Abstract
Hair cells in the adult mammalian cochlea cannot spontaneously regenerate after damage resulting in the permanency of hearing loss. Stem cells have been found to be present in the cochlea of young rodents; however, there has been little evidence for their existence into adulthood. We used nestin-CreERT2/tdTomato-reporter mice to trace the lineage of putative nestin-expressing cells and their progeny in the cochleae of adult mice. Nestin, an intermediate filament found in neural progenitor cells during early development and adulthood, is regarded as a multi-potent and neural stem cell marker. Other investigators have reported its presence in postnatal and young adult rodents; however, there are discrepancies amongst these reports. Using lineage tracing, we documented a robust population of tdTomato-expressing cells and evaluated these cells at a series of adult time points. Upon activation of the nestin promoter, tdTomato was observed just below and medial to the inner hair cell layer. All cells co-localized with the stem cell and cochlear-supporting-cell marker Sox2 as well as the supporting cell and Schwann cell marker Sox10; however, they did not co-localize with the Schwann cell marker Krox20, spiral ganglion marker NF200, or GFAP-expressing supporting cell marker. The cellular identity of this unique population of tdTomato-expressing cells in the adult cochlea of nestin-CreERT2/tdTomato mice remains unclear however these cells may represent a type of supporting cell on the neural aspect of the inner hair cell layer.
Keywords: inner ear, supporting cell, regeneration, stem cell, mouse, AB_10015251, AB_10013382, AB_2286684, AB_2195374, AB_306298, AB_10064079, AB_149792, AB_396354, AB_2251134, AB_2314882
Introduction
Many organs in the adult mammal maintain a population of progenitor cells for general maintenance and regeneration (Fuchs and Segre, 2000). The adult mammalian cochlea lacks the regenerative capacity to replace damaged or lost hair cells resulting in the permanent nature of most cases of sensorineural hearing loss. Non-mammalian vertebrates, in contrast, have the ability to replace lost hair cells through direct trans-differentiation of supporting cells or through mitotic division of supporting cells with subsequent differentiation into one hair cell and one supporting cell (Corwin and Cotanche, 1988; Ryals and Rubel, 1988).
The vestibular epithelia in mammals demonstrates some capacity for hair cell regeneration; however, the amount of functional restoration after regeneration of the sensory epithelia remains unclear (Forge et al., 1993; Walsh et al., 2000; Warchol et al., 1993). To explore reasons for cochlear and vestibular restorative differences, Oshima and colleagues collected cells from multiple areas of the inner ear at different postnatal time points to assess their ability to form spheres in vitro, a characteristic of stem cells (Oshima et al., 2007). Sphere-forming populations of putative stem cells were found in the utricular macula, saccular macula, ampullary crista, organ of Corti, spiral ganglion, and stria vascularis at early postnatal time points however during the second and third post natal weeks, there was a significant decrease in the sphere-forming ability of isolates from the auditory sensory epithelium as compared to the vestibular epithelium. This observation is consistent with reports of expression of the adult stem cell marker Lgr5 in some supporting cells of the organ of Corti from embryonic and neonatal mice (Chai et al., 2011). Cells expressing Lgr5 are proposed to have the capacity to generate hair cells in the developing inner ear (Shi et al., 2012) although their expression appears to diminish rapidly in the postnatal cochlea (Chai et al., 2011; Shi et al., 2012). In related work, Cox et al. (2014) have reported that neonatal mice have the capacity to regenerate hair cells spontaneously in vivo after hair cell ablation. New hair cells were derived by cell proliferation and direct trans-differentiation of the Lgr5-positive population of cochlear supporting cells. Although hair cell regeneration appeared to be restricted to the first postnatal week, this report provided further insight into the competency of the mammalian cochlea for some level of regeneration. In aggregate, these findings suggest that tissue-specific stem cells reside in the rodent cochlea but lose their ability to self-renew in early adolescence, in general agreement with the known absence of regenerative potential in the adult cochlea.
A number of investigators have evaluated nestin expression in the organ of Corti (Carricondo et al., 2010; Kojima et al., 2004; Lopez et al., 2004; Lou et al., 2007; Malgrange et al., 2002; Smeti et al., 2011; Watanabe et al., 2012). This neural and stem cell marker is a type VI-intermediate filament protein widely used to identify cells with stem cell characteristics in developing and adult tissues (Lendahl et al., 1990). It is expressed in numerous proliferating tissues and is thought to be a reliable marker of mitotically active cells (Kachinsky et al., 1995; Lendahl et al., 1990; Sejersen and Lendahl, 1993; Suzuki et al., 2010). During murine embryonic development, nestin expression is observed in proliferating central nervous system (CNS) regions starting on embryonic day (E)7.75 (Dahlstrand et al., 1995). Expression decreases significantly after birth with the majority of nestin expression in proliferating neural tissues restricted to the subventricular zone of the lateral ventricles and dentate gyrus of the hippocampus (Alvarez-Buylla et al., 2002; Cameron and McKay, 2001; Fukuda et al., 2003; Gage, 2002; Lagace et al., 2007). Upregulation of nestin-expressing cells occurs following injury to the CNS (Sahin Kaya et al., 1999) and retina (Xue et al., 2006) suggesting that nestin expression may mark cells with stem- or progenitor-like qualities. Several studies have documented nestin expression in the cochlea of developing and postnatal rodents. In all cases, nestin expression was observed in the early postnatal cochlea and down-regulated in the mature cochlea. In some of these reports, nestin-expressing cells in the postnatal rodent cochlea were multipotent tissue-specific stem cells. Interestingly, the persistence of nestin expression during maturation, as well as the localization of nestin-expressing cells within the auditory sensory epithelium (the organ of Corti) has varied between reports (Carricondo et al., 2010; Kojima et al., 2004; Lopez et al., 2004; Lou et al., 2007; Malgrange et al., 2002; Smeti et al., 2011; Watanabe et al., 2012).
In this study, we examined the temporal and spatial organization of putative nestin-expressing cells and their progeny in the adult cochlea using nestin-CreERT2/tdTomato-reporter mice. We identified a robust, unique population of tdTomato-expressing cells on the neural aspect of the inner hair cell layer. In addition, we found tdTomato-positive cells co-localized with the stem- and supporting-cell marker Sox2 and did not express proteins indicative of a Schwann, neural or glial cell fate. Our characterization of putative nestin-expressing cells and their progeny provides insight into the possible persistence of a population of stem cell-like cells in the adult mammalian cochlea.
Materials and Methods
Animal Use
Animal subject protection
All experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) at the University of Wisconsin-Madison. Animals were handled and cared for in compliance with the Animal Welfare Act and National Institutes of Health Guidelines.
Nestin-CreERT2/tdTomato-reporter mice were used to identify putative stem cells and their progeny in the adult mouse cochlea. The sample included 15 C57BL/6 mice ranging from ~8–26 weeks of age, 13 of which were used for quantitative analysis (Table 1).
Table 1.
