Abstract
Purpose
Renal cell carcinoma (RCC) is an uncommon tumor in the pediatric population. We examined a large national cancer database to determine outcomes for children with RCC and to identify variables affecting long-term survival.
Methods
The National Cancer Data Base (NCDB) was queried for patients age 0 to 17 years diagnosed with RCC from 1998–2011. Patient demographics, tumor stage and characteristics, management, and outcomes were evaluated.
Results
A total of 304 children met inclusion criteria. Overall, 39% of children had stage I disease, 16% stage II, 33% stage III, and 12% stage IV. One-year and five-year survival for all children was 87% and 70%, respectively. Eighty-six percent of patients underwent surgical resection. In comparison to children who underwent complete nephrectomy, patients undergoing partial nephrectomy had smaller tumors and were of lower clinical stages. Survival following partial resection was 100% at one and five years. Age and gender had no significant impact on survival. Survival was negatively impacted by increasing tumor size (P < 0.001), positive nodal status (P = 0.001), and higher pathologic stage (P < 0.001).
Conclusion
Children with renal cell carcinoma who undergo surgical resection have excellent one-year and five-year survival. Overall survival is significantly affected by pathologic stage, tumor size, and nodal status.
Keywords: renal cell carcinoma, transitional renal cell carcinoma, National Cancer Data Base, partial nephrectomy
Renal cell carcinoma (RCC) is an uncommon tumor in children, accounting for 0.1% to 0.3% of all neoplasms and 2% to 6% of all malignant renal tumors [1–3]. The incidence of RCC increases throughout childhood and among children is most commonly seen in the second decade of life [4]. The clinical behavior, genetic abnormalities, and pathologic characteristics of RCC in children are distinct from that in adults [5–7]. Argani et al [8,9] have suggested that pediatric RCC occurs as a result of genetic translocations, mostly commonly involving the TFE3 gene on locus Xp11.2 and less commonly involving the TFEB gene on locus 6p21. Recognition of this genetic basis has led to such pediatric and young adult RCCs being dubbed “translocation carcinomas”, a term that is now included in the AJCC (American Joint Committee on Cancer) pathological guides. Furthermore, the triad of gross hematuria, abdominal mass, and flank pain commonly described in adults is a less common presentation in children, occurring in only 9% of patients in one series [7].
In adult RCC, survival for AJCC/TNM stage I and II disease is high, but worsens with increasing stage, with a survival rate of only 15% for stage IV disease [10,11]. Several single center studies in pediatric RCC have identified predictors of poor survival including tumor stage, lymph node status, metastases, and grade [11–15]. However, these reports have wide variation in findings likely because of the small number of patients. For rare diseases, large databases are useful tools to define prognostic characteristics and to determine optimal management. To better understand the care of children with RCC, we examined a large national cancer database to describe characteristics of this tumor and determine prognostic indicators associated with increased survival.
1. Methods
1.1. Data source
The National Cancer Data Base (NCDB) is a voluntary patient registry sponsored by the American College of Surgeons and the American Cancer Society. This dataset contains clinical demographics and outcomes data on cancer treatment for patients in more than 1500 Commission on Cancer-accredited facilities.
1.2. Patient population
The NCDB was queried for all children ages 0 to 17 years who carried a diagnosis of RCC from 1998 to 2011 by International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) codes 8260, 8310, 8312, 8316, 8317, 8318, 8319, 8320, and 8510. We collected patient demographics, tumor characteristics, treatment, and outcomes. This dataset separates patients by histologic subtype of RCC, including papillary (8260), clear cell (8310), not otherwise specified (NOS, 8312), cyst associated (8316), chromophobe (8317), sarcomatoid (8318), collecting duct type (8319), and granular cell (8320). Accurate differentiation between adjuvant or neoadjuvant use of chemotherapy and radiation was not available for many patients, and these variables simply represent the use of “any” chemotherapy or radiation.
