Abstract
The nitric oxide synthase (NOS) family of enzymes form nitric oxide (NO) from arginine in the presence of oxygen. At reduced oxygen availability NO is also generated from nitrate in a two step process by bacterial and mammalian molybdopterin proteins, and also directly from nitrite by a variety of five-coordinated ferrous hemoproteins. The mammalian NO cycle also involves direct oxidation of NO to nitrite, and both NO and nitrite to nitrate by oxy-ferrous hemoproteins. The liver and blood are considered the sites of active mammalian NO metabolism and nitrite and nitrate concentrations in the liver and blood of several mammalian species, including human, have been determined. However, the large tissue mass of skeletal muscle had not been generally considered in the analysis of the NO cycle, in spite of its long-known presence of significant levels of active neuronal NOS (nNOS or NOS1). We hypothesized that skeletal muscle participates in the NO cycle and, due to its NO oxidizing heme protein, oxymyoglobin, has high concentrations of nitrate ions. We measured nitrite and nitrate concentrations in rat and mouse leg skeletal muscle and found unusually high concentrations of nitrate but similar levels of nitrite, when compared to the liver. The nitrate reservoir in muscle is easily accessible via the bloodstream and therefore nitrate is available for transport to internal organs where it can be reduced to nitrite and NO. Nitrate levels in skeletal muscle and blood in nNOS−/− mice were dramatically lower when compared with controls, which support further our hypothesis. Although the nitrate reductase activity of xanthine oxidoreductase in muscle is less than that of liver, the residual activity in muscle could be very important in view of its total mass and the high basal level of nitrate. We suggest that skeletal muscle participates in overall NO metabolism, serving as a nitrate reservoir, for direct formation of nitrite and NO, and for determining levels of nitrate in other organs.
Keywords: nitrate, nitrite, mammalian nitrate reductases, xanthine oxidoreductase, functional hyperemia
Introduction
In mammals nitric oxide (NO) is a signaling molecule with a broad array of effects – NO has been implicated in such diverse processes as vascular homeostasis, blood clotting, the immune response and neuronal signaling [1 - 4]. When oxygen supply in tissue is adequate, NO is produced mostly from enzymatic conversion of arginine to citrulline by the family of nitric oxide synthase, (NOS), enzymes. In conditions of reduced oxygen supply, reduction of nitrite either by the 5-coordinated ferrous heme of deoxyhemoglobin or other ferrous hemoproteins and molybdopterin-containing enzymes in several tissues provide additional NO [5 - 9]. Nitrite is supplied either by oxidation of NO excess in blood or by reduction of nitrate via molybdopterin-containing mammalian nitrate reductases, such as xanthine oxidoreductase (XOR) or aldehyde oxidase (AO) [10 - 16] or various salivary bacteria nitrate reductases [17; 18]. Nitrate is a final end product of either NO or nitrite oxidation by oxyheme proteins, such as oxyhemoglobin or oxymyoglobin, and also enters the body from diverse dietary sources. For details of the current knowledge of the NO cycle, see extensive review by van Faassen et al. [3].
Generally, only blood and the internal organs, especially the heart and liver, have been considered as active sites where the nitric oxide metabolites cycle [7; 9; 12; 19; 20]. Smooth muscle was recognized early as a target organ for NO effects, but very little has been presented about the possibility of skeletal muscle playing an important role in the NO cycle or its function. However, Murad et al. found in 1993 that neuronal NOS (nNOS or NOS1) is expressed in skeletal muscle tissue in significant quantities [21]. In fact, it was later shown that skeletal muscle contains two different active nNOS splice variants, nNOSμ an essential member of the dystrophin-associated protein complex in sarcolemma, and nNOSβ - located in the Golgi complex [22]. The exact roles of both NOS proteins in myocytes are still under debate. Interestingly, progression of dystrophin loss in some types of muscular dystrophies (especially Duchenne and Becker) is associated with increasing disruption of dystrophin-associated protein complex and dissociation of nNOSμ into the cytoplasm [23]. It is worth noting that sarcopenia - process of muscle mass loss with aging - has been recently connected with NOS deficiency in mice [24].
Also in recent years it has also been recognized that NO plays an important role in skeletal muscle function. It is produced in muscle tissue even at rest and during contraction its production substantially increases [25]. When NO released from isolated rat muscle into a bathing solution was measured, there was ~60pmol of NO/mg at resting conditions and the value almost tripled to ~150pmol of NO/mg during electrical stimulation [25]. When L-NMMA, a general NOS inhibitor was added, NO release was suppressed to about half of resting value and addition of arginine or SNP (an NO donor) doubled the released NO compared to resting conditions. The above values show that NOS activity of skeletal muscle is important, even at the resting conditions, which would likely make the skeletal muscle, as the largest organ in the mammalian body, the main site of production of NO and its metabolites. NO participates both in the regulation of resting and exercise-induced blood flow, possibly contributing to active functional hyperemia, and it is also involved in skeletal muscle metabolism by increasing glucose uptake into cells - for review on NO effects in skeletal muscle see [26]. Presumably, due to its action on mitochondrial respiratory chain complex IV, NO also improves muscle endurance capabilities and muscle repair – for more details on the variety of known NO functions in muscle cells see [27 -29].
