Abstract
The B-subunits of heat-labile enterotoxins LT-I (LT-IB) and LT-IIa (LT-IIaB) are strong adjuvants that bind to cell-surface receptors, including gangliosides GM1 and GD1b, respectively. LT-IIaB also binds TLR-2. We demonstrate for the first time that co-incubation with the B-subunits induces significant clustering of B cells after only 4 hrs, and B and T cells in 24 hrs. Clustering was dependent on intact B-subunits, but not on the TLR-2 binding activity of LT-IIaB, indicating it was ganglioside-mediated. Treatment of B cells with LT-IB, a mixture of LT-IB+LT-IIaB, but not LT-IIaB alone, caused a delay in T cell division following ovalbumin endocytosis. B cell receptor-mediated uptake in presence of each treatment caused an arrest, but with increased production of IL-2. Further, treatments differentially increased the proportion of macrophages expressing MHC class-II. These results highlight the outcomes of interplay between signals involving different receptors and implicate a novel mechanism of adjuvanticity.
Keywords: B cells, T cells, GM1, TLR-2, GD1, gangliosides, Enterotoxins, Adjuvant, Vaccine
1. Introduction
The family of E. coli heat-labile enterotoxins (LTs) includes type I (LT-I, also named LT) and type II (LT-II) enterotoxins. The enterotoxins share structural and some functional similarities, but each has unique properties. All the variants of LT-I (LTh-I, LTp-I, etc.) that have been identified, are classified as LT-I. There are, however, three antigenically distinctive types of LT-II (LT-IIa, LT-IIb, and LT-IIc) [1, 2]. Both types of LTs are composed of a toxic A-subunit (A1+A2) with ADP-ribosylase activity responsible for causing diarrhea, and five B-subunits forming a pore through which the A2-subunit interacts with the B pentamer [3]. The B-subunits of the LTs (LT-IB and LT-IIaB) are non-toxic and bind to gangliosides on the surface of mammalian cells. While LT-IB binds avidly to ganglioside GM1, LT-IIaB binds with high affinity to ganglioside GD1b, GD1a, GM1 (in decreasing order) and to Toll-like receptor 2 (TLR-2) [1, 3-7]. Gangliosides are ubiquitously found on most cells including cells of the immune system. TLR-2 is expressed on the surface of many cells including those involved in the innate and the adaptive immune response [8, 9].
A characteristic and unique property of LTs is their potent immunogenicity and adjuvant properties [1, 10-12]. These properties are manifested in part at the level of antigen presenting cells (APC) and T cells by a number of partially-defined mechanisms that include alteration of cytokine production, enhanced expression of co-stimulatory molecules, efficient antigen (Ag) uptake and presentation, and expansion of T cells [1, 10, 13-17]. Most of the stimulatory effects of LTs on the APC and T cells are mediated by the binding of the B-subunits to their respective receptors [1, 7, 13-15]. Thus, in contrast to a non-receptor binding mutant of LT-IB, incubation of mouse cells with wild type LT-IB results in increased expression of MHC class II, B7-2 (CD86), IL-2Rα (CD25), CD40 and ICAM-1 (CD54) on B cells [14]. Some of these events are mediated by increases in the levels of PI3K and MAP/ERK kinases [18]. The LT-IB stimulatory effect on CD25 expression, a marker of cell activation, is also shown in B cells and CD4+ T cells in cultures from the spleen and lymph nodes [15]. Immunization with LT-IB induces high levels of mucosal and systemic antibody responses [15]. LT-IB also modulates cytokine secretion by dendritic cells [13]. Further, the targeting of Ag which is chemically coupled or fused to LT-IB to the surface of APCs significantly enhances the presentation of that Ag to T cells and its immunogenicity [13, 19]. These findings are explained by the high affinity binding of LT-IB to GM1 on surface of APCs and the efficient delivery of the Ag to MHC-I and MHC-II compartments of Ag processing and presentation [13, 20]. Incubation of mouse splenic cells with LT-IB also results in enhanced levels of IL-4 and IL-5 and reduced level of IFN-γ [15]. The induction of this anti-inflammatory T helper 2 (Th2) cytokine profile by LT-IB alters the course of disease as shown in a mouse model of collagen-induced arthritis [21].
In comparison to LT-IB, LT-IIaB binds with high affinity to TLR-2 and GD1b on mouse and human monocytes, and induces secretion of TNF-α, IL-1, IL-6 and IL-8 by increasing activation of NF-kB [22]. LT-IIaB also induces migration of dendritic cells in nasal mucosa by increasing expression of CCR7, uptake and presentation of Ag, and inducing their maturation as indicated by elevated expression of CD80, CD86, and CD40 [7]. TLR-2 and GD1b binding mediates the stimulatory effects of LT-IIaB on dendritic cells [7]. LT-IIaB also augments proliferation of Ag-specific CD4+T cells and IgA and IgG antibodies following intranasal immunization with Ag [7]. Thus the LT-IIaB effects on immune cells result mainly in a proinflammatory immune response [22].
In accord with the studies above, a few micrograms or even nanograms of LTs or the closely related cholera toxin (CT) from Vibrio cholera, as well as their non-toxic B-subunit derivatives induce strong systemic and mucosal immune responses to themselves and to co-administered Ag. Although the adjuvanticity of LTs could be explained by a direct effect on APCs resulting in their activation, the mechanisms by which such minute amounts of the enterotoxins enhance immune responses to an unrelated Ag are unclear. It has been previously shown that binding of LT-IB or LT-IIaB to their respective receptors on immune cells is essential for their immunogenicity and adjuvant function [7, 15]. Nevertheless, this mechanism alone (i.e. local activation of APCs and resulting Ag-specific T cell responses by very small amounts of the binding proteins) would not be sufficient to explain the induction of a remarkable expansion of the antibody and T cell immune responses to the co-administered Ag, and the generation of long-term memory (2 years in the case of CT) even after single oral or systemic immunization [23-26]. In this regard, early studies on the adjuvanticity of CT to sheep red blood cells (SRBCs) showed that the remarkable expansion of the immune response to SRBCs was delayed as it occurred several days after a subsequent boost with the Ag [25]. A delay in the uptake of LT-IB conjugated to ovalbumin (OVA) has also been shown in B cells when compared to the binding of OVA alone to the BCR, which suggests it may play a role in its adjuvanticity [19]. Moreover, maximal expression of MHC class II on B cells occurred after a 48 hr incubation with LT-IB [14] compared to only 24 hrs with LPS or anti-BCR antibody. These results suggest that besides the effect of LT-IB on the activation of APC, the adjuvant properties of LT-IB toward other Ags are partly due to a delay in uptake and processing of that Ag, and to other unknown mechanisms. Therefore, we sought to determine additional effects of the enterotoxins B-subunits on B and T cells within a controlled in vitro system to allow visualization of various events involved in the response to a co-administered Ag, in this case OVA. These include the effects of the B-subunits LT-IB and LT-IIaB on cellular clustering, T cell division, IL-2 production, and the regulation of MHC class II in macrophages, each of which is an essential event involved in the enhancement of an immune response to Ag.
2. Materials and Methods
2.1 Cell lines
A20 WT B cells and A20 transfected PC-specific human μ heavy chain (A20μ WT) are murine B lymphoma cells described previously [27] (a generous gift from Prof. Jim Drake, Albany Medical College, NY). DO.11.10 is a T-cell hybridoma specific for OVA323-339 in the context of I-Ad. A20 WT and DO.11.10 cells were cultured in DMEM (Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum, 50 IU/ml penicillin, 50 μg/ml streptomycin, 2 mM L-glutamine and 50 μM 2-mercaptoethanol (2-ME). A20μ WT B cells were grown in medium supplemented with 0.6 mg/ml G418 (Sigma-Aldrich). RAW264.7 cell line was cultured in DMEM high glucose (Sigma-Aldrich) supplemented with 20 % FBS and penicillin/streptomycin as described above.
2.2 Antigens
Recombinant E. coli heat-labile enterotoxin B subunits (LT-IB) expressed in the yeast Pichia pastoris (Sigma-Aldrich, >90% pure), and highly purified recombinants LT-IIaB, LT-IIbB and LT-IIbB(S74D) were used. Recombinant preparation of the B-subunits of LT-II were obtained from E. coli and purified to > 90% by affinity chromatography using a His-Bind resin column and gel filtration chromatography [22, 28]. The preparations were essentially free of LPS (0.0064 ng/μg of protein). LT-IB and LT-IIaB were used at 5μg/ml and 2.5μg/ml, respectively, unless indicated otherwise. The choice of LT-IB and LT-IIaB doses in this study is based on their direct or indirect stimulatory effect on immune cells and their products, as shown previously [13, 14, 19, 22]. LT-IIbB and LT-IIbB(S74D) were used at 10 μg/ml. OVA was added at 500 μg/ml. PC-OVA was used at 30 μg/ml. Conjugation of PC-OVA was performed as it follows: Diazoniumphenyl-PC was prepared by dissolving 5 mmol of aminophenyl-PC (Toronto Research Chemicals, Canada) in 5 ml of 1 N HCl and adding 5 mmol of sodium nitrite (Sigma). The reaction was incubated for 15 min at room temperature. OVA (1.5 μmol) was dissolved in 0.1 M borate buffer with 0.15 M sodium chloride, pH 9.0. Diazoniumphenyl-PC (125 μmol) was coupled to OVA by incubation at 4 °C followed by concentration and dialysis against PBS, pH 7.4. Development of a yellow color confirmed a successful reaction. Concentration of the conjugate was determined by spectrophotometry and SDS-PAGE and was compared to a similar concentration of unconjugated OVA. Lipopolysaccharide (LPS) from E. coli 0111:B4 was purchased from Sigma Aldrich (USA).
