Abstract
Functional neuroimaging studies have consistently demonstrated abnormalities in fear and threat processing systems in youth with anxiety disorders; however, the structural neuroanatomy of these systems in children and adolescents remains largely unknown. Using voxel-based morphometry (VBM), gray matter volumes were compared between 38 medication-free patients with anxiety disorders (generalized anxiety disorder; social phobia; separation anxiety disorder, mean age: 14.4 ± 3 years) and 27 comparison subjects (mean age: 14.8 ± 4 years). Compared to healthy subjects, youth with anxiety disorders had larger gray matter volumes in the dorsal anterior cingulate and had decreased gray matter volumes in the inferior frontal gyrus (ventrolateral prefrontal cortex), postcentral gyrus, and cuneus/precuneus. These data suggest the presence of structural differences in regions previously implicated in the processing and regulation of fear in pediatric patients with anxiety disorders.
Keywords: children, adolescent, voxel-based morphometry (VBM), anxiety, amygdala, ventrolateral prefrontal cortex (VLPFC), cingulate cortex
1. Introduction
Childhood and adolescence represent vulnerable periods during which many psychiatric disorders first emerge and during which time structural and functional brain changes are rapidly occurring (Giedd et al., 1996; Uddin et al., 2011; Alexander-Bloch et al., 2013). It is during this developmental period that anxiety disorders, which are among the most common psychiatric disorders in youth (Costello et al., 1996; Beesdo et al., 2007; Merikangas et al., 2011) and affect 10% of children and adolescents, first manifest clinically (Costello et al., 1996). Importantly, pediatric anxiety disorders are associated with significant functional impairment (Kendall et al., 2010), increased risk of suicidal ideation and suicide attempt (Foley et al., 2006; Boden et al., 2007; Jacobson et al., 2008; Husky et al., 2012) and a greater likelihood of developing secondary mood, substance use and other anxiety disorders (Pine et al., 1998; Beesdo-Baum et al., 2012a).
Among the anxiety disorders affecting children and adolescents, the most common are generalized anxiety disorder (GAD), separation anxiety disorder (SAD) and social phobia (SoP) (Beesdo et al., 2010; Kessler et al., 2012). These disorders, which are often referred to as the “pediatric anxiety triad,” commonly co-occur (Walkup et al., 2008), exhibit parallel clinical courses (Beesdo et al., 2010; Beesdo et al., 2009a; Beesdo-Baum et al., 2012b) and share risk factors (Beesdo et al., 2010). Additionally, GAD, SAD and SoP respond to similar psychopharmacologic (e.g. selective serotonin reuptake inhibitors [SSRIs] and selective serotonin-norepinephrine reuptake inhibitors [SSNRIs]) (for review see Strawn et al., 2012a) and similar psychotherapeutic treatments (e.g. cognitive-behavioral therapy [CBT]) (Comptom et al., 2010; Kendall et al., 2010; Verduin and Kendall, 2003). Because of the shared features of these disorders, most (Birmaher et al., 1994; Fairbanks et al., 1997; RUPP, 2001; Birmaher et al., 2003; Walkup et al., 2002; Geller et al., 2007; Walkup et al., 2008) but not all (Rynn et al., 2001; Rynn et al., 2007) treatment studies have involved patients with the “pediatric anxiety triad” rather than individual disorders proper.
In addition to the common clinical features and risk factors, the triad disorders, share neuropathophysiologic features (Strawn et al., 2012b; Blackford and Pine, 2012). In this regard, the extant functional magnetic resonance imaging (fMRI) data from children and adolescents with GAD, SAD and SoP implicate dysfunction within central fear circuitry (Blackford and Pine, 2012). Specifically, most fMRI studies reveal abnormal activation of the amygdala in pediatric GAD (Monk et al., 2008; Beesdo et al., 2009b) and SoP (Guyer et al., 2008). Additionally, many studies suggest increased activation of the ventrolateral prefrontal cortex (VLPFC) in pediatric patients with GAD (Monk et al., 2006; McClure et al., 2007a; Guyer et al., 2008; Beesdo et al., 2009; Strawn et al., 2012c) and SoP (Guyer et al., 2008) while some studies of pediatric patients with GAD have also noted increased activation of the anterior cingulate cortex (ACC) (McClure et al., 2007). Interestingly, in adolescents with GAD (and some co-morbid SoP or SAD), glutamatergic tone within the ACC is also linked to severity of anxiety symptoms (Strawn et al., 2013b) suggesting neurochemical and neurofunctional dysregulation within this key structure which integrates both attentional and emotional processing (Yamasaki et al., 2002). More recently, several studies have demonstrated altered functional connectivity among these structures in adolescents with GAD and mixed anxiety disorders (Strawn et al., 2012c; McClure et al., 2007; Monk et al., 2008). In short, these studies reveal altered functional connectivity between the amygdala and VLPFC in anxious youth (Strawn et al., 2012c; McClure et al., 2007; Monk et al., 2008).