Resection in the NCDB was categorized as partial, complete, or radical nephrectomy, with clinical and pathologic staging defined using Union for International Cancer Control (UICC)/AJCC criteria [16]. In this staging system, stage I was defined as T1N0M0; II as T2N0M0; III as T1 or T2, N1, M0, or T3, N0 or N1, M0; and IV as T4, any N, M0, or any T, any N, M1. T1 was defined as a tumor ≤7 cm; T2 as >7 cm, but ≤10 cm; T3 as any tumor with extension into major veins or perinephric tissue; and T4 as any tumor invading beyond Gerota’s fascia. Any metastases in regional lymph nodes are termed N1. Partial nephrectomy was defined as a segmental or wedge resection; complete as a total or simple nephrectomy; and radical as complete nephrectomy, possibly including removal of portion of vena cava, adrenal gland(s), Gerota’s fascia, perinephric fat, or partial/total ureter. Patients categorized as nephrectomy not otherwise specified (NOS) or nephrectomy en bloc were excluded, as it could not be determined if these were subtotal or total nephrectomies.
This study was approved for exempt status by the Duke University Institutional Review Board.
1.3. Statistical analysis
Continuous nonparametric data were compiled as median (inter-quartile range (IQR)) and categorical data were compiled as frequency (percentage). There were missing data points for some variables; missing data are noted in Table 1. For analysis of resection types, patients with complete or radical nephrectomy were analyzed as one group. Patients with missing variables were not included in the unadjusted or survival analyses. For those with missing positive node data, the TNM pathologic N variable was used. Univariable analysis of resection type was performed using one-way ANOVA for parametric continuous variables, and the Kruskal–Wallis test for nonparametric continuous variables. Categorical variables were compared with Fisher’s exact test or the Chi-squared test as appropriate. Survival differences were analyzed using the Kaplan–Meier method, with significance determined by the log-rank test. A P value of less than 0.05 was considered statistically significant for all comparisons. All statistical analysis was performed using R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Table 1.
Demographics, tumor characteristics, and treatment for children with renal cell carcinoma. Other types of pathology include cyst associated, chromophobe, sarcomatoid, collecting duct type, granular cell, and medullary carcinoma. Variables with missing data are noted with adjusted total n. y = years, mi = miles, IQR = interquartile range, NOS = not otherwise specified.
| Variable (Total N = 304) | N (%) |
|---|---|
| Male | 159 (52%) |
| Age, y | |
| <5 | 23 (8%) |
| 5–8 | 40 (13%) |
| 9–13 | 98 (32%) |
| >13 | 143 (47%) |
| Race/ethnicity | |
| White | 166 (55%) |
| Black | 97 (32%) |
| Hispanic | 25 (8%) |
| Other | 12 (4%) |
| Missing | 4 |
| Insurance | |
| Private | 257 (90%) |
| Medicare/Medicaid | 21 (7%) |
| Uninsured | 8 (3%) |
| Missing | 18 |
| Distance to cancer center (mi) (IQR) | 16.1 (7.2, 36) |
| Tumor size | |
| ≤4 cm | 101 (36%) |
| 4.1–7 cm | 75 (27%) |
| 7.1–10 cm | 55 (20%) |
| >10 cm | 47 (17%) |
| Missing | 26 |
| Median tumor size, cm (IQR) | 5.5 (3, 8.9) |
| Histologic type | |
| Papillary | 48 (16%) |
| Clear cell | 38 (12%) |
| RCC NOS | 170 (56%) |
| Other | 48 (16%) |
| Pathologic stage | |
| I | 83 (39%) |
| II | 35 (16%) |
| III | 70 (33%) |
| IV | 26 (12%) |
| Missing | 90 |
| Surgical margins | |
| Negative | 232 (91%) |
| Positive margin | 22 (9%) |
| Missing | 50 |
| Positive nodes | 76 (30%) |
| Missing | 54 |
| Radiation | 12 (4%) |
| Missing | 4 |
| Chemotherapy | 58 (20%) |
| Missing | 9 |
2. Results
A total of 304 patients met inclusion criteria and were included in analysis. Of the overall cohort, 159 were male (52%) and 145 (48%) were female (Table 1). The median age at presentation was 13 years (IQR 9–16) and 55% (n = 166) were Caucasian.
