Abstract
Sepsis, a major clinical problem with high morbidity and mortality, is caused by overwhelming systemic host-inflammatory response. Toll-like receptors (TLRs) play a fundamental role in induction of hyperinflammation and tissue damage in sepsis. In this study, we demonstrate a protective role of TLR9 inhibition against the dysregulated inflammatory response and tissue injury in sepsis. TLR9 deficiency decreased the mortality of mice following cecal ligation and puncture (CLP) -induced sepsis. TLR9 knockout mice showed dampened p38 activation and augmented Akt phosphorylation in the spleen, lung and liver. In addition, TLR9 deficiency decreased the levels of inflammatory cytokines and attenuated splenic apoptosis after CLP. These results indicate that TLR9 inhibition might offer a novel therapeutic strategy for the management of sepsis.
Keywords: TLR9, Sepsis, Immune response, Cytokines
1. Introduction
Sepsis, a huge medical problem, is one of the leading causes of death in intensive care units throughout the world. It is generally admitted that sepsis is caused by excessive inflammatory response to invading pathogens. This uncontrolled systemic inflammation leads to tissue damage and multiple organ failure, which is proposed as the major contributor to the particularly high mortality in sepsis [1]. It has been estimated that sepsis is associated with a mortality rate ranging between 30%–70% [2]. Currently, the management of sepsis is largely supportive. Specific and efficacious therapeutic interventions targeting the underlying mechanism of sepsis have yet not been developed [3].
Once invaded, the host response to pathogens is initiated by the sensing of microbial structures through families of receptors called pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) which play pivotal roles both in the innate and adaptive immune responses. At least 10 TLRs in mammals have been described. The binding of microbial ligands to TLRs triggers the release of plenteous amounts of cytokines and chemokines, and subsequently initiate the inflammatory response [4]. Many reports have suggested that uncontrolled TLR response plays a major role in the pathogenesis and development of sepsis [5, 6]. Consistently, recent data show that TLR4 antagonism attenuated cardiac dysfunction and cytokine expression in the sepsis model induced by lipopolysaccharide [7]. After the procedure of cecal ligation and puncture (CLP), TLR2-deficient mice exhibited increased survival rates compared to wild type mice, indicating that TLR2 activation is involved in the progress of sepsis [8]. Plitas et al. showed that blockage of TLR9 signaling averts the uncontrolled immune response in CLP [9]. However, the mechanism underlying the beneficial effects of TLR9 inhibition in sepsis has not been elucidated clearly.
In this study, we demonstrated that TLR9 deficiency obstructs p38 activation and preserves Akt phosphorylation in septic mice. Additionally, TLR9 deficiency alters cytokine expression and prevents against splenic apoptosis in polymicrobial sepsis.
2. Materials and methods
2.1. Experimental animals
TLR9 knockout (KO) mice on a Balb/c background were generously provided by Dr. Shizuo Akira (Osaka University, Osaka, Japan) via Dr. Dennis Klinman (National Cancer Institute, Frederick, MD). Wild type (WT) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were maintained in the Division of Laboratory Animal Resources at East Tennessee State University (ETSU), a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All animal studies were approved by the ETSU Committee on Animal Care.
2.2. Cecal ligation and puncture (CLP)
Polymicrobial sepsis was induced using the CLP model, which closely emulates polymicrobial intraabdominal sepsis occurring in humans and has been used widely to study the immunopathological changes in sepsis [10, 11]. CLP was performed to induce sepsis in mice as described previously [10, 11]. Briefly, 8–10-week-old mice were anesthetized via inhalation with 2.5% isoflurane. A midline abdominal incision was made and the cecum was exteriorized, ligated distal to the ileocecal valve, and then punctured twice. A small amount of fecal material was extruded into the abdominal cavity. The abdominal wall and skin were sutured in layers with 3-0 silk. Sham-operated mice were treated identically except that the cecum was not ligated or punctured. Immediately following surgery, all mice were resuscitated with 1 ml physiological saline injected s.c. and returned to their cages with free access to food and water.
2.3. Western blot
Western blot analysis was performed as described previously [12]. Samples containing equal amounts of protein were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred onto Hybond ECL membranes (Amersham Pharmacia, NJ). The ECL membranes were incubated overnight at 4 °C with the appropriate primary antibody [anti-GAPDH, anti-phospho-Akt, anti-Akt, anti-phospho-p38 mitogen-activated protein kinase (MAPK), anti-p38 MAPK, anti-cleaved-caspase-3, anti-caspase-3, anti-Bcl-2, anti-Bax (Cell Signaling Technology, Beverly, MA)] followed by incubation with peroxidase-conjugated secondary antibodies (Cell Signaling Technology). The blot was exposed to the SuperSignal West Dura Extended Duration substrate (Pierce Biotechnology, Rockford, IL). The signals were quantified by scanning densitometry using a Bio-Image Analysis System (Bio-Rad).
2.4. TUNEL assay
Mouse spleens were fixed in 10% buffered formalin and embedded in paraffin. TUNEL staining for apoptotic nuclei was performed using an In Situ Cell Death Detection kit (Roche Diagnostic, IN) as described in our previous publication [13]. Briefly, sections were exposed for 10 min to a permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate). After washing, 50 µl of TUNEL reaction mixture was placed on the sections and then incubated in a humidified atmosphere for 60 min at 37 °C. 50 µl of substrate solution was added following convert-AP incubation. Finally, sections were counterstained with haematoxylin. Sections were examined with the light microscope using 40 × objective. The percentage of apoptotic cells was calculated (TUNEL-positive cells/ total cells) and averaged across at least five randomly chosen microscopic fields for each slide.
