Abstract
Objective
The Department of Veterans Affairs (VA) uses the 11-point pain numeric rating scale (NRS) to gather pain intensity information from veterans at outpatient appointments. Yet, little is known about how NRS scores may vary over time within individuals; NRS variability may have important ramifications for treatment planning. Our main objective was to describe variability in NRS scores within a one-month timeframe, as obtained during routine outpatient care in older patients with chronic pain treated in VA hospitals. A secondary objective was to explore for patient characteristics associated with within-month NRS score variability.
Design
Retrospective cohort study.
Subjects
National sample of veterans 65 years or older seen in VA in 2010 who had multiple elevated NRS scores indicating chronic pain.
Methods
VA datasets were used to identify the sample and demographic and clinical variables including NRS scores. For the main analysis, we identified subjects with 2 or more NRS scores obtained in each of 2 or more months in a 12 month period; we examined ranges in NRS scores across the first 2 qualifying months.
Results
Among 4,336 individuals in the main analysis cohort, the mean and median of the average NRS score range across the two months were 2.7 and 2.5, respectively. In multivariable models, main significant predictors of within-month NRS score variability were baseline pain intensity, overall medical comorbidity, and being divorced/separated.
Conclusions
The majority of patients in the sample had clinically meaningful variation in pain scores within a given month. This finding highlights the need for clinicians and their patients to consider multiple NRS scores when making chronic pain treatment decisions.
Keywords: Chronic pain, Veterans, Numeric Rating Scale, Aged
Introduction
The prevalence of chronic non-cancer pain (CNCP) is estimated to be 10% to 20% in the general population (1,2). CNCP is especially common among veterans; up to half of veterans treated in Department of Veterans Affairs (VA) primary care settings have CNCP (3–6). Moreover, CNCP is associated with deficits in social function and well-being (7), increased morbidity and mortality (7), decreased satisfaction with healthcare (8), and increased healthcare utilization (9). Older adults are at high risk for pain problems—and pain treatment may be complicated by other chronic medical and psychiatric conditions (10), as well as the need to take multiple medications (11).
Since 1998, as one part of a national strategy to improve pain care (“Pain as the 5th Vital Sign” (12,13)), the VA has collected pain intensity score information from veterans as part of routine care during most outpatient clinical encounters; this information is available to clinicians in the electronic medical record and can be used to facilitate clinical decision-making about pain. These data also recently became available to researchers through VA’s national Corporate Data Warehouse (CDW).
Specifically, veterans are administered the 11-point Numeric Rating Scale (NRS) (14). The version of the NRS used in the VA rates a patient’s pain intensity as experienced “today” on a scale of 0 to 10, with 0 representing no pain and 10 representing worst possible pain. The NRS remains a standard for briefly measuring pain intensity and has been validated in a number of clinical contexts and patient populations (15,16). However, relatively little is known about how the NRS performs in clinical settings when administered by clinical personnel. Several investigations of VA patient populations (17–19) have shown that the accuracy of the NRS in measuring pain as administered during routine care is modest; problems with accuracy may largely reflect nurses’ lack of adherence to administering standardized NRS items.
It is also unknown how NRS scores may vary over time. This information has potential clinical value. If variability over time is small, a single NRS score may be useful for understanding a patient’s recent pain experience or response to a treatment change. Conversely, if NRS variability over time is large, single NRS values will have less clinical utility. Furthermore, some pain or comorbid conditions may be associated with more constant levels of pain intensity, while other conditions may be associated with more variable levels of pain intensity. Clinicians may benefit from awareness of relationships among patient or disease characteristics and pain intensity variability when making treatment decisions based on pain intensity scores.
In our review of the literature, we were able to identify only one study which found that fibromyalgia patients who had greater variability in pain scores were more likely to respond to placebo in a drug trial (20); we identified no published manuscripts describing how NRS scores may vary over time in routine practice. This is potentially important, as chronic pain guidelines recommend that clinicians systematically monitor response to treatment and make changes in opioid and other pain treatment based on treatment response (21–23). Such guidelines recommend monitoring outcomes every 3 to 6 months when regimens are stable, but more frequently when treatment changes are made. Yet, in current primary care practice, where most chronic pain care is provided, if a change is made in treatment, it is not likely that multiple measurements will be available before a subsequent treatment decision is required; clinicians may have only one score on hand to incorporate into clinical decision-making.
The primary objective of this project was therefore to describe variability in NRS scores as obtained during routine clinical care in an older patient population in the VA within a one-month period (defined here as short-term variability). A secondary objective was to explore for patient-related demographic and clinical characteristics that may be associated with short-term variability in NRS scores.
Materials and Methods
This study was reviewed and approved by the Institutional Review Board of the Veterans Affairs Medical Center (VAMC) where the study was conducted. The study was considered exempt from requiring informed consent as it was a secondary analysis of existing data contained in VA administrative datasets.
Sources of Data
In the VA, pain data are recorded as structured vital sign data in the electronic health record. These data are readily linked with outpatient and inpatient utilization, pharmacy, diagnosis, and demographic data available in VA’s national CDW. The CDW is a rich, multilevel database that combines electronic health record data collected across 1,400 points of care for over 20 million veterans historically. VA researchers can access these data through the VA Informatics and Computing Infrastructure (VINCI). Within VINCI, project-specific databases are created and accessed using a suite of tools available to securely select, transform, and analyze data.
Sample
Our goal was to identify a national cohort of older (≥ age 65) veterans with indicators of chronic pain. A retrospective sample was obtained from the population of 5.9 million VA patients who had at least one ambulatory VA visit in 2010. Of these, 82% (4,834,884) had at least one NRS score recorded in the study year 2010. For each patient, all outpatient NRS scores available within a given month were averaged to produce a monthly pain score. If a given month included only one recorded pain score, this score was used as the respective monthly pain score for that patient. To be included in this study, we selected patients who had monthly pain scores from at least 3 different months within a 12-month period that were 4 or greater (defined as qualifying scores). Each candidate patient in the sample was assigned an index date, defined as the last day of the month in which the most recent qualifying monthly pain score was obtained.
