Abstract
Background
Early studies showed beneficial effects of phosphodiesterase 5 inhibitors (PDE5i) on cardiovascular function in heart failure (HF) patients, but the RELAX trial observed no improvement in exercise capacity with sildenafil treatment in subjects with HF and preserved ejection fraction (HFpEF).
Methods and Results
A subgroup of participants in the RELAX trial (n=48) underwent comprehensive noninvasive cardiovascular assessment before and after treatment with sildenafil or placebo in a prospective ancillary study. Left ventricular (LV) contractility was assessed by peak power index (PWR/EDV) and stroke work index (SW/EDV). Systemic arterial load was assessed by arterial elastance (Ea) and right ventricular afterload by pulmonary artery systolic pressure (PASP). Endothelial function was assessed by reactive hyperemia index (RHI) following upper arm cuff occlusion. Compared to placebo (n=25), sildenafil (n=23) decreased Ea (−0.29±0.28mmHg/ml vs +0.02±0.29, p=0.008) and tended to improve RHI (+0.30±0.45 vs −0.17±0.30, p=0.054). In contrast, LV contractility was reduced by 11–16% with sildenafil compared to placebo (ΔPWR/EDV −52±70 vs +0±40 mmHg/s, p=0.006; ΔSW/EDV +0.3±5.8 vs −6.0±5.1 mmHg, p=0.04). Sildenafil had no effect on PASP.
Conclusions
In subjects with HFpEF, sildenafil displayed opposing effects on ventricular and vascular function. We speculate that beneficial effects of PDE5i in the systemic vasculature and endothelium were insufficient to improve clinical status, or that the deleterious effects on left ventricular function offset any salutary vascular effects, contributing to the absence of benefit observed with sildenafil in subjects with HFpEF in the RELAX trial.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00094302.
Keywords: heart failure, diastolic heart failure, phosphodiesterase inhibitor heart failure, ventricular function, vascular function
Nearly half of people with heart failure (HF) have a preserved ejection fraction (HFpEF).1 The pathophysiology of HFpEF is complex, caused by multiple impairments in ventricular diastolic, systolic, chronotropic, endothelial, vascular and peripheral functions.2–8 Trials testing neurohormonal antagonists in HFpEF have produced neutral results to date, and novel HF therapies that target multiple ventricular-vascular domains may be more effective in this HF phenotype.9
Numerous lines of evidence point to abnormalities in nitric oxide-cyclic guanosine monophosphate (NO-cGMP) signaling as playing a key role in the pathophysiology of HFpEF.2, 10, 11 Phosphodiesterase 5 inhibitors (PDE5i) enhance cGMP by decreasing its degradation. Studies in HF with reduced EF (HFrEF) have reported beneficial effects from PDE5i on ventricular structure, function and exercise capacity,12–15 and a small, single center trial in HFpEF reported marked improvements in hemodynamics, ventricular function, and gas exchange with sildenafil therapy in HFpEF subjects with pulmonary hypertension and right ventricular dysfunction.16
The RELAX trial tested the hypothesis that the PDE5i sildenafil would improve exercise capacity and clinical status in people with HFpEF but observed no benefit compared to placebo.17 In order to better understand the effects of sildenafil on cardiovascular function in people with HFpEF, we performed a prospective RELAX ancillary study comprehensively examining ventricular, vascular function and endothelial function at rest and during exercise in participants enrolled in the parent trial.
Methods
RELAX was a multicenter randomized (1:1) placebo-controlled trial testing the impact of chronic PDE5i with sildenafil on exercise capacity in patients with HFpEF.17 The trial was conducted by the Heart Failure Clinical Research Network and funded by the National Heart, Lung, and Blood Institute. This prospective ancillary study was approved by the institutional review board and enrolled subjects participating in the parent study who provided separate written informed consent. Additional details on the rationale, study design and trial results for the RELAX trial have been published.17, 18
Study Participants
Consecutive HFpEF subjects participating at the Mayo Clinic site meeting the following criteria were enrolled: LVEF≥50%, peak oxygen consumption (peak VO2) ≤60% predicted, and evidence for pulmonary venous hypertension (elevated pulmonary capillary wedge pressure or NT-proBNP≥400 pg/mL). Detailed inclusion and exclusion criteria have been published.17, 18 Subjects with irregular heart rhythm at enrollment were excluded from this ancillary study.
Study Protocol
At the first visit, prior to administration of study drug, a comprehensive resting echocardiogram was performed in the upright position to derive baseline measures of ventricular systolic, diastolic and vascular function. Arterial tonometry was performed to characterize central aortic waveforms. Endothelium-dependent vasodilation was assessed by digital tonometry. Brachial arterial blood pressure (BP) was determined by auscultation. Heart rate (HR) was determined by 12 lead electrocardiography continuously during the test. After resting assessments, subjects underwent maximal effort upright cycle exercise testing with gas exchange analysis. Echocardiographic measurements and BP assessment were repeated at low level exercise (20 Watts) and again at peak exercise. Subjects then repeated this protocol after 12 weeks of treatment with sildenafil 20 mg tid or placebo, and again after 24 weeks of sildenafil titrated to 60 mg tid or placebo. All outcome measures were analyzed offline by persons blinded to study drug allocation and time of study (baseline, 12 weeks, and 24 weeks).
