Abstract
Background
Phlebotomy-induced anemia (PIA) is common in preterm infants. The hippocampus undergoes rapid differentiation during late fetal/early neonatal life and relies on adequate oxygen and iron to support oxidative metabolism necessary for development. Anemia shortchanges these two critical substrates, potentially altering hippocampal development and function.
Methods
PIA (hematocrit <25%) was induced in neonatal mice pups from postnatal day (P)3 to P14. Neurochemical concentrations in the hippocampus were determined using in vivo 1H NMR spectroscopy at 9.4T and compared with control animals at P14. Gene expression was assessed using qRT-PCR.
Results
PIA decreased brain iron concentration, increased hippocampal lactate and creatine concentrations, and decreased phosphoethanolamine (PE) concentration and the phosphocreatine/creatine ratio. Hippocampal transferrin receptor (Tfrc) gene expression was increased, while the expression of calcium/calmodulin-dependent protein kinase type II alpha (CamKIIα) was decreased in PIA mice.
Conclusion
This clinically relevant model of neonatal anemia alters hippocampal energy and phospholipid metabolism and gene expression during a critical developmental period. Low target hematocrits for preterm neonates in the NICU may have potential adverse neural implications.
Introduction
Preterm neonates become anemic in large part due to repeated blood sampling while in the NICU (1,2). Anemia poses two major risks to the still rapidly developing hippocampus-based memory system in preterm neonates: tissue hypoxia due to anemia and iron deficiency (ID) due to loss of total body iron by phlebotomy. Two clinical trials in preterm infants demonstrate that short term neurodevelopment may be at risk in infants randomized to lower target hematocrits (3,4). Preclinical models demonstrate that reduction of oxygen and iron availability during this critical period of hippocampal development potentially leads to acute metabolic changes that may alter development and lead to long-term consequences (5,6)
The brain has a set order and timing to the development of its various structures (7). Adequate metabolic substrates, including iron and oxygen, are necessary for proper structural development of a given brain region. Lack of these essential substrates can lead to alterations in energy metabolism, myelination, neuronal integrity, and connectivity. The hippocampus undergoes rapid growth and synaptogenesis during the last trimester of gestation through early postnatal life in humans and during the first three weeks postnatally in rodents (8). Fetal/neonatal iron deficiency anemia (IDA) induces long-lasting impairments in cognitive development, including learning and memory (9). Together, these factors indicate that potential critical periods may exist in early-life where iron (and oxygen) are necessary for development of a given brain region, which if missed, results in long-term neural dysfunction and behavioral abnormalities (10).
Two previous studies utilizing in vivo 1H nuclear magnetic resonance (NMR) spectroscopy by our group have shown negative effects of dietary fetal/neonatal IDA and chronic neonatal hypoxia on hippocampal metabolite profiles. Concentrations of 12 of 16 measured metabolites were altered in rats given an iron deficient diet from gestational day 3 to postnatal day (P)7. The metabolic changes indexed altered energy status, neurotransmission, and phospholipid metabolism (11). Chronic hypoxia from P3 to P28 in rats altered 10 of the 16 metabolites quantified (6). Changes were found in metabolites indexing energy metabolism, neurotransmission, neuronal integrity, and phospholipid metabolism. Some, but not all changes seen in the chronic hypoxia study were similar to the changes seen in the dietary IDA model.
It is unknown whether the rapid induction of anemia that occurs via phlebotomy in preterm infants generates the same degree of brain hypoxia and ID as more chronically induced dietary IDA. Therefore, it is unclear whether conclusions drawn from the extensive IDA literature (12) are relevant to phlebotomy-induced neonatal anemia. The objective of the current study was to use a developmentally and clinically appropriate model of phlebotomy-induced anemia and in vivo 1H NMR spectroscopy to determine whether anemia concurrent with rapid development of the hippocampus alters vital metabolic processes related to energy metabolism, phospholipid metabolism, and synaptic integrity.
Results
Hematocrit
Anemia was induced by subjecting mice in the PIA group to twice daily phlebotomy. Daily hematocrit was determined during the first bleeding session of the day from P3 through P13 to monitor the anemia in the PIA pups, (Figure 1a). On P3, pups in the PIA group had a mean ± SEM hematocrit of 42.0 ± 0.7% (n=9). Hematocrit reached the <25% threshold by P8 on average and required only once daily bleeding thereafter. The hematocrit of the PIA group at tissue collection was 21.1 ± 0.4% and that of the non-bled littermate controls was 34.1 ± 0.4% (n=12; p < 0.0001).