With increasing knowledge about NO metabolism pathways, greater attention has been given to sources of NO such as nitrite and, especially recently, nitrate. Both ions had been quantified using chemiluminescence in most of the internal organs [7; 9; 12; 19; 20], but we are not aware of any similar measurements done in skeletal muscle.
We hypothesized that, skeletal muscle is an important factor in NO homeostasis, due to co-localization of the functional nNOS isoforms and oxymyoglobin in the same compartment. Existence of active nNOS suggests the possibility of in situ formation of significant quantities of NO, while close proximity of myoglobin, mostly in its oxymyoglobin form, would lead to quick deactivation of excess NO and formation of nitrate. The presence of xanthine oxidoreductase, with its known nitrate reductase activity, may further enhance the importance of skeletal muscle as one of the possible major players in NO homeostasis. In this study we have found that rodent leg skeletal muscle contains unusually high concentrations of nitrate. We also measured the nitrate reductase activity of rat muscle xanthine oxidoreductase and found nitrate reduction, albeit at low levels, even at pH 7.4. Taken together, these findings support the hypothesis that skeletal muscle is an active and important compartment in the NO cycle.
Materials and Methods
Adult Wistar male rats (n=6, weight 250±50g, Charles River Laboratories, Wilmington, MA), adult nNOS−/− mice (n=5, background C57BL/6, The Jackson Laboratory, USA) and adult wild type (n=10, C57BL/6J, NCI Frederick, USA) were enclosed in an anesthesia box and anesthetized using 5% isoflurane mixture with air. Anesthetized animals were placed on a pad in supine position and anesthesia continued through a nose cone. The thoracic cavity was opened and ~9-10 ml of blood collected by cardiac puncture; representing about two third of total blood volume for an animal of this size. Approximately 1ml of blood was collected from the mouse. Heparin was used as an anticoagulant in nitrite and nitrate determinations. Immediately after its draw, blood was mixed with “stop” solution containing potassium ferricyanide, NEM and detergent in final ratio 2:1 as described in [30] in order to conserve nitrite from degradation by hemoglobin. Samples from liver and skeletal muscle from hind leg were collected shortly after the blood draw and placed into 250μl of stop solution for chemiluminescence analysis or flash frozen on dry ice for the Western blot and nitrate or nitrite reductase assays. All samples were stored at −80°C until analysis. Animals were housed in a 12-hour light/dark cycle environment with access to food and drinking water ad libitum. All animal procedures were carried out according to the recommendations in the Guide for the Care and Use of Laboratory Animals of NIH under NIDDK Animal Care and Use Commitee approved protocol.
Standard chemiluminescence nitrite and nitrate assays in mouse and rat tissues were carried out according to previously published procedures [30; 31]. Tissue samples were weighed, mixed with additional stop solution and homogenized using GentleMacs (Miltenyi Biotec Inc, Auburn, CA). Proteins were precipitated using methanol (dilution 1:3 sample:methanol) and samples centrifuged at 11000g for 5 min at 4°C to separate most of the protein. Supernatants were collected and used to determine nitrite/nitrate content by chemiluminescence (Sievers 280i Nitric Oxide Analyzer, GE Analytical Instruments, USA).
Nitrate reductase assays in rat liver and skeletal muscle tissues were performed according to the recently published procedures [14]. Due to insufficient amount of tissue samples available from mouse, we were restricted to rat tissues in these experiments. Briefly, tissue was homogenized using GentleMacs tissue dissociator (Miltenyi Biotech, Auburn, CA, USA), total protein in homogenate was determined using the bicinchoninic acid (BCA) assay kit (Pierce Rockford, IL) and adjusted as necessary to 7 mg/ml. Then either 100μM, 300μM or 500μM nitrate together with cofactor mix for nitrate reductases (AO/XOR) were added and aliquots were taken at 0 min, 30 min, 1h, 2h, 3h, 4h and 24h and analyzed by chemiluminescence for nitrite content. Cofactor mix consisted of 1 mM NADPH (Fluka), 2 mM UDP glucuronic acid (UDPGA), 0.5 mM glutathione (GSH), 0.5 mM NAD+ and NADH (all from Sigma) in 100 mM phosphate buffer pH 7.4 [14]. Experiments were performed at 37°C and samples were kept at 2% oxygen. Functional hyperemia in skeletal muscle occurs during intense exercise when oxygen availability in muscle tissue is decreased. Resulting reduced oxygen concentration depends on exercise intensity and duration. We choose 2% oxygen to perform the in vitro experiment, because we hypothesized that it could be close to the reduced oxygen tensions muscle may be subjected to during exercise. The experiment in liver homogenate was performed at the same oxygen levels for comparable conditions for XOR and AO.
To investigate if the observed the nitrate reduction in rat liver is a result of action of two known nitrate reductases, xanthine oxidoreductase (XOR) and aldehyde oxidase (AO), we used oxypurinol and raloxifene, inhibitors of XOR and AO, respectively. Inhibitors were added at time 0 to the tissue homogenate together with nitrate at a concentration of 300μM and the nitrate reduction assay proceeded as described above. We used 100μM oxypurinol and 50nM raloxifene.