2.3 Cell clustering assays
A20 WT B (1×106/ml) cells were cultured in the presence of 20 μg/ml LT-IB, 10 μg/ml LT-IIaB, and same doses of LT-IB+LT-IIaB, for 4 hrs, at 37°C. Cells were examined for the presence of clusters in 24-well plate by light microscopy. In each well and for each treatment, 4 identical measurements of areas in the center and 4 others at the edge of wells were randomly selected to count the number of clusters formed and the number of cells in these clusters. Data obtained from the center and the edges of each well were pooled. Only clusters that contained 5 cells or more were counted. In additional experiments, LT-IB and LT-IIaB were denatured by heating at 95°C. This causes disassembly of the pentameric B-subunits into monomers that fail to bind gangliosides [15, 29]. The B-subunits in either intact or disassembled forms were then added to A20 B cells which were cultured at 1×106/ml, and incubated for 4 hrs. Averages and SEM were calculated. Statistical significance was obtained by using the unpaired Student t-test with Welch correction, assuming populations have different standard deviation, and by determining two-tailed p value.
In other experiments, A20 WT B cells (0.5×106) were cultured in the presence of 20 μg/ml LT-IB, 10 μg/ml LT-IIaB, same doses of LT-IB+LT-IIaB, 10 μg/ml LT-IIbB or 10 μg/ml of LT-IIbB mutant (S74D), for 4-6 hrs. Cells were cultured in the absence or the presence of OVA. 0.8×106 OVA-specific DO.11.10 T cells were subsequently added in the presence of the Ags and incubated for approximately 24 hrs. To visualize the type of cells in the clusters, 0.5×106 A20 WT B cells were pulsed with low or higher doses of the enterotoxins B-subunits. Thus, A20 WT B cells were incubated with 5 μg/ml LT-IB, 2.5μg/ml LT-IIaB or a mixture of both, for 4-6 hrs. DO.11.10 were labeled with pre-optimized concentration of carboxyfluorescein succinimidyl ester (CFSE) (Life Technologies, USA) and 0.8×106 of these cells were added to B cells. Cells were incubated for 2hrs. Alternatively, A20 WT B cells were pulsed with 20 μg/ml of LT-IB or 10 μg/ml of LT-IIaB for 4-6 hrs. CFSE-DO.11.10 T cells were added in the same manner. Cells were then incubated for 24 hrs at 37°C.
2.4 Cell viability assays
Viability of B cells was measured following incubation of the Ags after 4 and 24 hrs, by treating the cells with 0.2% Trypan Bleu (TP), as well as Propidium Iodide (PI) for analysis of the cell cycle. PI staining was performed as previously described [30]. LT-IB was used at 5 μg/ml, LT-IIaB at 2.5 μg/ml, OVA at 500 μg/ml and LPS at 2 μg/ml. Measurement of the percentage of cells stained with TP and DNA stained with PI in the sub-G0 peak of the cell cycle indicated the presence of dead cells or cells undergoing programmed cell death, respectively.
2.5 Assays for the effects of the B-subunits of LT-I and LT-II on presentation of OVA
5×105 A20 WT or A20μ WT B cells were incubated with OVA or PC-OVA, respectively. LT-IB, LT-IIaB or a mixture of both were added either alone or with OVA. APCs were incubated for 4-6 hrs after which 8×105 CFSE-labelled DO.11.10 T cells were added in the presence of the Ags. Mixtures of cells were incubated for a total of 48 hrs at 37°C. Following incubation, supernatants were sampled to determine the concentration of IL-2. The remaining cells were collected and acquired by flow cytometry (Becton Dickenson). Cells were analyzed by FlowJo software (Tree Star, OR). The concentration of IL-2 in the cultures was determined using a mouse IL-2 kit (eBioscience, USA) following the manufacturer's instructions. Briefly, 96 well plates were coated with capture recombinant anti-mouse IL-2 antibody. Wells were blocked with the provided blocking solution. Pre-prepared recombinant mouse IL-2 concentration was 2× diluted in 2 rows and supernatants were added undiluted. Following overnight incubation at 4° C, biotin anti-mouse IL-2 detection antibody was added. Finally, IL-2 was detected by Avidin-HRP and TMB substrate solution. Plates were then read at 450 nm. Concentration of IL-2 in each sample was determined by extrapolation from the mouse IL-2 standard values. Statistical significance was obtained by using the unpaired Student t-test with Welch correction, assuming populations have different standard deviation, and by determining a two-tailed p value.
2.6 Effects of the B-subunits of LT-I and LT-IIa on growth and expression of MHC class II in the macrophage
RAW264.7 macrophages were cultured in DMEM medium at 37°C. At confluence, cells were harvested using Trypsin-EDTA solution (Sigma-Aldrich), washed and incubated for 24 hrs in 24-well cell culture plate with or without LT-IB, LT-IIaB or a mixture of both.
Lipopolysaccharide (LPS), as a model adjuvant, was added at 1 μg/ml. Cells were harvested with trypsin-EDTA (Sigma), washed thoroughly and suspended in phosphate buffered saline (PBS) containing 1% FBS to block Fc receptors. After incubation at room temperature for 45 min, cells were washed and labelled on ice for 40 minutes in PBS-0.5 %FBS-0.01% Azide with a predetermined concentration of FITC-IgG1-anti-mouse MHC class II antibody (BD Biosciences, CA). Cells were washed in PBS and analyzed by flow cytometry.
3. Results
3.1 Short-time incubation with the B-subunits of LTs induced B cell clustering
LT-IB acts directly on isolated mouse B cells to increase surface expression of CD25 and MHC class-II, after 48 hrs of incubation [18]. To investigate the short-time impact of LT-IB on B cells and to compare it to LT-IIaB and a mixture of LT-IB+LT-IIaB, cells were incubated with the B-subunits preparations for 4 hrs. LT-IB+LT-IIaB mix was used as a potentially new adjuvant proposed to stimulate the innate and adaptive immune responses of proteins especially those derived from highly virulent pathogens. Cell clustering was observed following incubation with LT-IB, LT-IIaB and the mix (Fig. 1A). Compared to the untreated control (medium), numerous small cell clusters were evident after treatment with LT-IB. LT-IIaB produced larger clusters than LT-IB (Fig.1A) and the mix induced a mixture of large and small clusters. To provide a quantitative measurement, the number of cell clusters and their size (number of cells in each cluster) were determined in 8 randomly selected fields of identical size (Fig. 1C). LT-IB produced significantly more clusters when compared to the untreated control or LT-IIaB (p<0.0001 and p<0.05), respectively) (Fig. 1C). Contrary to the observation in Fig, 1A, however, the differences in the size of clusters between LT-IB and LT-IIaB treated cells were not significant. Further, significantly more clusters were formed in the presence of the mix compared to each of LT-IB and LT-IIaB alone (p<0.05 and p<0.0001, respectively). While the mix induced clustering of more cells than LT-IB (p<0.0001), the number of cells was not significantly different from the cultures treated with LT-IIaB. Taken together, these data show that the B-subunits of LTs induced B cell clusters as early as 4 hrs after incubation. Further, incubation with the mix resulted in significantly larger number and larger sizes of clusters compared to LT-IB. The mix also induced larger number of clusters compared to LT-IIaB.
Fig. 1. Short-time incubation of B cells with the B-subunits of LTs induced cell clustering that is mostly ganglioside-mediated.
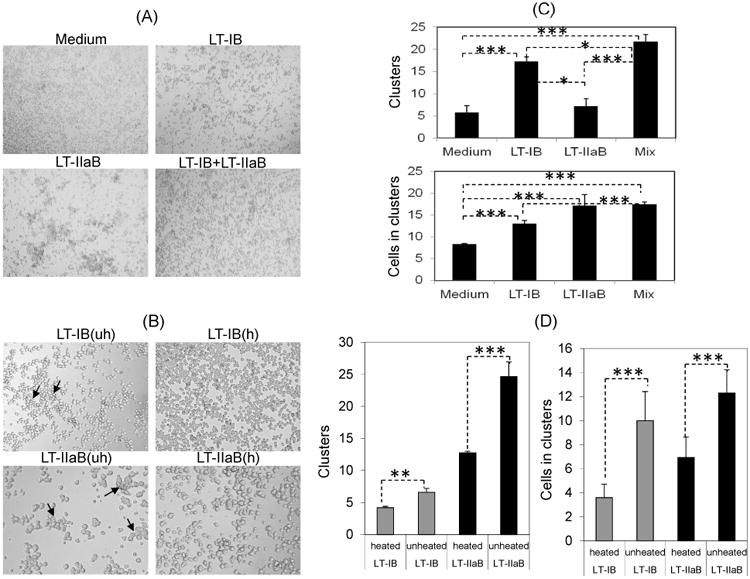
A20 WT B cells were cultured at 1×106/ml with 20 and 10 μg/ml LT-IB and LT-IIaB, respectively. Cells were also incubated with the same doses in a mixture (Mix) and examined 4hrs later by light microscopy. Images were taken at 4× magnification (A). (B), A20 B cells were incubated with either native (uh, unheated) or denatured (h, heated) forms of the B-subunits by heating at 95° C for 5 min. The average (+/- SEM) number of formed clusters and number of cells in the clusters were determined (C, D). *, **, and *** denote the difference in the means was significant (p<0.05), highly significant (p≤0.001) and extremely significant (p<0.0001), respectively. For significance of the values, the unpaired Student t-test with Welch correction was used, assuming populations have different standard deviation, and by determining two-tailed p value. Cells were examined by light microscopy (×10). Arrows indicate the presence of large clusters.
It has been demonstrated by our laboratory as well as others that most of the immunological and adjuvant properties of the B-subunits of LTs are mediated following binding to their respective receptors. This suggests that the observed cell clustering (Fig. 1A) might also be dependent on receptor binding. To address this, LT-IB and LT-IIaB were added to A20 B cells either in their native form or after heat-denaturing at 95°C (Fig. 1B). Fig. 1B shows that B cell clustering was considerably reduced following addition of heat-denatured LT-IB and LT-IIaB. The differences between cells treated with heated or unheated LT-IB and LT-IIaB were highly (p≤0.001) and extremely (p<0.0001) significant in the number of clusters and the number of cells in the clusters, respectively (Fig 1D). We conclude that the observed clustering of B cells by LT-IB and LT-IIaB were mostly mediated by binding to their respective gangliosides; GM1 for LT-IB, and GD1b for LT-IIaB. This is the first time the B-subunits of LTs have been shown to induce clustering of B cells or any other cell.