Figure 1.
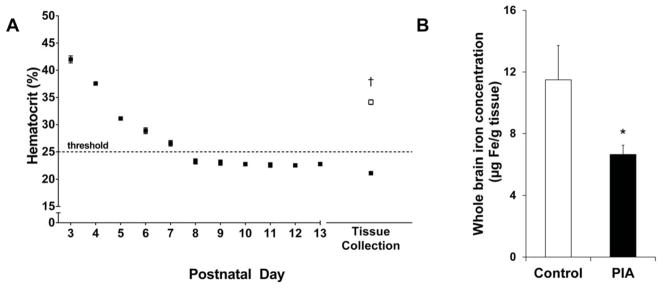
(A) Daily hematocrit values Hematocrit values were determined for PIA mice (black) each day from P3 through P13. The target threshold of 25% was reached by P8 on average. At tissue collection, hematocrit values were determined for both non-bled control mice (white) and PIA mice. (B) Whole brain iron concentration at P10. The PIA group (black) is 42% iron deficient as compared to the non-bled control group (white). Values are mean ± SEM. control, n=9; PIA, n=12. * p < 0.05, † p < 0.001 vs. the control group. PIA, phlebotomy-induced anemia.
Body Weight
Weight gain was measured daily and was linear throughout the experimental period with an average weight gain of 0.46 g/day for the control group and 0.40 g/day for the PIA group. The average weight of the PIA group on P14 (7.2 ± 0.2g) was not significantly different than the average weight of the control group (6.7 ± 0.2g).
Brain Iron Concentration
Whole brain iron concentration was determined on P10. The iron concentration of the PIA group (6.6 ± 0.6μg Fe/g brain tissue) was 42% lower than the control group (11.5 ± 2.2μg Fe/g brain tissue; p = 0.03; Figure 1b).
In vivo 1H NMR Spectroscopy
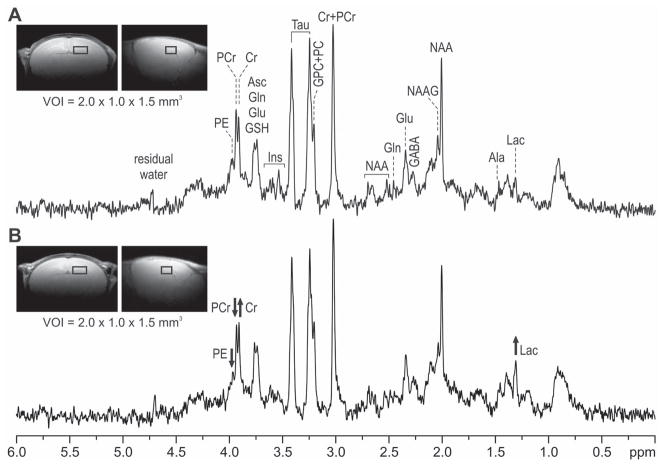
The hippocampal neurochemical profiles of the PIA and control mice were determined at P14 using 1H NMR spectroscopy (Figures 2 and 3). Spectra were obtained from 21 mice (control, n = 12; PIA, n = 9). As compared with their non-bled littermate controls, PIA mice had increased concentrations of lactate (+40.0%, p=0.003) and creatine (+9.2%, p=0.014) in the hippocampus. Phosphoethanolamine (−8.6%, p=0.01) concentration was decreased (Figure 3). The ratio of phosphocreatine to creatine (PCr/Cr) was lower in the PIA group as compared to the control group (−13.6%, p=0.019). Concentrations of other metabolites measured did not differ between PIA and control groups.
Figure 2. In vivo 1H NMR spectra from the hippocampus of a non-bled control (A) and a PIA (B) mouse at postnatal day 14.
The insets show coronal and sagittal MRIs with the position of VOI used for acquiring the spectra. STEAM, TE = 2 ms, TR = 5 s, number of scans = 240. Ala, alanine; Asc, ascorbate; Cr, creatine; Glu, glutamate; Gln, glutamine; GSH, glutathione; GPC, glycerophosphocholine; Lac, lactate; Ins, myo-inositol; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PC, phosphocholine; PCr, phosphocreatine; PE, phosphoethanolamine; Tau, taurine; VOI, volume of interest.