The median tumor size was 5.5 cm (IQR 3.0–8.9) and more than 60% were 7 cm or less in size. The most common histologic types were RCC NOS (56%), papillary (16%), and clear cell (12%). Eighty-three patients (39%) had pathologic stage I disease, 35 (16%) had stage II disease, 70 (33%) had stage III disease, and 26 (12%) had stage IV disease. Margins were negative in 91% of cases and positive (6 microscopic and 16 macroscopic) in 9% of cases. Chemotherapy was given to 58 children (20%), and 12 children received radiation therapy (4%). Three children died within 30 days (1%). Eight patients (4%) had unplanned readmissions. The median hospital length of stay was four days (IQR 3.5).
In terms of surgical procedures, 86% of patients underwent some type of resection, with 72% undergoing complete or radical nephrectomy and 14% partial nephrectomy; 39 patients did not undergo formal surgery (Table 2). There was no difference in gender or ethnicity between patients undergoing any type of resection and those who were not resected. Those undergoing partial nephrectomy were more likely to have tumors 4 cm or less in size and be classified as clinical stage I tumors (P = 0.001 and <0.001, respectively). Those that did not undergo surgery more often had stage IV disease (P < 0.001) and a greater proportion were treated with chemotherapy (P < 0.001) and radiation (P = 0.003). Seven patients had clinical stage I disease and did not undergo resection; there was no clear indication from the database as to why these patients were excluded from surgery.
Table 2.
Univariable analysis comparing various degrees of surgical resection in children with renal cell carcinoma. y = years, IQR = interquartile range.
| Variable | No Surgery | Partial Nephrectomy | Complete Nephrectomy | P value |
|---|---|---|---|---|
| N (%) | 39 (14) | 40 (14) | 204 (72) | |
| Age, y | 0.52 | |||
| <5 | 3 (8%) | 2 (5%) | 16 (8%) | |
| 5–8 | 6 (15%) | 5 (12.5%) | 28 (14%) | |
| 9–13 | 16 (41%) | 9 (22.5%) | 68 (33%) | |
| >13 | 14 (36%) | 24 (60%) | 92 (45%) | |
| Female | 14 (36%) | 24 (60%) | 94 (46) | 0.10 |
| Race/ethnicity | 0.11 | |||
| White | 14 (36%) | 27 (68%) | 114 (57%) | |
| Black | 18 (46%) | 8 (20%) | 66 (33%) | |
| Other | 7 (18%) | 5 (12%) | 20 (10%) | |
| Median tumor size, cm (IQR) | 3 (2, 7) | 3 (2, 5) | 6 (4, 9) | <0.001 |
| Tumor size | 0.001 | |||
| ≤4 cm | 12 (52%) | 25 (66%) | 60 (30%) | |
| 4.1–7 cm | 5 (22%) | 9 (24%) | 58 (29%) | |
| 7.1–10 cm | 3 (13%) | 3 (8%) | 44 (22%) | |
| >10 cm | 3 (13%) | 1 (3%) | 38 (19%) | |
| Clinical staging group | <0.001 | |||