Sample Information
Specimen information used for quantitative analysis include sample number (Mouse), sex, date of birth (DOB), date of first injection (DOI), number of days tissue collected after the last tamoxifen injection (ND), age in weeks at tissue harvest (ATH), and average number of cells per region for that sample (Cells/Region/Sample).
| Mouse | Sex | DOB | DOI | ND | ATH | Cells/Region/Sample |
|---|---|---|---|---|---|---|
| A1 | M | 2/3/12 | 3/25/12 | 1 | 8.1 | 14.5 |
| A2 | M | 2/3/12 | 3/25/12 | 1 | 8.1 | 14.83 |
| 7 | F | 12/9/11 | 4/7/12 | 7 | 18.9 | 20.07 |
| 8 | M | 12/17/11 | 4/7/12 | 7 | 17.7 | 23.17 |
| 10 | F | 12/9/11 | 4/7/12 | 14 | 19.9 | 24.56 |
| 11 | F | 12/17/11 | 4/7/12 | 14 | 18.7 | 21.06 |
| 12 | M | 12/17/11 | 4/7/12 | 14 | 18.7 | 30.32 |
| 13 | F | 12/9/11 | 4/7/12 | 35 | 22.9 | 28.27 |
| 14 | M | 12/31/11 | 4/7/12 | 35 | 19.7 | 25.39 |
| 15 | F | 12/17/11 | 4/7/12 | 35 | 21.7 | 29.37 |
| 16 | F | 12/9/11 | 4/7/12 | 56 | 25.9 | 24.89 |
| 17 | M | 12/9/11 | 4/7/12 | 56 | 25.9 | 26.00 |
| 18 | M | 12/9/11 | 4/7/12 | 56 | 25.9 | 25.83 |
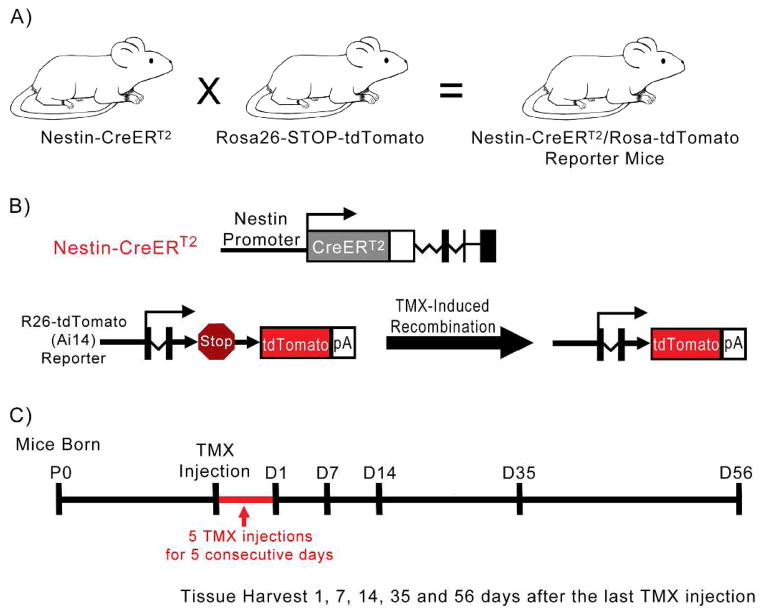
Generation of mice expressing CreERT2, and subsequent tdTomato, under nestin regulation was previously described (Lagace et al., 2007; Madisen et al., 2010). In brief, nestin-CreERT2 mice and Rosa-tdTomato (Ai14) reporter mice were bred to create transgenic offspring (Fig. 1A). CreERT2 is expressed under the control of a nestin promoter and remains in the cytoplasm of nestin-expressing cells. The expression of nestin-CreERT2 is inducible by means of tamoxifen (TMX) injection, which activates the estrogen receptor fused to Cre (CreERT2), allowing it to enter the nucleus, recombine the LoxP sites to remove a stop codon, and express tdTomato in tissues expressing Cre-recombinase (Fig. 1B). Genotyping for Cre and Rosa-tdTomato (Ai14) reporter mice was performed as described previously (Guo et al., 2011).
Figure 1.
Generation of transgenic mice and activation of tdTomato following tamoxifen (TMX) administration. A: Nestin-CreERT2 mice and Rosa-tdTomato (Ai14) reporter mice were bred to create transgenic offspring. B: CreERT2 is expressed under the control of the nestin promoter. The expression of nestin-CreERT2 is inducible by means of tamoxifen (TMX) injection, which activates the estrogen receptor fused to Cre (CreERT2), allowing it to enter the nucleus, recombine the LoxP sites to remove a stop codon, and express tdTomato in tissues expressing Cre-recombinase. C: Timeline of experiment. Mice were given 5 injections of tamoxifen (TMX) on 5 subsequent days, after which their tissue was harvested 1, 7, 14, 35 or 56 days following the last TMX injection.
In our experiment, 15 adult mice were given 5 injections of tamoxifen over 5 consecutive days (180 mg kg−1 intraperitoneal injection, 30 mg ml−1 in 10% EtOH mixed with sunflower oil) (Guo et al., 2011; Lagace et al., 2007). Cochlear tissue was harvested 1, 7, 14, 35, or 56 days post-tamoxifen injection (Fig. 1C; Table 1). Experimental cochleae were analyzed to ensure appropriate morphological appearance and expression of tdTomato. An adult control nestin-CreERT2/tdTomato-reporter mouse confirmed no tdTomato was expressed in the cochlea in the absence of tamoxifen.
Tissue Preparation
Mice were sacrificed by intraperitoneal injection of sodium pentobarbital and then transcardially perfused with saline, followed by 4% paraformaldehyde (Guo et al., 2011). Temporal bones containing the inner ear were removed and fixed in 4% paraformaldehyde for 24 hours, after which they were moved to a 4% solution of ethylenediaminetetraacetic acid (EDTA) for 3 days to decalcify the bone. Whole mount microdissections of the organ of Corti from the temporal bone were then performed and processed for immunohistochemistry.
Immunohistochemistry
Cochlear tissue sections were incubated at 4 °C for 30 minutes in a solution containing 0.1% Saponin (Sigma 47036) and 0.1% Tween 20 (Sigma P7949) in PBS, followed by incubation for 1 hour in a blocking solution consisting of 10% normal donkey serum, 0.2% Triton X-100 in 1xTBS (adapted from Hume et al. (2003)). Tissue sections were then exposed to primary antibodies diluted in the blocking solution overnight. Primary antibodies used in this study include: polyclonal Myosin VIIa, Glial fibrillary acidic protein (GFAP), Sox2, Sox10, neurofilament-200 (NF200), Krox20 and nestin (See Table 2 for details regarding all antibodies). The following day, samples were washed 3 times in PBS for 5 minutes, then exposed to fluorescent labeled secondary antibodies and DAPI (Sigma-Aldrich, 1:1000) in the blocking solution overnight. Secondary antibodies used include: donkey anti-goat Alexa 488 (Invitrogen, 1:500) and donkey anti-rabbit Alexa 647 (Invitrogen, 1:500). The following day, samples were washed 3 times in PBS for 5 minutes and mounted with Vectashield-mounting medium for analysis.
Table 2.
Primary antibodies used in this study
Complete list of antibodies, labeling, immunogens, manufacturers, species and dilutions.
| Antibody | Labeling | Immunogen | Manufacturer | Cat. No. | Species | Dilution | RRID |
|---|---|---|---|---|---|---|---|
| Anti-Myosin VIIA | Cochlear Hair Cells | Amino acids 877-1075 of human Myosin VIIA | Proteus Biosciences | 25-6790 | Rabbit Polyclonal | 1:100 | AB_10015251 |
| Anti-GFAP | Border Cells and Inner Phalangeal Cells (Supporting Cell Subtypes) | GFAP isolated from cow spinal cord | Dako (Denmark) | 70334 | Rabbit Polyclonal | 1:1000 | AB_10013382 |
| Anti-Sox2 | Supporting Cells and Stem Cells | Amino acids 277-293 of human Sox2 | Santa Cruz | sc-17320 | Goat Polyclonal | 1:100 | AB_2286684 |
| Anti-Sox10 | Supporting Cells and Schwann Cells | Amino acids 1-20 of human Sox10 | Santa Cruz | sc-17342 | Goat Polyclonal | 1:100 | AB_2195374 |
| Anti-NF200 | Spiral Ganglion Neurons | Recombinant fragment (Cow) | Abcam | Ab8135 | Rabbit Polyclonal | 1:1000 | AB_306298 |
| Anti-Krox20 | Schwann Cells | Amino acids 8-95 of Krox20 fused to GST | Covance | PRB-236P | Rabbit Polyclonal | 1:100 | AB_10064079 |
| Anti-Ki67 | Proliferating Cells | Human Ki67 C-terminus | Thermo Scientific | RM-9106-S1 | Rabbit Monoclonal | 1:250 | AB_149792 |
| Anti-Nestin | Nestin/Progenitor Cells | Rat (E15) spinal cord extracts | BD_Pharmagin | 556309 | Mouse Monoclonal | 1:100, 1:250, 1:500 | AB_396354 |
| Anti-Nestin | Nestin/Progenitor Cells | Full-length human recombinant nestin | Millipore | MAB5326 | Mouse Monoclonal | 1:100, 1:250. 1:500 | AB_2251134 |
| Anti-Nestin | Nestin/Progenitor Cells | EKE DQR FPR SPE EDQ Q; EKE RQE SLK SPE DED QQ; EVE EGP ERE QHQ ESL RS | Aves Labs | Cat#NES | Chicken Polyclonal | 1:100, 1:250, 1:500 | AB_2314882 |
Antibody Characterization
Rabbit polyclonal Myosin VIIa was generated from amino acids 877-1075 of human Myosin VIIa and specifically labels cochlear hair cells and their stereocilia (Hasson et al., 1995) (Proteus Biosciences Cat#25-6790, RRID: AB_10015251). This antibody was previously characterized by Burns et al. (2013), and found to recognize 230–250 kDa bands in immunoblots of temporal bone tissue from rats, which is consistent with the expected molecular weight of Myosin VIIa. The Myosin VIIa antibody used in this study showed a similar staining pattern in the cochlea that has previously been identified as cochlear hair cells (Burns et al., 2013).
