Abstract
The development of organ transplantation as a therapy for end-stage organ failure is among the most significant achievements of 20th century medicine, but chronic rejection remains a barrier to achieving long-term success. Current therapeutic regimens consist of immunosuppressive drugs that are efficient at delaying rejection but are associated with significant risks such as opportunistic infections, toxicity, and malignancy. Thus, the induction of specific immune tolerance to transplant antigens is the coveted aim of researchers. The use of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (ECDI)-treated, autoantigen-coupled syngeneic leukocytes has been developed as a specific immunotherapy in preclinical models of autoimmunity and is currently in a phase II clinical trial for the treatment of multiple sclerosis. In this review, we discuss the use of allogeneic ECDI-treated apoptotic donor leukocytes (allo-ECDI-SP) as a strategy for inducing antigen-specific tolerance in allogeneic transplantation. Allo-ECDI-SP therapy induces long-term systemic immune tolerance to transplant antigens by subverting alloimmune recognition and exploiting apoptotic cell uptake pathways to recapitulate innate mechanisms of peripheral tolerance. Lastly, we discuss potential indications and challenges for transitioning allo-ECDI-SP therapy into clinical practice.
Introduction
Organ transplantation is an invaluable component of therapeutic medicine for the treatment of end-stage disease and organ failure. Estimates for 2010 indicate that over 106 000 solid organ transplants were performed worldwide in nearly 100 countries, revealing how integral this recently emergent field has become to modern medicine (1). Although 1-year graft survival for renal transplantation has improved to roughly 90% over the past 2 decades due to advances in immunosuppression, long-term survival has remained relatively static due to chronic rejection. At present, therapy for transplant rejection is limited to chronic immunosuppression that is effective at preventing acute rejection, but is associated with significant risks including opportunistic infections, organ toxicity, metabolic derangement, and malignancy. Thus, developing a therapeutic regimen for transplant rejection that does not compromise the immune system, but can specifically constrain the deleterious response to allogeneic tissue is paramount for the future of transplant medicine. However, the complexity of allelic variation at the HLA loci and the propensity of the immune system for recognizing foreign HLA alleles have made the prospect of antigen-specific tolerance difficult to achieve.
According to a World Health Organization report, over 2500 new HLA alleles were identified between the years 2004 and 2010 (2); conversely, limiting dilution studies have determined that approximately 1–10% of the T cell repertoire can respond “directly” to donor-derived APCs presenting intact foreign peptide/MHC molecules (3,4). Understanding how T cells selected on self-restricted molecules can react to foreign MHC with such vigor has been the subject of intense investigation for decades. The evolutionary bias of TCRs for intra-species MHC molecules, TCR degeneracy, and polyspecificity of the TCR are mechanisms that have been cited as contributing to the high frequency of alloreactive T cells in the T cell repertoire (5). Recent investigation into the nature of alloreactivity has provided evidence that up to 50% of the alloresponse in GvHD is mediated by T cells that have undergone incomplete allelic exclusion and express dual TCRs (6,7). Moreover, increasing evidence suggests that higher primates and humans not previously exposed to primary allografts can harbor existing populations of virus-specific memory T cells that are cross-reactive and provide heterologous immunity to alloantigens (8). Additionally, the processing and representation of allogeneic peptides on endogenous MHC to T cells (indirect allorecognition) further increases the alloresponse by propagating additional cellular and humoral mechanisms. As a consequence of these factors, the reactivity of the T cell repertoire to foreign MHC is on the order of 100–1000 fold greater in magnitude than the T cell response to conventional antigens, and this presents a formidable barrier to the development of antigen-specific tolerance strategies to lead to acceptance of organ transplants.
Costimulation blockade strategies
The 1990s and first half of the following decade saw costimulation blockade emerge at the forefront of experimental strategies designed to induce transplant tolerance. T cell activation requires engagement of the TCR by cognate peptide/MHC in the presence of APC-derived costimulatory molecules, and signaling through the CD28/CD80/CD86 axis is the quintessential costimulatory pathway involved in T cell activation. Engagement of the TCR in the absence of CD28-mediated costimulation renders T cells anergic and functionally hyporesponsive to subsequent stimulation (9). Thus, multiple experimental strategies have attempted to exploit the two-signal hypothesis of T cell activation by depriving T cells of costimulatory signals following transplantation. CTLA-4 is a natural receptor for CD80 and CD86 that antagonizes T cell activation by limiting CD28 stimulation and delivering negative signals to the T cell. In spite of showing initial promise in laboratory settings, tolerance protocols using the fusion protein CTLA-4(Ig) has met with unexpected difficulties in clinical translation. Treatment with the CTLA-4(Ig) fusion protein Belatacept in the setting of renal transplantation was successful at promoting 1-year graft survival and superior renal function, but was also associated with a higher frequency of acute rejection, malignancy, and CNS posttransplant lymphoproliferative disorder when compared to cyclosporine in a Phase III clinical trial (10). CD154 is a potent T cell-derived signaling molecule that interacts with its receptor CD40 on APCs to induce APC activation and the expression of IL-12 and costimulatory molecules CD80/CD86 (11). MR1, an anti-murine CD154 antibody has been used in preclinical studies to promote transplant tolerance with great efficacy, especially when used in combination with donor-specific transfusion (DST). This tolerance occurs through a number of mechanisms, including T cell anergy and deletion through targeting the indirect antigen presentation pathway by phagocytosis of the infused donor cells (12–15). Surprisingly, the translation of this therapy into clinical settings was abruptly ended by the development of thrombotic events, due to the unexpected expression of CD154 on platelets in higher primates (16,17). Costimulatory blockade may inadvertently increase the likelihood of acute rejection as previously mentioned. This may be due to the low reliance of memory T cells on co-stimulation, their cross-reactivity for alloantigens, and a reduction in CD4+ CD25+ Foxp3+ regulatory T cells (TREGS) as a consequence of their dependence on co-stimulation (8,18–20). Since the level of intragraft Foxp3 expression is associated with a superior prognosis for graft survival, a better understanding of the disparity in the timing and requirement for co-stimulation in different T cell populations is needed to minimize complications (21).
