Abstract
PURPOSE
We evaluated the association between cardiovascular autonomic neuropathy (CAN), erectile dysfunction (ED) and lower urinary tract symptoms (LUTS) in men with type 1 diabetes (T1DM).
MATERIALS & METHODS
Male T1DM participants (n=635) in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications Study (DCCT/EDIC) were studied. CAN was assessed by standardized cardiovascular reflex tests including changes in R-R variation with deep-breathing, Valsalva maneuver (Valsalva ratio), and changes in supine-to-standing diastolic blood pressure. ED was assessed by a proxy item from the International Index of Erectile Function (IIEF), and LUTS by the American Urological Association Symptom Index (AUASI). Multivariable logistic regression models estimated the association between CAN, ED and/or LUTS, adjusting for time-weighted glycemic control, blood pressure, age, and other covariates.
RESULTS
Men who developed ED and/or LUTS during EDIC had significantly lower R-R variation and Valsalva ratio at DCCT closeout and EDIC year 16/17 compared to those without ED or LUTS. In adjusted analysis, participants with CAN had 2.65 greater odds of ED and LUTS (95% CI=1·47,4·79).
CONCLUSIONS
These data suggest that CAN predicts the development of urological complications in men with long-standing T1DM. Studies evaluating the mechanisms contributing to these interactions are warranted for targeting effective prevention or treatment.
Keywords: Autonomic Neuropathy, Diabetes, Erectile Dysfunction, Lower Urinary Tract Symptoms
INTRODUCTION
The autonomic nervous system plays a critical role in regulating the function of numerous systems/organs exerting its control through a broad network of afferent and efferent small nerve fibers, which are susceptible to hyperglycemia. Diabetes-induced damage of these fibers, known as autonomic neuropathy, can involve different systems including cardiovascular, gastrointestinal, urogenital, and sudomotor.1 The symptoms and signs vary broadly, from asymptomatic small-fiber dysfunction, to severe cases associated with high morbidity/mortality. Cardiovascular autonomic neuropathy (CAN), a prevalent form of diabetic autonomic neuropathy, is a significant cause of morbidity and mortality, due to high-risk of cardiac arrhythmias, silent myocardial ischemia and sudden death.1–3
The pathophysiology of ED and LUTS are complex, involving neural and vascular processes. Autonomic neurotransmission plays an important role in controlling the cavernosal and detrusor smooth muscle tone and function, and diabetes-induced autonomic dysfunction is instrumental in ED and bladder dysfunction pathophysiology.4 However, to date, well-designed human subject studies describing the relationship between various forms of autonomic neuropathy, including urological complications, are lacking. Prior observations resulted from studies that were limited in sample size lacking the power to adjust for important confounders, or used insensitive outcome measures.
The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) cohort is a large, well-characterized cohort of subjects with T1DM,5–7 continuously followed for ~30 years, with available data on numerous diabetic complications, risk factors and medication use. CAN was assessed longitudinally during DCCT and EDIC.8 ED and LUTS were assessed during EDIC as part of UroEDIC, an ancillary study of urological complications.9, 10 The objective of this study was to evaluate the associations among CAN, ED and LUTS in DCCT/EDIC participants. We hypothesized that participants with CAN at DCCT closeout were more likely to develop more advanced urologic complications, possibly mediated by autonomic nerve dysfunction.
MATERIALS & METHODS
Population and Setting
The DCCT/EDIC studies have been described in detail.5–7 Briefly, 1,441 subjects with T1DM with no or minimal diabetic retinopathy were enrolled in DCCT. Subjects were randomly assigned to either intensive or conventional treatment and were followed for a mean 6·5 years5. At the end of DCCT, all subjects returned to their own health care providers for diabetes care, but were asked to return for follow-up visits. Annual EDIC examinations began one year after completion of the DCCT, with 1,375 (96%) subjects consenting to participate in EDIC.6 Of 761 men enrolled, 746 completed the DCCT in 1993 and 720 (97%) elected to participate in the first annual examination of EDIC in 1994.
