Abstract
Background
Mammalian hearts exhibit positive inotropic responses to β-adrenergic stimulation as a consequence of protein kinase A (PKA)-mediated phosphorylation or as a result of increased beat frequency (the Bowditch effect). Several membrane and myofibrillar proteins are phosphorylated under these conditions, but the relative contributions of these to increased contractility are not known. Phosphorylation of cardiac myosin binding protein-C (cMyBP-C) by PKA accelerates the kinetics of force development in permeabilized heart muscle, but its role in vivo is unknown. Such understanding is important, since adrenergic responsiveness of the heart and the Bowditch effect are both depressed in heart failure.
Methods and Results
The roles of cMyBP-C phosphorylation were studied using mice in which either WT or nonphosphorylatable forms of cMyBP-C [ser273ala, ser282ala, ser302ala: cMyBP-C(t3SA)] were expressed at similar levels on a cMyBP-C null background. Force and [Ca2+]in measurements in isolated papillary muscles showed that the increased force and twitch kinetics due to increased pacing or β1-adrenergic stimulation were nearly absent in cMyBP-C(t3SA) myocardium, even though [Ca2+]intransients under each condition were similar to WT. Biochemical measurements confirmed that PKA phosphorylated ser273, ser282 and ser302 in WT cMyBP-C. In contrast, CaMKIIδ, which is activated by increased pacing, phosphorylated ser302 principally, ser282 to a lesser degree, and ser273 not at all.
Conclusions
Phosphorylation of cMyBP-C increases the force and kinetics of twitches in living cardiac muscle. Further, cMyBP-C is a principal mediator of increased contractility observed with β-adrenergic stimulation or increased pacing, due to PKA and CaMKIIδ phosphorylations of cMyB-C.
Keywords: myosin binding protein-C, phosphorylation, contractility
The force and kinetics of the cardiac twitch exhibit remarkable plasticity on a beat to beat basis as a means for matching cardiac output to circulatory demand. Such demand-driven inotropy is depressed in heart failure, causing the majority of symptoms in patients; therefore, better understanding of the underlying mechanisms should lead to new approaches for treating this disease. Cardiac inotropy has usually been understood to result from alterations in Ca2+ delivery to thin filament regulatory proteins during the myocardial twitch or alterations in thin filament responsiveness to myoplasmic Ca2+ as a consequence of post-translational modifications of thick or thin filament accessory proteins. However, the relative contributions of these mechanisms to cardiac function under resting conditions or under stress, such as β1-adrenergic stimulation, are not known. The present study was undertaken to determine the basis for cardiac inotropy in both to better understand this phenomenon and to suggest mechanisms of reduced function in heart failure.
Here, measurements of force and intracellular Ca2+ transients were done in intact ex vivo myocardial preparations from either wild-type mice or mutant mice expressing a phosphorylation-deficient form of the thick filament regulatory protein, cardiac myosin binding protein-C (cMyBP-C). cMyBP-C binds to the thick filament1 and represses myosin-actin interactions and thereby slow cross-bridge cycling when an individual is at rest.2 Thus, cMyBP-C may be a major modulator of cardiac inotropy. Previous studies of hypo-phosphorylated cMyBP-C used skinned (i.e. removed cellular membrane) myocardium at fixed concentrations of added calcium.3–8 The current study was undertaken to determine the roles of cMyBP-C in regulating cardiac contractility in living myocardium in the context of the time-varying Ca2+ transient during the twitch. Measurements were done as a function of increased stimulus frequency and in the presence and absence of β1-adrenergic stimulation. The results, together with measurements of phosphorylation of other myofilament proteins under these conditions, show that phosphorylation of cMyBP-C is the predominant proximate mediator of both pacing-dependent and β1-adrenergic-dependent potentiation of force and contraction kinetics. Remarkably, replacement of phosphorylatable serines in cMyBP-C with alanines blunted positive inotropic responses, even though the expected phosphorylations of other myofilament proteins and the expected increases in the amplitude and rates of the myoplasmic Ca2+ transients were observed to occur in both WT and mutant myocardium.
Methods
The experiments described here employed previously generated mouse lines in which non-PKA-phosphorylatable cMyBP-C (ser273ala, ser282ala, ser302ala) [the cMyBPC-C(t3SA) mouse] or WT cMyBP-C [the cMyBP-C(tWT) mouse] were expressed on a cMyBP-C null background.4 Expression levels in the lines used were 74% for cMyBP-C(t3SA) mice and 72% for cMyBP-C(tWT) mice, referenced to cMyBP-C expression in non-transgenic WT mice.4 The protocols for animal care and use were approved by the Animal Care and Use Committees of the UW School of Medicine and Public Health and Texas A&M Health Science Center College of Medicine.
[Ca2+]in and force were measured simultaneously in ex vivo intact papillary muscles to assess cross-bridge interactions in the context of the [Ca2+]in transient during a twitch.9 Pacing frequency was varied and 1 µM dobutamine (β1-adreneric agonist) was added to the bath at to mimic β1-adrenergic stimulation. Fura-2 AM was used to assess [Ca2+]in. Experiments were performed at room temperature to avoid rapid extrusion of Fura-2 AM from myocardial cells that occurs at higher temperatures.9 At room temperature, increasing the pacing frequency from 1 to 3 Hz produced a positive force-frequency relationship much like that observed when pacing frequency is increased from 1 to 7 Hz at 34°C.9
For measurements of cardiac function in vivo, echocardiography with tissue Doppler was used to assess myocardial contraction and relaxation velocities. Echocardiography was done using a previously developed protocol employing a VisualSonics Vevo-770 system with a 30 MHz probe.4
Isolated cMyBP-C(tWT) hearts were first subjected to Langendorf retrograde perfusion to removing endogenous circulating catecholamines and establish baseline phosphorylation of thick and thin filament proteins. Pacing frequency was increased and/or dobutamine was infused to determine patterns of myofibrillar protein phosphorylation under these conditions. Western-blotting with site-specific antibodies for cMyBP-C phosphorylations10 was done on myofibrils from the perfused hearts to determine which phosphorylatable serines were associated with the increased contractility due to increased pacing or β1-adrenergic stimulation.
Recombinant full length WT cMyBP-C, recombinant CaMK2δ, and CaMK2δ kinase inhibitors were used to test whether CaMK2δ mediates rate-dependent phosphorylation of cMyBP-C.
Phosphoprotein staining (Pro-Q Diamond) and total protein staining (Sypro-Ruby) were used to survey possible changes in phosphorylation of other myofilament proteins in cMyBP-C(t3SA) and cMyBP-C(tWT) papillary muscles and hearts under each experimental condition.
SPSS and R software were used to perform statistical analyses. The normality of a data set is determined by having both a normal appearing histogram and non-significant Shapiro test for normality. Normal data sets are analyzed by: an independent t-test when comparing two lines; a repeated measure ANOVA for increasing pacing frequency from 1-Hz to 3-Hz; a paired t-test for comparison between basal and post-dobutamine treatment; and ANOVA with post-hoc Tukey-HSD for multiple comparisons between groups. Data sets that do not have normal histrograms are analyzed by exact tests: a Mann-Whitney U test for two independent samples; a Friedman's 2-way analysis by rank for repeated measurements for increasing pacing frequency from 1-Hz to 3-Hz; and a Wilcoxon test for comparison between basal and post-dobutamine treatment. For all analyses, the choice of either a parametric test or an exact test did not impact the conclusions. Whenever possible, data were shown with Box-Whisker plots with the following percentiles: box-bottom 25th, median line-in-box, box-top 75th, bottom-whisker 10th and top-whisker 90th.