2.5. Determination of cytokine levels
Splenic CD4+ T cells were negatively selected by using the MagCellect™ Mouse CD4+ T Cell Isolation Kit (R&D Systems, Minneapolis, MN) [14]. Isolated CD4+ T cells were seeded at a final concentration of 2.5×106 cells/ml in 96-well plates with or without immobilized anti-CD3 (50 µl of 5 µg/ ml in PBS for 24 h at 4 °C, BD Biosciences Pharmingen, San Diego, CA) and soluble anti-CD28 (1 µg/ ml, BD Biosciences Pharmingen). The supernatants were collected after 24 h, 48 h and 72 h of cultivation. To determine the serum level of cytokines, blood was collected from all experimental and control mice. Samples were allowed to clot for 2 h at room temperature before centrifugation for 20 min at 2000×g. Then serum was removed and stored at −20°C for subsequent ELISA assay. The amount of cytokines was examined by using a Quantikine Mouse ELISA kit (R&D Systems, MN) [15].
2.6. Real-time quantitative RT-PCR
Total RNA was isolated from mouse splenic CD4+ T cells stimulated with anti-CD3 and anti-CD28 using a RNeasy Plus Mini Kit (QIAGEN Sciences, MD). The real-time PCR was performed as described previously [15]. Briefly, one microgram of RNA from each sample was used for reverse transcription and synthesis of cDNA using a Reaction ReadyTM first strand cDNA synthesis kit (SABiosciences, Frederick, MD). PCR was performed using RT2 real-timeTM SYBR Green Fluorescien PCR Master Mix (SABiosciences). GAPDH expression was used as internal control.
2.7. Statistical analysis
Student’s t-test was used to determine the significance of the differences between groups. Survival curves were generated using the Kaplan-Meier method, and the significance of difference was calculated by the log-tank test. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. TLR9 deficiency increases survival in sepsis
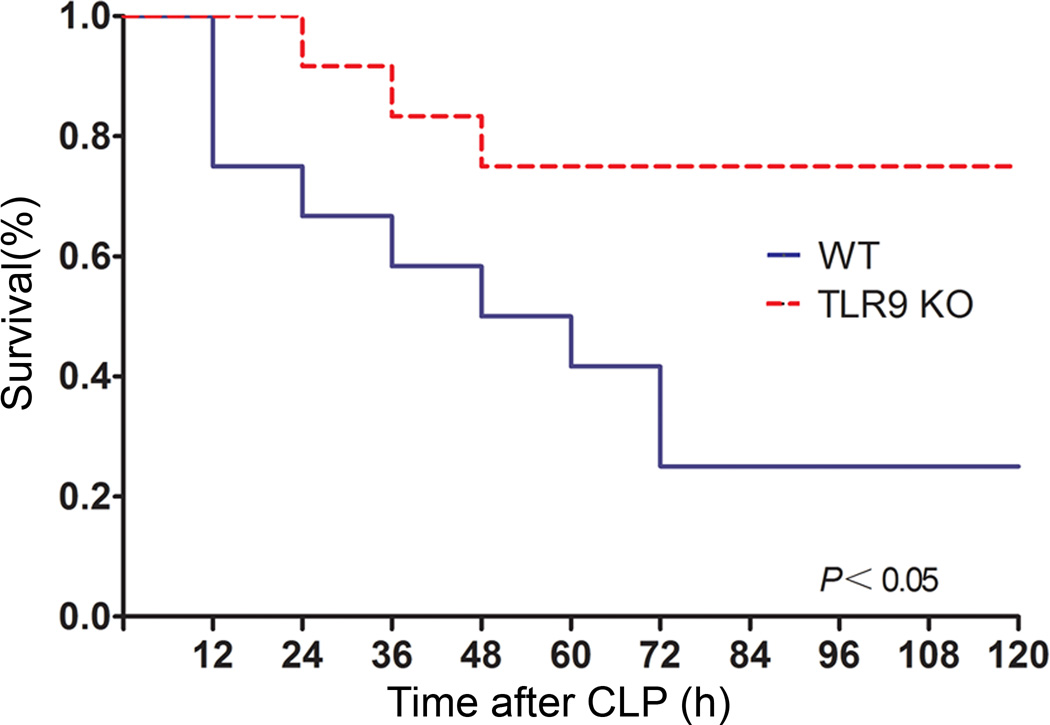
First, we investigated the effect of TLR9 deficiency on mortality in CLP-induced polymicrobial sepsis. We observed that TLR9 KO mice had significantly lower mortality than WT mice as shown in Fig. 1. The difference was most striking on the third day after CLP. Specifically, the survival rate of TLR9 KO mice was > 70% compared with < 20% of WT mice within 72 h following CLP. The similar results were obtained in inhibition of TLR9 by TLR9 antagonist in WT mice (data not shown). In the next set of experiments, we intended to disclose the underlying mechanism for the preventative effect of TLR9 inhibition in sepsis.
Fig. 1.
TLR9 KO mice are less susceptible to CLP-induced polymicrobial sepsis. WT and TLR9 KO mice (n = 20–24 per group) were subjected to CLP and then monitored for survival for up to 120 h.