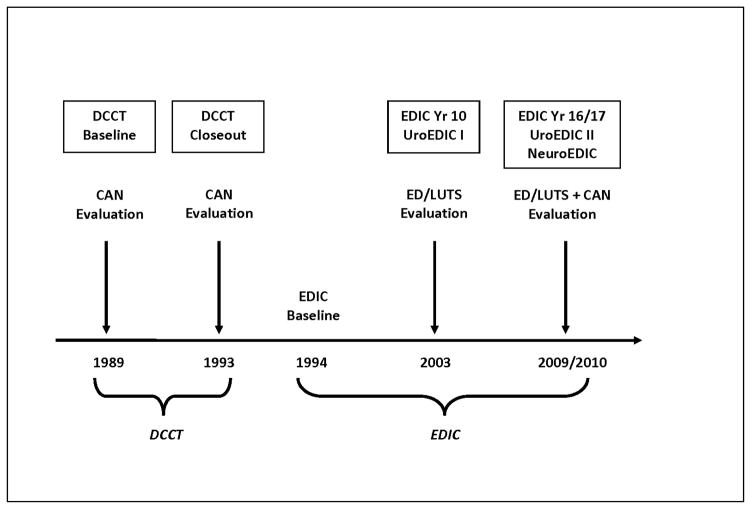
UroEDIC, an ancillary study designed to examine urologic complications of diabetes, included ED and LUTS evaluations in 2003, which were repeated in 2010. At EDIC year 17 (2010), 644 of 669 active men (96%) agreed to participate in UroEDIC and 635 had valid ED and LUTS information and comprise the cohort for the current analyses. Figure 1 summarizes the timeline for ED, LUTS and neuropathy data collection for DCCT/EDIC. All DCCT/EDIC procedures were approved by institutional review boards of all participating centers. Written informed consent was provided by all participants.
Figure 1.
Neuropathy and ED/LUTS data collection in DCCT/EDIC.
Erectile Dysfunction and LUTS Evaluations
Presence of ED was assessed with the validated International Index of Erectile Function (IIEF).11 Since 31% of participants responded, “Did not attempt intercourse” on questions in the Erectile Function domain, we used a proxy item to assess erectile function in the entire cohort regardless of sexual activity and presence/absence of a partner.9 A question in the Erectile Function domain asks participants: “Over the past 4 weeks, how would you rate your confidence that you get and keep your erection?” Those who answered ‘Very Low’ or ‘Low’ were classified as having ED. Those who answered ‘Moderate’, ’High’, or ‘Very High’ were classified as no ED. Among men who engaged in sexual intercourse during the preceding four weeks, this definition of ED correlated strongly with total IIEF ED domain scores (r=0·77, p<0·001) and ED bother (r=0·80, p<0·001).9 Further, sensitivity analyses were conducted to examine associations between both the quantitative and qualitative measures of CAN and ED/LUTS using the IIEF definition for ED both with and without the zero responses compared to the proxy item. There was little variation (similar magnitude and statistical significance in ORs) in findings between our primary exposures and outcomes using the IIEF measure vs. our proxy item, therefore all findings presented focus on the proxy item. In addition, a separate question queried use of oral medications and/or erectile aids/devices of all participants. Those who reported any use were categorized as having ED.
LUTS severity was determined with the American Urological Association Symptom Index (AUASI), a standardized seven-item questionnaire that quantifies the presence and frequency of: nocturia, frequency, urgency, weak urinary stream, intermittency, straining, and the sensation of incomplete emptying.12 Scores range from 0 to 35 with 8–35 indicating the presence of LUTS.12
Cardiovascular Autonomic Neuropathy Evaluations
Standardized and rigorously applied CAN evaluations, included cardiovascular autonomic reflex tests that assessed the R-R response to paced breathing (R-R variation), the Valsalva maneuver, and postural changes in blood pressure measured at baseline, biennially during DCCT, and at years 13/14 and 16/17 during EDIC.8, 13 R-R variation measures the magnitude of cardiac sinus arrhythmia – predominantly a function of the parasympathetic nervous system and is computed as a dimensionless circular mean vector of R-R intervals as described.3, 8, 14 The Valsalva ratio evaluates cardiovagal function in response to a standardized increase in intrathoracic pressure and is influenced by both parasympathetic and sympathetic activity.3, 8, 14 This battery of tests is well-suited to explore long-term changes in CAN function and has been recommended as the gold-standard for assessing CAN.3, 14 The standardized cut points for CAN measures used in DCCT/EDIC included R-R variation<15 and Valsalva ratio≤1·5.12 Abnormal CAN function was defined as: either R-R variation<15 or R-R variation between 15–19·9 plus either a Valsalva ratio≤1·5 or a supine-to-standing drop of 10 mm Hg in diastolic blood pressure.8
Other Evaluations
Hemoglobin A1c (HbA1c) level was measured at baseline and quarterly during DCCT and annually in EDIC using high-performance ion-exchange liquid chromatography.5 Retinopathy was assessed using fundus photographs centrally graded using the Early Treatment Diabetic Retinopathy Study scale. Albumin excretion rate (AER) was measured in half of the cohort annually. Nephropathy was defined as AER≥30 mg/24hr. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured quarterly during the DCCT and annually during EDIC. Hypertension was defined as sitting SBP≥140 mmHg and/or DBP≥90 mmHg or the use of antihypertensive medication. Time-weighted mean values for HbA1c, SBP, and DBP were used, representing the running means up to each study visit in the DCCT/EDIC. Total testosterone (TT) was measured at the University of Minnesota using a rapid LC-MS/MS platform (Liquid Chromatography-Tandem Mass Spectrophotometry). The lower and upper limits of detection of the TT assay are <0·25 nmol/L and 50 nmol/L respectively.