Rat liver and skeletal muscle homogenate was prepared by GentleMacs tissue dissociator with RIPA buffer (Sigma, Cat.# R0278) containing protease inhibitor cocktail (Sigma, Cat.# S8830), and then protein concentration was determined by BCA assay. Denatured samples (50μg) were run on SDS-PAGE and then transferred to nitrocellulose membrane. The membrane was incubated with primary antibodies (Anti-XOR:Santa Cruz Biotechnology, sc-20991; Anti-AO: Santa Cruz Biotechnology, sc-98500; Anti-GAPDH:Sigma-Aldrich, G9545) overnight at 4°C, then immunoblotted with anti-rabbit fluorescent antibody (Licor Biosciences, 926-32211) for 1 hour at 4°C. The blots were imaged using the Odyssey imaging system (Licor Biosciences).
Statistical significance of results was tested using one way ANOVA test.
Results
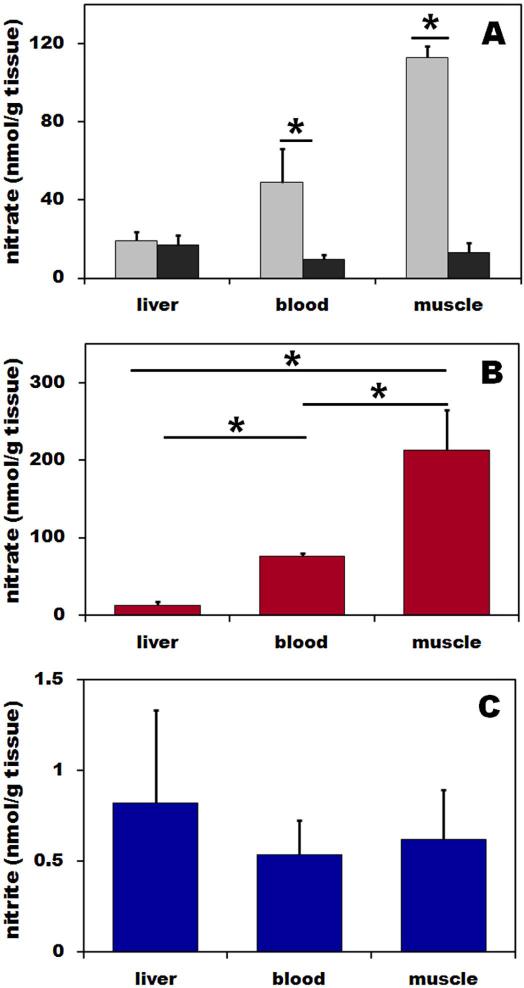
To examine possible mechanisms for the high nitrate levels in muscle we measured nitrate levels in wild type (C57BL/6) and nNOS−/−. Nitrate concentrations of wild type and nNOS−/− mice in skeletal muscle were 113±5.8 and 13.2±5, in blood 48.9±17.3 and 9.7±2.3, and in liver and16.9±5 nmol/g, respectively. These nitrate concentrations in skeletal muscle and blood of nNOS−/− mice were significantly lower than in control mice (p<0.05 for both cases), as seen in Figure 1A. Nitrite levels in all mice tissues were similar in wild type and nNOS−/− mice (data not shown).
Figure 1. (A) Nitrate distribution in skeletal muscle, blood and liver of wild type (grey bar) and nNOS−/− (black bars) mice, (B) nitrate, and (C) nitrite distributions in liver, blood and skeletal muscle of Wistar rat.
Nitric oxide metabolites in tissues homogenates and blood were determined using chemiluminescence method with tri-iodide or vanadium chloride reducing solutions for nitrite and nitrate, respectively. Tissues and blood from ten wild types and 5 nNOS−/− mice and nine rats were used in this experiment and final results were calculated as nitrite and nitrate content per gram of original tissue.
Nitrate concentrations in rat liver, blood and skeletal muscle were 12.7±4.6, 76.6±2.6 and 212.4±52.1 nmol/g tissue, respectively. Thus the nitrate concentration in muscle is significantly higher than in liver and blood (p<0.05) but nitrate level in liver was significantly lower than in blood (p<0.05) (Figure 1B).
We found that nitrite concentrations in rat liver, blood and skeletal muscle were, 0.8±0.5, 0.5±0.2 and 0.6±0.2 nmol/g tissue, respectively. As seen in Figure 1C, nitrite levels in skeletal muscle and liver were comparable and both values trended to be slightly higher (but not statistically significantly) than in blood.