Rabbit polyclonal GFAP was isolated from the cow spinal cord and is a marker for glial cells (Dako Cat#70334, RRID: AB_10013382). This antibody was previously characterized and found to have appropriate band size and expression pattern consistent with previous reports (Fuentes-Santamaria et al., 2013; Yamanaka et al., 2011). The GFAP antibody used in this study localized to the border cell and inner phalangeal area (under the inner hair cell row), and showed a similar expression pattern compared to other studies evaluating GFAP in the inner ear (Rio et al., 2002; Smeti et al., 2011).
Goat polyclonal Sox2 antibody was generated from amino acids 277-293 of human Sox2 and labels all cells in the nascent sensory epithelium during early development, and is later down-regulated to supporting cells of the inner ear (Hume et al., 2007; Jones and Warchol, 2009; Millimaki et al., 2010; Oesterle et al., 2008) (Santa Cruz Cat#sc-17320, RRID: AB_2286684). This antibody was previously characterized and found to have a band size and expression pattern consistent with previous reports (Jones and Warchol, 2009). The Sox2 antibody used in this study showed a similar staining pattern in the cochlea that has previously been identified as supporting cells (Hume et al., 2007).
Goat polyclonal Sox10 antibody was generated from amino acids 1-20 of human Sox10 and labels neural crest cells during early development, and glia cells in later development and throughout adulthood (Kuhlbrodt et al., 1998; Pusch et al., 1998; Southard-Smith et al., 1998) (Santa Cruz Cat#sc-17342, RRID: AB_2195374). In the inner ear, this antibody labels all cells in the otic vesicle epithelium during early development and is later down-regulated to the supporting cell layer (Breuskin et al., 2009). This antibody was previously characterized and found to have the appropriate band size and specificity (Commo et al., 2004; Davies, 2007; Watanabe et al., 2000). The Sox10 antibody used in this study showed a similar staining pattern in the cochlea that has previously been identified as supporting cells (Wakaoka et al., 2013).
Rabbit monoclonal Ki67 was obtained from Thermo Scientific (Cat# (SP6) RM-9106, RRID: AB_149792). This antibody labels proliferating cells during late G1, S, M, and G2 stages of the cell cycle (Taupin, 2007). This antibody was previously characterized and found to co-localize with BrdU-positive cells Garcia-Ovejero et al. (2013). In mouse cochlear tissue, Ki67 was not observed in the organ of Corti nor spiral ganglion. This was not unexpected given that terminal mitosis in the mouse otocyst occurs during development between E13.5 and E16 (Ruben, 1967).
Rabbit polyclonal NF200 was obtained from Abcam (Cat#Ab8135, RRID: AB_306298). This antibody recognized a single band of about 200 kDa on mouse brain lysates and cochlear tissues in western blot analysis. The NF200 antibody used in this study stained a comparable area of the cochlea previously identified as spiral ganglion neurons (Jyothi et al., 2010; Provenzano et al., 2011).
Rabbit polyclonal Krox20 was obtained from Covance (Cat#PRB-236P, RRID: AB_10064079). This antibody labels Schwann cells and co-expressed the supporting and Schwann cell marker Sox10. The Krox20 antibody used in this study localized to the spiral ganglion area reported to contain Schwann cells and showed a similar expression pattern compared to other studies evaluating Schwann cells in the inner ear (Knipper et al., 1998; Provenzano et al., 2011).
In an effort to confirm that cre was faithfully expressed with nestin, we immunostained for nestin. A number of methods and antibodies were employed in this endeavor including antigen retrieval, increasing the incubation times, and varying the permeabilization methods. Despite these efforts we were unable to successfully identify nestin expression in the mouse cochlea. The antibodies we employed were obtained from BD Pharmagin (Cat#556390, RRID: AB_396354), Millipore (Cat#MAB5326, RRID: AB_2251134), and Aves labs (Cat#NES, RRID: AB_2314882) and were characterized by (Naritsuka et al., 2009), (Farahani et al., 2011) and (White et al., 2010) respectively. These antibodies were reported to successfully label nestin-expressing progenitor cells in other tissues; however, as above they did not label nestin-expressing cells in adult mouse cochlear tissue.
Imaging and Analysis
Whole mount samples were imaged using a Nikon A1R-A1 confocal microscope with 60x and 10x objectives and a Lightsheet Z.1 by Carl Zeiss Microscopy GmbH with a 5x/0.16 EC Plan-NEOFLUAR objective. Images were exported into tiff-format, after which they were adjusted for brightness, contrast and sharpness using FUIJI ImageJ software (Schindelin et al., 2012). Figure panels were produced using Adobe Illustrator ™ software.
For quantification of tdTomato-positive cells, two to three 60x images were taken on the Nikon A1R-A1 confocal microscope of each region of the cochlea (apex, mid, base) for a total of 6–9 images per cochlea (12–18 high powered images per animal). TdTomato-positive cells were counted in each area of the cochlea and entered into a table for quantitative analysis (Table 1). TdTomato-positive cell counts from each high powered field were then averaged for each animal. Animals from the same time period after tamoxifen injection were averaged for statistical analysis for comparing counts at each time point. Statistical analysis of experimental data using a one-way analysis of variance (ANOVA) and post-hoc tests using Tukey HSD were performed using R statistical software (Team, 2008) and IBM SPSS ™ software. For all analyses, differences were considered significant if P < 0.05.
