Abstract
Early in the process of osteoarthritis (OA) the composition (water, proteoglycan [PG], and collagen) and structure of articular cartilage is altered leading to changes in its mechanical properties. A technique that can assess the composition and structure of the cartilage in vivo can provide insight in the mechanical integrity of articular cartilage and become a powerful tool for the early diagnosis of OA. Diffusion tensor imaging (DTI) has been proposed as a biomarker for cartilage composition and structure. DTI is sensitive to the PG content through the mean diffusivity (MD) and to the collagen architecture through the fractional anisotropy (FA). However, the acquisition of DTI of articular cartilage in vivo is challenging due to the short T2 of articular cartilage (~40 ms at 3 T) and the high resolution needed (0.5–0.7 mm in plane) to depict the cartilage anatomy. We describe the pulse sequences used for in vivo DTI of articular cartilage and discus general strategies for protocol optimization. We provide a comprehensive review of measurements of DTI of articular cartilage from ex vivo validation experiments to its recent clinical applications.
Keywords: Articular cartilage, diffusion-weighted imaging, diffusion tensor imaging, osteoarthritis, knee injury
INTRODUCTION
Articular cartilage covers the articular surfaces of the bones in the joints and provides efficient load transmission to the subchondral bone with almost frictionless gliding of the articular surfaces. Articular cartilage has a cartilage-to-cartilage dynamic coefficient of friction of 0.005 (200 times lower than the ice-to-ice dynamic friction coefficient) (1), support compression up to 20 MPa without wear (equivalent to a 2200 m immersion in water) (2), and last for the time of a life span (~80 years). These unique mechanical and tribological properties are a consequence of the cartilage composition and structure.
Articular cartilage is mostly composed by water (~70%) and a solid matrix composed principally of collagen (~20%) and proteoglycan (PG, ~7%) (Fig. 1). PG molecules are responsible for the high swelling pressure in the cartilage matrix and the extremely low water permeability, which is responsible for the time-dependent poroeslatic properties of the cartilage matrix. The collagen fibrils organize in an arch-like structure and are responsible for the tensile forces which balance the osmotic pressure generated by the PG and the shear forces acting on the cartilage. Thus, the mechanical properties of the cartilage matrix rely on the integrity of the extracellular matrix.
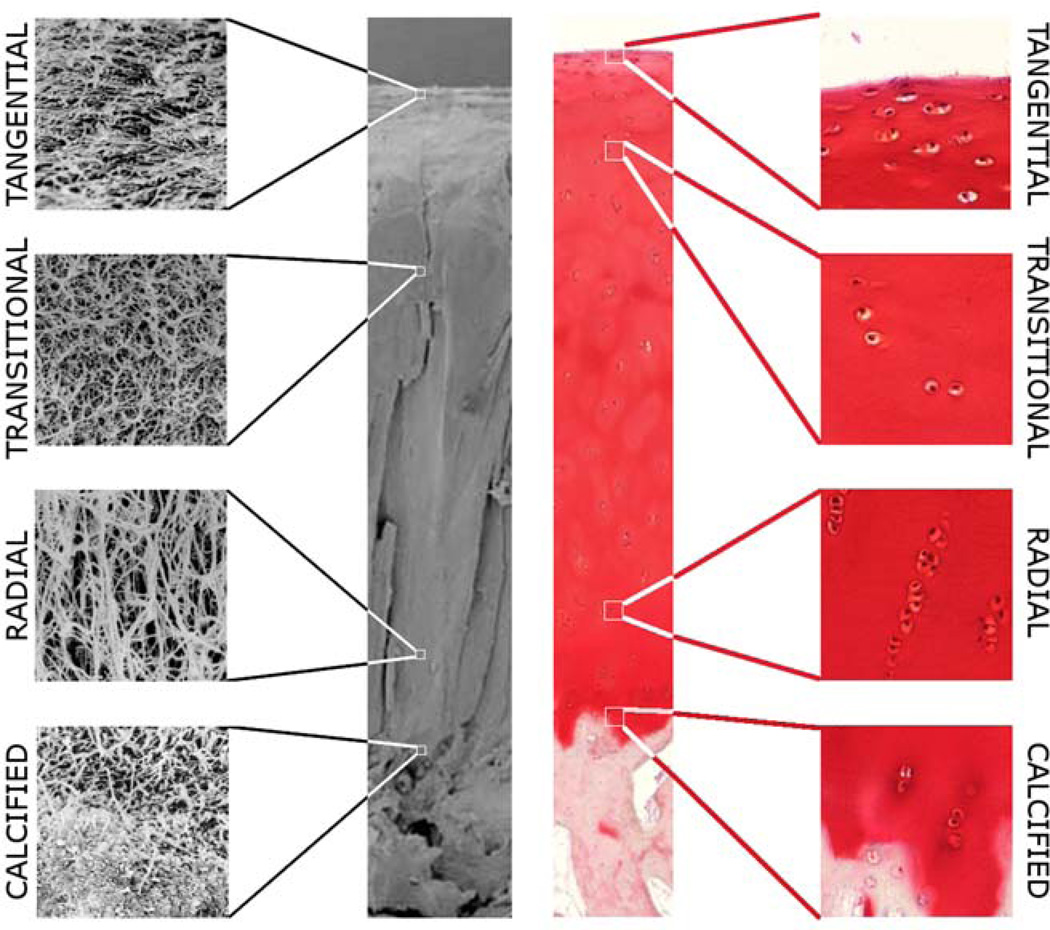
Figure 1.
Example of cartilage composition and structure in intact cartilage. Left side: Scanning electron microscopy (full view) and 6000× magnification fields of the collagen network showing the arrangement of collagen fibrils. The tangential zone shows thin collagen fibrils arranged parallel to the articular surface. The transitional zone presents random arrangement of slightly thicker collagen fibrils, while the radial zone contains thick collagen fibrils running perpendicular to the bone cartilage interface. The calcified zone shows the transition between the radial zone and the subchondral bone. Right side: Safranin-O stained of histology slide and 100× magnification showing chondrocytes. The intensity of the stain is estequiometric with the concentration of glycosaminoglycans in the cartilage matrix. Observe the increase in staining intensity from the articular surface to the calcified zone. The distribution of glycosaminoglycans provides an optimal gradient of osmotic pressure in the cartilage. The tangential zone is characterized by isolated ellipsoidal chondrocytes. In the transitional zone chondrocytes are still isolated but present a spherical shape. Chondrocytes in the radial zone piled up in columns.
Changes in cartilage composition occur early in the pathological process of OA. The molecular breakdown of the cartilage matrix causes an increases in its permeability changing its poroeslatic mechanical properties (2). The earliest signs of OA include loss of PG and disruption of the collagen architecture (3,4). Articular cartilage can stimulate PG synthesis but has very limited ability to repair damaged collagen, so changes in collagen are felt to be an indication of irreversibility (5–7). Measurement of collagen and PG is critical to understand the natural history of OA in vivo and accurately diagnose and stage OA (8).
Magnetic resonance imaging (MRI) has potential to assess the integrity of the cartilage matrix in vivo non-invasively. Most of MRI biomarkers for cartilage composition focus on PG: sodium (Na)-imaging (9,10), delayed Gadolinium enhanced MRI of the cartilage (dGEMRIC)(11), T1ρ relaxation time (12,13), and glycosaminoglycan chemical exchange dependent saturation transfer (gagCEST) (14). However, MRI biomarkers do not provide convincing assessment of collagen, with the T2 relaxation time (15,16) and magnetization transfer (MT) (17–19) only partially sensitive to the collagen.
Diffusion tensor imaging (DTI) can provide a comprehensive evaluation of articular cartilage by assessing both PG content and collagen architecture. The key idea behind the use of DTI is that the various components of the cartilage matrix have different effects on the motion of water molecules (20). The collagen network favors the motion of water along the collagen fibers inducing anisotropy in the motion of water, which can be measured with fractional anisotropy (FA) (20–27). PG molecules, on the other hand, do not show a preferred orientation and therefore restrict the motion of water molecules equally in all directions. Thus, PG content only affects the mean diffusivity (MD) (28–30). Measurement of diffusion is also interesting from the physiological point of view, since diffusion is an essential mechanism for mechanical function of articular cartilage and for the transport of nutrients to the chondrocytes and for the removal of their metabolic waste product.
Here we review the technical aspects of the acquisition of diffusion tensor imaging, its validation in ex vivo experiments and its translation for the clinical studies. This review is divided in five sections. In the first section we review the MRI techniques used to estimate the composition of articular cartilage, and discuss the advantages and disadvantages of diffusion measurements. We then describe in the second section the challenges of performing diffusion measurements of articular cartilage in vivo and discuss different diffusion-weighted MRI pulse sequences, which have demonstrated potential for this in vivo application. We provide some guidelines for protocol optimization in the third section. The fourth section is devoted to review the validation of diffusion measurements with ex vivo experiments. In the last section we review clinical applications of diffusion measurements of articular cartilage.
STATE OF THE ART COMPOSITIONAL CARTILAGE IMAGING: AVANCES AND LIMITATIONS
This section provides a very succinct overview of the current MRI techniques used to assess the cartilage composition and ultrastructure. This overview is intended to put into perspective the advantages and potential limitations of diffusion-measurements of articular cartilage.
Sodium (Na) imaging
Na MRI is based on the direct detection of Na+ ions in tissues using specific MRI acquisition sequences and coils. In Na-imaging the concentration of Na+ ions can be quantified. Since in cartilage Na+ ions balance the fixed negative charge of the glycosaminoglycans side chains of the PG molecules, measurement of the Na+ concentration is a biomarker for the PG content. In vivo application of Na-imaging have intrinsically low signal to noise ratio (SNR~20–40), and limited resolution (~2–10 mm isotropic) (31). Several in vivo studies have demonstrated lower sodium concentration in subjects with OA (Na-concentration <250 mM) as compared with a healthy population (~300 mM) (32–35). Due to the low resolution partial volume effects with the the low-sodium concentration synovial fluid (~140 mM) is a confounding factor. The use of an inversion recovery Na-imaging can suppress the signal of the synovial fluid (36,37) and improve the accuracy in the detection of early OA up to 78.7% (Kellgren-Lawrence [KL] score1–2) (35).
Delayed gadolinium enhanced MRI of articular cartilage (dGEMRIC)
The standard protocol for dGEMRIC involves intravenous injection of a double dose of gadopentetic acid (Gd-DTPA2−) and requires the subject to do exercise for 10 min (11). MRI is performed 90 min after the injection of the contrast agent to allow diffusion of the contrast agent in articular cartilage (38). Gd-DTPA2− is negatively charged and distributes in the cartilage matrix inversely proportional to the PG concentration. Williams et al. (39), analyzed the difference in the dGEMRIC index of patients with different radiographic grades of OA and observed a trend of decreased T1 with KL grade, although data from healthy subjects showed great variability. dGEMRIC After anterior cruciate ligament (ACL) rupture the cartilage dGEMRIC shows reduced T1 values in cartilage that with a trend of longer T1 relative to the time of injury (40). dGEMRIC can also monitor the process of cartilage remodeling after cartilage repair (41–43).
T2 relaxation time
The T2 relaxation time in articular cartilage depends on several factors like the water content (44,45), the proteoglycan content (45), and the collagen orientation (16,46,47). T2 relaxation time has shown correlation with the histological grade (48), although some ex vivo studies show that T2 might be insensitive to detect early stages of cartilage damage previous to cartilage fibrillation (48–50). T2 measurement can be performed in vivo with sequences that are available in most of the scanners and has good reproducibility (~5%), although influence of stimulated echoes and SNR need to be considered for an accurate T2 measurement (51–54). Clinical studies have found slightly increased T2 values in OA subjects as compared with healthy controls, (50,55) although T2 showed no difference between mild and advance OA subjects (55). The addition of a sequence for T2 mapping to the routine clinical protocol significantly improves the sensitivity to detect cartilage defect as assessed by arthroscopy (56, 57). The osteoarthritis initiative (OAI) provided data to demonstrate the association of increased T2 in articular cartilage with Body Mass Index (BMI), higher activity levels and elevated pain levels (58).
Spin-lattice relaxation time in the rotating frame (T1rho)
T1rho (the spin-lattice relaxation time in the rotating frame), is the relaxation time measured under the continuous irradiation of a low-power radio frequency (RF) field. Ex vivo studies at high field and high frequency spin-lock field (1.5–9 kHz) in native and depleted bovine cartilage have demonstrated that T1rho linearly increases with decreased GAG content (59,60). However, at low spin-lock frequency used in clinical scanners T1rho (400-500 Hz) only shows a moderate correlation with PG concentration (50,61,62). This is likely a consequence that at low spin lock frequencies the relaxation mechanism is still dominated by the dipolar-dipolar interaction as in T2 (63). Nontheless, T1rho has a higher dynamical range (30–120%) as compared to T2 (5–50%) (12). Clinical studies using T1rho and T2 showed increased T2 and T1rho in subjects with OA as compared to normal controls, however T1rho systematically demosntrated a larger dynamical range than T2 (13,64,65). T1rho has also shown value to detect early cartilage changes occurring as a consequence of ACL rupture and to monitor changes after ACL reconstruction within 1 year (66).
Glycosaminoglycan chemical-exchange-dependent saturation transfer (gagCEST)
This method uses the chemical-exchange-dependent saturation transfer (CEST) technique, which provide a mechanism for amplified detection of low concentration metabolites which contains residues with exchangeable protons such as the -NH and -OH spins of the glycosaminoglycans (67). gagCEST measurements are technically challenging due to the inherent sensitivity to B0 and B1 inhomogeneity and the presence of magnetization transfer effects with other macromolecules that bias the CEST quantification. This effects can be minimized with the simultaneous excitation of two symmetric frequencies (68). gagCEST has shown ability to monitor the process of cartilage remodeling after cartilage repair surgery (69).