| I | 7 (26%) | 20 (91%) | 45 (43%) | |
| II | 0 (0%) | 1 (4.5%) | 26 (25%) | |
| III | 0 (0%) | 1 (4.5%) | 20 (19%) | |
| IV | 20 (74%) | 0 (0%) | 13 (13%) | |
| Surgical margins | 0.08 | |||
| Negative | N/A | 36 (97%) | 182 (93%) | |
| Positive | N/A | 1 (3%) | 14 (7%) | |
| Chemotherapy | 20 (50%) | 2 (5%) | 31 (16%) | <0.001 |
| Radiation therapy | 6 (15%) | 0 (0%) | 6 (3%) | 0.003 |
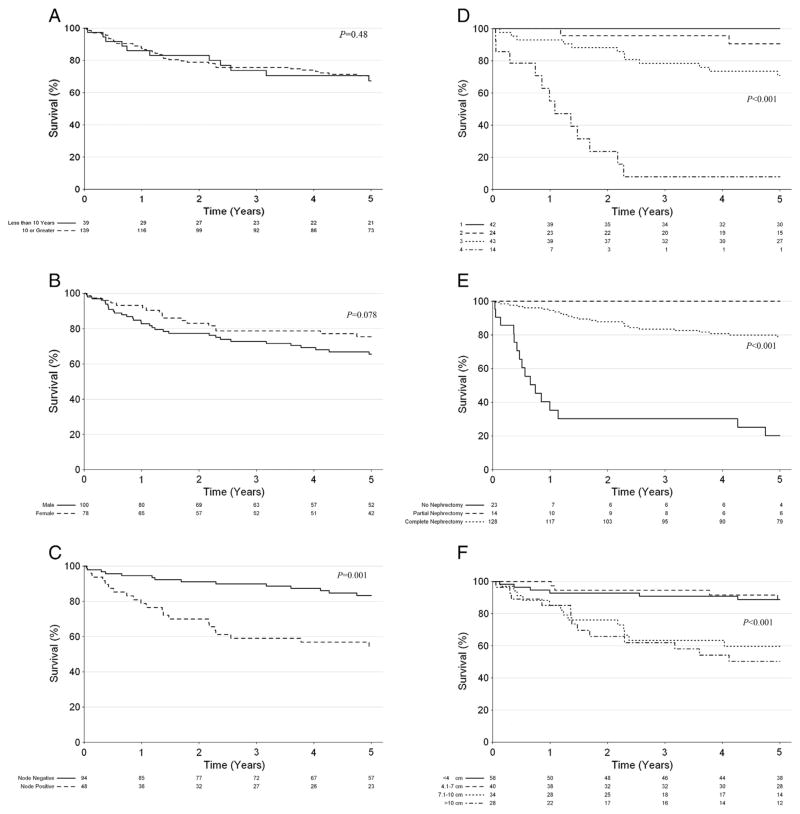
Several factors were analyzed for their effect on overall survival (Table 3), including age, gender, nodal status, pathologic stage, resection type, and tumor size (Fig. 1A–F). Overall one-year and five-year survival was 87% (95% CI: 0.82–0.92) and 70% (95% CI: 0.63–0.77), respectively. Age and gender did not significantly affect survival (P = 0.48 and 0.078, respectively). Five-year survival was decreased for children with positive nodes compared to children with negative nodes (55% vs. 83%, P = 0.001). Patients who did not undergo resection also had lower five-year survival (20%, 95% CI: 0.08–0.48), compared to partial nephrectomy (100%, 95% CI: 1.00–1.00), and complete nephrectomy (79%, 95% CI: 0.72–0.87) (P < 0.001). Survival was also negatively impacted by large tumor size (P < 0.001) and higher pathologic stage (P < 0.001). Five-year survival for stage I–IV RCC was 100%, 91%, 71%, and 8%, respectively.
Table 3.
One-year and five-year survival for children with renal cell carcinoma based on age, gender, nodal status, pathologic stage, resection type, and tumor size. CI = confidence interval, y = years.
| Variable | One-Year Survival (95% CI) | Five-Year Survival (95% CI) |
|---|---|---|
| Overall | 87% (0.82–0.92) | 70% (0.63–0.77) |
| Age (y) | ||
| <10 | 86% (0.75–0.98) | 67% (0.53–0.85) |
| ≥10 | 88% (0.82–0.93) | 70% (0.63–0.79) |
| Gender | ||
| Female | 93% (0.88–0.99) | 76% (0.66–0.86) |
| Male | 83% (0.76–0.91) | 66% (0.57–0.76) |
| Nodal status | ||
| Positive | 79% (0.68–0.91) | 55% (0.42–0.71) |
| Negative | 95% (0.90–0.99) | 83% (0.76–0.92) |
| Path stage | ||
| I | 100% (1.0–1.0) | 100% (1.0–1.0) |
| II | 100% (1.0–1.0) | 91% (0.79–1.0) |
| III | 93% (0.86–1.0) | 71% (0.58–0.86) |
| IV | 55% (0.34–0.90) | 8% (0.01–0.52) |
| Resection type | ||
| No resection | 35% (0.20–0.64) | 20% (0.08–0.48) |
| Partial nephrectomy | 100% (1.0–1.0) | 100% (1.0–1.0) |
| Complete nephrectomy | 95% (0.91–0.99) | 79% (0.72–0.87) |
| Tumor size (cm) | ||
| ≤4 | 93% (0.86–0.99) | 89% (0.81–0.98) |
| 4.1–7 | 100% (1.0–1.0) | 88% (0.78–1.0) |
| 7.1–10 | 85% (0.74–0.98) | 60% (0.45–0.80) |
| >10 | 85% (0.73–1.0) | 50% (0.34–0.74) |
Fig. 1.