While there is no gold standard for identifying a chronic pain patient population using large datasets, our operational definition of chronic pain is consistent with commonly used definitions for chronic pain of moderate or greater intensity and our and other investigators’ prior work using similar operational definitions for chronic pain (24–30). Use of similar methods facilitates potential comparisons across studies and populations. We chose a cutoff NRS score of 4 because of its consistency with VA clinical practice and policy regarding indication for further evaluation of pain; in addition, scores of 4 or greater are also indicative of moderate-to-severe pain (22,31,32). Finally, Tian et al (33) recently developed and validated an algorithm to identify chronic pain that combines pain intensity scores, International Classification of Diseases, Clinical Modification 9 (ICD-9-CM) diagnostic codes, and opioid prescription medications; in their analysis, addition of pain diagnoses resulted in 85% sensitivity and 98% specificity (ROC 0.98) using clinician documentation of pain condition as a standard. Therefore, we further refined our sample using ICD-9-CM diagnostic codes (Table 1) from outpatient and inpatient visits, selecting patients who had at least one pain diagnosis made by VA clinicians during the 12 months prior to the index date.
Table 1.
International Classification of Diseases, ninth edition, clinical modification (ICD-9-CM) codes used to identify pain and psychiatric diagnoses
| Pain | Chronic neck/joint pain | 716 – 719.99; 723 – 723.99; 729 – 729.09; 729.2 – 729.99 |
| Rheumatism/Arthritis/Gout | 274 – 274.99; 712 – 712.99; 714 – 716.99; 720 – 720.99; 729 – 729.09; 729.20 – 729.99 | |
| Fibromyalgia | 729.1 | |
| Chronic low back pain | 722 – 722.99; 724 – 724.99 | |
| Neuropathies1 | 250.6 – 256.69; 337.0 – 337.19; 354 – 354.99; 356 – 357.99; 377 – 377.99 | |
| Headache/Migraine | 346.9 – 349.99; 784.0 | |
| Inflammatory bowel disease | 558.9 | |
| Other musculoskeletal pain | 710 – 711.99; 713 – 713.99; 721 – 721.99; 725 – 728.99; 730 – 739.99 | |
Includes diabetic neuropathy and carpal tunnel syndrome
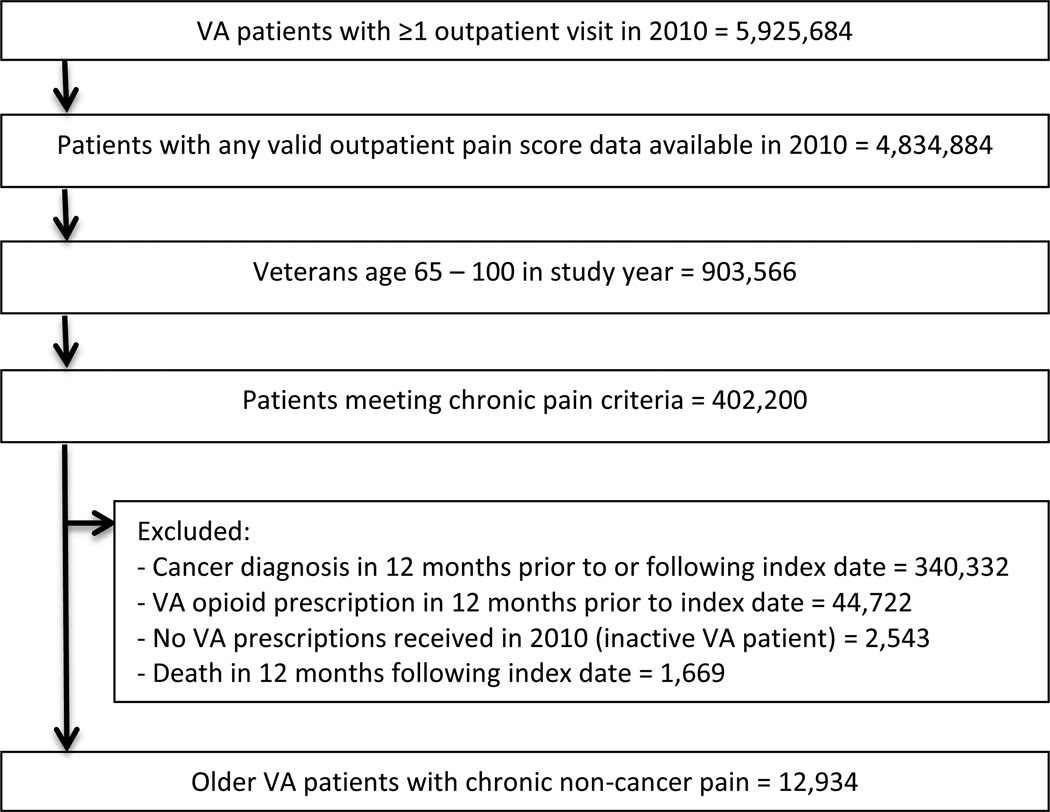
The current analysis is part of a larger research project in which we are seeking to understand associations between incident opioid use and pain scores over time. We thus excluded patients from our main cohort who had VA opioid prescriptions dispensed during the 12 months prior to the index date. We also excluded patients with documented ICD-9-CM cancer diagnoses in the 12 months prior to or after the index date—these diagnoses include malignant neoplasms, skin cancers, and carcinomas in situ: ICD-9-CM codes 140 through 208 and 230 through 239.2, inclusive. We also excluded patients who participated in a VA opioid substitution program in the 12 months prior to or following the index date and patients who died during the 12 months following the index date. After applying exclusion criteria, the final cohort included N=12,934 patients (Figure 1).
Figure 1.
