Abstract
NFAT transcription factors are key regulators of gene expression in immune cells. In addition, NFAT1‐induced genes play diverse roles in mediating the progression of various solid tumors. Here we show that NFAT1 induces the expression of the IL8 gene by binding to its promoter and leading to IL8 secretion. Thapsigargin stimulation of breast cancer cells induces IL8 expression in an NFAT‐dependent manner. Moreover, we show that NFAT1‐mediated IL8 production promotes the migration of primary human neutrophils in vitro and also promotes neutrophil infiltration in tumor xenografts. Furthermore, expression of active NFAT1 effectively suppresses the growth of nascent and established tumors by a non cell‐autonomous mechanism. Evaluation of breast tumor tissue reveals that while the levels of NFAT1 are similar in tumor cells and normal breast epithelium, cells in the tumor stroma express higher levels of NFAT1 compared to normal stroma. Elevated levels of NFAT1 also correlate with increased neutrophil infiltrate in breast tumors. These data point to a mechanism by which NFAT1 orchestrates the communication between breast cancer cells and host neutrophils during breast cancer progression.
Keywords: Nuclear factor of activated T cells 1, Interleukin, Breast cancer, Neutrophil infiltration
Highlights
NFAT1 is a transcriptional regulator of IL8 in breast cancer.
Active NFAT1 promotes IL8 production and neutrophil migration.
Active NFAT1 suppresses tumor growth in an IL8‐independent mechanism.
NFAT1 levels correlate with neutrophil infiltration in breast tumor samples.
1. Introduction
Nuclear Factor of Activated T cells (NFAT) transcription factors orchestrate key functions in the immune system and also play multiple roles in tumor progression. Although NFATs have been extensively studied in T lymphocytes, all immune cells, including B cells and dendritic cells, as well as epithelial cells, express NFAT (Mancini and Toker, 2009; Muller and Rao, 2010). The subcellular localization and transcriptional activities of NFAT1‐4 isoforms are tightly regulated by calcium flux, the calcium/calmodulin‐dependent phosphatase calcineurin, and maintenance and export kinases (Muller and Rao, 2010). Ligand‐mediated activation of cell surface receptors leads to an increase in cytoplasmic calcium through the endoplasmic reticulum and calcium‐release activated calcium (CRAC) channels on the plasma membrane, thereby activating calcineurin. Calcineurin dephosphorylates critical residues on NFATs leading to nuclear translocation. Maintenance and export kinases such as glycogen synthase kinase‐3, casein kinase 1 and the dual specificity tyrosine phosphorylation‐regulated kinases rephosphorylate nuclear NFATs thus relocalizing them to the cytoplasm for another round of activation (Muller and Rao, 2010).
In addition to immune cells, both epithelial cells and various types of solid tumors express one or more NFATs that modulate signaling pathways and phenotypes associated with malignancy. For example, NFAT isoforms differentially regulate cell growth and proliferation (Buchholz et al., 2006; Flockhart et al., 2009; Koenig et al., 2010; Robbs et al., 2008). Moreover, NFAT1 has been shown to promote the migration and invasion of breast cancer cells. For example, Akt1 inhibits the migration of breast cancer cells by regulating the turnover of NFAT1 (Yoeli‐Lerner et al., 2005). Moreover, the protein products of several NFAT target genes that modulate the migratory function of NFAT1 have been described, including autotaxin, cyclooxygenase‐2, and glypican‐6 (Chen and O'Connor, 2005; Yiu et al., 2011; Yiu and Toker, 2006). In addition, T‐cell‐expressed NFAT1 participates in tumor‐induced immunosuppression by regulating the expression of cytokines that promote T cell anergy (Abe et al., 2012).
Tumors maintain dynamic communication with the surrounding stromal cells and thereby create an immunologically permissive environment that also facilitates adequate supply of nutrients and oxygen. NFATs contribute to cellular processes that promote cancer progression, and some studies have examined the function of NFAT in the context of tumor–stromal interactions. For example, calcineurin/NFAT modulate the tumor microenvironment by regulating VEGF signaling and angiogenesis (Armesilla et al., 1999; Jinnin et al., 2008; Qin et al., 2006; Ryeom et al., 2008). VEGF activates NFAT, which promotes angiogenesis through the expression of tissue factor (Armesilla et al., 1999). Furthermore, NFAT drives the expression of VEGFR1 (Jinnin et al., 2008). Similarly, the Down syndrome critical region 1 gene DSCR1 encodes a regulator of calcineurin, whose splice variants differentially regulate angiogenesis through NFAT (Qin et al., 2006; Ryeom et al., 2008). NFAT2 has also been shown to promote tumor growth by cell‐autonomous and non‐cell‐autonomous mechanisms by promoting cell cycle progression, invasive capacity, and expression of mitogenic cytokines (Oikawa et al., 2013; Robbs et al., 2008; Tripathi et al., 2013). These reports highlight the intimate connection between NFAT and phenotypes that govern tumor initiation and progression.
