Abstract
Background
Migraine is associated with a number of cardio-metabolic risk factors including abnormalities in lipid metabolism. However, little is known about these associations among pregnant migraineurs. We conducted the present study to evaluate the extent to which altered lipid profiles are associated with history of migraine among pregnant women.
Methods
A cohort of 1,062 Peruvian women were interviewed at 24-28 weeks of gestation. Migraine status was classified based on the International Classification of Headache Disorders (ICHD-2) diagnostic criteria. Serum lipid concentrations were measured enzymatically using standardized assays. Multivariable logistic regression was used to estimate adjusted odds ratios (AORs) and 95% confidence intervals (CIs) as measures of associations of migraine status with varying concentrations of lipids and lipoproteins during pregnancy.
Results
Approximately 18.5% of the study participants were identified as migraineurs (196 of 1,062). Maternal serum total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), triglycerides, and total cholesterol:HDL ratio were all statistically significantly elevated among pregnant migraineurs compared with pregnant non-migraineurs. In multivariate adjusted models pregnant women with migraine had higher odds of elevated total cholesterol, LDL, and total cholesterol:HDL ratio as compared with pregnant women without migraine. For instance the AOR and 95% CI for successive quartiles of the total cholesterol associated with history of migraine were Q2 (219-247 mg/dL): 1.05 (0.64-1.70), Q3 (248-281mg/dL): 1.16 (0.72-1.86), and Q4 (≥282mg/dL): 1.87 (1.20-2.91) with the lowest quartile (<219 mg/dL) as the referent group (p-value for trend= 0.003). Obese women with elevated total cholesterol (≥282 mg/dL) were more likely to be migraineurs (OR=3.71; 95% CI 1.58-8.71) as compared with non-obese women with lower total cholesterol (< 219 mg/dL). Similar elevated odds of migraine were observed for obese women with elevated LDL cholesterol, elevated triglycerides and high total cholesterol:HDL ratio.
Conclusion
Pregnant migraineurs had elevated odds of dyslipidemia, particularly hypercholesterolemia, elevated LDL, and total cholesterol:HDL ratio as compared with pregnant non-migraineurs. The observed associations were more pronounced among obese migraineurs. Our findings add to the accumulating evidence of adverse cardiometabolic risk profiles among migraineurs and extends these associations to pregnant women.
Introduction
Migraine and cardiometabolic disease risk factors are highly prevalent conditions with considerable morbidities and socio-economic burden to society.1, 2 Although the association between migraine and the risk of ischemic stroke has been well established in previous studies of men and non-pregnant women,3, 4 the pathophysiologic mechanisms underlying observed associations are poorly understood. Possible mechanisms for the comorbidities include common vascular pathophysiology and accompaniment of cardiometabolic stroke risk factors such as altered lipid profiles.5, 6 Elevated serum or plasma concentrations of atherogenic lipids and lipoproteins have been independently associated with migraine and migraine severity in most,6-11 but not all,12, 13 prior studies. Furthermore, platelet apoptosis, platelet activation and aggregation, platelet-leukocyte aggregate (PLA) formation and endothelial dysfunction are common to both disorders.14, 15 While dyslipidemia has been documented in men and non-pregnant women migraineurs, to the best of our knowledge, there is no published report concerning this association among pregnant women. The prevalence of migraine is known to be high among reproductive age and pregnant women.16-18 It is also known that lipid metabolism is dramatically altered during pregnancy 19, 20. In light of (1) the high burden of migraine among pregnant women, (2) alterations in lipid metabolism during pregnancy, and (3) evidence of associations of migraine with altered lipid profiles among men and non-pregnant women, we sought to evaluate the extent to which history of migraine is associated with maternal serum lipid and lipoprotein concentrations among pregnant women. We also explored the extent to which maternal pre-pregnancy obesity status acts as a potential effect modifier of these associations.
Materials and Methods
Participants and Study Setting
This analysis used data initially gathered for the Study of Screening, Treatment and Effective Management of Gestational Diabetes Mellitus (STEM-GDM), an ongoing prospective study that evaluates the prevalence of GDM using the new diagnostic criteria proposed by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) among Peruvian women attending perinatal care at Instituto Nacional Materno Perinatal (INMP) in Lima, Peru. The INMP, overseen by the Peruvian Ministry of Health, is the primary referral hospital for maternal and perinatal care. Recruitment began in February 2013. Women who initiated prenatal care before 28 weeks' gestation were eligible to participate. Women were ineligible if they were younger than 18 years of age, did not speak and read Spanish, did not plan to carry the pregnancy to term or deliver at INMP, and/or were past 28 weeks' gestation.
Enrolled subjects were asked to participate in a structured interview that gathered information regarding sociodemographic, lifestyle, medical, and reproductive characteristics. Fasting blood specimens were collected between 24-28 weeks gestation, processed, and stored until analysis. Detailed information about maternal sociodemographic characteristics, lifestyle habits, reproductive history and medical history were collected using in-person interviews completed between 24-28 weeks gestation (25 weeks gestation, on average). Following the interview, a brief physical examination was administered by a trained research nurse who took anthropometric measures including standing height and weight. All participants provided informed consent and the research protocol was approved by the Institutional Review Boards of the INMP, Lima, Peru and the Harvard T. H. Chan School of Public Health Office of Human Research Administration, Boston, MA, USA.