Results
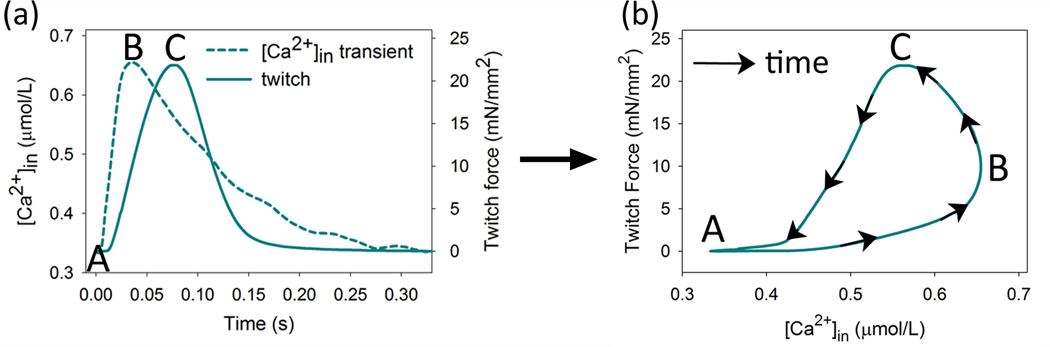
Initial experiments investigated the contributions of myofibrillar protein phosphorylations and variations in [Ca2+]in to the positive inotropy associated with increased pacing frequency or β1-adrenergic stimulation. For these measurements, experimental recordings of force and [Ca2+]in during cardiac twitches are reported as force vs [Ca2+]in (Figure 1 to allow direct comparisons of force or [Ca2+]in for each experimental condition. During a pacing-triggered twitch, the [Ca2+]in transient rises to its maximum prior to the force peak (Figure 1A), so that plotting force vs. [Ca2+]in yields a hysteresis loop due to the continuing rise in force during the initial decline in [Ca2+]in from its peak (Figure 1B). The hysteresis loop exhibits consistent phases: A to B, concordant rise, both force and [Ca2+]in increase; B to C, discordant rise, force increases while [Ca2+]in decreases; C to A, concordant relaxation, both force and [Ca2+]in decrease.9
Figure 1. Hysteresis in plots of force vs [Ca2+]in during the cardiac twitch.
(A) Upon stimulation, [Ca2+]in rises to a peak well before twitch force. (B) Plotting twitch force vs. [Ca2+]in during the time progression of a twitch forms a hysteresis loop. Labels on the loop indicate diastole, A; peak of [Ca2+]in, B; peak of twitch force C. The segments formed between these points are described in the text.
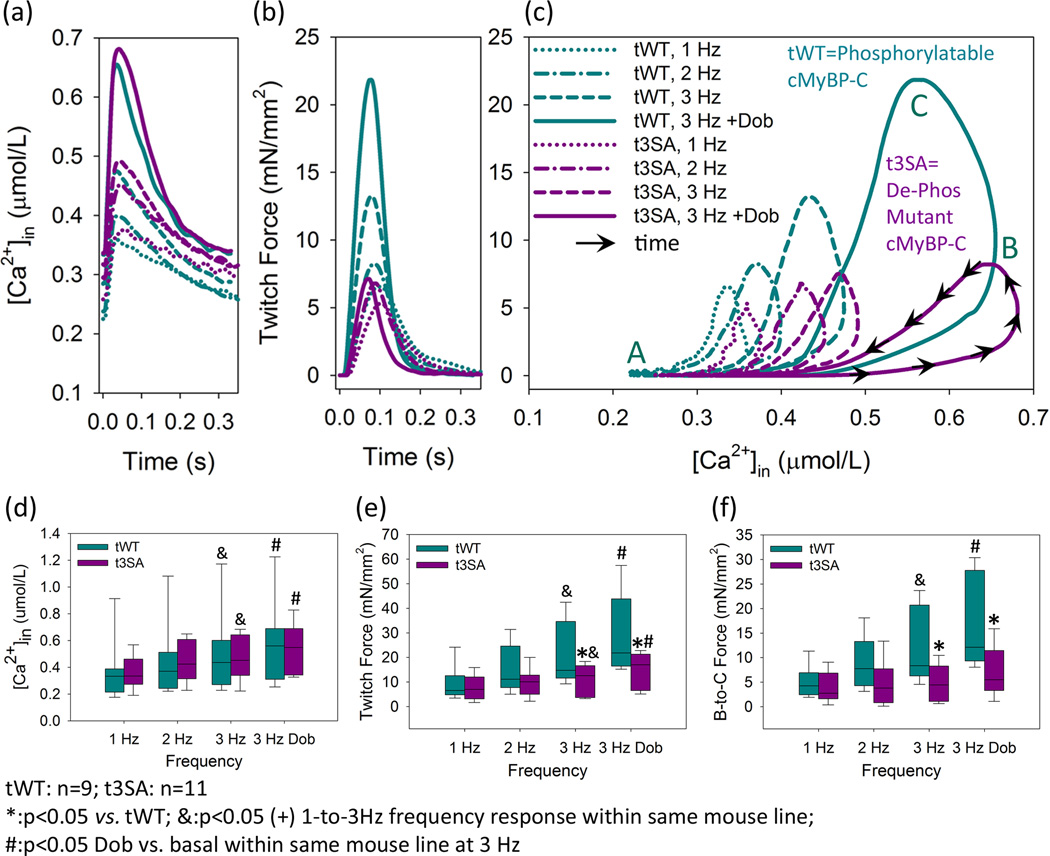
Increasing the pacing frequency from 1 to 3 Hz substantially increased twitch force, i.e., the Bowditch effect,11 in papillary muscles from cMyBP-C(tWT) hearts (Figures 2B,2C,2E). However, the Bowditch effect was severely blunted in cMyBP-C(t3SA) muscles expressing non-PKA-phosphorylatable cMyBP-C. Recordings of [Ca2+]in (Figures 2A,D) showed that there were similar progressive increases in the amplitudes of the [Ca2+]in transients in the two types of muscle as frequency was increased over this same range. Thus, the dramatically reduced effect of increasing stimulus frequency on twitch force in myocardium expressing phosphorylation-deficient cMyBP-C suggests that such phosphorylation is required for full expression of the positive inotropy that characterizes the Bowditch effect. Conversely, any direct activating effect on thin filaments due to the increased [Ca2+]in transients at higher frequencies only partially increases peak twitch force in phosphorylation-deficient papillary muscles.
Figure 2. Phosphorylation-deficient cMyBP-C blunts the positive inotropic responses to a β1-agonist or increased pacing frequency.
(A) Increased pacing frequency or dobutamine treatment cause similar increases in [Ca2+]in transients in cMyBP-C(tWT) and cMyBP-C(t3SA) myocardium. (B) Increased pacing or dobutamine substantially increase twitch force in cMyBP-C(tWT) but not cMyBP-C(t3SA) muscle. (C) Increased pacing or dobutamine causes much greater increases in the height of hysteresis loops from cMyBP-C(tWT) myocardium, predominantly in the B to C discordant rise segment. (D) Both mouse models exhibit similar increases in the amplitudes of [Ca2+]in transients in response to increased pacing or dobutamine. (E) cMyBP-C(t3SA) muscle exhibits depressed responses to increased pacing or dobutamine. However, both interventions increase force in both mouse models. (F) cMyBP-C(tWT) myocardium exhibits significant increases in the amplitudes of the discordant rise in response to either increased pacing or dobutamine. With identical interventions, cMyBP-C(t3SA) myocardium showed no significant changes in the discordant rise which is much less than cMyBP-C(tWT).
Next, the β1-adrenergic agonist dobutamine was infused into the solution bathing the papillary muscles. At a frequency of 3 Hz, dobutamine substantially further increased twitch force in WT myocardium (Figures 2B, 2C, 2E), an effect that was nearly absent in cMyBP-C(t3SA). In both instances, the [Ca2+]in transients were increased to a similar degree (Figures 2A,2D). Thus, the fullest potentiation of twitch force due to a β1-agonist requires phosphorylation of cMyBP-C and appears to include a much smaller component that is a direct effect of the concomitant increase in the myoplasmic [Ca2+]in transient. Thus, the increase in the myoplasmic [Ca2+]in transient due to dobutamine induced, on average, a small increase in twitch force in cMyBP-C(t3SA) myocardium. This increase is likely due to a direct activating effect of Ca2+ binding to the thin filament.
