Abstract
Background & Aims
New therapies for chronic hepatitis B (CHB) are urgently needed since current treatments rarely lead to cure. We evaluated whether the oral small molecule toll-like receptor (TLR7) agonist GS-9620 could induce durable anti-viral efficacy in woodchucks chronically infected with woodchuck hepatitis virus (WHV), a hepadnavirus closely related to human hepatitis B virus (HBV).
Methods
After evaluating the pharmacokinetics, pharmacodynamics and tolerability of oral GS-9620 in uninfected woodchucks, adult woodchucks chronically infected with WHV (n=7 per group) were dosed with GS-9620 or placebo for 4 or 8 weeks with different treatment schedules.
Results
GS-9620 treatment induced rapid, marked and sustained reduction in serum viral DNA (mean maximal 6.2 log10 reduction), and hepatic WHV DNA replicative intermediates, WHV cccDNA and WHV RNA, as well as loss of detectable serum WHV surface antigen (WHsAg). GS-9620 treatment also induced a sustained antibody response against WHsAg in a subset of animals. Strikingly, treatment reduced the incidence of hepatocellular carcinoma (HCC) from 71% in the placebo group to 8% in GS-9620-treated woodchucks with sustained viral load reduction. GS-9620 treatment was associated with reversible increases in serum liver enzymes and thrombocytopenia, and induced intrahepatic CD8+ T cell, NK cell, B cell and interferon (IFN) response transcriptional signatures.
Conclusions
The data demonstrate that short duration, finite treatment with the oral TLR7 agonist GS-9620 can induce a sustained antiviral response in the woodchuck model of CHB, and support investigation of this compound as a therapeutic approach to attain a functional cure in CHB patients.
Keywords: TLR7, Hepatitis B virus, Hepatocellular carcinoma, Immunomodulation, Seroconversion, Woodchuck animal model
INTRODUCTION
An estimated 350 million people have chronic hepatitis B virus infection (CHB), and over 500,000 people die each year due to HBV-associated liver diseases, such as cirrhosis and hepatocellular carcinoma (HCC) [1]. Current therapeutics for CHB are limited to nucleos(t)ides and interferon alfa (IFN-α); these reduce viral load, improve long term outcome, but rarely lead to a cure [2]. Immunologic control of CHB, recognized as a “functional cure,” is defined by control of viremia, loss of HBV surface antigenemia (HBsAg), and seroconversion to anti-HBs antibody (HBsAb) and occurs in only 2–3% of patients per year with anti-virals [2]. Functional cure with long-term interferon treatment occurs in <10% of patients at doses associated with treatment-limiting adverse effects [2]. There is therefore an urgent need for a curative therapy.
Natural infection with woodchuck hepatitis virus (WHV), a hepadnavirus closely related to the HBV, occurs in the Eastern woodchuck (Marmota monax). Chronic WHV infection is a model for studying CHB, hepadnavirus-associated HCC, and is used to evaluate HBV therapeutics [3]. Importantly, comparison of the intrahepatic transcriptional profiles in woodchucks and humans with chronic hepadnaviral infection identified important parallels in the anti-viral immune responses and demonstrated molecular similarities in HCC induced by WHV and HBV [4]. As this establishes the translational value of the WHV model, we utilized it to explore therapeutic immunomodulation with the small molecule TLR7 agonist GS-9620.
TLR7 is expressed predominantly in plasmacytoid dendritic cells (pDCs) and B lymphocytes and recognizes viral single-stranded RNA [5]. TLR7 activation induces innate and adaptive immune responses via induction of specific cytokines (including multiple IFN-α subtypes [6]) and chemokines, activation of B cells [7], and cross-priming of cytotoxic lymphocytes [8]. GS-9620 is a selective, oral small molecule TLR7 agonist that has activity in rodents, nonhuman primate and humans [9–12]. A single dosing regimen (three times a week for 8 weeks) of GS-9620 has previously been evaluated in a small number of chimpanzees (n=3) with CHB [10]. Here we report a placebo-controlled study in which the activity of different regimens and treatment durations of GS-9620 were evaluated in woodchucks chronically infected with WHV. Importantly, this model provided a unique opportunity to evaluate the antiviral activity of GS-9620 in a model of vertical transmission and to determine treatment impact on development of hepadnavirus-associated HCC.
MATERIALS AND METHODS
Investigational drug
GS-9620, (8-(3-(pyrrolidin-1-ylmethyl)benzyl)-4-amino-2-butoxy-7,8-dihydropteridin-6(5H)-one), a small molecule, selective TLR7 agonist, was manufactured by Gilead Sciences, Inc. [9]. Doses were administered in a liquid diet (1 mg/mL; 79002 Liquid Woodchuck Control Diet, Dyets, Inc., Bethlehem, PA) in a dose volume of 10 mL. Placebo-treated woodchucks were administered liquid diet alone.
Woodchuck TLR7 cloning and sequencing
Total RNA was isolated from woodchuck PBMC using RNeasy extraction kit (QIAGEN, Redwood City, CA) and DNA was synthesized using SuperScript® III First-Strand Synthesis System (Life Technologies, Chicago, IL) according to the manufacturer’s instructions. Woodchuck TLR7 (wTLR7) sequence was identified by a PCR-based strategy using various primers that were designed based on highly conserved sequences between human TLR7 (NM_016562.3) and mouse TLR7 (NM_133211.3). The 5’- and 3’-ends of wTLR7 were identified using 5’ and 3’RACE System kits respectively according to the manufacturer’s instructions (Life Technologies, Chicago, IL). All amplified PCR fragments were sequenced to obtain the complete wTLR7 sequence, deposited into NCBI (Accession TBD Prior to Publication). wTLR7 was synthesized and cloned into pUNO1-hTLR7-HA3x vector (InvivoGen, San Diego, CA) in place of human TLR7 to generate pUNO1-wTLR7-HA3x.
HEK293 TLR7 assay
Human embryonic kidney (HEK) 293 cells (CRL1573-ATCC) were seeded in 96 well plates in DMEM-GlutaMAX-I supplemented with 10% FBS. Eight hours post seeding, the cells were co-transfected with either human TLR7 (pUNO1-hTLR7-HA3x) or wTLR7 (pUNO1-wTLR7-HA3x) along with the NF-κB luciferase reporter pNiFty2-Luc (InvivoGen, San Diego, CA) using TransIT®-293 transfection reagent (Mirus, Pittsburgh, PA) according to the manufacturer’s instructions. Eighteen hours post-transfection the cells were stimulated with various concentrations of GS-9620 for 6 hours. Cells were lysed using ONE-Glo Luciferase assay system (Promega, Madison, WI) and NF-κB luciferase activity measured using a VICTOR × Light Luminescence plate reader (Perkin-Elmer, Waltham, MA).