Echocardiographic Assessment
All echocardiographic studies were recorded to optical disc and interpreted offline in a blinded fashion at the Mayo clinic core laboratory. Resting LV end systolic and end diastolic volumes (ESV, EDV), mass and EF were determined from biplane 2D echocardiography by trained cardiovascular sonographers according to current guidelines using the mean of ≥3 separate beats.19 Stroke volume (SV) was determined from the LV outflow pulse wave Doppler spectrum.20 Cardiac output (CO) was calculated as the product of SV and HR. Left ventricular contractility was assessed using load-independent indices as previously described: LV peak power index (PWR/EDV), determined as the product of peak LV systolic flow velocity and systolic BP divided by EDV, and LV stroke work index (SW/EDV), determined as the product of mean BP and SV divided by EDV.2, 20–22 LV diastolic function was assessed by transmitral E and A flow velocities, mitral annular tissue velocity averaged from the medial and lateral annuli (E′), and the E/E′ ratio.23 Pulmonary arterial systolic pressure (PASP) was estimated by the modified Bernoulli equation assuming a right atrial pressure of 10mmHg.19
Vascular assessment
Radial applanation tonometry (Millar Instruments) was performed at rest to derive central aortic BP waveforms (SphygmoCor device, Atcor Medical) as previously described.24 Arterial stiffness and wave reflection were quantified by the augmentation index (cAIx), defined by the ratio of central augmented pressure to central pulse pressure (cPP). Effective arterial elastance (Ea) was calculated as 0.9*cSBP/SV, systemic vascular resistance (SVR) as cMBP*80/CO, and total arterial compliance (TAC) as SV/cPP.2
Endothelial function was assessed by reactive hyperemic (RH) change in digital blood flow in response to upper arm cuff occlusion (EndoPAT 2000, Itamar-Medical, Caesarea, Israel) as previously described.2 Briefly, after 5 min of baseline recording, a BP cuff was inflated to supra-systolic pressure in the test arm for 5 minutes. The cuff was then rapidly deflated with peripheral arterial tonometry (PAT) tracings recorded in the test and control arms. The RH index (RHI) was determined as the PAT ratio between 60 seconds and 120 seconds after occlusion in the test arm relative to the control arm, divided by the respective values for the 140 seconds prior to cuff inflation. Endothelial dysfunction was defined categorically by RHI<2.0.2
Exercise Protocol
Details of the RELAX cardiopulmonary exercise test (CPXT) protocol and HFN CPXT core laboratory (Massachusetts General Hospital, Boston, MA) methodologies have been reported.18 Symptom-limited CPXT with simultaneous expired gas analysis was performed on an upright cycle ergometer during maximal effort exercise testing to derive peak oxygen consumption (peak VO2) and respiratory exchange ratio (RER).
Statistical Analysis
Data are presented as mean±SD, median (25th–75th percentiles), and number (%). Baseline characteristics were compared using t test, Wilcoxon rank-sum test, or χ2 test. Baseline corrected parameters were compared in the sildenafil and placebo groups using a generalized linear model for changes at 12 weeks relative to baseline and 24 weeks relative to baseline. Analyses were performed using SAS version 9.2; P<0.05 (2-sided) was considered statistically significant.
Results
Of the 216 subjects enrolled in RELAX, 48 (22%) participated in this prospective ancillary study, 25 randomized to placebo and 23 to sildenafil. Similar to the parent trial, subjects were older-aged with high prevalence of comorbidities including hypertension, diabetes, obesity, and coronary disease (Table 1). Plasma NT-proBNP levels were similarly elevated in subjects randomized to placebo and sildenafil. There were no statistically significant baseline differences in age, sex, HF severity, comorbidities, exam findings, laboratories or medications.
Table 1.
Baseline Characteristics
| Placebo (n=25) | Sildenafil (n=23) | p | |
|---|---|---|---|
| Clinical Characteristics | |||
| Age (years) | 71 (65, 77) | 69 (62, 72) | 0.2 |
| Female (%) | 56 | 61 | 0.7 |
| Body Mass Index (kg/m2) | 31.2 (28.4, 33.3) | 29.9 (27.4, 38.4) | 0.97 |
| NYHA class II/III (%) | 44/56 | 43/57 | 0.97 |
| Hypertension (%) | 84 | 74 | 0.4 |
| Diabetes (%) | 32 | 39 | 0.6 |
| Obese (%) | 64 | 48 | 0.3 |
| Coronary artery disease (%) | 32 | 35 | 0.8 |
| Atrial fibrillation/flutter history (%) | 24 | 30 | 0.6 |
| Exam | |||
| Jugular venous pressure (%) | 0.4 | ||
| < 8 cm | 48 | 61 | |
| 8–12 cm | 28 | 26 | |
| 13–16 cm | 20 | 4 | |
| > 16 cm | 4 | 9 | |
| Peripheral Edema (%) | 0.2 | ||
| None | 40 | 57 | |
| Trace | 44 | 35 | |
| Moderate | 16 | 9 | |
| Severe | 0 | 0 | |
| Laboratories | |||
| Hemoglobin (gm/dL) | 13.3 (12.3, 13.7) | 13.1 (12.4, 14.4) | 0.4 |
| Creatinine (mg/dL) | 1.0 (0.9, 1.1) | 1.0 (0.8, 1.3) | 0.7 |
| NT-proBNP (pg/mL) | 435 (132,618) | 353 (76,788) | 0.5 |
| Medications | |||
| ACEI/ARB (%) | 72 | 61 | 0.4 |
| Beta Blocker (%) | 80 | 74 | 0.6 |
| Diuretic (%) | 68 | 65 | 0.8 |
Subjects displayed well-controlled BP, normal LV chamber size, LV diastolic dysfunction (low E′, elevated E/e′ and E/A ratios), increased arterial stiffness (median cAIx 24%) and normal PASP (median 29 mmHg, Table 2). Endothelium-dependent vasodilation was assessed in 33 subjects (19 randomized to placebo, 14 sildenafil) and endothelial dysfunction (ED, RHI<2.0) was observed in 58%. There were no statistically significant differences in baseline ventricular-vascular structure and function, endothelial function, arterial tonometry or exercise capacity between subjects randomized to sildenafil vs placebo (Table 2).