Study flowchart
Measures
Dependent variables
Follow-up NRS scores over 12 months from index dates were obtained to examine short-term variability in outpatient NRS scores. Short-term variability was measured by averaging, for each individual, the within-month ranges of scores (for months with at least two scores). In order to ensure we had sufficient data (at least two measurements for each subject), for our primary analysis, we examined data from a subsample of veterans who had two or more months which each contained two or more scores; when there were more than two months with multiple scores, we examined only the first two months. We chose the first two scores in a given month because variability is sensitive to time, and the amount of time between scores might vary more when looking for highest scores.
Independent variables
Our independent variables were chosen based on prior research showing relationships among these variables and pain treatment or outcomes: pain and comorbid condition diagnoses and demographics have all been shown to predict pain prevalence and outcomes (34–41). As noted above, we were unable to identify prior studies examining associations among these variables and pain intensity variability in routine practice, but hypothesized we would detect such associations. Patient demographic variables included age (at index date), sex, race/ethnicity, marital status, and VA service-connected disability status. Ninety-three percent (12,043) of the cohort had at least one race designation on file. Asian, Pacific Islander, Native American, and other races represented cumulatively only 3% of the sample, so we collapsed these races into an “other” category. Available ethnicity categories included “not Hispanic or Latino,” “Hispanic or Latino,” “unknown,” and “declined.” We combined race and ethnicity to create 5 race/ethnicity categories: white (non-Hispanic), black (non-Hispanic), Hispanic/Latino, other (including multiple races), and unknown (including missing and declined).
Clinical variables included pain diagnoses obtained using ICD-9-CM codes recorded in the medical record in the 12 months prior to the index date (Table 1). Psychiatric diagnoses included major depression, schizophrenia, post-traumatic stress disorder (PTSD), panic or other anxiety disorder, substance use disorder including alcohol use disorder and nicotine use disorder. The baseline pain intensity score was defined as the average of all average monthly NRS pain scores beginning with the first qualifying monthly pain score and ending with the last qualifying monthly pain score prior to the index date. We measured overall medical comorbidity using the Selim index, which creates a score based on the presence of ICD-9-CM codes for 36 physical and mental conditions in the prior 12 months (42)(43). We also obtained counts of major surgeries to generate a dichotomous variable indicating whether a major surgery took place in the 12 months prior to the index date based on definitions from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP)(44).
Analysis
For the analyses, we excluded any pain scores that had been obtained during inpatient, residential, or nursing home stays and on the days of surgical and medical procedures. We also excluded scores taken on days with multiple pain scores. The rationale for these decisions was to remove scores reflecting particularly acute events so that the study would be focused on chronic pain. For example, multiple scores from an emergency department visit may be associated with acute injury or illness and with subsequent receipt of pain medications, and therefore may not be as representative of chronic pain.
The primary outcome for assessment of within-month variability was the average range of within-month pain scores, defined as high (>=2.5) vs. low (<2.5). Since the range is highly dependent on the number of scores available, our analyses fixed the amount of data used per individual. In particular, the main analysis used the first two scores within each of the first two months that had multiple scores to calculate an average within-month range. Because the distribution of the average NRS score range was highly skewed, we dichotomized at the median (2.5). We then used binomial regression (with log-link) to estimate the relative risk for having high within-month variability based on baseline pain score, age, sex, race/ethnicity, marital status, percent VA service-connected disability rating, Selim comorbidity score, occurrence of major surgery in previous year, nicotine or substance use disorder diagnosis in prior year, psychiatric disorder diagnoses (major depressive disorder, schizophrenia or bipolar disorder, post-traumatic stress disorder (PTSD), panic disorder or other anxiety disorder) in prior year, and pain diagnoses in prior year (Table 1). We collapsed Selim scores into 4 categories (0–5, 6–10, 11–15, 16–24) to reduce the impact of large Selim scores that were associated with relatively few individuals. We present relative risks for each predictor itself from univariable models as well as adjusted relative risks based on multivariable models containing all predictors. Sensitivity analyses were also conducted using the analogous outcome measure based on three months of data rather than two.
Results
Follow-up NRS pain scores were obtained for 12,934 individuals over the 12-month period following their index dates. On average, these patients had 5.4 months which included any pain scores. Of the 12,934 individuals, 4,912 (38%) did not have any months with multiple NRS scores in the follow-up period, 3,686 (28%) had exactly one month in which there were two or more scores, and 4,336 (34%) had two or more months which contained multiple pain scores; this latter group comprises our main study sample. Table 2 compares demographic and clinical characteristics of this latter group to the subjects who had been excluded from the sample due to having fewer than two months of multiple pain scores (N=8,598). We can see that patients in the main analysis subgroup (N=4,336) were more likely to be unmarried, have greater medical comorbidity, and to have had major surgery and more pain diagnoses given in the prior year versus the comparison group. Mean baseline pain score was slightly lower in the main study sample.
Table 2.