3.2. TLR9 deficiency dampens p38 activation in sepsis
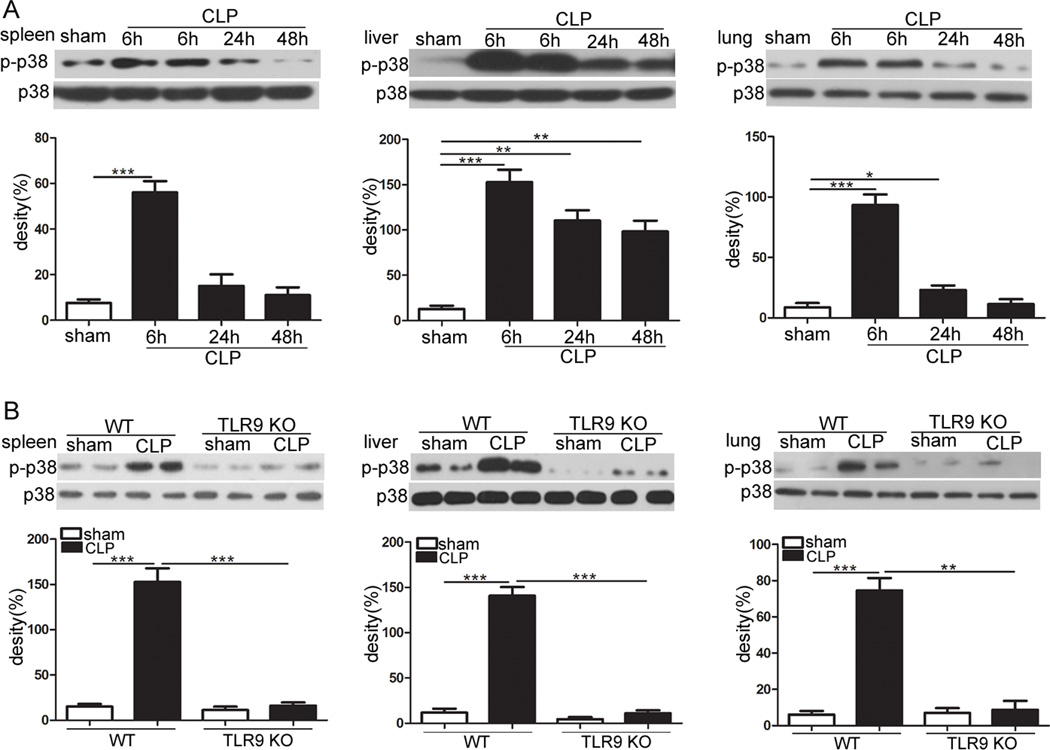
P38 MAPK has been considered to play a critical role in releasing of inflammatory mediators in sepsis [16, 17]. Hence, we studied p38 activation in the spleen, lung and liver of TLR9 KO and WT mice following CLP. At 6 h after CLP, most marked increases of p38 phosphorylation were observed in septic WT mice compared with control WT mice (Fig. 2a). Consequently, we assessed whether TLR9 deletion can regulate p38 activity in the septic organs. As shown in Fig. 2b, the activation of p38 was strikingly decreased in the spleen, lung and liver of septic mice lacking TLR9 as compared with WT littermates.
Fig. 2.
Deletion of TLR9 attenuates p38 activation upon sepsis. a WT mice underwent CLP procedure. Spleen, liver and lung were harvested at the indicated times after CLP. Levels of phosphorylated p38 were examined using Western blotting. b Age-matched WT and TLR9 KO mice were subjected to CLP. At 6 h after CLP, mice were sacrificed. Cellular lysates were extracted from spleen, liver and lung. Expression of phospho-p38 was determined by immunoblotting. The values are mean ± S.E.M. of three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001.
3.3. TLR9 deficiency preserves Akt signaling in sepsis
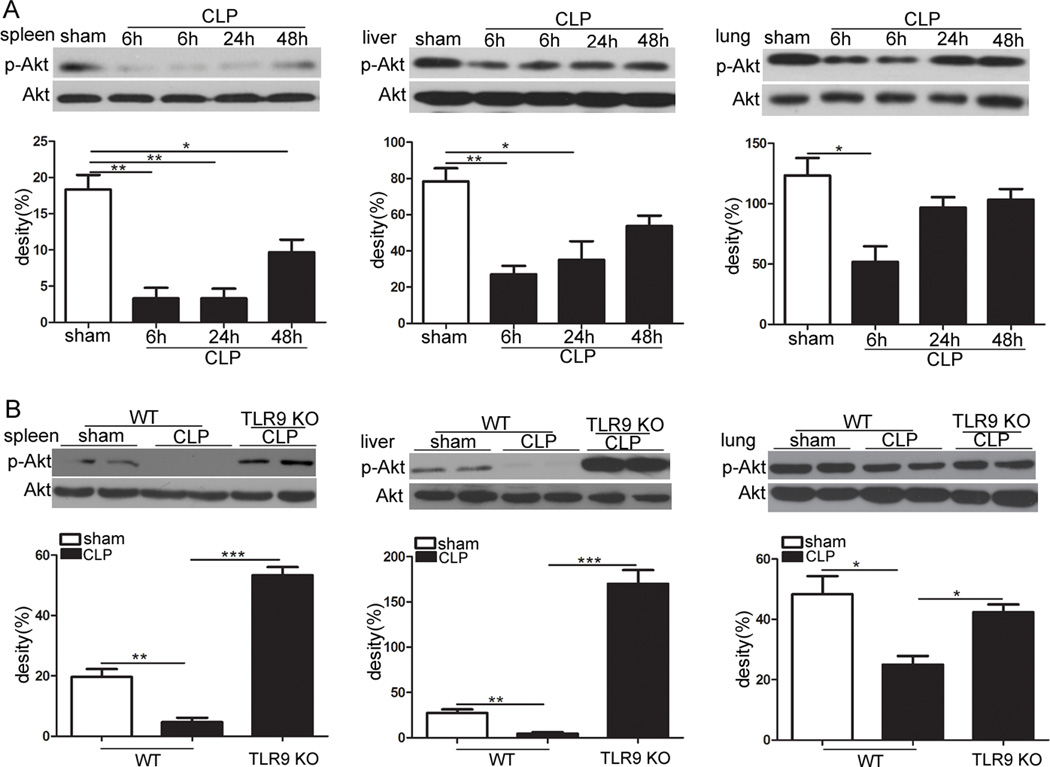
Akt is a key negative regulator of inflammatory response [18, 19]. In the present study, we tested whether Akt activation can be altered by CLP-induced sepsis. As shown in Fig. 3a, the levels of phosphorylated Akt were notably decreased in the spleen, lung and liver of WT septic mice especially at 6 h after the CLP procedure when compared to control animals. Intriguingly, we subsequently found that TLR9 KO mice subjected to CLP had greater activation of Akt when compared to their WT littermates (Fig. 3b).