Cell-based immunotherapy
As a result of these shortcomings, cell-based immunotherapy has reemerged at the forefront of experimental tolerance protocols, such as mixed hematopoietic chimerism (22) and the adoptive transfer of ex vivo expanded, donor-specific TREGS (23). A third form of cell-based tolerance that has proven successful in experimental settings is the use of drug conditioned (24) or chemically modified allogeneic APCs (25). This form of tolerance predates costimulation blockade and other forms of cell-based immunotherapy by many decades, dating back to the hapten-drug studies of the late 1920s (26), which demonstrated that chemical haptens coupled to the cellmembrane of leukocytes could be used to prevent hapten-induced contact dermatitis in an antigen specific manner (27,28). The induction of regulatory cells and the clonal inhibition of cellular and humoral immunity were demonstrated to be the mechanisms responsible for tolerance induced by coupled-cell administration (25). Many strategies employing drug-conditioned APCs, such as rapamycin-conditioned DCs (29) and vitamin D3-treated DCs (30), attempt to recapitulate these mechanisms by modifying the DC phenotype to favor the induction of transplant tolerance. However, a number of recent publications have called into question the ability of drug-modified DC protocols to induce transplant tolerance, instead suggesting that these strategies risk enhancing alloimmune responses and may promote graft rejection (31,32).
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (ECDI) is a hygroscopic, water-soluble chemical that has been used extensively by biochemists as a cross-linker for peptide synthesis and conjugation. Introduction of ECDI in a reaction mixture with peptides or proteins activates free carboxyl groups, catalyzing the formation of covalent peptide bonds between the active carboxyl group and primary amines. Studies comparing ECDI with other cross-linking agents have demonstrated the superiority of ECDI as a tissue fixative for immunohistochemistry and as a cross-linking agent for conjugating peptides to cellular membranes (33,34). When compared to other cross-linking agents, ECDI-treated cells demonstrated better viability when maintained at 4°C. However, within hours of in vivo administration ECDI-treated leukocytes undergo rapid apoptosis, although the precise mechanism of how this occurs remains unclear (35). Thus, the association of protein and peptide antigens (Ag) by covalent linkage to apoptotic leukocytes promotes a highly efficacious method for inducing Ag-specific immune tolerance in the context of autoimmune and allergic disease (36). Phagocytosis and representation of antigen covalently linked to the apoptotic ECDI-treated leukocyte appears to be the dominant mechanism by which T cells recognize the associated antigen, since antigen-conjugated allogeneic splenic leukocytes or red blood cells were able to induce tolerance as effectively as antigen-conjugated syngeneic leukocytes in a model of PLP139–151-induced EAE (35). Thus, ECDI-treated Ag-coupled leukocytes have the potential to regulate T cell responses to cryptic or undetermined peptide sequences within a protein antigen, and multiple antigens can be simultaneously targeted (37,38). This approach is useful for modulating multi-determinant T cell responses as in the context of autoimmune epitope spreading or allogeneic transplant rejection. Intravenous infusion of ECDI-treated Ag-coupled leukocytes can be used in a therapeutic application without inducing anaphylaxis (39), contrary to other antigen-specific strategies such as soluble peptide tolerance and altered peptide ligand therapy (40,41). In agreement with these pre-clinical studies, a human phase I clinical trial utilizing autologous patient peripheral blood leukocytes ECDI-coupled with a cocktail of encephalitogenic peptides has shown promising results indicating that this approach may be safe and efficacious as an immunotherapy for multiple sclerosis (42).
In the context of allogeneic transplantation, ECDI-treated apoptotic leukocyte treatment represents a promising therapy for the prevention of allograft rejection. As allogeneic leukocytes express donor antigens directly on the cell surface, tolerance can be induced to a full spectrum of allogeneic MHC and minor antigens by directly fixing the membrane with ECDI prior to i.v. infusion. Using this method, our labs have demonstrated that intravenous infusion of ECDI-treated apoptotic allogeneic splenocytes from the transplant donor into transplant recipients at one week before and 24 h after the transplant will induce long-lasting tolerance for the survival of minor antigen mismatched skin grafts, full-MHC mismatched heart allografts, and full-MHC mismatched pancreatic islets for the restoration of euglycemia in a model of streptozotocin-induced diabetes (43–46). This therapy is allo-specific (46). This approach is also flexible since B cells of donor origin can be expanded in vitro and subsequently treated with ECDI to induce tolerance. Additionally, the lysate from allogeneic donor cells can be conjugated to syngeneic splenocytes with ECDI to induce tolerance with no loss of efficacy (47). The potency of ECDI-treated allogeneic leukocytes is dependent upon their exploitation of apoptosis pathways to reshape the peripheral T cell repertoire by several mechanisms including anergy, deletion, and the induction of regulatory elements such as the expansion of Foxp3+ TREGS (46). Acting in concert, these mechanisms lead to effective induction of tolerance and long-term maintenance of tolerance. Furthermore, while antigen-coupled ECDI-treated leukocytes effectively restrain the function of naive T cells, emerging evidence suggest that they also down-regulate the function of memory T cells. In the autoimmune EAE model, antigen-coupled ECDI-treated leukocytes prevent adoptive transfer EAE induced by previously activated/memory autoreactive T cells (38). We are currently conducting similar experiments using pre-sensitized alloimmune transplant models, also demonstrating potential protective effect of ECDI-treated donor leukocytes in such recipients. Therefore, the potential of ECDI-treated donor leukocytes to constrain both naïve and memory T cells may significantly enhance its application in clinical transplantation where pre-sensitization is frequently encountered.