Animals
Protocols were approved by the Institutional Animal Care and Use Committee of Cornell University. Neonatal woodchucks were infected at 3 days of age with a WHV7P1 inoculum containing 5 × 106 WID50 of WHV strain WHV7-11 and were then raised to adulthood prior to use in this study. All infected woodchucks were monitored serologically through 1 year post infection. At study start all had antibody to WHV core antigen (anti-WHc antibody), were negative for antibody to WHV surface antigen (anti-WHs antibody; WHsAb), had serum WHV DNA levels ≥109 ge/mL and detectable WHV surface antigen (WHsAg) at pretreatment, and were free of HCC by ultrasound liver examination and serum gamma glutamyl transferase (GGT). Uninfected control woodchucks were negative for anti-WHc antibody, anti-WHs antibody, WHsAg, and WHV DNA.
Single-dose pharmacokinetic (PK) and pharmacodynamic (PD) study in uninfected woodchucks
Uninfected healthy, adult male woodchucks (n=3 per dose group) were orally administered 1, 5, or 10 mg/kg of GS-9620. Evaluations included clinical observations, clinical pathology, pharmacokinetics and pharmacodynamics. Serum levels of GS-9620 were measured over time to calculate maximum serum concentration (Cmax) and exposure, calculated as area under the serum concentration versus time curve (AUClast).
Efficacy study in woodchucks chronically infected with WHV
The study design is described in Table 1. Briefly, five groups of woodchucks (n=7 per group, mixed sex) were treated with GS-9620 for approximately 4 weeks (groups 1, 2 and 3) or 8 weeks (groups 4 and 5) and evaluations continued through 35 weeks (time of scheduled euthanasia). Woodchucks that developed HCC were euthanized; HCC was diagnosed by ultrasound and serum GGT and confirmed at necropsy. Woodchucks that died unexpectedly during the study were evaluated by necropsy. Serum GS-9620 levels were evaluated at week 4, 4 hours post-dose, the approximate time of peak exposure.
Table 1.
Design of GS-9620 efficacy study.
| Group | Animals (n=7/group) |
Intended Regimena | Dose (mg/kg) |
|---|---|---|---|
| 1 | WHV infected, placebo control | QOD × 4 weeks | 0 |
| 2 | Uninfected, GS-9620 treated | QOD × 4 weeks | 5 (5 doses) 2.5 (9 doses) |
| 3 | WHV infected, GS-9620 treated | QOD × 4 weeks | 5 (5 doses) 2.5 (9 doses) |
| 4 | QOD every other week (QOW) for 8 weeks | 5 (4 doses) 2.5 (12 doses) |
|
| 5 | QW × 8 every week for 8 weeks | 5 (8 doses) |
. QOD, every other day; QW, once a week; QOW, every other week.
Determination of GS-9620 in serum
Woodchuck serum was mixed with acetonitrile containing an internal standard. After protein precipitation and centrifugation, the supernatant was mixed with water with 0.1% formic acid. An aliquot was injected to a TSQ Ultra Quantum LC/MS/MS system (Thermo Finnigan, San Jose, CA).
Clinical pathology
Clinical pathology was assessed at pretreatment, approximately weekly during treatment (24 hours post-dose), at least 1 and 2 weeks after the last dose, and approximately monthly through the end of the 35 week study [13].
WHV serum viremia levels
WHV DNA was quantified by two different methods depending on concentration: dot blot hybridization or real time PCR assay on a 7500 Real Time PCR System instrument (Applied Biosystems, Foster City, CA) as described previously [14].
Hepatic levels of WHV nucleic acids
Liver levels of WHV DNA replicative intermediates (RI), WHV cccDNA, and WHV RNA were determined in liver biopsies collected 2 weeks prior to treatment and at select time-points during and after treatment. Biopsies were obtained under general anesthesia with a 16-gauge needle directed by ultrasound imaging. Biopsy specimens for WHV nucleic acid analyses were placed immediately in liquid nitrogen and stored at −70°C. WHV RNA was measured quantitatively by Northern blot hybridization as previously described [15, 16]. WHV DNA RI and WHV cccDNA was quantitatively determined by Southern blot hybridization as previously described [17]. WHV cccDNA levels were expressed as a percentage change from pre-treatment level.
WHV serology
WHsAg and anti-WHs antibody were assessed approximately weekly through week 10 and then monthly through week 35 using WHV-specific enzyme immunoassays [18]. Serum WHsAg was quantified by ELISA against a standard curve for purified WHsAg (lower sensitivity: ca. 30 ng/mL serum). Anti-WHsAb titer was quantified by ELISA as described [18].
Statistical analyses for repeat-dose study
Analysis of variance (ANOVA) models using SAS PROC GLM were performed to compare treatment effects of continuous PD response variables and serum and liver viral load variables. ANOVA was also performed to relate binary endpoints, such as HCC incidence with PD response and with serum and liver viral load variables. Pearson correlation coefficients, using SAS PROC CORR, were calculated for PD response variables with viral load reduction, liver WHV DNA RI, RNA, and cccDNA reduction.
Pharmacodynamic (PD) analyses
Methods for quantitative RT-PCR and RNA-Seq analysis are provided in the Supplementary Materials.
RESULTS
Single Dose Pharmacokinetics, Pharmacodynamics and Tolerability of GS-9620 in Uninfected Adult Woodchucks
After single oral doses of 1, 5 and 10 mg/kg, the average Cmax of GS-9620 was 4.8 ± 1.4 nM, 23.6 ± 27.0 nM and 660 ± 907 nM, respectively, with an average Tmax at 3.7, 5.5 and 3.3 hours, respectively. GS-9620 exposure was roughly dose-proportional at 1 and 5 mg/kg but greater than proportional at 10 mg/kg (Supplementary Fig. 2a). The pharmacodynamic (PD) response was determined by measuring induction of mRNA levels of the interferon-stimulated genes (ISGs) 2’5’-OAS and MxA in whole blood (Supplementary Fig. 2b and c, respectively). Reagents to measure woodchuck serum IFN-α protein were not available, however 2’5’-OAS and MxA RNA levels are sensitive and established markers of IFN-α induction in other species. In uninfected woodchucks, the average maximum fold increase from pretreatment was 6.7, 11.8 and 11.0 for 2’5’-OAS after GS-9620 doses of 1, 5 and 10 mg/kg, respectively, and 30.1, 35.6 and 45.2, respectively, for MxA, with peak induction at 24 hours (Supplementary Fig. 2b and c). GS-9620 demonstrated a similar dose responsive induction of 2’5’-OAS and MxA in PBMCs across other species, including cynomolgus monkeys, chimpanzees and humans (Supplementary Table 1) [10–12]. GS-9620 has comparable in vitro potency for woodchuck and human TLR7 (Supplementary Fig. 1).
GS-9620 induced transient increases in body temperature in at least some woodchucks at each dose level (1, 5 and 10 mg/kg). In addition, at the 5 and 10 mg/kg, GS-9620 induced transient hematological changes, including reversible decreases in platelets (26% and 75% decrease at 5 and 10 mg/kg, respectively) and lymphocytes (72% decrease at 10 mg/kg). Transient lymphocyte changes were consistent with trafficking and/or margination associated with TLR7 induced chemokines [19, 20]. Based on these single dose assessments, 5 mg/kg was chosen for the efficacy study.