While many studies in pediatric GAD, SoP and SAD have demonstrated functional abnormalities in central fear circuits, relatively few structural studies have examined these systems. In one study of adolescents with GAD (many of whom had other triad disorders), De Bellis and colleagues (2000) observed increased right amygdala volumes in adolescents with GAD (n=12) compared to healthy adolescents; however, in another cohort of anxious adolescents (n=17), left amygdala volumes were decreased compared to healthy comparison subjects (Milham et al., 2005). Additionally, a study of adolescents with GAD, using voxel-based morphometry (VBM), found increased gray matter in the superior temporal gyrus, a structure which shares numerous connections with the amygdala (De Bellis et al., 2000). Finally, two very recent studies have utilized VBM to evaluate gray matter volumes in anxious youth (Mueller et al., 2013) and in adolescents with a primary diagnosis of GAD (Strawn et al., 2013a). In the first, Mueller and colleagues (2013), using a region-of-interest (ROI) approach (ROIs: amygdala, hippocampus, insula and ACC), observed decreased gray matter volumes in adolescents with anxiety in the amygdala and right anterior hippocampus and noted increased gray matter volume in the right insula. In this study, a group-by-genotype effect for a Val66Met polymorphism in the brain-derived neurotropic factor gene was observed in the insula and ACC (Mueller et al., 2013). In another recent study of adolescents with GAD, in which we did not restrict our analyses to specific ROIs, we observed that, compared to healthy adolescents, youth with GAD had increased gray matter volumes in the right precuneus and right precentral gyrus and decreased gray matter volumes in the left orbital gyrus and posterior cingulate. However, we did not observe structural differences in the amygdala (Strawn et al., 2013b).
Given the prevalence and morbidity associated with the triad anxiety disorders in youth and rapidly accumulating data regarding the functional neurocircuitry of these conditions, understanding their structural basis may clarify potential pathogenic mechanisms and may inform the development of more effective or perhaps novel treatments (Strawn et al., 2012a). With this in mind, in the present study, gray matter volumes were compared between children and adolescents with GAD, SAD and SoP and healthy comparison subjects using VBM (Ashburner and Friston, 2000). Of note, the current cohort represents a completely independent sample from the GAD and healthy control groups previously reported (Strawn et al., 2013b). We hypothesized that structural abnormalities would be present in the amygdala and in regions that functionally modulate the amygdala (e.g. ACC, ventrolateral prefrontal cortex and medial prefrontal cortex).
2. Materials and methods
2.1 Participants
Participants included thirty eight children and adolescents, aged 7–19 years of age (mean age 14.4 ± 3.0 years; 28 female) with a DSM-IV primary diagnosis of GAD, SoP, and/or SAD and 27 matched healthy controls (14.8 ± 3.9 years; 15 female). There is no overlap in the current sample with the sample previously investigated by Strawn and colleagues (Strawn et al., 2013b). All patients and controls were medication-free at the time of testing, and were recruited via referrals from the University of Michigan Pediatric Anxiety Disorders Clinic and from advertisements posted in the local community. Study participants were administered the Kiddie-Schedule for Affective Disorders-Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997) by masters level, clinical social workers and diagnoses were confirmed by a board-certified psychiatrist (KLP). Additionally, anxiety symptom severity was assessed with the Multidimensional Anxiety Scale for Children (MASC, March et al., 1997) and the Pediatric Anxiety Rating Scale (PARS, RUPP, 2002); social anxiety symptoms were assessed with the Liebowitz Social Anxiety Scale-Child and Adolescent Version (LSAS-CA, Masia-Warner, 2003) and depressive symptoms were evaluated with the Children’s Depression Inventory (CDI, Kovacs, 1985). Exclusionary criteria for patient participants were: an IQ < 70, a lifetime diagnosis of bipolar disorder, schizophrenia, or a pervasive developmental disorder, and current diagnosis of major depressive disorder. Healthy comparison subjects were free of lifetime diagnosis of DSM-IV Axis I disorders. Legal guardians and participants provided written, informed consent and assent, respectively and this study was approved by the University of Michigan Institutional Review Board.
2.2 Image acquisition and analysis
A 3.0 T GE Signa Scanner (General Electric; Milwaukee, Wisconsin, USA) with a GE quad head coil was used to acquire high resolution, T1-weighted volumetric anatomical scans (3D spoiled-gradient echo sequence, 9 ms repetition time, 1.8 ms echo time, 500 ms inversion time, 15 degree flip angle, 256 × 256 matrix, 256 mm field of view; 124 slices, 1.2mm slice thickness). Whole-brain structural data were processed using the VBM toolbox (http://dbm.neuro.uni-jena.de/vbm/) in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) running on the MATLAB (Math Works, Natick, MA) platform. A customized tissue probability map was generated with the Template-O-Matic (TOM8) Toolbox (Wilke et al., 2008) using the matched-pairs approach to accurately reflect the specific brain morphometry for the age and gender of the pediatric population in this study. The anterior commissure was identified in each image and images were uniformly aligned to provide optimal starting estimates for subsequent spatial normalization. T1-weighted images were spatially normalized and segmented into gray matter, white matter, and cerebrospinal fluid (CSF) according to the unified segmentation model. Segmented images were resampled to a 1.5 × 1.5 × 1.5 mm resolution. Voxel values from the gray matter and white matter images were subsequently multiplied by the Jacobian determinants of the normalization matrix to produce modulated data accounting for global and regional differences in the absolute amount (volume) of gray matter (Ashburner and Friston, 2000). To ensure data quality, orientations of the native images were inspected, guided by boxplots and covariance matrices from the VBM8 toolbox, following methods previously published (Hajek et al., 2013). Finally, the modulated images were smoothed with a 10 mm Gaussian kernel of full width at half-maximum to create a local weighted average of the surrounding pixels.