Characteristics of VA patients with CNCP, comparing short-term variability subgroup to patients not included in short-term variability subgroup
| Short-term variability subgroup (N=4,336)* |
Not in short-term variability subgroup (N=8,598)** |
p-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years):Mean(SD) | 73.51 (7.42) | 72.93 (7.13) | <0.001 |
| Sex: Male | 4,154 (95.80) | 8,319 (96.76) | 0.006 |
| Race/Ethnicity | <0.001 | ||
| White(non-Hispanic) | 2,799 (64.55) | 5,687 (66.14) | |
| Black(non-Hispanic) | 862 (19.88) | 1,435 (16.69) | |
| Hispanic | 272 (6.27) | 484(5.63) | |
| Other | 188 (4.34) | 411 (4.78) | |
| Unknown | 215 (4.96) | 581 (6.76) | |
| Marital status | <0.001 | ||
| Married | 2,280 (52.58) | 5,126 (59.62) | |
| Divorced/Separated | 1,153 (26.59) | 1,995 (23.20) | |
| Single/Never Married | 268 (6.18) | 422 (4.91) | |
| Widowed | 635 (14.64) | 1,055 (12.27) | |
| Percent service-connected disability | <0.001 | ||
| Not service-connected | 1,731 (39.92) | 3,430 (39.89) | |
| Less than 50% | 551 (12.71) | 1,303 (15.15) | |
| 50% or more | 2,054 (47.37) | 3,865 (44.95) | |
| Clinical characteristics (12 months prior to index date): | |||
| Baseline pain intensity***: Mean(SD) | 5.34 (1.61) | 5.71 (1.57) | <0.001 |
| Selim comorbidity index score | <0.001 | ||
| 0–5 | 1,130 (26.06) | 3,785 (44.02) | |
| 6–10 | 2,098 (48.39) | 3,933 (45.74) | |
| 11–15 | 851 (19.63) | 761 (8.85) | |
| 16–24 | 257 (5.93) | 119 (1.38) | |
| Major surgery | 147 (3.39) | 189 (2.20) | <0.001 |
| Any Mental health diagnosis**** | 1,904 (43.91) | 3,357 (39.04) | <0.001 |
| Nicotine or substance use disorder | 739 (17.04) | 1,402 (16.31) | 0.287 |
| Pain diagnoses | |||
| Chronic neck/joint pain | 2,763 (63.72) | 5,114 (59.48) | <0.001 |
| Rheumatism/Arthritis/Gout | 2,905 (67.00) | 5,537 (64.40) | 0.003 |
| Fibromyalgia | 198 (4.57) | 306 (3.56) | 0.005 |
| Chronic lower back pain | 2,026 (46.73) | 3,974 (46.22) | 0.587 |
| Neuropathies | 1,228 (28.32) | 1,960 (22.80) | <0.001 |
| Headache/Migraine | 363 (8.37) | 614 (7.14) | 0.012 |
| Other musculoskeletal pain | 2,082 (48.02) | 3,535 (41.11) | <0.001 |
Short term variability subgroup = main study sample: Patients with two or more months which contained multiple numeric rating scale (NRS) pain intensity scores
Not in short term variability subgroup: Patients who had less than two months during the follow-up period in which there were two or more scores
Baseline pain intensity score is average of all monthly NRS pain scores beginning with first qualifying monthly pain score and ending with last qualifying monthly pain score prior to the index date.
Includes major depression, anxiety disorder, PTSD, bipolar disorder or schizophrenia
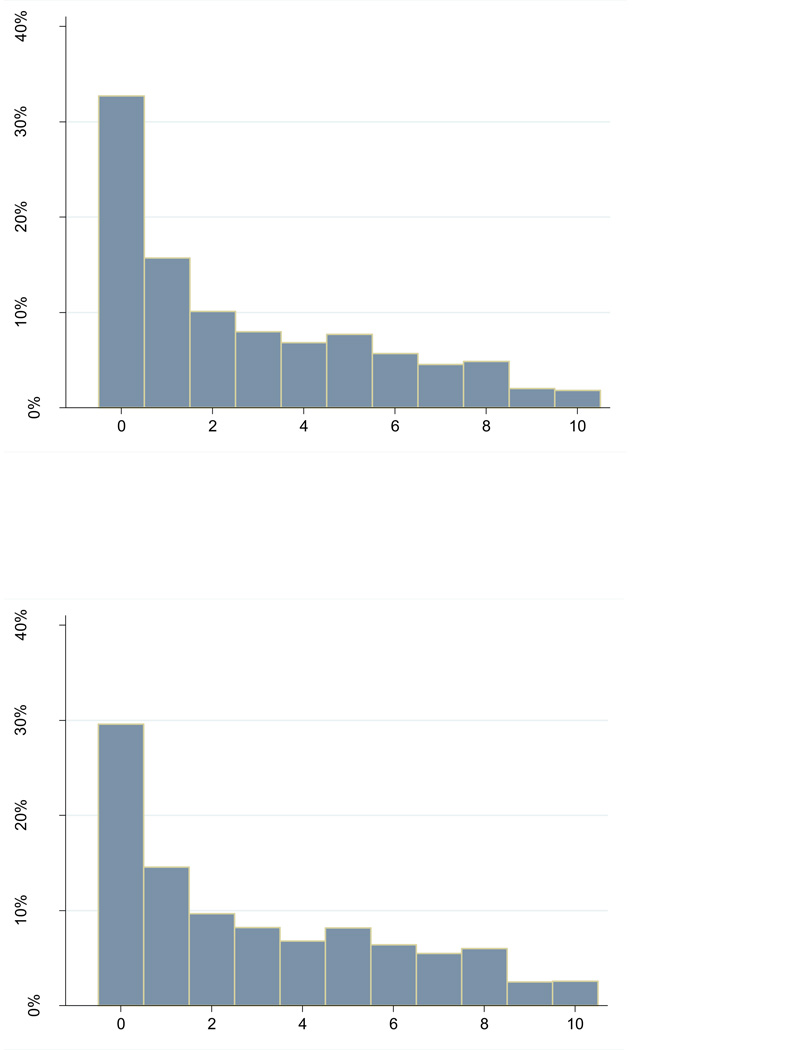
Figure 2 shows the distributions of the pain score ranges in the main study sample. For comparison we also examined the pain score ranges within the larger sample of all patients who had any months with two or more scores (N=8,022) (also Figure 2); in this latter case, we used all the scores obtained in months that had multiple scores. As can be seen, the two distributions are quite similar, but as expected, when we included more than 2 scores per month, the within-month ranges tend to be a little larger. In the main analysis, the mean and median of the pain score ranges (across 2 months), were 2.7 and 2.5, respectively.
Figure 2.