Diffusion-weighted MRI in perspective of the existing methods
The major advantage of DTI is that it can evaluate PG and collagen of articular cartilage without the need of an exogenous contrast agent (contrary to dGEMRIC) and at much higher resolution as compared to the Na-imaging. However, diffusion cannot provide quantitative estimation of the absolute PG or collagen composition. Contrary to T2 and MT, FA provides a measurement of collagen that does not depend on PG or water content. The major drawback of diffusion is that is technically challenging, since standard diffusion sequences do not provide the adequate image quality for diffusion in cartilage. Thus, we start by reviewing the diffusion pulse sequences available for in vivo diffusion measurements of articular cartilage.
MRI PULSE SEQUENCES FOR DIFFUSION MEASUREMENTS OF ARTICULAR CARTILAGE
Challenges of in vivo diffusion imaging of articular cartilage
DTI of articular cartilage is challenging because of the high resolution needed (<1 mm) and the characteristic low T2 relaxation times of articular cartilage (around 40 ms at 3 T). Also joints have a complex anatomy that causes changes in the magnetic field (B0) that can lead to susceptibility artifacts. Thus, most commonly used pulse sequences for diffusion have very limited applicability to measure diffusion of the articular cartilage.
Single shot sequences (e.g. diffusion-weighted echo-planar imaging (DW-EPI) (70) or diffusion-weighted turbo-spin echo (DW-TSE) sequences (71,72)) are by far the most used diffusion sequences in clinical scanners because of their insensitivity to macroscopic motion and its short acquisition times. However, single shot sequences have characteristic long echo times (~80 ms) and only allow the acquisition of a limited number of phase encoding steps per excitation leading to in-plane resolution larger than 1 mm. Thus, these sequences have limited application for DTI of articular cartilage (Fig. 2).
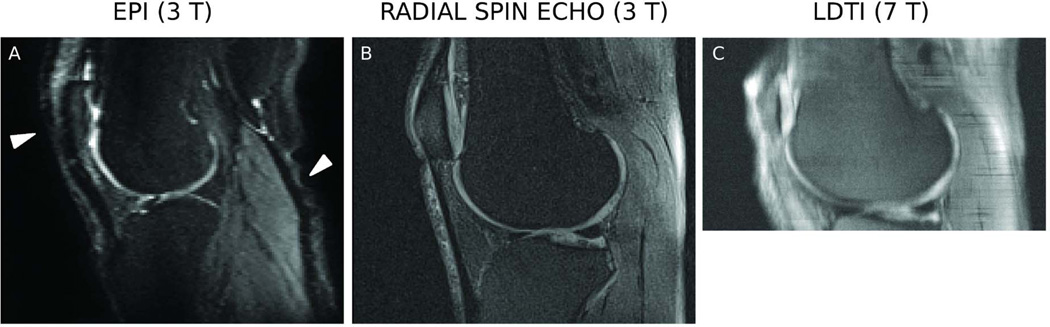
Figure 2.
Diffusion-weighted images acquired on a 39-years old asymptomatic male subject with different diffusion sequences. Clincal MRI of this subject (Sagittal and coronal PD and T1-weighted, not shown here) showed no signs of cartilage abnormality. A. Diffusion-weighted (b=400 s/mm2) images acquired with a DW-EPI sequence on a 3 T scanner (TE/TR = 56/5500 ms, slice thickness = 3 mm, 21 slices, field of view [FOV] = 176×176 mm2, in-plane resolution = 1.19×1.19 mm2, 6 averages, bandwidth = 1024 Hz/pixel, b-value = 0,400 s/mm2, phase samples = 123, parallel imagine acceleration factor (iPat) = 3, 36 reference lines, acquisition time = 2:34 min). Arrows in the EPI images indicate areas of geometric distortion. B. Diffusion-weighted images (b=350 s/mm2) acquired with a RAISED sequence on a 3 T scanner (TE/TR = 38/1500 ms, slice thickness = 3 mm, 21 slices, FOV = 154×154 mm2, in-plane resolution = 0.75×0.75 mm2, b-value = 0,350 s/mm2, bandwidth = 290 Hz/pixel, spokes = 114 [2.89 acceleration with respect to the Niquist condition], acquisition time = 2:50 min per b-value). C. Diffusion-weighted images acquired on a 7 T whole body scanner using a LSDTI sequence (TE/TR = 46/180 ms, slice thickness = 3 mm, 15 slices, FOV = 154×154 mm2, in-plane resolution = 0.6×0.6 mm2, b-value = 450 s/mm2, bandwidth = 500 Hz/pixel, rotation angle = 20°, acquisition time = 2:48 min per slice and 7 diffusion weightings).
Multiple shot sequences have much shorter echo times and therefore provide better SNR and allow for submillimeter image resolution. However, multiple-shot sequences have longer acquisition times than single shot sequences and are sensitive to macroscopic motion. Macroscopic motion during the diffusion sensitizing gradients results in phase errors, which causes inconsistent spatial encoding of the magnetization between the different shots (73). Thus, the use of multiple shot techniques requires the use of phase navigators to correct for motion-induced phases (80,81).
In the following we summarize all diffusion-weighted MRI pulse sequences that have been reported on the articular cartilage in vivo.
Single shot diffusion weighted echo planar imaging
Several authors have used DW-EPI to gain first experience on diffusion of articular cartilage (74–76). However, DW-EPI images of the knee are usually distorted due to the B0 inhomogeneity induced by the complex joint anatomy (74–76). Also, DW-EPI sequences only achieve limited in-plane resolution (over 1 mm), which is inadequate to assess cartilage structure (Fig. 2A).
Spin echo-based sequences
Diffusion-weighted spin echo (SE) sequences have attractive properties for diffusion imaging of articular cartilage, namely a high SNR efficiency and robustness against errors in the flip angle and inhomogeneity of the magnetic field (susceptibility). However, SE sequences are sensitive to coherent macroscopic motions and require long acquisition times due to the long repetition times necessary to allow the longitudinal magnetization to recover.
Different strategies have been applied to correct the sensitivity of SE sequences to motion and reduce the acquisition time. A modification of the SE which solves both issues is the line scan diffusion tensor imaging (LSDTI) pulse sequence (77), which is an extension for DTI of the line scan diffusion imaging sequence introduced by Gurbdjatsson et al (78). The LSDTI sequence acquires the images by exciting and refocusing a single line in space within each repetition time. Thus, the LSDTI sequence does not use phase encoding and is robust against macroscopic motion (Fig. 2C). Even more, the LSDTI sequence is much faster than the SE sequence, since lines can be subsequently acquired and the repetition time can be as low as 100–200 ms (i.e. around 3:00 min for 7 diffusion-weighted images in one slice). However, the LSDTI inherently suffers from low SNR, since the signal comes from one line and not from the whole imaging plane as in conventional SE sequences. The use of LSDTI for articular cartilage requires the use of ultra high fields (7 T) and dedicated knee coils.
A different strategy to optimize the acquisition of SE sequences for low fields is to use a 2D SE sequence with a non-Cartesian sampling of the k-space. The most simple non-Cartesian sampling is a radial acquisition of the k-space (79). Radial sampling allows undersampling rates of up to three times as compared to a fully sampled Cartesian image (up to five times as compared to fully sampled radial acquisition) without compromising the image quality or the parameter maps (Fig. 2B).
The radial SE sequence is also more robust against motion than conventional Cartesian SE sequences. However, radial sequences still need motion correction. The most common method for motion correction is the navigator echo acquisition (80,81). The navigator acquisition is an additional measurement performed after each diffusion-weighted preparation of the magnetization, which is always performed in the same way. The phase inconsistencies introduced by motion reflect in the phase of the navigator maps. By subtraction of the phases to the acquired data within each repetition, diffusion weighted images can be corrected to a great extent of non-linear macroscopic motions occurring during the diffusion encoding (82).
Steady state free precession sequences
Steady state free precession (SSFP) sequences can be adapted to DWI by introducing a diffusion gradient after each RF pulse. The most useful of these sequences for imaging of the musculoskeletal system is the diffusion-weighted fast imaging with steady precession (DW-FISP) sequence (83,84) (or the reversed fast imaging with steady precession [PSIF] sequence (85)), which allows the acquisition of high resolution images with short acquisition times due to the short repetition times used. SSFP sequences also have a high SNR efficiency. However, the echo in SSFP sequences is formed by the superposition of multiple coherence pathways. This produces a complicated signal dependence on sequence parameters (flip angle, echo time and repetition time), the T1 and T2 relaxation times, and the diffusion coefficient (86,87).
Miller et al. (82) used an standard 3D diffusion-weighted SSFP sequence with a 2D navigator to measure diffusion in articular cartilage (4:40 min per diffusion-weighted image). In this work Miller et al. demonstrated that the estimation of the diffusion constant with the SSFP sequences is very sensitive errors in the nominal flip angle (over 100% bias with a 10° error in the flip angle) and less to the initial estimation of the T1 and T2 relaxation times. Using standard T1 and T2 values for articular cartilage Miller et al. found the diffusion in cartilage to be 1.6×10−3 mm2/s in accordance with the values obtained ex vivo. However, since T1 and T2 values were assumed for the whole image diffusion in the synovial fluid presented unacceptable high values ranging from 4 to 6×10−3 mm2/s. Overestimation of diffusivity in the synovial fluid can be problematic in OA patients with cartilage fibrillation and subsequent infiltration of the synovial fluid in the cartilage matrix.
Different groups have worked to improve the calculation of diffusion parameters with SSFP sequences (88–90). A promising sequence for diffusion is the 3D double echo steady state (DESS) sequence, a variant of the SSFP sequence which acquires two echoes within each repetition time: a free induced decay (FID)-SSFP echo after each radio frequency pulse and a SFFP-echo previous to the next excitation (91). Staroswiecki et al (88) used a combination of 3D DESS images acquired with different repetition and echo times, flip angles and diffusion-weightings to calculate T1, T2, and the diffusion constant. Although the idea of quantifying so many parameters with only two acquisitions (two times 6:13 min) is very appealing, the measured diffusion constant showed poor precision with standard deviations up to 40% for typical cartilage values (T2=20–40 ms and D=1–1.5×10−3 mm2/s), which is twice the expected changes in diffusivity with cartilage degradation (around 20%).
Bieri et al. (89,90) proposed to use a 3D DESS acquisition in the fast acquisition regime (TR<<T2) with low flip angles, which results in improved SNR and T2 independence of the signal (4:00 min per diffusion direction, Fig. 3). Bieri et al. demonstrated that the quotient of the images from the two echoes (FID and SSFP echo) of two DESS acquisitions with and without diffusion-weighting depend on the diffusivity but not on the relaxation times (89). Bierei et al. reported a diffusivity on the articular cartilage between 1.0 and 1.7×10−3 mm2/s and a diffusivity in the synovial fluid of 2.6×10−3 mm2/s (89,90). The high SNR of this technique allows also high resolution imaging, so that application to small joints like the ankle becomes feasible (Fig. 3) (89).
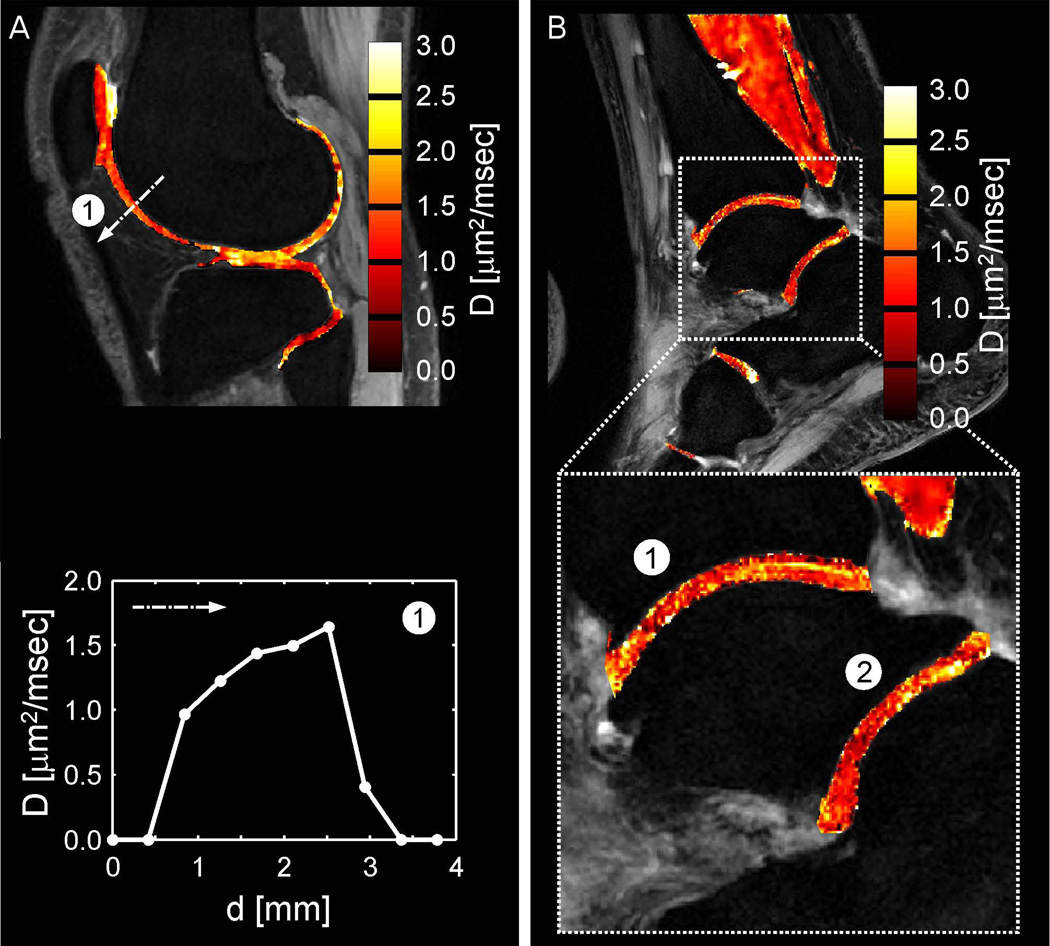
Figure 3.