Table 2.
Baseline Cardiovascular Structure and Function
| Placebo (n=25) | Sildenafil (n=23) | p | |
|---|---|---|---|
| Heart rate (bpm) | 61 (56, 75) | 62 (58, 75) | 0.8 |
| SBP (mmHg) | 122 (108, 128) | 118 (110, 128) | 0.8 |
| DBP (mmHg) | 64 (62, 70) | 70 (62, 74) | 0.15 |
| LV structure and function | |||
| EDV (ml, n=23/21) | 126 (113, 137) | 114 (101, 128) | 0.18 |
| LV mass (gm, n=22/18) | 167 (153, 225) | 167 (123, 207) | 0.4 |
| E velocity (cm/s, n=25/22) | 70 (60, 100) | 70 (60, 90) | 0.6 |
| DT (ms, n=24/22) | 215 (202, 251) | 221 (190, 251) | 0.8 |
| E/A ratio (n=23/20) | 1.0 (0.8, 2.1) | 1.1 (0.8, 1.3) | 0.7 |
| E′ (cm/s) | 6.5 (5.5, 7.0) | 6.0 (5.0, 6.5) | 0.2 |
| E/e′ ratio (n=25/22) | 11.7 (9.3, 15.0) | 12.5 (9.3, 20.0) | 0.6 |
| EF (%, n=24/23) | 60 (55, 60) | 60 (55, 60) | 0.6 |
| PWR/EDV (mmHg/sec, n=23/21) | 327 (277, 365) | 359 (319, 380) | 0.19 |
| SW/EDV (mmHg, n=23/21) | 50.7 (46.5, 55.2) | 54.2 (47.3, 56.8) | 0.3 |
| Vascular Function | |||
| Ea (mmHg/ml, n=24/21) | 1.5 (1.2, 1.7) | 1.7 (1.6, 1.9) | 0.07 |
| RHI (n=19/14) | 1.9 (1.6, 2.3) | 1.9 (1.5, 2.1) | 0.6 |
| ED (%) | 53 | 64 | 0.5 |
| SVR (DSC, n=24/21) | 1417 (1245, 1700) | 1688 (1385, 1828) | 0.11 |
| TAC (ml/mmHg, n=22/20) | 1.69 (1.40, 2.43) | 1.71 (1.33, 1.99) | 0.7 |
| cSBP (mmHg, n=23/22) | 110 (100, 121) | 109 (99, 118) | 0.7 |
| cAIx (%, n=23/22) | 24 (17, 29) | 24.5 (17, 29) | 0.98 |
| PASP (mmHg, n=19/11) | 30 (28, 43.4) | 28 (26.2, 41) | 0.3 |
| Exercise Capacity | |||
| Peak VO2 (ml/min/kg) | 12.6 (11.1, 14.3) | 11.6 (10.1, 15.7) | 0.97 |
| Peak RER | 1.07 (10.4, 1.11) | 1.09 (1.03, 1.17) | 0.4 |
| Exercise Hemodynamics and Reserve | |||
| ΔHR, low (bpm) | 13 (9, 18) | 16 (9, 18) | 0.8 |
| ΔHR, peak (bpm) | 47 (34, 53) | 52 (34, 70) | 0.13 |
| ΔSBP, low (mmHg) | 10 (2, 16) | 14 (6, 18) | 0.4 |
| ΔSBP, peak (mmHg) | 38 (28, 56) | 34 (22, 58) | 0.5 |
| ΔDBP, low (mmHg) | 2 (0, 4) | 0 (−2, 4) | 0.4 |
| ΔDBP, peak (mmHg) | 2 (−6, 8) | 6 (−2, 10) | 0.2 |
| ΔEF, low (%, n=23/23) | 5 (0, 5) | 0 (0, 5) | 0.06 |
| ΔEF, peak (%, n=21/21) | 10 (5, 10) | 5 (5, 10) | 0.19 |
Column 1 parentheses indicate number of placebo/sildenafil subjects with available data for each parameter; if no numbers are presented there was no data missing. P values are not adjusted for multiple comparisons.
Exercise capacity was severely depressed in HFpEF subjects (median peak VO2 12.6 ml/min/kg). There were similar increases in HR and BP at low level and peak exercise in subjects randomized to placebo and sildenafil at baseline (Table 2). As in prior studies,2 subjects with HFpEF displayed blunted increases in EF during low level and peak exercise. Subjects randomized to sildenafil tended to display less increase in EF during exercise at the baseline visit (Table 2).