Distributions of monthly pain score ranges
Range of first 2 NRS scores within first 2 months for 4,366 individuals who had 2 or more scores in 2 or more months (main analysis sample)
Range in all NRS scores within all months for 8,022 individuals who had 2 or more scores in any month
We next examined potential predictors of within-month variability using the main study sample (N=4,336). Table 3 provides the unadjusted (univariable) and adjusted (multivariable) relative risks for high within-month variability based on demographic and clinical characteristics. In particular we found that black and Hispanic patients (compared to whites), divorced/separated patients (compared to married individuals), those with high Selim scores, and those with diagnosis of headache/migraine pain were more likely to have high short-term variability. Also, the greater a patient’s baseline pain intensity, the greater the short-term variability in follow-up pain score.
Table 3.
Univariable and Multivariable models of short term variability subjects, N=4,336 [Outcome = Range of first two scores in first 2 months greater than 2.5 (median). Low group: 0–2, High group: 2.5–10]
| Univariable Risk Ratio (95% CI) |
p-value | Multivariable Risk Ratio (95% CI) |
p-value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years): Mean(SD) | 1.00 (1.00, 1.00) | 0.533 | 1.00 (1.00, 1.01) | 0.599 |
| Sex | ||||
| Male | 0.94 (0.83, 1.07) | 0.367 | 0.96 (0.84, 1.10) | 0.548 |
| Female | Reference | Reference | ||
| Race/Ethnicity | 0.005 | 0.006 | ||
| White, non-Hispanic | Reference | Reference | ||
| Black, non-Hispanic | 1.12 (1.04, 1.20) | 0.001 | 1.11 (1.04, 1.19) | 0.003 |
| Hispanic | 1.13 (1.02, 1.26) | 0.024 | 1.14 (1.03, 1.27) | 0.014 |
| Other | 1.08 (0.95, 1.23) | 0.251 | 1.10 (0.96, 1.25) | 0.160 |
| Unknown | 0.97 (0.85, 1.12) | 0.683 | 0.98 (0.85, 1.13) | 0.783 |
| Marital status | 0.045 | 0.057 | ||
| Married | Reference | Reference | ||
| Divorced/Separated | 1.09 (1.02, 1.17) | 0.007 | 1.10 (1.03, 1.17) | 0.007 |
| Single/Never Married | 1.04 (0.93, 1.17) | 0.479 | 1.04 (0.92, 1.18) | 0.497 |
| Widowed | 1.07 (0.99, 1.16) | 0.098 | 1.05 (0.97, 1.15) | 0.223 |
| Percent service-connected disability rating | 0.835 | 0.406 | ||
| Not service-connected | Reference | Reference | ||
| Less than 50% | 1.01 (0.92, 1.10) | 0.857 | 1.01 (0.93, 1.11) | 0.792 |
| 50% or more | 1.02 (0.96, 1.08) | 0.548 | 1.04 (0.98, 1.11) | 0.186 |
| Clinical characteristics: 12 months prior to index date | ||||
| Baseline average NRS: Mean* | 1.02 (1.00, 1.04) | 0.028 | 1.02 (1.00, 1.04) | 0.011 |
| Selim comorbidity index score | 0.001 | 0.002 | ||
| 0–5 | Reference | Reference | ||
| 6–10 | 0.96 (0.90, 1.03) | 0.231 | 0.96 (0.89, 1.03) | 0.211 |
| 11–15 | 1.02 (0.94, 1.10) | 0.682 | 1.01 (0.93, 1.10) | 0.801 |
| 16–24 | 1.19 (1.07, 1.32) | 0.002 | 1.17 (1.04, 1.31) | 0.007 |
| Major surgery | 1.07 (0.93, 1.24) | 0.340 | 1.08 (0.93, 1.24) | 0.304 |
| Any Mental health diagnosis** | 1.01 (0.95, 1.07) | 0.779 | 1.00 (0.94, 1.06) | 0.931 |
| Substance use disorder including nicotine | 1.00 (0.93, 1.08) | 0.929 | 0.99 (0.92, 1.07) | 0.870 |
| Pain diagnoses | ||||
| Chronic neck/joint pain | 1.06 (1.00, 1.12) | 0.064 | 1.02 (0.96, 1.09) | 0.447 |
| Rheumatism/Arthritis/Gout | 1.06 (0.99, 1.12) | 0.076 | 1.05 (0.98, 1.11) | 0.151 |
| Fibromyalgia | 1.05 (0.93, 1.19) | 0.436 | 1.05 (0.93, 1.20) | 0.415 |
| Chronic lower back pain | 0.96 (0.91, 1.01) | 0.134 | 0.95 (0.90, 1.01) | 0.101 |
| Neuropathies | 1.03 (0.96, 1.09) | 0.422 | 1.03 (0.96, 1.09) | 0.431 |
| Headache/Migraine | 1.10 (1.01, 1.21) | 0.037 | 1.11 (1.01, 1.21) | 0.030 |
| Other musculoskeletal pain | 1.04 (0.98, 1.10) | 0.171 | 1.03 (0.97, 1.09) | 0.382 |
For every 1 unit increase in baseline NRS, the estimated increase in risk of having high short-term pain score variability is 2%
Includes Major Depression, Anxiety, PTSD, Bipolar or Schizophrenia
For our planned sensitivity analysis, when restricting analyses to the 2,353 patients who had three or more months containing two or more scores, overall estimates remained similar, and marital status, Selim score and baseline NRS score remained significantly associated with greater within-month variability (p=0.008 for highest Selim category compared to lowest; p=0.041 for divorced/separated compared to married; and p<0.001 for baseline NRS score) (results not shown). However, in this model, race/ethnicity and headache/migraine pain diagnosis were no longer significant predictors of within-month pain score variability (p≥0.233 for each race category as compared to whites; p=0.490 for headache/migraine).