For the Bowditch effect and β1-adrenergic stimulation, the mechanism of twitch potentiation is thought to be increased activation of the myofibrils due to the increased amplitude of the [Ca2+]in transient,12, 13 but our results above do not support this idea, implying that the mechanism is much more complex. From our data, positive inotropy in tWT myocardium occurred primarily as a result of increases in the amplitude of the discordant rise segment (Figures 2C,F), which has previously been suggested to involve a cooperative activation process.9,14–16 In contrast, cMyBP-C(t3SA) myocardium exhibited much smaller increases in force with dobutamine or increased stimulus frequency despite increases in [Ca2+]in that were similar to cMyBP-C(WT) myocardium. The depressed force responses in cMyBP-C(t3SA) myocardium were associated with no significant increases in the discordant-rise segment when pacing was increased (Figures 2C,F). Overall, the increases in amplitude of [Ca2+]in transients accounted for a very small component of the positive inotropic responses to increased pacing or dobutamine. Thus, phosphorylation of cMyBP-C appears to be the predominant mediator of the force-frequency relationship and the inotropy due to a β1-adrenergic agonist, doubtless due to increased numbers of force-generating cross-bridges.
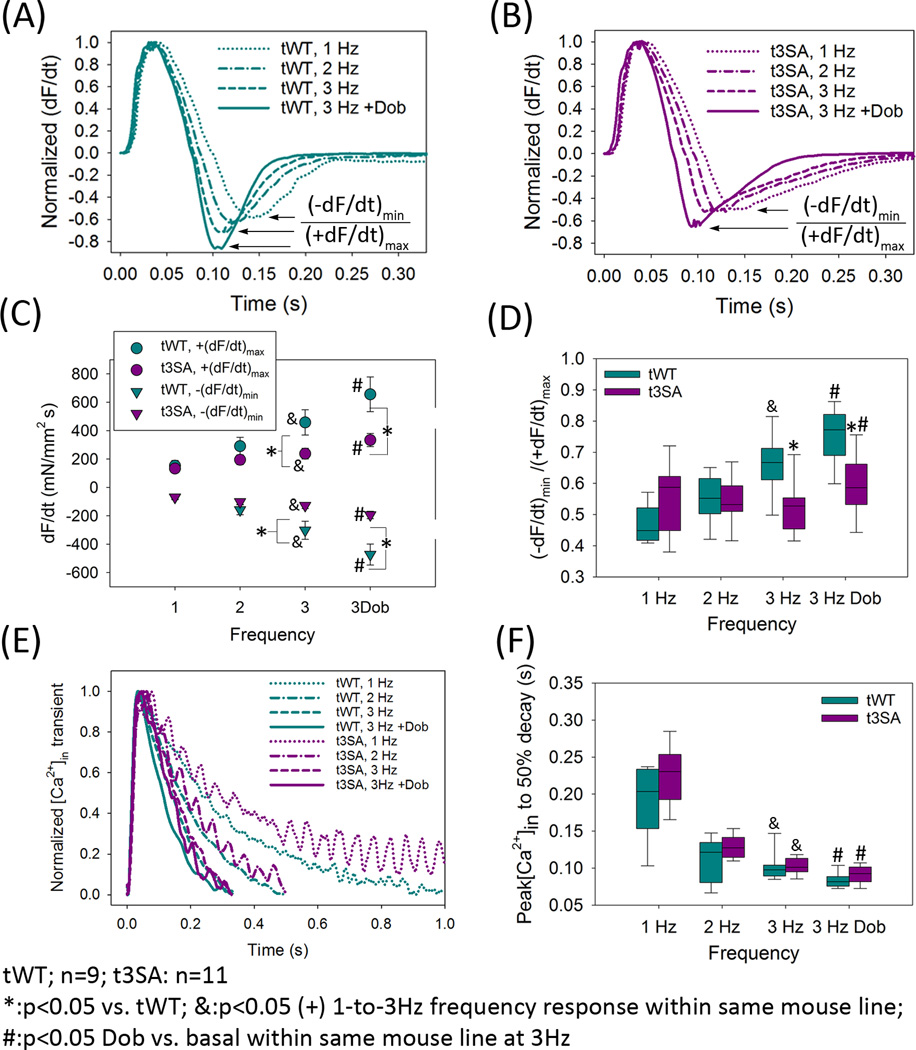
cMyBP-C(tWT) muscles also exhibited pacing-dependent acceleration of relaxation (Figure 3A) that was virtually absent in cMyBP-C(t3SA) (Figure 3B). Dobutamine increased the peak rates of force development (+dF/dt)max and relaxation (−dF/dt)min (Figure 3C) in both WT and cMyBP-C(t3SA) myocardium, but these effects were much greater in WT. A change in (+dF/dt)max might account for a change in (−dF/dt)min simply as a result of the corresponding change in peak force. Therefore, we used the ratio |(−dF/dt)min|/(+dF/dt)max to assess the effects of inotropic stimuli on the kinetics of relaxation. Since |(−dF/dt)min|/(+dF/dt)max is the ratio of peak relaxation rate to peak contraction rate, increases in |(−dF/dt)min|/(+dF/dt)max indicate acceleration of relaxation kinetics. Increased pacing frequency accelerated relaxation in cMyBP-C(tWT) but not in cMyBP-C(t3SA) muscles (Figures 3A,B,D). Dobutamine did accelerate relaxation in cMyBP-C(t3SA) but to a lesser degree than in WT (Figure 3D). The differing magnitudes of effects in the two mouse lines were not due to differences in the intracellular Ca2+ transients, since myocardium from both lines exhibited similar increases in peak [Ca2+]in and similar acceleration of transient decay times from peak to 50% maximal [Ca2+]in with either increased pacing frequency or dobutamine (Figures 2A,2D,3E–3F). These results strongly suggest that phosphorylation of cMyBP-C mediates the acceleration of relaxation by increasing the rate of cross-bridge cycling. This complex observation is addressed in detail in a companion paper and is not considered further here.
Figure 3. cMyBP-C phosphorylation accelerates relaxation.
(A) cMyBP-C(tWT) myocardium exhibits acceleration of relaxation in response to increased pacing or dobutamine, which is seen as increased (−dF/dt)min/(+dF/dt)max in normalized dF/dt traces. (B) cMyBP-C(t3SA) myocardium exhibits no change in (−dF/dt)min/(+dF/dt)max in response to increased pacing (upper arrow) but still exhibited a diminished increase in (−dF/dt)min/(+dF/dt)max in response to dobutamine. (C) Increased pacing increased absolute (+dF/dt)max for both lines. Increased pacing increased absolute (−dF/dt)min for both lines, although the effect was much greater in cMyBP-C(tWT) myocardium. cMyBP-C(t3SA) muscles also exhibited much smaller values of absolute (+dF/dt)max and (−dF/dt)min at 3 Hz with or without dobutamine. (D) Increased pacing causes acceleration of relaxation, seen as increased |(−dF/dt)min/(+dF/dt)max| only in cMyBP-C(tWT). Dobutamine treatment causes increased |(−dF/dt)min/(+dF/dt)max| in both mouse models, although the effect was greater in cMyBP-C(tWT) myocardium. (E) Normalized [Ca2+]in transients show that increased pacing or dobutamine accelerates the time course of [Ca2+]in decay similarly in both mouse models. (F) Increased pacing causes similar decreases in the time from the [Ca2+]in peak to 50% decay in both mouse models.