Apoptosis and innate immune recognition of allogeneic ECDI-treated leukocytes
Apoptosis has been largely implicated in the maintenance of peripheral tolerance, and defects in apoptotic clearance have been demonstrated to have significant consequences on immune homeostasis (48,49). Initial reports of the apoptosis-inducing consequence of ECDI demonstrated a 30–35% incidence of apoptosis in ECDI-treated allogeneic DCs cultured for 24 h in vitro (50), while subsequent experiments in vivo have revealed that the majority of ECDI-treated Ag-coupled syngeneic splenic leukocytes are fragmented within 3 h of tail vein injection (51). When donor ECDI-treated splenocytes pre-labeled with PKH-67 were administered to recipients, the majority of donor cells were rapidly internalized by recipient MHC class II expressing splenocytes (52). Studies from the EAE model using fluorescently labeled cells have shown that following i.v. infusion of ECDI-treated Ag-coupled leukocytes, the cells localize predominantly in the lungs, liver, and spleen within 1 h and are almost completely fragmented at 3 h post i.v. infusion (51). These apoptotic fragments were primarily associated with F4/80+ macrophages in the splenic marginal zone (51), an area between the red pulp and lymphoid follicles that positions marginal zone APCs to capture blood borne matter and represent Ags to lymphocytes in the white pulp (53). Marginal zone macrophages (MZM) are a population of professional APCs specialized in their ability to capture and clear cellular debris from the blood due to the expression of various scavenger receptors that recognize particulate antigens (54), polyanionic molecules (55), oxidized low-density lipoproteins (56), and dying cells (57). Targeted deletion of specific scavenger receptors such as DC-SIGN/SIGN-R1, SR-A, MARCO, and CD68 has been shown to augment autoantibody production in mouse strains susceptible to lupus (58,59), while the depletion of MZMs abrogates the apoptotic cell-induced upregulation of TGF-β and increases the expression of proinflammatory cytokines, antigen-specific T cell proliferation (59), and resistance to tolerance induction in a model of MOG-induced EAE (60). In spite of the association between the apoptotic cells and MZMs, genetic deletion of MARCO had no affect on tolerance induced by ECDI-treated, antigen-coupled leukocytes (61), nor did the depletion of splenic macrophages by clodronate liposomes (52). Upon further investigation, splenic DCs appear to be the critical APC population involved in mediating tolerance to allo-ECDI-SP since the administration of diphtheria toxin to DTR-CD11c transgenic mice at the time of allogeneic ECDI-treated splenocyte infusion prevented the establishment of tolerance (52). Although these data appear to be in conflict with observations regarding the role of splenic macrophages in ECDI-treated antigen-coupled cell therapy, MZMs have been reported to acquire and transfer antigen to splenic CD8α+ DCs for presentation to T cells (62); similarly, a recent publication by Mellor and colleagues provides evidence that antigen-bearing, CD11c+ CD8+ CD103+ marginal zone DCs are recruited to the follicles by metallophilic MZMs in a CCL22-dependent manner following phagocytosis of apoptotic cells (63). Thus, the phagocytosis of apoptotic cells by MZMs may facilitate presentation of antigens by DCs to the T cells in the splenic lymphoid follicles, thereby providing a conciliatory mechanism for our observations regarding these splenic populations. Nonetheless, the spleen has been demonstrated to be required for tolerance induction mediated by ECDI-treated, antigen-coupled leukocytes since splenectomized mice were not protected from PLP139–151 induced EAE following the administration of PLP139-coupled splenocytes (51).
Cytokines and negative costimulation in ECDI-treated cell tolerance
The unresponsiveness induced by ECDI-treated Ag-coupled leukocytes has been attributed to T cell anergy and deletion. CD4+ T cells receiving cognate signals via the TCR in the absence of APC-derived costimulation fail to sustain IL-2 production and become anergic to secondary stimulation (9,64). Experiments from autoimmune models have demonstrated that tolerance mediated by ECDI-treated Ag-coupled leukocytes is dependent upon low APC expression of CD80 and CD86 which favors binding to CTLA-4 over CD28 on T cells, and blockade of CTLA-4 signaling at the time of tolerization inhibited unresponsiveness in the EAE model of MS (65). Conversely, the PD-1/PD-L1/PD-L2 pathway has been strongly implicated as a target for immunotherapy in tolerance models due to its role in T cell exhaustion and anergy (66). Expression of the PD-1 receptor is induced on T cells following activation where it binds to its ligands PD-L1 and PD-L2 to negatively regulate T cell function. PD-L1 can also bind to CD80 and prevent signaling to CD28 (67), and antibody blockade against either PD-1 or PD-L1 can abrogate transplant tolerance (68) and the protection mediated by ECDI-treated Ag-coupled leukocytes in a model of type 1 diabetes (69). How administration of ECDI-treated Ag-coupled leukocytes establishes an environment wherein negative costimulation is the favored outcome may be a consequence of the immunoregulatory cytokine milieu induced by recognition of apoptotic debris.