Statistical analysis of processed gray matter images was carried out by means of an independent samples t-test. We applied a statistical threshold of p < .005 with a minimum cluster size of 60 voxels (equivalent to a 200 voxel cluster size when sampled at 1 × 1 × 1 mm) as previously applied in our prior independent study of gray matter voxel-based morphometry in a distinct, non-overlapping study of pediatric patients GAD (Strawn et al., 2013a) and in prior voxel-based morphometry studies of pediatric patients with mood disorders (Lisy et al, 2011; Adleman et al, 2012). Based on prior voxel-based morphometry studies of anxious youth, which have revealed large effect sizes and our sample size, this study was powered to detect large effect sizes with 78% power. Gray matter values from all anxious participants were extracted using 10-mm diameter spherical volumes of interest surrounding peak voxels exhibiting group differences (AD>HC or HC>AD) and correlated with MASC, PARS, and CDI scores in order to investigate associations, if any, between gray matter volumes and symptom severity. Additionally, given that prior studies of anxious youth suggest that the alteration of gray matter volumes in some regions may be influenced by the presence of SoP (Mueller et al., 2013), a post-hoc comparison of anxious patients and healthy controls which excluded those patients with a pure SoP was completed. This post-hoc analysis was augmented by a regression analysis of gray matter volumes with social anxiety symptom severity as reflected by the LSAS-CA score in the anxious youth.
Finally, to facilitate comparison of these data with previously reported ROI-based studies of the amygdala in anxious children and adolescents (De Bellis et al., 2000; Milham et al., 2005; Mueller et al., 2013), gray matter volumes were extracted from Anatomical Automatic Labeling (AAL) system for the amygdala ROI (Tzourio-Mazoyer et al., 2002) and corrected for multiple comparisons using small-volume correction with a Gaussian random field threshold at p < .05 and corrected for at least 10 contiguous voxels as previously described (Mueller et al., 2013). Amygdala gray matter volumes were then compared between patients with anxiety disorders and healthy comparison subjects using independent samples t-tests.
3. Results
3.1 Demographics
No group differences were observed for sex, age or race, although, symptom scores were significantly higher in the anxious patients than healthy comparison subjects for both anxiety symptoms inventories and also for the CDI (Table 1)
Table 1.
Demographic and clinical characteristics of the sample
| Anxiety patients (n = 38) |
Healthy comparison subjects (n =27) |
p | |
|---|---|---|---|
| Sex, female, n (%) | 28 (74) | 15 (56) | |
| Age (years, mean ± SD) | 14.4 ± 3.0 | 14.8 ± 3.9 | 0.305 |
| Race | 31C, 5AA, 2Multi | 16C, 2AA, 1As, 4Multi | |
|
| |||
| Anxiety Diagnoses | |||
| Generalized anxiety | 29 | 0 | |
| disorder | |||
| Social phobia | 23 | 0 | |
| Separation anxiety disorder | 15 | 0 | |
| Specific phobia | 8 | 0 | |
| ADHD | 3 | 0 | |
|
| |||
| PARS (mean ± SD) | 21.7 ± 3.9 | 1.8 ± 2.5 | p<.001 |
| MASC T Score (mean ± SD) | 62.3 ± 11.6 | 41.4 ± 9.9 | p<.001 |
| CDI T score (mean ± SD) | 56.5 ± 11.2 | 39 ± 4.5 | p<.001 |
C, Caucasian; AA, African American; As, Asian; Multi, Multiracial; PARS, Pediatric Anxiety Rating Scale; MASC, Multidimensional Anxiety Scale for Children; CDI, Children’s Depression Inventory.
3.2 Gray matter volumes in anxious and healthy youth
In comparison to healthy subjects, youth with anxiety disorders exhibited greater gray matter volumes in the left cingulate gyrus compared to healthy subjects (Figure 1A, Table 2, Cohen’s d: 0.83). Additionally, patients with anxiety disorders (compared to healthy subjects) exhibited less gray matter volume in the left inferior frontal gyrus, left postcentral gyrus and precuneus (Figure 1B, Table 2, Cohen’s d: 0.74, 0.54 and 0.62). To elucidate any contribution of SoP symptoms to neuroanatomic differences in regions where gray matter volume differences were detected between anxious and healthy youth, gray matter volumes within these regions were correlated with LSAS-CA scores and no statistically significant correlations were observed in (1) ACC gray matter volumes (r = −.16, p = .33); (2) precuneus/cuneus gray matter volumes (r = −.14, p = .41) or (3) inferior frontal gyrus gray matter volumes (r = −.07, p = .68) in the anxious patients. Additionally, a post-hoc comparison of anxious patients and healthy comparison subjects which excluded those patients with pure SoP (n = 3) did not affect the differences in gray matter volumes that were previously observed between anxious patients and healthy comparison subjects in the ACC, precuneus/cuneus gray or inferior frontal gyrus.
Fig 1.