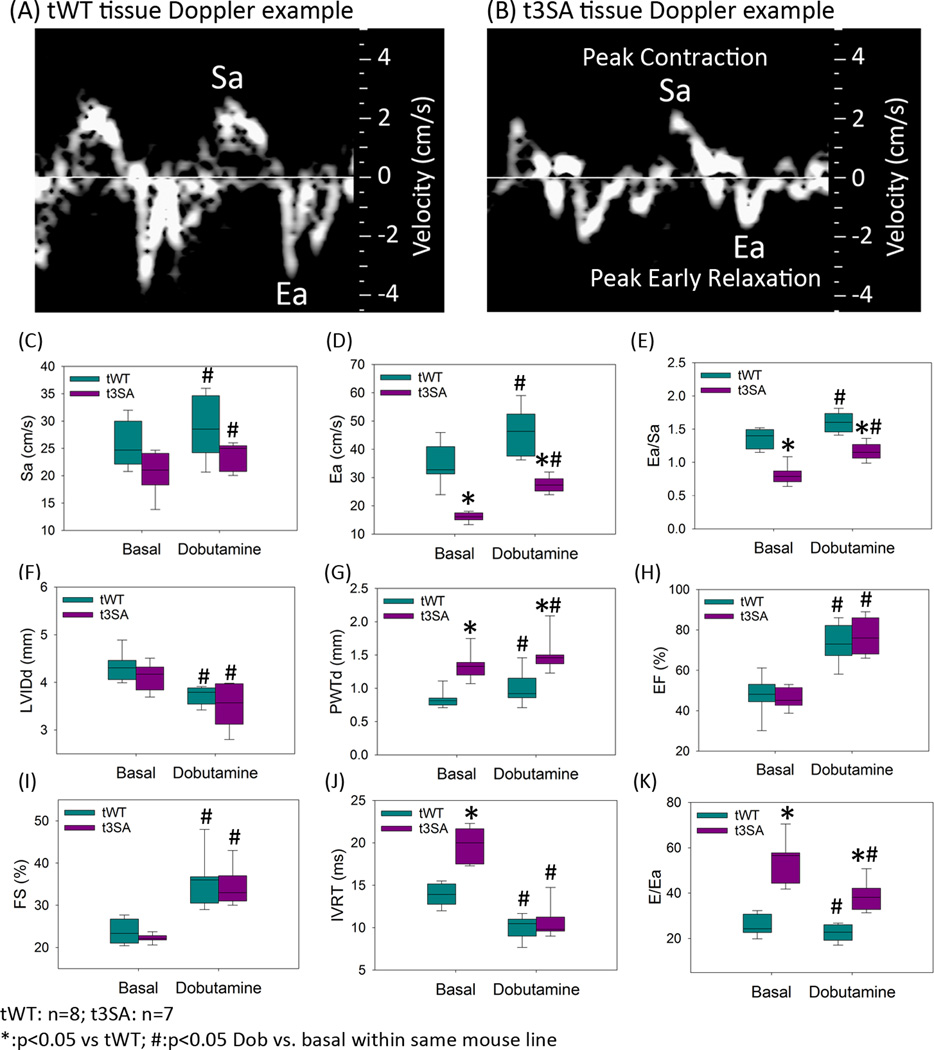
In vivo tissue Doppler echocardiography showed that cMyBP-C(t3SA) hearts exhibit slower rates of contraction and relaxation and a depressed ratio of the rates of relaxation to contraction in comparison to WT (Figure 4). Sa is the peak myocardial tissue Doppler velocity during systole and Ea is the peak myocardial tissue Doppler relaxation velocity during early diastole (Figures 4A,B). Sa17 is analogous to (+dP/dt)max and Ea18 is analogous to (−dP/dt)min; therefore, Sa, Ea, and Ea/Sa are in vivo analogues of (+dF/dt)max, (−dF/dt)min, and |(−dF/dt)min|/(+dF/dt)max measurements in papillary muscles. cMyBP-C(t3SA) hearts exhibited depressed Sa, Ea, and Ea/Sa (Figure 4C,D), providing an in vivo correlate to the depressed twitch kinetics observed in papillary muscles. Thus, the mechanistic insights obtained at room temperature from intact papillary muscles apply to hearts in vivo. Also, the phenotypes of cMyBP-C(t3SA) hearts in vivo correspond to the phenotypes of human heart failure with preserved ejection fraction (HFpEF),19 i.e., a combination of preserved ejection fraction and diastolic dysfunction (Figure 4F–K).
Figure 4. cMyBP-C(t3SA) hearts exhibit hypertrophy, slower relaxation velocity, and diastolic dysfunction.
Sa is peak myocardial contraction velocity by tissue Doppler; Ea is peak myocardial relaxation velocity during early diastole. Both lines exhibit similar basal heart rates (cMyBP-C(tWT) 506±18 BPM, n=8; cMyBP-C(t3SA) 453±30 BPM, n=7). Dobutamine treatment increased heart rates on both lines in similar fashion (cMyBP-C(tWT) 635±7 BPM, n=8; cMyBP-C(t3SA) 660±12 BPM, n=7) (A) In cMyBC-C(tWT) hearts, Ea > Sa. (B) In cMyBP-C(t3SA) hearts, Ea<Sa. (C-E) cMyBP-C(t3SA) hearts exhibit slower Ea resulting in depressed Ea/Sa (analogous to |(−dF/dt)min/(+dF/dt)max|). (F) Left ventricular inner diameters during diastole (LVIDd) are similar between MyBP-C(tWT) and MyBP-C(t3SA) hearts. (G) cMyBP-C(t3SA) hearts exhibit increased LV posterior wall thickness, indicating hypertrophy. (H,I) Both models show similar ejection fraction and fractional shortening. (J) cMyBP-C(t3SA) hearts exhibit prolonged iso-volumetric relaxation time (IVRT) in the basal state. IVRT was similar in both models when dobutamine was applied, likely due to an increase in heart rate and a probable increase in left atrial pressure. (4K) cMyBP-C(t3SA) hearts exhibited greater E/Ea (Doppler blood flow velocity across mitral valve at early filling / tissue Doppler velocity at medial mitral valve annulus during early filling) for all conditions, indicating persistent diastolic dysfunction.
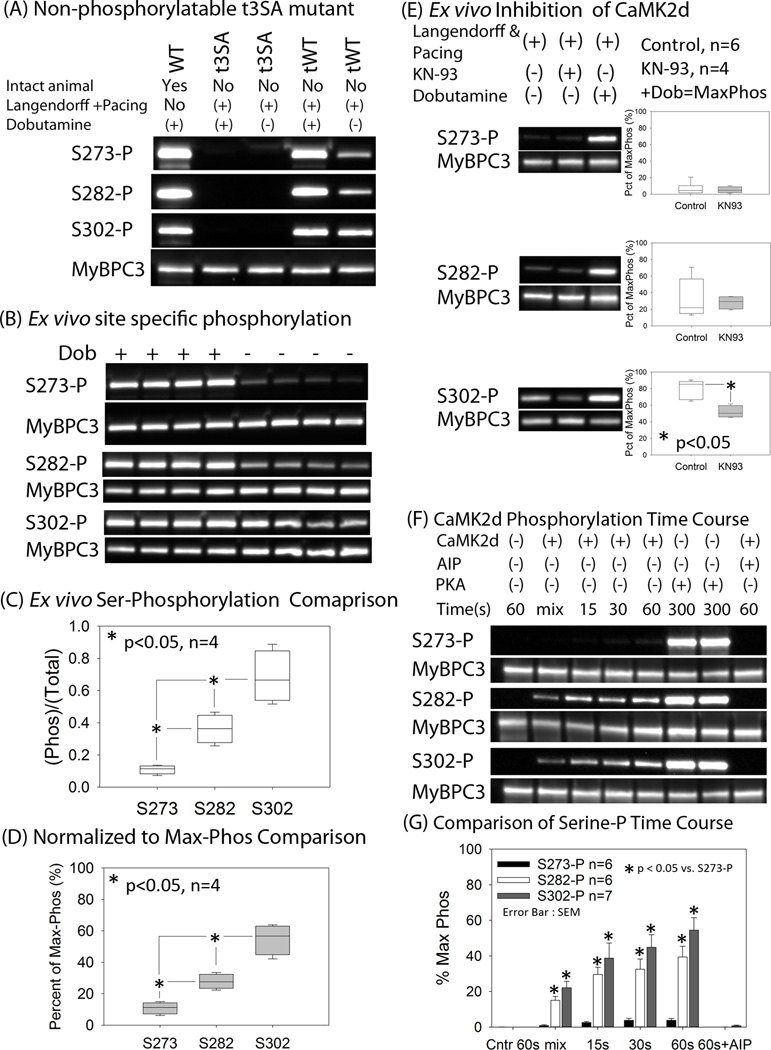
Western blotting with site-specific phosphoantibodies10 revealed that Ser302 is the predominant site of phosphorylation when pacing is increased, with no increase in phosphorylation of Ser273, i.e., Ser302-P > Ser282-P > Ser273-P (Figures 5A–D). In contrast, dobutamine resulted in robust phosphorylation of all three residues (Figures 5A–D), indicating that the kinases mediating these phosphorylations differ for the two interventions. Earlier studies showed that increasing the frequency of calcium pulses from 1 to 2.5 Hz activates CaMK2δ20 and that inhibition of CaMK2δ suppresses the phosphorylation of cMyBP-C.9 Thus, our results suggest that pacing-induced inotropy is mediated by CaMK2δ phosphorylation of cMyBP-C principally at Ser302. Consistent with this, pharmacologic inhibition of CaMK2δ depressed the pacing-induced increase in Ser302 phosphorylation (Figure 5E).