GS-9620 Efficacy Study in Woodchucks Chronically Infected with WHV
The efficacy study evaluated adult woodchucks (12–14 months of age) chronically infected with WHV. In order to model vertical transmission in humans, chronic infection in these adult animals was established by neonatal WHV infection (see Materials and Methods). As described in Table 1, five groups of woodchucks (n=7 per group, both males and females) were treated with either GS-9620 (groups 2–5) or placebo (group 1). Groups 3–5 comprised chronically infected animals treated with GS-9620 at different schedules. Control groups included WHV-infected animals treated with placebo (group 1), and uninfected animals treated with GS-9620 (group 2). Due to thrombocytopenia noted during the first 2 weeks of GS-9620 treatment in some animals (data not shown), dosing was interrupted for 9 to 10 days for groups 2 and 3, and then reinitiated with dose reduction to 2.5 mg/kg in groups 2, 3 and 4. Group 5, treated weekly, was not dose reduced. No further changes in dose or schedule occurred. Treatment duration was thus approximately 4 weeks (groups 1, 2 and 3) or 8 weeks (groups 4 and 5) and evaluations continued through 35 weeks (time of scheduled euthanasia).
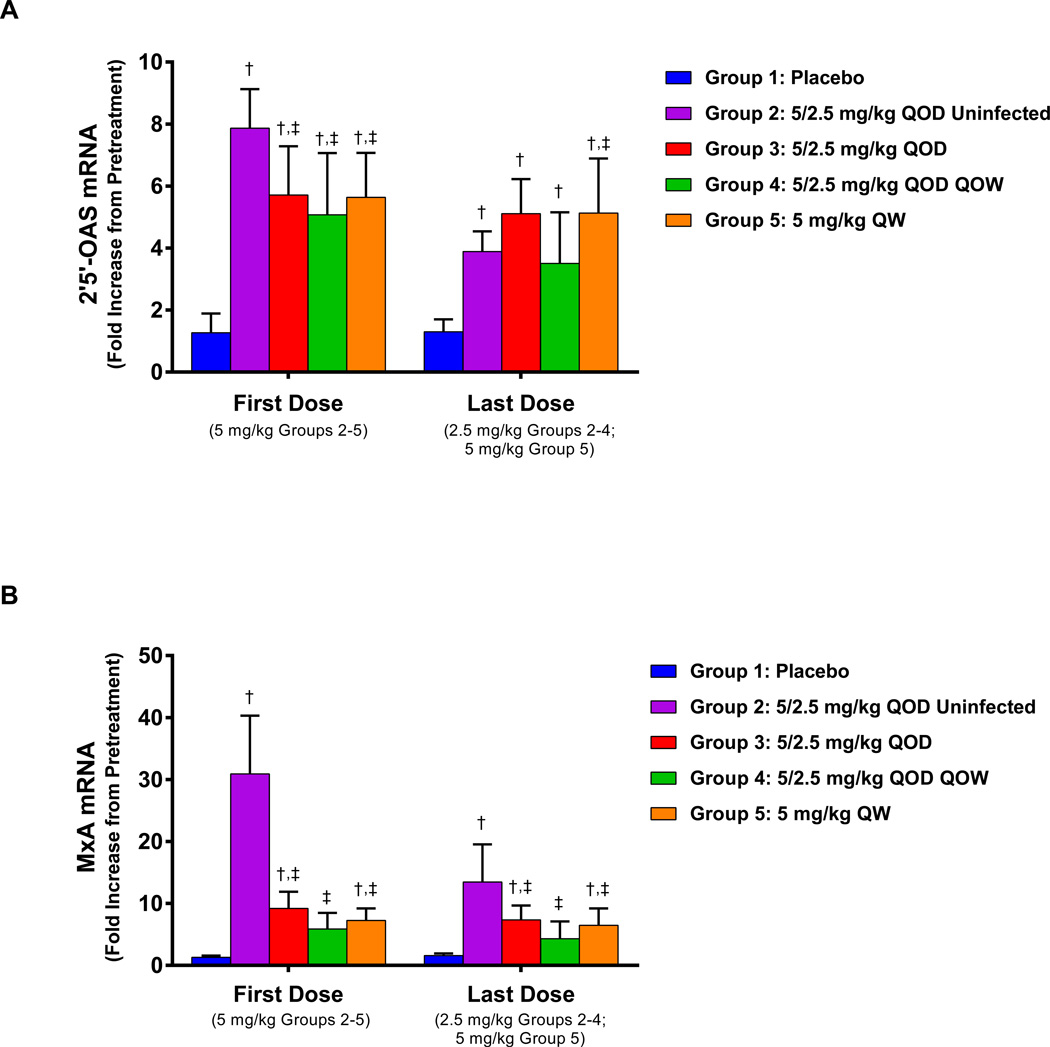
The serum exposure of GS-9620 at 5 and 2.5 mg/kg (Supplementary Table 2) was consistent with single-dose data in uninfected woodchucks (Supplementary Fig. 2a). GS-9620 administration induced statistically significant (p<0.05) increases in 2’5’-OAS and MxA RNA transcripts in all treated animals (Fig. 1, Supplementary Fig. 3) with the exception of four WHV-infected animals in group 4 (QOD × 8 weeks). Because of these four non-responding animals, group 4 generally had lower but still significant (p<0.05) induction of 2’5’-OAS, but did not have significant induction of MxA. Induction of 2’5’-OAS and MxA was statistically significantly higher in GS-9620-treated uninfected animals (group 2) versus WHV-infected woodchucks (groups 3, 4 and 5) (all p<0.05).
Fig. 1. Pharmacodynamic response to GS-9620 administration.
Mean fold increase in (A) 2′5′-OAS and (B) MxA transcript levels after the first and last doses of GS-9620 administration. † denotes statistical significance vs. group 1 (infected, placebo treated). ‡ denotes statistical significance vs. group 2 (uninfected, GS-9620 treated). QOD, every other day; QOW: every other week; QW: every week.