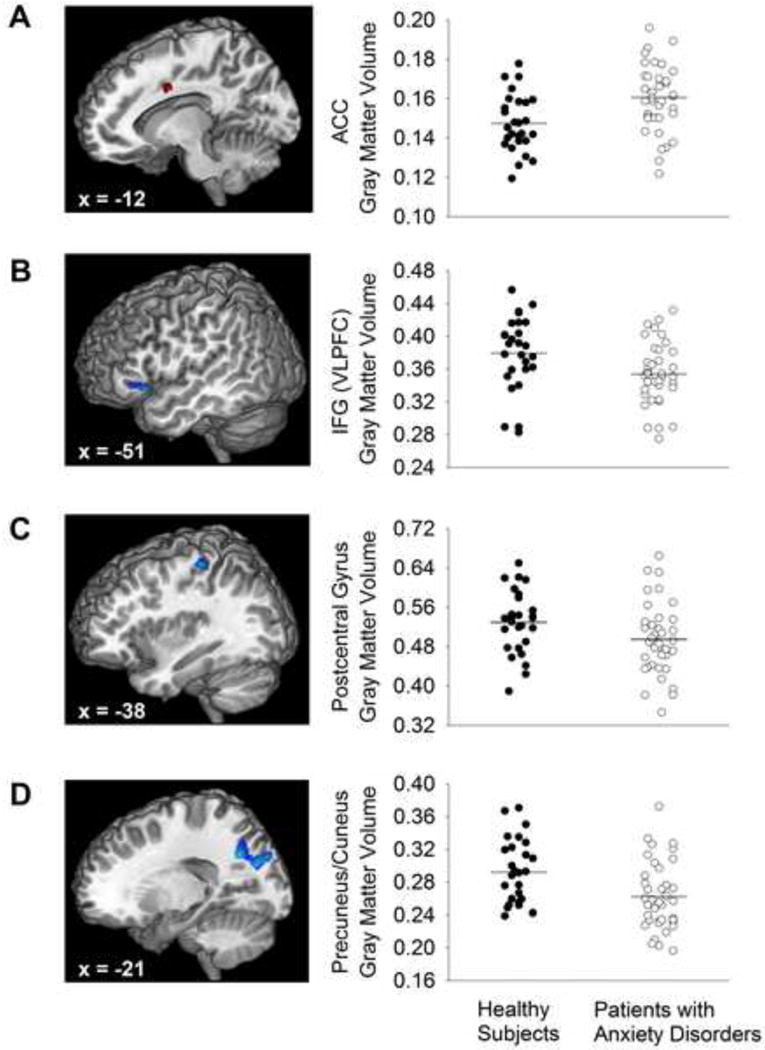
Compared to matched, healthy youth, patients with anxiety disorders had (A) increased gray matter volume in the dorsal anterior cingulate gyrus (ACC) and decreased gray matter volume in the (B) inferior frontal gyrus (IFG; ventrolateral prefrontal cortex, VLPFC), (C) postcentral gyrus, and (D) cuneus/precuneus. T-scores are represented by colors (positive [red]: AD>HC; negative [blue]: AD<HC). Additionally, scatter plots of the gray matter volumes for each structure/region with the mean gray matter volume is represented as a horizontal line.
TABLE 2.
Gray matter volume differences between patients with anxiety disorders (AD) and healthy comparison (HC) subjects: Whole-brain voxel-wise voxel-based morphormetry analysis
| Cluster Size | x | y | z | t value | p value | |
|---|---|---|---|---|---|---|
| AD > HC | ||||||
| Left cingulate gyrus | 79 | −15 | 3 | 36 | 3.4 | 0.001 |
|
| ||||||
| AD < HC | ||||||
| Left inferior frontal gyrus | 173 | −51 | 18 | −6 | −3.2 | 0.001 |
| Left cuneus/precuneus | 757 | −19.5 | −60 | 34.5 | −3.2 | 0.001 |
| Left postcentral gyrus | 94 | −38 | −29 | 59 | −3.1 | 0.002 |
Cluster size in number of contiguous voxels. Significance set at p < 0.005 (uncorrected) with clusters > 60 contiguous voxels.
3.3 Amygdala gray matter volumes in anxious and healthy youth
ROI-based analyses of the amygdala gray matter volumes demonstrated decreased gray matter volumes in the left amygdala (cluster size 83 voxels, p < 0.01) and in the right amygdala (cluster size 23 voxels, p < 0.04) in youth with anxiety compared to healthy subjects (Figure 2). Effect sizes for the right and left amygdala were 0.48 and 0.50, respectively.
Fig 2.