3.2 The cell clusters were present up to 24 hrs and contained B and T cells
To further examine the impact of the B subunits of LT-I and LT-IIaB on cells in cultures containing B and T cells, I-Ad–bearing A20 WT B cells and DO.11.10 T cells were incubated with the enterotoxin B-subunits with or without Ag (OVA). DO.11.10 T cells proliferate in response to OVA323-339 in the context of I-Ad. B cells were incubated with LT-IB, LT-IIaB, LT-IB+LT-IIaB in the presence or absence of OVA. In some experiments unlabeled DO.11.10 cells were added and cells were visualized after 24 hrs incubation by light microscopy (Fig. 2A). In other experiments, DO.11.10 cells were labeled with CFSE and incubated with B cells for 2 and 24 hrs. Regardless of the presence or absence of OVA, LT-IB and LT-IIaB induced significant cell clustering which was equally pronounced in the presence of a mixture of both enterotoxins B-subunits (Fig. 2A). While cell clustering was observed in the control cultures, these were much smaller than those found in cells treated with either LT-IB or LT-IIaB. To examine whether the clusters induced by LT-IIaB occurred following binding to TLR-2, LT-IIbB was compared with LT-IIbB(S74D), a derivative of LT-IIbB that does not bind to TLR-2 but retains binding activity to ganglioside GD1a [31] (Fig. 2A). Similar to LT-IIaB, LT-IIbB has high binding affinity for TLR-2 [5, 7]. Moreover, both LT-IIaB and LT-IIbB are similarly proinflammatory [22]. As shown, both enterotoxins B-subunits (LT-IIbB and LT-IIbB(S74D) induced the production of large cell clusters indicating that formation of cell clusters is not mediated solely by TLR-2 activation. This model is supported by the ability of LT-IB, which binds GM1 ganglioside, but not TLR-2, to induce formation of cell clusters. Therefore, binding of LT-IB and LT-IIaB to their respective gangliosides appears to be the main mechanism for induction of cell clusters.
Fig. 2. The B-subunits of LT-I and LT-IIa enterotoxins induced cell clustering of B and T cells.
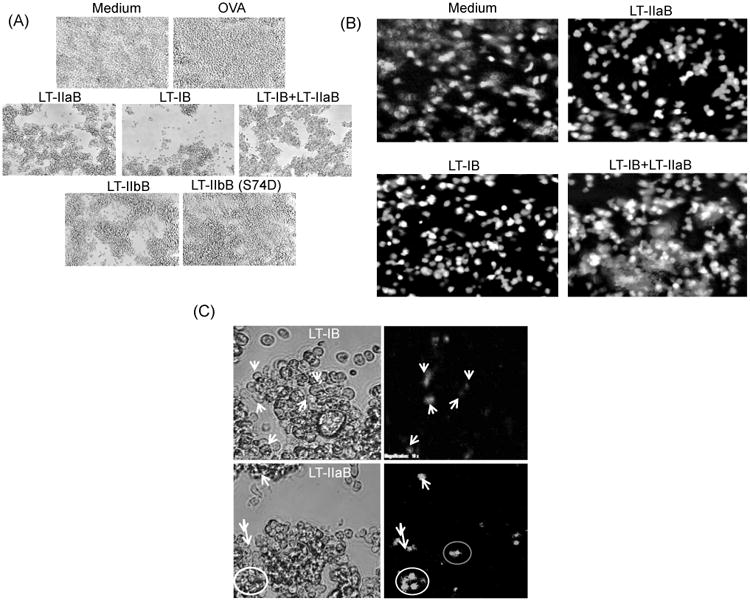
A20 WT B cells were cultured with or without OVA in presence of 20 μg/ml LT-IB, 10 μg/ml LT-IIaB, same doses of LT-IB+LT-IIaB, 10 μg/ml LT-IIbB or 10 μg/ml non-TLR-2 binding mutant of LT-IIbB, LT-IIbB(S74D), for 4-6 hrs. Unlabelled OVA-specific DO.11.10 T cells were added to the culture, and incubated for 24 hrs (A). Alternatively, A20 WT B cells were incubated for 4-6 hrs with lower doses of 5 μg/ml LT-IB, 2.5 μg/ml LT-IIaB, and same doses of LT-IB+LT-IIaB (B). CFSE-labeled T cells were then added and examined either after 2 hrs (B) or 24 hrs (C). Cells were examined by fluorescence microscopy [×20 (A and B), 10× cropped images (C)]. In (C), white arrows point to cells from same images taken in bright and overlapping fluorescence fields. Shown are cells incubated with the B-subunits without OVA. Almost identical cellular profile was found after cells were incubated in the presence of OVA (not shown). The data are representative of at least 2 similar experiments.
To further examine the type of cells in these clusters, A20 WT B cells were incubated with the B subunits of LTs in the presence or absence of OVA and with DO.11.10 T cells that had been labelled with CFSE. The cellular populations were examined after 2 hrs (Fig. 2B) and 24 hrs (Fig. 2C) of co-incubation. After 2 hrs, cell clusters were observed at the lower doses of the enterotoxins B-subunits (Fig. 2B). At the lower doses, LT-IB+LT-IIaB induced larger clusters that contained many CFSE+ T cells and unlabeled B cells (Fig. 2B). Similarly, cell clusters of B and T cells were still visible after 24 hrs at the higher doses of the enterotoxins B-subunits (Fig. 2C). In this case, CFSE-labeled T cells were also visible inside these clusters thus suggesting an active process. Clusters were also visible after 48hrs incubation with the cells (not shown). Similar patterns of cell clustering were observed in wells containing the B-subunits of LTs and OVA (not shown). Collectively, the data indicate that clustering of B and T cells by the B-subunits of LTs is a very early event that occurs subsequent to their binding to B cells and persists for an extended period of time.
3.3 The B-subunits of LT-I and LT-IIa did not significantly affect viability of B cells
Although release of DNA from dead cells is unlikely to be the cause for the observed B cell clustering by the B-subunits particularly after the short 4 hrs incubation time, the viability of the cells was examined with Trypan Bleu and PI staining (Fig. 3). After 4 hrs incubation, the cells were 100% viable in the presence of the individual B-subunits, while incubation with the mix resulted in 12% of cells stained with Trypan Bleu (Fig. 3A). PI staining of DNA in the sub-G0 peak of the cell cycle is indicative of cells undergoing apoptosis. As shown (Fig. 3B), the outcome after incubation with each of the individual B-subunits of LTs or a mixture of both was not significantly different from the controls. The percentage of B cells undergoing apoptosis in those treated with the B-subunits ranged from 27-30 % which was not different from the untreated and OVA-treated cells and still less by that induced by the B cell mitogen, LPS. Thus, the observed 12% of Trypan Bleu positive cells after the short 4hrs incubation with the mixture may not reflect actual death of the cells. It is possible that the mixture could have altered the charge in the membrane on the surface of some cells allowing penetration of the negatively charged dye.
Fig. 3. The B-subunits of LT-I and LT-IIa did not significantly affect the viability of B cells.
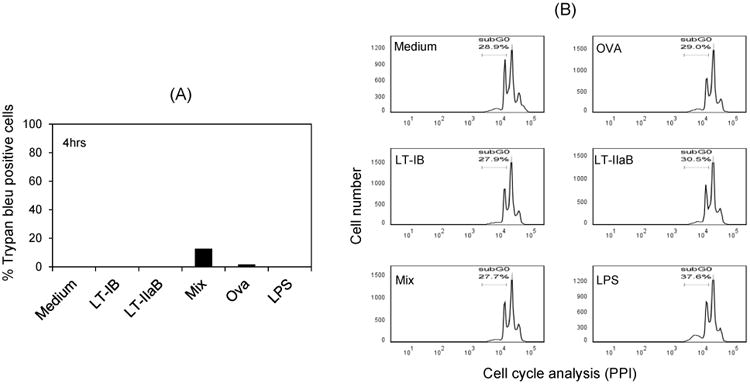
A20 WT B cells were incubated with each of B-subunits of LT-1 and LT-II individually or in a mixture for 4 and 24 hrs. Incubations with OVA or LPS were used as controls. Viability of the cells was evaluated by staining with Trypan Bleu (TB)(4hrs) or propidium iodide (PI) (24 hrs). The percentage of dead cells stained with TB (A) and the percentage of cells with fragmented DNA (sub G0) in the cell cycle are shown.
3.4 The B-subunits of LT-I and LT-IIa increased maintenance of CFSE+ T cells in culture
To examine the impact of the B-subunits of LTs on total T cells in the cultures, A20 WT B cells were pre-incubated with LT-IB, LT-IIaB and LT-IB+LT-IIaB in the presence of OVA. CFSE-labeled DO.11.10 T cells were subsequently added. Flow cytometry analysis revealed that the proportion of CFSE+ T cells was significantly higher in the presence of LT-IB+OVA (approx. 70%) and LT-IIaB+OVA (approx. 46%) compared to OVA alone (approx. 32%)(Fig. 4B). As DO.11.10 is a continuously dividing cell line, the increase in the proportion of CFSE+ cells could reflect retention of CFSE in these cells (see below). Further, in wells containing each of the enterotoxins B-subunits, the proportion of some CFSE+ T and unlabeled cells with higher FSC (a readout of cell size) increased compared to OVA alone and untreated control (Fig. 4B). This increase in cell size is also evident in the selected gate in the cultures incubated with LT-IB (Fig. 4A) where the spread of the cells from center to forward position in the FSC channel is noticeable. The increase in FSC of these cells suggests an increase in cell size due to their activation. In support, examination of these by light microscopy revealed some single large cells compared to untreated control and OVA-treated controls (not shown) although the nature of these cells could not be verified. Nevertheless, T cell hybrids have been documented to increase their size following Ag stimulation [32]. In a similar outcome to LT-IB and LT-IIaB, the proportion of CFSE+ T cells in wells incubated with LT-IB+LT-IIaB+OVA was significantly higher (approx. 61%) compared to OVA (approx. 32%). However, it is noted that the total number of B and T cells in the live gate was lower in this cell culture compared to the others. This effect can be seen by the increase in the density of the Low FSC/high SSC cells, most likely dead cells, compared to other treatments (Fig. 4A). The reason for the cell death is unclear; particularly that analysis of cells undergoing apoptosis after 24 hrs incubation with the B-subunits of LTs did not reveal a difference from other treatments including the untreated control culture (Fig. 3B). However, this response may reflect an increase in activation of these cells. After 48 hrs, there is a significant increase in the amount of IL-2 production (an indicator of cell activation) in this culture supernatant when compared to the levels of IL-2 found in the supernatants of cells treated solely with OVA (see below). Additional experiments were designed to examine a correlation between the increase in the proportion of DO.11.10 T cells, T cell division and production of IL-2.