A (left panel). Fusion of the derived diffusion map onto a conventional DESS image of normal appearing cartilage in the knee joint at 3.0T (derived from the nondiffusion-weighted DESS scan) and analysis of regional variations in the apparent molecular diffusion coefficient between deep and superficial femoral cartilage (along the dash-dotted line labeled with 1). B (right panel). Fusion of the derived diffusion map onto a conventional DESS image of normal appearing cartilage in the ankle joint at 3.0 T (derived from the nondiffusion-weighted DESS scan). For cartilage, D = 1.27±0.53 µm2/ms (tibiotalar joint, region 1) and D = 1.15±0.41 µm2/ms (talocalcaneal joint, region 2) is found. [Adapted from Figs. 7 and 8 of Bieri et al.(89)]
Spoiled Gradient Echo sequences
Spoiled gradient echoes have been also used for measurement of diffusion. Guha et al. recently used a stimulated diffusion-weighted preparation followed by a magnetization prepared angle modulated partitioned k-space spoiled gradient echo snapshots (MAPSS) readout (92,93). The MAPSS sequence is a variation of the spoiled gradient echo sequence in which the flip angle is modulated to obtain a uniform excitation profile through the evolution to the steady state. To preserve the diffusion-weighting of the magnetization the MRI signal need to be acquired during its transition to the steady state. Thus, a segmented acquisition of the k-space is necessary, which is achieved using an elliptical centric phase encoding order (92).
Additional pulse sequences
In this section, we restricted our discussion to those diffusion-weighted sequences which has been used to image the articular cartilage. However, there are many other diffusion sequences which may have potential for DTI of articular cartilage and might be worth investigating.
Diffusion-weighted sequences using a periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) acquisition may compensate for many of the limitations of the EPI sequences while providing good quality images (94). New generation scanners equipped with parallel transmit capabilities offer new opportunities for single shot sequences in articular cartilage. With a parallel transmit system a small field-of-view can be excited, so that high resolution images can be acquired with a reduced number of phase-encoding steps, so that high resolution diffusion-weighted images can be acquired using single shot sequences like EPI (Fig. 4). However, reduction of the field-of-view also involves a signal drop, which counteracts the signal gain due to the shortening of the echo time.
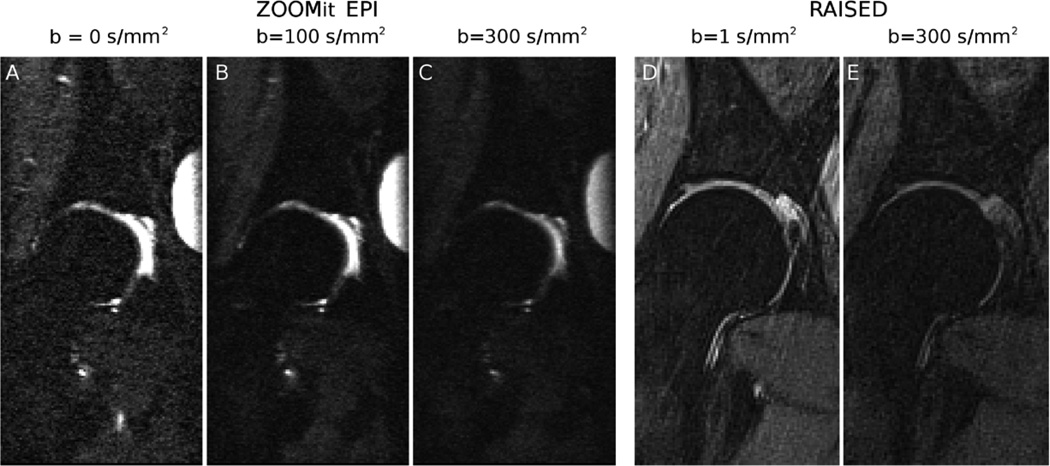
Figure 4.
Example of diffusion-weighted images of the femoral head on a 34-years old female asymptomatic subject. A–C. Imaging sequences include a DW-EPI acquired with the parallel transmit technology to selective excite a small region around the femur head (syngo ZOOMit; Siemens Healthcare, Erlangen, Germany). Parameter acquisition for the EPI were TE/TR = 71/6600 ms, slice thickness = 3.3 mm, echo-train length = 63, 13 slices, matrix size = 192×96 mm2, in-plane resolution = 0.80×0.80 mm2, bandwidth = 1024 Hz/pixel, b-value = 0,100,300 s/mm2 with averages of 1, 3, 5, partial Fourier = 5/8, acquisition time = 5:35 min. D–E. Diffusion-weighted images acquired on the same volunteer with a RAISED sequence (TE/TR = 38/1500 ms, slice thickness = 3 mm, 13 slices, FOV = 154×154 mm2, in-plane resolution = 0.80×0.80 mm2, b-value = 0,350 s/mm2, bandwidth = 290 Hz/pixel, spokes = 114 [2.64 acceleration with respect to the Niquist condition], acquisition time = 2:50 min per b-value).
PROTOCOL OPTIMIZATION FOR DIFFUSION IMAGING OF ARTICULAR CARTILAGE
In this section we present some general recommendations for protocol optimization, which can be applied to almost any diffusion-weighted pulse sequence (SSFP and DESS sequences require other considerations (89,95)). These recommendations aim to offer basic considerations for a protocol set up for DTI of articular cartilage and do not intend to provide systematic coverage of all technical aspects involved in the optimization of DTI acquisition, for which the reader should refer to the many excellent reviews on this topic, e.g. (73,96).
In practice we need to optimize a protocol within the constraints of a target resolution and/or a total acquisition time, while guaranteeing enough SNR on the diffusion-weighted images to avoid bias in the diffusion parameters due to the Rician noise. As a rule of thumb it is generally accepted that an SNR of at least 5 (the higher, the better) in the diffusion-weighted image with the highest b-value is necessary to avoid bias (96). However, in our experience SNR greater than 10 is necessary to avoid bias of the diffusion parameters, especially FA, when a reduced number of directions are used. The SNR can be measured with different methods depending on the pulse sequence, the use of filters, and the coil used (97).
Image resolution and matrix size
Since articular cartilage is only few millimeter thick, a resolution bellow 1 mm is mandatory to properly depict the cartilage anatomy. Ideally, resolution should be 0.5–0.6 mm. However, this image resolution can easily result in SNR bellow 10 so that slightly lower resolutions of 0.7 mm are sometimes needed to increase SNR (a 36% increase in SNR by reducing in plane resolution from 0.6 to 0.7 mm). The target resolution determines the matrix size for a given a field of view. For example, if we plan to measure the knee with a field of view in the phase direction of 154 mm with a 0.6 mm resolution we will need a 154/0.6 = 256 matrix.
Repetition time
The repetition time (i.e. the time between two-diffusion weighted preparations) should be ideally set to five times the T1 relaxation time of articular cartilage (~5–6 s at 3 T) to allow for full relaxation of the magnetization after each excitation. However, for multi shot sequences the use of this optimal repetition time can result in prohibitive large acquisition times, so that shorter repetition times of 1–2 s have to be considered. The relative loss in SNR for a given TR as compared with full recovery is e−TR/T1. For a T1 of 1 s the SNR loss with a TR of 1.5 s is only 22% (e−TR/T1 = e−1.5 = 0.22), i.e. 78% of the signal remains.
Echo time and diffusion weighting
Due to the short T2 of articular cartilage is critical to keep the TE as short as possible, while keeping enough diffusion-weighting of the images. As a rule of thumb the optimal diffusion weighting is 1.09/D, with D being the characteristic diffusion constant of cartilage, D=1.0–1.5×10−3 mm2/s. Thus, the optimal b-values are between 700 and 1000 s/mm2. However, a maximum b-value over 500 s/mm2 usually result in SNR<10 in the diffusion-weighted images, so that more moderate maximum b-values of the order of 300–450 s/mm2 are used. As an example, with a maximum gradient of 35 mT/m, which is available in most clinical scanners, the minimum echo time for a b-value of 720 s/mm2 is 50 ms, which means a remaining signal of e−bDe−TE/T2 ≈ 0.10, i.e. only 10% of the signal remains (assuming T2=40 ms and D=1.5×10−3 mm2/s). For multishot sequence the trade-off between diffusion weighting and echo time is even worse. For a DW-EPI using partial Fourier 5/8 and parallel imaging acceleration factor (iPat) of 2 the shortest echo time available for a b-value of 400 s/mm2 is of the order of 80 ms (assuming monopolar gradients and a matrix size of 192 × 256), which means that less than 5% of the signal is available at the echo time.
Diffusion directions
There have been many studies which analyzed the optimal diffusion directions for DTI (96). Different criteria have been proposed to find an optimal distribution of diffusion directions. A popular option is to minimize the condition number (98). The condition number is a dimensionless number equal or greater than 1 that measures how the acquisition errors propagate to the coefficients of the diffusion tensor. The lower the condition number the more precise is the estimation of diffusion coefficients. Minimization of the condition number for six diffusion direction resulted in the “downhill simplex scheme” (DMS6) gradient (condition number=1.32) (98). However, minimization of the condition number, especially for DTI schemas with low number of diffusion directions, strongly depends on the orientation of the sample. To provide robustness against rotational variance diffusion directions need to be evenly distributed in the sphere. Thus, an icosahedral distribution is also commonly used (condition number=1.58), since it shows better rotational invariance properties (96,98).
Depending on the acquisition time available intermediary b-values, additional directions or averages to improve the SNR can be performed. The optimal strategy depends on how many extra diffusion weighted-images can be acquired. If you can only acquire few more images, it is better to increase the number of diffusion directions, for larger number of diffusion weighted-images averaging or the acquisition of an intermediate b-value can be considered.
EX VIVO VALIDATION OF DWI AND DTI OF ARTICULAR CARTILAGE
Diffusion spectroscopy and diffusion-weighted imaging: dependence with cartilage composition and diffusion time
The first diffusion measurements in articular cartilage using MRI were performed by Burstein et al. (29). In their seminal work, Burstein et al. performed spectroscopic measurement of the diffusivity of small solutes (water, Na+, Li+ and CF3CO2−) in healthy calf cartilage at different diffusion times and temperatures. Interestingly, the diffusion constant of all these small solutes was the same (~60% of their values in solution), indicating the low influence of the fixed charge of the GAGs in their diffusivities. Burstein et al. (29) also investigated the change in diffusion properties by selective removal of the glycosaminoglycans (GAG) side chains of the PG with trypsin. Progressive GAG depletion resulted in an increase in the diffusion coefficient of water up to 20% (Table 1). By analyzing the change in the diffusivity with the diffusion time (Δ=25–2000 ms) they observed that intact and PG depleted samples showed the same change in diffusion with Δ. Authors concluded that the influence of GAGs to the diffusion properties of the tissue is homogeneous by 25 ms while the collagen was responsible for the diffusion properties of cartilage at long diffusion times.
Table 1.