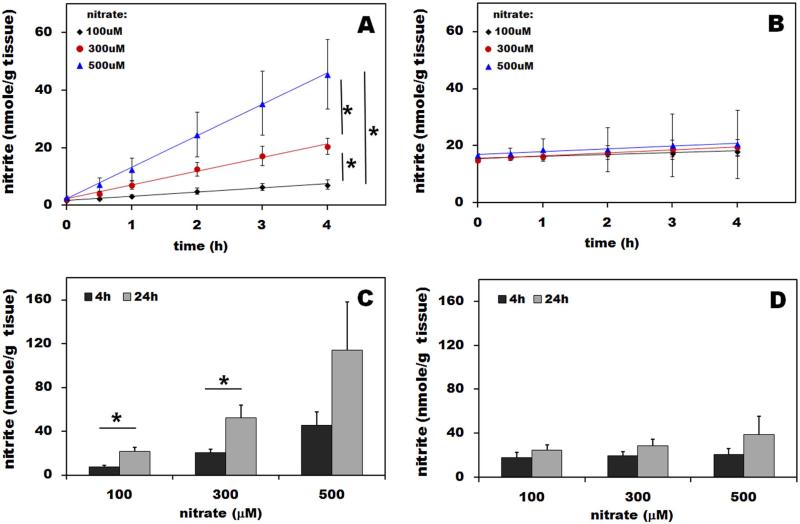
Results of nitrate reductase assays in rat skeletal muscle and liver homogenate determined at 2% oxygen are shown and compared in Figure 2A and B. After addition of nitrate to the final concentration of 100, 300 or 500μM, we measured nitrite concentration in the homogenate over the next 4 hours with a final point taken at 24h (Figure 2C and D). In liver, after addition of the different concentrations of nitrate, we observed a linear increase of nitrite in the homogenate over the next 4 hours (Figure 2A), with rates of 1.5, 4.8 and 10.9 nmol/(h x g tissue), respectively. Four hours after nitrate addition significantly different amounts of nitrite were found in liver homogenate with different starting nitrate levels (p<0.05). Figure 2B shows the results for skeletal muscle homogenate. Calculated rates of nitrate reduction to nitrite were 0.4, 0.5 and 0.9 nmol/(h x g tissue), numbers that are significantly lower than observed for liver. No significant differences in amount of accumulated nitrite were observed at different starting conditions. For clearer comparison of both organs, total nitrite in liver and muscle homogenates measured at 4h and 24h after addition of 100, 300 and 500μM nitrate are shown in Figures 2C and D. In liver, after initial addition of 100, 300 and 500 μM nitrate, nitrate reduction reaction leads to further accumulation of nitrite over 24 hours, with statistical significance reached for 100 and 300μM nitrate (p<0.05).
Figure 2. Nitrate reductase assay in rat liver (A) and skeletal muscle (B) homogenates.
The assay was performed according to published protocol [14]. Fresh liver homogenate was mixed with NADPH, UDP glucuronic acid, NAD+, NADH and glutathione in order to provide all necessary cofactors and reducing environment for XOR/AO. After addition of 100, 300 and 500μM nitrate, homogenate mixture was incubated at 37°C under the atmosphere containing 2% oxygen and aliquots were taken at 30 min, 1, 2, 3, 4 and 24 hours and analyzed by chemiluminescence to determine nitrite production/ gram liver tissue. Panel A and B summarize the resulting nitrite levels as a function of time during first 4 hours. Panel C and D compare total nitrate conversion into nitrite at 4 and 24h in liver (C) and skeletal muscle (D) after addition of 100, 300 and 500μM nitrate. Muscle and liver tissues from four rats were used in this experiment.
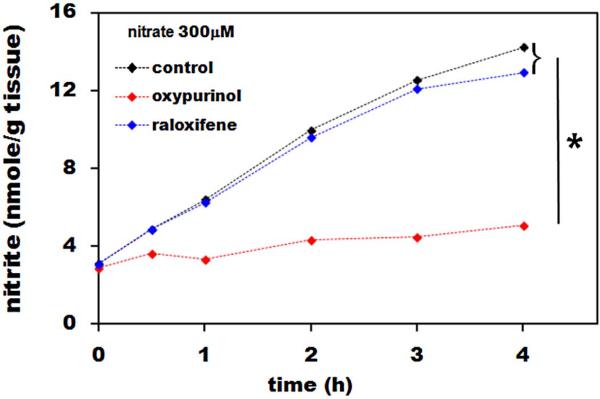
Figure 3A shows the effect of the XOR inhibitor, oxypurinol, and the AO inhibitor, raloxifene, on nitrate reduction rat in liver homogenate over 4 hours after addition of 300μM nitrate at pH 7.4. While oxypurinol drastically inhibited nitrate reduction (p<0.05), raloxifene had little effect, suggesting that most of the reduction was due to XOR.
Figure 3. Effect of XOR inhibitor, oxypurinol, and AO inhibitor, raloxifene on nitrate reduction in liver.
We used 100μM oxypurinol and 50nM raloxifene, added at time 0h together with 300μM nitrate and the nitrate reductase assay followed the same protocol as used to collect data without inhibitors addition. Liver homogenate from three different rats was used in this experiment.
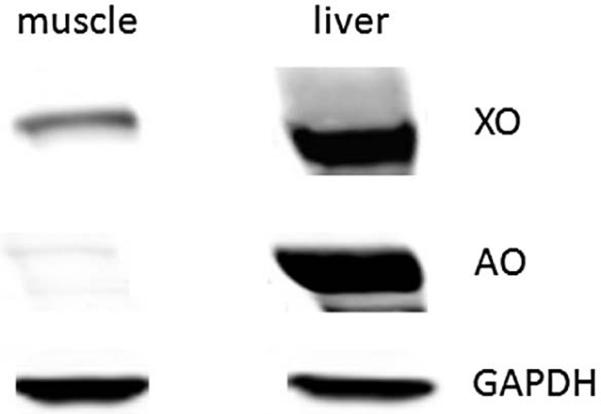
The presence of XOR and AO in rat skeletal muscle tissue and liver was checked using Western blot as presented in Figure 4. XOR is expressed at significant levels in muscle and liver tissues while AO expression level is very low, but detectable, in skeletal muscle tissue.