Fig. 4. The B-subunits of LT-1 and LT-IIa increased maintenance of DO.11.10 T cells.
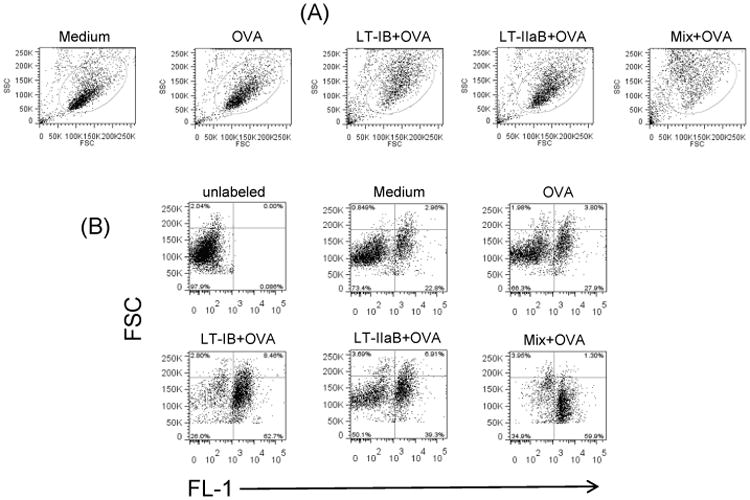
A20 WT B cells were cultured with OVA in presence of LT-IB, LT-IIaB or LT-IB + LT-IIaB (Mix), for 4-6 hrs. OVA-specific DO.11.10 T cells were CFSE labelled, added to the culture and incubated for 48 hrs. Cells were then analyzed by Flow cytometry. Dead cells were gated out based on their low forward and side scatter (A). Other cells were analyzed for the proportion of T cells in culture (B). The data is representative of 3 similar independent experiments.
3.5 The B-subunits of LTs cause a delay in T cell division following OVA endocytosis
It has been shown that the cycle of T cell hybrids (including DO.11.10 cells) is dramatically influenced by the presence of a variety of specific soluble Ags in Ag presentation assays [32]. Addition of Ag to these cultures resulted in T cell cycle delay/arrest where T cell hybrids normally undergo one division every 12-13 hrs [32].
To begin to dissect the impact of the enterotoxin B-subunits on T cell division in these cultures, A20 WT B cells which process Ag only by fluid phase endocytosis were used. A20 WT B cells express surface mouse Ig of unknown specificity. B cells were pulsed with LT-IB, LT-IIaB, LT-IB+LT-IIaB with or without OVA and the T cell division profile was subsequently analyzed. An increase in T cell division is recorded when T cells progress from generation (g)0 to g1-7 (Fig. 5A).
Fig. 5. The B-subunits of LT-I and LT-IIa caused delay and arrest in T cell division.
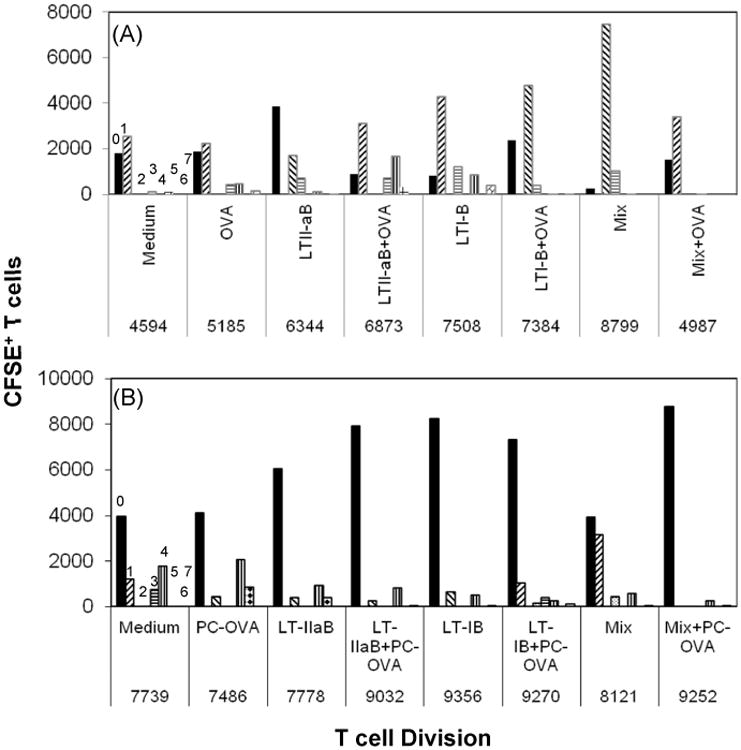
A20 WT (A) or A20μ WT B cells (B) were cultured with OVA (A) or PC-OVA (B) in presence of LT-IB, LT-IIaB or LT-IB+LT-IIaB (mix), for 4-6 hrs. OVA-specific DO.11.10 T cells were labelled with CFSE, added to the cell culture and incubated for 48 hrs. Cells were then analyzed by Flow cytometry. Dead cells were gated out based on their low forward and side scatter. CFSE+T cells were gated on and analyzed using FlowJo proliferation software. Numbers 0-7 indicate cell generation number. A shift from generation “0” to other generations is indicative of cell division. The value below each treatment represents number of total DO.11.10 T cells in all generations. The data is representative of 3 similar independent experiments for each of (A), and (B).
Incubation with OVA alone induced a small increase in the T cell division when compared to the untreated control, shown by the progression of cells to advanced generations. There was also a slight increase in the total number of CFSE+ T cells. Incubation with LT-IB+OVA or LT-IIaB+OVA increased T cell division when compared to OVA alone and was associated with an increase in the total number of CFSE+-low T cells, which is consistent with the results from the dot plot analysis (Fig. 4B). T cell division in wells containing LT-IB+OVA was slower compared to those containing LT-IIaB+OVA since most of the cells remained in g0 and g2. Further, comparison between the T cell division profiles of LT-IIaB with and without OVA showed higher division in LT-IIaB+OVA. This effect is revealed by a shift of the cells from g0 to more advanced generations. Nevertheless, the total number of CFSE+ T cells between these treatments did not noticeably increase. This may be due to reduced detection of CFSE+ T cells as a result of the increase in T cell division in the LT-IIaB+OVA culture. Conversely, LT-IB alone increased T cell division when compared to LT-IB+OVA. The total number of T cells remained the same in these cultures again suggesting CFSE dilution due to an accelerated T cell division in the LT-IB culture. Finally, like LT-IB and LT-IIaB, the incubation with a mixture of both without OVA increased T cell division and the total number of T cells, when compared to untreated or OVA-treated controls, suggesting increased survival of T cells in these cultures. Stimulation by the mix+OVA slightly increased T cell division, shown by a shift from g0 to g1. However, the total number of T cells did not noticeably increase over that of untreated or OVA-treated control, suggesting a loss of cells following activation (Fig. 4). It should be noted that T cell division in the presence of LT-IB+OVA, mix, and mix+OVA was slower compared to LT-IIaB+OVA or the other treatments, in that most of the cells did not advance further in their division. Collectively, these results show that stimulation by LT-IIaB+OVA results in higher division of OVA-responsive T cells following fluid phase endocytosis when compared to LT-IB+OVA, mix or mix+OVA.
3.6 The B-subunits of LTs caused arrest in T cell division following BCR-mediated uptake of OVA
To mimic in vivo events, the impact of the B subunits of LTs on T cell division was further investigated in cultures of B and T cells in which OVA is targeted to the BCR. A20μ WT B cells acquire and process Ag by the BCR [27]. The incubation with PC-OVA slightly increased T cell division, when compared to the untreated control, as shown by a shift from g1 to g3-5 (Fig. 5B). The total number of T cells in these cultures remained essentially the same. On the other hand, incubation with LT-IB, LT-IIaB, or the mix, with or without PC-OVA did not significantly alter T cell division. In fact, the majority of T cells appeared to be stuck at g0. The total number of CFSE+ T cells in these cultures, except for LT-IIaB, only slightly increased when compared to PC-OVA treated and the untreated control (Fig. 5B) suggesting T cell maintenance as a result of the block in cell division. Collectively, these data indicate profound impact of the B-subunits of LTs on T cell division in the absence of OVA. However, the results above do not necessarily indicate direct effects of the B-subunits on Ag presentation since T cell proliferation is an intrinsic property of the cell line. Nevertheless, a delay or a halt of T cell division would have significant effects on the mechanism of Ag presentation in vivo. To examine whether Ag presentation, measured by the amount of IL-2 production in these cultures are affected by the B-subunits, further experiments were performed below.
3.7 The delay/arrest in T cell division was associated with increased IL-2 production
To determine if incubation with the B-subunits of LT impacts production of IL-2 by T cells in these cultures (Fig. 6), A20 WT and A20μ WT B cells were pulsed with LT-IB, LT-IIaB or a mixture of both B subunits with or without OVA or PC-OVA, respectively, and supernatants from each well were collected and analyzed for the presence of IL-2.
Fig. 6. The B-subunits of LT-I and LT-IIa differentially enhanced IL-2 production.