Figure 3. Neurochemical profiles.
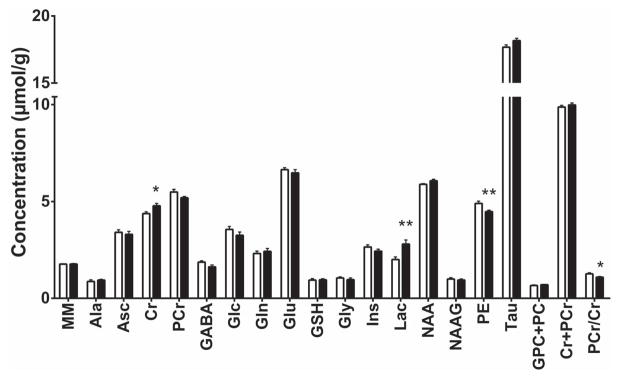
The concentration of each metabolite measured is shown for the control (white) and PIA (black) groups. Values are mean ± SEM. control, n = 9; PIA, n = 12. * p < 0.02, ** p < 0.01 vs. the control group. Ala, alanine; Asc, ascorbate; Cr, creatine; Glc, glucose; Gln, glutamine; Glu, glutamate; Gly, glycine; GSH, glutathione; GPC, glycerophosphocholine; Lac, lactate; Ins, myo-inositol; MM, macromolecules; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PC, phosphocholine; PCr, phosphocreatine; PE, phosphoethanolamine; Tau, taurine; PIA, phlebotomy-induced anemia. MM is expressed in arbitrary units, PCr/Cr ratio has no units.
Gene Expression
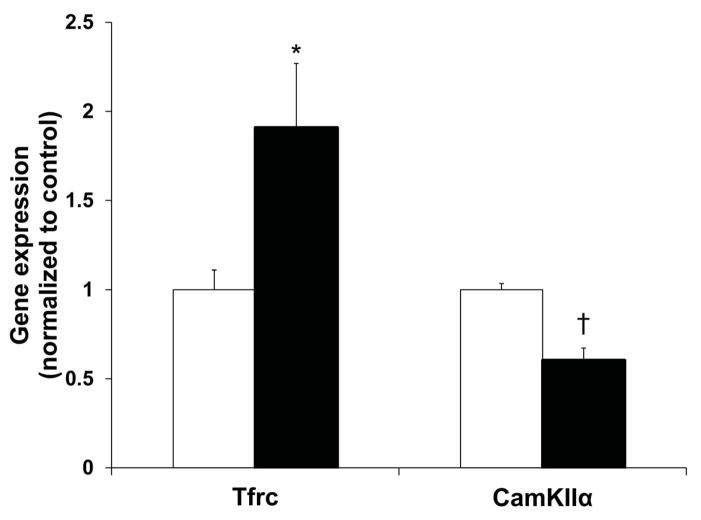
qRT-PCR was performed using P14 hippocampal tissues (Figure 4). Gene expression of transferrin receptor (Tfrc), an indicator of ID, was increased by 91% (p = 0.028) in the PIA group. Gene expression of calcium/calmodulin-dependent protein kinase type II alpha (CamKIIα), a marker of synaptic plasticity, was decreased 40% (p < 0.001) in the PIA group. No group differences were found for myelin basic protein (Mbp), proteolipid proteins (Plp1, Plp2), or monocarboxylate transporters (Mct1, Mct2).
Figure 4. Gene expression changes in the hippocampus at P14.
PIA mice (black) had increased expression of Tfrc and decreased expression of CamKIIα as compared to control mice (white). Values are mean ± SEM normalized to the control group (n = 5–7 per group). * p < 0.05, † p < 0.001 vs. the control group. PIA, phlebotomy-induced anemia; Tfrc, transferrin receptor; CamKIIα, calcium/calmodulin-dependent protein kinase type II alpha.