Results
TdTomato-expressing cells are observed in a consistent location and distribution in the cochlea of nestin-CreERT2/tdTomato mice
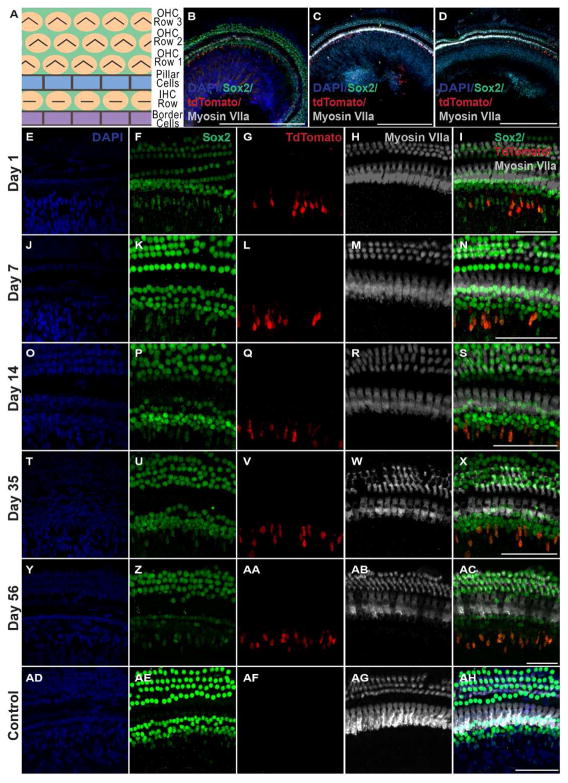
We used a transgenic line of nestin-CreERT2 mice with an inducible form of Cre recombinase, which allowed for Cre-mediated recombination only in nestin-expressing cells upon tamoxifen injection(Lagace et al., 2007). The specificity and efficiency of transgene expression in nestin-expressing cells in this mouse line has been validated (Gao et al., 2009; Guo et al., 2011; Lagace et al., 2007; Sun et al., 2014). We crossed nestin-CreERT2 mice with the tdTomato-reporter line (Madisen et al., 2010) so that, upon tamoxifen injection, tdTomato expression represents the nestin-expressing cells or their progenies that have Cre-recombination. The cochleae obtained from adult nestin-CreERT2/tdTomato-reporter mice appeared morphologically normal, with tdTomato observed on the neural (medial) aspect of the inner hair cell layer (Fig. 2). TdTomato-positive cells were observed throughout the cochlea for all time points examined (Fig. 3B–D). An adult negative control nestin-CreERT2/tdTomato-reporter mouse confirmed the absence of tdTomato-positive cells in the organ of Corti in the absence of tamoxifen (Fig. 3AF). Staining with the hair cell marker Myosin VIIa and the supporting cell marker Sox2 revealed three rows of outer hair cells and one row of inner hair cells along with a normal complement of supporting cells at each time point examined (Fig. 3I, N, S, X, AC, AH). As expected, tdTomato-positive cells appeared in the spiral ganglion. In addition, a distinct population of cells expressing tdTomato consistently appeared adjacent to the organ of Corti medial (on the neural aspect) to the inner hair cell layer (Fig. 3G, L, Q, V, AA), which were of interest in this study.
Figure 2.
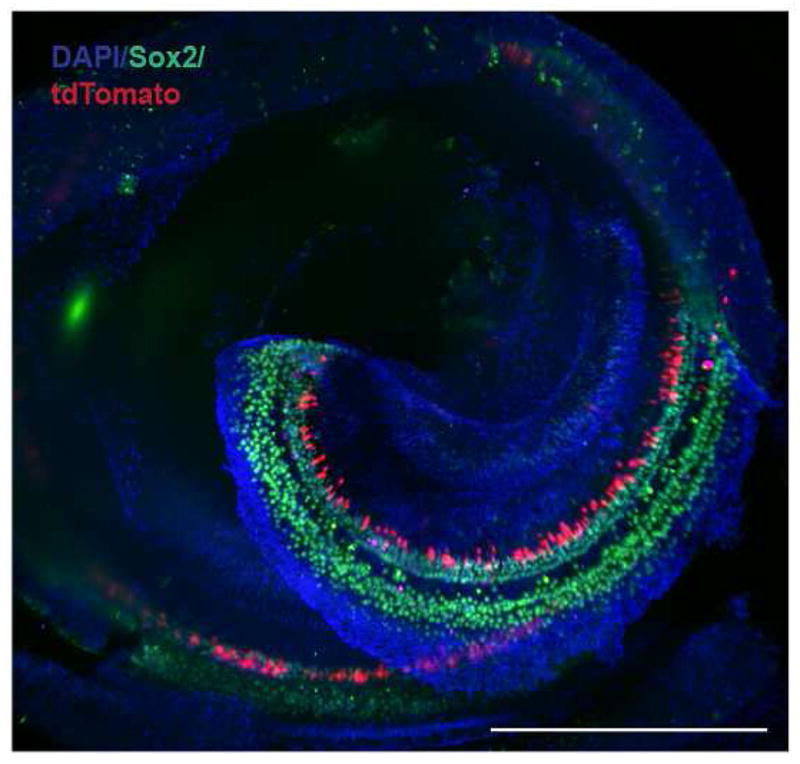
Cochlea from an adult nestin-CreERT2/tdTomato-reporter mouse 56 days post tamoxifen injection. Low power macroscopic imaging analysis showed a pattern of tdTomato expression on the neural aspect of Sox2-positive supporting cells. An apical-basal gradient was not observed in tdTomato-positive cells. Image was acquired on a Lightsheet Z.1 by Carl Zeiss Microscopy GmbH with a 5x/0.16 EC Plan-NEOFLUAR objective. Scale bar: 500μm.
Figure 3.
TdTomato-positive cells are found throughout the adult mammalian inner ear for all time points examined. A: Orientation of the organ of Corti. B–D: TdTomato-positive cells are observed throughout the cochlea in the apex (B), mid (C), and base (D) 56 days after the last tamoxifen injection. Images were acquired using a Nikon A1R-A1 confocal microscope. E-AH: High-power images showing tdTomato-positive cells in the normal mammalian inner ear 1 day, 7 days, 14 days, 35 days, and 56 days after the last tamoxifen injection (G, L, Q, V, AA respectively). The morphology of the tdTomato-positive cells appeared consistent, having oval cells bodies with extensions protruding upward towards the inner hair cell layer; however, some tdTomato-positive cells appeared to have slightly different morphologies with elongated oval bodies and/or widening protrusions near the apical end. Cochlear hair cells, labeled with Myosin VIIa, reveal a normal configuration with three rows of outer hair cells and one row of inner hair cells (H, M, R, W, AB, AG). TdTomato co-localizes with the stem cell and supporting cell marker Sox2 (I, N, S, X, AC). An adult control nestin-CreERT2/tdTomato-reporter mouse confirmed no tdTomato was expressed in the cochlea in the absence of tamoxifen (AF). Images were acquired using a Nikon A1R-A1 confocal microscope. Scale bar: 500 μm in B–D; 50 μm in E-AH.
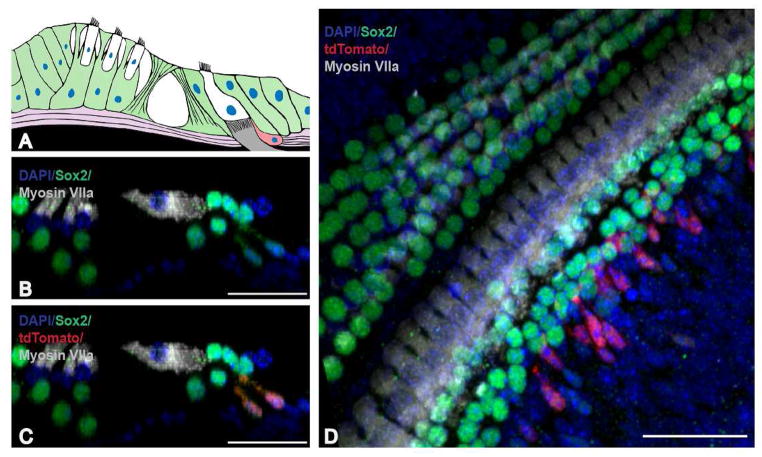
High magnification cross-sectional evaluation revealed the tdTomato-positive cells to be consistently located throughout the cochlea on the neural side (medial) and beneath the inner hair cell layer (closer to Rosenthal’s canal) (Fig. 4C, D). There did not appear to be any tdTomato-positive cells residing completely within the organ of Corti; however, apical protrusions of tdTomato-positive cells appeared to approach the inner most Sox2 expressing cell population, presumably the border cell population of supporting cells. In addition, the position and morphology of these cells was similar at each time point analyzed. A majority of these cells appeared oval at the base with an upward protrusion though the basal lamina towards the inner hair cell layer (Fig. 3G, L, Q, V, AA; Fig. 4C, D). Occasionally we observed that the protrusion at the apical end appeared to widen or flair to some extent (Fig. 5J). This type of morphology was seen in each region of the cochlea (apex, mid, base) and was observed in a seemingly random pattern across all time points.
Figure 4.
Cellular organization of tdTomato-positive cells in the normal mammalian inner ear. A: Orientation of the organ of Corti. B–C: Cross sectional view of a cochlea 35 days after the last tamoxifen injection reveals one row of inner hair cells and three rows of outer hair cells along with a normal compliment of cells labeled with the stem cell and supporting cell marker Sox2. D: Apical region of the cochlea 7 days after the last tamoxifen injection. TdTomato-positive cells are located below and medial to the inner hair cell layer and co-localize with the stem cell and supporting cell marker Sox2. Scale bar: 10 μm in B–D.