A–F. Kaplan–Meier estimates of survival of children with renal cell carcinoma by (A) age, (B) gender, (C) nodal status, (D) pathologic stage, (E) resection type, and (F) tumor size.
3. Discussion
Renal cell carcinoma is a rare malignancy in children, and the outcomes of this tumor remain poorly defined as most studies have been small single center or multicenter retrospective case series [7,17–19]. The current study examined a large, national cancer database and describes current demographics, management, and outcomes of this disease in the pediatric population.
Although partial nephrectomy for RCC is generally recommended for adult patients with tumors less than 7 cm in size, studies on the value of partial nephrectomy in children with RCC are limited [20]. Our study demonstrates that children with tumors 4 cm or less and lower stage can undergo partial nephrectomy with excellent short-term and long-term results, similar to adult patients. Tumors greater than 4 cm but less than 7 cm in size may also be amenable to partial nephrectomy, although an almost equal number of this tumor size underwent complete nephrectomy. Our study also aligns with the limited available published data on partial nephrectomy in children. Cook et al [17] reported on five cases of partial nephrectomy, who had a mean tumor size of 2.8 cm and were clinical stages I and II. Outcomes in this small case series were excellent, with 100% disease free on follow-up and 100% overall survival.
We found that lower pathologic stage and smaller tumor size are associated with improved survival in children, again in agreement with most published data. In the few published studies of RCC in children, tumor grade has also been shown to impact survival, but this variable was not available in the NCDB dataset [17]. Similar to adults, we found that the survival for children with RCC remains high for stage I and II, and drops precipitously for stage III and IV tumors [10,11,21]. Tumor stage in both adult and pediatric RCC appears to be the strongest prognostic indicator for survival [11,19,22,23].
The importance of nodal status in children with RCC is controversial, and our study demonstrates that the presence of nodal disease is associated with worse overall survival. In adult RCC, lymph node metastases are an important prognostic factor [11]. Similarly, Indolfi et al noted that children with regional lymph nodes involved by tumor have significant decreased survival, and they recommend lymph node dissection for node-positive patients [13,18,24,25]. In contrast, Geller and Dome [26] conducted a systematic review of the literature and of patients from their own institution, and noted that although lymph node involvement in adults with RCC was a negative prognostic factor, this association is not seen in pediatric RCC. The overall survival rate for children with local lymph node involvement and absent distant metastases in their series was nearly triple that reported for adults. Our study did not examine the outcomes of lymph node dissection, only the effect of nodal status on survival, and thus conclusions cannot be drawn regarding the role of lymph node dissection in management of pediatric RCC.
Although national databases such as the NCDB provide a large amount of clinical data for children with rare conditions, our findings should be considered within the context of the study’s limitation. Our review is not a population-based study, and therefore the overall disease incidence and prevalence cannot be extracted. Data in the NCDB is collected only from participating Commission on Cancer institutions, and thus may miss nonparticipating sites, such as many large children’s hospitals. Furthermore, as with all voluntary disease registries, data accuracy and quality may vary widely between participating institutions. As the NCDB is a standardized dataset of patients with different types of tumors, important data for one particular tumor may not be collected. For example, this dataset does not collect RCC-specific information such as tumor grade as well as associated inherited syndromes, such as von Hippel–Lindau disease and tuberous sclerosis. Future iterations of the database should be expanded to include specific variables considered particularly important for certain cancers. Finally, the use of chemotherapy and radiation is not well recorded in the NCDB, so limited conclusions can be drawn about the role of multimodality therapies.