Analytical Population
The analytical population was derived from participants who enrolled in the STEM-GDM Cohort between February 2013 and February 2014. During this period, a total of 1,088 women participated in the study. A total of 26 women were excluded from the present analysis because of insufficient serum sample or missing headache information. Thus, 1,062 women remained for analysis. The 26 women excluded from this analysis did not differ in regards to sociodemographic and lifestyle characteristics as compared with those included.
Migraine Assessment
At the time of enrollment (25 weeks' gestation, on average), participants completed a questionnaire administered by trained interviewers that included a structured migraine assessment questionnaire based on the International Classification of Headache Disorders-II (ICHD-II) criteria 21 as previously described16. Migraine was classified by those fulfilling the following criteria: at least 5 lifetime headache attacks lasting 4-72 hours, with at least 2 of the qualifying pain characteristics (unilateral location, pulsating quality, moderate or severe pain intensity, aggravation by routine physical exertion), at least one of the associated symptoms (nausea and/or vomiting, photo/phonophobia), and not readily attributable to another central nervous system disorder or head trauma (according to participant self-report). Those fulfilling all but one of the definitive migraine criteria were classified as probable migraine. Additionally any migraine was defined as the total group with either definitive or probable migraine combined. Herein, participants with any migraine are referred to as migraineurs 21. Finally migraine subgroups were classified according to aura status (i.e., migraine with or without aura).
Serum Lipid Measurement and Laboratory Analytical Procedures
At or near the time of interview, a 7 ml fasting blood sample was collected. Blood was fractionated using standard procedures and stored at −80°C until analyzed. Total cholesterol, triglycerides, HDL and LDL testing were performed on the ARCHITECT c System™ [ci8200, Abbott Diagnostics Division, Abbott Park IL]. Total Cholesterol was measured enzymatically [cholesterol esterase]. Triglycerides were measured by the glycerol phosphate oxidase assay using ACS grade glycerol calibrators traceable to gravimeteric reference methods. HDL was directly measured using a homogeneous method [accelerator selective detergent assay]. HDL calibrators are traceable to the CDC Abell-Kendall reference method. LDL was directly measured using a homogeneous method [liquid selective detergent assay] traceable to the National Reference System for Cholesterol [NRS/CHOL]. Lipids and lipoproteins were measured at Clinical Laboratory Research Core in the Pathology Service at the Massachusetts General, Boston, MA. All assays were performed according to manufacturer's specifications and were monitored using commercial quality-control materials and materials received for proficiency testing from the College of American Pathologists. Analytical interassay coefficients of variation for cholesterol, triglyceride, HDL, and LDL cholesterol were 1.2%, 2.5%, 2.6% and 1.5%, respectively. All laboratory assays were completed without knowledge of participants' medical history.
Other Covariates
Maternal pre-pregnancy body mass index (BMI) was calculated as weight in kilograms divided by height in square meters (kg/m2). Categories of pre-pregnancy BMI were characterized as normal weight (<25 kg kg/m2), overweight (25-29.9 kg/m2), and obese (≥30 kg/m2). Maternal age was categorized as follows: <19, 20-29, 30-34, ≥35 years. Other social and demographic variables were categorized as: maternal education (≤6, 7-12, and >12 completed years of schooling), parity (primiparous vs. multiparous), marital status (married or living with partner vs. single or living alone/divorced), difficulties to pay for basics like food (very hard/hard, somewhat hard, and not very hard), and difficulties to access medical care (very hard/hard, somewhat hard, and not very hard).
Statistical Analysis
We examined the frequency distributions of maternal characteristics according to migraine status. We also examined the distribution of continuous variables (e.g. each lipid and lipoproteins) and found them to be approximately normal, hence we used Student's t-test to evaluate unadjusted mean differences according to migraine status. When making comparisons for categorical variables, we used the Chi-square test or the Fisher's exact test where appropriate. Adjusted mean differences of lipid concentrations according to maternal migraine status were determined using multivariate linear regression procedures.
To estimate the relative association between migraine and maternal serum lipid concentrations, we categorized each subject according to quartiles of each analyte determined by the distribution of concentrations among non-migraineurs. We used the lowest quartile as the reference group, and estimated odds ratios (OR) and 95 % confidence intervals (95 % CI) for each of the remaining three quartiles. Given the lack of established thresholds to define normal lipid profiles in pregnancy and nature of relationship with migraine, we used quartiles. By using quartiles, we were able to increase the sensitivity of the study and avoid missing true associations and their magnitude effects.22 To assess confounding, we entered covariates into a logistic regression model one at a time, then compared the adjusted and unadjusted odds ratios 23. Final logistic regression models included covariates that altered unadjusted odds ratios by at least 10%, as well as those covariates of a priori interest (e.g., maternal parity and pre-pregnancy BMI). In final multivariable models, we adjusted for maternal age, parity, and pre-pregnancy BMI.
We explored the possibility of a nonlinear relation between total cholesterol, LDL cholesterol, total cholesterol:HDL ratio and migraine odds, using generalized additive logistic regression modeling procedures (GAM) 24. S-Plus (version 6.1, release 2, Insightful Inc. Seattle, WA) was used for these analyses. We also assessed the joint and independent associations of maternal pre-pregnancy obesity (pre-pregnancy BMI < 30 vs. ≥ 30 kg/m2) and dyslipidemia with migraine status (i.e., elevated serum total cholesterol, elevated LDL, etc.). We categorized women into four groups based on combinations of whether or not their serum concentration for each biological marker was elevated. For these analyses elevated total cholesterol was defined as concentrations ≥282 mg/dL (i.e., the highest quartile among non-migraineurs). Elevated triglyceride was defined as concentrations ≥253 mg/dl (i.e., the highest quartiles among non-migraineurs). For the obesity and elevated cholesterol analysis, the four resulting categories were as follows: non-obese and no elevated cholesterol, obese only, elevated cholesterol only, and both obese and elevated cholesterol. Non-obese women with no elevated cholesterol comprised the reference group, against which women in the other three categories were compared. Similar analyses were conducted to assess the joint and independent effects of other lipid variables and obesity status. The magnitude and direction of associations for migraineurs with and those without aura were similar (data not shown). All analyses, with the exception of our GAM analysis, were performed using Stata 12.0 statistical software (Stata, College Station, TX). P-values <0.05 were considered statistically significant. All reported confidence intervals were calculated at the 95% level. All reported p-values are two-tailed.
