH. Reproductive toxicity of 2-bromopropane in Sprague Dawley rats. Scandinavian journal of work, environment & health. 1997;23:281–288. doi: 10.5271/sjweh.221. [DOI] [PubMed] [Google Scholar]
- 34.Easley CAt, Ben-Yehudah A, Redinger CJ, Oliver SL, Varum ST, Eisinger VM, Carlisle DL, Donovan PJ, Schatten GP. mTOR-mediated activation of p70 S6K induces differentiation of pluripotent human embryonic stem cells. Cellular reprogramming. 2010;12:263–273. doi: 10.1089/cell.2010.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamkanfi M, Kanneganti TD. Caspase-7: a protease involved in apoptosis and inflammation. The international journal of biochemistry & cell biology. 2010;42:21–24. doi: 10.1016/j.biocel.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of Oxidative Stress on Male Reproduction. The world journal of men's health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Archives of toxicology. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 38.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxidative medicine and cellular longevity. 2008;1:15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maiorino M, Ursini F. Oxidative stress, spermatogenesis and fertility. Biological chemistry. 2002;383:591–597. doi: 10.1515/BC.2002.061. [DOI] [PubMed] [Google Scholar]
- 40.1,2-Dibromo-3-chloropropane, Report on carcinogens : carcinogen profiles / U.S. Dept. of Health and Human Services, Public Health Service. National Toxicology Program. 2011;12:134–135. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.