Effects of Sildenafil on Resting Ventricular-Vascular Function
After 12 weeks treatment with study drug (20 mg tid), subjects randomized to sildenafil displayed greater reduction in resting central systolic BP (cSBP) and tended to display greater reductions in resting Ea and SVR compared to controls (Table 3). Low dose sildenafil had no statistically significant effect on resting LV systolic or diastolic function, cAIx, or PASP after 12 weeks as compared to placebo. As in the main RELAX trial, there was no significant effect of sildenafil on peak VO2 compared to placebo after 12 or 24 weeks treatment (Table 3).
Table 3.
Sildenafil Effects on Resting Cardiovascular Function
| Placebo (n=25) | Sildenafil (n=23) | p | |
|---|---|---|---|
| Changes at 12 weeks | |||
| Δ HR (bpm) | 0 ± 10 | −2 ± 9 | 0.5 |
| Δ EDV (ml, n=22/18) | 3 ± 20 | 4 ± 20 | 0.9 |
| Changes at 24 weeks | |||
| Δ HR (bpm) | −1 ± 10 | −2 ± 10 | 0.6 |
| Δ EDV (ml, n=21/18) | 3 ± 19 | 12 ± 23 | 0.2 |
| Changes in vascular function, 12 weeks | |||
| Δ cSBP (mmHg, n=21/20) | 7 ± 17 | −9 ± 20 | 0.005 |
| Δ cAIx (%, n=21/20) | 1 ± 7 | −1 ± 10 | 0.4 |
| Δ Ea (mmHg/ml, n=23/18) | 0.08 ± 0.45 | −0.14 ± 0.25 | 0.17 |
| Δ SVR (dyne/s*cm5, n=23/18) | 73 ± 457 | −131 ± 300 | 0.2 |
| Δ TAC (ml/mmHg, n=19/15) | 0.01 ± 0.70 | 0.30 ± 0.60 | 0.3 |
| Δ RHI (n=16/12) | 0.05 ± 0.60 | 0.20 ± 0.51 | 0.5 |
| Δ PASP (mmHg, n=16/8) | −1 ± 8 | 0 ± 4 | 0.8 |
| Changes in vascular function, 24 weeks | |||
| Δ cSBP (mmHg, n=16/17) | 2 ± 16 | 1 ± 29 | 0.9 |
| Δ cAIx (%, n=16/17) | −1 ± 5 | 1 ± 11 | 0.6 |
| Δ Ea (mmHg/ml, n=22/18) | 0.02 ± 0.29 | −0.29 ± 0.28 | 0.008 |
| Δ SVR (dyne/s*cm5, n=22/18) | −27 ± 370 | −169 ± 375 | 0.4 |
| Δ TAC (ml/mmHg, n=15/15) | 0.04 ± 0.73 | 0.25 ± 0.61 | 0.7 |
| Δ RHI (n=15/14) | −0.17 ± 0.61 | 0.30 ± 0.45 | 0.054 |
| Δ PASP (mmHg, n=15/10) | 0 ± 6 | 3 ± 7 | 0.5 |
| Changes in LV systolic function, 12 weeks | |||
| Δ EF (%, n=24/22) | −1 ± 7 | 1 ± 4 | 0.08 |
| Δ SW/EDV (mmHg, n=22/18) | 0.6 ± 8.1 | −2.8 ± 4.5 | 0.2 |
| Δ PWR/EDV (mmHg/s, n=22/18) | 37 ± 80 | 18 ± 50 | 0.8 |
| Changes in LV systolic function, 24 weeks | |||
| Δ EF (%, n=23/21) | 0 ± 5 | 0 ± 4 | 0.95 |
| Δ SW/EDV (mmHg, n=21/18) | 0.3 ± 5.8 | −6.0 ± 5.1 | 0.006 |
| Δ PWR/EDV (mmHg/s, n=21/18) | 0 ± 40 | −52 ± 70 | 0.04 |
| Changes in LV diastolic function, 12 weeks | |||
| Δ E velocity (cm/s, n=24/20) | 1.7 ± 19 | 8.5 ± 18 | 0.3 |
| Δ DT (ms, n=22/19) | −4 ± 48 | 2 ± 38 | 0.7 |
| Δ E/A ratio (n=21/19) | −0.0 ± 0.5 | 0.2 ± 0.7 | 0.3 |
| Δ E′ (cm/s, n=24/19) | 0.1 ± 1.0 | 0.2 ± 1.0 | 0.9 |
| Δ E/E′ (n=23/19) | −0.0 ± 3.8 | 1.2 ± 3.8 | 0.3 |
| Changes in LV diastolic function, 24 weeks | |||
| Δ E velocity (cm/s, n=23/20) | −0.4 ± 19 | 12.5 ± 19 | 0.02 |
| Δ DT (ms, n=22/18) | −4 ± 39 | −8 ± 39 | 0.8 |
| Δ E/A ratio (20/18) | −0.0 ± 0.6 | 0.4 ± 0.8 | 0.07 |
| Δ E′ (cm/s, n=23/20) | −0.2 ± 0.0 | 0.4 ± 0.0 | 0.2 |
| Δ E/E′ (n=22/20) | 0.9 ± 4.9 | 1.9 ± 5.2 | 0.6 |
| Changes in Exercise Capacity, 12 weeks | |||
| Δ Peak VO2 (ml/min/kg) | −0.4 ± 1.5 | −0.7 ± 3.0 | 0.7 |
| Δ RER | 0.01 ± 0.08 | 0.02 ± 0.11 | 0.8 |
| Changes in Exercise Capacity, 24 weeks | |||
| Δ Peak VO2 (ml/min/kg, n=24/23) | −0.8 ± 1.7 | −0.6 ± 2.7 | 0.8 |
| Δ RER (n=23/23) | 0.01 ± 0.11 | 0.01 ± 0.11 | 0.5 |
Column 1 parentheses indicate number of placebo/sildenafil subjects with available data for each parameter; if no numbers are presented there was no data missing. P values are not adjusted for multiple comparisons.