Figure 5. CaMK2δ predominantly phosphorylates S302 during increased pacing.
A cooled CCD camera was used to directly record chemiluminescence from Western blots. (A) Hearts were surgically removed and then perfused on a Langendorff apparatus prior to treatments indicated. A wild-type mouse was treated with dobutamine in vivo to provide reference data. The western blot demonstrates that ser273, ser282, and ser302 are phosphorylated in cMyBP-C(tWT) myocardium under basal conditions and with dobutamine, which (as expected) is not evident in cMyBP-C(t3SA) myocardium. (B) Western blots show that Ser302 is the residue predominantly phosphorylated during pacing. (C) Analyses of site-specific phospho-ser/cMyBP-C ratios show that pacing increased cMyBP-C phosphorylation in the order Ser302-P > Ser282-P > Ser273-P, while dobutamine treatment similarly phosphorylated all three residues. (D) P-ser/cMyBP-C ratios were normalized to maximum phosphorylation due to dobutamine treatment of cMyBP-C(tWT) hearts to eliminate the possibility that different sensitivities of the three site specific antibodies would be a confounding factor. The normalized to maximum ((P-Ser/cMyBP-C)pacing/(P-Ser/cMyBP-C)dobutamine) ratios confirmed that Ser302-P > Ser282-P > Ser273-P with pacing. (E) Western blots were done on a separate set of Langendorff-perfused paced hearts: control, CaMK2δ inhibition with KN-93 (10 µmol/L), and dobutamine treatment. Using dobutamine treatment as the reference for maximal phosphorylation, KN-93 inhibition of CaMK2δ reduced only Ser302-P. (F) Pre-activated recombinant CaMK2δ was mixed with recombinant cMyBP-C at a calculated final [Ca2+] of 12 nmol/L to study the time course of phosphorylation at a low mean [Ca2+] mimicking diastole (see data supplement for details). (G) Using PKA-treated recombinant cMyBP-C as the reference for maximal phosphorylation, pre-activated CaMK2δ phosphorylated ser 282 to 37 ± 7% and ser 302 to 53 ± 8% in 60s. In fact, CaMK2δ starts to phosphorylate cMyBP-C immediately upon mixing. Autocamtide inhibitory peptide (AIP) at 10 µmol/L, a non-competitive inhibitor of CaMK2δ, inhibited phosphorylation of cMyBP-C at all sites.
Further experiments confirmed that CaMK2δ preferentially phosphorylates cMyBP-C at Ser302. A bacterial expression system was used to produce unphosphorylated recombinant cMyBP-C, which was then treated with recombinant CaMK2δ. This in vitro method for assessing site specificity of phosphorylation precludes potentially confounding effects on phosphorylation due to other endogenous kinases or basally phosphorylated cMyBP-C in intact muscle. CaMK2δ phosphorylated cMyBP-C at Ser302 within 15–30 seconds at [Ca2+] comparable to in vivo diastolic [Ca2+]in (Figure 5G). The results from Langendorff perfused hearts and in vitro studies indicate that CaMK2δ phosphorylates cMyBP-C at Ser302 when pacing frequency is increased, Ser282 to a much lesser degree, and Ser273 not at all, while PKA phosphorylates cMyBP-C at all three serines during adrenergic stimulation.
Experiments were conducted in solution on full-length recombinant WT cMyBP-C to explore the potential synergistic effects of CaMK2δ and PKA when both are present. CaMK2δ and PKA were added both singly and in combination for a reaction time of 5 minutes. CaMK2δ caused a greater amount of phosphorylation than PKA at Ser302, and together, the combined kinases caused greater phosphorylation than either kinase alone. Autocamtide inhibitory peptide (AIP), a specific inhibitor of CaMK2δ, was added to the kinase mixture, which reduced the effect of the combined kinases to the effect of PKA alone. These results suggest that CaMK2δ and PKA act independently: CaMK2δ specifically targets Ser302, while PKA targets all three but has a lesser effect on Ser302 than does CaMK2δ.
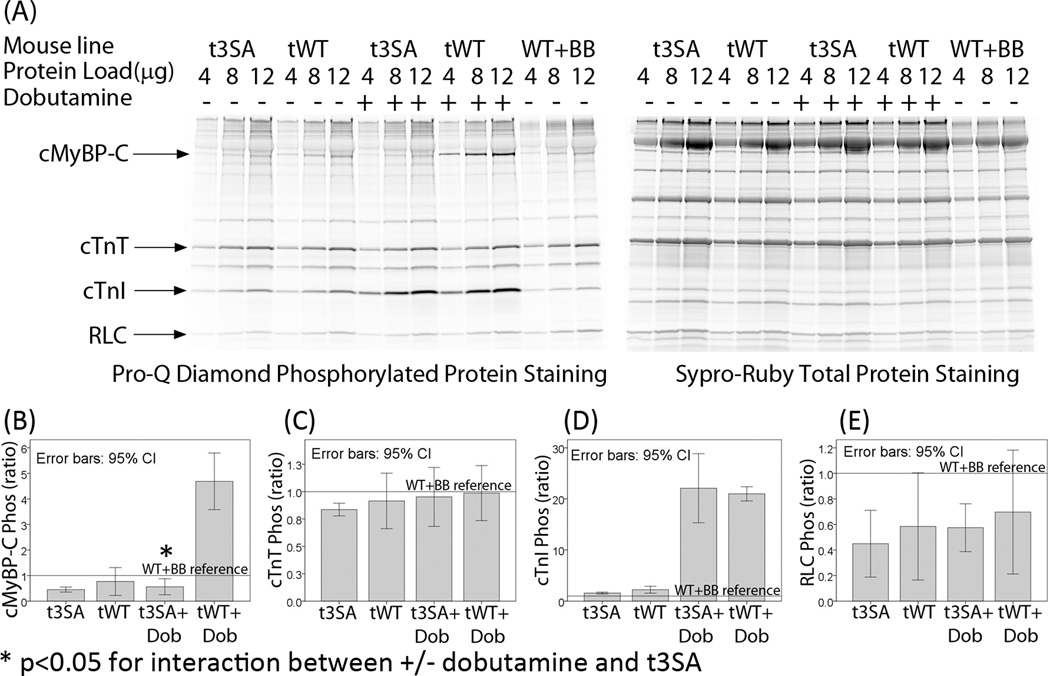
Phosphoprotein staining showed no differences in the phosphorylation of other myofilament proteins in WT and t3SA myocardium under each experimental condition. Myofibrils from both mouse lines exhibited similar basal levels of phosphorylation of cardiac troponin-I (cTnI), cardiac troponin-T (cTnT), and regulatory light chain (RLC) (Figure 6). Dobutamine treatment increased cTnI phosphorylation to similar levels in both cMyBP-C(tWT) and cMyBP-C(t3SA) myocardium (Figure 6). Therefore, the observed differences in functional responses to dobutamine are not due to differential phosphorylation of myofibrillar regulatory proteins other than cMyBP-C.