Discussion
Our study utilized a neurodevelopmentally appropriately timed phlebotomy-induced anemia (PIA) mouse model with comparable hematocrit levels to those found in preterm infants in the NICU (3,4) to investigate the effects of anemia on hippocampal neurochemistry and gene expression. Phlebotomy alone was enough to cause a substantial loss of total body iron and induce a 42% reduction in whole brain iron concentration (Figure 1b). Cerebral oxygen consumption compared to body weight is much lower in the mouse than in humans (13), so this effect could be even more detrimental to human infants in the NICU who have even greater metabolic demands to the developing brain. This degree of brain iron deficiency (ID) is also seen in dietary models of gestational/lactational ID, and causes profound genomic, metabolic, and structural changes in the hippocampus, accompanied by long-term learning and memory deficits in those models (6,14–19). The iron dose provided to the pups through their maternal milk was equivalent to approximately 3 mg/kg body weight daily (unpublished data), consistent with the American Academy of Pediatrics recommended iron supplementation dose for premature infants (20). Increased hippocampal gene expression of Tfrc, the carrier protein for iron uptake into the cell, confirms that the PIA mice are iron deficient in the hippocampus as well as the whole brain (Figure 4).
PIA significantly affected hippocampal neurochemistry and expression of both iron-dependent and synaptic plasticity genes at a time of rapid hippocampal growth and differentiation. The findings emphasize the potential neurodevelopmental implications of repeated phlebotomy and low target hematocrits in preterm infants between 26 and 40 weeks post-conceptional age. They are consistent with the findings of two clinical trials that demonstrated poorer short-term neurodevelopment in preterm infants randomized to lower target hematocrits and may offer insights into the specific neuropathology induced by PIA (3,4).
Since the PIA mice were both iron deficient as well as anemic, which causes significant tissue hypoxia, either factor could have driven the observed changes in neurochemistry and gene expression. A 2003 study by Rao et al. used a dietary model of iron deficiency anemia (IDA) and assessed neurochemical changes at several time points in the developing rat hippocampus including at P14 (11). A 2005 study by Raman et al. determined the effects of chronic hypoxia (10% O2 from P3 to P28) without dietary ID on hippocampal neurochemistry at P14 in rats (6). Our experiment can be compared with the results of these two studies to assess whether hypoxia, ID, or both drove the observed metabolic changes in the PIA model.
Phosphoethanolamine (PE), a precursor of myelin phospholipid phosphotidylethanolamine, as well as a constituent of cell membranes, was decreased in the PIA pups (Figure 3). A similar reduction in hippocampal PE concentration occurs in chronically hypoxic rat pups and is interpreted as decreased myelination or decreased cell turnover in a hypometabolic state (6). In contrast, dietary IDA increases hippocampal PE during development (6,21). The reduction in PE in PIA mice could be indicative of decreased myelination. Hypomyelination leads to long-term reductions in processing speed (22,23) and is common in dietary IDA (24). However, as we did not find changes in the myelin markers Mbp, Plp1, or Plp2, this favors the interpretation that the change in PE reflects a general decrease in phospholipid synthesis, including in cell membrane and synaptosome content and subsequent function (25). Alterations in membrane dynamics may result in poorer neurotransmitter release and synaptic function (11,25). Another recent study from our lab demonstrated persistently decreased hippocampal PE in formerly ID adult rats, despite iron supplementation beginning in the neonatal period (26).
PIA also altered oxidative metabolism in the hippocampus as evidenced by a 40% increase in lactate concentration. The finding is consistent with increased anaerobic metabolism and could be due to anemia, ID, or both. As the cytochromes of the electron transport chain contain iron, loss of brain iron can also lead to a suppression of the TCA cycle and a reliance on anaerobic glycolysis to support energy metabolism (27). Previous studies have demonstrated increased activity of lactate dehydrogenase, the enzyme responsible for lactate production, in the brain of adult rats with dietary IDA (28). Induction of ID by adding the iron chelator desferrioxamine (DFO) to the human cell line K-562 also results in an increase in lactate to compensate for the ATP loss in the TCA cycle (29). A decrease in the citrate/lactate ratio of cerebrospinal fluid has previously been shown in dietary IDA monkeys (30). While lactate can be used by the brain for energy, the finding of increased steady-state, intracellular lactate levels suggests an accumulation of this by-product of anaerobic glycolysis. The lack of compensatory increases in expression of lactate transporters Mct1 and Mct2 support the idea that the increase in intracellular lactate is not a positive adaptive response. The lack of increased Mct1 and Mct2 expression also support our contention that increased lactate in the PIA hippocampus likely represents local production in the CNS and is not due to increased transport from the blood across the blood brain barrier.