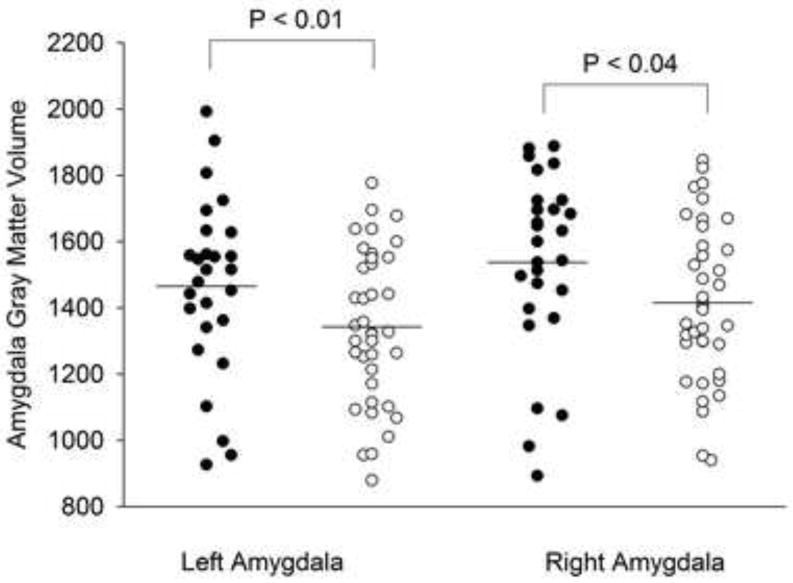
Gray matter volumes were reduced in the right (cluster size 23 voxels, p=0.04) and in the left amygdala (cluster size 83 voxels, p=0.01) in patients with anxiety (white circles) disorders compared to healthy youth (black circles). Mean gray matter volume is represented as a horizontal line.
3.4 Relationships between anxiety symptoms and gray matter volumes
No significant correlations were found within the anxious patients between gray matter volume of the three regions differentiating patients from healthy subjects with MASC (all rs < .12, all p values non-significant), PARS (all rs < .09, all p values non-significant), or CDI scores (all rs < 0.3, all p values non-significant).
4. Discussion
The neuroanatomic differences in gray matter volumes described herein, in children and adolescents with triad anxiety disorders, implicate a number of structures that subserve cognitive modulation of anxiety, generation of fear responses and threat perception, yet also replicate and extend the results of prior studies of separate, independent cohorts of anxious youth (Mueller et al., 2013; Strawn et al., 2013a). Compared to healthy subjects, children and adolescents with anxiety disorders had larger gray matter volumes in the dACC and had decreased gray matter volumes in the inferior frontal gyrus (VLPFC), postcentral gyrus, and cuneus/precuneus. In anxious youth, there was no relationship between the severity of anxiety symptoms (as measured by either the MASC or the PARS) and structural differences in these regions.
Our findings of increased gray matter volumes in the dACC replicate a recent VBM study of adolescents with triad anxiety disorders in which gray matter volumes in this region are increased in those anxious patients with a functional single nucleotide polymorphism (SNP), resulting in a Val66Met substation in brain-derived neurotrophic factor (BDNF) (Mueller et al., 2013). Interestingly, the structural aspects or functional responses of several brain structures implicated in anxiety disorders may be modulated by this SNP (Bueler et al., 2006; Egan et al., 2003), consistent with the observation that BDNF directs neuronal survival and migration (Huang and Reichardt, 2003). Additionally, data from adults with anxiety disorders suggest that the dACC coordinates the processing of conditioned fear responses (Milad et al., 2007) and, in adults with anxiety disorders, structure—function relationships have been observed for the dACC. In this regard, in anxious adults, cortical thickness positively correlates with conditioned fear responses and may play a pivotal role in the interpretation of these fear responses (Milad et al., 2007). Recently, Maier and colleagues (2012), attempted to further elucidate the role of the dACC in mediating the “conscious, negative appraisal of threat situations including, as an extreme variant catastrophizing” versus “fear learning” (Maier et al., 2012) and their findings suggest that the dACC is primarily involved in appraisal and to a lesser extent fear learning. This is both consistent with prior observations that the dACC is functionally activated during the processing of fear responses (Vogt et al., 2007) and is consistent with our data suggesting increased gray matter volumes in this region, in youth with anxiety disorders—conditions fundamentally characterized by dysfunction in threat appraisal.
In the youth with anxiety disorders, we also observed decreased gray matter volumes in the inferior frontal gyrus (VLPFC), a finding consistent with one prior VBM study of children and adolescents with anxiety disorders in which subthreshold decreases in this region were noted (Milham et al., 2005). This finding is noteworthy in that the VLPFC subserves a number of regulatory functions, including modulation of amygdala activity (Monk et al., 2008) and responds in tandem with the amygdala to emotional probes (Blair et al., 2008). Moreover, the VLPFC is responsible for voluntary aspects of affect regulation (Phillips et al., 2008) and, in lower animals, is responsible for extinction processes during fear conditioning paradigms (Tian et al., 2011) and is consistently hyperactivated in fMRI studies of pediatric and adult patients with anxiety disorders (reviewed in Etkin and Wagner, 2007). The VLPFC has also received attention in pediatric patients with anxiety disorders with regard to its putative compensatory role insofar as three studies of anxious adolescents have observed an inverse relationship between activation of the VLPFC and the severity of anxiety symptoms (Monk et al., 2006; Monk et al., 2008; Strawn et al., 2012c). Also, consistent with the notion that this region plays a compensatory function in the central fear circuit are findings of treatment-associated increases in activation of the VLPFC in both adults and youth. For example, treatment with the SSRI paroxetine is associated with increases in metabolic activity in this region in adults with anxiety disorders (Sim et al., 2010) and CBT in adults with anxiety disorders results in increased connectivity between the inferior frontal gyrus and the amygdala as well as the anterior cingulate cortex (Kircher et al., 2013). Additionally, one small study of adolescents with GAD revealed that successful treatment with either the SSRI fluoxetine or CBT was associated with increases in functional activation within the VLPFC (Maslowsky et al., 2010). Thus, our findings of decreased gray matter volumes in the VLPFC in pediatric anxiety disorders parallel abnormal basal and task-related functional activation as well as treatment-associated increases in activation in youth and adults with anxiety disorders and suggest a neurostructural basis for these neurofunctional phenomena. Nonetheless, it remains to be determined whether the structural abnormalities in the VLPFC observed in the present study represent a risk factor for pediatric anxiety disorders or a marker of disease progression.