Tolerability of GS-9620 Treatment in Woodchucks Chronically Infected with WHV
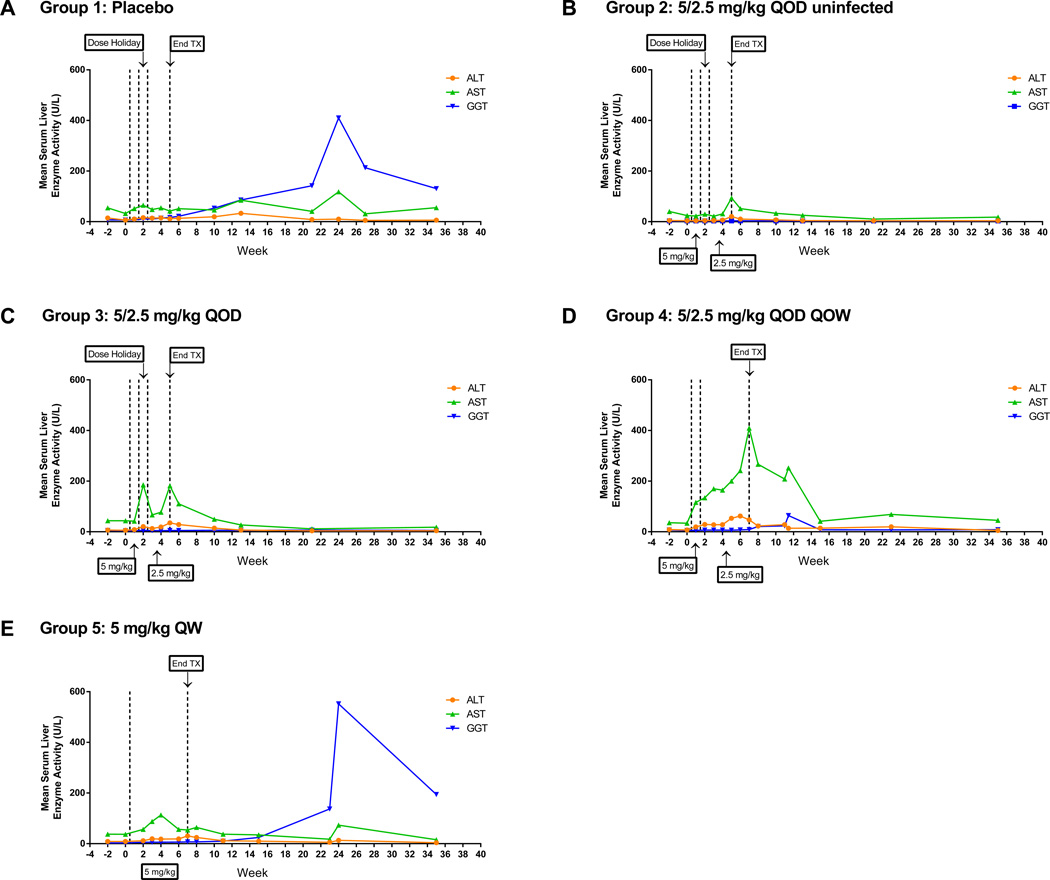
The most common adverse effects were transient, dose-dependent thrombocytopenia and increases in serum liver enzymes (AST, ALT, GGT and SDH). Reductions in platelets occurred primarily at 5 mg/kg and, when present, recovered between weekly treatments. Increases in serum liver enzymes occurred in all study groups including placebo-treated WHV-infected woodchucks, but were more consistent in WHV-infected, GS-9620-treated woodchucks (Fig. 2, Supplementary Fig. 8). The increases in liver enzymes observed in placebo-treated, WHV-infected woodchucks were likely related to WHV infection, as described previously [21]. In GS-9620-treated, WHV-infected woodchucks, transient serum liver enzyme increases generally occurred mainly during the treatment period and coincided with viral load reduction (Fig. 2, Supplementary Fig. 8). Liver enzyme increases were likely due to a combination of spontaneous increases associated with WHV infection as noted in placebo-treated woodchucks (group 1), possible GS-9620 effects as observed in GS-9620-treated, uninfected woodchucks (group 2), or hepatocellular effects related to an antiviral immune response.
Fig. 2. Serum ALT, AST and GGT levels in all study groups.
Group mean serum liver enzyme levels (U/L) over the course of the study. The vertical outer dotted lines represent the treatment period and, when present, the inner vertical dotted lines represent dose holiday. Tx: treatment. The increases in GGT in groups 1 and 5 correlated with the development of HCC in five animals in group 1 and two animals in group 5. Individual animal data is shown in Supplementary Fig. 8.
No deaths occurred in GS-9620-treated uninfected woodchucks (group 2) or in GS-9620-treated, WHV-infected woodchucks treated for approximately 4 weeks (group 3). Two woodchucks in the placebo-treated WHV-infected group and one GS-9620-treated WHV-infected animal in Group 5 were euthanized due to HCC. A non-treatment related death occurred in group 5 from hemorrhage caused by a liver biopsy procedure. Mortality or euthanasia occurred in two other WHV-infected woodchucks treated with GS-9620 for 8 weeks, one at the end of treatment (group 5) and one during the post-treatment evaluation period (group 4). The former animal had microscopic findings consistent with a bacterial/viral infection that likely contributed to the death. The other animal, based on its clinical chemistries and liver pathology (chronic inflammation, necrosis and nodular regeneration) had diminished liver function which contributed to its poor clinical condition leading to euthanasia. Because of the complex disease pathogenesis of chronic WHV infection in woodchucks and inflammatory processes associated with chronic infection, the role of GS-9620 treatment in these two mortalities is undetermined.
Antiviral Efficacy of GS-9620 Treatment in Woodchucks Chronically Infected with WHV
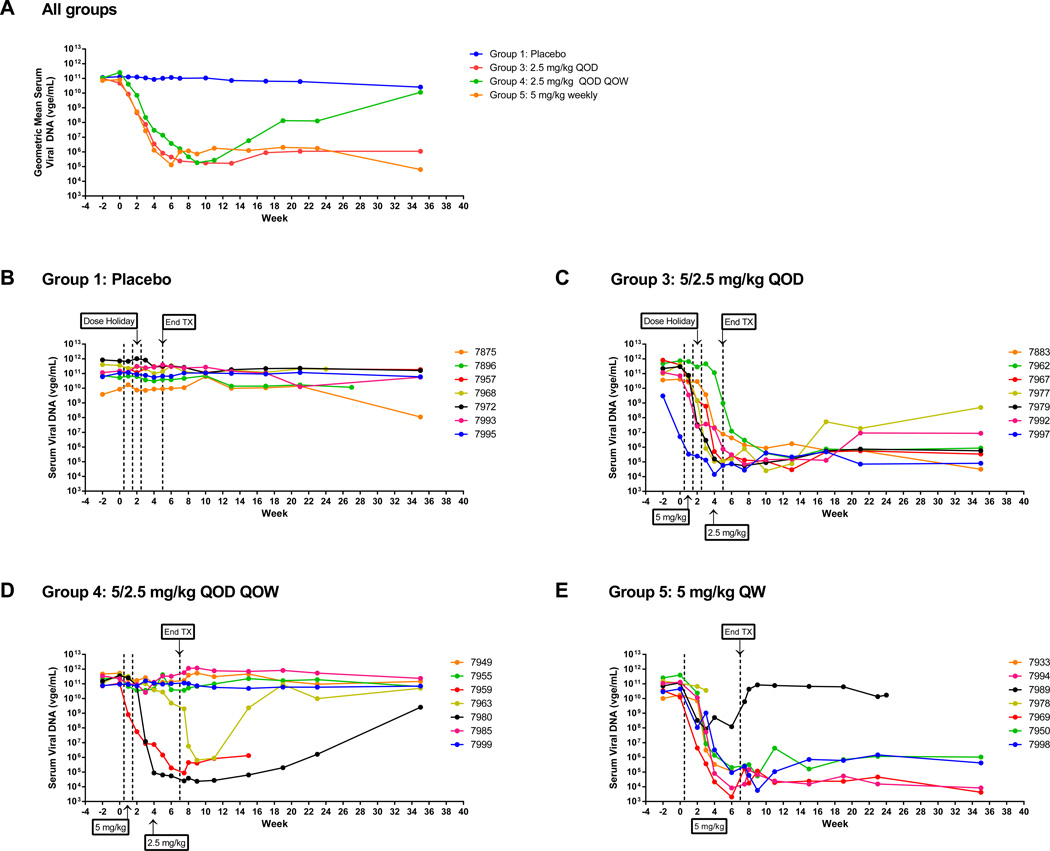
GS-9620 treatment induced marked, sustained reductions in serum WHV DNA noted as early as the first week of treatment. GS-9620 induced reductions in viremia occurred in nearly all GS-9620-treated woodchucks by week 4 (Fig. 3 and Table 2). In contrast, the placebo group had no changes in serum WHV DNA. WHV-infected woodchucks treated QOD for 4 weeks (group 3) had a uniformly marked antiviral response with a mean maximal viral load reduction of 6.2 log10. At the end of the study (week 35), this group had a mean 4.8 log10 reduction in viral load. Mean viral load in this group during weeks 3–35 were significantly reduced compared to pretreatment (all p<0.05) and the placebo control group at weeks 2–35 (all p<0.05). Similar efficacy occurred animals that completed weekly (QW) GS-9620 treatment for 8 weeks (group 5) (Fig. 3 and Table 2). In this group (group 5), GS-9620 treatment caused statistically significant reductions in viral load compared to pretreatment and the placebo control group at weeks 2–35 (all p<0.05) with a mean maximal viral load reduction of 6.1 log10 and a sustained mean 5.0 log10 viral load reduction at the end of the study (week 35). Strikingly, after completion of only 4 or 8 weeks of GS-9620 treatment, suppression of viral load was sustained through the end of the study (week 35) in all woodchucks in group 3 (QOD × 4 weeks), and in 4 of 5 woodchucks in group 5 (QW × 8 weeks) that completed treatment (Fig. 3c and e).