IL-10 is a regulatory cytokine that has a non-redundant role in immune homeostasis and inflammation (70,71). Early studies on IL-10 reported an inhibitory effect of this cytokine on the expression of CD80 by macrophages, without affecting MHC class II presentation (72), and IL-10 has also been shown to induce expression of the negative costimulatory ligand PD-L1 in a STAT3-dependent manner (73). In agreement with these studies, splenic macrophages were shown to express IL-10 following infusion of ECDI-treated Ag-coupled leukocytes, and both MZM and splenic CD8α+ DCs uptaking apoptotic debris demonstrated an IL-10 dependent increase in their expression of PD-L1 with no significant upregulation of CD40 or CD80 (51,52). Moreover, the inhibition of either IL-10 or PD-L1 in mice administered ECDI-treated leukocytes prevented tolerance in the context of both autoimmunity and allogeneic islet transplantation (46,51,52). Thus antigen is presented to T cells specific for cross-presented alloantigens (indirect allospecificity) in the context of low costimulation and the provision of inhibitory signals. Surprisingly, experiments examining T cells of indirect allospecificity showed significant activation after allo-ECDI-SP treatment. The administration of ECDI-treated BALB/c-SP induced TCR transgenic TEa CD4+ T cells (specific for a BALB/c I-Eαd peptide complexed with the B6 MHCII I-Ab molecule) to undergo robust proliferation and produce IFN-γ. Following transplantation of the allogeneic islets and a second infusion of allo-ECDI-SP (52), these TEa T cells were later depleted by IFN-γ-dependent mechanisms, a finding that is consistent with a requirement for T cell deletion in the establishment of transplant tolerance (74). IL-10 and IFN-γ have been reported to condition DCs to express lower TNF-α and IL-12p40 while increasing levels of indoleamine 2,3 dioxygenase (IDO) (75). Additionally, both IDO and IFN-γ are critical for transplant tolerance mediated by allo-ECDI-SP, and recent experiments have elucidated splenic myeloid derived suppressor cells as a source of IDO and IFN-γ in the splenic environment following treatment (45,52). In lieu of the silencing effect of allo-ECDI-SP on the indirect allorecognition pathway, graft reactive B cells do not undergo class switching to produce alloantibodies, and effector cell infiltration is largely reduced in the allograft of tolerized recipients (45). This suppression of the indirect CD4+ T cell response may be the most critical outcome of allo-ECDI-SP therapy, confirming reports that the indirect alloresponse rapidly becomes the dominant pathway involved in graft rejection (76).
Modulation of the direct allorecognition pathway can occur by T cells directly interacting with allo-ECDI-SP (50), and this is in agreement with prior reports that T cells with cognate TCRs could be tolerized by directly engaging the peptide-MHC molecules. Indeed, antibody blockade of MHC molecules on ECDI-treated, antigen-coupled leukocytes prevented the induction of T cell unresponsiveness in an in vitro culture system (9). As treatment of cells with ECDI prevents the expression of costimulatory molecules CD40, CD80, and CD86 (77), presentation by ECDI-treated-SP results in the provision of TCR signals in the absence of costimulation. Experiments utilizing allo-ECDI-SP cultured with allogeneic T cells demonstrated a 30–50% reduction in cluster formation concomitant with impaired T cell proliferation and IFN-γ production (50,64). Thus, for CD4+ T cells from the direct allorecognition pathway, intravenous infusion of allo-ECDI-SP may directly engage these cells and subsequently induce their anergy. Although one potential advantage of targeting T cells from the direct allorecognition pathway is the inactivation of graft-reactive CD8+ T cells, both in vitro and in vivo evidence support an argument for unresponsiveness in the CD8+ T cell compartment being mediated indirectly through the CD4+ T cells. In a culture assay examining the lytic ability of cytotoxic T cells following encounter with allo-ECDI-SP, cytotoxic T cells lysed stimulator cells in a secondary MLR if CD4+ T cells were absent from the MLR, but lysis was reduced if CD4+ T cells previously cultured with allo-ECDISP were added to the MLR (78). In an H-Y model of skin graft rejection, tolerance to the MHC class-I restricted H-Y antigens failed to protect the graft from rejection while tolerance to the MHC class-II restricted epitope Dby prevented graft rejection and limited the activation, proliferation, and function of H-Y specific CD8+ T cells (79). Interestingly, a recent publication by Pettigrew and colleagues provides evidence for the involvement of CD4+ T cells of the indirect lineage in providing T cell help to directly alloreactive CD8+ T cells. The authors found that recipient dendritic cells could present processed and intact donor allogeneic MHC to activate CD8+ T cells of direct specificity, but only if the recipient DCs also expressed recipient MHC class II (80). Therefore, inhibition of the direct and indirect CD4+ T cell response to alloantigens by ECDI-SP treatment may be sufficient to limit CD8+ T cell activation in the context of allogeneic transplantation. Additionally, active suppression by TREGS may also limit the activation of graft-reactive T cells (81). Although the study by Corlett et al (78) did not examine the phenotype of the CD4+ T cells that inhibit CD8+ T cell activation following exposure to allo-ECDI-SP, CD4+ CD25− T cells isolated from human PBLs and co-cultured with allo-ECDI-SP were induced to express a Foxp3+ TREG phenotype and demonstrated suppressive function when added to a primary MLR. Thus, tolerance in the CD8+ compartment following allo-ECDI-SP therapy may be mediated by active regulation involving Foxp3+ TREGS in addition to the absence of functional CD4+ T helper responses, possibly as a consequence of altered CD154 expression (79).