Fig. 3.
TLR9 deficiency enhances Akt activation in polymicrobial sepsis. a WT mice were subjected to CLP. Spleen, liver and lung were harvested at the indicated times after CLP. Levels of phosphorylated Akt were evaluated using Western blotting. b Age-matched WT and TLR9 KO mice were subjected to CLP. Spleen, liver and lung were harvested at 6 h after CLP. Expression of phospho-Akt was determined by immunoblotting. The values are mean ± S.E.M. of three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001.
3.4. TLR9 deficiency suppresses CLP-induced cytokine release
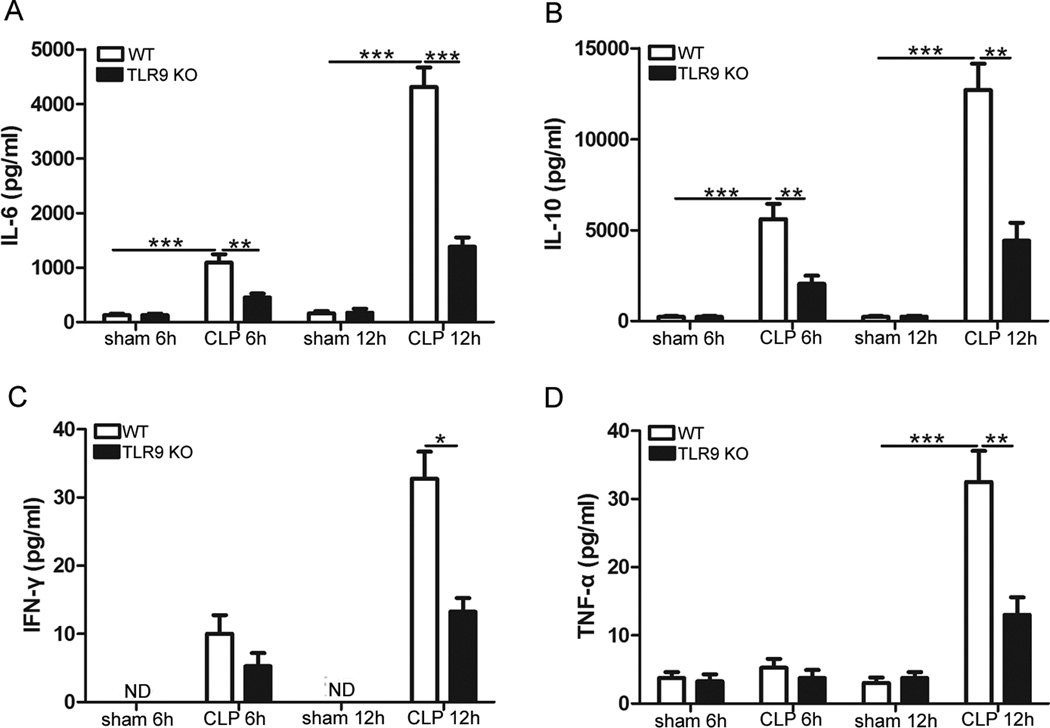
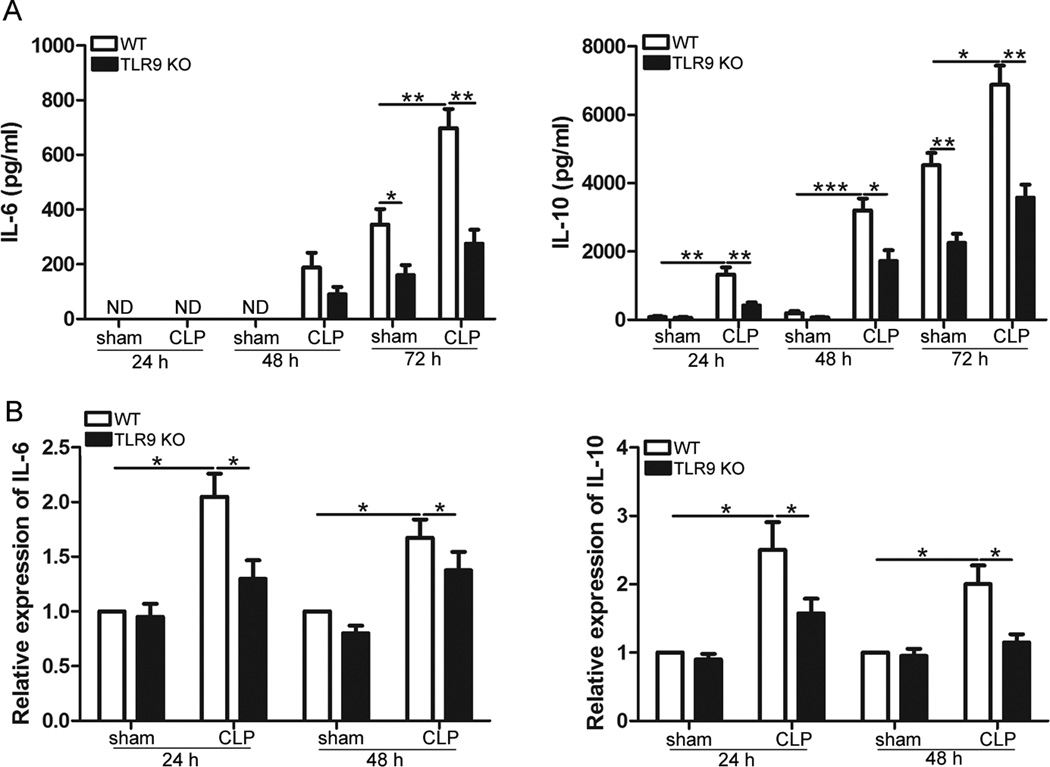
We next examined the effect of TLR9 ablation on the cytokine production following CLP. TLR9 KO mice and WT mice were subjected to CLP, and cytokine levels were measured in the sera 6 h and 12 h, respectively, after CLP. In WT mice, the concentrations of IL-6, IL-10, IFN-_ and TNF-_ were higher in the sera of mice subjected to CLP procedure (Fig. 4). Compared with WT septic littermates, TLR9 KO mice exhibited significantly decreased cytokine levels in the sera (Fig. 4). These results suggest that TLR9 deficiency dampens cytokine responses to polymicrobial sepsis.
Fig. 4.
TLR9 deficiency suppresses cytokine response to CLP. Age-matched WT and TLR9 KO mice were subjected to CLP. At the indicated times after CLP, serum samples were collected. Levels of IL-6, IL-10, IFN-γ and TNF-γ in the sera were determined by ELISA. Data are mean ± SD for 5 mice per group. * p < 0.05; ** p < 0.01; *** p < 0.001.
3.5. Effect of TLR9 deficiency on the levels of cytokines in the spleen of septic mice