Fig. 3. Serum viral DNA levels in WHV-infected woodchucks.
(A) Group geometric means and (B–E) individual animal levels. Geometric means were calculated from animals that completed treatment and had an antiviral response to GS-9620 in the treatment groups (≥1 log10 reduction in serum viral load at more than one time point evaluated; Table 2). Group 2 animals were not infected with WHV and were therefore not included in the plots. Tx: treatment.
Table 2.
Summary of efficacy parameters and hepatocellular arcinoma (HCC) incidence in GS-9620 treated woodchucks.
| Group | Mean Maximal Viral Load Log Reduction in Animals with Viral Responsea |
Number of Animals | HCC Incidence | |||
|---|---|---|---|---|---|---|
| Sustained Viral Responseb |
Loss of Serum WHsAg |
Anti-WHs Antibody |
All Animals |
Animals with Sustained Response |
||
| 1 | N/A | 0/7 | 0/7 | 0/7 | 5/7 | N/A |
| 2 | N/A | N/A | N/A | N/A | N/A | N/A |
| 3 | 6.2 ± 1.1 (n=7) |
7/7 | 7/7 | 3/7 | 0/7 | 0/7 |
| 4 | 6.0 ± 0.8 (n=3)c |
1/3 | 2/3 | 1/3 | 0/3 | 0/1 |
| 5 | 6.1 ± 1.8 (n=5)d |
4/5 | 4/5 | 3/5 | 2/5 | 1/4 |
. Animals with a viral response were defined as any animal that had ≥1 log10 reduction in serum viral load at more than one time point evaluated; data shown are from the animals with a viral response that completed treatment (mortality occurred in two animals in group 5 during the treatment phase). The serum viral load was determined by PCR and the limit of detection for the assay was 1×103 virus genome copies (vge)/mL [14].
. Sustained viral response were defined as a ≥ 2 log10 reduction in serum viral load at the end of study (week 35).
. Three group 4 animals (n=7 total) were defined as having a viral response to treatment.
. Group 5 values were derived from the 5 of 7 animals that completed the 8 weeks of treatment.
In woodchucks treated QOD every other week (QOD QOW) (group 4), three of seven animals had marked decreases in viral load (Fig. 3d). Two of these three animals survived to week 35; one maintained a 2 log10 decrease in viral load, whereas viremia returned to pretreatment levels by week 18 in the other animal (Fig. 3d). These three woodchucks had a PD response comparable to GS-9620-treated WHV-infected woodchucks in groups 3 and 5, whereas the four woodchucks in this group that did not have a reduction in viral load had a lower PD response based on MxA and 2’5’-OAS RNA induction (Supplementary Fig. 3d, 3i and 3k). It is not clear why these four animals had lower PD responses to GS-9620, although drug exposure (Supplementary Table 2), animal gender and relative tumor burden (Supplementary Fig. 8) do not appear to be contributing factors.
GS-9620 Treatment Reduced the Hepatic Levels of WHV Nucleic Acids
Compared with placebo-treated woodchucks, levels of hepatic WHV DNA replicative intermediates (RI), WHV cccDNA, and WHV RNA were significantly (all p<0.005) decreased in groups 3 and 5 at week 35, but not in group 4 (Fig. 4, Supplementary Figs. 4–6). MxA and 2’5’-OAS RNA induction negatively correlated with the maximal reduction in serum WHV DNA, liver WHV RI, and WHV cccDNA (all p< 0.0001; r2 >0.55) and positively correlated with liver WHV RNA (p<0.0001; r2 >0.48).
Fig. 4. Intrahepatic levels of (A) WHV DNA replicative intermediates (RI), (B) WHV cccDNA, and (C) WHV RNA in WHV-infected woodchucks.
Geometric means were calculated from animals that completed treatment and had an antiviral response to GS-9620 in the treatment groups (≥1 log10 reduction in serum viral load at more than one time point evaluated; Table 2). Group 2 animals were not infected with WHV and were therefore not included in the plots. The p-values denote the level of statistical significance at week 35 relative to the placebo group.
GS-9620 Treatment Reduced Serum WHsAg Levels
GS-9620 treatment caused pronounced and sustained reductions in WHsAg (Fig. 5a and b, Supplementary Fig. 7). Through week 35, GS-9620 treatment of group 3 woodchucks (QOD × 4 weeks) induced complete loss of detectable (<30 ng/mL) WHsAg in all animals (7 of 7 woodchucks). Loss of detectable WHsAg also occurred in two of the three group 4 woodchucks (QOD × 8 weeks) with a viral response to treatment, and four of five group 5 woodchucks (QW × 8 weeks) that completed treatment. Loss of detectable WHsAg occurred as early as 2 weeks after the initiation of treatment. Mean WHsAg was significantly reduced in groups 3 and 5 at weeks 3–35 and weeks 2–35, respectively, when compared with the pretreatment level (all p<0.05), and at weeks 1–35 and 2–35, respectively, when compared with the placebo control (all p<0.05).
Fig. 5. Serum WHsAg and anti-WHs antibody levels in WHV-infected woodchucks.
