Abstract
Purpose
To analyze the Memorial Sloan Kettering Cancer Center 23-year experience with surgical resection and utilization of concurrent adrenalectomy and lymphadenectomy for locally advanced non-metastatic renal cell carcinoma.
Material and Methods
Retrospective review of 802 patients who underwent nephrectomy, with or without concurrent adrenalectomy or lymphadenectomy, for locally advanced renal cell carcinoma defined as stage ≥T3 and M0. Patients who had undergone adjuvant treatment within 3 months of surgery, had <3 months of follow-up, or had bilateral renal masses at presentation were excluded. Five- and 10-year progression-free and overall survivals were estimated using the Kaplan-Meier method. Differences between groups were analyzed by the log-rank test.
Results
A total of 596 (74%) and 206 (26%) patients underwent radical and partial nephrectomy, respectively. Renal cell carcinoma progressed in 189 patients and 104 died from it. Median follow-up for patients who did not progress was 4.6 years. Symptoms at presentation, American Society of Anesthesiologists classification, tumor stage, histologic subtype, grade, and lymph node status were significantly associated with progression-free and overall survival. On multivariate analysis, adrenalectomy utilization decreased over time with odds ratio .82/year, whereas lymphadenectomy increased with odds ratio 1.16/year. Larger tumors were associated with a higher likelihood of concurrent adrenalectomy and lymphadenectomy.
Conclusions
In our series of patients with locally advanced non-metastatic renal cell carcinoma, those who are in good health, asymptomatic upon presentation, have T3 tumors, and negative lymph nodes had favorable survival. Further, there has been a trend toward more selective use of adrenalectomy and increased use of lymphadenectomy.
Keywords: kidney neoplasms, partial nephrectomy, radical nephrectomy, renal cell carcinoma, survival
INTRODUCTION
In the United States, kidney cancer is the sixth most common cancer in men and the eighth most common cancer in women.1 The increased availability of cross-sectional imaging has led to earlier detection of small kidney tumors and a concurrent rise in the incidence of RCC.2 However, mortality from kidney cancer also continues to rise.3 At the time of diagnosis, approximately 20% of patients have locally advanced disease, 25% have metastatic disease, and up to 50% will ultimately develop metastases.4
We previously reported MSKCC 15- year experience with resection for clinically localized renal cortical tumors,2 and herein we now report a 23-year experience with surgical resection alone for locally advanced nmRCC classified as pathologic T3 or T4.5 We also closely examine the use of ipsilateral adrenalectomy and regional LND in this patient population over the same period to look for trends in utilization of these adjunct procedures at our institution and whether they mirror the published literature.6,7
MATERIAL and METHODS
After institutional review board approval, we conducted a retrospective review of all patients with pathologically confirmed, locally advanced (ie, classified as ≥T3 and M0) nmRCC who underwent nephrectomy at MSKCC from 1989 to 2012. Of 938 such patients treated at our institution, patients were excluded from analysis if they had received adjuvant chemotherapy or radiation therapy within 3 months of surgery (n = 47), had less than 3 months’ follow-up (n = 61), or had bilateral renal masses at diagnosis (n = 28). A total of 802 patients were found to be evaluable. We identified 596 patients (74%) who underwent RN and 206 (26%) who underwent PN. Tumor classification was determined according to the 2002 American Joint Committee on Cancer (AJCC) staging system.5 In cases of conventional clear cell carcinoma (n = 576), Fuhrman grading was used and assigned as low (grades 1–2) or high (grades 3–4).8 Data reviewed included patient age, sex, race, tumor laterality, histology, year of surgery, type of surgery, surgical approach, whether patients underwent concomitant adrenalectomy or LND, and disease progression status. Disease progression was defined as the development of local recurrence or distant metastases.
Statistical Methods
Survival time was calculated from the date of nephrectomy. A progression event included the first instance of local or metastatic recurrence. The probability of freedom from progression of RCC was estimated using Kaplan-Meier methods, censoring patients who did not have progression at their date of last follow-up. Differences in recurrence-free probabilities and survival probabilities by various tumor features were tested by the log-rank test. Multivariable Cox proportional hazards regression was used to investigate tumor features associated with progression and overall OS, with adjustment for age, ASA physical status classification I/II vs. III/IV. All tumor features of interest were included in the multivariable model without any variable selection.