The spleen is one of the most important immune organs that contribute to the exaggerated inflammation in sepsis. We, therefore, studied the effect of TLR9 deficiency on the cytokine response of the spleen to CLP-induced sepsis. As shown in Fig. 5, CLP procedure led to significant increases in protein and mRNA levels of IL-10 and IL-6. Of note, CLP-induced alterations of cytokines were remarkably suppressed by TLR9 deficiency both at the transcriptional and protein levels. For splenic CD4+ T cells cultured in vitro for 72 h, there was no significant difference in the transcriptional levels of IL-6 and IL-10 between WT and TLR9 KO septic mice (data not shown).
Fig. 5.
TLR9 deficiency dampens cytokine production in the spleen after CLP. Age-matched WT and TLR9 KO mice were subjected to CLP. At 6 h after CLP, mouse spleens were harvested. Splenic CD4+ T cells were isolated and cultured with anti -CD3/anti-CD28 antibodies for the indicated times. Levels of IL-6 and IL-10 in the supernatants were determined by ELISA (a). RNA was extracted from CD4+ T cells and quantitative real-time PCR was performed (b). Data are mean ± SD for 4 mice per group. * p < 0.05; ** p < 0.01; *** p < 0.001.
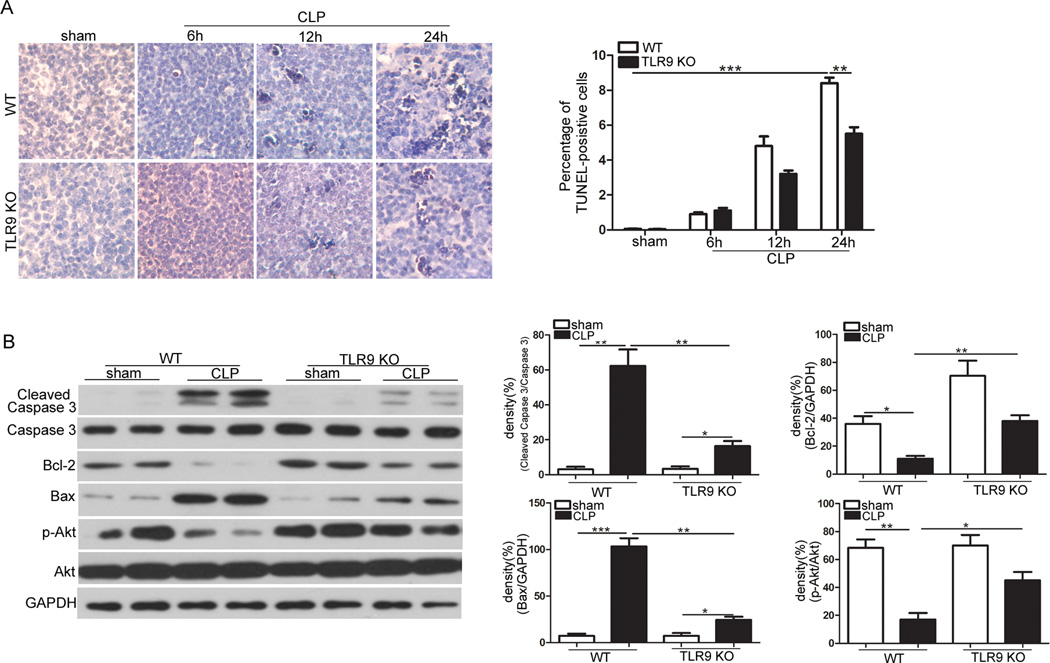
3.6. TLR9 deficiency prevents splenic apoptosis induced by CLP
Sepsis provokes apoptosis of lymphocytes in lymphoid organs including the spleen, which is correlated with poor outcome [20, 21]. To investigate whether TLR9 deficiency protects splenocytes against apoptosis in sepsis, TUNEL staining was performed. At 24 h, we found that CLP resulted in markedly-increased percent of TUNEL-positive cells (Fig. 6a). TLR9 deletion significantly attenuated CLP-induced reduction of splenocytes. Furthermore, cleaved caspase-3, Bcl-2 and Bax, good indicators of apoptosis, were also evaluated in the current study. Fig. 6b shows that CLP provoked expression of cleaved caspase-3 and Bax, but decreased the level of Bcl-2 protein as shown by Western blot analysis. Importantly, these alterations were greatly prevented by TLR9 ablation.