Further evidence of impaired oxidative metabolism in the PIA pups is reflected in the increased concentration of creatine and the decreased PCr/Cr ratio in the PIA pups, which collectively indicate decreased phosphorylation potential. IDA can decrease oxidative ATP production because the enzymes involved in oxidative phosphorylation contain iron and are decreased in ID (31). Gestational/lactational dietary ID induces a decrease in cytochrome c oxidase activity of 42% in the P10 hippocampus (27).
The PIA mice had decreased levels of gene expression for calcium/calmodulin-dependent protein kinase type II alpha (CamKIIα) in the hippocampus on P14 (Figure 4). CamKIIα is an enzyme crucial to the calcium signaling pathways leading to plasticity in glutamatergic synapses. CamKIIα is required for hippocampal LTP as well as spatial learning. Gene expression of this enzyme is reduced in dietary IDA models, accompanied by poorer performance on spatial learning tasks (17,19). Further behavioral testing will be needed to determine if PIA leads to similar functional behavioral deficits consistent with the reduction in CamKIIα expression.
Overall, the metabolic changes in the hippocampus of the anemic neonatal PIA mouse appear to be driven by both hypoxia and ID. The combined effect results in a unique neurometabolic profile. Fewer metabolites were affected in the PIA model than in either of the previous dietary IDA and chronic hypoxia NMR spectroscopy studies (6,11), most likely because the duration of exposure to adverse conditions was shorter in the present study. In our previous study (11), IDA was induced at G3 and continued until P14 (i.e., 32 days). In the current study the phlebotomy began on P3 and reached the target hematocrit at P8; thus the animals were anemic for only 7 days. However, the degree of brain acidosis as indexed by the increased lactate concentration was more severe in the PIA model. This may reflect the steeper decline in oxygen delivery to the brain over time and may have put more stress on compensatory mechanisms (e.g., deamination of amino acids; alternate fuel sources) than the neonatal brain can achieve. Increased lactate concentration is also relevant from a translational perspective as many physicians tolerate similar hematocrits as long as the infant is not tachycardic or systemically acidotic. It is possible that elevated lactate levels in the brain precede systemic lactic acidosis. While the anemia is relatively acute in real-time (i.e., 7 days), developmentally the study days span the equivalent of the last trimester in humans during which the hippocampus begins to differentiate and rapid myelination begins. This more prolonged exposure to anemia on the developmental time scale makes it possible that critical periods of brain development were missed and that long-term neurochemical and neurodevelopmental changes could occur.
Several limitations exist in the current study. Only one brain area was studied, so we cannot assess effects across brain areas or draw conclusions about how the PIA protocol affected brain structures differentially. Additionally, there was no long-term assessment to determine behavioral or brain structural consequences. Clearly, the amount of dietary iron was not sufficient to replenish the iron pool lost by phlebotomy to supply the needs of both the reconstituting red cell mass and the developing brain. Previous studies suggest that iron is prioritized to red cells over other organs including the brain (32–34). Whether a higher dose of iron in the maternal diet would have protected the neonatal brain in the PIA mice is unknown.
Translationally, there has been a decades-long debate regarding target hematocrits in preterm infants. Two randomized controlled trials have been performed to assess whether higher or lower hematocrit thresholds are associated with better outcomes (3,4). Of note, the target hematocrit of the lower hematocrit group in both trials was in the range that we induced in our study. Both trials demonstrated a tendency toward poorer early neurodevelopment in the more anemic group (3,4), although one of the studies demonstrated better long-term development in the more anemic group (4). A third multi-center trial is currently underway to provide greater clarity regarding the question. Our model confirms it is feasible for acute PIA to lead to harmful effects on brain development. The current study in a developmentally appropriately-timed mouse model provides some clues to the fundamental metabolic changes that occur with PIA in a brain area subserving learning and memory. Other models of anemia and hypoxia have shown that the induction of metabolic changes indicative of altered energy and phospholipid metabolism result in long-term structural and functional abnormalities in the hippocampus (4,10–15). Future directions include investigating the effects of exogenous recombinant human erythropoietin (rhEpo) administration. Epo stimulates red blood cell production and thus should alleviate the anemia, thereby lessening some of the hypoxic effects. However, in an iron limited state rhEpo may exacerbate brain ID as iron is preferentially shunted to red blood cells at the expense of the brain.