Statistical Analysis
The distribution of demographic and clinical characteristics, markers of diabetes control/treatment, blood pressure control and neuropathy measures was examined by ED and LUTS status (No ED or LUTS, ED only, LUTS only, ED and LUTS). The Kruskal-Wallis test assessed differences in quantitative variables, and the contingency Chi-square test assessed categorical variables. Multivariable logistic regression models were used to estimate the association between quantitative and qualitative neuropathy measures and ED and/or LUTS at EDIC year 17, after adjustment for DCCT cohort assignment, DCCT/EDIC time-weighted HbA1c, DCCT/EDIC time-weighted SBP, and the following year 17 characteristics: age, BMI, smoking status, drinking status, and any antihypertensive medication use. Effects nominally significant at p≤0·05 are cited. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
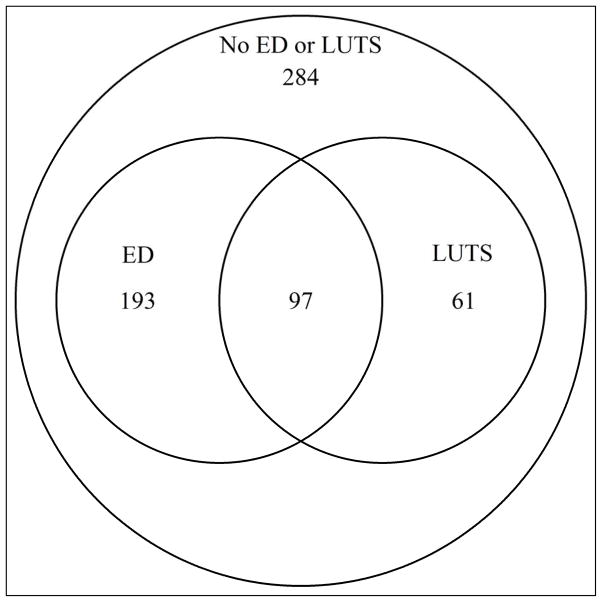
The 635 men in the study had a mean age of 52±7 years, diabetes duration of 29±5 years, and DCCT/EDIC mean HbA1c of 7·9±1.0% (63mmol/mol). DCCT/EDIC mean SBP was 121±8 mmHg, body mass index (BMI) 30±5, and 12% were smokers (Table 1). At EDIC year 17, ED only was reported by 193 (30%), LUTS only by 61 (10%), and ED and LUTS by 97 (15%) participants (Figure 2). Compared with subjects without ED or LUTS, those with either condition were significantly older, had higher DCCT/EDIC HbA1c, higher DCCT/EDIC SBP, higher prevalence of nephropathy, and were more likely to be drinkers. Total testosterone levels were similar among all participants (Table 1).
Table 1.