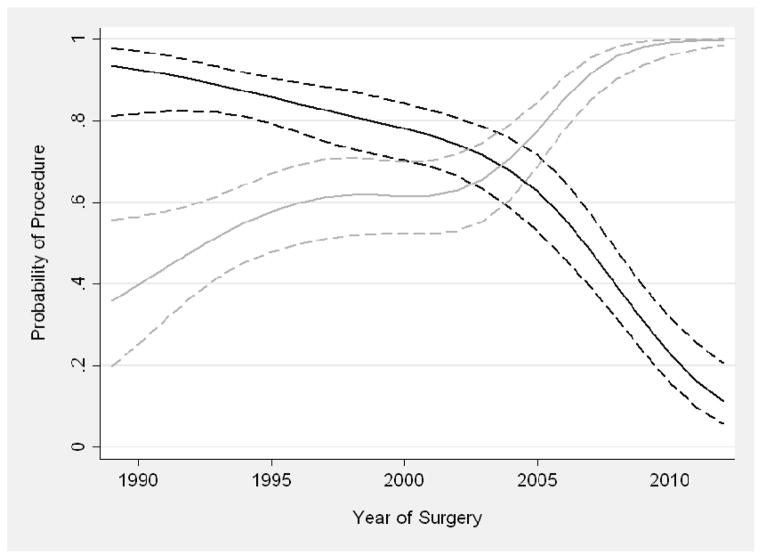
Year of surgery was entered as a continuous variable into a multivariable logistic regression model to test for trends in the utilization of adrenalectomy and LND over time; covariates were age, presentation, and tumor size. The utilization of these procedures was analyzed in the subgroup of patients who underwent RN (n = 596) because these procedures were conducted primarily for these patients. The predicted probability of each procedure was plotted over time. Year of surgery was included in the model with restricted cubic splines (knots at the tertiles) to allow for a nonlinear association. Statistical analyses were conducted using Stata 12.0 (StataCorp LP, College Station, TX). LND and adrenalectomies were done at the discretion of the surgeon with reliance on preoperative imaging and/or palpable disease. The extent of LND most commonly included regional and ipsilateral great vessels.
RESULTS
Characteristics for all 802 patients are given in Table 1. The median age at nephrectomy was 64 years, and 71% (n = 566) of patients were men. The majority of patients underwent open surgery, with 69% (n = 551) having undergone an open RN and 21% (n = 169) an open PN. LND was performed in 61% (n = 440) of cases; however, data were not available for 86 patients because of incomplete medical records. LND most commonly included regional lymph nodes with extension to the ipsilateral great vessels. Adrenalectomy was performed in 52% (n = 416) of cases. Of the 565 patients who underwent an LND or adrenalectomy, 45 (8.0%) had LN involvement alone, 10 (1.7%) had adrenal involvement alone, and 3 (<1%) had both LN and adrenal involvement. There were 189 (23.6%) patients who experienced cancer progression. Median follow-up for entire cohort was 4.2 years; at last follow-up, 273 of the 802 (34.0%) patients had died, 104 of kidney cancer. The median follow-up for patients who did not progress was 4.6 years. No patient had recurrence in the ipsilateral adrenal gland at last follow-up.
Table 1.