Previous studies have demonstrated that NFAT1 is a key regulator of breast cancer cell migration through the specific induction of genes that enhance these phenotypes (Chen and O'Connor, 2005; Yiu and Toker, 2006). Here we have investigated the mechanism by which NFAT1 modulates communication between tumor and host cells in breast cancer. We show that NFAT1 promotes the transcriptional induction of IL8 and that this stimulates neutrophil migration, leading to increased intratumoral neutrophil infiltration in breast cancer xenograft tumors.
2. Results
2.1. NFAT1 regulates the expression of IL8 in MDA‐MB‐231 breast cancer cells
NFAT1 contributes to cell‐autonomous processes such as migration, but its role in tumor–stromal interactions is not completely understood. To evaluate NFAT1‐mediated transcriptional induction of soluble factors that contribute to tumor–stromal interactions, MDA‐MB‐231 human breast cancer cells were infected with inducible NFAT1 shRNA and mRNA collected 72 h after induction with doxycycline. Using quantitative RT‐PCR, mRNA copy numbers of selected secreted factors known to play important roles in the tumor microenvironment were determined (Supplementary Table 1). The analysis reveals that certain genes are not expressed in MDA‐MB‐231 cells (mRNA copy number per cell <1; not shown); others are expressed at a low to moderate (1‐10 mRNA copy number per cell) or high levels (>10 mRNA copy number per cell). Interestingly, a reproducible decrease in IL8 mRNA is observed upon NFAT1 silencing. To validate the RT‐PCR analysis, two distinct NFAT1 shRNA sequences were used, and we show that their induction attenuates IL8 mRNA (Figure 1A) and protein expression (Figure 1B) in MDA‐MB‐231 cells. A concomitant decrease in secreted IL8 upon NFAT1 silencing is also observed as measured by ELISA (Figure 1C). These data indicate that NFAT1 promotes IL8 expression.
Figure 1.
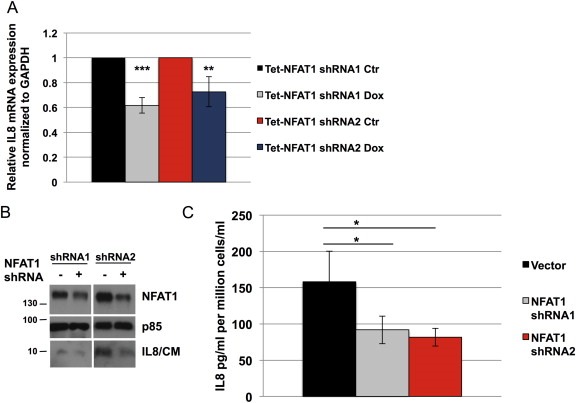
Silencing NFAT1 decreases IL8 expression. A, RT‐qPCR of IL8 mRNA expression in MDA‐MB‐231 cells in response to the silencing of NFAT1 by shRNA sequences #1 and #2 (doxycycline 300 ng/ml, 72 h). B–C, MDA‐MB‐231 cells transduced with NFAT1 shRNA 1 and shRNA 2 and treated with doxycycline (300 ng/ml, 72 h), and IL8 expression assessed by immunoblotting (B) and ELISA (C; CM: conditioned media). Statistical significance was determined by Student's unpaired t‐test (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001. All results are representative of at least 3 independent experiments.
2.2. NFAT1 activity and ER stress induce IL8 transcription
We next evaluated the mechanism by which NFAT1 regulates IL8 expression. To this end, we used a constitutively active mutant of NFAT1 containing multiple serine to alanine mutations on the regulatory domain, exposing the nuclear localization sequence and rendering NFAT1 unresponsive to kinases that regulate its nuclear export. Expression of a doxycycline‐inducible, constitutively active NFAT1 mutant significantly increases IL8 mRNA (Figure 2A). This induction is accompanied by an increase in secreted IL8 protein in both MDA‐MB‐231 (Figure 2B and C) as well as in non‐tumorigenic MCF10A and Ras‐transformed MCF10A‐Ras cells (Supplementary Figure S1).
Figure 2.