Discussion
To our knowledge, this study is the first to examine variability over time in pain intensity scores obtained during routine outpatient practice from a large national sample of patients. We found a median change in within-month pain score of 2. We also found that the main predictors of within-month variability in pain score were baseline level of pain intensity, overall medical comorbidity, and being divorced or separated. While black and Hispanic patients were more likely to have greater variability in scores in our main model, this difference became non-significant in our sensitivity analysis which incorporated three months of data rather than two.
In our main analysis, although approximately 30% of the months evaluated had within-month NRS ranges of 0, more than 50% of the months had ranges in NRS scores that were greater than 2. A change of 2 in NRS represents a 36% change based on a mean baseline score of 5.5 in the sample. Using varying reference standards, several studies (45,46) suggest that changes of 2 or of 30%, in NRS scores constitute clinically important changes. Thus, the majority of patients in the sample had clinically meaningful variation in pain scores within a given month. There are a number of potential sources for this variation including fluctuations in the intensity of pain or the conditions contributing to pain, application of new interventions for pain, or factors related to NRS administration (18,47). While our study findings support the potential for NRS scores from routine practice to be useful for understanding pain outcomes, the results suggest that synthesis of NRS scores obtained over multiple time points is indicated when NRS scores are to be used in clinical decision-making or as outcomes in research.
The results specifically highlight the need for clinicians to keep in mind that short-term fluctuations in pain intensity in ambulatory patients are likely to be common, and that they should avoiding making chronic pain treatment decisions based on scores obtained from single visits. Furthermore, providing basic education to chronic pain patients that the severity of their pain is expected to fluctuate, and discussing with them that measuring pain intensity over time, and in the context of what is perhaps a usual level of pain intensity for the patient, may be more useful for clinical decision making (48).
Our results further support that pain intensity may not be a particularly useful measure by itself or for monitoring outcomes of chronic pain treatment in outpatient settings. VA’s Pain as the 5th Vital Sign initiative was initially conceived primarily as a mechanism for screening patients for unidentified, unrelieved pain (12), not as a treatment monitoring mechanism. Indeed, measures of function are highly recommended for monitoring outcomes over time and for guiding treatment of chronic pain (48). Unfortunately, functional measures are not currently available in national VA datasets. Incorporation of systematically-obtained, serial, patient-reported measures of pain (intensity and function) into routine care in a way that can facilitate decision-making based on data from multiple time points is clearly indicated.
Our study results identified certain demographic and clinical factors that predict greater variability in pain NRS scores. These include higher baseline pain intensity, race/ethnicity, marital status, medical comorbidity, and diagnosis of headache/migraine. Several studies have demonstrated race/ethnicity differences and disparities in screening, ratings of pain, and treatment of pain (49–52). However we note that race/ethnicity was not significantly associated with NRS variability in our planned sensitivity analysis which examined variability using three months of data. Here, we suggest that some factors (e.g., multiple treatment changes or disease progression) may have a greater influence on NRS scores when examining NRS scores over longer time frames. Clearly more research is needed in this particular area.
While we detected significant relationships between certain variables and short-term variability in NRS scores, we note that the magnitude of the differences we found were fairly small—in the 10% range; to what extent these differences may be meaningful in practice is debatable. The variable demonstrating the greatest increase (17%) in probability of greater variability was overall medical comorbidity—this suggests perhaps that comorbid medical conditions may contribute substantively to the experience of pain intensity. Somewhat contrary to our expectations, mental health diagnoses were not associated with increased variability; prior research has demonstrated that mental health comorbidity is associated with worse pain outcomes as well as propensity to be prescribed opioids (53,54). Taken together with our findings, this research suggests that the factors that predict short-term variability in pain intensity may be different than the factors that affect overall pain prognosis, and that the factors that may most strongly predict short-term variability may not be captured in administrative healthcare datasets.
There are a number of important limitations to this study—the first regards generalizability of our findings. For this study’s main analysis, the sample was restricted to those patients who were seen frequently enough to have multiple pain intensity scores obtained in two or more months. As can be seen in Table 2, there were differences when comparing these patients to patients less frequently administered the NRS. In post-hoc analyses, we examined clinical and demographic characteristics when comparing our main study sample to patients who had been excluded from the overall cohort due to having been prescribed an opioid in the year prior to the index date and to those excluded due to cancer. In these comparisons (results not shown), there were few meaningful differences seen aside from a higher prevalence of major surgery in the year prior to index dates in the prior opioid subgroup and the cancer subgroup, and a higher prevalence of back pain in the prior opioid subgroup. Overall, these comparisons suggest that in many ways our main study sample was representative of the larger group of older patients with chronic pain treated in VA, but that frequency of administration of NRS scores (likely reflecting morbidity and care utilization) and prior major surgery may have impacts on short-term variability in NRS scores that our study did not detect.
There are additional limitations: Although we used methods similar to those of prior studies, there is no gold standard for identifying a chronic pain patient population using a large administrative dataset. We also note that we were not able to account for the impact of new pain treatments that may have been initiated between subjects’ first and second pain scores. Our sensitivity analysis did attempt to account for effects of differences in how we defined the study period, and did show somewhat different results. This finding suggests that variability in pain intensity scores may change over time within individuals. Further examination of this result would be important, but was beyond the scope of this study. Although researchers have examined validity and reliability of NRS in a number of patient populations, we had no way of ascertaining to what extent cognitive characteristics of patients or characteristics of administering NRS to this older patient population may have influenced the NRS data obtained. Finally, in our models, we had limited data available from VA administrative datasets to explore for correlates of variability.
Acknowledgments
The authors wish to acknowledge the assistance of Holly B. Williams BA with literature review and synthesis.
Funding Sources
This material is based upon work supported by the National Institutes of Health, National Institute on Aging, R03-AG042756 (PI: Dobscha) and the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service, CIN 13-404 (PI: Dobscha). Dr. Dobscha is the Director of the Center to Improve Veteran Involvement in Care (CIVIC) at the Portland VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, National Institutes of Health, or United States government.
Footnotes
Disclosures
There are no relevant conflicts of interest to report for any of the authors.