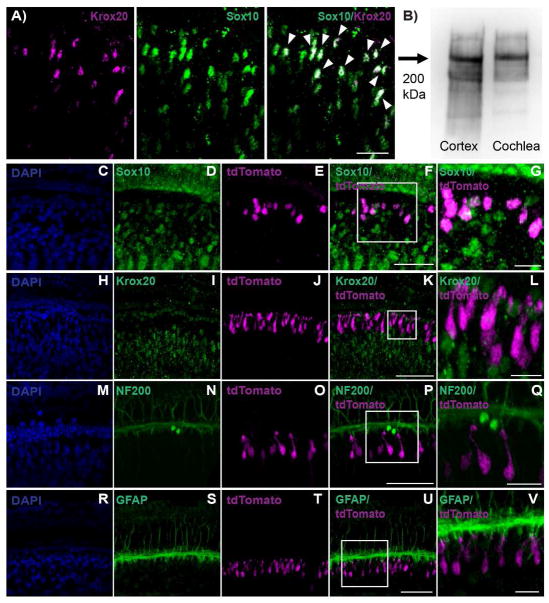
Figure 5.
Characterization of tdTomato-positive cells. A: In adult murine cochlear tissue, cells expressing Krox20 co-expressed with the Schwann cell and supporting cell marker Sox10 (arrows indicate double-layered cells with overlap shown in white). A smaller percentage of Sox10-positive cells expressed Krox20, suggesting Krox20 is a more precise Schwann cell marker B: NF200 antibody recognized a single band of about 200 kDa using adult murine brain lysates and cochlear tissues. C–G: 1 day after the last tamoxifen injection. The supporting cell and Schwann cell marker Sox10 co-localized with all tdTomato-positive cells. H–L: 14 days after the last tamoxifen injection. Krox20, a Schwann cell marker, did not co-localize with tdTomato-positive cells. M–Q: 14 days after the last tamoxifen injection. The spiral ganglion cell marker NF200 did not co-localize with tdTomato-positive cells. R–V: 7 days after the last tamoxifen injection. GFAP (expressed in border and inner phalangeal cell sub-populations of supporting cells) did not co-localize with tdTomato-positive cells. Images were acquired using a Nikon A1R-A1 confocal microscope. Scale bar: 10 μm in A; 50 μm in panels F, K, P, U; and 10μm in panels G, L, Q, V.
We next sought to determine if the number of tdTomato-positive cells increased over time. The number of tdTomato-positive cells in each high powered field (apex, mid and base) was counted for each ear of 13 mice and averaged across time points. The mean number of tdTomato-positive cells increased considerably from day 1 after the last tamoxifen injection to day 7, after which there was a slight increase in the average number of cells per region which peaked at day 35 (Fig. 6A). Statistical analysis of experimental data using a one-way analysis of variance (ANOVA) revealed P < 0.006. Post-hoc tests using Tukey HSD revealed no significant difference for the number of tdTomato-positive cells between days 1 and 7 post tamoxifen injection, and no significant difference between days 7 and 56 (Fig. 6A, B). There was a small but significant increase in the number of tdTomato-positive cells between day 1 and days 14 to 56. To determine if the observed increase in the number of tdTomato-positive cells was due to cell proliferation, we stained cochlear tissues with antibodies to Ki67. We found no evidence of Ki67 positive cells in any locations in the cochlea or spiral ganglion.
Figure 6.
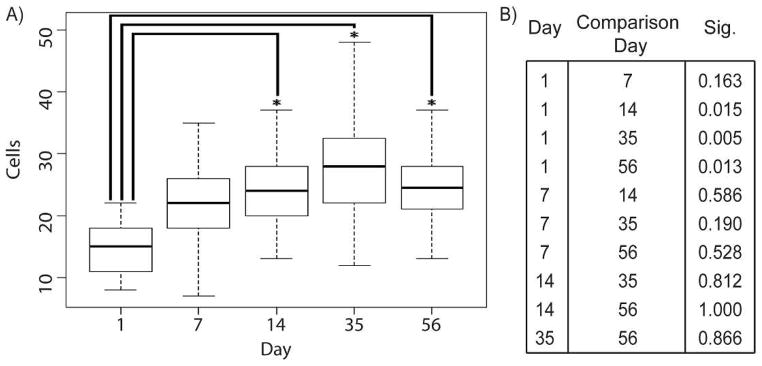
Statistical analysis. A: Box plot displaying the averages and spread of data (number of cells per high powered field) for each time point (Day). The mean number of tdTomato-positive cells increased from day 1 after the last tamoxifen injection to day 35, after which there was a slight decrease at day 56. B: Post-hoc tests using Tukey HSD comparing each time point (Day) to a comparison time point (Comparison Day) and the significance. There was no significant difference for the number of tdTomato-positive cells between days 1 and 7 post tamoxifen injection, and no significant difference between days 7 and 56. The only statistically significant difference seen was between day 1 and days 14 to 56. For all analyses, differences were considered significant if P < 0.05.
Characterization of tdTomato-positive cells
We did not observe co-expression of Myosin VIIa, a hair cell marker, in tdTomato-positive cells in any specimen. We therefore sought to identify if the tdTomato-positive cells in our experimental animals co-expressed supporting cell markers. Antibodies to a panel of supporting cell markers were tested for reactivity. We found reliable staining for the stem cell and supporting cell marker Sox2 which appeared to co-localize with all tdTomato-positive cells (Fig. 3I, N, S, X, AC; Fig. 4B, C, D). Additionally, the Schwann cell and supporting cell marker Sox10 co-localized with all tdTomato-positive cells (Fig. 5G). The expression of Sox2 and Sox10 appeared to have some level of variability amongst the population of tdTomato-positive cells. In general, weaker Sox10 expression was observed in Sox10 cells that co-localized tdTomato compared to cells expressing Sox10 alone. In addition, weak to strong expression of Sox2 was observed in cells that co-localized tdTomato. Although the expression levels of Sox2 and Sox10 cells that co-localized with tdTomato varied, tdTomato-positive cells consistently co-expressed these supporting cell markers on some level.
To determine if the population of tdTomato-positive cells might be Schwann cells, spiral ganglion neurons, or glia, we stained for Krox20, NF200 and GFAP respectively. All antibodies utilized in this study were previously characterized except for Krox20 and NF200. Krox20 is expressed in Schwann cells (Kipanyula et al., 2013; Provenzano et al., 2011) and NF200 is expressed in spiral ganglion neurons (Jyothi et al., 2010; Provenzano et al., 2011). Immunohistochemical analysis of adult mouse cochlear tissue found the Krox20 antibody co-expressed with the supporting and Schwann cell marker Sox10 (Fig. 5A). Not all cells co-localized Krox20 and Sox10, suggesting Krox20 is a more specific Schwann cell marker. In addition, the NF200 antibody recognized a single band of about 200 kDa in adult mouse brain lysates and cochlear tissue upon western blot analysis (Fig. 5B).
TdTomato cells did not co-localize with the Schwann cell marker Krox20 (Fig. 5L:). Krox20 localized to the area surrounding the spiral ganglion in a comparable region of the cochlea reported to contain Schwann cells (Knipper et al., 1998; Provenzano et al., 2011) (Fig. 5I). In addition, tdTomato-positive cells did not co-localize with NF200, which labels spiral ganglion neurons in the inner ear (Fig. 5Q). NF200 localized to the area just under the inner hair cell layer, and found to have a staining pattern in the cochlea consistent with reports of its expression in spiral ganglion neurons (Jyothi et al., 2010; Provenzano et al., 2011) (Fig. 5N). Last, tdTomato-positive cells did not co-localize with the glia cell marker GFAP (Fig. 5V). GFAP is reported to be expressed by inner border cells and inner phalangeal cells in the organ of Corti (Rio et al., 2002; Smeti et al., 2011). GFAP expression localized to a region comparable to inner border cells and inner phalangeal cells, with tdTomato-positive cells located below and medial to these cells (Fig. 5U; Fig. 7).