Characteristics of DCCT/EDIC male participants at EDIC year 17 (N=635) by ED and LUTS status
| No LUTS or ED N=284 | ED only N=193 | LUTS only N=61 | ED & LUTS N=97 | p-value | |
|---|---|---|---|---|---|
| Sociodemographic/Clinical | |||||
| Attained Age (years) | 49·3±6·3 | 52·9±6·2 | 51·4±6·7 | 55·7±5·7 | <0·0001 |
| Married | 217 (77) | 139 (72) | 45 (76) | 76 (80) | 0·5 |
| Current Cigarette Smoker | 25 (9) | 26 (14) | 5 (8) | 17 (18) | 0·07 |
| Current Drinker | 136 (48) | 103 (54) | 40 (68) | 44 (46) | 0·03 |
| Body Mass Index (BMI) (kg/m2) | 28·5±4·4 | 29·5±5·0 | 28·2±3·6 | 28·9±5·6 | 0·08 |
| BMI Category (kg/m2) | |||||
| Normal (BMI<25) | 63 (23) | 32 (17) | 11 (19) | 26 (28) | 0·1 |
| Overweight (25≤BMI<30) | 124 (44) | 76 (40) | 30 (52) | 34 (37) | |
| Obese (BMI≥30) | 92 (33) | 82 (43) | 17 (29) | 33 (35) | |
| Total Testosterone (ng/dL) | 553·3±196·0 | 538·8±238·4 | 548·8±208·8 | 546·1±202·4 | 0·6 |
| Diabetes Control and Treatment | |||||
| Primary Prevention Cohort | 149 (52) | 87 (45) | 37 (61) | 42 (43) | 0·07 |
| Intensive Treatment Group | 144 (51) | 96 (50) | 29 (48) | 46 (47) | 0·9 |
| Duration of diabetes (years) | 29·4±4·9 | 29·6±4·5 | 28·4±4·7 | 30·4±5·2 | 0·06 |
| DCCT/EDIC time-weighted HbA1c (%) | 7·8±0·9 | 8·2±1·0 | 7·8±1·0 | 8·1±1·0 | 0·0001 |
| DCCT/EDIC time-weighted HbA1c (mmol/mol) | 62±10 | 66±11 | 62±11 | 65±11 | |
| Diabetic Complications | |||||
| Retinopathy* | 44 (15) | 45 (23) | 12 (20) | 25 (26) | 0·07 |
| Nephropathy** | |||||
| None (AER<30) | 236 (86) | 134 (72) | 43 (74) | 55 (61) | <0·0001 |
| Microalbuminuria (30≤AER<300) | 29 (11) | 39 (21) | 6 (10) | 28 (31) | |
| Albuminuria (AER≥300) | 10 (4) | 14 (7) | 9 (16) | 7 (8) | |
| eGFR<60 | 5 (2) | 15 (8) | 3 (5) | 4 (4) | 0·02 |
| Blood Pressure Control | |||||
| DCCT/EDIC time-weighted SBP (mmHg) | 119·4±7·4 | 122·0±6·9 | 121·2±7·0 | 122·2±8·5 | 0·0002 |
| DCCT/EDIC time-weighted DBP (mmHg) | 75·9±4·8 | 76·6±4·4 | 76·0±5·0 | 75·0±4·7 | 0·1 |
| Hypertension# | 181 (64) | 145 (76) | 38 (64) | 73 (77) | 0·02 |
| Antihypertensive Use§ | |||||
| Beta-blockers | 21 (7) | 30 (16) | 2 (3) | 18 (19) | 0·0007 |
| ACE inhibitors or ARB blockers | 155 (55) | 131 (68) | 32 (54) | 66 (69) | 0·005 |
| Ca-channel blockers | 15 (5) | 21 (11) | 6 (10) | 13 (14) | 0·04 |
Data are Mean±Std or N (%). P-values based the Kruskal-Wallis test for quantitative variables or the Contingency chi-square for qualitative variables.
Sample sizes may vary due to missing data.
Retinopathy defined through EDIC year 14 using the Early Treatment Diabetic Retinopathy Study on a scale of 0–23 (<12 Non Proliferative or None, ≥12 Proliferative).
AER= AER=Albumin Excretion Rate (mg/24hr) at EDIC year 17/18. Estimated GFR (eGFR) at EDIC year 16 using the CKD-EPI equation.
Hypertension defined as sitting systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP)≥90 mmHg or the use of antihypertensive medication.
ACE=Angiotensin-converting enzyme; ARB=Angiotensin receptor blockers.
Figure 2.
ED and/or LUTS Status in Male Participants of UroEDIC II at EDIC Year 17.
The prevalence of CAN using the composite definition was 37%. Abnormal R-R variation was present in 33% (Table 2). In unadjusted analyses, participants with ED or LUTS at EDIC year 17 had lower R-R variation at DCCT closeout (39·7±19·2 and 41·3±15·7 respectively) compared with participants without ED or LUTS (41·7±19·1) while R-R variation at DCCT closeout was lowest among participants with both ED and LUTS (30.4±15.2) p<0·0001 (Table 2). The prevalence of R-R variation<15 at DCCT closeout was highest among participants who developed ED and/or LUTS at EDIC year 17 (p=0·01). Similar differences were observed when comparing the Valsalva ratio at DCCT closeout among participants with or without ED or LUTS at year 17 (p=0·01). (Table 2)
Table 2.