Results
A total of 1,062 pregnant women between the ages of 18 and 45 years (mean age=28.6 years, standard deviation=6.1 years) participated in the study. The majority of the cohort were married or living with their partner (87.4%) while one third of the cohort (31.9%) reported being employed during pregnancy. Approximately 49% of participants reported difficulties paying for basics (48.5%) while 64.7% reported food insecurities and 88.5% reported difficulties in accessing medical care. Approximately 18.5% of the cohort fulfilled migraine criteria (196 of 1,062). Characteristics of participants according to migraine status are presented in Table 1. Pregnant women with migraine were more likely to be multiparous as compared with non-migraineurs. Other characteristics including maternal age, marital status, education, employment status, gestational age, and pre-pregnancy BMI were similar for pregnant women with and without a history of migraines.
Table 1. Maternal socioeconomic and anthropometric characteristics according to categories of maternal migraines status, in Lima, Peru, 2014 (N = 1062).
| No Migraine (N = 866) n (%) |
Yes Migraine (N = 196) n (%) |
P-value* | |
|---|---|---|---|
| Maternal age (years) | 28.5 ± 6.3 | 29.0 ±5.5 | 0.37† |
| Maternal age (years) | |||
| <19 | 41 (4.7) | 2 (1.0) | 0.08 |
| 20-29 | 452 (52.2) | 109 (55.6) | |
| 30-34 | 202 (23.3) | 49 (25.0) | |
| ≥35 | 171 (19.8) | 36 (18.4) | |
| Primiparous | 314 (36.3) | 47 (24.0) | 0.001 |
| Single or living alone/divorced | 117 (13.5) | 17 (8.7) | 0.15 |
| Employed during pregnancy | 279 (32.2) | 60 (30.6) | 0.66 |
| Smoking during pregnancy | 12 (1.4) | 0 (0.0) | --- |
| Drinking during pregnancy | 20 (2.3) | 7 (3.6) | 0.31 |
| Women education in years | |||
| ≤6 | 25 (2.9) | 9 (4.6) | 0.45 |
| 7-12 | 465 (53.7) | 101 (51.5) | |
| >12 | 376 (43.4) | 86 (43.9) | |
| Difficulties to pay basics | |||
| Very hard/hard | 128 (14.8) | 31 (15.8) | 0.54 |
| Somewhat hard | 293 (33.8) | 62 (31.6) | |
| Not very hard | 444 (51.3) | 102 (52.0) | |
| Difficulties to pay medical care | |||
| Very hard/hard | 161 (18.6) | 43 (21.9) | 0.65 |
| Somewhat hard | 604 (69.8) | 131 (66.8) | |
| Not very hard | 100 (11.5) | 22 (11.2) | |
| Pre-pregnancy BMI (kg/m2) | 25.0 ± 3.7 | 25.4 ± 4.4 | 0.22† |
| Pre-pregnancy BMI (kg/m2) | |||
| <25 (Normal weight) | 485 (56.0) | 104 (53.1) | 0.06 |
| 25-29.9 (Overweight) | 298 (34.4) | 62 (31.6) | |
| ≥30 (Obese) | 83 (9.6) | 30 (15.3) | |
| Pregnancy BMI at OGTT (kg/m2) | 27.5 ± 3.7 | 27.6 ± 3.9 | 0.73† |
| Gestational age at interview (weeks) | 25.5 ± 1.2 | 25.5 ± 1.2 | 0.85† |
Data were presented in mean ± standard deviation (SD) or number (%).
Chi-square test /Fisher's Exact test ;
Student's t test
Maternal serum total cholesterol and LDL cholesterol concentrations were significantly elevated among migraineurs as compared with non-migraineurs (Table 2). Mean HDL and triglyceride concentrations were similar across study groups. After adjusting for maternal age, parity and pre-pregnancy BMI, adjusted mean total cholesterol concentrations were 12.3 mg/dL higher among migraineurs (95% CI 3.6-21.0 mg/dL, p-value=0.005) as compared with non-migraineurs. Similarly, LDL cholesterol concentrations were 9.4 mg/dL higher in migraineurs (95% CI 2.8-16.1 mg/dL, p-value=0.005) as compared with non-migraineurs. When a Bonferroni correction for potential multiple-comparison was applied, total cholesterol and LDL cholesterol concentrations remained statistically significantly elevated among migraineurs as compared with non-migraineurs at p-value<0.01.