Regulatory cells in ECDI-treated cell tolerance
Regulatory T cells
TREGS are critical to the induction and maintenance of peripheral immune tolerance, and the expansion of this population has significant potential to mediate tolerance to allogeneic tissue (82). Recent insight into the biology of TREGs suggest that the establishment of transplant tolerance depends on the TREG homing to graft draining lymph nodes and the allogeneic tissue itself where TREGS can suppress the activation of naïve conventional T cells (TCONV) and the function of effector T cells, respectively (83). In vivo, TREGS have been shown to be critical for the induction of transplant tolerance mediated by allo-ECDI-SP, and are preferentially expanded in frequency in the secondary lymphoid organs and grafts of tolerized transplant recipients. In a model of allogeneic islet transplantation, CD25 depletion at the time of allo-ECDI-SP treatment prevented the establishment of tolerance to the islet grafts, although CD25 depletion during long-term tolerance maintenance did not have a lasting detrimental effect on graft retention (46). Whether or not these TREGS are expanded from an existing pool of natural TREGS or derived from TCONV has not been thoroughly examined, but CD4+ T cells isolated from human PBLs can be induced to express Foxp3 during culture with allo-ECDI-treated PBLs. These TREGS exhibited decreased levels of classic TREG activation markers such as CTLA-4 and GITR, arguing for their induction from TCONV precursors rather than expansion from nTREGS (77). Furthermore, the requirement for TGF-β at the time of tolerance induction to allo-ECDI-SP supports the conversion of TREGS from TCONV cells. Little is known regarding the specificity of the TREG response mediated by allo-ECDI-SP. Although TREGS were previously thought to mediate suppression in a non-specific bystander manner, transgene expression of indirect alloreactivity by TCR DNA transfer into TREGS has been shown to favor transplant tolerance (84), and a recent publication by the Rudensky lab has reported that continued expression of the TCR is critical for TREG mediated suppression (85), thereby supporting an argument for the importance of antigenspecificity in TREG function. In spite of the observations that TREGS can be induced by direct stimulation with allo-ECDISP using human PBLs, in the murine model, 4C Tg CD4+ T cells displaying a TCR specific for allogeneic MHC (I-Ad) were not observed to upregulate FoxP3 expression during tolerance induction by allo-ECDI-SP, nor were TEa CD4+ T cells of indirect specificity (52). However, studies from the EAE model have demonstrated that a transferred population of CD4+ CD25+ splenocytes derived from PLP139–151-SP treated donors conferred better protection from PLP139–151 mediated EAE disease when compared to CD4+ CD25+ splenocytes derived from OVA323–339-SP treated donors (51), consistent with a role for antigen-specificity in TREG mediated suppression. Therefore, additional untested donor-reactive TCR specificities may better support TREG induction or expansion. Nonetheless, significant questions remain regarding the biology of TREGS, their specificity, and the mechanism of their contribution to ECDI-treated SP tolerance therapy.
Myeloid-derived suppressor cells (MDSCs)
MDSCs are a heterogeneous population of activated but immature myeloid cells that are identified by their co-expression of Gr1 and CD11b. First described in cancer, these cells are potent inhibitors of T cell proliferation and function, and are known to suppress immune function under a number of inflammatory conditions (86). These cells are activated by a number of factors including TGF-β and IFN-γ, both of which are required for tolerance by allo-ECDI-SP. Treatment with allo-ECDI-SP induced a splenic population of MDSCs that produced significant levels of IFN-γ-dependent IDO and suppressed CD8+ T cell proliferation when compared to control mice. Moreover, MDSCs were found to be present in the cardiac allograft and suppressed the infiltration of CD8+ T cells and other effectors. MDSCs present in the graft were also found to produce IL-10 and to recruit TREGS in a CCL4-dependent manner, and depletion of MDSCs restored CD8+ T cell infiltration and graft rejection (87). Thus, a critical role for MDSCs in allo-ECDI-SP therapy may be the recruitment of TREGS into the graft, which subsequently suppress graft infiltration by CD8+ T cells. This argument is consistent with an early requirement for TREGS following tolerance induction, since the direct CD8+ T cell response is activated by passenger leukocytes from the graft that do not persist long-term.
Based on the above-described mechanisms involved in tolerance induced allo-ECDI-SP, there are several potential advantages of this strategy over other forms of cell-based therapies such as DST: (1) unlike DST, allo-ECDI-SP in principle does not require concomitant co-stimulation blockade, as the allo-ECDI-SP uniquely lack the ability to provide adequate co-stimulation signals themselves to the interacting T cells (52); (2) DST carries a potential risk for recipient sensitization, especially in recipients with preexisting alloimmunity (88); in contrast, allo-ECDI-SP does not display a similar risk of sensitization, and may in fact provide further graft protection in recipients with preexisting alloimmunity (Luo, unpublished data). Lastly, our unpublished work showed that in direct comparison with other methods of inducing cell death such as γ-irradiation or paraformaldehyde fixation, ECDI treatment is significantly more efficient for inducing tolerance, likely due to its ability to arrest the treated cells in the apoptotic stage rather than allowing them to progress to the necrotic stage which counters tolerance efficacy.
Conclusions
In summary, ECDI-treated apoptotic donor cells are highly effective in tempering allo-specific immune responses via a multitude of mechanisms employing negative costimulation, inhibitory cytokines, and regulatory cell populations (Figure 1). The efficacy of this form of donor cell-based immunotherapy in allo-sensitized recipients warrants further detailed investigation. Ongoing studies in nonhuman primates will undoubtedly inform future clinical trial design using this form of negative donor vaccination for transplant tolerance induction in humans. Lastly, establishing a source for unlimited production of donor antigens and an immune modulatory cell-free synthetic particle delivery system of donor antigens will likely significantly streamline the manufacturing of clinical-grade negative donor vaccines, and thus provide an appropriate potential path for moving this therapy toward clinical translation.
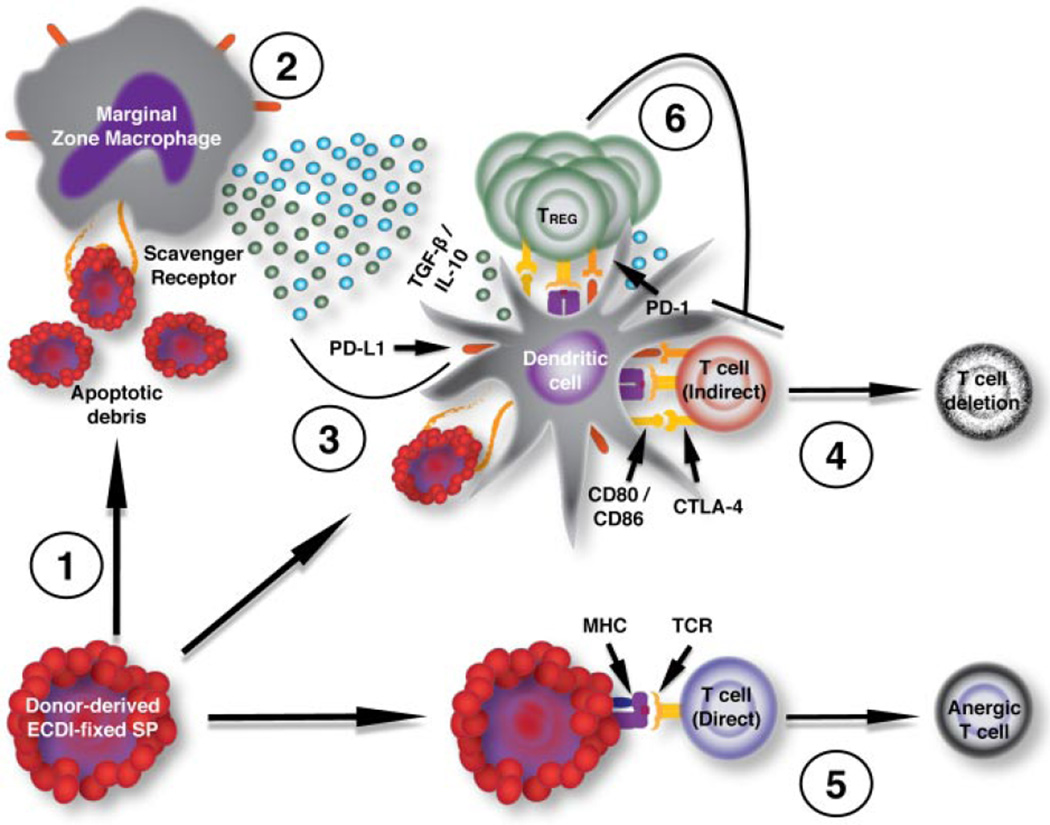
Figure 1. Proposed mechanisms of Allo-ECDI-SP tolerance.