Figure 6. Except for cMyBP-C, there are no detectable differences in phosphorylation of myofilament regulatory proteins by dobutamine treatment.
Myofibrils were isolated from Langendorff perfused hearts for analysis of protein phosphorylation; a metoprolol treated WT mouse heart was used for reference (WT+BB). Metoprolol, a selective β1-adrenergic receptor blocker, was infused initially to minimize phosphorylation of myofibrillar proteins. The ratio [(Pro-Q slope of experiment)/(Pro-Q slope of WT+BB)]/[(Sypro-Ruby slope of experiment)/(Sypro-Ruby slope of WT+BB)] was used to compare levels of phosphorylation. Unlike previous figures, 95% confidence interval (CI) error bars are used instead of standard error of mean. Use of 95% CI error bar is intended to provide easier visualization of significant differences instead of using multiple characters to denote significant differences on multiple comparisons. Non-overlapping 95% (CI) indicate statistically significant differences. (A) Pro-Q Diamond staining of phosphorylated proteins showed that cTnT, cTnI, and RLC phosphorylation levels were similar between cMyBP-C(tWT) and cMyBP-C(t3SA) hearts under comparable experimental conditions. Staining with Sypro-Ruby verified that total myofibril protein loading were similar for each mouse model and condition. (B) cMyBP-C phosphorylation levels were not statistically different between the two mouse models under basal conditions. Dobutamine increased phosphorylation of cMyBP-C in cMyBP-C(tWT) but not cMyBP-C(t3SA) hearts. cMyBP-C is the only protein that showed significant interaction between +/− dobutamine treatment and t3SA mutation. (C) cTnT phosphorylation levels were similar in both mouse models. (D) Basal cTnI phosphorylation levels were similar under basal conditions, and dobutamine induced similar increases in cTnI phosphorylation in both mouse models. (E) RLC phosphorylation levels were similar in both mouse models.
A cMyBP-C phosphorylation mimetic mouse was also generated to determine whether replacement of the three PKA phosphorylatable serines with aspartic acid to mimic constitutive phosphorylation would alter the responsiveness to increased pacing. cMyBP-C with Ser273D, Ser282D, and Ser302D was expressed on the cMyBP-C null background, creating the cMyBP-C(t3SD) mouse. Increasing pacing from 1 to 2.5 Hz in 0.5 Hz increments yielded the unexpected finding of a positive force-frequency response in cMyBP-C(t3SD) myocardium (Data Supplement Figure S1). The existence of additional phosphorylatable sites could provide an explanation for a positive force/frequency relationship in cMyBP-C(t3SD) myocardium.21 However, such a site or sites must not be accessible in cMyBP-C(t3SA) myocardium since t3SA exhibits a severely depressed force-frequency relationship, as reported here. Thus, it may be that the introduction of glutamates induces conformational changes in cMyBP-C that are not observed in t3SA or tWT myocardium. Other mechanistic interpretations involving cMyBP-C are possible and are the subject of planned detailed investigations.
Discussion
The study was undertaken to determine the contributions of cMyBP-C phosphorylation in relation to increased [Ca2+]in or post-translational modifications of other myofibrillar proteins in mediating positive inotropy due to increased pacing or β1-adrenergic stimulation. Both heart failure with reduced ejection fraction (HFrEF) and HFpEF exhibit reduced inotropic reserve, which is evidenced by depressed inotropic responses to increased heart rate and β1-adrenergic stimulation. Thus, elucidating the mechanisms of positive inotropy in response to these physiological interventions may provide insights into the mechanisms of dysfunction in heart failure and potential targets for therapeutic interventions.
The approaches employed to study mechanisms of inotropy utilized intact myocardium expressing only non-PKA-phosphorylatable mutant cMyBP-C(3SA) or phosphorylatable WT cMyBP-C(tWT), both to similar levels on a cMyBP-C null background. The key findings are: (1) cMyBP-C(tWT) myocardium exhibited the expected steep force-frequency relationship (the Bowditch effect);11 while cMyBP-C(t3SA) myocardium exhibited dramatically smaller increases in twitch force when pacing frequency was increased; (2) dobutamine increased twitch force in cMyBP-C(tWT) and cMyBP-C (t3SA) myocardium, but the increase was significantly blunted in the latter; (3) for each experimental condition, the amplitude and kinetics of the [Ca2+]in transient did not differ between cMyBP-C(tWT) and cMyBP-C(t3SA) myocardium,; and (4) phosphorylation levels for myofibrillar proteins other than MyBP-C were similar between cMyBP-C(tWT) and cMyBP-C(t3SA) myocardium under each condition studied. Together, these results indicate that positive inotropy due to the physiological interventions studied here is primarily mediated by phosphorylation of cMyBP-C with a smaller component due to increased binding of Ca2+ to thin filament proteins secondary to increased [Ca2+]in under these conditions.
Based on different patterns of serine phosphorylation, the kinases mediating cMyBP-C phosphorylation appear to differ for pacing-induced and β1-agonist-induced inotropy. Here we show that CaMK2δ phosphorylation of cMyBP-C at Ser302 appears to be the primary mechanism for pacing-induced inotropy, although Ser282 is also phosphorylated to a much lesser degree. Activation of CaMK2δ has previously been shown to contribute to the Bowditch effect by increasing delivery of Ca2+ to the myoplasm as a result of phosphorylation of L-type Ca2+ channels22, 23 and ryanodine receptors RyR2.24 An increased frequency of calcium pulses, similar to the transients in successive twitches, has been shown to activate CaMK2δ,20 and once activated, CaMK2δ can remain active even despite a low average [Ca2+]in.20 These properties make CaMK2δ an ideal sensor for increasing heart rate to affect inotropy. In our experiments, pacing induced cMyBP-C phosphorylation at Ser302, while inhibition of CaMK2δ decreased Ser302 phosphorylation. Activated recombinant CaMK2δ phosphorylated recombinant full length cMyBP-C at Ser302 at low calcium (< 100 nmol/L) similar to [Ca2+]in during diastole in less than 30 seconds. Thus, our data suggest that cMyBP-C contributes to pacing-induced inotropy through activation of CaMK2δ.
Although the present study has identified CaMK2δ as the dominant mediator of cMyBP-C phosphorylation at Ser302 as the proximate basis for the positive force/frequency response, there are likely to be modulators of this mechanism in the heart in vivo. For example, reactive oxygen species when present can activate CaMK2δ,25 and angiotesin-II26 or aldosterone.25 In addition, protein kinase D (PKD) can phosphorylate cMyBP-C at Ser302.8 Regardless of the identities of upstream signals as additional modulators of Ser302 phosphorylation, the present results show that pacing or adrenergic agonist-induced phosphorylation of Ser302 increases contractility.
Our conclusion that the force-frequency relationship in healthy myocardium is mediated by CaMK2δ phosphorylation of cMyBP-C is consistent with earlier results,24 which showed depressed force-frequency relationships in mice expressing mutant ryanodine receptors (S2814A) that could not be phosphorylated by CaMK2δ. However, the mutant hearts in their study showed a marked residual positive force-frequency relationship,24 unlike our results showing near-elimination of the force frequency relationship when non-phosphorylatable cMyBP-C is expressed. The residual force-frequency relationship in their experiments on S2814A myocardium was associated with a significant residual increase in myoplasmic Ca2+ when pacing frequency was increased,24 which presumably increased CaMK2δ activity above basal levels and partially phosphorylated cMyBP-C. Conversely, they showed that inhibition of CaMK2δ in WT mice with KN93 almost completely eliminated the positive force-frequency relationship,24 which is consistent with our finding that cMyBP-C(t3SA) papillary muscles exhibited minimal positive inotropy when pacing frequency was increased. In a different study, autocamtide inhibitory peptide inhibition of CaMK2δ depressed the force-frequency response, decreased cMyBP-C phosphorylation, and did not significantly change Ca2+ transients in intact papillary muscles.9 These results lend further support to the idea that CaMK2δ phosphorylation of cMyBP-C is a principal mediator of the Bowditch effect. Our finding that the Ca2+ transient increased similarly in cMyBP-C(tWT) and cMyBP-C(t3SA) myocardium shows that the increased twitch force at higher pacing frequencies in cMyBP-C(tWT) myocardium is due mainly to the phosphorylation of cMyBP-C and to a much lesser degree the increased activation of the thin filaments as a consequence of increased Ca2+ binding to troponin.