Table 2. Summary of lipid profiles according to migraine status.
| Serum Lipid/Lipoproteins | No Migraine (N = 866) |
Yes Migraine (N = 196) |
P-value |
|---|---|---|---|
| Total cholesterol (mg/dL) | |||
| Mean ± SD | 254.7 ± 52.4 | 267.3 ± 56.3 | 0.003 |
| HDL cholesterol (mg/dL) | |||
| Mean ± SD | 65.7 ± 15.0 | 66.7 ± 15.5 | 0.431 |
| LDL cholesterol (mg/dL) | |||
| Mean ± SD | 145.6 ± 41.5 | 155.3 ± 42.9 | 0.004 |
| Triglyceride (mg/dL) | |||
| Mean ± SD | 219.4 ± 74.4 | 229.4± 80.6 | 0.092 |
| Total cholesterol:HDL ratio | |||
| Mean ± SD | 4.0 ± 0.9 | 4.1 ± 0.8 | 0.074 |
P-value from Student's t test (comparison of means)
SD=standard deviation
A linear relation was seen across levels of maternal total cholesterol, LDL, and total cholesterol:HDL ratio for migraine risk (Figure 1). In multivariate adjusted models migraineurs had higher odds of elevated total cholesterol, LDL cholesterol, and total cholesterol:HDL ratio as compared with non-migraineurs (Table 3). For instance, the AOR and 95% CI for successive quartiles of the total cholesterol associated with history of migraine were Q2 (219-247 mg/dL): 1.05 (0.64-1.70), Q3 (248-281mg/dL): 1.16 (0.72-1.86), and Q4 (≥282mg/dL): 1.87 (1.20-2.91) with the lowest quartile (<219 mg/dL) as the referent group (p-value for trend= 0.003). The AOR and 95% CI for successive quartiles of the LDL cholesterol associated with history of migraine were Q2 (117-140 mg/dL): 1.25 (0.77-2.02), Q3 (141-169 mg/dL): 1.30 (0.81-2.09), Q4 (≥170 mg/dL): 1.70 (1.08-2.69) as compared with the lowest quartile (<117 mg/dL) (p-trend=0.023). Associations were notably stronger for total cholesterol:HDL ratios with the history of migraine. The AORs and 95% CI for successive quartiles of total cholesterol:HDL ratios associated with history of migraine were Q2 (3.4-3.8): 1.65 (1.01-2.68), Q3 (3.9-4.4): 1.50 (0.93-2.41), Q4 (≥4.5): 1.73 (1.07-2.78) as compared with the lowest quartile (<3.4) (p-trend=0.0504). There was no clear evidence of associations of migraine with HDL or triglyceride concentrations.
Figure 1.
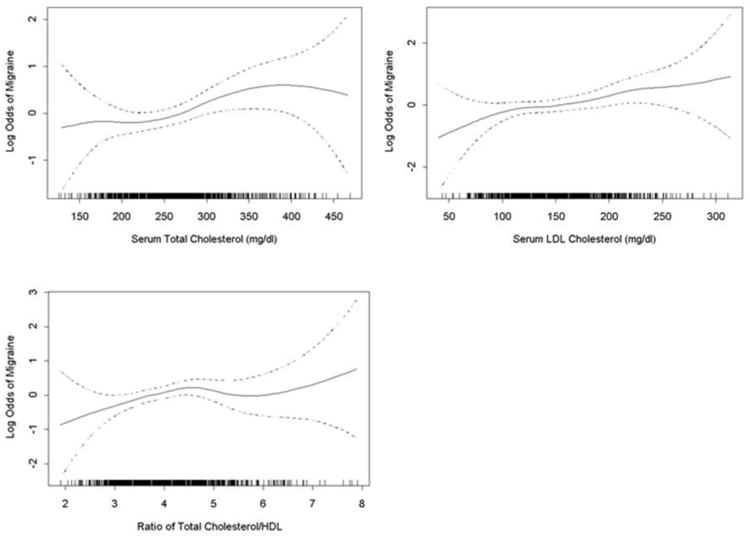
Relation between (a) maternal serum total cholesterol, or (b)LDL cholesterol concentrations or (c) ratio of total cholesterol/HDL cholesterol and the log odds of risk of migraine (solid line), with 95% confidence interval (dotted lines). The vertical bars along the X axis indicate distribution of study subjects. These generalized additive logistic regression models were adjusted for maternal age, parity and pre-pregnancy body mass index.
Table 3. Odds ratios (OR) and 95% confidence intervals (CI) of migraine status according to quartiles of serum lipids and lipoproteins.