Characteristics of 802 Patients Who Underwent Nephrectomy at Memorial Sloan Kettering Cancer Center from 1989 to 2012
| Patient and Disease Characteristics | Surgical Characteristics | ||
|---|---|---|---|
| Median age(yrs) at surgery | 64 (55, 72) | Year of surgery | |
| Sex | 1989–1994 | 102 (13%) | |
| Male | 566 (71%) | 1995–1999 | 140 (17%) |
| Female | 236 (29%) | 2000–2004 | 173 (22%) |
| Race | 2005–2009 | 258 (32%) | |
| White | 719 (90%) | 2010–2012 | 129 (16%) |
| Black | 39 (5%) | Surgery type | |
| Other or unknown | 44 (5%) | Partial | 206 (26%) |
| ASA classification | Radical | 596 (74%) | |
| 1 | 27 (3%) | Surgical approach | |
| 2 | 401 (50%) | Open | 720 (90%) |
| 3 | 354 (44%) | Laparoscopic | 61 (7.6%) |
| 4 | 14 (2%) | Robotic | 21 (2.6%) |
| Unknown | 6 (<1%) | Adrenalectomy performed | 416 (52%) |
| Presentation | LND performed, n = 716b | 440 (61%) | |
| Incidental discovery | 460 (57%) | Tumor laterality | |
| Local or systemic symptoms | 335 (42%) | Left | 404 (50%) |
| Right | 398 (50%) | ||
| Unknown | 7 (1%) | ||
| Pathologic classification | |||
| T3a | 515 (64%) | ||
| T3b | 262 (33%) | ||
| T4 | 25 (3%) | ||
| Pathologic gradea | |||
| Low | 237 (41%) | ||
| High | 301 (52%) | ||
| Unknown | 38 (7%) | ||
| Tumor histology | |||
| Chromophobe | 74 (9%) | ||
| Clear cell | 575 (72%) | ||
| Oncocytoma | 47 (6%) | ||
| Papillary | 59 (7%) | ||
| Unclassified/Other | 47 (6%) | ||
Data are presented as frequency (percent) or median (interquartile range).
For patients with conventional (clear cell) tumor histology.
Lymph node dissection information was available for only 716 patients.
Kaplan-Meier plots of PFS and OS are given in Figures 1 to 4, and estimates at 5 and 8 years after nephrectomy are listed in Table 2. All variables analyzed (tumor stage, histology, grade, and LN status) were significantly associated with both progression and survival on univariate analysis. Similar results were found using a multivariable Cox proportional hazards regression model (Table 3).
Figure 1.
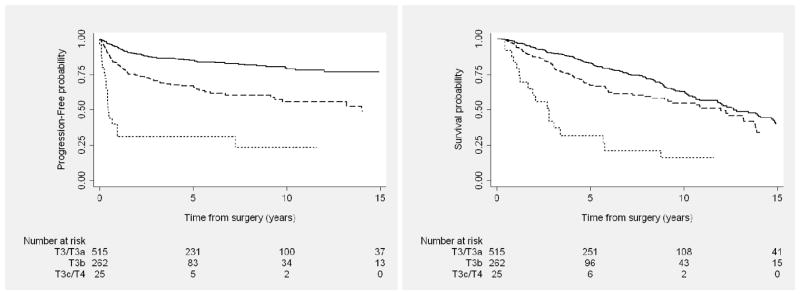
Progression-free (Left) and overall (Right) survival outcomes among patients with locally advanced kidney cancer after nephrectomy, by tumor classification. Solid line represents T3/T3a; dashed line, T3b; dotted line, T3c/T4.
Figure 4.
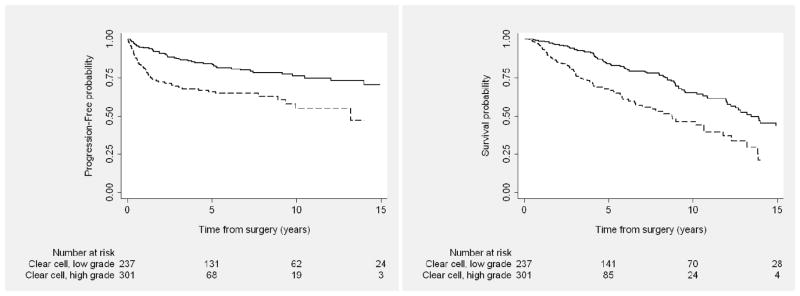
Progression-free (Left) and overall (Right) survival outcomes among patients with locally advanced clear cell kidney cancer after nephrectomy, by grade. Solid line represents low grade; dashed line, high grade.
Table 2.