Figure 4. Representative Western blot of XOR and AO in skeletal muscle and liver tissues.
Skeletal muscle and liver homogenate samples (50μg) were run on SDS-PAGE and transferred onto nitrocellulose membrane for immunoblotting. Primary antibodies (anti-XOR, -AO, and –GAPDH) were incubated overnight at 4°C and then anti-rabbit fluorescent antibodies were used for imaging with the Odyssey imaging system. Tissues from four rats were used in independent experiments.
Discussion
There are many reports on nitrite and/or nitrate concentration measurements in organs and blood of different animal species and humans. Our data in liver and blood in control wild type animals (mice and rats) are in good agreement with these reports [32 - 34]. To our knowledge, values of nitrite and/or nitrate in skeletal muscle have not been reported so far. These new results on nitrate concentrations in rodent skeletal muscle tissue suggest that this tissue, constituting one of the largest organs in the mammalian body, could play a major role in mammalian nitrogen metabolism.
It is likely that the unexpectedly high nitrate content of rodent skeletal muscle tissue is due to the nNOSμ and nNOSβ activity of this tissue. When nitrate levels were compared in skeletal muscle tissue of wild type and nNOS−/− mice, lack of the nNOS enzyme markedly reduced the amount of nitrate measured in skeletal muscle and blood compared to wild type animals, as documented in Figure 1A. Nitrate levels in nNOS−/− mice presumably originate from dietary contribution and oxidation of NO produced by other NOS isoforms – eNOS (NOS3) and iNOS (NOS2).We postulate that the high levels of nitrate result from local NO production and its oxidation, as skeletal muscle contains high concentrations of oxymyoglobin, which avidly reacts with NO to form nitrate and metmyoglobin. In fact, the role of myoglobin as an NO scavenger in skeletal muscle to prevent its toxicity had been proposed [35; 36]. Muscle cells also contain robust metmyoglobin reductase systems, which reduce metmyoglobin back to myoglobin to keep it functional as an oxygen transporter within the myocyte [37; 38], and available for further reaction with NO.
However, another more direct pathway of nitrate formation within muscle tissue is also possible. NO is not the only possible end product in the reaction cycle of the nNOS enzyme. In fact, depending on the path by which ferric heme releases NO and returns to the initial ferrous oxidation state, two end products are possible – NO and nitrate ions. The details of this alternative process, termed the “futile” cycle, are described elsewhere [39 - 41]. Since nitrate was long seen only as a “waste” end product without much physiological importance, this pathway has not been of much interest until now. However, in the light of recent developments showing that nitrate is in fact an active member of the NO cycle, we believe more attention should be given to the possibility that NOS enzymes, and in particular the nNOS enzyme, could be a significant source of nitrate in skeletal muscle tissue (or other tissues, such as brain), due to the relative abundance of the NOS isoforms. Indeed, nNOS enzymes appear to be equally abundant in virtually all tissues, as measured by mRNA levels [42]. Thus it may be better to designate it as NOS1 rather than nNOS in reference to its ubiquitous distribution and potential function.
When analyzing the data in Figure 1B in terms of distributions of nitrate and nitrite ions among skeletal muscle, blood and liver, it is striking that nitrate concentrations in skeletal muscle are about 3-fold over those found in blood and about 17-fold higher than in liver. This distribution is not reflected by nitrite ions, levels of which are slightly higher in muscle and liver than in blood, although the differences are not statistically significant. Therefore, there is a well pronounced nitrate gradient from skeletal muscle through blood to other tissues, such as liver, and a slight nitrite gradient from these organs (liver and skeletal muscle) to blood. This distribution leads us to the hypothesis that skeletal muscle mass serves as an endogenous reservoir of nitrate ions that are then distributed via the bloodstream into other internal organs, and in particular liver, where they can be converted by actions of XOR or AO into nitrite and further reduced into NO.
The origin of the nitrate “stock” in skeletal muscle is either oxidation of NO from nNOS by oxymyoglobin or direct nitrate synthesis during the nNOS “futile” cycle. To support this hypothesis, we measured nitrate reduction by XOR and AO in skeletal muscle and compared it with nitrate reduction in liver, an organ that had been shown several times as a locus for nitrate reduction in mammals [9; 12; 14]. The results are plotted in Figure 2. Comparison of data shown in Figure 2A (liver) and 2B (skeletal muscle) leads to conclusion that liver is much more potent in reducing nitrate than the skeletal muscle, likely due to the much higher XOR expression levels in liver tissue (Figure 4). An inhibitor study with oxypurinol (XOR inhibitor) confirmed that XOR is the active protein responsible for nitrate reduction in liver, as shown in Figure 3. Results with an AO inhibitor, raloxifene, also shown in Figure 3, suggest that this protein does not contribute to nitrate reduction under these conditions. However, it is worth noting that although the nitrate reductase activity of skeletal muscle tissue is low, when combined with its large total mass in body and high nitrate levels, muscle could still contribute to total nitrite and NO formation even under resting conditions. Also, when skeletal muscle is active, a significant drop in pH occurs within the tissue, due to the formation of lactic acid. It has been shown that XOR nitrate reductase activity is highly pH-dependent, with higher activity occurring at lower pH [12]. We believe that this pathway is important and deserves additional investigation.