| No Migraine (N = 866) n (%) |
Yes Migraine (N = 196) n (%) |
Unadjusted OR (95%CI) |
Adjusted OR (95%CI) |
|
|---|---|---|---|---|
| Total cholesterol (mg/dL) | ||||
| Q1 (<219) | 210 (24.3) | 37 (18.9) | 1.00 (REF) | 1.00 (REF) |
| Q2 (219-247) | 216 (24.9) | 40 (20.4) | 1.05 (0.65-1.71) | 1.05 (0.64-1.70) |
| Q3 (248-281) | 220 (25.4) | 46 (23.5) | 1.19 (0.74-1.90) | 1.16 (0.72-1.86) |
| Q4 (≥282) | 220 (25.4) | 73 (37.2) | 1.88 (1.21-2.92) | 1.87 (1.20-2.91) |
| P value for trend | 0.002 | 0.003 | ||
| HDL cholesterol (mg/dL) | ||||
| Q1 (<56) | 209 (24.1) | 45 (23.0) | 0.84 (0.54-1.29) | 0.80 (0.52-1.24) |
| Q2 (56-63) | 203 (23.4) | 51 (26.0) | 0.98 (0.64-1.49) | 0.95 (0.62-1.45) |
| Q3 (64-72) | 228 (26.3) | 42 (21.4) | 0.72 (0.46-1.11) | 0.70 (0.45-1.08) |
| Q4 (≥73) | 226 (26.1) | 58 (29.6) | 1.00 (REF) | 1.00 (REF) |
| P value for trend | 0.713 | 0.560 | ||
| LDL cholesterol (mg/dL) | ||||
| Q1 (<117) | 208 (24.0) | 35 (17.9) | 1.00 (REF) | 1.00 (REF) |
| Q2 (117-140) | 219 (25.3) | 47 (24.0) | 1.28 (0.79-2.05) | 1.25 (0.77-2.02) |
| Q3 (141-169) | 221 (25.5) | 50 (25.5) | 1.34 (0.84-2.15) | 1.30 (0.81-2.09) |
| Q4 (≥170) | 218 (25.2) | 64 (32.6) | 1.74 (1.11-2.75) | 1.70 (1.08-2.69) |
| P value for trend | 0.017 | 0.023 | ||
| Triglyceride (mg/dL) | ||||
| Q1 (<170) | 212 (24.5) | 46 (23.5) | 1.00 (REF) | 1.00 (REF) |
| Q2 (170-206) | 218 (25.2) | 43 (21.9) | 0.91 (0.58-1.44) | 0.88 (0.56-1.40) |
| Q3 (207-252) | 218 (25.2) | 38 (19.4) | 0.80 (0.50-1.28) | 0.79 (0.49-1.26) |
| Q4 (≥253) | 218 (25.2) | 69 (35.2) | 1.46 (0.96-2.22) | 1.39 (0.91-2.14) |
| P value for trend | 0.087 | 0.133 | ||
| Total cholesterol:HDL ratio | ||||
| Q1 (<3.4) | 214 (24.7) | 32 (16.3) | 1.00 (REF) | 1.00 (REF) |
| Q2 (3.4-3.8) | 204 (23.6) | 50 (25.5) | 1.64 (1.01-2.66) | 1.65 (1.01-2.68) |
| Q3 (3.9-4.4) | 229 (26.4) | 55 (28.1) | 1.61 (0.99-2.58) | 1.50 (0.93-2.41) |
| Q4 (≥4.5) | 219 (25.3) | 59 (30.1) | 1.80 (1.13-2.88) | 1.73 (1.07-2.78) |
| P value for trend | 0.026 | 0.054 |
Adjusted for maternal age, parity and pre-pregnancy BMI.
Separate models were fitted for each lipid or lipoprotein
We next examined the independent and joint associations of maternal elevated lipid concentrations and pre-pregnancy obesity status with risk of migraine. As shown in Table 4, obese women with elevated total cholesterol (≥282 mg/dL) were more likely to be migraineurs (OR=3.71; 95% CI 1.58-8.71) as compared with non-obese women with lower total cholesterol (< 219 mg/dL). This apparent interaction was not statistically significant (p=0.493). Similar elevated odds were observed for obese women with elevated LDL cholesterol (≥170 mg/dL) (OR=2.71 95%CI: 1.21-6.10), obese women with elevated triglycerides (≥253 mg/dL) (OR=2.36; 95%CI:1.17-4.79) and obese women with high total cholesterol:HDL ratio (>4.5) (OR=2.62; 95%CI: 1.41-4.88) as compared with non-obese women with lower LDL cholesterol (<117 mg/dL), non-obese women with lower triglycerides (<170 mg/dL), and obese women with lower total cholesterol:HDL ratio (<3.4), respectively. The interactions were not statistically significant.
Table 4. Odds ratios (OR) and 95% confidence intervals (CI) of migraine status according to serum lipid/lipoproteins and pre-pregnancy obesity status.
| Elevated biomarker level & Pre-pregnancy Obesity | No Migraine (N = 866) n (%) |
Yes Migraine (N = 196) n (%) |
Unadjusted OR (95%CI) |
Adjusted OR (95%CI) |
|---|---|---|---|---|
| Total cholesterol | ||||
| No & No | 577 (66.6) | 103 (52.6) | 1.00 (REF) | 1.00 (REF) |
| Yes & No | 206 (23.8) | 63 (32.1) | 1.71 (1.21- 43) | 1.70 (1.20-2.42) |
| No & Yes | 69 (8.0) | 20 (10.2) | 1.62 (0.95-2.79) | 1.52 (0.88-2.63) |
| Yes & Yes | 14 (1.6) | 10 (5.1) | 4.00 (1.73-9.25) | 3.71 (1.58-8.71) |
| P value for interaction term | 0.483 | 0.493 | ||
| HDL cholesterol | ||||
| Yes & No | 596 (68.8) | 130 (66.3) | 1.00 (REF) | 1.00 (REF) |
| No & No | 187 (21.6) | 36 (18.4) | 0.88 (0.59-1.32) | 0.86 (0.57-1.30) |
| Yes & Yes | 61 (7.0) | 21 (10.7) | 1.58 (0.93-2.68) | 1.46 (0.85-2.50) |
| No & Yes | 22 (2.5) | 9 (4.6) | 1.88 (0.84-4.17) | 1.75 (0.78-3.94) |
| P value for interaction term | 0.562 | 0.519 | ||
| LDL cholesterol | ||||
| No & No | 583 (67.3) | 112 (57.1) | 1.00 (REF) | 1.00 (REF) |
| Yes & No | 200 (23.1) | 54 (27.5) | 1.41 (0.98-2.02) | 1.39 (0.96-2.00) |
| No & Yes | 65 (7.5) | 20 (10.2) | 1.60 (0.93-2.75) | 1.49 (0.86-2.57) |
| Yes & Yes | 18 (2.1) | 10 (5.1) | 2.89 (1.30-6.43) | 2.71 (1.21-6.10) |
| P value for interaction term | 0.620 | 0.592 | ||
| Triglyceride | ||||
| No & No | 592 (68.4) | 110 (56.1) | 1.00 (REF) | 1.00 (REF) |
| Yes & No | 191 (22.1) | 56 (28.6) | 1.58 (1.10-2.26) | 1.57 (1.09-2.26) |
| No & Yes | 56 (6.5) | 17 (8.7) | 1.63 (0.92-2.92) | 1.56 (0.87-2.81) |
| Yes & Yes | 27 (3.1) | 13 (6.6) | 2.59 (1.30-5.18) | 2.36 (1.17-4.79) |
| P value for interaction term | 0.991 | 0.939 | ||
| Total cholesterol:HDL ratio | ||||
| No & No | 194 (22.4) | 28 (14.3) | 1.00 (REF) | 1.00 (REF) |
| Yes & No | 589 (68.0) | 138 (70.4) | 1.62 (1.05-2.51) | 1.58 (1.02-2.46) |