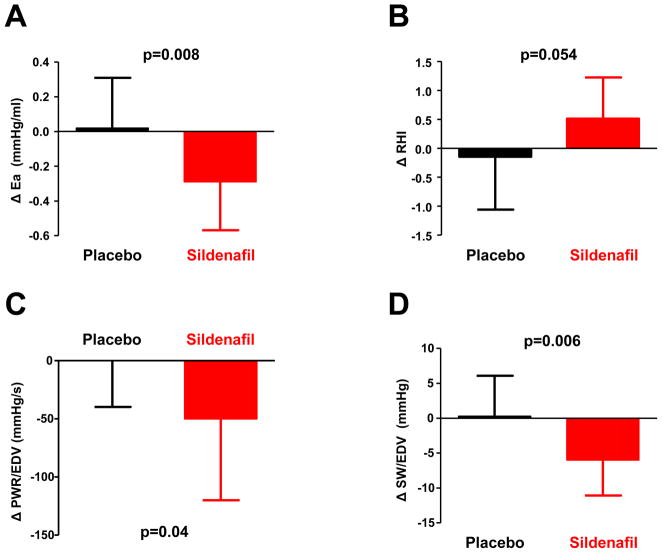
After 24 weeks of treatment with study drug titrated to 60 mg tid, subjects randomized to sildenafil displayed greater reductions in Ea and tended to show greater increases in RHI (Table 3, Figure). Improvements in Ea and RHI were not correlated with changes in peak VO2 (p>0.2). There were no statistically significant differences in central BP, cAIx or SVR. Higher dose sildenafil treatment had no significant effect on LV EF, but resting LV stroke work index (SW/EDV) and peak power index (PWR/EDV) decreased by 11% and 16% respectively (Table 3, Figure). The change in LV peak power index correlated directly with the change in peak VO2 compared to baseline, as patients with a larger decrease in contractility displayed greater decreases in peak VO2 (Supplemental Figure). High dose sildenafil had no significant effect on PASP but increased early LV diastolic filling velocity (E) compared to controls (Table 3). Sildenafil had no significant effect on other measures of LV diastolic function at 12 or 24 weeks.
Figure.
[A] Effective arterial elastance (Ea) was reduced at rest after 24 weeks treatment in subjects randomized to sildenafil (red) compared to placebo. [B] 24 week changes in endothelial function, assessed as the reactive hyperemia index (RHI) in placebo and sildenafil. Left ventricular contractility, assessed as [C] peak power index (PWR/EDV) and [D] stroke work index (SW/EDV) was reduced after 24 weeks treatment with sildenafil (red) as compared to placebo (black).
Effects of Sildenafil on Ventricular-Vascular Reserve Function
Subjects in this study were not selected based upon adequate echocardiographic images, and this fact, coupled with the technical limitations with 2D and Doppler imaging in the upright position during exercise, and the strict criteria for high quality of images/signals for quantitative analysis by the core laboratory, resulted in high rates of missingness for most variables during exercise. Thus assessment of reserve function was limited to measurement of changes in blood pressure and EF during low level and peak exercise. Adjusted to reserve responses observed in the baseline exercise test at study entry, there was greater enhancement in EF with exercise after 12 weeks in subjects randomized to sildenafil, coupled with a trend toward less increase in systolic BP (12 weeks, Table 4). After 24 weeks, there was less increase in systolic BP in subjects randomized to sildenafil at low level exercise, but no statistically significant difference in the change in EF with exercise (Table 4). Sildenafil tended to decrease peak exercise heart rate at both 12 and 24 weeks but this was not significant compared to placebo.
Table 4.