Diffusion measurements of artificially degraded cartilage1
| Method | D/MD1 (native) |
D/MD1 (treated) |
Diff. (%) |
FA (native) |
FA (treated) |
Diff. (%) |
Treatment [Doses] |
Time | N | GAG loss | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spect | 0.72–1.42 | 0.83–1.56 | 10–15 | – | – | – | Trypsin | – | – | – | (29) |
| [25 mg/ml] | |||||||||||
| Spect | 1.35 | 1.62 | 20 | – | – | – | Trypsin | 5–180 min | – | 0–13 µmol | (29) |
| [25 mg/ml] | |||||||||||
| DWI | 1.27 | 1.49 | 17 | – | – | – | Trypsin | – | 1 | – | (29) |
| [25 mg/ml] | |||||||||||
| Spect | – | – | 25 | – | – | – | Trypsin | 5–180 min | 34 | 60–80% | (30) |
| [25 mg/ml] | |||||||||||
| Spect | – | – | 30 | – | – | – | Hyaluronidase | 2–8 h | 10 | 60–87% | (30) |
| [100 units/ml] | |||||||||||
| Spect | – | – | <5 | – | – | – | Retinoic acid | 4 days | 16 | 10–70% | (30) |
| [0.0025–10 µg/ml] | |||||||||||
| Spect | – | – | 15 | – | – | – | Collagenase | 1 day | 10 | 5–50% | (30) |
| [0–225 units/ml] | |||||||||||
| DWI | 1.05±0.02 | 1.12±0.07 | 7 | – | – | – | EDTA | 1 day | 3 | 2.5±0.8% | (103) |
| (1D) | [204 mg/ml] | ||||||||||
| DWI | 1.08±0.08 | 1.18±0.08 | 9 | – | – | – | Gu-HCL | 1 day | 9 | 69±16% | (103) |
| (1D) | [382 mg/ml] | ||||||||||
| DWI | 1.07±0.02 | 1.08±0.02 | 1 | – | – | – | NaCl | 1 day | 5 | 0.7±0.4% | (103) |
| (1D) | [8.77 mg/ml] | ||||||||||
| Spect | 1.35±0.22 | 2.00±0.22 | 41 | – | – | – | Trypsin | 15–205 min | 2 | – | (99) |
| [25 mg/l] | |||||||||||
| DWI | 1.40±0.19 | 1.80±0.19 | 29 | – | – | – | Trypsin | 4 h | 1 | – | (99) |
| [25 mg/l] | |||||||||||
| DWI | 1.33±0.22 | 1.84±0.14 | 38 | – | – | – | Trypsin | 4 h | 2 | – | (99) |
| [25 mg/l] | |||||||||||
| DWI | 0.93±0.10 | 1.13±0.06 | 22 | – | – | – | Trypsin | 18 h | 120 | 62.8% | (100) |
| [Trypsin] | [1 mg/ml] | [Trypsin] | [Trypsin] | ||||||||
| 1.04±0.07 | 12 | Collagenase | 20 h | 13.2% | |||||||
| [collagen.] | [30 units/ml] | [collagen.] | [collagen] | ||||||||
| DWI | 0.93±0.10 | 0.96±0.10 | 3 | – | – | – | Trypsin | 6 h | 120 | – | (102) |
| [Trypsin] | [1 mg/ml] | [Trypsin] | |||||||||
| 0.97±0.09 | 4 | Collagenase | 18 h | ||||||||
| [collagen.] | [30 units/ml] | [collagen.] | |||||||||
| DWI | 1.01±0.09 | 1.25±0.08 | 24 | Trypsin | 24 h | 36 | 77% | (101) | |||
| [1 mg/ml] | [Trypsin] | ||||||||||
| DTI3 | 1.80±0.18 [AS] | 1.9 [AS] | 10–15 | 0.10 [AS] | 0.05 [AS] | – | Trypsin | 14 h | 15 | ~100%2 | (21) |
| 0.79±0.15 [BCI] | 1.0 [BCI] | 0.50 [BCI] | 0.20 [BCI] | [100 mg/ml] | |||||||
| DTI4 | 1.54±0.14 | 1.69±0.09 | 10 | 0.10±0.01 | 0.09±0.02 | −10 | Trypsin | 24–48 h | 6 | 68% | (22) |
| 1.43±0.14 | 1.65±0.14 | 15 | 0.10±0.01 | 0.10±0.01 | 0 | [25 mg/ml] | 75% | ||||
| 1.23±0.14 | 1.39±0.17 | 13 | 0.15±0.02 | 0.15±0.02 | 0 | 76% | |||||
| DTI5 | 1.34±0.02 | 1.44±0.07 | 7 | 0.10±0.01 | 0.11±0.01 | +10 | Trypsin | 24 h | 3 | 96% | (22) |
| 1.32±0.03 | 1.47±0.07 | 11 | 0.11±0.02 | 0.12±0.02 | +9 | [25 mg/ml] | 99% | ||||
| 1.21±0.03 | 1.43±0.17 | 18 | 0.16±0.02 | 0.19±0.03 | +19 | Collagenase | 9 days | 98% | |||
| [60 nM] | |||||||||||
| DTI | – | 0.25±0.03 | 20.6 | – | 0.03 | 0 | Trypsin | 6–96 h | 12 | −40 – −1205 | (24) |
| [96h trypsin] | [96 h trypsin] | [0.1 mg/ml] | |||||||||
| 0.07±0.04 | 0.03 | ||||||||||
| [Controls] | [Controls] |
The diffusion coefficient (D) for spectroscopy (Spect.) and diffusion-weighted imaging (DWI). MD was used for diffusion-tensor imaging (DTI) acquisitions (in units of 10−3 mm2/s). 1D indicates that imaging was performed in one dimension. Diff. = Relative difference. Bold face indicates significant (P<0.05) difference if provided in the reference. N=number of samples. Ref. = Reference
PG loss measured as the change in absorbance at 475 nm in safranin-O slides. After treatment absorbance changed 2 to almost 0, no direct quantification was reported.
AS=Articular surface; BCI=Bone cartilage interface.
Analysis performed by dividing the cartilage into three layers. Data presented for the 1/3 superficial, 1/3 middle and 1/3 deep layers.
Semiquantitative assessment of the PG loss based on the change in the staining of the safranin-O slides. Reproducibility of the method ±9 units.
Knauss et al. also analyzed the change of the diffusion constant with the diffusion time for short diffusion ranges (Δ=1–600 ms) (28). In their experiments they analyzed samples under varying compression from 0 to 41.8 atm. Using an in house built spectrometer with gradients up to 25 T/m they found that for low diffusion times (Δ≤13 ms) the diffusion in cartilage is determined only by the water content. For long diffusion times (Δ~500 ms) the diffusion in articular cartilage showed increased restriction. In agreement with Burstein et al, Knauss et al. (28) concluded that the restriction of diffusion for long Δ is due to the collagen network. In their experiments Knauss et al. showed that cartilage degradation with collagenase, which selectively degrades collagen, produced an increase in diffusivity of up to +40%.
Several groups have investigated the effect of artificial cartilage degradation on diffusion using many different degradation mechanisms (Table 1) (99–104). All these experiments consistently demonstrated an increase on diffusion properties after cartilage degradation. Lin et al. (100–102) investigated the value of a multiparametric MRI approach to classify samples of nasal bovine cartilage as intact or degraded. Lin et al. (100–102) measured the magnetization transfer ratio, the diffusion coefficient, and T1 and T2 relaxation times in intact, mild degraded (6 h in trypsin), and extensive degraded (24 h in trypsin) samples of bovine nasal cartilage. T1 was the single best predictor of cartilage followed closely by the diffusion constant (area under the curve of 0.97 (T1) and 0.97 (diffusion) for extensive degradation and 0.60 (T1) and 0.58 (diffusion) for mild degradation) (101). A combination of T1, the diffusion coefficient, and the magnetization transfer ratio led to the best classification, with an area under the receiver operating characteristic curve of 1 for severe cartilage damage and 0.94 for mild cartilage damage (101).
Diffusion-weighted imaging and spectroscopy: relationship with mechanical and tribological properties
Since the diffusion of interstitial water during compression determines the time-dependent mechanical properties of the cartilage matrix, it seems natural to expect a relationship between diffusion constant and the mechanical properties. Juráš et al. (105) analyzed the change in several MRI parameters (T1, T2 and diffusion constant) before and after compression (15% strain) and found a 11.5% decrease in diffusion from (0.96±0.40)×10−3 mm2/s to (0.85±0.39)×10−3 mm2/s. In a subsequent study Juráš et al. (106) correlated the diffusion constant with the mechanical properties measured with indentation (instantaneous and equilibrium modulus and relaxation time) found a positive correlation between the diffusion coefficient and the equilibrium modulus (r = −0.52) and the relaxation time (r = −0.73). More recently, Aoki et al. (107) found significant correlations between the diffusion coefficient in articular cartilage and the viscoelasticity (r2=0.69, P<0.01) and mechanical relaxation time (r2=0.75, P<0.01) as measured with an indentation experiment.
Two studies by Greene and collaborators (108,109) have analyzed the dynamical change of the diffusion coefficient under compression. They found evidence that the axial porosity during loading can play an important role in redirecting the flow of the interstitial fluid to the articular surface, thus providing an additional mechanism for cartilage lubrication.
Diffusion-weighted imaging: detection of early cartilage damage
Diffusion measurements have shown potential to detect artificial cartilage degradation. However, the value of diffusion to detect actual cartilage damage on human cartilage with signs of OA needs to be assessed. Mlynárik et al. (110) investigated the potential of different MRI parameters (including dGEMRIC, T2 and the diffusion coefficient) to detect cartilage damage in articular cartilage obtained from patients undergoing knee replacement surgery. Maps of the diffusion constant were correlated with fourteen histological slices with signs of OA. Increased diffusion coefficient was found in 10 slices (1.15–1.60×10−3 mm2/s) as compared with areas of intact cartilage (0.75–1.20×10−3 mm2/s), which means a sensitivity of 71.4%. In four slices the diffusion coefficient showed values in the range of healthy cartilage (0.85–1.25×10−3 mm2/s).
Diffusion-weighted spectroscopy: detection of diffusion anisotropy
First diffusion experiments to investigate the anisotropy in diffusion did not observe differences in diffusion direction (28,29,111). These first experiments were performed with spectroscopy measurements and the signal from different cartilage zones and different collagen orientation was averaged masking the anisotropy in articular cartilage. The most appropriate method to investigate the anisotropy of the diffusion in articular cartilage is DTI1, since it can allows assessing the anisotropy in diffusion at each voxel of the image.
Diffusion tensor imaging: Dependence with cartilage composition
The first DTI measurements in the articular cartilage demonstrated evident diffusion anisotropy in the cartilage matrix (20). Fillidoro et al. (20) showed a trend of increased FA and decreased MD towards the bone-cartilage interface. Filidoro et al observed that the orientation of the first eigenvector (i.e. the direction with the largest diffusion coefficient) showed a distinct zonal pattern which corresponds with the expected zonal arrangement of the collagen matrix in articular cartilage (20).
Meder et al. (21) analyzed the effect of artificial degradation of articular cartilage in the diffusion parameters (Table 1). Complete removal of the PG resulted in a 10–15% increase in MD and no change in the FA. Deng et al. (22) reproduced these findings on cartilage samples degraded with trypsin and a combination of trypsin and collagenase. Both samples treated with trypsin and trypsin and collagenase showed a significant increase in MD. After treatment with trypsin FA values remained unchanged. Interestingly, FA values after degradation with trypsin and collagenase were slightly although significantly increased (Table 1).
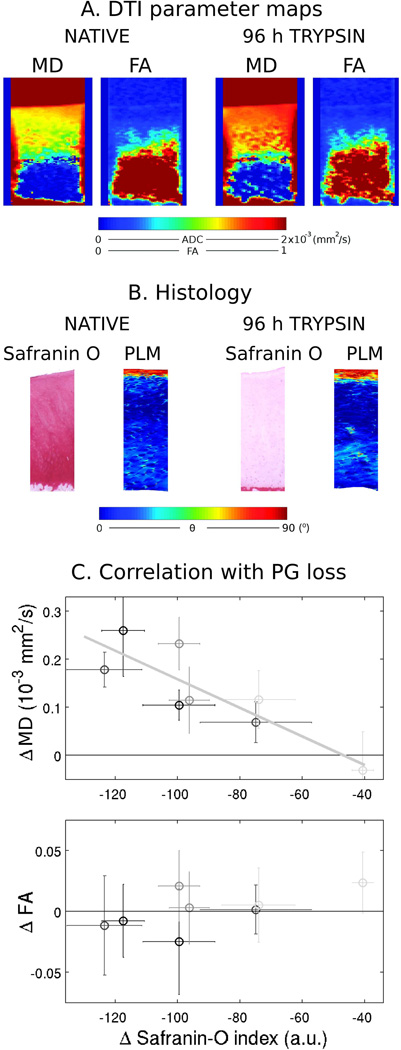
More recently, Raya et al. (24) analyzed the effect in the DTI parameters of progressive GAG depletion (Fig. 5). Osteochondral samples were imaged before and after treatment with a dilute trypsin solution. Histology with safranin-O (for GAG estimation) and picrosirius red (to assess the orientation of the collagen architecture with polarized light microscopy [PLM]) were available before and after cartilage treatment with trypsin. Raya et al. (24) found a significant correlation between the MD and the GAG loss as measured from safranin-O slices (r2=0.86, P<0.007). FA and first eigenvector as well as PLM showed only small changes in the order of magnitude of measurement errors, not correlating with PG loss. A region of interest analysis of the data revealed a depth-dependent correlation between the change in the MD and the degree of GAG-loss likely reflecting compositional differences in articular cartilage with depth.
Figure 5.
Change of diffusion parameters with progressive PG depletion. A. Diffusion parameter maps (MD and FA) of a sample before and after a 96 h treatment with a low dose (0.1 mg/ml) of trypsin for 96 hours. PG depletion resulted in increased MD but no change of FA. B. Histology analysis of the same sample before and after treatment with trypsin. Histology included safranin-O sensitive to the PG content and polarized light microscopy (PLM) for analysis of the collagen structure. Treatment with trypsin led to decrease in the intensity of the safranin-O staining, but no change in the collagen architecture. C. Correlation between the change in MD (r2 = −0.86, P<0.007) and FA (no significant) with the loss in PG content measured on safranin-O histology slides. Error bars represent the 2σ intervals of the difference to baseline. Light to dark gray encodes increasing trypsin incubation times (6, 48, 72 and 96 h). [Adapted from data of Raya et al. (24)].