Our results also show that the greatest part of cardiac inotropy due to β-adrenergic stimulation is due to phosphorylation of cMyBP-C, presumably by PKA, at all three phosphorylatable serines in the cMyBP-C motif, with only a small fraction of the inotropic response associated with the increased delivery of Ca2+ alone. This observation provides insight into the long-standing conundrum presented by the observation that β-adrenergic stimulation increases twitch force despite a decrease in Ca2+ binding affinity of troponin when PKA phosphorylates cTnI,27 as well as an additive decrease in Ca2+ sensitivity of force due to phosphorylation of cMyBP-C.28 The net increase in force is due to phosphorylation of cMyBP-C and the resulting increases in the rate of cross-bridge binding. The observation that adrenergic inotropy is greater than pacing induced inotropy can be related to the observation that PKA phosphorylation involves more serines, i.e., positive inotropy appears to scale with the degree of phosphorylation of cMyBP-C.
Our results show that each cMyBP-C phosphorylation site can be phosphorylated independently. Sadayappan, et al, have suggested that ser282 phosphorylation is in some way permissive for ser302 phosphorylation,5 which they determined by generating a ser282ala mutant cMyBP-C. In contrast, our data show that ser302 is the residue predominantly phosphorylated during increased pacing, suggesting that phosphorylation of ser282 is not necessary for phosphorylation of ser302 in vivo, at least by CaMK2δ. Copeland, et al, found that ser302 can be phosphorylated at levels ~3-fold higher than ser28229 and Gresham et al, showed that PKA can phosphorylate both Ser273 and Ser302 in the presence of a Ser282Ala mutation.6 Both studies provide further support of our conclusion that phosphorylation of Ser282 is not permissive to phosphorylation at the other two sites.
The relevance of these observations in isolated muscle to cardiac function was confirmed with in vivo Doppler measurements showing that cMyBP-C(tWT) myocardium has a faster contraction velocity (Sa), a faster relaxation velocity (Ea), and an increased Ea/Sa ratio compared to cMyBP-C(t3SA) myocardium under basal conditions and during either increased pacing or β1-agonist stimulation. The combination of results showing that paced ex vivo hearts exhibit predominantly Ser302 phosphorylation, that inhibition of CaMK2δ decreased Ser302 phosphorylation, and recombinant CaMK2δ robustly phosphorylated recombinant full length cMyBP-C mainly at ser302 strongly supports our hypothesis that activation of CaMK2δ leading to phosphorylation of cMyBP-C mediates the positive force-frequency response.
cMyBP-C(tWT) myocadium exhibited much greater increases in (+dF/dt)max and (−dF/dt)min than cMyBP-C(t3SA) in response to increased pacing frequency or dobutamine even though peak calcium and calcium decay kinetics were similar in both muscle types in each instance. Because of the absence of differences in the intracellular calcium transients, we conclude that the differences in maximum rates of contraction and relaxation are due to CaMK2δ or PKA phosphorylation of cMyBP-C to cause faster cross-bridge cycling kinetics. This conclusion is consistent with our prior study demonstrating that PKA treatment of skinned myocardium from cMyBP-C(t3SA) mice does not accelerate cross-bridge cycling kinetics.4
The more robust force-frequency and β1-adrenergic responses observed in cMyBP-C(tWT) myocardium can be accounted for by effects of cMyBP-C phosphorylation to increase the rate of myosin binding to actin and thus the number of strongly-bound cross-bridges. Consistent with this idea, phosphorylation of cMyBP-C induces displacement of myosin toward actin,30, 31 which would increase the probability of myosin binding to actin. cMyBP-C binding to actin also appears to restrict structural torsion dynamics of thin filaments, an effect that is relieved by phosphorylation of cMyBP-C.32 Either or both of these structural changes have the potential to promote cross-bridge interactions and to increase the rate of myosin binding to actin. Force can also increase due to enhancement of positive cooperativity in the binding of myosin to actin. Initial Ca2+ binding to troponin is thought to drive tropomyosin (Tm) from a Tmblocked to a Tmoff state in which cross-bridges bind weakly to actin.33 The subsequent transition of cross-bridges from weakly to strongly-bound states switches Tmoff to a Tmon state, a process that is accompanied by force development and promotion of cooperative binding of cross-bridges to actin.33–35 The activation dependence of the rate of force development has been proposed by some to manifest the longer time taken at low levels of activation for the cooperative spread of cross-bridge binding along the thin filament from the initial site of cross-bridge binding.36 Such a model supposes that the rate of cross-bridge interaction is constant and maximal, so that force development at low activation is slowed only by the relatively slow spread of cooperative cross-bridge binding to the thin filament. In the present context, the dramatically reduced inotropy in cMyBP-C(t3SA) myocardium was related to much lower forces generated in the discordant rise segment of the force-[Ca2+]in loop, i.e., the cooperative phase of the twitch in which force continues to increase despite declining [Ca2+]in.
While the present study shows clearly that phosphorylation of the phosphorylatable serines within the M-domain of cMyBP-C has profound physiological effects, some care in extrapolating these results to other systems or conditions is warranted. However, published data showing positive force/frequency responses in mouse hearts under a wide range of conditions lend confidence in the conclusions drawn here. The living papillary muscle has intact membranes, calcium handling proteins, preserved myofilament lattice structure, and plausible time courses of force and calcium during the twitch. Also, our earlier studies showed that pacing papillary muscle at room temperature at 1–3 Hz yields the same overall force frequency effects as pacing at 34°C and 1–7 Hz.9 Rottman et al, have shown that mouse hearts in vivo exhibit robust, positive force/frequency relationships from 150 to 700 BPM in both conscious and anesthetized mice.37 Since pacing in our study overlaps the lower range, it seems reasonable to conclude that our findings at room temperature are broadly applicable to normal physiology.
Physiological differences can potentially limit the applicability of the present results from mice to humans. Mice have much higher resting heart rates (350–600) than humans (60–70),38 much smaller hearts with higher overall metabolism, and faster contractile protein isoforms. An overarching consideration in the selection of mice for this study is the ability to perform gene deletion and mutant gene expression in murine hearts, but studies in higher animal models will be necessary to confirm the degree to which the mechanisms reported here are also operable in the human heart.
Supplementary Material
Acknowledgments
Sources of Funding
Supported by NIH R37-82900 and NIH P01 HL094291 to RLM and NIH K08HL114877 to CWT.
Disclosures
RL Moss is a consultant to Myokardia, Inc., South San Francisco, CA.