Effects of Sildenafil on Exercise Reserve Responses
| Placebo (N=25) | Sildenafil (N=23) | P value* | P-value** | |
|---|---|---|---|---|
| 12 Weeks | ||||
|
| ||||
| Baseline SBP, low (mmHg, n=25/22) | 131 ± 18 | 138 ± 18 | ||
| Week 12 SBP, low (mmHg, n=25/22) | 133 ± 16 | 129 ± 23 | ||
| ΔSBP, low | 2 ± 21 | −9 ± 16 | 0.15 | 0.3 |
|
| ||||
| Baseline SBP, peak (mmHg, n=25/23) | 163 ± 23 | 169 ± 24 | ||
| Week 12 SBP, peak (mmHg, n=25/23) | 166 ± 24 | 165 ± 24 | ||
| ΔSBP, peak | 3 ± 19 | −3 ± 16 | 0.3 | 0.4 |
|
| ||||
| Baseline DBP, low (mmHg, n=25/22) | 72 ± 9 | 75 ± 13 | ||
| Week 12 DBP, low (mmHg, n=25/22) | 72 ± 8 | 72 ± 10 | ||
| ΔDBP, low | −0.2 ± 9 | −3 ± 13 | 0.6 | 0.7 |
|
| ||||
| Baseline DBP, peak (mmHg, n=25/23) | 71 ± 13 | 77 ± 15 | ||
| Week 12 DBP, peak (mmHg, n=25/23) | 73 ± 14 | 74 ± 12 | ||
| ΔDBP, peak | 2 ± 13 | −3 ± 12 | 0.4 | 0.4 |
|
| ||||
| Baseline HR, low (mmHg, n=25/23) | 80 ± 12 | 83 ± 16 | ||
| Week 12 HR, low (mmHg, n=25/23) | 79 ± 11 | 79 ± 17 | ||
| ΔHR, low | −1 ± 9 | −4 ± 9 | 0.3 | 0.4 |
|
| ||||
| Baseline HR, peak (mmHg, n=25/23) | 110 ± 19 | 119 ± 25 | ||
| Week 12 HR, peak (mmHg, n=25/23) | 109 ± 18 | 108 ± 19 | ||
| ΔHR, peak | −1 ± 13 | −11 ± 19 | 0.11 | 0.08 |
|
| ||||
| Baseline EF, low (mmHg, n=22/20) | 62 ± 6 | 61 ± 6 | ||
| Week 12 EF, low (mmHg, n=22/20) | 61 ± 6 | 63 ± 5 | ||
| ΔEF, low | −1 ± 6 | 2 ± 4 | 0.054 | 0.09 |
|
| ||||
| Baseline EF, peak (mmHg, n=19/18) | 68 ± 6 | 66 ± 6 | ||
| Week 12 EF, peak (mmHg, n=19/18) | 64 ± 7 | 68 ± 6 | ||
| ΔEF, peak | −3 ± 6 | 2 ± 4 | 0.008 | 0.01 |
| 24 Weeks | ||||
|
| ||||
| Baseline SBP, low (mmHg, n=24/23) | 130 ± 18 | 140 ± 19 | ||
| Week 24 SBP, low (mmHg, n=24/23) | 133 ± 16 | 130 ± 28 | ||
| ΔSBP, low | 3 ± 16 | −9 ± 19 | 0.047 | 0.07 |
|
| ||||
| Baseline SBP, peak (mmHg, n=24/23) | 163 ± 24 | 169 ± 24 | ||
| Week 24 SBP, peak (mmHg, n=24/23) | 162 ± 25 | 163 ± 30 | ||
| ΔSBP, peak | −0.2 ± 19 | −5 ± 22 | 0.5 | 0.5 |
|
| ||||
| Baseline DBP, low (mmHg, n=24/23) | 72 ± 9 | 75 ± 13 | ||
| Week 24 DBP, low (mmHg, n=24/23) | 71 ± 11 | 70 ± 9 | ||
| ΔDBP, low | −1 ± 10 | −5 ± 13 | 0.5 | 0.4 |
|
| ||||
| Baseline DBP, peak (mmHg, n=24/23) | 70 ± 12 | 77 ± 15 | ||
| Week 24 DBP, peak (mmHg, n=24/23) | 71 ± 15 | 72 ± 13 | ||
| ΔDBP, peak | 1 ± 12 | −6 ± 12 | 0.2 | 0.2 |
|
| ||||
| Baseline HR, low (mmHg, n=24/23) | 80 ± 12 | 83 ± 16 | ||
| Week 24 HR, low (mmHg, n=24/23) | 79 ± 10 | 77 ± 14 | ||
| ΔHR, low | −1 ± 9 | −6 ± 10 | 0.07 | 0.09 |
|
| ||||
| Baseline HR, peak (mmHg, n=24/23) | 109 ± 19 | 119 ± 25 | ||
| Week 24 HR, peak (mmHg, n=24/23) | 110 ± 19 | 107 ± 24 | ||
| ΔHR, peak | 1 ± 13 | −12 ± 19 | 0.02 | 0.01 |
|
| ||||
| Baseline EF, low (mmHg, n=25/22) | 62 ± 5 | 61 ± 6 | ||
| Week 24 EF, low (mmHg, n=25/22) | 62 ± 6 | 62 ± 6 | ||
| ΔEF, low | −0.2 ± 6 | 0.3 ± 5 | 0.99 | 0.93 |
|
| ||||
| Baseline EF, peak (mmHg, n=20/18) | 68 ± 6 | 66 ± 6 | ||
| Week 24 EF, peak (mmHg, n=20/18) | 66 ± 6 | 66 ± 6 | ||
| ΔEF, peak | −1 ± 6 | 1 ± 6 | 0.5 | 0.6 |
P-values adjusted for baseline
P-values adjusted for age and baseline
Column 1 parentheses indicate number of placebo/sildenafil subjects with available data for each parameter.