Measures of CAN at DCCT closeout and EDIC year 16/17 by ED and LUTS status at year 17
| All Men N=635 | No LUTS or ED N=284 | ED only N=193 | LUTS only N=61 | ED & LUTS N=97 | p-value | |
|---|---|---|---|---|---|---|
| CAN Measures* | ||||||
| DCCT Closeout | ||||||
| R-R Variation | 39·4±18·6 | 41·7±19·1 | 39·7±19·2 | 41·3±15·7 | 30·4±15·2 | <0·0001 |
| R-R Variation <15 | 39 (7) | 11 (4) | 13 (7) | 3 (6) | 12 (14) | 0·01 |
| Valsalva Ratio | 2·0±0·4 | 2·0±0·4 | 1·9±0·4 | 2·0±0·4 | 1·9±0·3 | 0·01 |
| Valsalva Ratio ≤1.5 | 55 (10) | 20 (8) | 22 (13) | 5 (9) | 8 (10) | 0·4 |
| Abnormal CAN Function** | 45 (8) | 14 (5) | 15 (8) | 4 (7) | 12 (14) | 0·06 |
| EDIC Year 16/17 | ||||||
| R-R Variation | 24·8±17·1 | 28·5±16·8 | 21·8±16·9 | 27·4±16·6 | 17·7±15·7 | <0·0001 |
| R-R Variation <15 | 197 (33) | 58 (21) | 79 (42) | 11 (19) | 49 (56) | <0·0001 |
| Valsalva Ratio | 1·7±0·3 | 1·8±0·3 | 1·6±0·3 | 1·8±0·4 | 1·6±0·3 | <0·0001 |
| Valsalva Ratio ≤1.5 | 181 (33) | 62 (24) | 63 (37) | 19 (35) | 37 (48) | 0·0005 |
| Abnormal CAN Function** | 230 (37) | 70 (25) | 85 (45) | 19 (33) | 56 (62) | <0·0001 |
Data are Mean±Std or N (%). P-values based the Kruskall-Wallis test for quantitative variables or the Contingency chi-square for qualitative variables.
Sample sizes vary based on availability of CAN data.
Abnormal CAN function defined as either R-R variation<15 or R-R variation between 15–19·9 plus either a Valsalva ratio≤1·5 or a supine-to-standing drop of 10 mm Hg in diastolic blood pressure(7).
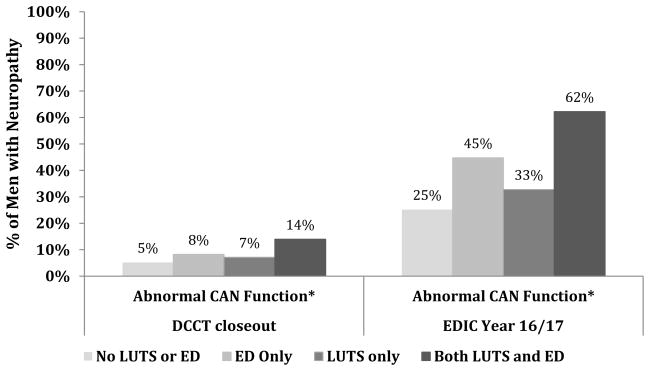
At EDIC year 16/17, R-R variation and Valsalva ratios demonstrated a substantial decline from DCCT closeout (Table 2). Additionally, both R-R variation and Valsalva ratios were significantly lower in participants with ED and/or LUTS compared with those without ED and LUTS with the lowest values observed among men with both ED and LUTS (p<0·0001 for all) (Table 2). Similarly, the prevalence of an abnormal composite CAN at EDIC year 16/17 was higher in participants with ED and/or LUTS compared with those without ED and LUTS (p<0·0001; Figure 3).
Figure 3.
Prevalence of CAN at DCCT closeout and EDIC year 16/17 by ED and LUTS status at EDIC year 17. Abnormal CAN function defined as either R-R variation<15 or R-R variation between 15–19·9 plus either a Valsalva ratio≤1·5 or a supine-to-standing drop of 10 mm Hg in diastolic blood pressure7.
Table 3 presents adjusted odds of ED and LUTS based on quantitative CAN measures at DCCT closeout and EDIC year 16/17. Men with lower R-R variation at DCCT closeout had significantly increased odds of having both ED and LUTS at EDIC year 17; each 5-unit change in R-R variation was associated with an 11% increased odds of ED and LUTS. The same relationship was seen at year 16/17. Stronger associations were seen for each ½-unit change in Valsalva ratio (at both time points) for ED and LUTS. Lower R-R variation at either time point was not associated with presence of ED only or LUTS only. Additionally, lower Valsalva ratio at DCCT closeout was associated with 47% increased odds of ED only at year 17.
Table 3.