Innate immune responses are required for allo-ECDI-SP tolerance induction. The splenic marginal zone is the primary interface between the splenic non-lymphoid compartment and the lymphoid compartment. It is composed of B cells and macrophages important for capturing exogenous Ags and debris, which may be processed for subsequent presentation to T cells in T cell zones. For efficient tolerance, allo-ECDI-SP must be delivered (1) via i.v. administration. Once within the marginal sinus, the ECDI-SP rapidly degrade via apoptotic pathways (2), with debris and cells recognized and rapidly taken up via scavenger receptors on MZMs and DCs either directly from the marginal zone sinus or via membrane transfer. The uptake of allo-ECDI-SP triggers the production and secretion of soluble mediators including IL-10 and TGF-β, which have multifarious functions including the regulation of costimulatory molecules, such as PD-L1, on APCs (3). The immunoregulatory milieu provided by the MZM response to the apoptotic allo-ECDI-SP conditions DCs to present antigen to T cells in the context of low CD80/CD86 expression and increased PD-L1 expression, thereby favoring costimulation through the inhibitory receptors such as CTLA-4 and PD-1 (4). T cells of the indirect allorecognition pathway recognizing cognate peptide/MHC ligands on host APCs undergo deletion in this context (4), while T cells directly engaging peptide/MHC ligands on allo-ECDI-SP become anergic (5). Regulatory T cells of the Foxp3+ lineage expand in the presence of TGF-β to inhibit further priming in the secondary lymphoid organs and effector responses in the transplanted tissue (6).
Acknowledgments
This work was supported by NIH and JDRF grants to SDM and XL. DPM was supported by NIH NIDDK Postdoctoral Training Grant T32DK077662.
Abbreviations
- Ag
antigen
- Allo-ECDI-SP
allogeneic ECDI-treated splenocytes/leukocytes
- APC
antigen presenting cell
- CTLA-4
cytotoxic T lymphocyte antigen-4
- DC
dendritic cell
- DST
donor specific transfusion
- EAE
experimental autoimmune encephalomyelitis
- ECDI
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- ECDI-SP
ECDI-treated splenocytes/leukocytes
- GvHD
graft versus host disease
- HLA
human leukocyte antigen
- IDO
indoleamine 2,3 dioxygenase
- MDSC
myeloid derived suppressor cell
- MHC
major histocompatibility complex
- MLR
mixed lymphocyte reaction
- MS
multiple sclerosis
- MZM
marginal zone macrophage
- OVA323-339
ovalbumin protein peptide sequence 323–339
- PBL
peripheral blood leukocyte
- PD-1
programmed cell death 1
- PD-L1/2
programmed death ligand 1/2
- PLP139-151
proteolipid protein peptide sequence 139–151
- TCONV
conventional T cells
- TCR
T cell receptor
- TREG
regulatory T cell
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Grinyo JM. Why is organ transplantation clinically important? Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh SG, Albert ED, Bodmer WF, et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010;75:291–455. doi: 10.1111/j.1399-0039.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindahl KF, Wilson DB. Histocompatibility antigen-activated cytotoxic T lymphocytes. I. Estimates of the absolute frequency of killer cells generated in vitro. J Exp Med. 1977;145:500–507. doi: 10.1084/jem.145.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindahl KF, Wilson DB. Histocompatibility antigen-activated cytotoxic T lymphocytes. II. Estimates of the frequency and specificity of precursors. J Exp Med. 1977;145:508–522. doi: 10.1084/jem.145.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felix NJ, Allen PM. Specificity of T-cell alloreactivity. Nat Rev Immunol. 2007;7:942–953. doi: 10.1038/nri2200. [DOI] [PubMed] [Google Scholar]
- 6.Morris GP, Allen PM. Cutting edge: Highly alloreactive dual TCR T cells play a dominant role in graft-versus-host disease. J Immunol. 2009;182:6639–6643. doi: 10.4049/jimmunol.0900638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris GP, Uy GL, Donermeyer D, Dipersio JF, Allen PM. Dual receptor T cells mediate pathologic alloreactivity in patients with acute graft-versus-host disease. Sci Transl Med. 2013;5:188ra174. doi: 10.1126/scitranslmed.3005452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 11.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 12.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol. 2000;164:512–521. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- 13.Phillips NE, Greiner DL, Mordes JP, Rossini AA. Costimulatory blockade induces hyporesponsiveness in T cells that recognize alloantigen via indirect antigen presentation. Transplant. 2006;82:1085–1092. doi: 10.1097/01.tp.0000235521.83772.29. [DOI] [PubMed] [Google Scholar]
- 14.Kishimoto K, Yuan X, Auchincloss H, Jr, Sharpe AH, Mandelbrot DA, Sayegh MH. Mechanism of action of donor-specific transfusion in inducing tolerance: role of donor MHC molecules, donor co-stimulatory molecules, and indirect antigen presentation. J Am Soc Nephrol. 2004;15:2423–2428. doi: 10.1097/01.ASN.0000137883.20961.2D. [DOI] [PubMed] [Google Scholar]
- 15.Zheng XX, Markees TG, Hancock WW, et al. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J Immunol. 1999;162:4983–4990. [PubMed] [Google Scholar]