Progression-Free Probability and Survival Probability after Nephrectomy, According to Tumor Features and Nodal Status
| Pa | Progression-Free Probability (95% CI) | Pa | Overall Survival Probability (95% CI) | |||
|---|---|---|---|---|---|---|
| 5 Years | 8 Years | 5 Years | 8 Years | |||
| Presentation | <.01 | <.01 | ||||
| Incidental discovery | 87% (83%, 90%) | 84% (79%, 87%) | 84% (79%, 87%) | 73% (67%, 78%) | ||
| Local or systemic symptoms | 65% (59%, 70%) | 60% (53%, 65%) | 68% (62%, 73%) | 59% (53%, 65%) | ||
| Tumor classification | <.01 | <.01 | ||||
| T3 | 79% (75%, 82%) | 75% (71%, 78%) | 78% (74%, 81%) | 68% (63%, 72%) | ||
| T4 | 31% (14%, 50%) | 23% (8%, 44%) | 32% (14%, 51%) | 21% (7%, 41%) | ||
| Tumor histology | <.01 | <.01 | ||||
| Chromophobe | 90% (78%, 95%) | 90% (78%, 95%) | 85% (72%, 92%) | 70% (54%, 82%) | ||
| Conventional (clear cell) | 74% (70%, 78%) | 69% (64%, 73%) | 75% (70%, 78%) | 66% (61%, 71%) | ||
| Oncocytoma | 100% (NA, NA) | 100% (NA, NA) | 97% (81%, 100%) | 86% (67%, 95%) | ||
| Papillary | 85% (72%, 92%) | 82% (68%, 90%) | 80% (67%, 89%) | 69% (52%, 81%) | ||
| Unclassified/Other | 59% (42%, 72%) | 59% (42%, 72%) | 54% (35%, 69%) | 35% (13%, 59%) | ||
| Tumor gradeb | <.01 | <.01 | ||||
| Low (1–2) | 84% (78%, 88%) | 78% (72%, 84%) | 84% (78%, 89%) | 77% (70%, 83%) | ||
| High (3–4) | 66% (59%, 72%) | 63% (55%, 70%) | 67% (60%, 74%) | 53% (43%, 61%) | ||
| Lymph node status | <.01 | <.01 | ||||
| Negative (N0) | 72% (67%, 77%) | 69% (63%, 74%) | 72% (67%, 77%) | 61% (55%, 67%) | ||
| Positive (N1) | 27% (15%, 41%) | 24% (12%, 38%) | 52% (36%, 66%) | 39% (23%, 54%) | ||
| LND not done (NX) | 88% (84%, 91%) | 84% (78%, 88%) | 83% (78%, 87%) | 75% (69%, 80%) | ||
P values were obtained using the log-rank test.
For patients with conventional (clear cell) tumor histology.
Table 3.
Predictors of Progression and Mortality after Nephrectomy (n = 789), Estimated Using a Multivariable Cox Proportional Hazards Regression Model
| Progression | Overall Survival | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Predictor | Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P |
| Age at surgery (per 10 years) | .92 | .80, 1.05 | .2 | 1.48 | 1.31, 1.67 | <.01 |
|
| ||||||
| Year of surgery (per 5 years) | .92 | .79, 1.07 | .3 | 1.06 | .91, 1.23 | .5 |
|
| ||||||
| ASA classification | ||||||
| ≤ 2 | Reference | Reference | ||||
| > 2 | 1.58 | 1.12, 2.22 | <.01 | 1.44 | 1.08, 1.90 | .01 |
|
| ||||||
| Presentation | ||||||
| Incidental discovery | Reference | Reference | ||||
| Local or systemic symptoms | 2.28 | 1.65, 3.16 | <.01 | 1.57 | 1.21, 2.03 | <.01 |
|
| ||||||
| Tumor classification | ||||||
| T3a/b/c | Reference | Reference | ||||
| T4 | 2.68 | 1.55, 4.65 | <.01 | 3.37 | 1.99, 5.72 | <.01 |
|
| ||||||
| Tumor histology | <.01 | <.01 | ||||
| Chromophobe, papillary, oncocytoma | Reference | Reference | ||||
| Conventional (clear cell) | 2.20 | 1.24, 3.91 | 1.01 | .71, 1.44 | ||
| Unclassified/Other | 5.93 | 2.92, 12.03 | 3.03 | 1.71, 5.35 | ||
|
| ||||||
| Tumor gradea | ||||||
| Low (1–2) | Reference | Reference | ||||
| High (3–4) | 1.62 | 1.10, 2.37 | .01 | 1.48 | 1.07, 2.06 | .02 |
|
| ||||||
| Lymph node status | <.01 | <.01 | ||||
| Negative | Reference | Reference | ||||
| Positive | 3.12 | 2.01, 4.86 | 1.48 | .94, 2.33 | ||
| LND not performed | .63 | .45, .90 | 0.68 | .51, .89 | ||
Long-term survival data were available for only 789 patients.