Adjusted odds of ED and/or LUTS at year 17 by quantitative CAN measures at DCCT closeout and EDIC year 16/17
| Characteristic | ED only (N=193) vs. No LUTS or ED (N=284) | LUTS only (N=61) vs. No LUTS or ED (N=284) | ED & LUTS (N=97) vs. No LUTS or ED (N=284) |
|---|---|---|---|
| CAN Measures* | Odds ratio (95% CI) | ||
| At DCCT Closeout | |||
| R-R Variation | 0·96 (0·91–1·02) | 0·98 (0·90–1·07) | 1·11 (1·01–1·22) |
| Valsalva Ratio | 1·47 (1·10–1·96) | 1·31 (0·90–1·92) | 1·50 (1·01–2·21) |
| At EDIC Year 16/17 | |||
| R-R Variation | 1·01 (0·94–1·08) | 0·98 (0·89–1·08) | 1·12 (1·01–1·25) |
| Valsalva Ratio | 1·43 (0·99–2·08) | 0·90 (0·56–1·45) | 2·11 (1·17–3·78) |
Data are odds ratios (95% confidence intervals) from separate multivariable logistic regression models with ED/LUTS status as the dependent variable and CAN measures as the independent variables. The odds ratios were evaluated according to a 5-unit decrease in R-R Variation or a ½-unit decrease in Valsalva ratio. Adjustments were made for DCCT cohort assignment, DCCT/EDIC time-weighted HbA1c, DCCT/EDIC time-weighted systolic blood pressure, and the following EDIC year 17 characteristics: age, BMI, smoking status, drinking status, and any antihypertensive medication use.
Sample sizes vary based on availability of CAN data.
Table 4 presents adjusted odds of ED and LUTS based on qualitative CAN measures at DCCT closeout and EDIC year 16/17. Abnormal R-R variation, Valsalva ratio, and CAN at year 16/17 were associated with increased odds of ED and LUTS. No other associations were observed.
Table 4.
Adjusted odds of ED and/or LUTS by qualitative CAN measures at DCCT closeout and EDIC year 16/17
| Characteristic | ED only (N=193) vs. No LUTS or ED (N=284) | LUTS only (N=61) vs. No LUTS or ED (N=284) | ED & LUTS (N=97) vs. No LUTS or ED (N=284) |
|---|---|---|---|
| CAN Measures* | |||
| At DCCT Closeout | |||
| R-R Variation <15 | 1·47 (0·58–3·71) | 1·67 (0·42–6.65) | 2·64 (0·95–7·28) |
| Valsalva Ratio ≤1·5 | 1·83 (0·90–3·72) | 1·08 (0·37–3·17) | 0·87 (0·31–2·44) |
| Abnormal CAN Function** | 1·31 (0·57–3·03) | 1·42 (0·42–4·79) | 2·10 (0·80–5·52) |
| At EDIC Year 16/17 | |||
| R-R Variation <15 | 1·38 (0·86–2·23) | 0·55 (0·24–1·26) | 2·40 (1·30–4·43) |
| Valsalva Ratio ≤1·5 | 1·33 (0·82–2·15) | 1·60 (0·80–3·19) | 1·89 (1·01–3·55) |
| Abnormal CAN Function** | 1·27 (0·80–2·01) | 1·15 (0·58–2·29) | 2·65 (1·47–4·79) |
Data are odds ratios (95% confidence intervals) from separate multivariable logistic regression models with ED/LUTS status as the dependent variable and CAN measures as the independent variables. The odds ratios were evaluated according to the presence or absence of an R-R<15, Valsalva≤1·5, or abnormal CAN function. Adjustments were made for DCCT cohort assignment, DCCT/EDIC time-weighted HbA1c, DCCT/EDIC time-weighted systolic blood pressure, and the following EDIC year 17 characteristics: age, BMI, smoking status, drinking status, and any antihypertensive medication use.
Sample sizes vary based on availability of CAN data.
Abnormal CAN function defined as either R-R variation<15 or R-R variation between 15–19·9 plus either a Valsalva ratio≤1·5 or a supine-to-standing drop of 10 mm Hg in diastolic blood pressure(7).
DISCUSSION
This study examines the association between CAN and ED and LUTS in a large cohort of male participants with T1DM in the DCCT/EDIC study. We found a strong association between CAN, ED and LUTS suggesting that CAN may be a useful surrogate biomarker of more generalized autonomic neuropathy and may predict the development of ED and LUTS in men with long-standing T1DM. Although experimental evidence suggests that autonomic dysfunction may be an important factor in the etiology of ED and possibly LUTS, these findings are the first to systematically demonstrate a link between CAN and ED/LUTS in a large cohort of well-characterized men with T1DM.