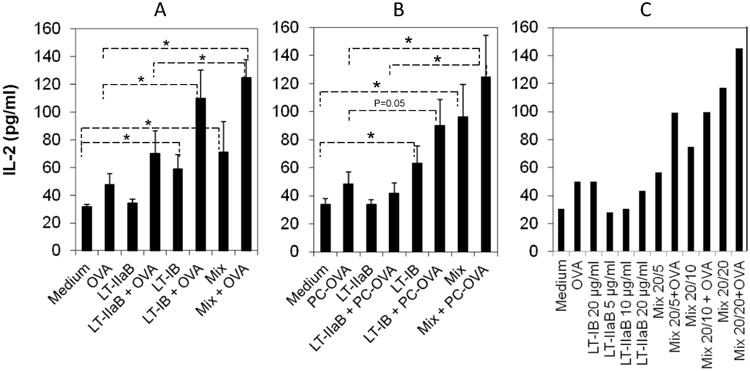
A20 WT (A,C) or A20μ WT B cells (B) were cultured with or without OVA (A, C) or PC-OVA (B) in presence of LT-IB, LT-IIaB or LT-IB+LT-IIaB (mix), for 4-6 hrs. The antigen doses were 5μg/ml for LT-IB and 2.5 μg/ml for LT-IIaB (A, B) or as indicated (C). OVA-specific DO.11.10 T cells were labelled with CFSE, added to culture and incubated for 48 hrs. Supernatant from cultures treated with or without the antigens were sampled, and concentration of IL-2 determined by extrapolation from IL-2 standard incubated on the same plate. Shown are IL-2 values representing average of 3 independent experiments with SEM (A, B), or one experiment (C). Average (+/- SEM) of IL-2 represents values from 3 independent experiments. For significance of the values, the unpaired Student t-test with Welch correction was used, assuming populations have different standard deviation, and by determining two-tailed p value. *, denotes the difference in the means was significant (p<0.05).
In the A20 WT cultures, the IL-2 levels were mainly compared to 2 controls untreated or OVA treated. When compared to treatment with OVA alone, the level of IL-2 was 2.3 folds higher for LT-IB+OVA treated cultures, which is statistically significant (p<0.05). On the other hand, IL-2 levels in LT-IIaB+OVA treated cultures were only 1.49 folds higher than cultures treated with OVA alone (Fig. 6A). A significant increase of IL-2 at about 2.64 folds was also detected in supernatant from cells incubated with mix+OVA compared to OVA alone (p<0.05). This result was also significantly higher than the level of IL-2 in cultures treated with LT-IIaB+OVA (p<0.05), but not significantly higher than those in cultures treated with LT-IB+OVA. Therefore, in response to OVA, LT-IB and the mix, but not LT-IIaB significantly increased IL-2 production. It should be noted that although the increase in the IL-2 level by LT-IIaB+OVA did not attain statistical significance, a higher production of IL-2 over that in the untreated or OVA treated controls is an indication of T cell activation. Further, in cultures treated with the B-subunits without OVA, the IL-2 level in the LT-IB culture was significantly higher compared to the untreated control (1.86 folds, p<0.05) whereas LT-IIaB did not increase IL-2 above that of the untreated control. Additionally, the IL-2 level on cultures containing the mix was significantly higher (2.24 folds, p<0.05) compared to the untreated control. There was no significant difference between the level of IL-2 in the mix and mix+OVA (p>0.05). Collectively, the data above show that LT-IB and the mix in the presence or absence of OVA stimulated significant production of IL-2. Finally, to ensure that a threshold of IL-2 production was attained, A20 WT B cells were incubated with higher doses of the B-subunits (Fig, 6C). Comparison between the IL-2 levels in cultures incubated with low and higher doses of the enterotoxins B-subunits showed no larger increase in IL-2 production (Fig. 6A and Fig. 6C). This indicates that a threshold of IL-2 production in these cells was attained by the lower doses.
The impact of the B-subunits of LTs on the level of IL-2 was further investigated in the A20μ WT B cell cultures (Fig. 6B). The samples were compared for statistical significance mainly to either untreated or PC-OVA treated. Compared to PC-OVA, LT-IB+PC-OVA and mix+PC-OVA increased IL-2 by 1.87 folds and 2.6 folds, respectively. This increase was significant (p<0.05) for the mix+PC-OVA, but not quite significant for the LT-IB+PC-OVA (p=0.05). Again, the higher increase of IL-2 production by LT-IB+PC-OVA though not quite significant is however an indication of T cell activation. The level of IL-2 in the mix+PC-OVA culture was significantly higher (p<0.05) than that in the LT-IIaB+PC-OVA, but not that in the LT-IB+PC-OVA culture (p>0.05). However, the mix induced higher level of IL-2 compared to LT-IB alone. Thus, the presence of LT-IIaB with LT-IB may reflect a more efficient way to stimulate T cells. In comparison to LT-IB+PC-OVA, incubation with LT-IIaB+PC-OVA or LT-IIaB alone did not increase the level of IL-2 over that detected in PC-OVA or the untreated control, respectively. Like in the A20 WT cultures above, incubation with LT-IB or the mix without PC-OVA significantly increased the level of IL-2 by 1.86 and 2.83 folds (p<0.05) compared to the untreated sample, respectively. Collectively, these data indicate that LT-IB and the mix mediate similar levels of IL-2 with or without OVA.
3.8 The B-subunits of LT-IB and LT-IIaB differentially increase the proportion of macrophages expressing MHC class II
While the study above analyzed the impact of LT-IB and LT-IIaB on B cells which are mainly involved in the adaptive immune response, we also compared effects of the enterotoxins B-subunits on macrophages in the context of their adjuvant properties. For this purpose, we examined the means by which LT-IB, LT-IIaB, and LT-IB+LT-IIaB regulate surface expression of MHC class-II on RAW264.7 macrophages. Further, effects of the B-subunits on the macrophages were compared to those exerted by LPS. In contrast to B cells, unstimulated macrophages express very low levels of MHC class-II. Similar to B cells, however, macrophages express TLR-2 as well as ganglioside receptors. The cells were incubated with the stimulants for 48 hrs, after which they were analyzed for change in morphology (increase in cell size) and for expression of MHC class II. In the absence of a stimulant, two major populations can be seen, low and high FSC (an indicator of cell size) representing approx. 45% and 55%, respectively (Fig. 7). The number of cells that expressed MHC class II was low in both populations. After LPS stimulation, a noticeable decrease in the proportion of high FSC population occurred. As a result, the proportion of the low FSC population was higher and was associated with an increase in the proportion of cells expressing MHC class II. The decrease in the proportion of cells of high FSC may be a consequence of activation and expression of MHC class II. In support, agents that cause activation of macrophages such IFN-γ and LPS cause an arrest in the cell cycle associated with increased surface expression of MHC class II and secretion of proinflammatory cytokines [33]. Incubation with LT-IB reproduced the LPS effects on cell morphology and MHC class II with a further increase in the proportion of cells expressing MHC class II (approx. 7%, 10%, and 17% for medium, LPS and LT-IB, respectively). By comparison, LT-IIaB at the low dose of 2.5 μg/ml although it reproduced a morphological change of the cells similar to LPS, it did not increase the proportion of MHC class-II expressing cells above those in the untreated control. This result suggests that the potent proinflammatory property of LT-IIaB on monocytes/macrophages [22] is independent of the events that regulate MHC class II expression, but in concert with the cell morphological change that is reportedly followed by secretion of proinflammatory cytokines. However, when LT-IIaB was incubated with LT-IB in a mixture, the proportion of class II-expressing cells increased similar to the effects of LPS. Collectively, these results show that the effects of LT-IB and LT-IB+LT-IIaB on MHC class II are similar to those of LPS.
Fig. 7. The B-subunits of LT-IB and LT-IIaB differentially increased the proportion of macrophages expressing MHC class II.
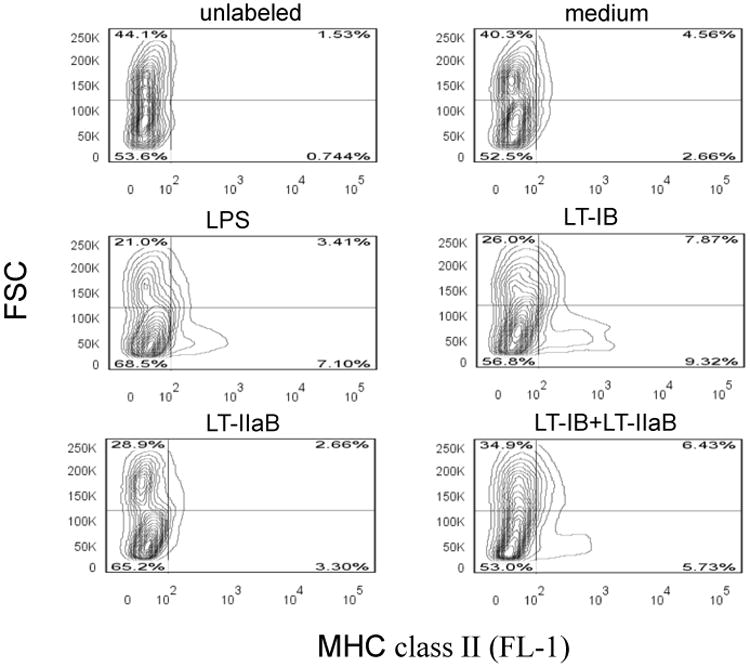
RAW264.7 cells were incubated with 2 μg/ml LPS, 5 μg/ml LT-IB, 2.5 μg/ml LT-IIaB or a mixture of both (LT-IB+LT-IIaB), for 48 hrs. Cells were then harvested and labelled with FITC-anti-MHC class II antibody and analyzed by flow cytometry. Shown is a contour plot displaying different cell populations in the FSC channel and the proportion of cells expressing MHC class-II in the FL-1 channel. The quadrants were set so to analyze each population as shown in the FSC channel.