For patients with conventional (clear cell) tumor histology.
Ten-year PFS rate for all patients with clear cell RCC was 65% (95% confidence interval [CI], 60%–70%). When stratifying by AJCC tumor classification, 10-year PFS for those with T3 clear cell tumors was 72% (95% CI, 67%–76%) versus 23% (95% CI, 8%–44%) for patients with T4 tumors. By grade, 10-year PFS rate was 76% (95% CI, 69%–82%) for those with low-grade versus 55% (95% CI, 44%–65%) for high-grade clear cell tumors. For patients who underwent concurrent LND and were found to be LN negative, median PFS was 36.8 months and median OS was 49.5 months; for LN-positive patients, median PFS was 7.4 months and median OS was 39.4 months.
Table 4 summarizes characteristics of patients who underwent RN, further broken down by adrenalectomy (yes/no) and LND (yes/no). This summary includes only patients who underwent a radical procedure because these additional procedures were conducted primarily among these patients. Of the 206 patients who underwent a PN, 30 (14.6%) had an LND, 10 (4.9%) had an adrenalectomy, and 4 (1.9%) had both procedures. As shown in Table 5, on multivariable analysis, the utilization of adrenalectomy decreased over time (OR .82 for each year; 95% CI, .79–.86; P < .01), whereas the utilization of LND increased (OR 1.16 for each year; 95% CI, 1.12–1.21; P < .01) (Figure 5). Patients with larger tumors were significantly more likely to undergo LND (OR 1.24 per cm; 95% CI, 1.16–1.33; P < .01). Patients who were symptomatic at presentation were also more likely to undergo LND, although this was not statistically significant on multivariable analysis (OR 1.28 for symptomatic vs. asymptomatic; 95% CI, .83–1.98; P = .3).
Table 4.
Characteristics of Patients Who Underwent Radical Nephrectomy, Stratified by Whether an Adrenalectomy or Lymph Node Dissection Was Performed
| Adrenalectomy | Lymph Node Dissection | |||
|---|---|---|---|---|
| No (n = 190) | Yes (n = 406) | No (n = 172) | Yes (n = 410) | |
| Median age (yrs) at surgery | 64 (55, 72) | 63 (55, 71) | 65 (56, 74) | 62 (54, 70) |
| ASA physical status classification | ||||
| 1 | 2 (1%) | 19 (5%) | 8 (5%) | 13 (3%) |
| 2 | 82 (43%) | 238 (59%) | 104 (60%) | 216 (53%) |
| 3 | 99 (52%) | 139 (34%) | 56 (33%) | 169 (41%) |
| 4 | 6 (3%) | 5 (1%) | 3 (2%) | 7 (2%) |
| Unknown | 1 (1%) | 5 (1%) | 1 (1%) | 5 (1%) |
| Presentation | ||||
| Incidental discovery | 118 (62%) | 177 (44%) | 99 (58%) | 186 (45%) |
| Local or systemic symptoms | 70 (37%) | 225 (55%) | 72 (42%) | 219 (53%) |
| Unknown | 2 (1%) | 4 (1%) | 1 (1%) | 5 (1%) |
| Tumor classification | ||||
| T3/T3a | 124 (65%) | 228 (56%) | 127 (74%) | 215 (52%) |
| T3b | 63 (33%) | 157 (39%) | 44 (26%) | 172 (42%) |