With regard to the main finding of this study, the unusually high nitrate levels in skeletal muscle, these results impact the generally accepted paradigms about nitrate sources and its circulation within the body. Currently, dietary nitrate is often considered to be the main source of nitrate and nitrite in the body [43]. According to current knowledge, dietary nitrate absorbed into blood in the GI tract is transported through the whole body and can slowly diffuse into tissues – the half-life of nitrate in blood is about 6-8 hours – before being excreted by the kidneys. Also, the most abundant protein in blood, oxyhemoglobin in red blood cells, is a well-known and efficient nitrite and NO oxidase, and thus blood could also be a very significant source of nitrate generation from nitrite and NO oxidations, in addition to its role in dietary nitrate transport.
In addition to this source, as we report here, there is also a much larger but previously unknown pool of nitrate originating from metabolic processes in the skeletal muscle that exceeds the blood nitrate pool, both in the concentration and total stored nitrate amount. By comparison, an adult human has about 5 liters of blood, but about 10-15kg of skeletal muscle. If we use our measured values for liver and skeletal muscle in rat, this would translate into total 2.5μmol of nitrite and 382μM of nitrate in blood and 7-10.5μM of nitrite and 2.12 – 3.18mmol of nitrate available in skeletal muscle at any given time. This rough estimation shows that skeletal muscle tissue is highly likely to be one of the most important storage pools of nitrate in mammals.
Our hypothesis, that skeletal muscle is the largest pool of endogenous nitrate in the body, does not contradict or negate the importance of exogenous, dietary nitrate as an important source of body nitrate, with blood as its main transporter from various parts of GI tract into internal organs. Nitrate is likely transported from skeletal muscle into blood by passive diffusion, based on the approximately 3-fold concentration gradient between nitrate in blood and skeletal muscle (Figure 1B). Also, because of the existence of secondary nitrate gradients, from blood into liver and other organ tissues, there is no need for active transport systems for blood nitrate to reach various internal organ tissues. The passive diffusion of nitrate through endothelium into the intercellular space in tissues driven by these gradients could be the main mechanism of nitrate distribution between various body compartments.
However, an alternative hypothesis, that high concentration of nitrate in the skeletal muscle is a result of its accumulation in this tissue due to the active nitrate transport from blood, cannot be completely ruled out. An active nitrate transporter, sialin, has recently had been described in salivary glands [44]. Even if this pathway exists in muscle, it would not change our proposal that muscle tissue is a very important reservoir of body nitrate.
Further, acidosis or hypoxia during physical activity predicts increased nitrate reduction by XOR in muscle tissue and open the possibility of local nitrate reduction within the muscle cell, possibly to NO. Indeed, when nitrate reductase activity of liver and muscle homogenates were tested, increased nitrite formation from nitrate was observed at pH 6 compared to pH 7.4 (data not shown). However, even this low XOR activity, when combined with the large mass of skeletal muscles and the high concentrations of nitrate could be enough to significantly support internal organs and tissue homeostasis at normal basal levels of function.
Conclusions
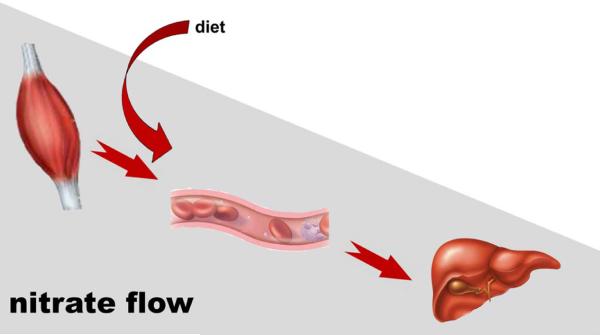
The hypotheses presented above in our view represents an intrinsic mechanism for nitrite/nitrate homeostasis in the mammalian body that is acting in synergy with variable levels of dietary nitrate and nitrite, as well as the significant contribution of the symbiotic bacterial flora that have been intensively discussed in the recent literature [1]. However, the bacterial contribution to nitrate/nitrite metabolism is also dietary-dependent and therefore can be considered only as an additional mechanism that is highly dependent on extended factors. Our data show that mammals have their own mechanisms to synthesize, store and distribute nitrate that is independent of symbiotic bacteria. Unexpectedly, skeletal muscle appears to be the organ governing intrinsic nitrate homeostasis, being its main production site and distribution regulator. The proposed endogenous diffusion-driven nitrate flow through the mammalian body based on the data collected in rat is schematically depicted in Figure 5.
Figure 5. Exogenous nitrate flow through mammalian body.