4. Discussion
We postulate that early events governing the interactions of LT enterotoxins with immune B and T cells play a pivotal role in their adjuvanticity towards co-administered Ags. We demonstrate that co- incubation with the B-subunits of LTs results in significant clustering of B cells and B and T cells. B cell clustering was observed as early as 4 hrs after the addition of the B-subunits, and was likely mediated by gangliosides, but not TLR-2. Further, co-incubation with the B-subunits resulted in a delay or blockage of T cell division which was inversely related to the production of IL-2. Moreover, co-incubation of specific B-subunits with macrophages differentially increased the proportion of MHC class-II+ cells. These results highlight the impact of signals derived from gangliosides, BCR, and TLR-2 on immune cells and shed new insights on the mechanism behind the adjuvant properties of the B-subunits of LTs.
A large body of evidence exists demonstrating the potent immunogenicity and adjuvanticity of E. coli heat-labile enterotoxins (LTs), their non-toxic derivatives, and the structurally and functionally related CT from Vibrio cholera [1, 10-12, 34]. An essential part of their potent immunogenicity and adjuvant properties lies in their high affinity binding to their respective receptors on immune cells. However, it is unclear how a minute amount of these enterotoxins administered in PBS, and in the absence of any other adjuvants, leads to a remarkable expansion of the antibody and T cell response to a co-administered Ag, including generation of long-lasting immunological memory [23-26]. Due to the enterotoxins' high affinity binding to their receptors on APCs, it would be expected that injection of a minute amount of the enterotoxins in vivo would result in binding to only a small number of these cells. An even smaller number of APCs would simultaneously bind both the enterotoxin and co-administered soluble Ag. Adding to this, the enterotoxin would be ‘consumed’ by binding to other cells not involved in professional Ag uptake and processing, such as T cells. Hence, any effect the enterotoxins have on the APCs that result in their activation would likely involve only a few cells. Alternatively, a mechanism that involves bystander activation of B and T cells, a delay in the immune response to co-administered Ag, and a local aggregation of immune cells near the site of inoculation would likely result in a more effective immune response to that Ag.
In this regard, early events of cell clustering between the APCs and T cells have been shown to dictate the immunogenicity of Ags in vivo [35]. Significant differences in cell motility, meandering, number of clusters, and number of cells in the clusters were observed in lymph nodes between animals fed “no Ag” and those fed OVA+CT as an adjuvant. Also, subtle but noticeable differences were present between animals fed OVA and OVA+CT, particularly in the size of the clusters; clusters in the lymph nodes from animals fed OVA+CT were larger and contained higher number of cells. Although the authors attributed these findings to the effect of priming with Ag (OVA+CT) versus tolerance (OVA), they stated that no clusters were formed by the adjuvant CT alone although no data was presented to support their claim. Our data suggest that CT, independent of OVA, probably contributed to formation of clusters in that study. Nevertheless, OVA priming would be more effective when CT is used as an adjuvant. An alternative explanation would be that the B-subunits of LTs and CT act differently on immune cells.
Fig. 1 and Fig. 2 in this report show cell clusters in the B-subunits treated samples 4 hrs after incubation with B cells, and 2 and 24 hrs following introduction of T cells to the culture. Overall, the results indicate that formation of the cell clusters occurs very early following binding of the B-subunits to A20 WT B cells before any significant increase in the expression of surface co-stimulatory and adhesion molecules. A20 WT B cells express ICAM-1 (CD54), MHC class II, CD80 and CD86 [36]. T cells also express the adhesion molecule CD54. CD54 is an unlikely candidate for cell clustering, however, since significant expression of CD54 occurs over the course of 2–3 days of culture following activation by mitogens [37] and 48 hrs is required for LT-IB to increase expression of CD54 on mouse B cells [14]. The data acquired using LT-IIbB and the TLR-2 binding deficient mutant LT-IIbB (S74D) suggest that clustering was not mediated solely by TLR-2 activation (Fig. 2). It is thus likely that LT-IIbB and LT-IIaB, like LT-IB, mediate cell clustering following binding to gangliosides; GD1a for LT-IIbB, GD1b for LT-IIaB and GM1 for LT-IB.
The question is, therefore, how do the B-subunits increase adhesiveness of cells at such early time (4 hrs)? In this context, early events that lead to internalization of Ag and initiation of the signaling cascade include translocation of a number of surface receptors into specialized membrane structures named ‘detergent-insoluble glycolipid-enriched membranes’ (DIGs) or rafts [38-40]. Gangliosides including GM1 and GD1b are raft constituents [41]. Further, TLR-2 translocates to lipid rafts after its ligation. Binding of LT-IB or CT/CTB on cells has been shown to result in patching and capping which can be visualized within minutes after co-incubation, and is observed in B and T cells (8, 42, 43). Capping of cell membrane receptors induces redistribution of membrane proteins some of which are associated with signaling molecules that regulate the cytoskeleton. Gangliosides can influence signaling molecules associated with the cytoskeleton [42, 43]. Thus, aggregation of CTB bound to GM1 lipid rafts in Jurkat T cells increases their adhesiveness by regulating the affinity of integrins to their ligands and cytoskeleton dynamics [43]. This increases the resistance of T cells to shear flow detachment and stretching, induces F actin polymerization, and inhibits T cell mobility including migration and chemotaxis [43]. The leukocyte function-associated antigen-1 (LFA-1) is an important adhesion molecule for lymphocyte migration and the initiation of an immune response. Its ligand is CD54. Both LFA-1 and CD54 are expressed on B and T cells. Cross-linking of lipid rafts on thymocytes or T lymphocytes by CT regulates avidity of LFA-1 and induces its clustering on these cells [44]. Thus, independent of T cell activation by Ag, cross-linking of lipid rafts modulates adhesiveness of cells that enhance cell-cell interactions. Similarly, the incubation of LT-IB with unprimed mouse splenic cells resulted in increased expression of co-stimulatory molecules on B cells, including CD54 [14]. Interestingly, the expression of most of these molecules required the presence of both B and T cells [14], pointing to the importance of early events that enhance interactions of the APC and T cell by LT-IB. We propose that clustering of the APC and T cells occurs immediately following LT-IB and LT-IIaB binding to their respective ganglioside receptors. This resulted in increased adhesiveness of the cells, and the interactions persisted throughout the response to OVA. By this later time, co-stimulatory and adhesion molecules contributed to further enhancement of B and T cell interactions.
The impacts of the B-subunits on T cell division and IL-2 production in cultures of B and T cells (Fig. 5 and Fig. 6) revealed an inverse relationship between T cell division rate and increased IL-2 production. This was particularly noticeable following BCR-mediated uptake of OVA in the presence or absence of the B-subunits. In this regard, it is important to highlight some of the known characteristics of several mouse T cell hybrids in their response to Ag. Thus while natural T cells undergo cell division following cognate interaction with the APC, T cell hybrids respond by a delay or complete blockage in their cell division [32]. The DNA blockage is evident 24 hrs after stimulation of the APC with Ag and persists up to 48hrs [32].
Most of the cells are stuck at the G1 and S cell cycle, which may be reversible after a lag period of 4-5 days. This inhibits growth of the cells resulting in an increase in cell size. Most importantly, the blockage in T cell division is inversely related to the production of IL-2 [32]. These observations have been shown for several T cell hybrids co-incubated with natural mouse B cells (thus expressing BCR of wide-range specificity) which were stimulated with a number of Ags, including OVA and hen egg lysozyme [32].
The question then is how do the B subunits of LTs impact T cell division and IL-2 production with or without OVA? Crosslinking of membrane gangliosides or TLR-2 by the enterotoxins B-subunits would be expected to result in rearrangement of membrane proteins that lead to intracellular signals. Indeed, binding of LT-IB to GM1 on isolated B cells increases levels of PI3K and MAP/ERK kinases [18] which is translated by an increased expression of MHC class II and CD25 [13, 18]. LT-IB also increases expression of several other costimulatory molecules on mouse splenic B cells [14]. Similarly, binding of LT-IIaB to GD1b and TLR-2 on surface of monocytes stimulates NF-kB and results in secretion of pro-inflammatory cytokines [7]. Moreover, crosslinking of these receptors affects signaling molecules that are associated with the cytoskeleton [42]. For OVA in the fluid phase, such an impact on the cytoskeleton would conceivably enhance its uptake and presentation. However, in a previous study it was found that incubation of A20 WT B cell with LT-IB did not noticeably enhance OVA presentation when added in the fluid phase [19]. In the latter study, the response of DO.11.10 T cells to OVA was evaluated by measuring the level of proliferation of IL-2-responsive HT-2 cells. However, the direct impact of LT-IB on DO.11.10 T cell division was not evaluated, and the response to IL-2 was measured after 24 hrs instead of 48 hrs as in the current study. By 24 hrs the stimulatory effects of LT-IB on IL-2 production would have not been complete. It is interesting in this regard that T cell division in cultures treated with LT-IB+OVA or LT-IB+LT-IIaB+OVA were slower when compared to LT-IIaB+OVA, and only the IL-2 levels in the former treatments were significantly higher when compared to OVA (Fig. 5A and Fig. 6A). This points to inverse relationship between the delay or the blockage in T cell division of the hybrids and production of IL-2 [32]. This was more evident in cultures treated with LT-IB+LT-IIaB+OVA where most of the cells appeared to be stuck at early generations, exhibited poor growth, and yet produced significant amount of IL-2 (Fig. 5A and Fig. 6A).
The impact of LT-IB and LT-IIaB on the presentation of OVA following BCR uptake (Fig. 5B and Fig. 6B) mimicked the behavior of mouse T cell hybrids in response to Ag [32]. Interestingly, the injection of mice with a mixture of LT-IB and HIV gag p24 protein also resulted in a blockage of T cell division in response to gag p24, compared to when gag p24 was chemically conjugated to LT-IB (17). With these findings in mind, it remains to elucidate the mechanism by which the B subunits impact T cell division and IL-2 production in the presence of OVA. It is known that ligation of the BCR by Ag results in its partial translocation into GM1-containing DIGS [45, 46]. Normally this process results in the initiation of downstream signals that activate B cells although internalization of the BCR-Ag complex can proceed independent of signaling from membrane rafts [46]. Thus, the partial translocation of the BCR-Ag complex into lipid rafts would not significantly impact redistribution of membrane proteins or the signaling initiated by LT-IB binding to GM1. Nevertheless, BCR partial translocation into DIGS would further increase signaling that would impact adhesiveness of B and T cells and this would further increase the blockage in T cell division and level of IL-2 production.