| T3a/T4 | 3 (2%) | 21 (5%) | 1 (1%) | 23 (6%) |
| Tumor gradea | ||||
| Low | 57 (39%) | 136 (43%) | 78 (62%) | 115 (35%) |
| High | 85 (57%) | 148 (47%) | 31 (25%) | 189 (58%) |
| Unknown | 6 (4%) | 31 (10%) | 17 (13%) | 20 (6%) |
| Tumor histology | ||||
| Chromophobe | 12 (6%) | 36 (8.9%) | 12 (7%) | 35 (9%) |
| Clear cell | 147 (77%) | 315 (78%) | 126 (73%) | 323 (79%) |
| Oncocytoma | 7 (4%) | 15 (4%) | 17 (10%) | 5 (1%) |
| Papillary | 14 (7%) | 25 (6%) | 15 (9%) | 24 (6%) |
| Unclassified/Other | 10 (5%) | 15 (4%) | 2 (1%) | 23 (6%) |
| Year of surgeryb | ||||
| 1989–1994 (n = 95) | 8 (4%) | 93 (23%) | 48 (28%) | 53 (13%) |
| 1995–1999 (n = 116) | 17 (9%) | 110 (27%) | 54 (31%) | 73 (18%) |
| 2000–2004 (n = 126) | 48 (25%) | 95 (23%) | 48 (28%) | 95 (23%) |
| 2005–2009 (n = 148/n = 144) | 63 (33%) | 89 (22%) | 22 (13%) | 126 (31%) |
| 2010–2012 (n = 90/n = 72) | 54 (28%) | 19 (5%) | 0 (0%) | 63 (15%) |
| Surgical approach | ||||
| Open | 156 (82%) | 395 (97%) | 154 (90%) | 385 (94%) |
| Laparoscopic | 27 (14%) | 11 (3%) | 18 (10%) | 18 (4%) |
| Robotic | 7 (4%) | 0 (0%) | 0 (0%) | 7 (2%) |
For patients with conventional (clear cell) tumor histology.
Percentages are rounded to the nearest integer. Percentages add up to 100% across rows rather than columns.
Table 5.
Logistic Regression to Evaluate Predictors of Adrenalectomy and Lymph Node Dissection Among Patients Treated with Radical Nephrectomy (n = 596)
| Adrenalectomy (n = 590) | Lymph Node Dissection (n = 576) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictor | Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value |
| Year of surgery | .82 | .79, .86 | <.0001 | 1.16 | 1.12, 1.21 | <.0001 |
| Age at surgery (yrs) | .99 | .97, 1.01 | .4 | .98 | .96, 1.00 | .02 |
| Symptomatic at presentationa | 1.69 | 1.12, 2.56 | .013 | 1.28 | .83, 1.95 | .3 |
| Tumor size (cm) | 1.18 | 1.10, 1.26 | <.0001 | 1.24 | 1.16, 1.33 | <.0001 |
Local or systemic symptoms versus no symptoms (detected incidentally).
Figure 5.
Predicted probability of adrenalectomy (black) and lymph node dissection (gray) over time in patients undergoing a radical procedure, adjusted for age, symptoms at presentation, and size of tumor. Dashed lines are 95% confidence intervals.
DISCUSSION
We report our institutional experience with locally advanced nmRCC treated with surgical resection alone over a 23-year period. Consistent with previous reports,9–13 patients with a symptomatic presentation and advanced final pathologic features (histology, grade, stage, and LN status) experienced significantly worse rates of PFS and OS on univariable and multivariable analyses. Patients with unclassified/other histologies had the worse rates of PFS and OS.