Our findings of decreased gray matter volumes in the precuneus/cuneus are consistent with prior VBM studies of adolescents with anxiety disorders in which subthreshold decreases in gray matter volume in the precuneus were noted (Milam et al., 2005) and are also consistent with findings in our prior VBM study of a separate cohort of youth with GAD (Strawn et al., 2013a) in which gray matter volumes were decreased in the posterior cingulate, an area which is structurally contiguous with the precuneus/cuneus. The precuneus is involved in reflective, self-related processing (Kjaer et al., 2002, Lou et al., 2004), awareness of self versus other and conscious informational processing (Kjaer et al., 2001, Vogt and Laureys, 2005) and is implicated in the processing of episodic memory (Dorfel et al., 2009, Lundstrom et al., 2005, Lundstrom et al., 2003) and anxious attachment (Suslow et al., 2009). Thus, it is not surprising that we would observe structural deficits in this region in pediatric patients with anxiety disorders. Additionally, our findings in this sample of children and adolescents with triad anxiety disorders complement our prior functional connectivity findings in adolescents with GAD (of whom several had co-morbid SAD and SoP), in which functional connectivity between the amygdala and precuneus/cuneus was decreased, compared to healthy comparison subjects (Strawn et al., 2012c) and are consistent with observations of decreased activity in this region in youth who are at risk for anxiety disorders (e.g. those with behavioral inhibition) in response to reward uncertainty (Bar-Haim et al., 2008).
It is noteworthy that in this large sample of anxious youth, our post-hoc ROI analysis of the amygdala gray matter volumes revealed decreased volumes in anxious patients compared to healthy participants, consistent with a ROI-based VBM study of pediatric triad disorders in whcih decreased amygdala volumes were observed in anxious patients compared to controls (Mueller et al., 2013). Prior smaller studies of mixed anxiety disorder samples (i.e., triad anxiety disorders) have also observed reduced amygdala volumes relative to comparison subjects (Milham et al., 2005). However, findings regarding the amygdala have been inconsistent in pediatric anxiety disorders with 1 study of pediatric patients with GAD (of whom many had other triad disorders), having reported right and total amygdala volumes were larger in anxious patients (DeBellis et al., 2000) and a prior VBM analysis of youth with GAD—which did not include an ROI-approach—failed to detect differences in amygdala gray matter (Strawn et al., 2013a). Additionally, with regard to accumulating data suggesting structural differences in the amygdala in anxious youth, it is worth mentioning that most (McClure et al., 2007; Beesdo et al., 2009; Guyer et al., 2008; Monk et al., 2008) but not all (Strawn et al., 2012c) studies of children and adolescents with anxiety disorders have demonstrated functional hyperactivity of this region. Also, fMRI studies of individuals who were characterized as having behavioral inhibition early in life (i.e., are at risk for developing anxiety disorders), have observed increased threat-associated fronto-amygdala connectivity compared to non-behaviorally inhibited young adults suggesting that this behavioral risk factor for anxiety disorders “predicts differences as young adults in threat and attention-related fronto-amygdala connectivity….while connectivity strength, in turn, moderates the relation[ship] between early behavioral inhibition” and the development of anxiety disorders (Hardee et al., 2013). Last, “lesion studies” involving patients with Urbach-Wiethe disease (lipoid proteinosis), which is associated with amygdala destruction, demonstrate that affected patients exhibit accentuated fear responses and threat vigilance (Terburg et al., 2012). Certainly, given functional findings in youth with triad anxiety disorders as well as youth who are at risk for anxiety disorders, it will be important to determine whether these structural abnormalities in the amygdala described herein represent a risk factor for pediatric anxiety disorders, a marker of disease progression or potentially, a biomarker of treatment response. While this study adds significantly to the extant and developing literature concerning the neurostructural basis of anxiety in pediatric patients with triad anxiety disorders and represents one of the largest VBM studies of pediatric anxiety disorders, there are several important limitations. First, there are inherent limitations of VBM, including the fact that precision may be reduced by the smoothing process, thus attenuating regional volumetric differences which, as a consequence, may not be well localized. Additionally, as we noted with our prior VBM study of youth with anxiety disorders (Strawn et al., 2013a), “specific anatomic patterns could result in group-specific misregistration and thus, VBM may be more sensitive to misregistrated shape differences from the spatial normalization step than alternative, manual approaches” (Strawn et al., 2013a; Mechelli et al., 2005). Second, VBM analyses have frequently relied on cluster size permutation for cluster size estimates and these estimates may be influenced by intrinsic attributes of the data or processing techniques (e.g., resolution, smoothing kernel, minimum cluster level). Moreover, the matrix sizes of typical structural analyses preclude typical “multiple comparison” approaches utilized in functional neuroimaging data (e.g. Monte Carlo based approaches) and thus, we have erred on the side of prior VBM analyses for our cluster-size specific thresholds in the current analysis. Third, in this study, we have not evaluated the potential genetic factors which may have contributed the regional differences between anxious patients and healthy comparison subjects. Importantly, BDNF-driven structural differences in these regions may be modulated by the Val66Met substation described above and it has been suggested that some of the “inconsistency in [structural] findings [in pediatric anxiety disorders] can be related not only to unstable data in small samples, but also to genetic modulation” (Mueller et al., 2013). Fourth, although we have suggested functional interpretations of the structural abnormalities observed in these anxious youth, additional work is needed to elucidate the relationship between structural abnormalities, deficits of emotion regulation and functional engagement of emotion processing circuits. In this regard, we did not observe a significant relationship between neurostructural abnormalities and symptom severity. This lack of a structure-function relationship could be the result of type II error or may relate to an association between brain structure and clinical symptom expression that is mediated by abnormalities in psychological processes such as the regulation of emotion. Certainly, combined VBM–fMRI studies will help to determine the extent to which these structural abnormalities are associated with neurofunctional deficits.