In conclusion, more than half of children with renal cell carcinoma present with stage I and II disease and have excellent survival following surgical resection. The long-term survival of these children is most affected by tumor size, lymph node status, and pathologic stage. Future clinical studies of children with RCC may benefit from the use of improved large national datasets to test important clinical questions, such as the role of adjuvant or neoadjuvant therapies in this condition.
Acknowledgments
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Appendix A. Discussions
Presenter: Kristy Rialon, MD, Durham, NC
Discussant: Dr. Ken Gow (Seattle, WA) This is a nice study. Two questions, one of which was I noticed on your abstract you had a different number of patients. And the second question was did you look at different types of histologies.
Response: Dr. Rialon: The reason there was a different number is because we had originally only included RCC not otherwise specified. And in this presentation, we went back and included the other different types of histologies, so including papillary and clear cell and some of the other less common histologies.
Moderator: I had a question regarding the apparent disparity in racial disparity noted in the abstract. So what is your idea in terms of why that observation was even expected, if there was access to care issues that might have presented with more advanced disease but that wouldn’t necessarily preclude surgical management?
Response: Dr. Rialon: When we redid the analysis with the larger subset of patients we did not find that there was a racial disparity. It was not statistically significant.
References
- 1.Booth CM. Renal parenchymal carcinoma in children. Br J Surg. 1986;73:313–7. doi: 10.1002/bjs.1800730422. [DOI] [PubMed] [Google Scholar]
- 2.Young JL, Miller RW. Incidence of malignant tumors in U. S. children. J Pediatr. 1975;86:254–8. doi: 10.1016/s0022-3476(75)80484-7. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Eisner MP, Kosary CL, et al. [Accessed August 3, 2014];SEER Cancer Statistics Review, 1975–2000. 2003 http://seer.cancer.gov/csr/1975_2000/
- 4.Hartman DS, Davis CJ, Madewell JE, et al. Primary malignant renal tumors in the second decade of life: Wilms tumor versus renal cell carcinoma. J Urol. 1982;127:888–91. doi: 10.1016/s0022-5347(17)54116-6. [DOI] [PubMed] [Google Scholar]
- 5.Freedman AL, Vates TS, Stewart T, et al. Renal cell carcinoma in children: the Detroit experience. J Urol. 1996;155:1708–10. [PubMed] [Google Scholar]
- 6.Dehner LP, Leestma JE, Price EB. Renal cell carcinoma in children: a clinicopathologic study of 15 cases and review of the literature. J Pediatr. 1970;76:358–68. doi: 10.1016/s0022-3476(70)80474-7. [DOI] [PubMed] [Google Scholar]
- 7.Aronson DC, Medary I, Finlay JL, et al. Renal cell carcinoma in childhood and adolescence: a retrospective survey for prognostic factors in 22 cases. J Pediatr Surg. 1996;31:183–6. doi: 10.1016/s0022-3468(96)90344-9. [DOI] [PubMed] [Google Scholar]
- 8.Argani P, Hicks J, De Marzo AM, et al. Xp11 translocation renal cell carcinoma (RCC): extended immunohistochemical profile emphasizing novel RCC markers. Am J Surg Pathol. 2010;34:1295–303. doi: 10.1097/PAS.0b013e3181e8ce5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argani P, Hawkins A, Griffin CA, et al. A distinctive pediatric renal neoplasm characterized by epithelioid morphology, basement membrane production, focal HMB45 immunoreactivity, and t(6;11)(p21. 1;q12) chromosome translocation. Am J Pathol. 2001;158:2089–96. doi: 10.1016/S0002-9440(10)64680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall FF, Stewart AK, Menck HR. The National Cancer Data Base: report on kidney cancers. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1997;80:2167–74. [PubMed] [Google Scholar]