De Cássio Zequi et al14 evaluated medical records of 145 patients who underwent PN or RN for RCC (T1–4, N0–2, M0–1) at their institution. ASA classification was found to be statistically significant and an effective prognostic factor for both cancer-specific survival and OS. However, the authors did not report on whether their patients received adjuvant therapies, leaving open the possibility of treatment selection bias, because patients with good health are more likely to receive additional treatments. In our cohort, we noted that high ASA classification was not only associated with worse OS but also with disease progression. Many mechanisms have been proposed for this observation, including a pro-neoplastic state due to chronic immunosuppression related to renal failure–associated uremia.15
Filson et al,6 in their review of the National Cancer Institute’s United States Kidney Cancer Study (2002–2007), noted that concurrent adrenalectomy was performed in 24% of RN cases, most of which involved larger tumors in symptomatic patients. The authors noted a decline of concurrent ipsilateral adrenalectomy over time, which they mostly attributed to improved quality of preoperative cross-sectional imaging. Similarly, in a Mayo Clinic retrospective review, routine ipsilateral adrenalectomy in patients with locally advanced RCC did not offer an oncologic benefit and placed the patients at risk for metastasis in a solitary adrenal gland.7 Our current practice is to perform an adrenalectomy for bulky tumors and for patients with radiographic or intraoperative evidence of adrenal involvement. Interestingly, in our study, none of our patients experienced recurrence in the ipsilateral adrenal gland after being spared.
A retrospective review by the Mayo Clinic demonstrated that high-risk RCC was associated with an increased likelihood of metastatic retroperitoneal lymphadenopathy16,17; however, the role of lymphadenectomy continues to be debated in the urologic literature.18 The European Organization for Research and Treatment of Cancer (EORTC) 30881 randomized phase 3 trial showed no benefit to lymphadenectomy19; however, this trial has been criticized for inclusion of patients with low-stage and -grade disease who had a very low likelihood of developing nodal metastases.18 In our series, we note that the use of lymphadenectomy increased with time, especially for younger patients and those with large tumors. Patients who did not undergo an LND (Nx) had better PFS in comparison with N0 patients, which is likely due to selection bias by surgeons judging those patients to be at lower risk for nodal metastatic disease based on preoperative imaging.
In our retrospective series, adrenalectomies and lymphadenectomies were most commonly performed during open surgery because patients with LN or adrenal gland involvement are more likely to undergo that approach. We believe that the finding of metastatic RCC, whether in regional LNs or adrenal glands, provides improved staging and prognostic information, and allows for better patient stratification in future clinical trials.
Our study is limited by its retrospective nature and difficulty in accounting for the effect of targeted therapy in prolonging survival in patients with metastatic RCC. We were unable to stratify patients by MSKCC criteria for patients with advanced RCC20 due to lack of laboratory data on all patients. Similarly as our experience spanned 23 years, there was no uniform follow up protocol for our patients. Also as we show that presence of disease in lymph nodes is associated with worsened survival, we do not have the actual lymph nodes count and data on local recurrence for patients who underwent lymphadenectomy.
CONCLUSIONS
In our experience, patients with locally advanced nmRCC who are asymptomatic upon presentation and have T3, low-grade disease, few comorbidities, and negative LN have favorable survival outcomes compared with symptomatic T4 patients. Further, there has been a trend in recent years toward more selective use of adrenalectomy and increased use of lymphadenectomy in patients with locally advanced RCC, largely because of careful case selection and improved preoperative imaging.
Figure 2.
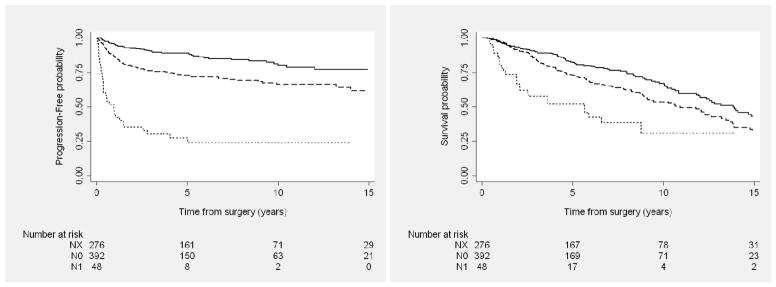
Progression-free (Left) and overall (Right) survival outcomes among patients with locally advanced kidney cancer after nephrectomy, by nodal status. Solid line represents NX; dashed line, N0; dotted line, N+.
Figure 3.