Discussion
This prospective study examined the effects of low dose (20 mg tid for 12 weeks) and high dose (60 mg tid from 12 to 24 weeks) sildenafil on cardiovascular function at rest and with submaximal and peak exercise in 48 subjects participating in the RELAX trial. Compared to placebo, subjects randomized to sildenafil displayed greater systemic afterload reduction and tended to show improved endothelium-dependent vasodilation. While there was no change in resting EF, there were modest reductions in left ventricular contractility assessed using two load-independent measures of systolic function, and the degree of reduction in PWR/EDV was correlated with reductions in peak VO2. There was no significant effect on PASP. Sildenafil tended to reduce the increase in BP during exercise, and this was coupled to slightly greater increases in EF with low dose but not high dose sildenafil. From these data we speculate that either the beneficial effects of sildenafil in the systemic vasculature and endothelium were insufficient to improve clinical status, or that the deleterious effects of sildenafil on left ventricular function offset any salutary vascular effects, contributing to the absence of benefit observed with sildenafil in subjects with HFpEF in the RELAX trial.17
The pathophysiology of HFpEF is complex—related to global impairments in systolic and diastolic function in the left and right ventricles, abnormal vasodilation, pulmonary hypertension, endothelial dysfunction, chronotropic incompetence, and abnormalities in the skeletal muscle and periphery.8 Clinical trials to date testing neurohormonal antagonists have been neutral in HFpEF, and novel therapies that target multiple components in the pathophysiology may be more effective.9 Phosphodiesterase inhibitors such as sildenafil have been shown in other patient populations to improve pulmonary vascular resistance, right ventricular function, ventricular remodeling, endothelial function, quality of life, and exercise capacity, suggesting numerous reasons for patients with HFpEF to benefit from this class of medicine.12–15, 18, 25
In a single center study of 44 patients with HFpEF and severe pulmonary vascular disease, 12 months of treatment with sildenafil improved RV-PA coupling and reduced PA and biventricular filling pressures.16 However, in the larger, multicenter RELAX trial, sildenafil use did not improve exercise capacity, plasma biomarkers or clinical status in patients with typical HFpEF as observed in the community.17
The current results may provide greater insight into the reasons for lack of benefit from sildenafil in HFpEF.17 Modest systemic arterial vasodilation was observed, which was coupled with a trend toward improved endothelium-dependent vasodilation after 24 weeks treatment, similar to prior observations of PDE5i in HFrEF.15, 25 Endothelial dysfunction has recently been proposed to play a major role in the pathophysiology of HFpEF, contributing to both ventricular and vascular dysfunction.10 However, 42% of HFpEF participants in this ancillary study did not display digital endothelial dysfunction, suggesting that abnormal flow mediated dilation is not necessary to cause the disease. The prevalence of endothelial dysfunction in this cohort is similar to a prior study evaluating microvascular function in HFpEF,2 but is higher than 2 other studies assessing large vessel flow mediated dilation.26, 27 The discrepancy between these studies may relate to fundamental differences in the vascular beds studied and their assessment methods.28 It may be that more chronic, sustained improvements in endothelial function are required to improve ventricular stiffness and remodeling, that different pharmacologic approaches to enhance cGMP signaling will be more effective, or that the degree of improvement in endothelial and vascular function achieved in RELAX was insufficient to improve aerobic capacity.
An alternative interpretation is that the deleterious effects on LV systolic function might have offset beneficial effects on the endothelium and vasculature. LV contractility, assessed by two load-independent measures, was modestly but significantly reduced with sildenafil. The mechanisms for this effect remain unclear, but previous studies have shown that enhanced NO-cGMP signaling can act as a negative inotrope.20, 29, 30 Intracellular cGMP-PKG may serve as a “brake” to oppose cAMP-PKA mediated effects on calcium handling and myofilament interaction. This is achieved in the myocyte by activation of dual substrate PDEs that hydrolyze both cAMP and cGMP,31 and through PKG, which directly counteracts multiple PKA effects that enhance contractile function.29, 32 The change in LV peak power index was correlated with changes in exercise capacity, supporting the plausibility of a mechanistic relationship.
Sildenafil has been shown to reduce PA pressures in HF,12, 13, 16 but in the current study there was no effect of sildenafil on resting PASP. This may relate in part to the absence of significant PH in the study population, as well as to the imprecision of echocardiography to estimate PA pressures. The presence of PH was not required for entry into RELAX, and it is possible that effects on ventricular-vascular function would have been different in a more diseased population with significant pulmonary vascular disease. Indeed, prior studies have shown that PDE5i may exert a positive inotropic effect in the right ventricle in groups with more severe PH.33 One theoretical concern with pulmonary vasodilators in HFpEF is the development of left atrial hypertension with reduction in PA resistance.34 There was greater increase in transmitral E velocity with high dose sildenafil, indicating an increase in the left atrium-LV pressure gradient, though the change in E/e′ did not differ between groups. If the magnitude of left atrial pressure elevation were matched to any reduction in PA resistance, there could be a neutral effect on PASP, but this hypothesis cannot be addressed with these noninvasive data.