Methods
Animal Preparation
The study was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota. WT C57/B6 mice were used for all experiments. Mice were given ad libitum access to food and water and kept on a 12-hour light/dark cycle. The environment was temperature and humidity controlled, with conditions monitored by Research Animal Resources (RAR). All mothers were fed a standard diet with 200ppm iron (Harlan; Indianapolis, IN) throughout the experiment. Litters were culled to 8 pups, with 4 pups in the non-bled control group and 4 in the PIA group. This reduced variability in food availability and mothering conditions, thereby minimizing differences in weight gain amongst litters.
Phlebotomy-Induced Anemia
The unique phlebotomy model in this study was based on neonatal blood volume calculations published by the Sola-Visner lab (35). The PIA pups were bled via facial venipuncture. A micropipette was used to accurately measure the amount of blood drawn. Each morning, pups were weighed and the quantity of blood drawn from each bled pup (5.25 μL/g) was calculated based on body weight. The PIA pups were bled twice per day until they reached the designated hematocrit threshold of 25%. Thereafter, they were bled once per day to maintain the desired severity of anemia. Phlebotomy occurred from P3 to P14. Glass microhematocrit tubes were used to determine the hematocrit. The hematocrit was determined by spinning the microhematocrit tubes in a centrifuge at 10,000 rpm for five minutes and then quantified using a hematocrit card reader. The control pups were pricked with a needle through the scruff of their neck in order to subject all mice to a similar needle stick while ensuring that the control pups did not bleed.
Tissue Collection
Mice received an overdose of pentobarbital (120 mg/kg i.p.) and brains were quickly removed from the skull and placed on filter paper atop a metal block partially submerged in ice. Hippocampi were dissected, flash frozen in liquid nitrogen, and stored at −80°C until further processing.
Iron Assay
Whole brain iron concentration was determined using a previously published microanalysis protocol to measure non-heme iron in animal tissues (36). Briefly, tissue homogenates were treated with hydrochloric acid and trichloroacetic acid. Heating to 95°C released non-heme iron precipitate. After centrifugation, ferrozine was added to the supernatant where it interacted with the iron and reducing agent thioglycolic acid. Results were quantified using spectrophotometry and a standard iron curve.
In vivo 1H NMR Spectroscopy
Spontaneously breathing P14 mice pups (control, n=9; PIA, n=12) were anesthetized by isoflurane (3% for induction and 1–2% for maintenance) in a 1:1 mixture of O2 and N2O. The respiration was continuously monitored (SA Instruments, Inc., Stony Brook, NY) and maintained at 60–90 breaths/min by adjusting the level of isoflurane in the gas mix. All experiments were performed using a 9.4 T/31cm horizontal bore magnet (Varian/Magnex Scientific; Yarnton, UK) interfaced to Varian INOVA/Direct Drive consoles (Agilent/Varian; Palo Alto, CA) using previously published protocols (6,11). The homogeneity of the B0 magnetic field was adjusted by FASTMAP automatic shimming (37). The 1H NMR spectra were acquired using ultra-short echo-time STEAM sequence (TE = 2 ms, TR = 5 s) combined with outer volume suppression and VAPOR water suppression (38). The spectra were collected from the 3 μL volumes of interest (VOI=2.0 x 1.0 x 1.5 mm3 centered in the left hippocampus). Multi-slice fast spin-echo MRI (echo train length = 8, echo spacing = 12 ms, field of view = 2 cm × 2 cm, slice thickness = 1 mm) in sagittal and axial orientations were used for precise positioning of the VOI. Imaging alternated throughout each day between PIA and control mice and mice not being imaged were kept with their mother and littermates in their home cage. No differences in metabolite concentrations were found between males and females and thus data from both sexes were combined in the analysis. Study of a single mouse did not exceed 60 minutes.