| No & Yes | 20 (2.3) | 4 (2.0) | 1.39 (0.44-4.35) | 1.32 (0.42-4.19) |
| Yes & Yes | 63 (7.3) | 26 (13.3) | 2.86 (1.56-5.23) | 2.62 (1.41-4.88) |
| P value for interaction term | 0.706 | 0.727 |
Adjusted for maternal age and parity. Separate models were fitted for each lipid or lipoprotein
Definition: High total cholesterol: ≥282 mg/dL (Q4); low total cholesterol: <282 mg/dL (Q1-Q3);
Low HDL cholesterol: <56mg/dL (Q1); high HDL cholesterol: ≥56 mg/dL (Q2-Q4);
High LDL cholesterol: ≥170 mg/dL (Q4); low LDL cholesterol: <170 mg/dL (Q1-Q3);
High triglyceride: ≥253 mg/dL (Q4); low triglyceride: <253 mg/dL (Q1-Q3);
High total cholesterol:HDL ratio: ≥3.4 (Q2-Q4); low total cholesterol:HDL ratio: <3.4 (Q1).
Discussion
In this cross-sectional study of pregnant women we found that elevated total cholesterol, LDL, total cholesterol:HDL ratio were all associated with history of migraine. In multivariate adjusted models pregnant women with migraine had higher odds of elevated total cholesterol, LDL, and total cholesterol:HDL ratio as compared with non-migraineurs. These associations were particularly pronounced among obese migraineurs.
Our findings add to the limited body of evidence of adverse cardiometabolic risk outcomes among pregnant migraineurs. In uncomplicated pregnancies, lipid profiles are known to change dramatically throughout gestation.19,20, 25 For instance, overall maternal serum lipid concentrations reported in the present cohort were substantially higher than concentrations reported among non-pregnant Peruvian women. 26 In a population-based study of risk factors for non-communicable disorders among adult Peruvians: total cholesterol total serum cholesterol was 186 mg/dL, the corresponding value for pregnant women in the present cohort of pregnant women was 275 mg/dL. Other lipid and lipoprotein concentrations were also lower in pregnant versus non-pregnant Peruvian women: HDL (65.9 vs 48.5 mg/dL); LDL (147 vs 114 mg/dL), triglycerides (221 vs 104 mg/dL).26 These observations, taken together with results from prior studies showing increased risk of stroke in migraineurs with aura27 suggest that risks for adverse cardiometabolic outcomes are increased among women with prior diagnosis of migraine.
To our best knowledge, this is the first study to examine the relation between pregnancy lipid concentrations among migraineurs. Our findings are consistent with the majority of studies evaluating lipid profiles in men or non-pregnant women.6, 8-10 In a cross-sectional analysis of 27,626 women aged ≥45 years, Kurth et al. evaluated the association of migraine and migraine aura status with elevated levels of biomarkers of cardiovascular risk including lipid profile in a cohort of US health care professionals, largely non-reproductive aged women (mean age 54 years). In this study, compared with women with no migraine history, women who reported any history of migraine had modestly increased odds of elevated total cholesterol (OR=1.09; 95%CI: 1.01-1.18) and elevated LDL cholesterol (OR=1.14; 95%CI: 1.05-1.23). The odds did not meaningfully differ according to migraine aura status and migraine frequency.9 Similarly, in a population-based data of 1,809 participants ≥ 50 years of age, Monastero et al. found that migraine was associated with increased odds (OR=1.60, 95% CI:1.1-2.3) of elevated total cholesterol (≥ 220 mg/dL).8 Tana et al., in their study of 52 migraineurs (17 with and 36 without aura) found that increased frequency and intensity of migraine attacks were statistically significantly associated with elevated total cholesterol and LDL cholesterol concentrations.6 Among treated migraineurs, a significant decrease in the number and intensity of migraine attacks was associated with a significant reduction of total cholesterol and LDL cholesterol (p-value< 0.001). No significant difference was found in the evaluated parameters for the subgroups of migraine with and without aura.6 Our results, however, did not corroborate the findings reported by some investigators.12, 13 For example, among obese adolescents with migraine who underwent an intervention program for weight loss, patients with persistence of migraine had significantly higher body mass index, triglyceride, total cholesterol and LDL cholesterol (all p-value < 0.05) values when compared with those became migraine-free. However, none of the biomarkers was significantly associated with persistent migraine after further adjusted for confounders.13
We found that obese pregnant women with migraine had elevated lipid profiles (total cholesterol, LDL cholesterol, total cholesterol:HDL ratio) as compared with lean non-migraineurs. This observation is in general agreement with prior studies. In an Austrian clinic-based study, obese migraineurs were statistically significantly more likely to have elevated mean LDL cholesterol (112.3 ± 32.0 mg/dL) compared with normal weight non-migraineurs (87.3 ± 28.9 mg/dL).10 Similarly, obese migraineurs had elevated mean total cholesterol (187.6± 42.8 mg/dL) compared with normal weight non-migraineurs (176.4 ± 32.8 mg/dL). These findings are not surprising given the well-established relationship between obesity and migraine.28,29, 30 For instance, using the National Comorbidity Survey Replication Peterlin et al. demonstrated that obese individuals are 1.81-times as likely (1.81; 95% CI: 1.27–2.57) to experience episodic migraine as compared with normal weight controls.29 Taken together, the current data indicate that migraine-associated alterations in lipid profiles in pregnant women share common features with obesity-related lipid alterations.10