- 16.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. NatMed. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172:5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 19.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+ CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 20.Riella LV, Liu T, Yang J, et al. Deleterious effect of CTLA4-Ig on a Treg-dependent transplant model. Am J Transplant. 2012;12:846–855. doi: 10.1111/j.1600-6143.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 21.Mansour H, Homs S, Desvaux D, et al. Intragraft levels of Foxp3 mRNA predict progression in renal transplants with borderline change. J Am Soc Nephrol. 2008;19:2277–2281. doi: 10.1681/ASN.2008030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scandling JD, Busque S, Shizuru JA, Engleman EG, Strober S. Induced immune tolerance for kidney transplantation. New Eng J Med. 2011;365:1359–1360. doi: 10.1056/NEJMc1107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 25.Miller SD, Wetzig RP, Claman HN. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J Exp Med. 1979;149:758–773. doi: 10.1084/jem.149.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulzberger MB. Hypersensitivities to arsphenamine in guinea pigs: Experiments in prevention and in desensitization. Arch Derm Syph. 1929;20:669–697. [Google Scholar]
- 27.Chase MW. Inhibition of experimental drug allergy by prior feeding of the sensitizing agent. Proc Soc Exp Biol Med. 1946;61:257–259. doi: 10.3181/00379727-61-15294p. [DOI] [PubMed] [Google Scholar]
- 28.Battisto JR, Bloom BR. Dual immunological unresponsiveness induced by cell membrane coupled hapten or antigen. Nature. 1966;212:156–157. doi: 10.1038/212156a0. [DOI] [PubMed] [Google Scholar]
- 29.Ezzelarab MB, Zahorchak AF, Lu L, et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant. 2013;13:1989–2005. doi: 10.1111/ajt.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 31.Macedo C, Turnquist HR, Castillo-Rama M, et al. Rapamycin augments human DC IL-12p70 and IL-27 secretion to promote allogeneic Type 1 polarization modulated by NK cells. Am J Transplant. 2013;13:2322–2333. doi: 10.1111/ajt.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth LA, Ratnasothy K, Moreau A, et al. Tolerogenic donor-derived dendritic cells risk sensitization in vivo owing to processing and presentation by recipient APCs. J Immunol. 2013;190:4848–4860. doi: 10.4049/jimmunol.1200870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panula P, Happola O, Airaksinen MS, Auvinen S, Virkamaki A. Carbodiimide as a tissue fixative in histamine immunohistochemistry and its application in developmental neurobiology. J Histochem Cytochem. 1988;36:259–269. doi: 10.1177/36.3.3343510. [DOI] [PubMed] [Google Scholar]
- 34.Johnson HM, Brenner K, Hall HE. The use of a water-soluble carbodiimide as a coupling reagent in the passive hemagglutination test. J Immunol. 1966;97:791–796. [PubMed] [Google Scholar]
- 35.Turley DM, Miller SD. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 36.Getts DR, McCarthy DP, Miller SD. Exploiting apoptosis for therapeutic tolerance induction. J Immunol. 2013;191:5341–5346. doi: 10.4049/jimmunol.1302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith CE, Miller SD. Multi-peptide coupled-cell tolerance ameliorates ongoing relapsing EAE associated with multiple pathogenic autoreactivities. J Autoimmun. 2006;27:218–231. doi: 10.1016/j.jaut.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith CE, Eagar TN, Strominger JL, Miller SD. Differential induction of IgE-mediated anaphylaxis after soluble vs. cell-bound tolerogenic peptide therapy of autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2005;102:9595–9600. doi: 10.1073/pnas.0504131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedotti R, Mitchell D, Wedemeyer J, et al. An unexpected version of horror autotoxicus: Anaphylactic shock to a self-peptide. NatImmunol. 2001;2:216–222. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- 41.Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. NatMed. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 42.Lutterotti A, Yusef S, Sputtek A, et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: A phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5:188ra75. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Busker AE, Miller SD, Melvold RW. Induction of allograft tolerance to the H-Y antigen in adult C57BL/6 mice: Differential effects on delayed-type hypersensitivity and cytolytic T-lymphocyte activity. Cell Immunol. 1990;125:225–234. doi: 10.1016/0008-8749(90)90076-4. [DOI] [PubMed] [Google Scholar]
- 44.Elliott C, Wang K, Miller SD, Melvold R. Ethylcarbodiimide as an agent for induction of specific transplant tolerance. Transplant. 1994;58:966–968. doi: 10.1097/00007890-199410270-00023. [DOI] [PubMed] [Google Scholar]
- 45.Chen G, Kheradmand T, Bryant J, et al. Intragraft CD11b(+) IDO(+) cells mediate cardiac allograft tolerance by ECDI-fixed donor splenocyte infusions. Am J Transplant. 2012 doi: 10.1111/j.1600-6143.2012.04203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo X, Pothoven KL, McCarthy D, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105:14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S, Zhang X, Zhang L, et al. Preemptive tolerogenic delivery of donor antigens for permanent allogeneic islet graft protection. Cell Transplant. 2014 doi: 10.3727/096368914X681027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Brien BA, Huang Y, Geng X, Dutz JP, Finegood DT. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes. 2002;51:2481–2488. doi: 10.2337/diabetes.51.8.2481. [DOI] [PubMed] [Google Scholar]
- 49.Hanayama R, Tanaka M, Miyasaka K, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 50.Kaneko K, Morelli AE, Wang Z, Thomson AW. Alloantigen presentation by ethylcarbodiimide-treated dendritic cells induces T cell hyporesponsiveness, and prolongs organ graft survival. Clin Immunol. 2003;108:190–198. doi: 10.1016/s1521-6616(03)00141-4. [DOI] [PubMed] [Google Scholar]