- 11.Ficarra V, Righetti R, Pilloni S, et al. Prognostic factors in patients with renal cell carcinoma: retrospective analysis of 675 cases. Eur Urol. 2002;41:190–8. doi: 10.1016/s0302-2838(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 12.Ghavamian R, Cheville JC, Lohse CM, et al. Renal cell carcinoma in the solitary kidney: an analysis of complications and outcome after nephron sparing surgery. J Urol. 2002;168:454–9. doi: 10.1016/s0022-5347(05)64657-5. [DOI] [PubMed] [Google Scholar]
- 13.Selle B, Furtwangler R, Graf N, et al. Population-based study of renal cell carcinoma in children in Germany, 1980–2005: more frequently localized tumors and underlying disorders compared with adult counterparts. Cancer. 2006;107:2906–14. doi: 10.1002/cncr.22346. [DOI] [PubMed] [Google Scholar]
- 14.Lack EE, Cassady JR, Sallan SE. Renal cell carcinoma in childhood and adolescence: a clinical and pathological study of 17 cases. J Urol. 1985;133:822–8. doi: 10.1016/s0022-5347(17)49242-1. [DOI] [PubMed] [Google Scholar]
- 15.Castellanos RD, Aron BS, Evans AT. Renal adenocarcinoma in children: incidence, therapy and prognosis. J Urol. 1974;111:534–7. doi: 10.1016/s0022-5347(17)60009-0. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compson CC, et al., editors. AJCC cancer staging manual. 7. New York, NY: Springer; 2010. pp. 479–89. [Google Scholar]
- 17.Cook A, Lorenzo AJ, Salle JL, et al. Pediatric renal cell carcinoma: single institution 25-year case series and initial experience with partial nephrectomy. J Urol. 2006;175:1456–60. doi: 10.1016/S0022-5347(05)00671-3. discussion 1460. [DOI] [PubMed] [Google Scholar]
- 18.Estrada CR, Suthar AM, Eaton SH, et al. Renal cell carcinoma: Children’s Hospital Boston experience. Urology. 2005;66:1296–300. doi: 10.1016/j.urology.2005.06.104. [DOI] [PubMed] [Google Scholar]
- 19.Helmy T, Sarhan O, Sarhan M, et al. Renal cell carcinoma in children: single-center experience. J Pediatr Surg. 2009;44:1750–3. doi: 10.1016/j.jpedsurg.2009.02.073. [DOI] [PubMed] [Google Scholar]
- 20.Leibovich BC, Blute M, Cheville JC, et al. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171(3):1066–70. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 21.Guinan P, Sobin LH, Algaba F, et al. TNM staging of renal cell carcinoma: Workgroup No. 3. Union International Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80:992–3. doi: 10.1002/(sici)1097-0142(19970901)80:5<992::aid-cncr26>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 22.Medeiros LJ, Gelb AB, Weiss LM. Renal cell carcinoma. Prognostic significance of morphologic parameters in 121 cases. Cancer. 1988;61:1639–51. doi: 10.1002/1097-0142(19880415)61:8<1639::aid-cncr2820610823>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Van Brussel JP, Mickisch GH. Prognostic factors in renal cell and bladder cancer. BJU Int. 1999;83:902–8. doi: 10.1046/j.1464-410x.1999.00120.x. quiz 908–909. [DOI] [PubMed] [Google Scholar]
- 24.Ebert A, Gravou C, Stumpfl M, et al. Renal cell carcinoma in childhood. Case report and review. Urologe A. 2003;42:263–8. doi: 10.1007/s00120-002-0212-4. [DOI] [PubMed] [Google Scholar]
- 25.Indolfi P, Bisogno G, Cecchetto G, et al. Local lymph node involvement in pediatric renal cell carcinoma: a report from the Italian TREP project. Pediatr Blood Cancer. 2008;51:475–8. doi: 10.1002/pbc.21652. [DOI] [PubMed] [Google Scholar]
- 26.Geller JI, Dome JS. Local lymph node involvement does not predict poor outcome in pediatric renal cell carcinoma. Cancer. 2004;101:1575–83. doi: 10.1002/cncr.20548. [DOI] [PubMed] [Google Scholar]