Fig. 6.
TLR9 deficiency protects splenocytes against sepsis-induced apoptosis. a Age-matched WT and TLR9 KO mice (n=5 per group) underwent CLP operation. Spleens were harvested at the indicated times after CLP. Apoptotic cells in spleens examined by TUNEL staining. TUNEL data are represented as mean ± SD. b Age-matched WT and TLR9 KO mice underwent CLP operation. Spleens were harvested at 6 after CLP. Cellular lysates were isolated. Expression of cleaved caspase-3, Bcl-2, Bax and phospho-Akt were determined by immunoblotting. The values are mean ± S.E.M. of three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001.
4. Discussion
TLRs play a crucial role in a number of pathological processes including sepsis. Based on this notion, previous studies put focus on the development of drugs (TLR agonists or antagonists) for improving sepsis management [22]. Especially TLR4 antagonists have attracted much interest into their efficacy and safety in patients with severe sepsis. By contrast, here we discovered that blockade of TLR9 dampened excessive inflammatory response in sepsis and decreased the mortality of septic animals, implicating that TLR9 inhibition may form the basis of a new strategy for the clinical treatment of sepsis.
Of the identified TLRs in mammals, TLR9 is located in the endosomal compartment and recognizes unmethylated CpG motifs in viral and bacterial DNA [4]. In recent years, TLR9 has entered the focus of sepsis-associated researches, and been demonstrated to be an important player in the pathogenesis of sepsis. For example, previous publications show that both pharmacological and genetical inhibition of TLR9 decreased mortality after polymicrobial sepsis and protected from acute kidney injury, a frequent and serious complication of sepsis [23, 24]. Additionally, TLR9 is suggested to be a key mediator for the induction of vascular dysfunction in polymicrobial sepsis [25]. In the colon ascendens stent peritonitis-induced sepsis model, TLR9 deficient mice exhibited significant reduction of cardiac inflammation and a sustained hear function compared with their WT counterparts [26]. Consistently, administration of CpG-ODN, a TLR9 agonist, induced increased levels of inflammatory cytokines and nuclear factor _B activity in hearts [27].
The mechanisms by which TLR9 inhibition restrains the excessive inflammatory response in sepsis need to be elucidated. It is well appreciated that p38 is a critical mediator of the release of proinflammatory cytokines [28, 29]. Importantly, TLR9 activation positively regulates p38 activity in multiple conditions [30, 31]. In this regard, we investigated whether TLR9 deficiency affect activation of p38 in CLP-induced sepsis. Our results demonstrating dampened p38 phosphorylation by TLR9 deficiency indicate that decreased p38 activation may be involved in the protection of TLR9 inhibition against inflammatory response in sepsis. PI3K/Akt signaling pathway is a negative regulator of TLR-mediated innate immune response [32]. It has been shown that PI3K/Akt pathway plays a pivotal role in the negative regulation of inflammatory process in sepsis [33]. An earlier study on Akt transgenic mice documented a marked improvement in sepsis survival after CLP [34]. In the current study, we observed preserved Akt phophorylation in septic TLR9 KO mice, indicating that activation of PI3K/Akt pathway may be an additional mechanism for the protective effect of TLR9 inhibition against sepsis. Negative regulation of Akt activity by TLR9 was also noted in our previous publication [35] and the mechanisms underlying this would be investigated in our future work.
Sepsis provokes immune cell apoptosis, which has been identified as one of the key pathways involved in the pathophysiology of sepsis [36]. Indeed, the notion that prevention of apoptosis is a promising therapy in sepsis has been supported by numerous experimental studies which documented improved survival by suppressing apoptosis in the sepsis models [34, 37]. Getts et al recently demonstrated that inflammatory monocytes undergo apoptosis in the spleen with the sodium thioglycollate (TG) peritoneal inflammation mouse model of colitis [38]. Even in CLP sepsis model, methods that interfere with the progress of apoptosis have been demonstrated to prevent cell apoptosis and protect against polymicrobial endotoxic shock, further indicating that apoptosis promotes the induction of sepsis [39,40]. In the current study, we observed that TLR9 ablation prevented apoptosis in the spleen after CLP as demonstrated by decreased percent of TUNEL-positive cells, lowered expression levels of cleaved caspase-3 and Bax, but increased Bcl-2 protein when compared to WT littermates. Therefore, prevention of apoptosis might be involved in the protective mechanisms of TLR9 deficiency against septic damage. Simultaneously, we found enhanced Akt activation in the spleen of TLR9 KO mice. Thus, there is a possibility that activation of PI3K/Akt signaling pathway is involved in the protection ofTLR9 deficiency against splenic apoptosis. Congruously, previous studies reported that inhibition of Akt promotes apoptosis but overexpression of Akt prevents against apoptosis in the spleen of septic animals [33, 34].