Figure 7.
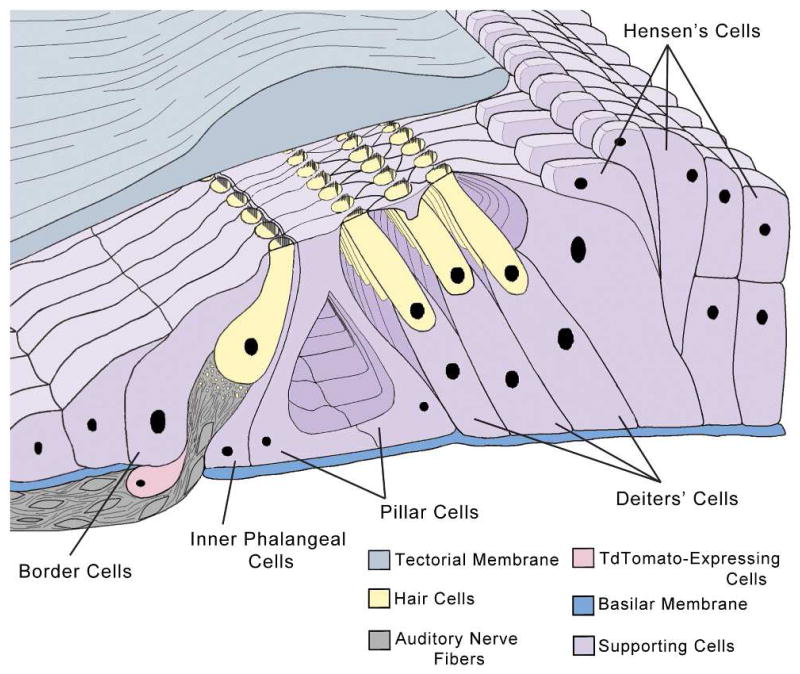
The cellular organization of the organ of Corti consists of several supporting cells including: Deiters’, Hensen’s, pillar, border, and inner phalangeal cells, in addition to inner and outer mechanosensory hair cells. TdTomato-positive cells appeared under and medial to the inner hair cell layer in the neural aspect.
We stained for relevant antibodies to nestin antigens in an effort to confirm cre-positive cells in the cochlea are nestin-positive, thus demonstrating tdTomato-expressing cells are indeed progeny of nestin-expressing cells. A number of methods and antibodies were employed in this endeavor; however, we were unsuccessful in these attempts to verify the fidelity of cre for nestin expression in our mouse model. Other investigators have encountered this challenge when staining mouse cochlear tissue for nestin (Watanabe et al., 2012).
Discussion
In this study, we examined tdTomato-positive cells in the organ of Corti of adult transgenic mice expressing an inducible form of CreERT2 under the control of a nestin promoter. In this model tamoxifen-mediated activation of Cre-recombinase leads to tdTomato fluorescence in nestin-expressing cells. Harvesting tissue shortly after tamoxifen activation can be used to evaluate ongoing expression of nestin. Harvesting tissue at later time points after nestin promoter activation allows for lineage tracing as has been demonstrated in the subventricular and subgranular zones of the brain in mice (Lagace et al., 2007). We used this technique to evaluate nestin expression in the adult murine inner ear and characterize the fate of nestin-expressing cells and their descendants.
A number of earlier studies have described nestin-expressing cells in the murine inner ear however their persistence during maturation and their cochlear localization has varied between reports (Lopez et al., 2004; Smeti et al., 2011; Watanabe et al., 2012). Using nestin-GFP transgenic mice, Lopez et al. (2004) observed nestin-GFP expression in border cells, inner phalangeal cells, Deiters’ cells, Satellite cells and in the greater epithelial ridge in postnatal day (P)5 cochleae. A mild GFP signal was also seen in outer hair cells although inner hair cells were GFP-negative. In P60 adult mice, nestin-GFP was down-regulated and localized to only a few Deiters’ cells under the outer hair cells. Smeti and colleagues (2011), in contrast, found nestin-GFP expression to be restricted to supporting cells near the inner hair cell area, particularly the border and inner phalangeal cells on P3 with mild expression of nestin in a small number of inner hair cells and outer hair cells. In 8-week-old mice, nestin-GFP expression was restricted to spiral ganglion cells and nerve fibers projecting towards the inner hair cells. Most recently, Watanabe et al. (2012) have reported that nestin expression is restricted to supporting cells lateral to the outer hair cell region using nestin β-gal mice, an observation more consistent with that made by Lopez et al. (2004). The number of β-gal positive cells decreased as the cochlea matured, with nestin-expressing cells becoming confined to the apical region of the cochlea by 3 weeks of age and minimally detectable in the apex by P90. Research evaluating nestin expression in the inner ear of rats has been more congruent (Carricondo et al., 2010; Kojima et al., 2004; Lou et al., 2007; Malgrange et al., 2002). All four studies on the topic in rats utilized immunostaining to evaluate the spatial and temporal expression of nestin in the organ of Corti and found nestin expression restricted to a region surrounding the basilar membrane and external edge of the spiral limbus (below the inner hair cell layer) in postnatal and adult animals (Carricondo et al., 2010; Kojima et al., 2004; Lou et al., 2007; Malgrange et al., 2002). In aggregate, in both mouse and rat studies it is clear that nestin expression is consistently found in the developing and young rodent cochlea; however, the localization and persistence of nestin-expressing cells in the adult rodent inner ear is less clear.
By using lineage tracing, we could document tdTomato-fluorescence in cells in adult mice ~ 8–26 weeks of age. The tdTomato-expressing cells were located beneath the inner hair cell layer at a level closer to Rosenthal’s canal and also medial to the inner hair cell layer in a region similar to that described in other studies (Carricondo et al., 2010; Kojima et al., 2004; Lou et al., 2007; Malgrange et al., 2002; Smeti et al., 2011). Importantly, the localization and morphology of these cells was consistent at each time point we examined with oval cell bodies having an upward protrusion through the basal lamina and into the organ of Corti in a vast majority of these cells.
We observed an increase in the number of tdTomato-positive cells following tamoxifen administration, with a significant difference in numbers of tdTomato-positive cells between day 1 and days 14 to 56. The increased numbers of tdTomato-positive cells during these intervals may reflect either delayed labeling or potentially some level of proliferation of this cell population over time. However we did not observe adjacent pairs of tdTomato-positive cells at later time points, which one might expect if this cell population was actively proliferating. Furthermore, we did not observe any tdTomato-positive cells that co-expressed Ki67. It is possible that proliferation of these tdTomato-positive cells are a rare event in healthy animals and the cell cycle window is too short to capture by Ki67 staining. It is also possible that the increased numbers of tdTomato positive cells over time was due to delayed labeling after tamoxifen administration.
It is unclear if the tdTomato-positive cells we have observed in the cochlea posses stem/progenitor characteristics. Nestin expression designates a progenitor fate in the central nervous system and other organ systems. While it appears the tdTomato-positive cells we have identified do not actively proliferate in the cochlea, these cells may represent a quiescent stem cell population. In adult tissues, stem cells may remain in a quiescent state until there is a need for proliferation such as in the event of injury. It is also possible that nestin-positive cells are not stem cells, but rather committed progenitors that are destined to become one particular differentiated cell type. Additional studies incorporating neonatal time points will help determine whether this cell population can give rise to differentiated cell types in the cochlea (such as hair and/or supporting cells) or proliferate in response to injury are ongoing. These studies will address more definitively whether nestin expression correlates with a stem or progenitor fate in the cochlea.