REFERENCES
- 1.Verhaak PF, Kerssens JJ, Dekker J, Sorbi MJ, Bensing JM. Prevalence of chronic benign pain disorder among adults: A review of the literature. Pain. 1998;77(3):231–239. doi: 10.1016/S0304-3959(98)00117-1. [DOI] [PubMed] [Google Scholar]
- 2.Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92(1–2):195–200. doi: 10.1016/s0304-3959(00)00483-8. [DOI] [PubMed] [Google Scholar]
- 3.Kerns RD, Otis J, Rosenberg R, Reid MC. Veterans' reports of pain and associations with ratings of health, health-risk behaviors, affective distress, and use of the healthcare system. J Rehabil Res Dev. 2003;40(5):371–379. doi: 10.1682/jrrd.2003.09.0371. [DOI] [PubMed] [Google Scholar]
- 4.Kazis LE, Miller DR, Clark J, Skinner K, Lee A, Rogers W, Spiro A, 3rd, Payne S, Fincke G, Selim A, Linzer M. Health-related quality of life in patients served by the Department of Veterans Affairs: Results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626–632. doi: 10.1001/archinte.158.6.626. [DOI] [PubMed] [Google Scholar]
- 5.Crosby FE, Colestro J, Ventura MR, Graham K. Survey of pain among veterans in Western New York. Pain Manag Nurs. 2006;7(1):12–22. doi: 10.1016/j.pmn.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage. 2002;23(2):131–137. doi: 10.1016/s0885-3924(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 7.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: A World Health Organization Study in Primary Care. JAMA. 1998;280(2):147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 8.Bair MJ, Kroenke K, Sutherland JM, McCoy KD, Harris H, McHorney CA. Effects of depression and pain severity on satisfaction in medical outpatients: Analysis of the Medical Outcomes Study. J Rehabil Res Dev. 2007;44(2):143–152. doi: 10.1682/jrrd.2006.06.0061. [DOI] [PubMed] [Google Scholar]
- 9.Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain. 2002;18(6):355–365. doi: 10.1097/00002508-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Reid MC, Bennett DA, Chen WG, Eldadah BA, Farrar JT, Ferrell B, Gallagher RM, Hanlon JT, Herr K, Horn SD, Inturrisi CE, Lemtouni S, Lin YW, Michaud K, Morrison RS, Neogi T, Porter LL, Solomon DH, Von Korff M, Weiss K, Witter J, Zacharoff KL. Improving the pharmacologic management of pain in older adults: Identifying the research gaps and methods to address them. Pain Medicine. 2011;12(9):1336–1357. doi: 10.1111/j.1526-4637.2011.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber JB, Gibson SJ. Treatment of chronic non-malignant pain in the elderly: safety considerations. Drug Saf. 2009;32(6):457–474. doi: 10.2165/00002018-200932060-00003. [DOI] [PubMed] [Google Scholar]
- 12.Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. [Accessed January 17, 2014];Pain as the 5th Vital Sign Toolkit. Available at: http://www1.va.gov/PAINMANAGEMENT/docs/TOOLKIT.pdf.
- 13.Department of Veterans Affairs. VHA Directive 2009-053 Pain Management. [Accessed February 27, 2014];2009 Available at: http://www.va.gov/PAINMANAGEMENT/docs/VHA09PainDirective.pdf.
- 14.Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 16.Jensen MP, Karoly P, O'Riordan EF, F B, Jr, Burns RS. The subjective experience of acute pain. An assessment of the utility of 10 indices. Clin J Pain. 1989;5(2):153–159. doi: 10.1097/00002508-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453–1458. doi: 10.1007/s11606-007-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenz KA, Sherbourne CD, Shugarman LR, Rubenstein LV, Wen L, Cohen A, Goebel JR, Hagenmeier E, Simon B, Lanto A, Asch SM. How reliable is pain as the fifth vital sign? J Am Board Fam Med. 2009;22(3):291–298. doi: 10.3122/jabfm.2009.03.080162. [DOI] [PubMed] [Google Scholar]
- 19.Goulet JL, Brandt C, Crystal S, Fiellin DA, Gibert C, Gordon AJ, Kerns RD, Maisto S, Justice AC. Agreement between electronic medical record-based and self-administered pain numeric rating scale: clinical and research implications. Med Care. 2013;51(3):245–250. doi: 10.1097/MLR.0b013e318277f1ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, Gracely RH, Clauw DJ. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis Rheum. 2005;52(11):3670–3674. doi: 10.1002/art.21407. [DOI] [PubMed] [Google Scholar]
- 21.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O'Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Department of Veterans Affairs and Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. [Accessed August 19, 2014];2010 Available at: http://www.healthquality.va.gov/Chronic_Opioid_Therapy_COT.asp.