- 51.Getts DR, Turley DM, Smith CE, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol. 2011;187:2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kheradmand T, Wang S, Bryant J, et al. Ethylenecarbodiimide-fixed donor splenocyte infusions differentially target direct and indirect pathways of allorecognition for induction of transplant tolerance. J Immunol. 2012;189:804–812. doi: 10.4049/jimmunol.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 54.Arredouani MS, Palecanda A, Koziel H, et al. MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J Immunol. 2005;175:6058–6064. doi: 10.4049/jimmunol.175.9.6058. [DOI] [PubMed] [Google Scholar]
- 55.Sasaki T, Horiuchi S, Yamazaki M, Yui S. Stimulation of macrophage DNA synthesis by polyanionic substances through binding to the macrophage scavenger receptor. Biol Pharm Bull. 1996;19:449–455. doi: 10.1248/bpb.19.449. [DOI] [PubMed] [Google Scholar]
- 56.Sakai M, Miyazaki A, Hakamata H, et al. The scavenger receptor serves as a route for internalization of lysophosphatidylcholine in oxidized low density lipoprotein-induced macrophage proliferation. J Biol Chem. 1996;271:27346–27352. doi: 10.1074/jbc.271.44.27346. [DOI] [PubMed] [Google Scholar]
- 57.Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci U S A. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wermeling F, Chen Y, Pikkarainen T, et al. Class A scavenger receptors regulate tolerance against apoptotic cells, and autoantibodies against these receptors are predictive of systemic lupus. J Exp Med. 2007;204:2259–2265. doi: 10.1084/jem.20070600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117:5403–5412. doi: 10.1182/blood-2010-11-320028. [DOI] [PubMed] [Google Scholar]
- 60.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Getts DR, Martin AJ, McCarthy DP, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Backer R, Schwandt T, Greuter M, et al. Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells. Proc Natl Acad Sci U S A. 2010;107:216–221. doi: 10.1073/pnas.0909541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ravishankar B, Shinde R, Liu H, et al. Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc Natl Acad Sci U S A. 2014;111:4215–4220. doi: 10.1073/pnas.1320924111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ibrahim MA, Coode WK, Takei F, Ellis JS, Chain BM, Katz DR. Chemically modified antigen-presenting cells induce T lymphocyte allospecific hyporesponsiveness. J Immunol. 1991;147:4086–4093. [PubMed] [Google Scholar]
- 65.Eagar TN, Karandikar NJ, Bluestone J, Miller SD. The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. Eur J Immunol. 2002;32:972–981. doi: 10.1002/1521-4141(200204)32:4<972::AID-IMMU972>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 66.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B 7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanaka K, Albin MJ, Yuan X, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179:5204–5210. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fife BT, Guleria I, Gubbels Bupp M, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shouval DS, Biswas A, Goettel JA, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the upregulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 73.Rodriguez-Garcia M, Porichis F, de Jong OG, et al. Expression of PD-L1 and PD-L2 on human macrophages is up-regulated by HIV-1 and differentially modulated by IL-10. J Leukoc Biol. 2011;89:507–515. doi: 10.1189/jlb.0610327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 75.Yanagawa Y, Iwabuchi K, Onoe K. Co-operative action of interleukin-10 and interferon-gamma to regulate dendritic cell functions. Immunology. 2009;127:345–353. doi: 10.1111/j.1365-2567.2008.02986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brennan TV, Jaigirdar A, Hoang V, et al. Preferential priming of alloreactive T cells with indirect reactivity. Am J Transplant. 2009;9:709–718. doi: 10.1111/j.1600-6143.2009.02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Albert MH, Yu XZ, Magg T. Ethylenecarbodiimide-coupled allogeneic antigen presenting cells induce human CD4+ regulatory T cells. Clin Immunol. 2008;129:381–393. doi: 10.1016/j.clim.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corlett L, Davies DH. Reduced lysis by CD8+ cytotoxic T cells in mixed lymphocyte reactions induced via CD4+ T cells exposed to chemically modified antigen presenting cells. Immunology. 1995;84:488–494. [PMC free article] [PubMed] [Google Scholar]
- 79.Martin AJ, McCarthy D, Waltenbaugh C, Goings G, Luo X, Miller SD. Ethylenecarbodiimide-treated splenocytes carrying male CD4 epitopes confer Hya transplant protection by inhibiting CD154 upregulation. J Immunol. 2010;185:3326–3336. doi: 10.4049/jimmunol.1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sivaganesh S, Harper SJ, Conlon TM, et al. Copresentation of intact and processed MHC alloantigen by recipient dendritic cells enables delivery of linked help to alloreactive CD8 T cells by indirect-pathway CD4 T cells. J Immunol. 2013;190:5829–5838. doi: 10.4049/jimmunol.1300458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee K, Nguyen V, Lee KM, Kang SM, Tang Q. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am J Transplant. 2014;14:27–38. doi: 10.1111/ajt.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 83.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsang JY, Tanriver Y, Jiang S, et al. Conferring indirect allospecificity on CD4 + CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bryant J, Lerret NM, Wang JJ, et al. Preemptive donor apoptotic cell infusions induce IFN-gamma-producing myeloid-derived suppressor cells for cardiac allograft protection. J Immunol. 2014;192:6092–6101. doi: 10.4049/jimmunol.1302771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol. 2011;186:214–221. doi: 10.4049/jimmunol.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]