The location of tdTomato-positive cells medial to the inner hair cells suggests either a supporting or neuronal cell fate. We found that the tdTomato-positive cells also express both Sox2 and Sox10, although expression of the latter is more variable and less robust. These transcription factors are expressed in both supporting cells in the mature organ of Corti (Millimaki et al., 2010; Oesterle et al., 2008; Watanabe et al., 2000) and in the spiral ganglion (Neves et al., 2007; Wakaoka et al., 2013). Consequently, based on Sox2 and Sox10 expression alone, we could not establish whether tdTomato-positive cells are supporting cells at the medial aspect of the organ of Corti or alternatively peripherally located elements of the spiral ganglion cell population.
To attempt to resolve this question, we used additional antibodies to assess expression of Krox20, GFAP and NF200. We found that the tdTomato-positive cells do not co-express GFAP, a marker for glia cells including non-myelinating Schwann cells. GFAP expression in the organ of Corti has also been found in supporting cells around the inner and outer hair cell area, including inner phalangeal cells, border cells and Deiters’ cells after P15 (Rio et al., 2002; Smeti et al., 2011). Lack of co-expression of tdTomato and GFAP suggests that the tdTomato-positive cells are neither border cells nor inner phalangeal cells, the two most likely supporting cells found in this area. These cells also did not express NF200, a marker for spiral ganglion neurons, or Krox20, a Schwann cell marker (Garbay et al., 2000; Hansen et al., 2001). These observations imply that the tdTomato-positive cell population we identified is not a subpopulation of spiral ganglion neurons, Schwann cells or GFAP-expressing supporting cells. Future development of new and better cell type-specific markers will allow us to clarify whether these cells belong to a unique cell population distinctive from Schwann or supporting cell types.
It is possible that the cre expression in the mouse line that we employed may not faithfully report nestin expression at the time of tamoxifen administration. A number of findings would support that this is not the case. First, the fidelity of cre expression in cells expressing nestin has been evaluated (Sun et al., 2014) and the nestin cre mouse line that we used in this study (K line) was found to be superior to others (lines H & 4) in limiting ectopic expression of the reporter. Furthermore, we did not find any evidence of a leak in the promoter activity in our negative control animals. In addition, we found tdTomato expression to occur in the mouse cochlea in a similar area as reported by other investigators using a different, constitutive transgenic nestin reporter line (Smeti et al., 2011). Collectively these findings provide support for the fidelity of the reporter line that we used for nestin expression in the mouse inner ear.
In summary, while evidence suggests that the putative stem cell population in the mammalian inner ear diminishes in mature mammals, our results raise the possibility that cells expressing a marker of a stem cell fate persist into adulthood. By using a lineage tracing technique, we were able to define the spatial and temporal expression of nestin in mice of similar or older ages as compared to earlier reports (Lopez et al., 2004; Smeti et al., 2011; Watanabe et al., 2012). We could also show that tdTomato-positive cells have supporting cell characteristics based on co-expression of Sox2 and Sox10 however the subtype of supporting cells remains unclear. Further investigations are needed to compare nestin-CreERT2/tdTomato-reporter mice to nestin-GFP mice. In addition, the proliferative capabilities and potency of this cell population should be evaluated. Advancing our understanding of these cells and their function may facilitate approaches to target this cell population as a treatment for hearing loss.
Acknowledgments
Grant Sponsor: National Institutes of Health (NIH); Grant numbers: NIH/NIDCD 1 R03 DC012432-01, 1 R01 DC013912-01, NIH/NIMH R01MH080434, and NIH P30 HD003352. University of Wisconsin-Madison; KL2 award and Type I pilot from the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant TL1 UL1TR000427.
We thank N.E. Patzlaff, J.J. Fong, Y. Xing, G.E. Leverson, J.R. Jones, E.M. Jobe, R.A. Bradley and R.J.H. Smith for their assistance throughout the duration of this project.
Footnotes
Conflict of Interest
The authors declare no potential conflict of interest.
Author Contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: CC, WX, XZ, SG. Acquisition of data: CC, WX. Analysis and interpretation of data: CC, WX, PT, SG. Drafting of the manuscript: CC. Critical revision of the manuscript for important intellectual content: CC. WX, PT, XZ, SG. Statistical analysis: CC. Obtained funding: XY, SG. Administrative, technical, and material support: PT, XY. Study supervision: CC, SG.
Literature Cited
- Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain research bulletin. 2002;57(6):751–758. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- Breuskin I, Bodson M, Thelen N, Thiry M, Borgs L, Nguyen L, Lefebvre PP, Malgrange B. Sox10 promotes the survival of cochlear progenitors during the establishment of the organ of Corti. Developmental biology. 2009;335(2):327–339. doi: 10.1016/j.ydbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Burns JC, Collado MS, Oliver ER, Corwin JT. Specializations of intercellular junctions are associated with the presence and absence of hair cell regeneration in ears from six vertebrate classes. The Journal of comparative neurology. 2013;521(6):1430–1448. doi: 10.1002/cne.23250. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. The Journal of comparative neurology. 2001;435(4):406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Carricondo F, Iglesias MC, Rodriguez F, Poch-Broto J, Gil-Loyzaga P. In vitro long-term development of cultured inner ear stem cells of newborn rat. Cell and tissue research. 2010;342(1):13–19. doi: 10.1007/s00441-010-1039-8. [DOI] [PubMed] [Google Scholar]
- Chai R, Xia A, Wang T, Jan TA, Hayashi T, Bermingham-McDonogh O, Cheng AG. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. Journal of the Association for Research in Otolaryngology: JARO. 2011;12(4):455–469. doi: 10.1007/s10162-011-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commo S, Gaillard O, Thibaut S, Bernard BA. Absence of TRP-2 in melanogenic melanocytes of human hair. Pigment cell research/sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2004;17(5):488–497. doi: 10.1111/j.1600-0749.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240(4860):1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen DH, Chalasani K, Steigelman KA, Fang J, Cheng AG, Zuo J. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014;141(4):816–829. doi: 10.1242/dev.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain research Developmental brain research. 1995;84(1):109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- Davies D. Temporal and spatial regulation of alpha6 integrin expression during the development of the cochlear-vestibular ganglion. The Journal of comparative neurology. 2007;502(5):673–682. doi: 10.1002/cne.21302. [DOI] [PubMed] [Google Scholar]
- Farahani RM, Simonian M, Hunter N. Blueprint of an ancestral neurosensory organ revealed in glial networks in human dental pulp. The Journal of comparative neurology. 2011;519(16):3306–3326. doi: 10.1002/cne.22701. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259(5101):1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Segre JA. A new lease on life. Cell. 2000;100:143–155. doi: 10.1016/s0092-8674(00)81691-8. [DOI] [PubMed] [Google Scholar]
- Fuentes-Santamaria V, Alvarado JC, Gabaldon-Ull MC, Manuel Juiz J. Upregulation of insulin-like growth factor and interleukin 1beta occurs in neurons but not in glial cells in the cochlear nucleus following cochlear ablation. The Journal of comparative neurology. 2013;521(15):3478–3499. doi: 10.1002/cne.23362. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23(28):9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(3):612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nature neuroscience. 2009;12(9):1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbay B, Heape AM, Sargueil F, Cassagne C. Myelin synthesis in the peripheral nervous system. Progress in neurobiology. 2000;61(3):267–304. doi: 10.1016/s0301-0082(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Arevalo-Martin A, Paniagua-Torija B, Sierra-Palomares Y, Molina-Holgado E. A cell population that strongly expresses the CB1 cannabinoid receptor in the ependyma of the rat spinal cord. The Journal of comparative neurology. 2013;521(1):233–251. doi: 10.1002/cne.23184. [DOI] [PubMed] [Google Scholar]