In comparison to LT-IB, the events that lead to the observed effects on T cell division and IL-2 production by LT-IIaB are less clear. In vivo, LT-IIaB has multiple effects on APCs including increase in uptake and level of OVA-specific antibody response after intranasal immunization [7, 22]. LT-IIaB exerts its adjuvant function following binding to GD1b and TLR-2 [5, 7]. FRET and fluorescent microscopic examination suggests that LT-IIbB stimulates formation of a multimolecular complex of B-pentamer, ganglioside and TLR-2 [31, 47]. As mouse B cells also express TLR-2 [8], interaction of LT-IIaB with TLR-2 and GD1b on B cells would likely occur. Because GD1b is an integral part of DIGS, and ligation of TLR-2 results in its translocation to DIGS, the mechanisms suggested above for LT-IB may also apply to LT-IIaB with minor differences. In this case, engagement of these receptors by LT-IIaB would enhance B cell Ag presentation of OVA by fluid phase endocytosis in the absence of BCR involvement (Fig. 5A and Fig. 6A). On the other hand, recruitment of the BCR and TLR-2 into GD1b DIGS would result in a strong signal from the three receptors that inhibit T cell division as well as secretion of IL-2 (Fig. 5B and Fig. 6B). Thus, studies that compared TLR and BCR signaling showed significant overlap between these pathways [8]. In this regard, the coordination of signals from the BCR with those from other receptors may play an important role in the outcome of immune responses to Ag [48]. Thus, BCR ligation followed by concurrent ligation of MHC II and CD40 induced significant mobilization of Ca2+. In contrast, simultaneous ligation of the BCR and CD40 results in a significant decrease in MHC II-mediated mobilization of intracellular Ca2+ compared to prior ligation of either receptor alone [48]. It is possible that LT-IIaB adjuvanticity involves the innate immune response directly, and that its enhancement of the antibody response to Ag, in vivo, is a result of bystander activation of B cells by cytokines secreted from DCs and macrophages [7, 22].
An interesting finding in the current study is that most of the observed impact on T cell division and IL-2 production discussed above can also be reproduced following direct stimulation by the B-subunits without OVA (Fig. 5, and Fig. 6). In this regard, incubation of unprimed mouse splenic cells with LT-IB stimulated production of IL-2 and IFN-γ [14]. Moreover, LT-IB increased expression of MHC class II, B7-2, ICAM-1 and CD25 on B cells in these cultures [14]. However, only CD25 and MHC class-II were upregulated when purified B cells were examined [18]. This indicates the requirement for other cells and/or their cytokines for the Ag-independent activation of B cells. DO.11.10 T cell hybrids have also been shown to secrete IL-2 following stimulation by conventional mitogens [49]. The absence of a significant difference between LT-IB and LT-IB+LT-IIaB in the production of IL-2 (Fig. 6) indicates that LT-IB mostly mediates production of this cytokine. However, it remains to be determined whether LT-IB could interact directly with T cells after binding the APC. This possibility is unlikely due to the high affinity binding of this protein to its receptor. More likely, however, events that govern B cell activation by LT-IB dictate the outcome of T cell activation including secretion of IL-2 and a blockage or a delay in DO.11.10 T cell division.
LT-IB and LT-IB+LT-IIaB had similar effects to LPS on the morphology and the proportion of macrophages that express MHC class-II (Fig. 7). In comparison, incubation with LT-IIaB alone was unable to increase the proportion of MHC class-II+ cells. In vivo, macrophages have multiple functions, including removal of tissue debris following microbial infection, secretion of proinflammatory mediators that stimulate other cells, and as Ag presenting cells. These functions are intimately related to their cell cycle, with an inverse relationship between proliferation and surface expression of MHC class-II or secretion of proinflammatory mediators [33]. In this regard, the RAW 264.7 cell line has often been used as a model for secretion of proinflammatory cytokines after stimulation with agents like LPS. LT-IIaB binds to TLR-2 and ganglioside GD1b [7, 47]. Binding of bacterial lipoproteins to TLR-2 stimulates secretion of proinflammatory mediators [9]. LT-IIaB is also a potent proinflammatory stimulant [22]. In contrast to LT-IIaB, LT-IB which binds to ganglioside GM1 ganglioside increased the proportion of MHC class-II+ cells (Fig. 7). This suggests that the inability of LT-IIaB to increase expression of MHC class II on macrophages, yet its ability to stimulate proinflammatory cytokines, were due to opposing signals following binding gangliosides and TLR-2. The outcome of such opposing signals can be seen in the reduction of MHC class II+ macrophages in the LT-IB+LT-IIaB treated culture, compared to those treated with LT-IB alone (Fig. 7). Thus, binding to gangliosides appears to stimulate higher proportion of cells expressing MHC class II, as shown for LT-IB (Fig. 7).
Although the data in this study does not imply cause-and-effect, there are a number of strong indicators that support relevance of the observed cell clustering and the delay/arrest of T cell division, and the IL-2 production caused by the B-subunits with respect to the immune response in vivo. First, there is direct evidence in vivo that links T cell priming in the immune response to an Ag with the persistence, number, and size of clusters [35]. Second, the injection of LT-IB, in vivo, has been shown to result in a blockage of T cell division to a co-administered Ag (17). Third, an increase in stable interactions between APC and T cells (i.e. the “immunological synapse”) is linked to improved immunogenicity and to a process that is mimicked by most conventional adjuvants. Fourth, our data demonstrate that cluster formation and the impact of the B subunits of LTs on T cell division and IL-2 production can also be reproduced in the absence of the co-administered Ag. Hence, the limitations that could be introduced in interpreting the data following the usage of a specific Ag are eliminated.
5. Conclusion
We demonstrate, for the first time, that incubation of the B-subunits with B cells induces significant B cell clustering as early as 4hrs, and B and T cells up to 24 hrs. Incubations of B cells with non-receptor binding B-subunits of LT-1 and LT-IIa significantly reduced cell clustering. Further, the co-incubations with B cells caused a delay or arrest in T cell division following endocytosis or BCR uptake of antigen, respectively. This was associated with increased production of IL-2. LT-IB and LT-IIaB also increased number of MHC class II+ macrophages. Our data suggest that clustering of APCs and/or delay in T cell division could be mechanisms that mediate the potent adjuvanticity of LT-IB and LT-IIaB, in vivo. Conversely, would this mechanism implicate a strategy designed by microbial agents to delay the immune response to their advantage?
Highlights.
Incubation with the B-subunits results in clustering of immune cells and delay/arrest in T cell division
Cell clustering is mostly gangliosides but not TLR-2 mediated
Cell clustering occurs 4 hrs after incubation thus before upregulation of adhesion markers
Delay in T division IL-2 production and MHC-II are differentially regulated by LT-IB and LT-IIaB
Delay/arrest in antigen presentation by the B-subunits also occurs in absence of antigen (OVA)
Acknowledgments
Research contributed by T.O. Nashar to this study was supported by grants from the US Department of Education (P031B120901) and US National Institute of Health RCMI core laboratory facilities grant (G12MD00758-23), and a fellowship to S. Elkassas from the Egyptian Ministry of Education. Research contributed by T.D. Connell and G. Hajishengallis is supported by NIH grant R01 DE017138.