Migraine is a neurovascular disorder in which changes in cortical excitability, neuro-inflammation and vascular endothelial dysfunction contribute to its pathophysiology.31 Hyperlipidemia may induce platelet activation, aggregation and triggering neurogenic inflammation.14 Activated platelets further induce changes in platelet serotonin and serum serotonin level 32 and help initiate a cascade of prostaglandins and leukotrienes (LT) --- potent prostaglandins (such as PGE2). These alterations have been implicated to lead to vasodilatation and trigger migraine attacks.33 Results from preliminary genetic studies support these observations. For instance, Mochi et al. investigated the polymorphism of LDL cholesterol receptor gene (that plays an important role in cholesterol homeostasis) and concluded that the allelic distributions of tri-allelic (TA) polymorphism was significantly different between migraine without aura and non-migraine controls.34
The strengths of our study include the relatively large number of women with information from the detailed standardized questionnaire allowing us to apply ICHD-II criteria for migraine diagnosis. Fasting samples were collected to minimize the influence of postprandial lipemia. However, some limitations must be considered when interpreting the results of our study. First, the cross-sectional data collection design of our study does not allow us to determine whether altered lipid concentrations preceded migraine or was a consequence of migraine. Second, despite adjustment for potential confounders, residual and unmeasurable confounding is possible since our study is observational.
In conclusion, we have shown that pregnant migraineurs had elevated odds of increased total cholesterol, LDL, and total cholesterol:HDL ratio compared with pregnant non-migraineurs. The observed associations were more pronounced among obese migraineurs. These results, when taken into consideration with other studies of men and non-pregnant women 6-11 indicate the adverse cardiometabolic risk outcomes associated with migraine may extend to include pregnant women. If confirmed in other pregnancy cohorts, these results may be instrumental in motivating clinicians to have heightened vigilance for detecting and managing modifiable cardiovascular risk factors among pregnant migraineurs. Future longitudinal studies are needed to confirm and extend the findings from this study.
Acknowledgments
This research was supported by Roche Diagnostic Operations Inc. (project number 208617-5074547) and the National Institutes of Health (NIH) (R01HD-055566). The funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors wish to thank the dedicated staff members of Instituto Especializado Materno Perinatal, Peru and Asociacion Civil Proyectos en Salud (PROESA), Peru for their expert technical assistance with this research.
References
- 1.Stovner L, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 2.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidetti D, Rota E, Morelli N, Immovilli P. Migraine and stroke: “vascular” comorbidity. Frontiers in Neurology. 2014;5:193. doi: 10.3389/fneur.2014.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector JT, Kahn SR, Jones MR, Jayakumar M, Dalal D, Nazarian S. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med. 2010;123:612–624. doi: 10.1016/j.amjmed.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nothiger R, Steinmann-Zwicky M. Genetics of sex determination in eukaryotes. Results Probl Cell Differ. 1987;14:271–300. doi: 10.1007/978-3-540-47783-9_17. [DOI] [PubMed] [Google Scholar]
- 6.Tana C, Santilli F, Martelletti P, et al. Correlation between Migraine Severity and Cholesterol Levels. Pain Practice. 2014 doi: 10.1111/papr.12229. [DOI] [PubMed] [Google Scholar]
- 7.Scher AI, Terwindt GM, Picavet HS, Verschuren WM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology. 2005;64:614–620. doi: 10.1212/01.WNL.0000151857.43225.49. [DOI] [PubMed] [Google Scholar]
- 8.Monastero R, Pipia C, Cefalu AB, et al. Association between plasma lipid levels and migraine in subjects aged > or =50 years: preliminary data from the Zabut Aging Project. Neurological Sciences. 2008;29(Suppl 1):S179–181. doi: 10.1007/s10072-008-0919-0. [DOI] [PubMed] [Google Scholar]
- 9.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–291. doi: 10.1001/jama.296.3.283. [DOI] [PubMed] [Google Scholar]
- 10.Gruber HJ, Bernecker C, Pailer S, et al. Lipid profile in normal weight migraineurs - evidence for cardiovascular risk. Eur J Neurol. 2010;17:419–425. doi: 10.1111/j.1468-1331.2009.02861.x. [DOI] [PubMed] [Google Scholar]