Limitations
Ventricular-vascular function was assessed in this study using noninvasive techniques that have greater variability than invasive methods. Owing to the difficulties in obtaining diagnostic quality echocardiography at rest and particularly during exercise, there was missing data, especially at peak exercise, which increases the risk of Type 2 error. Myocardial strain imaging was not performed as part of this study. Right atrial pressure was not estimated based upon caval dimension or collapsibility in this study, but rather assumed to uniformly be equal to 10 mmHg. While the clinical characteristics in this ancillary study resemble those in the community, some markers of disease severity such as E/e′ ratio and PASP were numerically lower that median values in the parent RELAX trial, suggesting a less advanced stage of HFpEF, which may limit the generalizability of these findings to patients with more advanced HFpEF, especially patients with significant pulmonary vascular disease. Patients who were in atrial fibrillation were excluded and these results may not apply to that cohort. The aim of this study was to comprehensively assess ventricular, vascular and endothelial function, requiring a large number of comparisons. Statistical adjustments were not made for multiple comparisons in this study, because many of the ventricular-vascular parameters are correlated with one another, and correction techniques such as the Bonferroni method assume the observations are independent. Multivariable analysis was not performed because of the missing data that varied between measured parameters of cardiac and vascular function, resulting in low power and higher risk of Type II error.
Conclusions
In patients with HFpEF, treatment with sildenafil was associated with systemic vasodilation, a trend toward improved endothelium-dependent vasodilation, and depressed resting left ventricular contractility, with no effect on estimated pulmonary artery pressure. We speculate that beneficial effects of PDE5i in the systemic vasculature and endothelium were insufficient to improve clinical status, or that the deleterious effects on left ventricular function offset any salutary vascular effects, contributing to the absence of benefit observed with sildenafil in subjects with HFpEF in the RELAX trial.
Acknowledgments
This study was funded by the NHLBI Heart Failure Research Network (U10HL084904, U10HL084907 and PO1HL 076611) and an American Heart Association National Clinical Research Program Grant (10CRP2600071).
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: Exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 7.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 9.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 11.van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase g activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 12.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 13.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 14.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pde5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: Results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 15.Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50:2136–2144. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 16.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: A target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 17.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, Lewinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E. Phosphdiesterase-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure (relax) trial: Rationale and design. Circ Heart Fail. 2012;5:653–659. doi: 10.1161/CIRCHEARTFAILURE.112.969071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: A report from the american society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits beta-adrenergic-stimulated cardiac contractility in humans. Circulation. 2005;112:2642–2649. doi: 10.1161/CIRCULATIONAHA.105.540500. [DOI] [PubMed] [Google Scholar]
- 21.Sharir T, Feldman MD, Haber H, Feldman AM, Marmor A, Becker LC, Kass DA. Ventricular systolic assessment in patients with dilated cardiomyopathy by preload-adjusted maximal power. Validation and noninvasive application. Circulation. 1994;89:2045–2053. doi: 10.1161/01.cir.89.5.2045. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama M, Chen CH, Nevo E, Fetics B, Wong E, Kass DA. Optimal preload adjustment of maximal ventricular power index varies with cardiac chamber size. Am Heart J. 1998;136:281–288. doi: 10.1053/hj.1998.v136.89584. [DOI] [PubMed] [Google Scholar]
- 23.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from olmsted county, minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borlaug BA, Olson TP, Abdelmoneim Mohamed S, Melenovsky V, Sorrell VL, Noonan K, Lin G, Redfield MM. A randomized pilot study of aortic waveform guided therapy in chronic heart failure. J Am Heart Assoc. 2014;3:e000745. doi: 10.1161/JAHA.113.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz SD, Balidemaj K, Homma S, Wu H, Wang J, Maybaum S. Acute type 5 phosphodiesterase inhibition with sildenafil enhances flow-mediated vasodilation in patients with chronic heart failure. J Am Coll Cardiol. 2000;36:845–851. doi: 10.1016/s0735-1097(00)00790-7. [DOI] [PubMed] [Google Scholar]
- 26.Haykowsky MJ, Herrington DM, Brubaker PH, Morgan TM, Hundley WG, Kitzman DW. Relationship of flow-mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. J Gerontol A Biol Sci Med Sci. 2013;68:161–167. doi: 10.1093/gerona/gls099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hundley WG, Bayram E, Hamilton CA, Hamilton EA, Morgan TM, Darty SN, Stewart KP, Link KM, Herrington DM, Kitzman DW. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol. 2007;292:H1427–1434. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 28.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: The framingham heart study. Hypertension. 2011;57:390–396. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: Ten years after, and continuing. Circ Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 30.Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA, Moens AL, Champion HC, Kass DA. Compartmentalization of cardiac beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation. 2007;115:2159–2167. doi: 10.1161/CIRCULATIONAHA.106.643536. [DOI] [PubMed] [Google Scholar]
- 31.Rivet-Bastide M, Vandecasteele G, Hatem S, Verde I, Benardeau A, Mercadier JJ, Fischmeister R. Cgmp-stimulated cyclic nucleotide phosphodiesterase regulates the basal calcium current in human atrial myocytes. J Clin Invest. 1997;99:2710–2718. doi: 10.1172/JCI119460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hare JM, Loh E, Creager MA, Colucci WS. Nitric oxide inhibits the positive inotropic response to b-adrenergic stimulation in humans with left ventricular dysfunction. Circulation. 1995;92:2198–2203. doi: 10.1161/01.cir.92.8.2198. [DOI] [PubMed] [Google Scholar]
- 33.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 34.Boilson BA, Schirger JA, Borlaug BA. Caveat medicus! Pulmonary hypertension in the elderly: A word of caution. Eur J Heart Fail. 2010;12:89–93. doi: 10.1093/eurjhf/hfp171. [DOI] [PubMed] [Google Scholar]