Nitrate is distributed nonhomogeneously, as represented by gray rectangle. Skeletal muscle-specific isoforms of nNOS, abundant in myocytes, produce either directly nitrate [39] or NO, which is rapidly oxidized into nitrate by oxymyoglobin present in muscle cells. This creates a large endogenous nitrate reservoir in skeletal muscle tissue. Diffusion-driven flows of nitrate from the skeletal muscle through bloodstream into liver are shown by red arrows. Nitrate is then reduced into nitrite in tissues of internal organs. In the current work we were concentrating on the liver as an organ with well described nitrate reductase activity, but actual nitrate levels in other organs were also measured and all fall well within the range found in liver. There is an important dietary nitrate input into blood, but the magnitude of this effect is highly variable as it depends on consumed diet.
NO, the most potent regulator of vascular homeostasis, has become one of the central molecules for proposed treatments for cardiovascular diseases. Many metabolic diseases, such as diabetes or the metabolic syndrome, also appear to be caused by or connected with deregulation or dysfunction of NO metabolism. It is also known that nNOS missing from the dystrophin protein complex in the skeletal muscle is associated with most forms of muscular dystrophies and likely also with sarcopenia [23; 24]. Better understanding of the mammalian NO cycle and its components may open new ways for developing more effective strategies for treatments of these diseases - see [45; 46]. In this view, our identification of the largest renewable pool of nitrate in mammalian body, the skeletal muscle tissue, may be very relevant.
Highlights.
Skeletal muscle is the major endogenous nitrate source and reservoir in mammals.
Blood serve as a transporter for nitrate from muscle into internal organs.
Abbreviations
- NO
nitric oxide
- NOS
nitric oxide synthase
- nNOS
neuronal NOS (NOS1)
- eNOS
endothelial NOS (NOS3)
- XOR
xanthine oxidoreductase
- AO
aldehyde oxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
BP, JWP and ANS designed the experiments and wrote the manuscript. BP, JWP and KMS performed the research and analyzed the data. SD and CTN bread nNOS−/− mice and provided help with animal experiment design. All authors contributed to data interpretation and commented on manuscript.
Competing financial interests:
BP, JWP, KMS, SD and CTN declare no conflict of interest. ANS is listed as a co-inventor on several patents issued to the National Institutes of Health for the use of nitrite salts for the treatment of cardiovascular diseases. He receives royalties based on NIH licensing of these patents for clinical development but no other compensation. These arrangements do not affect his adherence to NO journal policies.
References
- 1.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg JO, Weitzberg E. NO generation from inorganic nitrate and nitrite: Role in physiology, nutrition and therapeutics. Arch Pharm Res. 2009;32:1119–26. doi: 10.1007/s12272-009-1803-z. [DOI] [PubMed] [Google Scholar]
- 3.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weitzberg E, Hezel M, Lundberg JO. Nitrate-nitrite-nitric oxide pathway: implications for anesthesiology and intensive care. Anesthesiology. 2010;113:1460–75. doi: 10.1097/ALN.0b013e3181fcf3cc. [DOI] [PubMed] [Google Scholar]
- 5.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–9. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 6.Schechter AN, Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. N Engl J Med. 2003;348:1483–5. doi: 10.1056/NEJMcibr023045. [DOI] [PubMed] [Google Scholar]
- 7.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 8.Webb AJ, Milsom AB, Rathod KS, Chu WL, Qureshi S, Lovell MJ, Lecomte FM, Perrett D, Raimondo C, Khoshbin E, Ahmed Z, Uppal R, Benjamin N, Hobbs AJ, Ahluwalia A. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ Res. 2008;103:957–64. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem. 2008;283:17855–63. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendel RR. Cell biology of molybdenum. Biofactors. 2009;35:429–34. doi: 10.1002/biof.55. [DOI] [PubMed] [Google Scholar]
- 11.Mendel RR, Kruse T. Cell biology of molybdenum in plants and humans. Biochim Biophys Acta. 2012;1823:1568–79. doi: 10.1016/j.bbamcr.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry. 2003;42:1150–9. doi: 10.1021/bi026385a. [DOI] [PubMed] [Google Scholar]
- 13.Thatcher GR, Nicolescu AC, Bennett BM, Toader V. Nitrates and NO release: contemporary aspects in biological and medicinal chemistry. Free Radic Biol Med. 2004;37:1122–43. doi: 10.1016/j.freeradbiomed.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–7. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 15.Maia LB, Moura JJ. Nitrite reduction by xanthine oxidase family enzymes: a new class of nitrite reductases. J Biol Inorg Chem. 2011;16:443–60. doi: 10.1007/s00775-010-0741-z. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Kundu TK, Zweier JL. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J Biol Chem. 2009;284:33850–8. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–7. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med. 2013;55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126:325–34. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendgen-Cotta UB, Kelm M, Rassaf T. Myoglobin's novel role in nitrite-induced hypoxic vasodilation. Trends Cardiovasc Med. 2014;24:69–74. doi: 10.1016/j.tcm.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Nakane M, Schmidt HH, Pollock JS, Forstermann U, Murad F. Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett. 1993;316:175–80. doi: 10.1016/0014-5793(93)81210-q. [DOI] [PubMed] [Google Scholar]