Abbreviations
- BCR
B cell receptor
- DIGs
detergent-insoluble glycolipid-enriched membranes
- μ
human IgM
- OVA
ovalbumin
- Toll-like receptor
TLR
Footnotes
All authors agree on the content and declare no financial and commercial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Connell TD. Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev Vaccines. 2007;6:821–834. doi: 10.1586/14760584.6.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nawar HF, Greene CJ, Lee CH, Mandell LM, Hajishengallis G, Connell TD. LT-IIc, a new member of the type II heat-labile enterotoxin family, exhibits potent immunomodulatory properties that are different from those induced by LT-IIa or LT-IIb. Vaccine. 2011;29:721–727. doi: 10.1016/j.vaccine.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sixma TK, Kalk KH, van Zanten BA, Dauter Z, Kingma J, Witholt B, Hol WG. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J Mol Biol. 1993;230:890–918. doi: 10.1006/jmbi.1993.1209. [DOI] [PubMed] [Google Scholar]
- 4.Sixma TK, Pronk SE, Kalk KH, van Zanten BA, Berghuis AM, Hol WG. Lactose binding to heat-labile enterotoxin revealed by X-ray crystallography. Nature. 1992;355:561–564. doi: 10.1038/355561a0. [DOI] [PubMed] [Google Scholar]
- 5.Hajishengallis G, Tapping RI, Martin MH, Nawar H, Lyle EA, Russell MW, Connell TD. Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infect Immun. 2005;73:1343–1349. doi: 10.1128/IAI.73.3.1343-1349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973;8:851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CH, Masso-Welch P, Hajishengallis G, Connell TD. TLR2-dependent modulation of dendritic cells by LT-IIa-B5, a novel mucosal adjuvant derived from a type II heat-labile enterotoxin. J Leukoc Biol. 2011;90:911–921. doi: 10.1189/jlb.0511236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekeredjian-Ding I, Jego G. Toll-like receptors--sentries in the B-cell response. Immunology. 2009;128:311–323. doi: 10.1111/j.1365-2567.2009.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr Opin Immunol. 2002;14:103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 10.Nashar TO, Williams NA, Hirst TR. Importance of receptor binding in the immunogenicity, adjuvanticity and therapeutic properties of cholera toxin and Escherichia coli heat-labile enterotoxin. Med Microbiol Immunol. 1998;187:3–10. doi: 10.1007/s004300050068. [DOI] [PubMed] [Google Scholar]
- 11.Williams NA, Hirst TR, Nashar TO. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95–101. doi: 10.1016/s0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]
- 12.Freytag LC, Clements JD. Bacterial toxins as mucosal adjuvants. Curr Top Microbiol Immunol. 1999;236:215–236. doi: 10.1007/978-3-642-59951-4_11. [DOI] [PubMed] [Google Scholar]
- 13.Nashar TO, Betteridge ZE, Mitchell RN. Evidence for a role of ganglioside GM1 in antigen presentation: binding enhances presentation of Escherichia coli enterotoxin B subunit (EtxB) to CD4 (+) T cells. Int Immunol. 2001;13:541–551. doi: 10.1093/intimm/13.4.541. [DOI] [PubMed] [Google Scholar]
- 14.Nashar TO, Hirst TR, Williams NA. Modulation of B-cell activation by the B subunit of Escherichia coli enterotoxin: receptor interaction up-regulates MHC class II, B7, CD40, CD25 and ICAM-1. Immunology. 1997;91:572–578. doi: 10.1046/j.1365-2567.1997.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nashar TO, Webb HM, Eaglestone S, Williams NA, Hirst TR. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci U S A. 1996;93:226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arce S, Nawar HF, Russell MW, Connell TD. Differential binding of Escherichia coli enterotoxins LT-IIa and LT-IIb and of cholera toxin elicits differences in apoptosis, proliferation, and activation of lymphoid cells. Infect Immun. 2005;73:2718–2727. doi: 10.1128/IAI.73.5.2718-2727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karmarcha M, Nashar TO. E. coli Heat-labile Enterotoxin B Subunit as a Platform for the Delivery of HIV Gag p24 Antigen. J Clin Cell Immunol. 2013;4:1–7. doi: 10.4172/2155-9899.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bone H, Eckholdt S, Williams NA. Modulation of B lymphocyte signalling by the B subunit of Escherichia coli heat-labile enterotoxin. Int Immunol. 2002;14:647–658. doi: 10.1093/intimm/dxf029. [DOI] [PubMed] [Google Scholar]
- 19.Nashar TO, Betteridge ZE, Mitchell RN. Antigen binding to GM1 ganglioside results in delayed presentation: minimal effects of GM1 on presentation of antigens internalized via other pathways. Immunology. 2002;106:60–70. doi: 10.1046/j.1365-2567.2002.01397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hearn AR, de Haan L, Pemberton AJ, Hirst TR, Rivett AJ. Trafficking of exogenous peptides into proteasome-dependent major histocompatibility complex class I pathway following enterotoxin B subunit-mediated delivery. J Biol Chem. 2004;279:51315–51322. doi: 10.1074/jbc.M408279200. [DOI] [PubMed] [Google Scholar]
- 21.Williams NA, Stasiuk LM, Nashar TO, Richards CM, Lang AK, Day MJ, Hirst TR. Prevention of autoimmune disease due to lymphocyte modulation by the B-subunit of Escherichia coli heat-labile enterotoxin. Proc Natl Acad Sci U S A. 1997;94:5290–5295. doi: 10.1073/pnas.94.10.5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajishengallis G, Nawar H, Tapping RI, Russell MW, Connell TD. The Type II heat-labile enterotoxins LT-IIa and LT-IIb and their respective B pentamers differentially induce and regulate cytokine production in human monocytic cells. Infect Immun. 2004;72:6351–6358. doi: 10.1128/IAI.72.11.6351-6358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 24.Lycke N, Holmgren J. Intestinal mucosal memory and presence of memory cells in lamina propria and Peyer's patches in mice 2 years after oral immunization with cholera toxin. Scand J Immunol. 1986;23:611–616. doi: 10.1111/j.1365-3083.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- 25.Kateley JR, Kasarov L, Friedman H. Modulation of in vivo antibody responses by cholera toxin. J Immunol. 1975;114:81–84. [PubMed] [Google Scholar]
- 26.Vajdy M, Lycke NY. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology. 1992;75:488–492. [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw AC, Mitchell RN, Weaver YK, Campos-Torres J, Abbas AK, Leder P. Mutations of immunoglobulin transmembrane and cytoplasmic domains: effects on intracellular signaling and antigen presentation. Cell. 1990;63:381–392. doi: 10.1016/0092-8674(90)90171-a. [DOI] [PubMed] [Google Scholar]
- 28.Cody V, Pace J, Nawar HF, King-Lyons N, Liang S, Connell TD, Hajishengallis G. Structure-activity correlations of variant forms of the B pentamer of Escherichia coli type II heat-labile enterotoxin LT-IIb with Toll-like receptor 2 binding. Acta Crystallogr D Biol Crystallogr. 2012;68:1604–1612. doi: 10.1107/S0907444912038917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathias-Santos C, Rodrigues JF, Sbrogio-Almeida ME, Connell TD, Ferreira LC. Distinctive immunomodulatory and inflammatory properties of the Escherichia coli type II heat-labile enterotoxin LT-IIa and its B pentamer following intradermal administration. Clin Vaccine Immunol. 2011;18:1243–1251. doi: 10.1128/CVI.00012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nashar TO, Williams NA, Hirst TR. Cross-linking of cell surface ganglioside GM1 induces the selective apoptosis of mature CD8+ T lymphocytes. Int Immunol. 1996;8:731–736. doi: 10.1093/intimm/8.5.731. [DOI] [PubMed] [Google Scholar]
- 31.Liang S, Hosur KB, Lu S, Nawar HF, Weber BR, Tapping RI, Connell TD, Hajishengallis G. Mapping of a microbial protein domain involved in binding and activation of the TLR2/TLR1 heterodimer. J Immunol. 2009;182:2978–2985. doi: 10.4049/jimmunol.0803737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashwell JD, Cunningham RE, Noguchi PD, Hernandez D. Cell growth cycle block of T cell hybridomas upon activation with antigen. J Exp Med. 1987;165:173–194. doi: 10.1084/jem.165.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xaus J, Comalada M, Valledor AF, Cardo M, Herrero C, Soler C, Lloberas J, Celada A. Molecular mechanisms involved in macrophage survival, proliferation, activation or apoptosis. Immunobiology. 2001;204:543–550. doi: 10.1078/0171-2985-00091. [DOI] [PubMed] [Google Scholar]
- 34.Lycke N, Bemark M. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins. Mucosal Immunol. 2010;3:556–566. doi: 10.1038/mi.2010.54. [DOI] [PubMed] [Google Scholar]
- 35.Zinselmeyer BH, Dempster J, Gurney AM, Wokosin D, Miller M, Ho H, Millington OR, Smith KM, Rush CM, Parker I, Cahalan M, Brewer JM, Garside P. In situ characterization of CD4+ T cell behavior in mucosal and systemic lymphoid tissues during the induction of oral priming and tolerance. J Exp Med. 2005;201:1815–1823. doi: 10.1084/jem.20050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo A, Ishikawa F, Nakano H, Nakazaki H, Kobayashi K, Kakiuchi T. Enhancement of B7-1 (CD80) expression on B-lymphoma cells by irradiation. Immunology. 1999;96:642–648. doi: 10.1046/j.1365-2567.1999.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz TF, Mitterer M, Neumayer HP, Vogetseder W, Dierich MP. Involvement in the initiation of T cell responses and structural features of an 85-kDa membrane activation antigen. Eur J Immunol. 1988;18:1253–1258. doi: 10.1002/eji.1830180816. [DOI] [PubMed] [Google Scholar]
- 38.Xavier R, Seed B. Membrane compartmentation and the response to antigen. Curr Opin Immunol. 1999;11:265–269. doi: 10.1016/s0952-7915(99)80043-0. [DOI] [PubMed] [Google Scholar]
- 39.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 40.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 41.Wimer-Mackin S, Holmes RK, Wolf AA, Lencer WI, Jobling MG. Characterization of receptor-mediated signal transduction by Escherichia coli type IIa heat-labile enterotoxin in the polarized human intestinal cell line T84. Infect Immun. 2001;69:7205–7212. doi: 10.1128/IAI.69.12.7205-7212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta. 2014;1838:532–545. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell JS, Brown WS, Woodside DG, Vanderslice P, McIntyre BW. Clustering T-cell GM1 lipid rafts increases cellular resistance to shear on fibronectin through changes in integrin affinity and cytoskeletal dynamics. Immunol Cell Biol. 2009;87:324–336. doi: 10.1038/icb.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krauss K, Altevogt P. Integrin leukocyte function-associated antigen-1-mediated cell binding can be activated by clustering of membrane rafts. J Biol Chem. 1999;274:36921–36927. doi: 10.1074/jbc.274.52.36921. [DOI] [PubMed] [Google Scholar]
- 45.Cheng PC, Dykstra ML, Mitchell RN, Pierce SK. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J Exp Med. 1999;190:1549–1560. doi: 10.1084/jem.190.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caballero A, Katkere B, Wen XY, Drake L, Nashar TO, Drake JR. Functional and structural requirements for the internalization of distinct BCR-ligand complexes. Eur J Immunol. 2006;36:3131–3145. doi: 10.1002/eji.200636447. [DOI] [PubMed] [Google Scholar]
- 47.Liang S, Wang M, Tapping RI, Stepensky V, Nawar HF, Triantafilou M, Triantafilou K, Connell TD, Hajishengallis G. Ganglioside GD1a is an essential coreceptor for Toll-like receptor 2 signaling in response to the B subunit of type IIb enterotoxin. J Biol Chem. 2007;282:7532–7542. doi: 10.1074/jbc.M611722200. [DOI] [PubMed] [Google Scholar]
- 48.Nashar TO, Drake JR. Dynamics of MHC class II-activating signals in murine resting B cells. J Immunol. 2006;176:827–838. doi: 10.4049/jimmunol.176.2.827. [DOI] [PubMed] [Google Scholar]
- 49.Zlotnik A, Daine B. Activation of IL 1-dependent and IL 1-independent T cell lines by calcium ionophore and phorbol ester. J Immunol. 1986;136:1033–1037. [PubMed] [Google Scholar]