In the present study, the inhibition of TLR9 deficiency on induction of sepsis is unlikely dependent on its limit on bacteria spread, since there was no significant difference in the levels of both blood and peritoneal bacteria between WT and TLR9 KO septic mice (data not shown). This is consistent with the notion that death in early sepsis (days 2 to 4) is mainly due to hyper-inflammation but not bacterial overgrowth which plays a major role in late sepsis (days 14 to 16) [11]. Additionally, the mechanisms of the protective effect of TLR9 deficiency might also be related to the attenuation of vascular dysfunction [25] or improvement of cardiac dysfunction [27] due to its down-regulation of inflammatory cytokines.
In summary, TLR9 ablation suppressed p38 activation, augmented Akt phosphorylation, restrained cytokine expression, prevented apoptosis of splenocytes and improved survival in sepsis. These results implicate the beneficial effectTLR9 inhibition in sepsis and open a novel promising target for the management of sepsis.
Highlights.
-
►
TLR9 deficiency decreased the mortality of mice following CLP-induced sepsis.
-
►
TLR9 KO mice showed dampened p38 activation in the spleen, lung and liver.
-
►
TLR9 deficiency augmented Akt phosphorylation in the spleen, lung and liver.
-
►
TLR9 deficiency decreased the levels of inflammatory cytokines and attenuated splenic apoptosis after CLP.
Acknowledgments
This work was supported in part by research grants NIGMS94740 and NIGMS114716 from the National Institutes of Health to D. Yin. This research was supported in part by the National Institutes of Health grant C06RR0306551. This work was also supported in part by the National Natural Science Foundation of China 81401051 to D. Hu.
Abbreviations
- TLR9
toll like receptor 9
- CLP
Cecal ligation and puncture
- MAPK
mitogen-activated protein kinase
- TUNEL
terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Wittebole X, Castanares-Zapatero D, Laterre PF. Toll-like receptor 4 modulation as a strategy to treat sepsis. Mediators Inflamm. 2010;2010:568396. doi: 10.1155/2010/568396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennessy EJ, Parker AE, O'Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 7.Ehrentraut S, Lohner R, Schwederski M, Ehrentraut H, Boehm O, Noga S, Langhoff P, Baumgarten G, Meyer R, Knuefermann P. In vivo Toll-like receptor 4 antagonism restores cardiac function during endotoxemia. Shock. 2011;36:613–620. doi: 10.1097/SHK.0b013e318235805f. [DOI] [PubMed] [Google Scholar]
- 8.Bergt S, Wagner NM, Heidrich M, Butschkau A, Noldge-Schomburg GE, Vollmar B, Roesner JP. Hydrocortisone reduces the beneficial effects of toll-like receptor 2 deficiency on survival in a mouse model of polymicrobial sepsis. Shock. 2013;40:414–419. doi: 10.1097/SHK.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 9.Plitas G, Burt BM, Nguyen HM, Bamboat ZM, DeMatteo RP. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med. 2008;205:1277–1283. doi: 10.1084/jem.20080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemeth ZH, Csoka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Hasko G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brudecki L, Ferguson DA, Yin D, Lesage GD, McCall CE, El Gazzar M. Hematopoietic stem-progenitor cells restore immunoreactivity and improve survival in late sepsis. Infect Immun. 2012;80:602–611. doi: 10.1128/IAI.05480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Hu D, Fan H, Zhang Y, LeSage GD, Caudle Y, Stuart C, Liu Z, Yin D. beta-Arrestin 2 negatively regulates Toll-like receptor 4 (TLR4)-triggered inflammatory signaling via targeting p38 MAPK and interleukin 10. J Biol Chem. 2014;289:23075–23085. doi: 10.1074/jbc.M114.591495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu D, Wan L, Chen M, Caudle Y, LeSage G, Li Q, Yin D. Essential role of IL-10/STAT3 in chronic stress-induced immune suppression. Brain Behav Immun. 2014;36:118–127. doi: 10.1016/j.bbi.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu D, Denney J, Liang M, Javer A, Yang X, Zhu R, Yin D. Stimulatory Toll-like receptor 2 suppresses restraint stress-induced immune suppression. Cell Immunol. 2013;283:18–24. doi: 10.1016/j.cellimm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Woodruff M, Miao J, Hanley G, Stuart C, Zeng X, Prabhakar S, Moorman J, Zhao B, Yin D. Toll-like receptor 4 mediates chronic restraint stress-induced immune suppression. J Neuroimmunol. 2008;194:115–122. doi: 10.1016/j.jneuroim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M, Wu J, Martin CM, Kvietys PR, Rui T. Important role of p38 MAP kinase/NF-kappaB signaling pathway in the sepsis-induced conversion of cardiac myocytes to a proinflammatory phenotype. Am J Physiol Heart Circ Physiol. 2008;294:H994–H1001. doi: 10.1152/ajpheart.01044.2007. [DOI] [PubMed] [Google Scholar]