- 22.Percival JM, Anderson KN, Huang P, Adams ME, Froehner SC. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest. 2010;120:816–26. doi: 10.1172/JCI40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehmsen J, Poon E, Davies K. The dystrophin-associated protein complex. J Cell Sci. 2002;115:2801–3. doi: 10.1242/jcs.115.14.2801. [DOI] [PubMed] [Google Scholar]
- 24.Samengo G, Avik A, Fedor B, Whittaker D, Myung KH, Wehling-Henricks M, Tidball JG. Age-related loss of nitric oxide synthase in skeletal muscle causes reductions in calpain S-nitrosylation that increase myofibril degradation and sarcopenia. Aging Cell. 2012;11:1036–45. doi: 10.1111/acel.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol (1985) 1994;77:2519–21. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- 26.McConell GK, Rattigan S, Lee-Young RS, Wadley GD, Merry TL. Skeletal muscle nitric oxide signaling and exercise: a focus on glucose metabolism. Am J Physiol Endocrinol Metab. 2012;303:E301–7. doi: 10.1152/ajpendo.00667.2011. [DOI] [PubMed] [Google Scholar]
- 27.Suhr F, Gehlert S, Grau M, Bloch W. Skeletal Muscle Function during Exercise- Fine-Tuning of Diverse Subsystems by Nitric Oxide. Int J Mol Sci. 2013;14:7109–39. doi: 10.3390/ijms14047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 29.De Palma C, Clementi E. Nitric oxide in myogenesis and therapeutic muscle repair. Mol Neurobiol. 2012;46:682–92. doi: 10.1007/s12035-012-8311-8. [DOI] [PubMed] [Google Scholar]
- 30.Piknova B, Schechter AN. Measurement of nitrite in blood samples using the ferricyanide-based hemoglobin oxidation assay. Methods Mol Biol. 2011;704:39–56. doi: 10.1007/978-1-61737-964-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinder AG, Rogers SC, Khalatbari A, Ingram TE, James PE. The measurement of nitric oxide and its metabolites in biological samples by ozone-based chemiluminescence. Methods Mol Biol. 2008;476:11–28. doi: 10.1007/978-1-59745-129-1_2. [DOI] [PubMed] [Google Scholar]
- 32.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–6. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 33.Hall CN, Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide. 2009;21:92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milsom AB, Fernandez BO, Garcia-Saura MF, Rodriguez J, Feelisch M. Contributions of nitric oxide synthases, dietary nitrite/nitrate, and other sources to the formation of NO signaling products. Antioxid Redox Signal. 2012;17:422–32. doi: 10.1089/ars.2011.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunori M. Nitric oxide moves myoglobin centre stage. Trends Biochem Sci. 2001;26:209–10. doi: 10.1016/s0968-0004(01)01824-2. [DOI] [PubMed] [Google Scholar]
- 36.Wittenberg JB, Wittenberg BA. Myoglobin function reassessed. J Exp Biol. 2003;206:2011–20. doi: 10.1242/jeb.00243. [DOI] [PubMed] [Google Scholar]
- 37.Taylor D, Hochstein P. Reduction of metmyoglobin in myocytes. J Mol Cell Cardiol. 1982;14:133–40. doi: 10.1016/0022-2828(82)90111-0. [DOI] [PubMed] [Google Scholar]
- 38.Echevarne C, Renerre M, Labas R. Metmyoglobin reductase activity in bovine muscles. Meat Sci. 1990;27:161–72. doi: 10.1016/0309-1740(90)90063-C. [DOI] [PubMed] [Google Scholar]
- 39.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–70. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 40.Tejero J, Santolini J, Stuehr DJ. Fast ferrous heme-NO oxidation in nitric oxide synthases. FEBS J. 2009;276:4505–14. doi: 10.1111/j.1742-4658.2009.07157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Poulos TL. Structure-function studies on nitric oxide synthases. J Inorg Biochem. 2005;99:293–305. doi: 10.1016/j.jinorgbio.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, 3rd, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner DA, Young VR, Tannenbaum SR, Schultz DS, Deen WM. Mammalian nitrate biochemistry: metabolism and endogenous synthesis. IARC Sci Publ. 1984:247–53. [PubMed] [Google Scholar]
- 44.Qin L, Liu X, Sun Q, Fan Z, Xia D, Ding G, Ong HL, Adams D, Gahl WA, Zheng C, Qi S, Jin L, Zhang C, Gu L, He J, Deng D, Ambudkar IS, Wang S. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc Natl Acad Sci U S A. 2012;109:13434–9. doi: 10.1073/pnas.1116633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai Z, Wu Z, Yang Y, Wang J, Satterfield MC, Meininger CJ, Bazer FW, Wu G. Nitric oxide and energy metabolism in mammals. Biofactors. 2013;39:383–91. doi: 10.1002/biof.1099. [DOI] [PubMed] [Google Scholar]
- 46.Weitzberg E, Lundberg JO. Novel aspects of dietary nitrate and human health. Annu Rev Nutr. 2013;33:129–59. doi: 10.1146/annurev-nutr-071812-161159. [DOI] [PubMed] [Google Scholar]