Diffusion tensor imaging: dependence with the collagen architecture
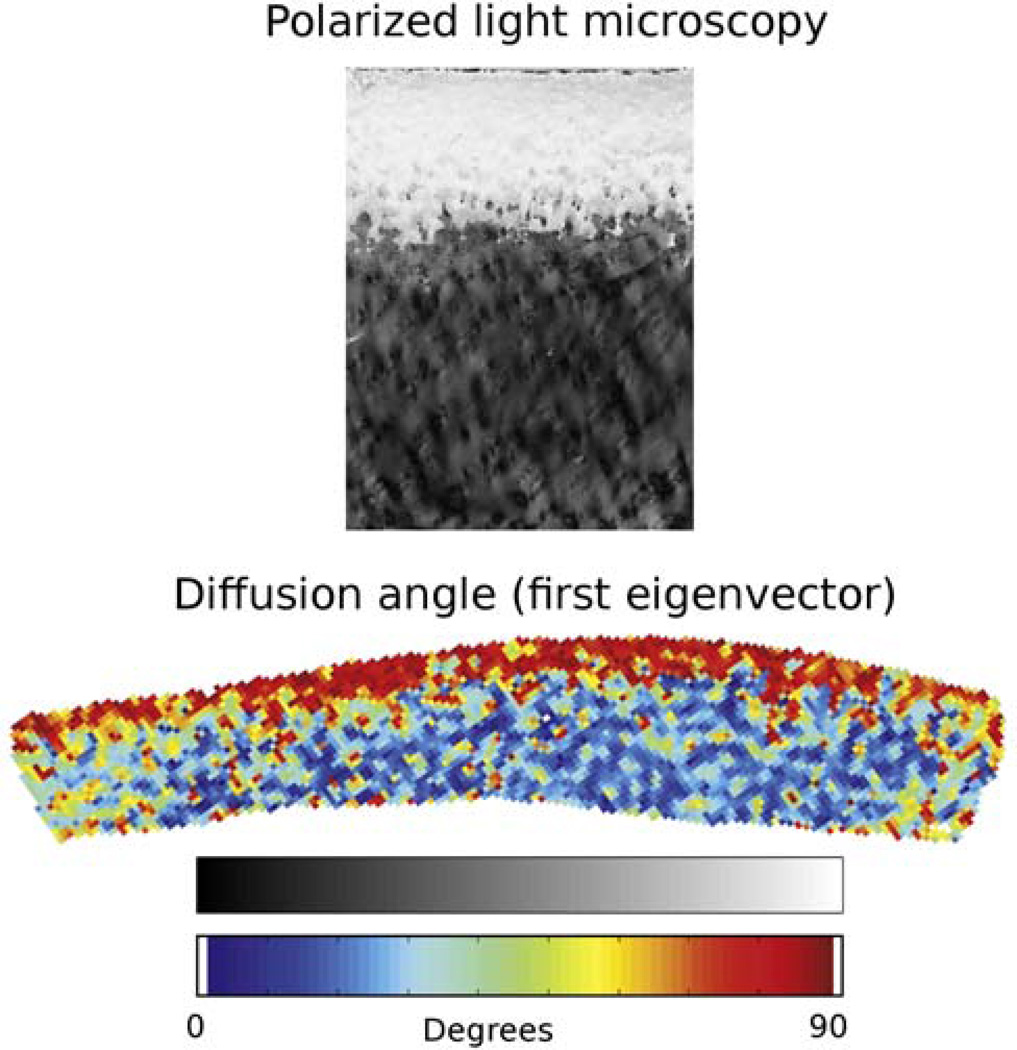
To better understand the origin of the diffusion anisotropy in articular cartilage, de Visser et al. (23) investigated the correlation between the orientation of the first eigenvector measured with DTI and the collagen orientation measured with PLM (Fig. 6). From a total of five samples three showed a good quantitative correlation with PLM and two showed only qualitative similarity. In a different study Raya et al. (25) found an excellent correlation between the orientation of the first eigenvector with the zonal arrangement of the collagen (radial (P<0.01, r2=0.89), transitional (P<0.01, r2=0.87) and tangential (r2=0.11)) as measured with scanning electron microscopy. The effect of cartilage compression in the DTI parameters has been also subject of investigation. de Visser et al. (112) performed the first study providing measurement of the cartilage under loading conditions (strain up to 30%). In this study de Visser et al. (112) reported increased FA in the 15% most superficial cartilage and decrease in MD in the 30% most superficial cartilage. The first eigenvector showed also rearrangement. With increased strain a progressive thickening of the tangential zone with higher alignment of the first eigenvector parallel to the articular surface was observed. Similar results were observed by Raya et al. on human cartilage samples (25,113). High resolution DTI has been used to provide accurate morphological information to calibrate new computational models of cartilage biomechanics (114,115).
Figure 6.
Correlation of DTI measurement with the polarized light microscopy (PLM) in a sample of bovine articular cartilage (sample B of reference (112)). Top. Map of PLM polarization angle α (the angle between the “fast” optical axis and the normal to AS). White corresponds to α = 90°; black, to α = 0°. Bottom. Map of the diffusion angle θ (the angle between the principal diffusion eigenvector and the normal to AS) for the same sample. The signal intensity scale is shown down, with white corresponding to θ = 0° and black to θ = 90°. The signal from the surrounding water and bone has been removed by thresholding. [Adapted from Figs. 2 and 3 of de Visser et al. (23)]
Diffusion tensor imaging: diagnosis of early cartilage damage
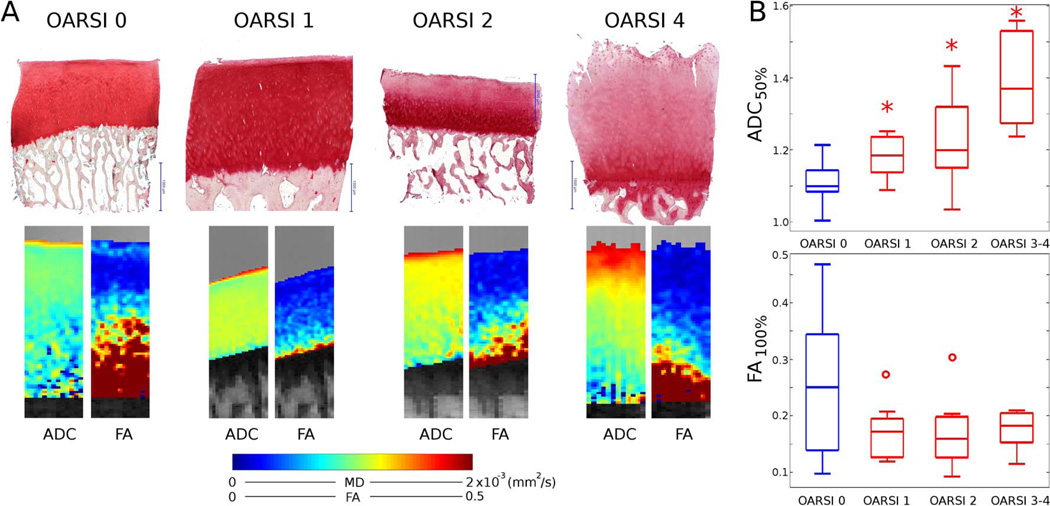
Raya et al. (26) investigated the value of DTI to detect early cartilage degeneration ex vivo in samples with early cartilage damage (Fig. 7). Cartilage damage was assessed in safranin O histology slides with the histology OARSI score, which ranges from 0 (healthy) to 6 (bone eburnation). The study included 43 samples with OARSI grades of 0 (n=14), 1 (n=11), 2 (n=12), 3 (n=4), and 4 (n=2) (Fig. 8). With increasing OARSI grade an increase in MD was observed from the articular surface propagating down to the bone-cartilage interface. The FA showed a slightly but significant decrease in the deep cartilage. DTI showed excellent performance in the detection of cartilage damage (accuracy, 95%; 41 of 43 samples) and good performance in the grading of cartilage damage (accuracy, 74%; 32 of 43 samples).
Figure 7.
Example of diffusion parameters of samples with different degrees of cartilage damage. A. Histology with safranin-O and diffusion maps (MD and FA) of samples with different grades of cartilage damage as shown by the OARSI scores. Maps show a clear trend of increased MD with increasing OARSI score and a less pronounced decrease in the FA. B. Box plot of the averaged MD over the 50% most superficial cartilage and averaged FA over the whole cartilage depth. Blue indicates average over intact cartilage (n=14 OARSI 0) and red average over cartilage with sign of degradation (OARSI 1 n = 11; OARSI 2 n = 12; OARSI ≥ 3 n = 6). Stars represent statistical significance with respect to the OARSI 0 group. Circles indicate outliers.
Figure 8.
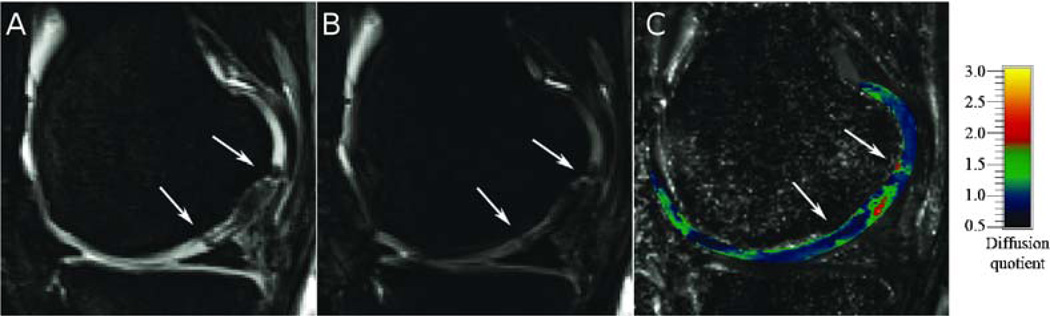
This three images show direct comparison of not diffusion-weighted image (A) with diffusion-weighted (B) image and corresponding diffusion-quotient map (C). Intensity of grayscaled part of left and central images was modified for better representation. Intensity of pseudo-colored cartilage part of images was not modified and can be evaluated using colorbar. [Adapted from Mamish et al.(116)]
In conclusion, ex vivo experiments have shown the potential of diffusion to detect very early cartilage changes occurring in articular cartilage in OA. Ex vivo experiments provide interpretation of DTI parameters in terms of cartilage composition and structure. While the PG only contributes to the MD, the collagen network is responsible for the anisotropy in diffusion measured by the FA. Ex vivo experiments have shown excellent correlation between the change in PG content and the change in diffusion coefficient. FA is not affected by the degree of PG extraction and is shown to change with cartilage compression. These ex vivo experiments provide the basis for the interpretation of the diffusion measurements performed in vivo.
FIRST IN VIVO STUDIES OF DIFFUSION OF ARTICULAR CARTILAGE
Diffusion-weighted imaging
Semiquantitative evaluation for cartilage repair
A first application of DWI has been to study cartilage repair using a diffusion-weighted SSFP sequence (116–119). In these first studies, a semi quantitative evaluation of the images was performed to avoid the complex calculation of the diffusion coefficient. Two images with and without diffusion-sensitizing gradients were acquired and the quotient their signal intensities, which they called the diffusion quotient, was used as a semiquantitative measure of the diffusion coefficient. However, as showed by Buxton and Wu (86,87) the diffusion quotient is a complex function of the diffusion constant, the T1 relaxation time, the repetition time, and the flip angle. Therefore, change in the diffusion quotient does not have a clear interpretation in terms of the diffusion doefficient.
In a first study Mamish et al. (116) investigated the change in diffusion quotient in a cohort of matrix autologous chondrocyte transplantation (MACT) patients (Fig. 8). Mamish et al. (116) divided the patients into two groups depending on the time from surgery. Group 1 included patients examined between 3 and 13 months after surgery and group 2 included patients examined between 19 to 42 months after surgery. Both groups showed significant increased diffusion quotients in cartilage repair (+36% and +11% for groups 1 and 2) as compared with the surrounding intact cartilage. After one year the patients in group 1 showed significantly decreased diffusion quotient while diffusion quotient did not change in patients of group 2 (118).
Welsch et al. (117) analyzed the diffusion quotient in MACT and microfracture (MFX) patients in the femoral condyle. MACT and MFX patients presented increased diffusion quotients with respect to intact cartilage and no difference in diffusion quotient was found between MACT and MFX patients. Interestingly, Welsch et al. found a positive correlation between the clinical symptoms evaluated using the Lysholm score and the diffusion quotient (Pearson’s correlation coefficient = −0.557; p = 0.011). This negative correlation indicates that the lower diffusion quotient led to better knee function. The high SNR of the SSFP sequences has allowed to measure the diffusion quotient in the tibiotalar joint (119,120). Apprich et al. measured patients that underwent MACT and MFX on the ankle at 48±22 and 60±23 months after surgery respectively. The diffusion coefficient of the cartilage repair of MACT subjects at 48 months was not significantly different from the diffusion quotient of the reference cartilage. However, MFX patients presented significantly higher diffusion quotient in the cartilage at 59 months (119,120). There were a correlation between the diffusion quotient and the clinical symptoms measured by the American orthopedic foot and ankle society (AOFAS) score for MFX (Pearson’s correlation coefficient = −0.648; p = 0.043).
Knee injury
Xu et al. (121) provided first in vivo evidence that diffusion have potential for the diagnosis of early cartilage degeneration after cartilage injury (30 asymptomatic controls and 32 subjects with cartilage injury). Inclusion criteria were pain in the medial or lateral compartment, a positive McMurray sign and an injury not older than 30 days at the time of MRI. Xu et al. (121) found significant increases between 26 and 30% in the diffusion constant depending on the cartilage plate (Table 2).
Table 2.
In vivo quantitative diffusion measurements of articular cartilage
| Sequence1 | Diffusion Directions |
Cartilage2 | D/MD3 (native) |
D/MD3 (OA/injury) |
Diff.4 (%) |
FA (native) |
FA (OA) |
Diff.4 (%) |
N5 | Ref.6 |
|---|---|---|---|---|---|---|---|---|---|---|
| 3D-SSFP | 1 | P | 1.60 | – | – | – | – | – | 3 | (95) |
| EPI | 6 | TF | 1.80–1.407 | – | – | 0.25–0.308 | – | – | 5 | (75) |
| TF | 1.70–1.408 | 0.25–0.309 | ||||||||
| 3D-DESS | 3 | P,F,T | 1.22–1.68 | – | – | – | – | – | 1 | (90) |
| 3D-DESS | 1 | P | 1.39±0.22 | – | – | – | – | – | 1 | (89) |
| F,T | 1.00–1.50 | |||||||||
| S | 2.60±0.24 | |||||||||
| A | 1.15–1.27 | |||||||||
| 3D-DESS | 1 | P,F,T | 1.00–3.5 | – | – | – | – | – | 4 | (88) |
| EPI | 1? | P | 1.17±0.31 | – | – | – | – | – | 30 | (122) |
| EPI | 1? | P | 1.44±0.16 | 1.78±0.32 | 24 | – | – | – | 30/32 | (121) |
| MCF | 1.43±0.18 | 1.76±0.41 | 23 | |||||||
| LCF | 1.45±0.16 | 1.80±0.47 | 24 | |||||||
| MT | 1.44±0.20 | 1.88±0.36 | 31 | |||||||
| LT | 1.43±0.17 | 1.80±0.31 | 26 | |||||||
| LSDTI | 6 | P | 1.00±0.10 | 1.29±0.16 | 29 | 0.30±0.04 | 0.22±0.05 | −26 | 15/10 | (77) |
| P9 | 1.20±0.14 | 1.45±0.14 | 21 | 0.37±0.06 | 0.26±0.07 | −30 | ||||
| P10 | 0.89±0.09 | 1.12±0.16 | 26 | 0.22±0.09 | 0.19±0.16 | −13 | ||||
| LSDTI | 6 | P | 1.08±0.18 | 1.43±0.18 | 32 | 0.29±0.12 | 0.21±0.13 | −28 | 10/3 | (26) |
| TF | 0.87±0.10 | 1.21±0.07 | 39 | 0.32±0.08 | 0.27±0.02 | −15 | ||||
| MCF | 1.12±0.08 | 1.10±0.06 | −2 | 0.29±0.07 | 0.29±0.04 | 0 | ||||
| LF | 1.06±0.14 | 1.31±0.13 | 24 | 0.29±0.07 | 0.21±0.06 | −28 | ||||
| MCT | 1.03±0.17 | 0.89±0.06 | 26 | 0.35±0.05 | 0.30±0.03 | −17 | ||||
| LT | 1.00±0.16 | 1.58±0.07 | 58 | 0.36±0.08 | 0.30±0.03 | −42 |
SSFP = Steady state free precession. EPI = Echo planar imaging. DESS = Double echo steady state. LSDTI = Line scan diffusion tensor sequence
P=Patella; F=Femur; T=Tibia; A=Ankle; S=Synovial fluid; TF= femoral trochlea; MCF=Medial femoral condyle; LCF=Lateral femoral condyle; MT=Medial tibial; LT=Lateral tibial.