Geometric mean serum WHsAg in animals that (A) completed treatment, and (B) had a sustained viral response. Sustained antiviral response was defined as ≥2 log10 reduction in viral load at Week 35 (Table 2). Note that the animals that were not sustained antiviral responders, also did not have a robust reduction in serum WHsAg (Supplementary Fig. 8). Group 2 animals were not infected with WHV and were therefore not included in the plots. (C) Seroconversion to anti-WHs antibody in animals in groups 3, 4, and 5. Placebo-treated woodchucks had no detectable antibody response.
GS-9620 Treatment Induced Anti-WHs Antibody Seroconversion in a Subset of Animals
Seroconversion to WHsAb was observed in a subset of GS-9620-treated woodchucks, but not in placebo-treated woodchucks (Fig. 5c). Anti-WHsAb was detected in 3 of 7 woodchucks in group 3, and 3 of 5 woodchucks in group 5. In these animals, anti-WHsAb was first noted as early as week 2, and as late as week 21. Anti-WHsAb was also detected in 1 of the 3 woodchucks in group 4 that had viral load reduction in responses to treatment. Anti-WHsAb titers in most woodchucks increased gradually through week 35.
GS-9620 Treatment Reduces the Incidence of Hepatocellular Carcinoma in Woodchucks Chronically Infected with WHV
The incidence of HCC was markedly reduced in GS-9620 treated woodchucks (Table 2). In placebo-treated WHV-infected woodchucks, HCC occurred in 5 of 7 (71%) woodchucks, consistent with the previous observation that nearly all chronic WHV carriers develop HCC over time [22]. In contrast, for all GS-9620 treated woodchucks that completed treatment and had a viral load reduction in response to treatment, the incidence was 2 of 15 (13%). Importantly, in GS-9620 treated woodchucks that had a sustained reduction in viral load, the incidence of HCC was only 1 of 12 (8%) (Table 2).
Intrahepatic Transcriptional Response to GS-9620 Treatment
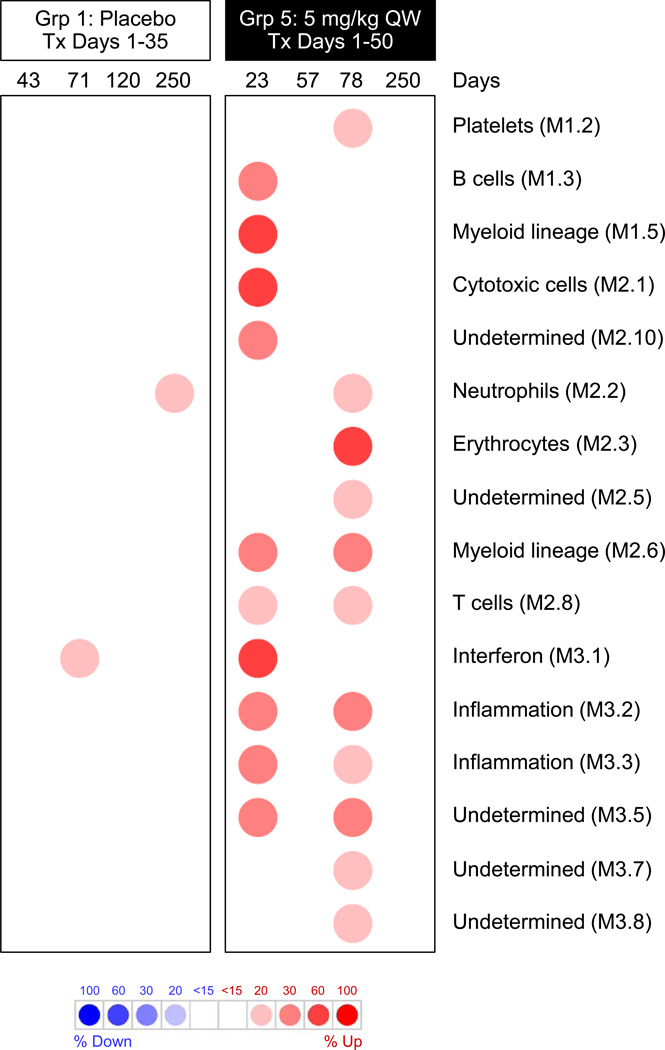
As outlined in Supplementary Fig. 9, intrahepatic transcriptional profiles of placebo-treated (group 1) and GS-9620-treated (group 5; 5 mg/kg QW) animals were determined by RNA-Seq at various times during the study, and were characterized by a gene module approach [23] and Ingenuity Pathway Analysis (IPA). Modular analysis (Fig. 6) revealed that GS-9620 treatment induced cytotoxic cell (CD8+ T cell/NK cell) (Module, M2.1), T cell (M2.8), myeloid cell lineage (M1.5 and M2.6), B cell (M1.3), and IFN response (M3.1) intrahepatic transcriptional signatures. In contrast, there was modest induction of only neutrophil (M2.2) and IFN response (M3.1) gene signatures in the liver of placebo-treated animals (Fig. 6). The moderate intrahepatic induction of these two gene signatures was likely related to WHV infection, as described previously [4]. The induction of intrahepatic CD8+ T, NK and B cell transcriptional signatures by GS-9620-treatment was confirmed by IPA (Supplementary Fig. 10), and is consistent with differential expression of T cell (CD3D and CD8A), NK cell (NKG7) and B cell (CD79B) markers (Supplementary Fig. 11). Taken together with the elevation in liver enzymes associated with GS-9620 treatment (Fig. 2) and the differential expression of perforin (PRF1) (Supplementary Fig. 11), these data suggest that the antiviral response induced by GS-9620 was likely mediated, at least in part, by the cytolytic activity of CD8+ T cells and/or NK cells. In addition, the intrahepatic induction of IFN-γ-stimulated genes (CXCL9, ubiquitin D; UBD) (Supplementary Fig. 11) as well as various IFN-α/β-stimulated genes (M3.1, Fig. 6) [4], suggests that non-cytolytic mechanisms may also play an important role in the antiviral response to GS-9620 treatment.
Fig. 6. Characterization of intrahepatic transcriptional signature associated with GS-9620 treatment.
Modular analysis of transcriptional signatures in the liver of placebo-treated (group 1) and GS-9620-treated (group 5; 5 mg/kg QW) animals relative to pre-treatment (day -14). Columns represent different study days. Spot intensity (red: over-expressed; blue: under-expressed) denotes the percentage of transcripts significantly changed in each module (M) and is defined by the scale bar. Only modules for which >15% of module genes were differentially expressed (absolute fold-change > 1.5 with a Benjamini-Hochberg corrected FDR<0.05, and passed a low expression threshold) are displayed. The functional interpretation of each module (taken from reference [23]) is displayed on the right.