- 17.Obata T, Brown GE, Yaffe MB. MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit Care Med. 2000;28:N67–77. doi: 10.1097/00003246-200004001-00008. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y, Yang Y, Li Y, Xu L, Wang Y, Guo Z, Song H, Yang M, Luo B, Zheng A, Li P, Zhang Y, Ji G, Yu Y. Ephedrine hydrochloride inhibits PGN-induced inflammatory responses by promoting IL-10 production and decreasing proinflammatory cytokine secretion via the PI3K/Akt/GSK3beta pathway. Cell Mol Immunol. 2013;10:330–337. doi: 10.1038/cmi.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koroglu TF, Yilmaz O, Gokmen N, Tugyan K, Baskin H, Egrilmez MY. Erythropoietin prevents lymphoid apoptosis but has no effect on survival in experimental sepsis. Pediatr Res. 2013;74:148–153. doi: 10.1038/pr.2013.86. [DOI] [PubMed] [Google Scholar]
- 21.Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A. In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106:2295–2301. doi: 10.1182/blood-2004-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savva A, Roger T. Targeting toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front Immunol. 2013;4:387. doi: 10.3389/fimmu.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasuda H, Leelahavanichkul A, Tsunoda S, Dear JW, Takahashi Y, Ito S, Hu X, Zhou H, Doi K, Childs R, Klinman DM, Yuen PS, Star RA. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F1050–F1058. doi: 10.1152/ajprenal.00461.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Li Y, Hu Z, Su J, Huo Y, Tan B, Wang X, Liu Y. Small interfering RNA targeting Toll-like receptor 9 protects mice against polymicrobial septic acute kidney injury. Nephron Exp Nephrol. 2012;122:51–61. doi: 10.1159/000346953. [DOI] [PubMed] [Google Scholar]
- 25.Ehrentraut SF, Dorr A, Ehrentraut H, Lohner R, Lee SH, Hoeft A, Baumgarten G, Knuefermann P, Boehm O, Meyer R. Vascular dysfunction following polymicrobial sepsis: role of pattern recognition receptors. PLoS One. 2012;7:e44531. doi: 10.1371/journal.pone.0044531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohner R, Schwederski M, Narath C, Klein J, Duerr GD, Torno A, Knuefermann P, Hoeft A, Baumgarten G, Meyer R, Boehm O. Toll-like receptor 9 promotes cardiac inflammation and heart failure during polymicrobial sepsis. Mediators Inflamm. 2013;2013:261049. doi: 10.1155/2013/261049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knuefermann P, Schwederski M, Velten M, Krings P, Ehrentraut H, Rudiger M, Boehm O, Fink K, Dreiner U, Grohe C, Hoeft A, Baumgarten G, Koch A, Zacharowski K, Meyer R. Bacterial DNA induces myocardial inflammation and reduces cardiomyocyte contractility: role of toll-like receptor 9. Cardiovasc Res. 2008;78:26–35. doi: 10.1093/cvr/cvn011. [DOI] [PubMed] [Google Scholar]
- 28.Kraatz J, Clair L, Rodriguez JL, West MA. Macrophage TNF secretion in endotoxin tolerance: role of SAPK, p38, and MAPK. J Surg Res. 1999;83:158–164. doi: 10.1006/jsre.1999.5587. [DOI] [PubMed] [Google Scholar]
- 29.Branger J, van den Blink B, Weijer S, Madwed J, Bos CL, Gupta A, Yong CL, Polmar SH, Olszyna DP, Hack CE, van Deventer SJ, Peppelenbosch MP, van der Poll T. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J Immunol. 2002;168:4070–4077. doi: 10.4049/jimmunol.168.8.4070. [DOI] [PubMed] [Google Scholar]
- 30.De Nardo D, De Nardo CM, Nguyen T, Hamilton JA, Scholz GM. Signaling crosstalk during sequential TLR4 and TLR9 activation amplifies the inflammatory response of mouse macrophages. J Immunol. 2009;183:8110–8118. doi: 10.4049/jimmunol.0901031. [DOI] [PubMed] [Google Scholar]
- 31.Xin BM, Wang XX, Jin W, Yan HM, Cui B, Zhang XW, Hua F, Yang HZ, Hu ZW. Activation of Toll-like receptor 9 attenuates unilateral ureteral obstruction-induced renal fibrosis. Acta Pharmacol Sin. 2010;31:1583–1592. doi: 10.1038/aps.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 33.Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 34.Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. Akt decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol. 2004;172:7583–7591. doi: 10.4049/jimmunol.172.12.7583. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Shi W, Li H, Sun X, Fan X, Lesage G, Li Y, Zhang Y, Zhang X, Yin D. Critical role of toll-like receptor 9 in morphine and Mycobacterium tuberculosis-Induced apoptosis in mice. PLoS One. 2010;5:e9205. doi: 10.1371/journal.pone.0009205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell JA, Boyd J, Nakada T, Thair S, Walley KR. Molecular mechanisms of sepsis. Contrib Microbiol. 2011;17:48–85. doi: 10.1159/000324009. [DOI] [PubMed] [Google Scholar]
- 37.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 38.Getts DR, Terry RL, Getts MT, Deffrasnes C, Müller M, Vreden CV, Ashhurst TM, et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med. 2014 Jan 15;6(219):219ra7. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda N, Takano Y, Kageyama S, Hatakeyama N, Shakunaga K, Kitajima I, Yamazaki M, Hattori Y. Silencing of caspase-8 and caspase-3 by RNA interference prevents vascular endothelial cell injury in mice with endotoxic shock. Cardiovasc Res. 2007;76:132–140. doi: 10.1016/j.cardiores.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Groesdonk HV, Wagner F, Hoffarth B, Georgieff M, Senftleben U. Enhancement of NF-kappaB activation in lymphocytes prevents T cell apoptosis and improves survival in murine sepsis. J Immunol. 2007;179:8083–8089. doi: 10.4049/jimmunol.179.12.8083. [DOI] [PubMed] [Google Scholar]