- Guo W, Allan AM, Zong R, Zhang L, Johnson EB, Schaller EG, Murthy AC, Goggin SL, Eisch AJ, Oostra BA, Nelson DL, Jin P, Zhao X. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nature medicine. 2011;17(5):559–565. doi: 10.1038/nm.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MR, Vijapurkar U, Koland JG, Green SH. Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hearing research. 2001;161(1–2):87–98. doi: 10.1016/s0378-5955(01)00360-4. [DOI] [PubMed] [Google Scholar]
- Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(21):9815–9819. doi: 10.1073/pnas.92.21.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume CR, Bratt DL, Oesterle EC. Expression of LHX3 and SOX2 during mouse inner ear development. Gene expression patterns: GEP. 2007;7(7):798–807. doi: 10.1016/j.modgep.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume CR, Kirkegaard M, Oesterle EC. ErbB expression: the mouse inner ear and maturation of the mitogenic response to heregulin. Journal of the Association for Research in Otolaryngology: JARO. 2003;4(3):422–443. doi: 10.1007/s10162-002-3008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JM, Warchol ME. Expression of the Gata3 transcription factor in the acoustic ganglion of the developing avian inner ear. The Journal of comparative neurology. 2009;516(6):507–518. doi: 10.1002/cne.22128. [DOI] [PubMed] [Google Scholar]
- Jyothi V, Li M, Kilpatrick LA, Smythe N, LaRue AC, Zhou D, Schulte BA, Schmiedt RA, Lang H. Unmyelinated auditory type I spiral ganglion neurons in congenic Ly5.1 mice. The Journal of comparative neurology. 2010;518(16):3254–3271. doi: 10.1002/cne.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachinsky AM, Dominov JA, Miller JB. Intermediate filaments in cardiac myogenesis: nestin in the developing mouse heart. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1995;43(8):843–847. doi: 10.1177/43.8.7542682. [DOI] [PubMed] [Google Scholar]
- Kipanyula MJ, Woodhoo A, Rahman M, Payne D, Jessen KR, Mirsky R. Calcineurin-nuclear factor of activated T cells regulation of Krox-20 expression in Schwann cells requires elevation of intracellular cyclic AMP. Journal of neuroscience research. 2013;91(1):105–115. doi: 10.1002/jnr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, Bandtlow C, Gestwa L, Kopschall I, Rohbock K, Wiechers B, Zenner HP, Zimmermann U. Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nerve prior to cochlea function. Development. 1998;125(18):3709–3718. doi: 10.1242/dev.125.18.3709. [DOI] [PubMed] [Google Scholar]
- Kojima K, Takebayashi S, Nakagawa T, Iwai K, Ito J. Nestin expression in the developing rat cochlea sensory epithelia. Acta oto-laryngologica Supplementum. 2004;(551):14–17. doi: 10.1080/03655230310016744. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18(1):237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, DiLeone RJ, Greer CA, Mandyam CD, Eisch AJ. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(46):12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lopez IA, Zhao PM, Yamaguchi M, de Vellis J, Espinosa-Jeffrey A. Stem/progenitor cells in the postnatal inner ear of the GFP-nestin transgenic mouse. Int J Dev Neurosci. 2004;22(4):205–213. doi: 10.1016/j.ijdevneu.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Lou X, Zhang Y, Yuan C. Multipotent stem cells from the young rat inner ear. Neuroscience letters. 2007;416(1):28–33. doi: 10.1016/j.neulet.2006.12.061. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature neuroscience. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgrange B, Belachew S, Thiry M, Nguyen L, Rogister B, Alvarez ML, Rigo JM, Van De Water TR, Moonen G, Lefebvre PP. Proliferative generation of mammalian auditory hair cells in culture. Mechanisms of development. 2002;112(1–2):79–88. doi: 10.1016/s0925-4773(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Developmental biology. 2010;338(2):262–269. doi: 10.1016/j.ydbio.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naritsuka H, Sakai K, Hashikawa T, Mori K, Yamaguchi M. Perisomatic-targeting granule cells in the mouse olfactory bulb. The Journal of comparative neurology. 2009;515(4):409–426. doi: 10.1002/cne.22063. [DOI] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. The Journal of comparative neurology. 2007;503(4):487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. Journal of the Association for Research in Otolaryngology: JARO. 2008;9(1):65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano MJ, Minner SA, Zander K, Clark JJ, Kane CJ, Green SH, Hansen MR. p75(NTR) expression and nuclear localization of p75(NTR) intracellular domain in spiral ganglion Schwann cells following deafness correlate with cell proliferation. Molecular and cellular neurosciences. 2011;47(4):306–315. doi: 10.1016/j.mcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch C, Hustert E, Pfeifer D, Sudbeck P, Kist R, Roe B, Wang Z, Balling R, Blin N, Scherer G. The SOX10/Sox10 gene from human and mouse: sequence, expression, and transactivation by the encoded HMG domain transcription factor. Human genetics. 1998;103(2):115–123. doi: 10.1007/s004390050793. [DOI] [PubMed] [Google Scholar]
- Rio C, Dikkes P, Liberman MC, Corfas G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. The Journal of comparative neurology. 2002;442(2):156–162. doi: 10.1002/cne.10085. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta oto-laryngologica:Suppl. 1967;220:221–244. [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Sahin Kaya S, Mahmood A, Li Y, Yavuz E, Chopp M. Expression of nestin after traumatic brain injury in rat brain. Brain research. 1999;840(1–2):153–157. doi: 10.1016/s0006-8993(99)01757-6. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. Journal of cell science. 1993;106 (Pt 4):1291–1300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- Shi F, Kempfle JS, Edge AS. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(28):9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeti I, Savary E, Capelle V, Hugnot JP, Uziel A, Zine A. Expression of candidate markers for stem/progenitor cells in the inner ears of developing and adult GFAP and nestin promoter-GFP transgenic mice. Gene expression patterns: GEP. 2011;11(1–2):22–32. doi: 10.1016/j.gep.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Southard-Smith EM, Kos L, Pavan WJ. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nature genetics. 1998;18(1):60–64. doi: 10.1038/ng0198-60. [DOI] [PubMed] [Google Scholar]
- Sun MY, Yetman MJ, Lee TC, Chen Y, Jankowsky JL. Specificity and efficiency of reporter expression in adult neural progenitors vary substantially among nestin-CreER(T2) lines. The Journal of comparative neurology. 2014;522(5):1191–1208. doi: 10.1002/cne.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2010;58(8):721–730. doi: 10.1369/jhc.2010.955609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain research reviews. 2007;53(1):198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Team RDC. A Language and Environment for Statistical Computing. Vienna, Austria: 2008. [Google Scholar]
- Wakaoka T, Motohashi T, Hayashi H, Kuze B, Aoki M, Mizuta K, Kunisada T, Ito Y. Tracing Sox10-expressing cells elucidates the dynamic development of the mouse inner ear. Hearing research. 2013;302:17–25. doi: 10.1016/j.heares.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Walsh RM, Hackney CM, Furness DN. Regeneration of the mammalian vestibular sensory epithelium following gentamicin-induced damage. The Journal of otolaryngology. 2000;29(6):351–360. [PubMed] [Google Scholar]
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259(5101):1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Takeda K, Katori Y, Ikeda K, Oshima T, Yasumoto K, Saito H, Takasaka T, Shibahara S. Expression of the Sox10 gene during mouse inner ear development. Brain research Molecular brain research. 2000;84(1–2):141–145. doi: 10.1016/s0169-328x(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Morell MH, Miller JM, Kanicki AC, O’Shea KS, Altschuler RA, Raphael Y. Nestin-expressing cells in the developing, mature and noise-exposed cochlear epithelium. Molecular and cellular neurosciences. 2012;49(2):104–109. doi: 10.1016/j.mcn.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE, McTigue DM, Jakeman LB. Regional heterogeneity in astrocyte responses following contusive spinal cord injury in mice. The Journal of comparative neurology. 2010;518(8):1370–1390. doi: 10.1002/cne.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue LP, Lu J, Cao Q, Kaur C, Ling EA. Nestin expression in Muller glial cells in postnatal rat retina and its upregulation following optic nerve transection. Neuroscience. 2006;143(1):117–127. doi: 10.1016/j.neuroscience.2006.07.044. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Kobayashi K, Okubo M, Fukuoka T, Noguchi K. Increase of close homolog of cell adhesion molecule L1 in primary afferent by nerve injury and the contribution to neuropathic pain. The Journal of comparative neurology. 2011;519(8):1597–1615. doi: 10.1002/cne.22588. [DOI] [PubMed] [Google Scholar]