Our findings confirm previously reported associations between CAN and ED observed in a cohort of ~9,000 Italian diabetic men,15 in which, the strongest risk factor for ED was the presence of autonomic neuropathy (OR [95%CI], 5·0 [3·9–6·4])15. In contrast to our study, the bedside testing performed by Fedele et al was not comprehensive or standardized. Several studies have also described possible associations between neuropathic complications and BPH/LUTS.16, 17,18 However these were limited by insensitive neuropathy evaluations and/or small sample. Another study specifically assessed the association of bladder dysfunction and autonomic neuropathy as detected by sympathetic skin response. Although the sample size was small and a certain degree of bladder dysfunction was observed in all patients with diabetes, there was a trend for worse indices in those patients without a sympathetic skin response.18
The observed association between ED/LUTS and CAN has biological plausibility. ED pathogenesis in diabetes is multifactorial, but experimental evidence suggests an important role for neuropathy. This is mediated through impaired nitric oxide (NO) release and NO-synthase function of nonadrenergic noncholinergic neurons, decreased smooth muscle relaxation of the corpus cavernosum, impaired sensation of the glans, and abnormal motor function of skeletal muscles participating in erection.19 These act in concert with endothelial cell dysfunction, chronic inflammation and accelerated atherosclerosis, contributing to both ED and a higher cardiovascular disease risk. Bladder dysfunction in diabetes is also multifactorial and can include an alteration in the physiology of the detrusor smooth muscle cell, the innervation or function of the neuronal component, or urothelial dysfunction.20 Several biological interrelationships between ED and LUTS have been postulated including 1) NOS/NO levels decreased or altered in the prostate and penile smooth muscle, 2) autonomic hyperactivity effects on LUTS, prostate growth and ED, 3) increased Rho-kinase activation/endothelin activity, and 4) prostate and penile ischemia.21
Our data provide new insight into the mechanisms of ED and LUTS in T1DM, confirming the role of CAN, as participants who developed ED/LUTS during EDIC had significantly lower R-R variation at DCCT closeout. These data also provide strong evidence that autonomic dysfunction in T1DM is a complex systemic phenomena, which eventually may involve multiple autonomic visceral pathways. Interestingly, the association with CAN was strongest in the group with both ED and LUTS, suggesting that men with ED/LUTS may be a high-risk subphenotype of diabetic autonomic dysfunction. These associations persisted after adjusting for known risk factors such as age, glycemic control, blood pressure, use of antihypertensive medications, smoking or drinking. This has important clinical and prognostic implications. First, these findings demonstrate that asymptomatic changes consistent with CAN predict the development of ED/LUTS, which could guide intervention/prevention strategies to mitigate urological complications. Second, the onset/progression of ED/LUTS associated with CAN may be a potential harbinger of CVD. CVD remains the major cause of mortality among patients with T1DM.7 ED is a well-recognized index of cardiovascular risk and a predictor of coronary artery disease factors.22 Furthermore, several studies have reported an association between BPH/LUTS and CVD, which demonstrate greater LUTS among men with one 23 or more 23–25 cardiovascular risk factors. Similarly, CAN complicates T1DM and is an independent CVD risk factor in affected patients.2, 3, 26
This study has several limitations including: reliance on a single item definition of ED and having concomitant CAN, ED and LUTS evaluations at one point. However, this cohort was quite young at DCCT closeout and the prevalence of ED/LUTS was likely very low. In addition, given the almost exclusively Caucasian nature of this cohort, we cannot extrapolate this work to other races. The strengths of this study include: the large sample size of men with T1DM; standardized, high quality, repeated CAN evaluations and the collection of validated measures of ED/LUTS. Additionally, this cohort of participants with T1DM has been followed for ~30 years and has been carefully characterized for many other risk factors, including blood pressure, lipids, BMI, smoking, and micro and macrovascular complications.
CONCLUSIONS
The association between CAN, ED and LUTS in the DCCT/EDIC cohort suggests that CAN may be a useful surrogate biomarker of more generalized autonomic neuropathy that plays a role in ED and LUTS development in men with long-standing T1DM. Future studies examining the progressive decline in sexual and urinary function associated with presence of CAN as a marker of CVD are warranted.
Supplementary Material
Acknowledgments
A complete list of participants in the DCCT/EDIC research group can be found in New England Journal of Medicine, 2011;365:2366–2376.
Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), OmniPod® Insulin Management System (Bedford, MA), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi-Aventis (Bridgewater NJ).
Funding/Support: The DCCT/EDIC has been supported by cooperative agreement grants (1982–93, 2012–2017), and contracts (1982–2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and Clinical Translational Science Center Program (2006-present), Bethesda, Maryland, USA.