DISCUSSION
Treatment of WHV-infected woodchucks with GS-9620 resulted in rapid, marked and sustained antiviral response. GS-9620 pharmacodynamic responses were consistent with activation of TLR7 and correlated statistically with antiviral efficacy. Importantly, the magnitude of ISG induction in chronically infected woodchucks was similar to that induced in humans at well tolerated doses (Supplementary Table 1) [11]. Strikingly, in most chronically infected animals GS-9620 induced a multi-log reduction in viral load, WHsAg loss and anti-WHs antibody seroconversion, and reduced cccDNA levels and HCC incidence. Specifically, reductions in viral load occurred in 15 of 19 woodchucks that completed treatment (regardless of regimen) and viral load reductions were sustained through the end of the study in 12 of these 15 woodchucks. Sustained WHsAg loss occurred in 13 of 15 woodchucks that completed treatment. Importantly, in 8 of 13 treated woodchucks with WHsAg loss, induction of WHsAb occurred and persisted. In GS-9620 treated woodchucks that had a sustained reduction in viral load, the incidence of HCC was less than 10% compared to a rate of over 70% in the placebo group.
The antiviral responses induced by GS-9620 are clearly differentiated from those observed with nucleos(t)ides evaluated in the woodchuck model of CHB. While the duration of viral load reduction was markedly longer with GS-9620 compared to similar treatment with nucleos(t)ides, the most important finding was that, in contrast to GS-9620, nucleos(t)ide treatment induced a sustained loss of WHsAg in only a small minority of WHV-infected woodchucks and induction of anti-WHsAb was rare [3, 14]. Other therapeutic strategies in this model have included therapeutic vaccination, IL-12 gene therapy and inhibition of PD-L1 [24–26]. Although, in some instances, these therapies have yielded transient reductions in viral load, WHsAg and induction of anti-WHsAb, no single agent has been reported to induce a comparable antiviral response to GS-9620. Most notably, while treatment of chronically infected woodchucks with recombinant woodchuck IFN-α strongly reduced serum WHV DNA and WHsAg in some treated animals, this was a transient response that was not accompanied by anti-WHsAb seroconversion in most woodchucks (Menne S, unpublished data).
Similar to a previous report in HBV-infected chimpanzees [10], GS-9620 treatment in woodchucks was associated with thrombocytopenia and liver enzyme increases. These adverse effects were transient, reversed with dose holiday, had reduced incidence and severity with lower doses, and were monitorable by routine clinical laboratory parameters. Increases in liver enzymes were noted in some animals in all groups, including placebo-treated animals, and were reversible in most cases without dose reduction or treatment cessation. The mechanism by which GS-9620 treatment caused platelet reductions in woodchucks is not clear. However, recent reports show that TLR7 is expressed in platelets, that TLR7 activation can induce a reduction in platelet count with no influence on thrombotic properties, and these effects enhanced host immune response and survival to viral infection [27]. As elevations in liver enzymes noted in GS-9620 treated animals were temporally associated with reductions in hepatic cccDNA, liver enzyme increases may represent immune-mediated killing of infected hepatocytes. This is consistent with the induction of an intrahepatic cytotoxic CD8+ T cell and/or NK cell transcriptional signature during GS-9620 treatment. Notably, transient increases in liver enzymes often precede clearance of acute hepadnavirus infection in woodchucks, chimpanzees and humans, and are thought to represent development of effective intrahepatic antiviral immunity [28–30].
The results demonstrate that short duration treatment with GS-9620 can induce a durable therapeutic anti-viral immune response during chronic hepadnavirus infection. In a previous study in chimpanzees chronically infected with HBV, GS-9620 treatment caused a sustained, albeit less marked reduction in viral load and HBsAg [10]. While studies are ongoing to define the cellular and molecular characteristics of the antiviral response to GS-9620, it likely involves local induction of IFN-α, as well as induction of antiviral NK and CD8+ T cell responses [8, 31, 32]. In addition, direct activation of B cells by GS-9620 may also be an important mechanism that contributes to viral clearance [33].
In summary, short duration treatment with GS-9620 induced a sustained antiviral response and anti-WHs antibody seroconversion in the woodchuck model of CHB. These data suggest that GS-9620 may have the potential to therapeutically induce sustained immunological control of chronic HBV infection in patients.
Supplementary Material
Acknowledgements
The authors acknowledge Mary A. Ascenzi, Lou Ann Graham, and Erin Graham from Cornell University, as well as Yun Wang and Junming Yang from Georgetown University. Becky Norquist provided medical writing services to Gilead Sciences.
Conflict of interest: This study was sponsored by Gilead Sciences, Inc. D Tumas, R Halcomb, G Wolfgang, A Fosdick, J Zheng, L Li, P Yue, S Daffis and S Fletcher are employees of Gilead Sciences, Inc.
Financial Support: This work was supported by contract N01-AI-05399 to the College of Veterinary Medicine, Cornell University and contract HHSN272201000011I, task order HHSN27200001 (D01) to Georgetown University Medical Center from the National Institute of Allergy and Infectious Diseases (NIAID).
Abbreviations
- CHB
chronic hepatitis B
- TLR7
toll-like receptor 7
- WHV
woodchuck hepatitis virus
- HBV
hepatitis B virus
- WHsAg
WHV surface antigen
- HCC
hepatocellular carcinoma
- IFN-α
interferon-alfa
- HBsAg
hepatitis B surface antigen
- HBsAb
anti-HBs antibody
- pDC
plasmacytoid dendritic cell
- HEK
Human embryonic kidney
- WHsAb
anti-WHs antibody
- ge/ml
genome equivalents per milliliter
- GGT
gamma glutamyl transferase
- PK
pharmacokinetic
- PD
pharmacodynamic
- Cmax
maximum serum concentration
- AUClast
area under the serum concentration versus time curve
- RI
replicative intermediate
- 2'5'-OAS
2',5'-oligoadenylate synthetase
- MxA
myxovirus resistance A
- FDR
false discovery rate
- DEG
differentially expressed gene
- IPA
Ingenuity Pathway Analysis
- Tmax
time of maximum serum concentration
- ISG
interferon-stimulated gene
- QOD
every other day
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- SDH
sorbitol dehydrogenase
- QW
every week
- QOW
every other week
- M
module
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author's Contributions: Participated in research design: S. Menne, D. Tumas, R. Halcolmb, G. Wolfgang, B. Tennant. Conducted experiments: B. Baldwin, C. Bellezza (woodchuck procedures), P. Cote (serum WHsAg and anti-WHsAb quantitative data), S. Menne, K. Liu, L. Thampi, D. AlDeghaither, (pharmacodynamics sample analysis, serum WHV DNA quantitative data, hepatic WHV nucleic acid quantitative data), J. Zheng (pharmacokinetic sample analysis), S. Daffis (HEK293 TLR evaluation), L. Li, P. Yue, S. Fletcher (RNA-Seq bioinformatics analysis). Performed data analysis: A. Fosdick, S. Menne, D. Tumas, J. Zheng, G. Wolfgang, S. Fletcher. Wrote or contributed to the writing of the manuscript: A. Fosdick, D. Tumas, S. Menne, S. Fletcher.
RNA-Seq Data Archiving The RNA-Seq data has been deposited in the NCBI GEO under accession number (Accession TBD Prior to Publication).