Metabolite Quantification
In vivo 1H NMR spectra were analyzed by LCModel (39) with the spectrum of fast-relaxing macromolecules included in the basis set. Unsuppressed water signal was used as an internal reference assuming 85% brain water content. The following 17 metabolites were included in the final analysis: alanine (Ala), ascorbate (Asc), creatine (Cr), phosphocreatine (PCr), GABA, glucose (Glc), glutamate (Glu), glutamine (Gln), glutathione (GSH), lactate (Lac), myo-inositol (Ins), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphoethanolamine (PE), taurine (Tau) and the sum of glycerophosphocholine (GPC) and phosphocholine (PC). The ratio for PCr/Cr was also calculated.
Gene Expression Analysis
RNA was extracted from the left hippocampus using the RNAqueous Total RNA Isolation Kit (Ambion; Austin, TX). The kit protocol was followed and samples were stored at −80°C. Quality of samples was verified using a NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific; Waltham, MA). Samples not meeting quality standards were further processed using the RNA Clean and Concentrator-5 Kit (Zymo Research; Irvine, CA). cDNA was generated using 1μg total RNA per sample and the High-Capacity RNA-to-cDNA kit (Invitrogen; Carlsbad, CA). Again the kit protocol was followed, samples were diluted 20x with TE buffer (10mM Tris, 1mM EDTA), and stored at −20°C. RT-qPCR was run in singleplex with duplicate wells using Taqman qPCR Universal Mix and Taqman Gene Expression Assay probes (Applied Biosystems; Carlsbad, CA). As a control, ribosomal protein s18 was used. Thermocycling was performed with the MX3000P instrument (Strategene; La Jolla, CA).
Statistical Analysis
All data are presented as mean ± SEM. Significance between groups for all metrics other than metabolite concentration was determined using a two-tailed unpaired t-test and results were considered significant at a threshold of α = 0.05. In order to reduce the chance for a false positive result in the NMR spectroscopy experiments due to multiple testing, a false discovery rate method with Benjamini-Hochberg procedure was applied. Using a q-value of 0.1, results were considered significant at a threshold of α = 0.02.
Acknowledgments
Statement of Financial Support: National Institutes of Health (NIH) (Bethesda, MD) Grant P01HL046925-16 awarded to John Widness (University of Iowa, Iowa City, IA) with Project 4 Lead to Michael K. Georgieff (University of Minnesota, Minneapolis, MN), NIH Grants P30 NS076408 and P41 EB015894 awarded to Kamil Ugurbil, Center for Magnetic Resonance Research (CMRR), (University of Minnesota), and support to CMRR from the W.M. KECK Foundation (Los Angeles, CA).
Footnotes
The authors have no financial ties or conflicts of interest to disclose.
References
- 1.Ramasethu J, Luban NLC. Red blood cell transfusions in the newborn. Semin Neonatol. 1999;4:5–16. [Google Scholar]
- 2.Widness JA. Pathophysiology, Diagnosis, and Prevention of Neonatal Anemia. NeoReviews. 2000;1:e61–8. [Google Scholar]
- 3.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–7. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Bell EF, Strauss RG, Widness JA, et al. Randomized Trial of Liberal Versus Restrictive Guidelines for Red Blood Cell Transfusion in Preterm Infants. Pediatrics. 2005;115:1685–91. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fretham SJB, Carlson ES, Wobken J, Tran PV, Petryk A, Georgieff MK. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus. 2012;22:1691–702. doi: 10.1002/hipo.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Dev Brain Res. 2005;156:202–9. doi: 10.1016/j.devbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Thompson RA, Nelson CA. Developmental science and the media. Early brain development. Am Psychol. 2001;56:5–15. doi: 10.1037/0003-066x.56.1.5. [DOI] [PubMed] [Google Scholar]
- 8.Pokorný J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Res Bull. 1981;7:113–20. doi: 10.1016/0361-9230(81)90075-7. [DOI] [PubMed] [Google Scholar]