These results provide an exciting structural brain framework for future studies aimed at understanding the functional neurophysiology and neurochemistry of these structures in youth with triad anxiety disorders. Moreover, these finding of neurostructural abnormalities in youth close to the onset of their illness, set the stage for structural and functional neuroimaging studies of youth at high risk for anxiety disorders, such as patients with a family history of anxiety disorders, or youth with psychological risk factors for the development of an anxiety disorder such as behavioral inhibition. As such, if the structural abnormalities described herein are present before the onset of illness, they might serve as neurostructural biomarkers for the development of anxiety disorders and thus may inform primary prevention strategies for these conditions. Such neurobiologically-informed primary prevention strategies could not only reduce the morbidity associated with the anxiety disorders in children and adolescents, but could circumvent the development of secondary disorders (e.g., major depressive disorder, substance use disorders) and their sequelae (e.g., suicidality).
5. Conclusions
In this study, anxious youth demonstrated neuroanatomic differences in gray matter volumes in an ensemble of structures which have been functionally implicated in the neuropathophysiology of anxiety disorders (e.g. dorsal ACC [dACC], inferior frontal gyrus, postcentral gyrus, and precuneus) and structures which—because of their known roles—are central to the psychpathophysiology of anxiety, in terms of generating fear responses and appraising threat. Additionally, our ROI-based analyses suggest decreased gray matter volumes in the amygdala of youth with anxiety disorders compared to healthy subjects. Importantly, these structures have been implicated in fMRI studies of anxious adolescents or adults and, in the case of the non-amygdala structures, share extensive connections with the amygdala.
Highlights.
The neuroanatomy of fear and threat processing systems in youth is largely unknown.
Neurostructural studies of pediatric anxiety may improve risk detection.
Anterior cingulate gray matter volumes are increased in anxious youth.
In anxious youth, gray matter volumes in the inferior frontal gyrus, cuneus/precuneus and amygdala are decreased.
Acknowledgments
Funding
This research was supported by the National Institute of Mental Health grant R01MH086517 (to KLP and CSM, Co-PIs).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors do not believe there to be any real or potential conflicts of interest.
References
- Adleman NE, Fromm SJ, Razdan V, et al. Cross-sectional and longitudinal abnormalities in brain structure in children with severe mood dysregulation or bipolar disorder. J Child Psychol Psychiatry. 2012;11:1149–56. doi: 10.1111/j.1469-7610.2012.02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J. The convergence of maturational change and structural covariance in human cortical networks. Journal of Neuroscience. 2013;33:2889–2899. doi: 10.1523/JNEUROSCI.3554-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol Sci. 2009;20:1009–18. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr Clin N Am. 2009a;32:483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Lau JYF, Guyer AE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009b;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Pine DS, Lieb R, Wittchen HU. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry. 2010;67:47–57. doi: 10.1001/archgenpsychiatry.2009.177. [DOI] [PubMed] [Google Scholar]
- Beesdo-Baum K, Pine DS, Lieb R, Wittchen HU. Mental disorders in adolescence and young adulthood: homotypic and heterotypic longitudinal associations. Annual Meeting of the American College of Neuropsychopharmacology; Hollywood, Fl. 2012a. [Google Scholar]
- Beesdo-Baum K, Knappe S. Developmental epidemiology of anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012b;21:457–78. doi: 10.1016/j.chc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Pine DS. Neural Substrates of Childhood Anxiety Disorders A Review of Neuroimaging Findings. Child Adolesc Psychiatr Clin N Am. 2012;21:501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Shaywitz J, Smith BW, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, Horwood LJ. Anxiety disorders and suicidal behaviours in adolescence and young adulthood: findings from a longitudinal study. Psychological Medicine. 2007;37:431–40. doi: 10.1017/S0033291706009147. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Angold A, Burns B, et al. The great smoky mountains study of youth:goals, design, methods and the prevalence of DSM-III-R disorders. Archives of General Psychiatry. 1996;53:1129–1136. doi: 10.1001/archpsyc.1996.01830120067012. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, et al. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2002;51:553–62. doi: 10.1016/s0006-3223(01)01375-0. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. Journal of Comparative Neurology. 1996;366:223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65:1303–12. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek T, Cullis J, Novak T, et al. Brain structural signature of familial predisposition for bipolar disorder: replicable evidence for involvement of the right inferior frontal gyrus. Biological Psychiatry. 