References
- 1.Luther PK, Bennett PM, Knupp C, Craig R, Padron R, Harris SP, Patel J, Moss RL. Understanding the organisation and role of myosin binding protein C in normal striated muscle by comparison with MyBP-C knockout cardiac muscle. J Mol Biol. 2008;384:60–72. doi: 10.1016/j.jmb.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: its role in physiology and disease. Circ Res. 2004;94:1279–1289. doi: 10.1161/01.RES.0000127175.21818.C2. [DOI] [PubMed] [Google Scholar]
- 3.Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW, 2nd, Klevitsky R, Seidman CE, Seidman JG, Robbins J. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res. 2005;97:1156–1163. doi: 10.1161/01.RES.0000190605.79013.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong CW, Stelzer JE, Greaser ML, Powers PA, Moss RL. Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ Res. 2008;103:974–982. doi: 10.1161/CIRCRESAHA.108.177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadayappan S, Gulick J, Osinska H, Barefield D, Cuello F, Avkiran M, Lasko VM, Lorenz JN, Maillet M, Martin JL, Brown JH, Bers DM, Molkentin JD, James J, Robbins J. A critical function for Ser-282 in cardiac Myosin binding protein-C phosphorylation and cardiac function. Circ Res. 2011;109:141–150. doi: 10.1161/CIRCRESAHA.111.242560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gresham KS, Mamidi R, Stelzer JE. The contribution of cardiac myosin binding protein-c Ser282 phosphorylation to the rate of force generation and in vivo cardiac contractility. J Physiol. 2014;592:3747–3765. doi: 10.1113/jphysiol.2014.276022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuello F, Bardswell SC, Haworth RS, Ehler E, Sadayappan S, Kentish JC, Avkiran M. Novel role for p90 ribosomal S6 kinase in the regulation of cardiac myofilament phosphorylation. J Biol Chem. 2011;286:5300–5310. doi: 10.1074/jbc.M110.202713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardswell SC, Cuello F, Rowland AJ, Sadayappan S, Robbins J, Gautel M, Walker JW, Kentish JC, Avkiran M. Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling. J Biol Chem. 2010;285:5674–5682. doi: 10.1074/jbc.M109.066456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong CW, Gaffin RD, Zawieja DC, Muthuchamy M. Roles of phosphorylation of myosin binding protein-C and troponin I in mouse cardiac muscle twitch dynamics. J Physiol. 2004;558:927–941. doi: 10.1113/jphysiol.2004.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadayappan S, Gulick J, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Robbins J. Cardiac myosin binding protein-C phosphorylation in a {beta}-myosin heavy chain background. Circulation. 2009;119:1253–1262. doi: 10.1161/CIRCULATIONAHA.108.798983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation. 1992;85:1743–1750. doi: 10.1161/01.cir.85.5.1743. [DOI] [PubMed] [Google Scholar]
- 12.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 13.Hasenfuss G, Reinecke H, Studer R, Pieske B, Meyer M, Drexler H, Just H. Calcium cycling proteins and force-frequency relationship in heart failure. Basic Res Cardiol. 1996;91(Suppl 2):17–22. doi: 10.1007/BF00795357. [DOI] [PubMed] [Google Scholar]
- 14.Tong CW, Kolomenskii A, Lioubimov VA, Schuessler HA, Trache A, Granger HJ, Muthuchamy M. Measurements of the cross-bridge attachment/detachment process within intact sarcomeres by surface plasmon resonance. Biochemistry. 2001;40:13915–13924. doi: 10.1021/bi0101648. [DOI] [PubMed] [Google Scholar]
- 15.Fitzsimons DP, Patel JR, Moss RL. Cross-bridge interaction kinetics in rat myocardium are accelerated by strong binding of myosin to the thin filament. J Physiol. 2001;530:263–272. doi: 10.1111/j.1469-7793.2001.0263l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kad NM, Kim S, Warshaw DM, VanBuren P, Baker JE. Single-myosin crossbridge interactions with actin filaments regulated by troponin-tropomyosin. Proc Natl Acad Sci U S A. 2005;102:16990–16995. doi: 10.1073/pnas.0506326102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo JS, Kim DH, Kim WJ, Song JM, Kang DH, Song JK. Peak systolic velocity of mitral annular longitudinal movement measured by pulsed tissue Doppler imaging as an index of global left ventricular contractility. Am J Physiol Heart Circ Physiol. 2010;298:H1608–H1615. doi: 10.1152/ajpheart.01231.2009. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Sun H, Kopelen HA, Middleton KJ, Khoury DS. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol. 2001;37:278–285. doi: 10.1016/s0735-1097(00)01056-1. [DOI] [PubMed] [Google Scholar]
- 19.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 20.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 21.Kooij V, Holewinski RJ, Murphy AM, Van Eyk JE. Characterization of the cardiac myosin binding protein-C phosphoproteome in healthy and failing human hearts. J Mol Cell Cardiol. 2013;60:116–120. doi: 10.1016/j.yjmcc.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. The Journal of cell biology. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Colbran RJ, Anderson ME. Calmodulin kinase is a molecular switch for cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2001;98:2877–2881. doi: 10.1073/pnas.051449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc Natl Acad Sci U S A. 2010;107:10274–10279. doi: 10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luczak ED, Anderson ME. CaMKII oxidative activation and the pathogenesis of cardiac disease. J Mol Cell Cardiol. 2014;73:112–116. doi: 10.1016/j.yjmcc.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, Neef S, Sowa T, Gao Z, Luczak ED, Stefansdottir H, Behunin AC, Li N, El-Accaoui RN, Yang B, Swaminathan PD, Weiss RM, Wehrens XH, Song LS, Dobrev D, Maier LS, Anderson ME. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation. 2013;128:1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wattanapermpool J, Guo X, Solaro RJ. The unique amino-terminal peptide of cardiac troponin I regulates myofibrillar activity only when it is phosphorylated. J Mol Cell Cardiol. 1995;27:1383–1391. doi: 10.1006/jmcc.1995.0131. [DOI] [PubMed] [Google Scholar]
- 28.Chen PP, Patel JR, Rybakova IN, Walker JW, Moss RL. Protein kinase A-induced myofilament desensitization to Ca2+ as a result of phosphorylation of cardiac myosin-binding protein C. J Gen Physiol. 2010;136:615–627. doi: 10.1085/jgp.201010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copeland O, Sadayappan S, Messer AE, Steinen GJ, van der Velden J, Marston SB. Analysis of cardiac myosin binding protein-C phosphorylation in human heart muscle. J Mol Cell Cardiol. 2010;49:1003–1011. doi: 10.1016/j.yjmcc.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Colson BA, Bekyarova T, Locher MR, Fitzsimons DP, Irving TC, Moss RL. Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium. Circ Res. 2008;103:244–251. doi: 10.1161/CIRCRESAHA.108.178996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colson BA, Patel JR, Chen PP, Bekyarova T, Abdalla MI, Tong CW, Fitzsimons DP, Irving TC, Moss RL. Myosin binding protein-C phosphorylation is the principal mediator of protein kinase A effects on thick filament structure in myocardium. J Mol Cell Cardiol. 2012;53:609–616. doi: 10.1016/j.yjmcc.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colson BA, Rybakova IN, Prochniewicz E, Moss RL, Thomas DD. Cardiac myosin binding protein-C restricts intrafilament torsional dynamics of actin in a phosphorylation-dependent manner. Proc Natl Acad Sci U S A. 2012;109:20437–20442. doi: 10.1073/pnas.1213027109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geeves MA, Lehrer SS. Dynamics of the muscle thin filament regulatory switch: the size of the cooperative unit. Biophys J. 1994;67:273–282. doi: 10.1016/S0006-3495(94)80478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss RL, Razumova M, Fitzsimons DP. Myosin crossbridge activation of cardiac thin filaments: implications for myocardial function in health and disease. Circ Res. 2004;94:1290–1300. doi: 10.1161/01.RES.0000127125.61647.4F. [DOI] [PubMed] [Google Scholar]
- 35.Vibert P, Craig R, Lehman W. Steric-model for activation of muscle thin filaments. J Mol Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- 36.Razumova MV, Bukatina AE, Campbell KB. Different myofilament nearest-neighbor interactions have distinctive effects on contractile behavior. Biophys J. 2000;78:3120–3137. doi: 10.1016/S0006-3495(00)76849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rottman JN, Ni G, Brown M. Echocardiographic evaluation of ventricular function in mice. Echocardiography. 2007;24:83–89. doi: 10.1111/j.1540-8175.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 38.Hamlin RL, Altschuld RA. Extrapolation from mouse to man. Circ Cardiovasc Imaging. 2011;4:2–4. doi: 10.1161/CIRCIMAGING.110.961979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.