The diffusion coefficient (D) for 1 diffusion direction and MD for 3 or 6 directions (units of 10−3 mm2/s).
Diff. = Relative difference. Bold face in the difference indicates significant difference.
N=number of subjects. For clinical studies including patients the number is given as healthy subjects/patients.
Ref. = Reference.
Images acquired after 10 min standing. Values indicate the range from the articular surface to the bone cartilage interface.
Images acquired after 1 h rest. Values indicate the range from the articular surface to the bone cartilage interface.
Average in the 50% more superficial cartilage
Average in the 50% deeper cartilage
In conclusion, studies using diffusion-weighted imaging have demonstrated the sensitivity of diffusion to detect cartilage remodeling after MXF and MACT, and showed good correlation with clinical symptoms in two studies. Also diffusion-weighted imaging has potential to detect early cartilage damage after knee injury. Cartilage damage after knee injury are common and involve damage of both the collagen and the PG. Articular cartilage can to some extent repair PG loss, but has little ability to repair collagen. Since DTI of articular cartilage provide information on both the collagen and the proteoglycan content it may provide important information to understand the pathology of posttraumatic osteoarthritis.
Diffusion tensor imaging
Healthy volunteers
Two studies used an EPI sequence in a 3T magnet to image healthy volunteers. Azuma et al. (75) investigated DTI of articular cartilage in the femoral trochlea of five volunteers. Volunteers were scanned twice, once after 10 min standing and a second scan after 60 min resting. Azuma et al. observed a slightly increase in MD in the 10% of the most superficial cartilage (data interpolation was used) and no change in FA after 60 min resting (Table 2) (75). In an independent study Zhu et al. (122) imaged the patellar cartilage of 30 healthy subjects. However in this study EPI images had insufficient quality with evident geometric distortion, and signs of N/2 ghosting.
Diagnosis of osteoartritis
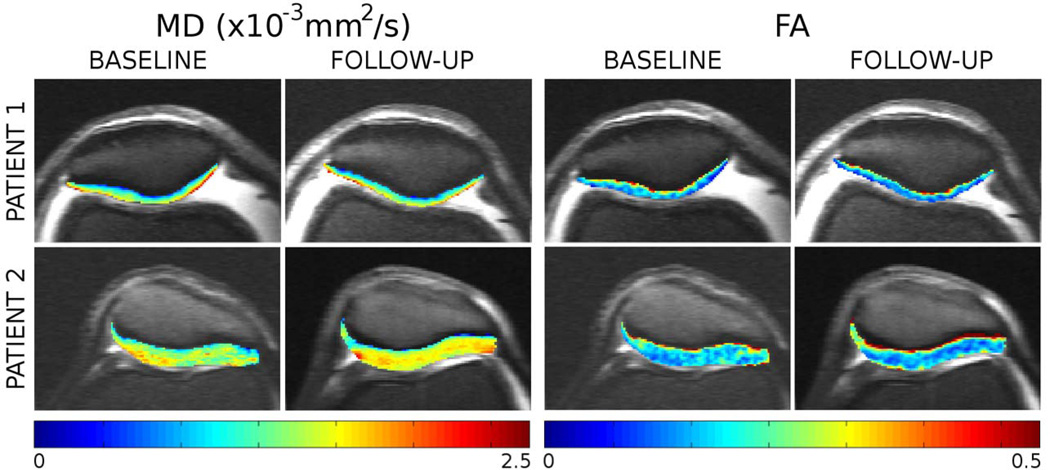
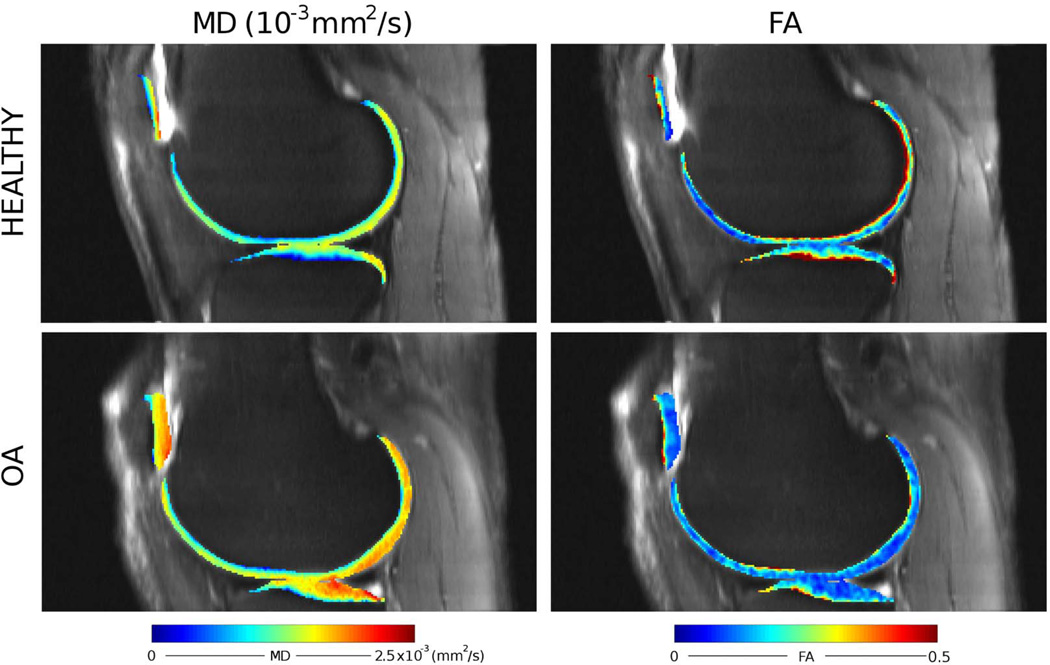
Raya et al. (77) performed the first in vivo study with healthy and OA subjects using a LSDTI sequence on a 7 T whole-body scanner with a dedicated one channel transmit and 28 channel receive knee coil. The LSDTI sequence was able to deliver very good image quality and provided high-quality DTI maps (image resolution 0.6×0.6×2 mm3). OA subjects showed significantly increased MD and decreased FA as compared with the healthy controls. The increase of MD was more pronounced in the superior 50% of the articular cartilage, whereas the decrease in FA was only significant in the deeper 50% of the articular cartilage (Table 2). The ability of each MR imaging parameter to help discriminate healthy subjects from subjects with OA was assessed by using receiver operating characteristic curve analysis. For MD, a specificity of 100% and a sensitivity of 80% were achieved with an optimal threshold of 1.2×10−3 mm2/s. For FA an optimal threshold of 0.25 led to a specificity of 88% and sensitivity of 80%. In the same study T2 showed poor differentiation between groups (optimal threshold=22.9 ms, specificity=69%, sensitivity=60%). Follow up of the patients included in this study demonstrated the ability of detect longitudinal changes with the DTI parameters (Fig. 9).
Figure 9.
Example of changes in DTI measurements on two OA patients. Patient 1 (KL 1, 61 y, 14 mo follow up) showed little change in DTI (MD +3.5%, FA-10.8%). Patient 2 (KL 2, 64 y, 20 mo follow up), who showed larger MD in the baseline also showed the largest changes in MD (+17.3%, twice the reproducibility error, +8.1%), and moderate change in FA (−17.1%, slightly under twice the reproducibility in FA, 9.7%).
Using the same sequence Raya et al. (27) investigated the value of DTI to detect OA in all knee compartments. DTI was measured in the sagittal plane of 10 healthy controls with a mean age of 30.6±4.2 years and five OA subjects (mean age, 66.3±9.1 years). Three healthy volunteers and two OA subjects were examined twice to assess the test-retest reproducibility, which was assessed with the coefficient of variation. Averaged mean diffusivity (MD) and fractional anisotropy (FA) were calculated in each cartilage region (femoral trochlea, lateral and medial femoral condyles, patella, and lateral and medial tibia). Test-retest reproducibility was better for MD (2.9±0.6 %) than for FA (5.6±1.8 %). Despite the low number of subjects included, significant differences in MD could be found in the lateral tibiofemoral compartment and the patellofemoral compartment (Fig. 10, Table 2). Differences in FA between healthy controls and OA subjects were lower and were only significant in the lateral tibia.
Figure 10.
Example of the MD and FA maps acquired on a healthy volunteer (29 year old male) and an OA subject (62 year old male subject with Kellgren Lawrence grade 2). The background image is the SNR map of the LSDTI image without diffusion weighting. MD showed higher values in the posterior condyle and in the posterior areas of the tibial cartilage as compared to the healthy volunteer. The OA subject also presented lower FA in these areas [Adapted from Raya et al. (27)].
Guha et al. (93) using a stimulated echo diffusion preparation with MAPSS readout measured 22 subjects with KL scores 0 and 1 and 22 subject with KL greater or equal than 2. They observed significantly increased mean diffusivity in the patellofemoral compartment in the KL≥2 group.
FUTURE PERSPECTIVE
Clinical applications of DWI or DTI of articular cartilage have been only possible in the last years. There is still need to find optimal acquisition techniques that allow measurement of articular cartilage in vivo at 3 T within reasonable acquisition times. Despite the promising preliminary results there is still a need to validate diffusion measurements on large collective of patients to finally assess their value to diagnose, and grade OA as well as predict OA progression.
In our opinion, there are still some aspects of the diffusion that still need to be clarify under well controlled experimental conditions in the laboratory. For example the relationship between the cartilage mechanical properties and the diffusion measurements has only been superficially assessed. For cartilage repair, the potential of diffusion to differentiate between fibrous and hyaline-like cartilage still needs to be assessed.
SUMMARY
In this review we concentrate on DTI of articular cartilage as a biomarker for the integrity of articular cartilage. Ex vivo experiments have provide broad evidence that diffusion is sensitive to the PG loss, and that the collagen network is responsible for the diffusion anisotropy. First ex vivo experiments have shown that diffusion parameters correlate with the mechanical properties of cartilage.
In vivo diffusion measurement of articular cartilage requires the use of dedicated diffusion-weighted pulse sequences. In the last years different approaches have shown value to measure diffusion in vivo, both at high fields (7 T) or a lower fields (3 T). Although the amount of clinical studies is still limited, all studies systematically show the potential of DTI to diagnose OA in early and mild populations with high accuracy (>90%). Diffusion weighted-imaging was also able to trace cartilage remodeling after cartilage repair surgery. Overall, the review of the existing literature shows that diffusion measurement of articular cartilage is feasible in vivo in clinical scanners and has potential to become an important biomarker to assess the integrity of articular cartilage in early stages of disease.
Acknowledgments
Grant Support:
Research reported in this manuscript was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Insititute of Health (NIH) under award number R21AR066897. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
For diffusion measurements in articular cartilage, which only involve moderate b-values (<1000 s/mm2), water diffusion is well described by a Gaussian model and the DTI framework offers an optimal approach to characterize the diffusion properties of articular cartilage.