- 23.American Pain Society. Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain. 2009 [Google Scholar]
- 24.Deyo RA, Smith DH, Johnson ES, Donovan M, Tillotson CJ, Yang X, Petrik AF, Dobscha SK. Opioids for back pain patients: primary care prescribing patterns and use of services. J Am Board Fam Med. 2011;24(6):717–727. doi: 10.3122/jabfm.2011.06.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morasco BJ, Corson K, Turk DC, Dobscha SK. Association between substance use disorder status and pain-related function following 12 months of treatment in primary care patients with musculoskeletal pain. J Pain. 2011;12(3):352–359. doi: 10.1016/j.jpain.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010;151(3):625–632. doi: 10.1016/j.pain.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macey TA, Morasco BJ, Duckart JP, Dobscha SK. Patterns and correlates of prescription opioid use in OEF/OIF veterans with chronic noncancer pain. Pain Med. 2011;12:1502–1509. doi: 10.1111/j.1526-4637.2011.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobscha SK, Corson K, Perrin NA, Hanson GC, Leibowitz RQ, Doak MN, Dickinson KC, Sullivan MD, Gerrity MS. Collaborative care for chronic pain in primary care: a cluster randomized trial. JAMA. 2009;301(12):1242–1252. doi: 10.1001/jama.2009.377. [DOI] [PubMed] [Google Scholar]
- 29.Haskell SG, Brandt CA, Krebs EE, Skanderson M, Kerns RD, Goulet JL. Pain among Veterans of Operations Enduring Freedom and Iraqi Freedom: Do women and men differ? Pain medicine (Malden, Mass. 2009;10(7):1167–1173. doi: 10.1111/j.1526-4637.2009.00714.x. [DOI] [PubMed] [Google Scholar]
- 30.Von Korff M, Dworkin SF, Le Resche L. Graded chronic pain status: an epidemiologic evaluation. Pain. 1990;40(3):279–291. doi: 10.1016/0304-3959(90)91125-3. [DOI] [PubMed] [Google Scholar]
- 31.Cleeland CS, Reyes-Gibby CC, Schall M, Nolan K, Paice J, Rosenberg JM, Tollett JH, Kerns RD. Rapid improvement in pain management: the Veterans Health Administration and the Institute for Healthcare Improvement Collaborative. Clin J Pain. 2003;19(5):298–305. doi: 10.1097/00002508-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 32.National Pain Management Coordinating Committee, Veterans Health Administration. Washington, DC: U S Department of Veterans Affairs, National Pain Management Coordinating Committee; 2000. [Accessed August 29, 2014]. Pain as the 5th vital sign toolkit, revised edition. Available at: http://www.va.gov/painmanagement/docs/toolkit.pdf. [Google Scholar]
- 33.Tian TY, Zlateva I, Anderson DR. Using electronic health records data to identify patients with chronic pain in a primary care setting. J Am Med Inform Assoc. 2013;20(e2):e275–e280. doi: 10.1136/amiajnl-2013-001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, regular prescription opioid use. Arch Intern Med. 2006;166(19):2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan MD, Von Korff M, Banta-Green C, Merrill JO, Saunders K. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149(2):345–353. doi: 10.1016/j.pain.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. The Clinical journal of pain. 2010;26(1):1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113(3):331–339. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12(9):964–973. doi: 10.1016/j.jpain.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ang DC, Bair MJ, Damush TM, Wu J, Tu W, Kroenke K. Predictors of pain outcomes in patients with chronic musculoskeletal pain co-morbid with depression: results from a randomized controlled trial. Pain Med. 2010;11(4):482–491. doi: 10.1111/j.1526-4637.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 41.Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain. 2012;13(3):228–234. doi: 10.1016/j.jpain.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selim AJ, Fincke G, Ren XS, Lee A, Rogers WH, Miller DR, Skinner KM, Linzer M, Kazis LE. Comorbidity assessments based on patient report: results from the Veterans Health Study. J Ambul Care Manage. 2004;27(3):281–295. doi: 10.1097/00004479-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Copeland LA, Zeber JE, Wang CP, Parchman ML, Lawrence VA, Valenstein M, Miller AL. Patterns of primary care and mortality among patients with schizophrenia or diabetes: a cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv Res. 2009;9:127. doi: 10.1186/1472-6963-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American College of Surgeons National Surgical Quality Improvement Program. User Guide for the Participant Use Data File. [Accessed January 27, 2014];2007 Available at: http://site.acsnsqip.org/wp-content/uploads/2012/03/ACS-NSQIP-Participant-User-Data-File-User-Guide_06.pdf. [Google Scholar]
- 45.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 46.Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30(11):1331–1334. doi: 10.1097/01.brs.0000164099.92112.29. [DOI] [PubMed] [Google Scholar]
- 47.Shugarman LR, Goebel JR, Lanto A, Asch SM, Sherbourne CD, Lee ML, Rubenstein LV, Wen L, Meredith L, Lorenz KA. Nursing staff, patient, and environmental factors associated with accurate pain assessment. J Pain Symptom Manage. 2010;40(5):723–733. doi: 10.1016/j.jpainsymman.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 48.National VA Pain Outcomes Working Group. VHA Pain Outcomes Toolkit. Washington, DC: U.S. Department of Veterans Affairs; 2003. National VA Pain Management Coordinating Committee, Veterans Health Administration. [Google Scholar]
- 49.Saha S, Freeman M, Toure J, Tippens K, Weeks C. Racial and Ethnic Disparities in the VA Healthcare System: A Systematic Review. Washington, DC: U.S. Department of Veterans Affairs, Evidence Synthesis Pilot Program; 2007. [PubMed] [Google Scholar]
- 50.Dobscha SK, Soleck GD, Dickinson KC, Burgess DJ, Lasarev MR, Lee ES, McFarland BH. Associations between race and ethnicity and treatment for chronic pain in the VA. J Pain. 2009;10(10):1078–1087. doi: 10.1016/j.jpain.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 51.Burgess DJ, Gravely AA, Nelson DB, van Ryn M, Bair MJ, Kerns RD, Higgins DM, Partin MR. A national study of racial differences in pain screening rates in the VA health care system. Clin J Pain. 2013;29(2):118–123. doi: 10.1097/AJP.0b013e31826a86ae. [DOI] [PubMed] [Google Scholar]
- 52.Burgess DJ, Nelson DB, Gravely AA, Bair MJ, Kerns RD, Higgins DM, van Ryn M, Farmer M, Partin MR. Racial differences in prescription of opioid analgesics for chronic noncancer pain in a national sample of veterans. J Pain. 2014;15(4):447–455. doi: 10.1016/j.jpain.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138(2):440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dobscha SK, Morasco BJ, Duckart JP, Macey TA, Deyo RA. Correlates of prescription opioid initiation and long-term opioid use in Veterans with persistent pain. Clin J Pain. 2013;29(2):102–108. doi: 10.1097/AJP.0b013e3182490bdb. [DOI] [PMC free article] [PubMed] [Google Scholar]