- 11.Rist PM, Tzourio C, Kurth T. Associations between lipid levels and migraine: cross-sectional analysis in the epidemiology of vascular ageing study. Cephalalgia. 2011;31:1459–1465. doi: 10.1177/0333102411421682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahromi SR, Abolhasani M, Meysamie A, Togha M. The effect of body fat mass and fat free mass on migraine headache. Iranian Journal of Neurology. 2013;12:23–27. [PMC free article] [PubMed] [Google Scholar]
- 13.Verrotti A, Carotenuto M, Altieri L, et al. Migraine and obesity: metabolic parameters and response to a weight loss programme. Pediatric Obesity. 2014 doi: 10.1111/ijpo.245. [DOI] [PubMed] [Google Scholar]
- 14.Sener A, Ozsavci D, Oba R, Demirel GY, Uras F, Yardimci KT. Do platelet apoptosis, activation, aggregation, lipid peroxidation and platelet-leukocyte aggregate formation occur simultaneously in hyperlipidemia? Clinical Biochemistry. 2005;38:1081–1087. doi: 10.1016/j.clinbiochem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Tietjen GE, Khubchandani J. Platelet dysfunction and stroke in the female migraineur. Curr Pain Headache Rep. 2009;13:386–391. doi: 10.1007/s11916-009-0063-4. [DOI] [PubMed] [Google Scholar]
- 16.Adeney KL, Flores JL, Perez JC, Sanchez SE, Williams MA. Prevalence and correlates of migraine among women attending a prenatal care clinic in Lima, Peru. Cephalalgia. 2006;26:1089–1096. doi: 10.1111/j.1468-2982.2006.01171.x. [DOI] [PubMed] [Google Scholar]
- 17.Melhado EM, Maciel JA, Jr, Guerreiro CA. Headache during gestation: evaluation of 1101 women. Can J Neurol Sci. 2007;34:187–192. doi: 10.1017/s0317167100006028. [DOI] [PubMed] [Google Scholar]
- 18.Nezvalova-Henriksen K, Spigset O, Nordeng H. Maternal characteristics and migraine pharmacotherapy during pregnancy: cross-sectional analysis of data from a large cohort study. Cephalalgia. 2009;29:1267–1276. doi: 10.1111/j.1468-2982.2009.01869.x. [DOI] [PubMed] [Google Scholar]
- 19.Williams MA. Pregnancy Complications. In: Buck-Louis G, P R, editors. Reproductive and Perinatal Epidemiology. Oxford University Press; 2011. [Google Scholar]
- 20.Williams MA, Mittendorf R. Maternal Morbidity. In: Goldman MB, HM, editors. Women and Health. Academic Press Inc.; 2000. pp. 172–181. [Google Scholar]
- 21.IHS. Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders, 2nd edition. Cephalalgia. 2004;13(Suppl 1):S5–S12. doi: 10.1111/j.1468-2982.2003.00824.x. 2004. [DOI] [PubMed] [Google Scholar]
- 22.Koepsell TD, Weiss NS. Epidemiologic methods : Studying the occurrence of illness. Oxford, New York: Oxford University Press; 2003. [Google Scholar]
- 23.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 24.Hastie T, Tibshirani R. Generalized Additive Models. London: Chapman-Hall; 1990. [DOI] [PubMed] [Google Scholar]
- 25.Bartels A, Egan N, Broadhurst DI, et al. Maternal serum cholesterol levels are elevated from the 1st trimester of pregnancy: a cross-sectional study. J Obstet Gynaecol. 2012;32:747–752. doi: 10.3109/01443615.2012.714017. [DOI] [PubMed] [Google Scholar]
- 26.Gelaye B, Revilla L, Lopez T, et al. Association between insulin resistance and c-reactive protein among Peruvian adults. Diabetology & Metabolic Syndrome. 2010;2:30. doi: 10.1186/1758-5996-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scher AI, Launer LJ. Migraine: migraine with aura increases the risk of stroke. Nat Rev Neurol. 2010;6:128–129. doi: 10.1038/nrneurol.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bigal ME, Liberman JN, Lipton RB. Obesity and migraine: a population study. Neurology. 2006;66:545–550. doi: 10.1212/01.wnl.0000197218.05284.82. [DOI] [PubMed] [Google Scholar]
- 29.Peterlin BL, Rosso AL, Williams MA, et al. Episodic migraine and obesity and the influence of age, race, and sex. Neurology. 2013;81:1314–1321. doi: 10.1212/WNL.0b013e3182a824f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vo M, Ainalem A, Qiu C, Peterlin BL, Aurora SK, Williams MA. Body mass index and adult weight gain among reproductive age women with migraine. Headache. 2011;51:559–569. doi: 10.1111/j.1526-4610.2010.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamed SA. The vascular risk associations with migraine: relation to migraine susceptibility and progression. Atherosclerosis. 2009;205:15–22. doi: 10.1016/j.atherosclerosis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 32.McNicol A, Israels SJ. Platelet dense granules: structure, function and implications for haemostasis. Thrombosis Research. 1999;95:1–18. doi: 10.1016/s0049-3848(99)00015-8. [DOI] [PubMed] [Google Scholar]
- 33.Antonova M, Wienecke T, Olesen J, Ashina M. Prostaglandin E(2) induces immediate migraine-like attack in migraine patients without aura. Cephalalgia. 2012;32:822–833. doi: 10.1177/0333102412451360. [DOI] [PubMed] [Google Scholar]
- 34.Mochi M, Cevoli S, Cortelli P, et al. Investigation of an LDLR gene polymorphism (19p13.2) in susceptibility to migraine without aura. J Neurol Sci. 2003;213:7–10. doi: 10.1016/s0022-510x(03)00124-2. [DOI] [PubMed] [Google Scholar]