References
- 1.Charnley J. The lubrication of animal joints in relation to surgical reconstruction by arthroplasty. Ann Rheum Dis. 1960;19:10–19. doi: 10.1136/ard.19.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mox VC, Gu WY, Chen FH. Structure and function of articular cartilage. In: Mow VC, Huiskes R, editors. Basic Orthopedics biomechanics and mechano-biology. 3rd ed. Philadelphia, PA, US: Lippincott Willians & Wilkins; 2005. pp. 123–180. [Google Scholar]
- 3.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977;36(2):121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maroudas AI. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976;260(5554):808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- 5.Maroudas A, Baylissb MT, Uchitel-Kaushanskya N, Schneidermana R, Gilav E. Aggrecan turnover in human articular cartilage: Use of aspartic acid racemization as a marker of molecular age. Arch Biochem Biophys. 1998;350:61–71. doi: 10.1006/abbi.1997.0492. [DOI] [PubMed] [Google Scholar]
- 6.Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 7.Stubendorff JJ, Lammentausta E, Struglics A, Lindberg L, Heinegård D, Dahlberg LE. Is cartilage sGAG content related to early changes in cartilage disease? Implications for interpretation of dGEMRIC. Osteoarthritis Cartilage. 2012;20(5):396–404. doi: 10.1016/j.joca.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland JB, Reddy R. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR in Biomedicine. 2006;19(7):781–821. doi: 10.1002/nbm.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insko EK, Reddy R, Leigh JS. High resolution, short echo time sodium imaging of articular cartilage. J Magn Reson Imaging. 1997;7(6):1056–1059. doi: 10.1002/jmri.1880070618. [DOI] [PubMed] [Google Scholar]
- 10.Reddy R, Li S, Noyszewski E, Kneeland J, Leigh J. In vivo sodium multiple quantum spectroscopy of human articular cartilage. Magn Reson Med. 1997;38(2):207–214. doi: 10.1002/mrm.1910380208. [DOI] [PubMed] [Google Scholar]
- 11.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36(5):665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 12.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9(12):1388–1394. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 13.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23(4):547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 14.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proc Natl Acad Sci U S A. 2008;105(7):2266–2270. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dardzinski B, Mosher T, Li S, Van Slyke M, Smith M. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205(2):546–550. doi: 10.1148/radiology.205.2.9356643. [DOI] [PubMed] [Google Scholar]
- 16.Nieminen MT, Rieppo J, Toyras J, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46(3):487–493. doi: 10.1002/mrm.1218. [DOI] [PubMed] [Google Scholar]
- 17.Kim DK, Ceckler TL, Hascall VC, Calabro A, Balaban RS. Analysis of water-macromolecule proton magnetization transfer in articular cartilage. Magn Reson Med. 1993;29(2):211–215. doi: 10.1002/mrm.1910290209. [DOI] [PubMed] [Google Scholar]
- 18.Gray ML, Burstein D, Lesperance LM, Gehrke L. Magnetization transfer in cartilage and its constituent macromolecules. Magn Reson Med. 1995;34(3):319–325. doi: 10.1002/mrm.1910340307. [DOI] [PubMed] [Google Scholar]
- 19.Seo GS, Aoki J, Moriya H, et al. Hyaline cartilage: in vivo and in vitro assessment with magnetization transfer imaging. Radiology. 1996;201(2):525–530. doi: 10.1148/radiology.201.2.8888253. [DOI] [PubMed] [Google Scholar]
- 20.Filidoro L, Dietrich O, Weber J, et al. High-resolution diffusion tensor imaging of human patellar cartilage: feasibility and preliminary findings. Magn Reson Med. 2005;53(5):993–998. doi: 10.1002/mrm.20469. [DOI] [PubMed] [Google Scholar]
- 21.Meder R, de Visser SK, Bowden JC, Bostrom T, Pope JM. Diffusion tensor imaging of articular cartilage as a measure of tissue microstructure. Osteoarthritis Cartilage. 2006;14(9):875–881. doi: 10.1016/j.joca.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Deng X, Farley M, Nieminen MT, Gray M, Burstein D. Diffusion tensor imaging of native and degenerated human articular cartilage. Magn Reson Imag. 2007;25(2):168–171. doi: 10.1016/j.mri.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 23.de Visser SK, Bowden JC, Wentrup-Byrne E, et al. Anisotropy of collagen fibre alignment in bovine cartilage: comparison of polarised light microscopy and spatially resolved diffusion-tensor measurements. Osteoarthritis Cartilage. 2008;16(6):689–697. doi: 10.1016/j.joca.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Raya JG, Melkus G, Adam-Neumair S, et al. Change of Diffusion Tensor Imaging Parameters in Articular Cartilage with Progressive Proteoglycan Extraction. Invest Radiol. 2011;46(6):401–409. doi: 10.1097/RLI.0b013e3182145aa8. [DOI] [PubMed] [Google Scholar]
- 25.Raya JG, Arnoldi A, Filidoro L, et al. Ultra high field diffusion tensor imaging of articular cartilage correlated with histology and scanning electron microscopy. Magn Reson Mater Phy. 2011;24(4):247–258. doi: 10.1007/s10334-011-0259-6. [DOI] [PubMed] [Google Scholar]
- 26.Raya JG, Melkus G, Adam-Neumair S, et al. Diffusion-tensor imaging of human articular cartilage specimens with early signs of cartilage damage. Radiology. 2013;266(3):831–841. doi: 10.1148/radiol.12120954. [DOI] [PubMed] [Google Scholar]
- 27.Raya JG, Dettmann E, Notohamiprodjo M, Krasnokutsky S, Abramson S, Glaser C. Feasibility of in vivo diffusion tensor imaging of articular cartilage with coverage of all cartilage regions. Eur Radiol. 2014 doi: 10.1007/s00330-014-3155-4. [DOI] [PubMed] [Google Scholar]
- 28.Knauss R, Schiller J, Fleischer G, Karger J, Arnold K. Self-diffusion of water in cartilage and cartilage components as studied by pulsed field gradient NMR. Magn Reson Med. 1999;41(2):285–292. doi: 10.1002/(sici)1522-2594(199902)41:2<285::aid-mrm11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Burstein D, Gray ML, Hartman AL, Gipe R, Foy BD. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J Orthop Res. 1993;11(4):465–478. doi: 10.1002/jor.1100110402. [DOI] [PubMed] [Google Scholar]
- 30.Xia Y, Farquhar T, Burton-Wurster N, Vernier-Singer M, Lust G, Jelinski L. Self-diffusion monitors degraded cartilage. Arch Biochem Biophys. 1995;323(2):323–328. doi: 10.1006/abbi.1995.9958. [DOI] [PubMed] [Google Scholar]
- 31.Madelin G, Regatte RR. Biomedical applications of sodium MRI in vivo. J Magn Reson Imaging. 2013;38(3):511–529. doi: 10.1002/jmri.24168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy R, Insko EK, Noyszewski EA, Dandora R, Kneeland JB, Leigh JS. Sodium MRI of human articular cartilage in vivo. Magn Reson Med. 1998;39(5):697–701. doi: 10.1002/mrm.1910390505. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro E, Borthakur A, Gougoutas A, Reddy R. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn Reson Med. 2002;47(2):284–291. doi: 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheaton AJ, Borthakur A, Shapiro EM, et al. Proteoglycan loss in human knee cartilage: quantitation with sodium MR imaging--feasibility study. Radiology. 2004;231(3):900–905. doi: 10.1148/radiol.2313030521. [DOI] [PubMed] [Google Scholar]
- 35.Madelin G, Babb J, Xia D, et al. Articular cartilage: evaluation with fluid-suppressed 7.0-T sodium MR imaging in subjects with and subjects without osteoarthritis. Radiology. 2013;268(2):481–491. doi: 10.1148/radiol.13121511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madelin G, Babb JS, Xia D, Chang G, Jerschow A, Regatte RR. Reproducibility and repeatability of quantitative sodium magnetic resonance imaging in vivo in articular cartilage at 3 T and 7 T. Magn Reson Med. 2012;68(3):841–849. doi: 10.1002/mrm.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madelin G, Lee JS, Inati S, Jerschow A, Regatte RR. Sodium inversion recovery MRI of the knee joint in vivo at 7T. J Magn Reson. 2010;207(1):42–52. doi: 10.1016/j.jmr.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiderius CJ, Svensson J, Leander P, Ola T, Dahlberg L. dGEMRIC (delayed gadolinium-enhanced MRI of cartilage) indicates adaptive capacity of human knee cartilage. Magn Reson Med. 2004;51(2):286–290. doi: 10.1002/mrm.10714. [DOI] [PubMed] [Google Scholar]
- 39.Williams A, Sharma L, McKenzie CA, Prasad PV, Burstein D. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage in knee osteoarthritis: findings at different radiographic stages of disease and relationship to malalignment. Arthritis Rheum. 2005;52(11):3528–3535. doi: 10.1002/art.21388. [DOI] [PubMed] [Google Scholar]
- 40.Neuman P, Tjörnstrand J, Svensson J, et al. Longitudinal assessment of femoral knee cartilage quality using contrast enhanced MRI (dGEMRIC) in patients with anterior cruciate ligament injury – comparison with asymptomatic volunteers. Osteoarthritis Cartilage. 2011;19(8):977–983. doi: 10.1016/j.joca.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Gillis A, Bashir A, McKeon B, Scheller A, Gray ML, Burstein D. Magnetic resonance imaging of relative glycosaminoglycan distribution in patients with autologous chondrocyte transplants. Invest Radiol. 2001;36(12):743–748. doi: 10.1097/00004424-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Kurkijärvi JE, Mattila L, Ojala RO, et al. Evaluation of cartilage repair in the distal femur after autologous chondrocyte transplantation using T2 relaxation time and dGEMRIC. Osteoarthritis Cartilage. 2007;15(4):372–378. doi: 10.1016/j.joca.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Trattnig S, Marlovits S, Gebetsroither S, et al. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) for in vivo evaluation of reparative cartilage after matrix-associated autologous chondrocyte transplantation at 3.0T: Preliminary results. J Magn Reson Imaging. 2007;26(4):974–982. doi: 10.1002/jmri.21091. [DOI] [PubMed] [Google Scholar]
- 44.Lüsse S, Claassen H, Gehrke T, et al. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging. 2000;18(4):423–430. doi: 10.1016/s0730-725x(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 45.Wayne J, Kraft K, Shields K, Yin C, Owen J, Disler D. MR imaging of normal and matrix-depleted cartilage: correlation with biomechanical function and biochemical composition. Radiology. 2003;228(2):493–499. doi: 10.1148/radiol.2282012012. [DOI] [PubMed] [Google Scholar]
- 46.Xia Y. Relaxation anisotropy in cartilage by NMR microscopy (muMRI) at 14-microm resolution. Magn Reson Med. 1998;39(6):941–949. doi: 10.1002/mrm.1910390612. [DOI] [PubMed] [Google Scholar]
- 47.Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol. 2000;35(10):602–621. doi: 10.1097/00004424-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 48.David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22(5):673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 49.Mlynárik V, Trattnig S, Huber M, Zembsch A, Imhof H. The role of relaxation times in monitoring proteoglycan depletion in articular cartilage. J Magn Reson Imaging. 1999;10(4):497–502. doi: 10.1002/(sici)1522-2586(199910)10:4<497::aid-jmri1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Cheng J, Lin K, et al. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29(3):324–334. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glaser C, Mendlik T, Dinges J, et al. Global and regional reproducibility of T2 relaxation time measurements in human patellar cartilage. Magn Reson Med. 2006;56(3):527–534. doi: 10.1002/mrm.21005. [DOI] [PubMed] [Google Scholar]
- 52.Raya JG, Horng A, Dietrich O, et al. Voxel-based reproducibility of T2 relaxation time in patellar cartilage at 1.5 T with a new validated 3D rigid registration algorithm. Magn Reson Mater Phy. 2009;22(4):229–239. doi: 10.1007/s10334-009-0168-0. [DOI] [PubMed] [Google Scholar]
- 53.Raya J, Dietrich O, Horng A, Weber J, Reiser M, Glaser C. T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn Reson Med. 2010;63(1):181–193. doi: 10.1002/mrm.22178. [DOI] [PubMed] [Google Scholar]
- 54.Mosher TJ, Zhang Z, Reddy R, et al. Knee Articular Cartilage Damage in Osteoarthritis: Analysis of MR Image Biomarker Reproducibility in ACRIN-PA 4001 Multicenter Trial. Radiology. 2011;258(3):832–842. doi: 10.1148/radiol.10101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: Comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hannila I, Nieminen MT, Rauvala E, Tervonen O, Ojala R. Patellar cartilage lesions: comparison of magnetic resonance imaging and T2 relaxation-time mapping. Acta Radiol. 2007;48(4):444–448. doi: 10.1080/02841850701280817. [DOI] [PubMed] [Google Scholar]
- 57.Kijowski R, Blankenbaker DG, Muñoz Del Rio A, Baer GS, Graf BK. Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology. 2013;267(2):503–513. doi: 10.1148/radiol.12121413. [DOI] [PubMed] [Google Scholar]
- 58.Eckstein F, Kwoh CK, Link TM investigators. O. Imaging research results from the osteoarthritis initiative (OAI): a review and lessons learned 10 years after start of enrolment. Ann Rheum Dis. 2014;73(7):1289–1300. doi: 10.1136/annrheumdis-2014-205310. [DOI] [PubMed] [Google Scholar]