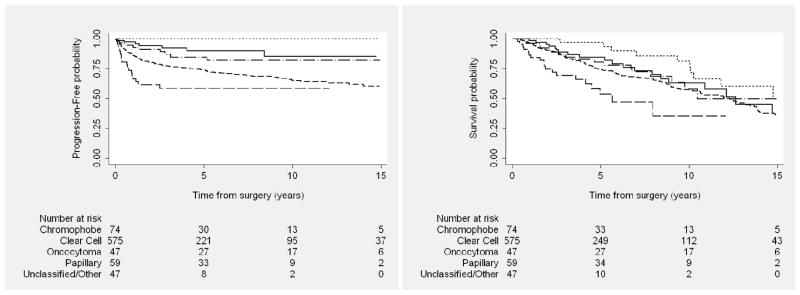
Progression-free (Left) and overall (Right) survival outcomes among patients with locally advanced kidney cancer after nephrectomy, by histology. Solid line represents chromophobe; short dashed line, clear cell; dotted line, oncocytoma; dash dot line, papillary; long dashed line, unclassified/other.
Acknowledgments
Source of Funding: This investigation was supported by the Hanson Family Renal Cancer Research Fund and the Sidney Kimmel Center for Prostate and Urologic Cancers.
Abbreviations and Acronyms
- ASA
American Society of Anesthesiologists
- LN
lymph node
- LND
lymph node dissection
- MSKCC
Memorial Sloan Kettering Cancer Center
- nmRCC
non-metastatic renal cell carcinoma
- OS
overall survival
- OR
odds ratio
- PFS
progression-free survival
- PN
partial nephrectomy
- RCC
renal cell carcinoma
- RN
radical nephrectomy
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Russo P, Jang TL, Pettus JA, et al. Survival rates after resection for localized kidney cancer: 1989 to 2004. Cancer. 2008;113:84–96. doi: 10.1002/cncr.23520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–4. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 4.Flanigan RC, Campbell SC, Clark JI, et al. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385–90. doi: 10.1007/s11864-003-0039-2. [DOI] [PubMed] [Google Scholar]
- 5.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6. New York, NY: Springer; 2002. [Google Scholar]
- 6.Filson CP, Miller DC, Colt JS, et al. Surgical approach and the use of lymphadenectomy and adrenalectomy among patients undergoing radical nephrectomy for renal cell carcinoma. Urol Oncol. 2012;30:856–63. doi: 10.1016/j.urolonc.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weight CJ, Kim SP, Lohse CM, et al. Routine adrenalectomy in patients with locally advanced renal cell cancer does not offer oncologic benefit and places a significant portion of patients at risk for an asynchronous metastasis in a solitary adrenal gland. Eur Urol. 2011;60:458–64. doi: 10.1016/j.eururo.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–63. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Kattan MW, Reuter V, Motzer RJ, et al. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63–7. [PubMed] [Google Scholar]
- 10.Sorbellini M, Kattan MW, Snyder ME, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173:48–51. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 11.Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–71. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 12.Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–66. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 13.Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 14.De Cássio Zequi S, de Campos ECR, Guimarães GC, et al. The use of the American Society of Anesthesiology Classification as a prognostic factor in patients with renal cell carcinoma. Urol Int. 2010;84:67–72. doi: 10.1159/000273469. [DOI] [PubMed] [Google Scholar]
- 15.Russo P. End stage and chronic kidney disease: associations with renal cancer. Front Oncol. 2012;2:28. doi: 10.3389/fonc.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blute ML, Leibovich BC, Cheville JC, et al. A protocol for performing extended lymph node dissection using primary tumor pathological features for patients treated with radical nephrectomy for clear cell renal cell carcinoma. J Urol. 2004;172:465–9. doi: 10.1097/01.ju.0000129815.91927.85. [DOI] [PubMed] [Google Scholar]
- 17.Crispen PL, Breau RH, Allmer C, et al. Lymph node dissection at the time of radical nephrectomy for high-risk clear cell renal cell carcinoma: indications and recommendations for surgical templates. Eur Urol. 2011;59:18–23. doi: 10.1016/j.eururo.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Delacroix SE, Chapin BF, Wood CG. The role of lymph node dissection in renal cell carcinoma. Urol Clin North Am. 2011;38:419–28. vi. doi: 10.1016/j.ucl.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Blom JHM, van Poppel H, Maréchal JM, et al. Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. 2009;55:28–34. doi: 10.1016/j.eururo.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]