2013;73:144–152. doi: 10.1016/j.biopsych.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee JE, Benson BE, Bar-Haim Y, Mogg K, et al. Patterns of neural connectivity during an attention bias task moderates associations between early childhood temperament and internalizing symptoms in young adulthood. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.01.036. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husky MM, Olfson M, He JP, et al. Twelve-month suicidal symptoms and use of services among adolescents: results from the National Comorbidity Survey. Psychiatr Serv. 2012;63:989–96. doi: 10.1176/appi.ps.201200058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall PC, Compton SN, Walkup JT, et al. Clinical characteristics of anxiety disordered youth. Journal of Anxiety Disorders. 2010;24:360–365. doi: 10.1016/j.janxdis.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Costello EJ, et al. Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69:372–80. doi: 10.1001/archgenpsychiatry.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher T, Arolt V, Jansen A, et al. Effect of cognitive-behavioral therapy on neural correlates of fear conditioning in panic disorder. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.07.026. (in press) [DOI] [PubMed] [Google Scholar]
- Kovacs M. The children’s depression inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Liebowitz MR. Social Phobia. Mod Probl Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Lisy ME, Jarvis KB, Delbello MP, et al. Progressive neurostructural changes in adolescent and adult patients with bipolar disorder. Bipolar Disorders. 2011;13:396–405. doi: 10.1111/j.1399-5618.2011.00927.x. [DOI] [PubMed] [Google Scholar]
- Maier S, Szalkowski A, Kamphausen S, et al. Clarifying the role of the rostral dmPFC/dACC in fear/anxiety: learning, appraisal or expression? PLoS One. 2012;7:e50120. doi: 10.1371/journal.pone.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Curr Med Imag Rev. 2005;1:1–9. [Google Scholar]
- Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the national comorbidity survey replication-adolescent supplement (NCS-A) Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:554–65. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Masia-Warner C, Storch EA, Pincus DB, Klein RG, Heimberg RG, Liebowitz MR. The Liebowitz social anxiety scale for children and adolescents: an initial psychometric investigation. J Am Acad Child Adolesc Psychiatry. 2003;42:1076–84. doi: 10.1097/01.CHI.0000070249.24125.89. [DOI] [PubMed] [Google Scholar]
- Mickley Steinmetz KR, Addis DR, Kensinger EA. The effect of arousal on the emotional memory network depends on valence. Neuroimage. 2010;15:318–24. doi: 10.1016/j.neuroimage.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol Psychiatry. 2005;57(9):961–6. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Aouidad A, Gorodetsky E, et al. Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val66Met Polymorphism? Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:184–195. doi: 10.1016/j.jaac.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Research Units on Pediatric Psychopharmacology Anxiety Study Group. The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Sim HB, Kang EH, Yu BH. Changes in Cerebral Cortex and Limbic Brain Functions after Short-Term Paroxetine Treatment in Panic Disorder: An [F]FDG-PET Pilot Study. Psychiatry Investig. 2010;7:215–219. doi: 10.4306/pi.2010.7.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Wehry AM, Chu WJ, et al. Neuroanatomic abnormalities in adolescents with generalized anxiety disorder: a voxel-based morphometry study. Depression and Anxiety. 2013a doi: 10.1002/da.22089. (in press) [DOI] [PubMed] [Google Scholar]
- Strawn JR, Chu WJ, Whitsel RM, Weber WA, et al. A pilot study of anterior cingulate cortex neurochemistry in adolescents with generalized anxiety disorder. Neuropsychobiology. 2013b;67:224–229. doi: 10.1159/000347090. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child & Adolescent Psychiatric Clinics of North America. 2012a;21:527–39. doi: 10.1016/j.chc.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Wehry AM, DelBello MP, et al. Establishing the neurobiologic basis of treatment in children and adolescents with generalized anxiety disorder. Depression & Anxiety. 2012b;29:328–39. doi: 10.1002/da.21913. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Bitter SM, Weber WA, et al. Neurocircuitry of generalized anxiety disorder in adolescents: a pilot functional neuroimaging and functional connectivity study. Depression & Anxiety. 2012c;29:939–47. doi: 10.1002/da.21961. [DOI] [PubMed] [Google Scholar]
- Suslow T, Kugel H, Rauch AV, et al. Attachment avoidance modulates neural response to masked facial emotion. HumBrain Mapp. 2009;30:3553–3562. doi: 10.1002/hbm.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburg D, Morgan BE, Montoya ER, Hooge IT. Hypervigilance for fear after basolateral amygdala damage in humans. Transl Psychiatry. 2012;2:e115. doi: 10.1038/tp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99:11447–51. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31:18578–89. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: A toolbox for creating customized pediatric templates. Neuroimage. 2008;41:903–913. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]