- 59.Akella SV, Regatte RR, Gougoutas AJ, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46(3):419–423. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 60.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38(6):863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 61.Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51(3):503–509. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 62.Keenan KE, Besier TF, Pauly JM, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1ρ and T2 MRI. Osteoarthritis Cartilage. 2011;19(2):171–179. doi: 10.1016/j.joca.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mlynárik V, Szomolányi P, Toffanin R, Vittur F, Trattnig S. Transverse relaxation mechanisms in articular cartilage. J Magn Reson. 2004;169(2):300–307. doi: 10.1016/j.jmr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Regatte RR, Akella SV, Wheaton AJ, et al. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11(7):741–749. doi: 10.1016/j.acra.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 65.Schooler J, Kumar D, Nardo L, et al. Longitudinal evaluation of T1ρ and T2 spatial distribution in osteoarthritic and healthy medial knee cartilage. Osteoarthritis Cartilage. 2014;22(1):51–62. doi: 10.1016/j.joca.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X, Cheng J, Lin K, et al. Cartilage in Anterior Cruciate Ligament–Reconstructed Knees: MR Imaging T1ρ and T2—Initial Experience with 1-year Follow-up. Radiology. 2011;258(2):505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proc Natl Acad Sci U S A. 2008;105(7):2266–2270. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JS, Parasoglou P, Xia D, Jerschow A, Regatte RR. Uniform magnetization transfer in chemical exchange saturation transfer magnetic resonance imaging. Sci Rep. 2013;3:1707. doi: 10.1038/srep01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmitt B, Zbýň Š, Stelzeneder D, et al. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology. 2011;260(1):257–264. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 70.Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J. Echo-planar imaging of intravoxel incoherent motion. Radiology. 1990;177:407–414. doi: 10.1148/radiology.177.2.2217777. [DOI] [PubMed] [Google Scholar]
- 71.Norris DG. Ultrafast low-angle RARE: U-FLARE. Magn Reson Med. 1991;17(2):539–542. doi: 10.1002/mrm.1910170224. [DOI] [PubMed] [Google Scholar]
- 72.Alsop DC. Phase insensitive preparation of single-shot RARE: application to diffusion imaging in humans. Magn Reson Med. 1997;38(4):527–538. doi: 10.1002/mrm.1910380404. [DOI] [PubMed] [Google Scholar]
- 73.Jones DK. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23(7):803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- 74.Gold GE, Butts K, Fechner KP, et al. In vivo diffusion-weighted imaging of cartilage; 6th Scientific Meeting and Exhibition of the International Society of Magnetic Resonance in teh Medicine; Sidney, Australia. 1998. p. 1066. [Google Scholar]
- 75.Azuma T, Nakai R, Takizawa O, Tsutsumi S. In vivo structural analysis of articular cartilage using diffusion tensor magnetic resonance imaging. Magn Reson Imaging. 2009;27(9):1242–1248. doi: 10.1016/j.mri.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 76.Zhu SC, Shi DP, Xuan A. Human patellar cartilage: echo planar diffusion-weighted MR imaging findings at 3.0 T. Clin Imag. 2012;36:199–202. doi: 10.1016/j.clinimag.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 77.Raya JG, Horng A, Dietrich O, et al. Articular cartilage: in vivo diffusion-tensor imaging. Radiology. 2012;262(2):550–559. doi: 10.1148/radiol.11110821. [DOI] [PubMed] [Google Scholar]
- 78.Gudbjartsson H, Maier SE, Mulkern RV, Morocz IA, Patz S, Jolesz FA. Line scan diffusion imaging. Magn Reson Med. 1996;36(4):509–519. doi: 10.1002/mrm.1910360403. [DOI] [PubMed] [Google Scholar]
- 79.Lauterbur PC. Image formation by induced local interactions: Examples employing nuclear magnetic resonance. Nature. 1973;242:190–191. [PubMed] [Google Scholar]
- 80.Ordidge RJ, Helpern JA, Qing ZX, Knight RA, Nagesh V. Correction of motional artifacts in diffusion-weighted MR images using navigator echoes. Magn Reson Imaging. 1994;12(3):455–446. doi: 10.1016/0730-725x(94)92539-9. [DOI] [PubMed] [Google Scholar]
- 81.Anderson AW, Gore JC. Analysis and correction of motion artifacts in diffusion weighted imaging. Magn Reson Med. 1994;32(3):379–387. doi: 10.1002/mrm.1910320313. [DOI] [PubMed] [Google Scholar]
- 82.Miller KL, Pauly JM. Nonlinear phase correction for navigated diffusion imaging. Magn Reson Med. 2003;50(2):343–353. doi: 10.1002/mrm.10531. [DOI] [PubMed] [Google Scholar]
- 83.Hawkes RC, Patz S. Rapid Fourier imaging using steady-state free precesion. Magn Reson Med. 1987;4(1):9–23. doi: 10.1002/mrm.1910040103. [DOI] [PubMed] [Google Scholar]
- 84.Gynell ML. The application of steady-state free precession in rapid 2DFT NMR imaging: FAST and CE-FAST sequences. Magn Reson Imag. 1988;6(4):415–419. doi: 10.1016/0730-725x(88)90478-x. [DOI] [PubMed] [Google Scholar]
- 85.Oppelt A, Graumann R, Barfuβ H, Fischer H, Hartl W, Schajor W. FISP: a new fast MRI sequence. Electromedica. 1986;54:15–18. [Google Scholar]
- 86.Wu EX, Buxton RB. Effect of diffusion on the steady-state magnetization with pulsed field gradients. J Magn Reson. 1990;90(2):243–253. [Google Scholar]
- 87.Buxton RB. The diffusion sensitivity of fast steady-state free precession imaging. Magn Reson Med. 1993;29(2):235–243. doi: 10.1002/mrm.1910290212. [DOI] [PubMed] [Google Scholar]
- 88.Staroswiecki E, Granlund KL, Alley MT, Gold GE, Hargreaves BA. Simultaneous estimation of T(2) and apparent diffusion coefficient in human articular cartilage in vivo with a modified three-dimensional double echo steady state (DESS) sequence at 3 T. Magn Reson Med. 2012;67(4):1086–1096. doi: 10.1002/mrm.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bieri O, Ganter C, Scheffler K. Quantitative in vivo diffusion imaging of cartilage using double echo steady-state free precession. Magn Reson Med. 2012;68(3):720–729. doi: 10.1002/mrm.23275. [DOI] [PubMed] [Google Scholar]
- 90.Bieri O, Ganter C, Welsch GH, Trattnig S, Mamisch TC, Scheffler K. Fast diffusion-weighted steady state free precession imaging of in vivo knee cartilage. Magn Reson Med. 2012;67(3):691–700. doi: 10.1002/mrm.23061. [DOI] [PubMed] [Google Scholar]
- 91.Bruder H, Fischer H, Graumann R, Deimling M. A new steady-state imaging sequence for simultaneous acquisition of two MR images with clearly different contrasts. Magn Reson Med. 1988;7(1):35–42. doi: 10.1002/mrm.1910070105. [DOI] [PubMed] [Google Scholar]
- 92.Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59(2):298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guha A, Wyatt C, Karampinos D, Nardo L, Link TM, Majumdar S. Clinical feasibility of a stimulated echo based diffusion sequence and correlation between T1rho and diffusion values; Proceedings of the 23rd meeting of the International Society of Magnetic Resonance on the Medicine (ISMRM); Milan, Italy. 2014. p. 3983. [Google Scholar]
- 94.Pipe JG, Farthing VG, Forbes KP. Multishot diffusion-weighted FSE using PROPELLER MRI. Magn Reson Med. 2002;47(1):42–52. doi: 10.1002/mrm.10014. [DOI] [PubMed] [Google Scholar]
- 95.Miller KL, Hargreaves BA, Gold GE, Pauly JM. Steady-state diffusion-weighted imaging of in vivo knee cartilage. Magn Reson Med. 2004;5(1):394–398. doi: 10.1002/mrm.10696. [DOI] [PubMed] [Google Scholar]
- 96.Jones DK. Optimal approaches for MR acquisition. In: Jones DK, editor. Diffusion MRI: Theory, methods and applications. New York: Oxford University Press; 2011. pp. 250–271. [Google Scholar]
- 97.Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007;26(2):375–385. doi: 10.1002/jmri.20969. [DOI] [PubMed] [Google Scholar]
- 98.Skare S, Hedehus M, Moseley ME, Li TQ. Condition number as a measure of noise performance of diffusion tensor data acquisition schemes with MRI. J Magn Reson. 2000;147:340–352. doi: 10.1006/jmre.2000.2209. [DOI] [PubMed] [Google Scholar]
- 99.Berg A, Singer T, Moser E. High-resolution diffusivity imaging at 3.0 T for the detection of degenerative changes: a trypsin-based arthritis model. Invest Radiol. 2003;38(7):460–466. doi: 10.1097/01.rli.0000078762.72335.57. [DOI] [PubMed] [Google Scholar]
- 100.Lin PC, Reiter DA, Spencer RG. Classification of degraded cartilage through multiparametric MRI analysis. J Magn Reson. 2009;201(1):61–71. doi: 10.1016/j.jmr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin PC, Irrechukwu O, Roque R, Hancock B, Fishbein KW, Spencer RG. Multivariate analysis of cartilage degradation using the support vector machine algorithm. Magn Reson Med. 2012;67(6):1815–1826. doi: 10.1002/mrm.23189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin PC, Reiter DA, Spencer RG. Sensitivity and specificity of univariate MRI analysis of experimentally degraded cartilage. Magn Reson Med. 2009;62(5):1311–1318. doi: 10.1002/mrm.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Toffanin R, Mlynarik V, Russo S, Szomolanyi P, Piras A, Vittur F. Proteoglycan depletion and magnetic resonance parameters of articular cartilage. Arch Biochem Biophys. 2001;390(2):235–242. doi: 10.1006/abbi.2001.2338. [DOI] [PubMed] [Google Scholar]
- 104.Xia Y, Farquhar T, Burton-Wurster N, Ray E, Jelinski LW. Diffusion and relaxation mapping of cartilage-bone plugs and excised disks using microscopic magnetic resonance imaging. Magn Reson Med. 1994;31(3):273–282. doi: 10.1002/mrm.1910310306. [DOI] [PubMed] [Google Scholar]
- 105.Juráš V, Bittšanský M, Majdišová Z, Trattnig S. In-vitro evaluation of pre- and post-compression states of human articular cartilage using MRI at 3 Tesla. Measurement science review. 2007;7(2):39–42. [Google Scholar]
- 106.Juráš V, Bittšanský M, Majdišová Z, et al. In vitro determination of biomechanical properties of human articular cartilage in osteoarthritis using multi-parametric MRI. J Magn Reson. 2009;197(1):40–47. doi: 10.1016/j.jmr.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 107.Aoki T, Watanabe A, Nitta N, Numano T, Fukushi M, Niitsu M. Correlation between apparent diffusion coefficient and viscoelasticity of articular cartilage in a porcine model. Skeletal Radiol. 2012;41(9):1087–1092. doi: 10.1007/s00256-011-1340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Greene GW, Zappone B, Söderman O, et al. Anisotropic dynamic changes in the pore network structure, fluid diffusion and fluid flow in articular cartilage under compression. Biomaterials. 2010;31(12):3117–3128. doi: 10.1016/j.biomaterials.2010.01.102. [DOI] [PubMed] [Google Scholar]
- 109.Greene GW, Zappone B, Zhao B, et al. Changes in pore morphology and fluid transport in compressed articular cartilage and the implications for joint lubrication. Biomaterials. 2008;29(33):4455–4462. doi: 10.1016/j.biomaterials.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 110.Mlynárik V, Sulzbacher I, Bittsanský M, Fuiko R, Trattnig S. Investigation of apparent diffusion constant as an indicator of early degenerative disease in articular cartilage. J Magn Reson Imaging. 2003;17(4):440–444. doi: 10.1002/jmri.10276. [DOI] [PubMed] [Google Scholar]
- 111.Henkelman RM, Stanisz GJ, Kim JK, Bronskill MJ. Anisotropy of NMR properties of tissues. Magn Reson Med. 1994;32(5):592–601. doi: 10.1002/mrm.1910320508. [DOI] [PubMed] [Google Scholar]
- 112.de Visser SK, Crawford RW, Pope JM. Structural adaptations in compressed articular cartilage measured by diffusion tensor imaging. Osteoarthritis Cartilage. 2008;16(1):689–697. doi: 10.1016/j.joca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 113.Raya JG, Melkus G, Adam-Neumair S, et al. Multiparametric assessment of healthy and OA articular cartilage under loading at 17.6 T; 19th congress of the International Society of the Magnetic Resonance in the Medicine (ISMRM); Montreal, Canada. 2011. p. 3207. [Google Scholar]
- 114.Pierce DM, Trobin W, Raya JG, et al. DT-MRI Based Computation of Collagen Fiber Deformation in Human Articular Cartilage: A Feasibility Study. 2010;38(7):2447–2463. doi: 10.1007/s10439-010-9990-9. [DOI] [PubMed] [Google Scholar]
- 115.Pierce DM, Ricken T, Holzapfel GA. Modeling sample/patient-specific structural and diffusional response of cartilage employing DT-MRI. Int J Numer Methods Biomed Eng. 2013;29(8):807–821. doi: 10.1002/cnm.2524. [DOI] [PubMed] [Google Scholar]
- 116.Mamisch TC, Menzel MI, Welsch GC, et al. Steady-state diffusion imaging for MR in-vivo evaluation of reparative cartilage after matrix-associated autologous chondrocyte transplantation at 3 tesla—Preliminary results. Eur J Radiol. 2008;65:72–79. doi: 10.1016/j.ejrad.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 117.Welsch GC, Trattnig S, Domayer S, Marlovits S, White LM, Mamisch TC. Multimodal approach in the use of clinical scoring, morphological MRI and biochemical T2-mapping and diffusion-weighted imaging in their ability to assess differences between cartilage repair tissue after microfracture therapy and matrix-associated autologous chondrocyte transplantation: a pilot study. Osteoarthritis Cartilage. 2009;17:1219–1227. doi: 10.1016/j.joca.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 118.Friederich KM, Mamisch TC, Plank C, et al. Diffusion-weighted imaging for the follow-up of patients after matrix-associated autologous chondrocyte transplantation. Eur J Radiol. 2010;73:622–628. doi: 10.1016/j.ejrad.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 119.Apprich S, Trattnig S, Welsch GH, et al. Assessment of articular cartilage repair tissue after matrix-associated autologous chondrocyte transplantation or the microfracture technique in the ankle joint using diffusion-weighted imaging at 3 Tesla. Osteoarthritis Cartilage. 2012;20:703–711. doi: 10.1016/j.joca.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 120.Quirbach S, Trattnig S, Marlovits S, et al. Initial results of in vivo high-resolution morphological and biochemical cartilage imaging of patients after matrix-associated autologous chondrocyte transplantation (MACT) of the ankle. Skeletal Radiol. 2009;38(8):751–760. doi: 10.1007/s00256-009-0682-1. [DOI] [PubMed] [Google Scholar]
- 121.Xu J, Xie G, Di Y, Bai M, Zhao X. Value of T2-mapping and DWI in the diagnosis of early knee cartilage injury. J Radiol Case Rep. 2011;5(2):13–18. doi: 10.3941/jrcr.v5i2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu SC, Shi DP, Xuan A. Human patellar cartilage: echo planar diffusion-weighted MR imaging findings at 3.0 T. Clin Imaging. 2012;36(3):199–202. doi: 10.1016/j.clinimag.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 123.Raya JG, Dettmann E, Golestani A, Block KT. In vivo DTI of Articular Cartialge at 3T with a Spin Echo Radial Diffusion Tensor Imaging (RAISED) Sequence; 21st conference of the International Society of Magnetic Resonance in the Medicine; Salt Lake City, Utah, US. 2013. p. 3530. [Google Scholar]