REFERENCES
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Kwon H, Lok AS. Hepatitis B therapy. Nat Rev Gastroenterol Hepatol. 2011;8:275–284. doi: 10.1038/nrgastro.2011.33. [DOI] [PubMed] [Google Scholar]
- 3.Menne S, Cote PJ. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J Gastroenterol. 2007;13:104–124. doi: 10.3748/wjg.v13.i1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher SP, Chin DJ, Ji Y, Iniguez AL, Taillon B, Swinney DC, et al. Transcriptomic analysis of the woodchuck model of chronic hepatitis B. Hepatology. 2012;56:820–830. doi: 10.1002/hep.25730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 6.Birmachu W, Gleason RM, Bulbulian BJ, Riter CL, Vasilakos JP, Lipson KE, et al. Transcriptional networks in plasmacytoid dendritic cells stimulated with synthetic TLR 7 agonists. BMC Immunol. 2007;8:26. doi: 10.1186/1471-2172-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanten JA, Vasilakos JP, Riter CL, Neys L, Lipson KE, Alkan SS, et al. Comparison of human B cell activation by TLR7 and TLR9 agonists. BMC Immunol. 2008;9:39. doi: 10.1186/1471-2172-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh JZ, Kurche JS, Burchill MA, Kedl RM. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood. 2011;118:3028–3038. doi: 10.1182/blood-2011-04-348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roethle PA, McFadden RM, Yang H, Hrvatin P, Hui H, Graupe M, et al. Identification and optimization of pteridinone Toll-like receptor 7 (TLR7) agonists for the oral treatment of viral hepatitis. J Med Chem. 2013;56:7324–7333. doi: 10.1021/jm400815m. [DOI] [PubMed] [Google Scholar]
- 10.Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508–1517. 1517, e1501–e1510. doi: 10.1053/j.gastro.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopatin U, Wolfgang G, Tumas D, Frey CR, Ohmstede C, Hesselgesser J, et al. Safety, pharmacokinetics and pharmacodynamics of GS-9620, an oral Toll-like receptor 7 agonist. Antivir Ther. 2013;18:409–418. doi: 10.3851/IMP2548. [DOI] [PubMed] [Google Scholar]
- 12.Fosdick A, Zheng J, Pflanz S, Frey CR, Hesselgesser J, Halcomb RL, et al. Pharmacokinetic and pharmacodynamic properties of GS-9620, a novel Toll-like receptor 7 agonist, demonstrate interferon-stimulated gene induction without detectable serum interferon at low oral doses. J Pharmacol Exp Ther. 2014;348:96–105. doi: 10.1124/jpet.113.207878. [DOI] [PubMed] [Google Scholar]
- 13.Hornbuckle WE, Graham ES, Roth L, Baldwin BH, Wickenden C, Tennant BC. Laboratory assessment of hepatic injury in the woodchuck (Marmota monax) Laboratory animal science. 1985;35:376–381. [PubMed] [Google Scholar]
- 14.Menne S, Butler SD, George AL, Tochkov IA, Zhu Y, Xiong S, et al. Antiviral effects of lamivudine, emtricitabine, adefovir dipivoxil, and tenofovir disoproxil fumarate administered orally alone and in combination to woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob Agents Chemother. 2008;52:3617–3632. doi: 10.1128/AAC.00654-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tennant BC, Baldwin BH, Graham LA, Ascenzi MA, Hornbuckle WE, Rowland PH, et al. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepatitis B virus infection. Hepatology. 1998;28:179–191. doi: 10.1002/hep.510280124. [DOI] [PubMed] [Google Scholar]
- 16.Peek SF, Cote PJ, Jacob JR, Toshkov IA, Hornbuckle WE, Baldwin BH, et al. Antiviral activity of clevudine [L-FMAU, (1-(2-fluoro-5-methyl-beta, L-arabinofuranosyl) uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax) Hepatology. 2001;33:254–266. doi: 10.1053/jhep.2001.20899. [DOI] [PubMed] [Google Scholar]
- 17.Jacob JR, Korba BE, Cote PJ, Toshkov I, Delaney WEt, Gerin JL, et al. Suppression of lamivudine-resistant B-domain mutants by adefovir dipivoxil in the woodchuck hepatitis virus model. Antiviral Res. 2004;63:115–121. doi: 10.1016/j.antiviral.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Cote PJ, Roneker C, Cass K, Schodel F, Peterson D, Tennant B, et al. New enzyme immunoassays for the serologic detection of woodchuck hepatitis virus infection. Viral Immunol. 1993;6:161–169. doi: 10.1089/vim.1993.6.161. [DOI] [PubMed] [Google Scholar]
- 19.Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006 doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 20.Perkins H, Khodai T, Mechiche H, Colman P, Burden F, Laxton C, et al. Therapy with TLR7 Agonists Induces Lymphopenia: Correlating Pharmacology to Mechanism in a Mouse Model. J Clin Immunol. 2012;32:1082–1092. doi: 10.1007/s10875-012-9687-y. [DOI] [PubMed] [Google Scholar]
- 21.McKenzie E, Sun J, Jackson M, Gruwel ML. Non-invasive detection of cellular proliferation in the woodchuck model of hepatocellular carcinoma. Hepatology. 2005;42:343A. [Google Scholar]
- 22.Tennant BC, Toshkov IA, Peek SF, Jacob JR, Menne S, Hornbuckle WE, et al. Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology. 2004;127:S283–S293. doi: 10.1053/j.gastro.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Chaussabel D, Quinn C, Shen J, Patel P, Glaser C, Baldwin N, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29:150–164. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roggendorf M, Schulte I, Xu Y, Lu M. Therapeutic vaccination in chronic hepatitis B: preclinical studies in the woodchuck model. J Viral Hepat. 2007;14(Suppl 1):51–57. doi: 10.1111/j.1365-2893.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 25.Crettaz J, Otano I, Ochoa L, Benito A, Paneda A, Aurrekoetxea I, et al. Treatment of chronic viral hepatitis in woodchucks by prolonged intrahepatic expression of interleukin-12. J Virol. 2009;83:2663–2674. doi: 10.1128/JVI.02384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Zhang E, Ma Z, Wu W, Kosinska A, Zhang X, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS pathogens. 2014;10:e1003856. doi: 10.1371/journal.ppat.1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, et al. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802. doi: 10.1182/blood-2013-11-536003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Menne S, Baldwin BH, Tennant BC, Gerin JL, Cote PJ. Kinetics of viremia and acute liver injury in relation to outcome of neonatal woodchuck hepatitis virus infection. J Med Virol. 2004;72:406–415. doi: 10.1002/jmv.20019. [DOI] [PubMed] [Google Scholar]
- 29.Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289–1300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 31.Gorski KS, Waller EL, Bjornton-Severson J, Hanten JA, Riter CL, Kieper WC, et al. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int Immunol. 2006;18:1115–1126. doi: 10.1093/intimm/dxl046. [DOI] [PubMed] [Google Scholar]
- 32.Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci U S A. 2013;110:E3216–E3224. doi: 10.1073/pnas.1312348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.