Footnotes
Trial Registration: clinicaltrials.gov NCT00360815 and NCT00360893.
Additional statement for collaborators: Additional support for this DCCT/EDIC collaborative study was provided by an R01 grant (2009–2013) with the National Institute of Diabetes and Digestive and Kidney Disease (5R01DK083927-03).
References
- 1.Pop-Busui R. Cardiac Autonomic Neuropathy in Diabetes A clinical perspective. Diabetes Care. 2010;33:434. doi: 10.2337/dc09-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pop-Busui R, Evans GW, Gerstein HC, et al. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:1578. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spallone V, Ziegler D, Freeman R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes/metabolism research and reviews. 2011;27:639. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 4.Norman EC, Mary AC. Erectile Dysfunction and Diabetes Mellitus: Mechanistic Considerations from Studies in Experimental Models. Current Diabetes Reviews. 2007;3:149. doi: 10.2174/157339907781368977. [DOI] [PubMed] [Google Scholar]
- 5.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pop-Busui R, Low P, Waberski B, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the DCCT/EDIC Study. Circulation. 2009;119:2886. doi: 10.1161/CIRCULATIONAHA.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wessells H, Penson DF, Cleary P, et al. Effect of Intensive Glycemic Therapy on Erectile Function in Men With Type 1 Diabetes. The Journal of Urology. 2011;185:1828. doi: 10.1016/j.juro.2010.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Den Eeden SK, Sarma AV, Rutledge BN, et al. Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care. 2009;32:664. doi: 10.2337/dc07-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 12.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. Journal of Urology. 1992;148:1549. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 13.The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT) Diabetologia. 1998;41:416. doi: 10.1007/s001250050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low PA, Denq JC, Opfer-Gehrking TL, et al. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Fedele D, Coscelli C, Santeusanio F, et al. Erectile dysfunction in diabetic subjects in Italy. Gruppo Italiano Studio Deficit Erettile nei Diabetici. Diabetes Care. 1998;21:1973. doi: 10.2337/diacare.21.11.1973. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi C, Sakakibara R, Uchiyama T, et al. Overactive bladder in diabetes: A peripheral or central mechanism? 2007;26 doi: 10.1002/nau.20404. [DOI] [PubMed] [Google Scholar]
- 17.Kebapci N, Yenilmez A, Efe B, et al. Bladder dysfunction in type 2 diabetic patients. 2007;26 doi: 10.1002/nau.20422. [DOI] [PubMed] [Google Scholar]
- 18.Ueda T, Yoshimura N, Yoshida O. Diabetic Cystopathy: Relationship to Autonomic Neuropathy Detected by Sympathetic Skin Response. The Journal of Urology. 1997;157:580. doi: 10.1016/s0022-5347(01)65209-1. [DOI] [PubMed] [Google Scholar]
- 19.Kempler P, Amarenco G, Freeman R, et al. Management strategies for gastrointestinal, erectile, bladder, and sudomotor dysfunction in patients with diabetes. 2011;27 doi: 10.1002/dmrr.1223. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura N, Chancellor MB, Andersson KE, et al. Recent advances in understanding the biology of diabetes_associated bladder complications and novel therapy. BJU international. 2005;95:733. doi: 10.1111/j.1464-410X.2005.05392.x. [DOI] [PubMed] [Google Scholar]
- 21.McVary KT, McKenna KE. The relationship between erectile dysfunction and lower urinary tract symptoms: epidemiological, clinical, and basic science evidence. Current Urology Reports. 2004;5:251. doi: 10.1007/s11934-004-0047-1. [DOI] [PubMed] [Google Scholar]
- 22.Araujo Ab, Hall SA, Ganz P, Chiu GR, et al. Does erectile dysfunction contribute to cardiovascular disease risk prediction beyond the Framingham risk score? doi: 10.1016/j.jacc.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbons EP, Colen J, Nelson JB, et al. Correlation between risk factors for vascular disease and the American Urological Association Symptom Score. BJU international. 2007;99:97. doi: 10.1111/j.1464-410X.2007.06548.x. [DOI] [PubMed] [Google Scholar]
- 24.NIH state-of-the-science conference statement on prevention of fecal and urinary incontinence in adults. NIH Consens State Sci Statements. 2007;24:1. [PubMed] [Google Scholar]
- 25.Ponholzer A, Temml C, Wehrberger C, et al. The Association Between Vascular Risk Factors and Lower Urinary Tract Symptoms in Both Sexes. European Urology. 2006;50:581. doi: 10.1016/j.eururo.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.