- 9.Fretham SJB, Carlson ES, Georgieff MK. The role of iron in learning and memory. Adv Nutr Bethesda Md. 2011;2:112–21. doi: 10.3945/an.110.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callahan LSN, Thibert KA, Wobken JD, Georgieff MK. Early-life iron deficiency anemia alters the development and long-term expression of parvalbumin and perineuronal nets in the rat hippocampus. Dev Neurosci. 2013;35:427–36. doi: 10.1159/000354178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–21. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- 12.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69 (Suppl 1):S43–8. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol. 1998;Suppl 27:177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–20. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- 15.Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci. 2010;32:238–48. doi: 10.1159/000314341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felt BT, Beard JL, Schallert T, et al. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171:261–70. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt AT, Waldow KJ, Grove WM, Salinas JA, Georgieff MK. Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behav Neurosci. 2007;121:475–82. doi: 10.1037/0735-7044.121.3.475. [DOI] [PubMed] [Google Scholar]
- 18.Jorgenson LA, Sun M, O’Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- 19.Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17:679–91. doi: 10.1002/hipo.20307. [DOI] [PubMed] [Google Scholar]
- 20.Baker RD, Greer FR. Clinical Report—Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0–3 Years of Age) Pediatrics. 2010;126:1–11. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- 21.Van de Looij Y, Chatagner A, Hüppi PS, Gruetter R, Sizonenko SV. Longitudinal MR assessment of hypoxic ischemic injury in the immature rat brain. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2011;65:305–12. doi: 10.1002/mrm.22617. [DOI] [PubMed] [Google Scholar]
- 22.Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brainstem responses. Am J Clin Nutr. 1998;68:683–90. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- 23.Algarín C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res. 2003;53:217–23. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- 24.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Georgieff MK, Innis SM. Controversial nutrients that potentially affect preterm neurodevelopment: essential fatty acids and iron. Pediatr Res. 2005;57:99R–103R. doi: 10.1203/01.PDR.0000160542.69840.0F. [DOI] [PubMed] [Google Scholar]
- 26.Rao R, Tkac I, Unger EL, et al. Iron supplementation dose for perinatal iron deficiency differentially alters the neurochemistry of the frontal cortex and hippocampus in adult rats. Pediatr Res. 2013;73:31–7. doi: 10.1038/pr.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–76. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Kariya F, Kaihatsu K, et al. Effects of chronic iron deficiency anemia on brain metabolism. Jpn J Physiol. 1995;45:257–63. doi: 10.2170/jjphysiol.45.257. [DOI] [PubMed] [Google Scholar]
- 29.Oexle H, Gnaiger E, Weiss G. Iron-dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochim Biophys Acta. 1999;1413:99–107. doi: 10.1016/s0005-2728(99)00088-2. [DOI] [PubMed] [Google Scholar]
- 30.Rao R, Ennis K, Oz G, Lubach GR, Georgieff MK, Coe CL. Metabolomic Analysis of Cerebrospinal Fluid Indicates Iron Deficiency Compromises Cerebral Energy Metabolism in the Infant Monkey. Neurochem Res. 2012;38:573–80. doi: 10.1007/s11064-012-0950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dallman PR, Schwartz HC. Myoglobin and cytochrome response during repair of iron deficiency in the rat. J Clin Invest. 1965;44:1631–8. doi: 10.1172/JCI105269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georgieff MK, Schmidt RL, Mills MM, Radmer WJ, Widness JA. Fetal iron and cytochrome c status after intrauterine hypoxemia and erythropoietin administration. Am J Physiol. 1992;262:R485–91. doi: 10.1152/ajpregu.1992.262.3.R485. [DOI] [PubMed] [Google Scholar]
- 33.Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr. 1992;121:109–14. doi: 10.1016/s0022-3476(05)82554-5. [DOI] [PubMed] [Google Scholar]
- 34.Guiang SF, 3rd, Georgieff MK, Lambert DJ, Schmidt RL, Widness JA. Intravenous iron supplementation effect on tissue iron and hemoproteins in chronically phlebotomized lambs. Am J Physiol. 1997;273:R2124–31. doi: 10.1152/ajpregu.1997.273.6.R2124. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z-J, Hoffmeister KM, Hu Z, et al. Expansion of the neonatal platelet mass is achieved via an extension of platelet lifespan. Blood. 2014;123:3381–9. doi: 10.1182/blood-2013-06-508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rebouche CJ, Wilcox CL, Widness JA. Microanalysis of non-heme iron in animal tissues. J Biochem Biophys Methods. 2004;58:239–51. doi: 10.1016/j.jbbm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Gruetter R, Tkác I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2000;43:319–23. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 1999;41:649–56. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 39.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]