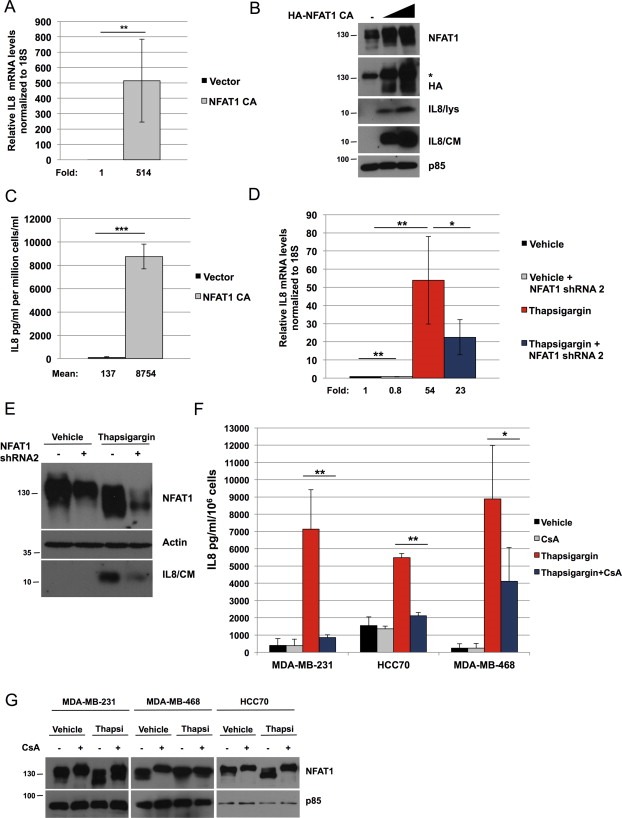
NFAT1 promotes the expression of IL8. A, MDA‐MB‐231 cells transduced with active NFAT1 (doxycycline 200 ng/ml, 48 h) and IL8 mRNA measured by RT‐qPCR, normalized to 18S. B–C, MDA‐MB‐231 cells transduced with active NFAT1 (doxycycline 100 or 400 ng/ml, 72 h), and IL8 expression determined by immunoblot (B; lys: lysate; CM: conditioned media; *denotes an unspecific band) and ELISA (C; n = 3). D–E, MDA‐MB‐231 cells transduced with NFAT1 shRNA #2 or vector control, and treated with vehicle or thapsigargin for 24 h, and IL8 expression assed by RT‐qPCR (D) or immunoblotting (E; CM: conditioned media). F, indicated triple‐negative cell lines were pre‐treated with cyclosporin A (CsA, 1 μM, 1 h) or vehicle, and then treated with thapsigargin (thapsi, 50 nM) for 24 h, after which conditioned media was filtered and used for ELISA to measure IL8. G, the indicated cells lines were pre‐treated with cyclosporin A (CsA, 1 μM, 24 h) or vehicle and thapsigargin (thapsi, 200 nM, 24 h), and lysates immunoblotted with the indicated antibodies. Statistical significance was determined by Student's unpaired t‐test. *p < 0.05; **p < 0.01; ***p < 0.001. All results are representative of at least 3 independent experiments.
Previous studies have demonstrated a role for ER stress in the induction of IL8 (Bobrovnikova‐Marjon et al., 2004; Marjon et al., 2004; Yu et al., 2001). Consistent with the notion that NFAT1 mediates IL8 induction, stimulation of cells with the ER stress‐inducing agent thapsigargin markedly enhances IL8 mRNA (Figure 2D) and secreted IL8 (Figure 2E), and this is attenuated by NFAT1 shRNA. Thapsigargin‐stimulated IL8 expression is observed also in MDA‐MB‐468 and HCC70 triple negative breast cancer (TNBC) cell lines (Figure 2F). However, the non‐TNBC lines MCF7, SKBR3 and T47D do not display a similar phenotype (Supplementary Figure S2A), suggesting that ER stress‐mediated, NFAT‐dependent IL8 induction may be particularly pronounced in this breast cancer molecular subtype. IL8 induction in TNBC lines is NFAT‐mediated, since it is markedly decreased by the calcineurin inhibitor cyclosporin A (Figure 2F). In both TNBC and non‐TNBC cell lines, thapsigargin promotes an increase in NFAT1 electrophoretic mobility shift, indicative of dephosphorylation and activation (Figure 2G, Supplementary Figure S2B). In addition, the ER stress markers C/EBP‐homologous protein (CHOP) and 78‐kDa glucose‐regulated protein (GRP78) are upregulated in response to thapsigargin treatment in breast cancer cell lines irrespective of TN status (Supplementary Figure S2C). Therefore, NFAT1 contributes to ER stress‐induced expression of IL8.
2.3. NFAT1 binds to the proximal IL8 promoter to enhance gene expression
Sequence analysis of the IL8 promoter reveals an evolutionarily conserved sequence conforming to the minimal NFAT‐binding element (TGGAAT; Figure 3A). A 1.4‐kb fragment containing the NFAT consensus motif upstream of the IL8 promoter translational start site was cloned into a luciferase reporter plasmid. Co‐expression of active NFAT1 strongly induces the expression of the wild‐type IL8 reporter (Figure 3B). Point mutations in the NFAT1 consensus sequence abrogate the increase in luciferase activity induced by NFAT1 expression, indicating that this conserved motif on the IL8 promoter harbors an NFAT1‐binding site (Figure 3B). To determine binding of endogenous NFAT1 to the IL8 promoter, we performed chromatin immunoprecipitation using primers spanning the putative NFAT1 consensus sequence, or primers spanning a 150‐bp part of the IL8 coding region. NFAT1 occupies the IL8 promoter under basal conditions, and this is increased following thapsigargin treatment, and blocked in the presence of cyclosporin A (Figure 3C). Together, these data demonstrate that IL8 is an endogenous target of NFAT1 in MDA‐MB‐231 breast cancer cells.
Figure 3.
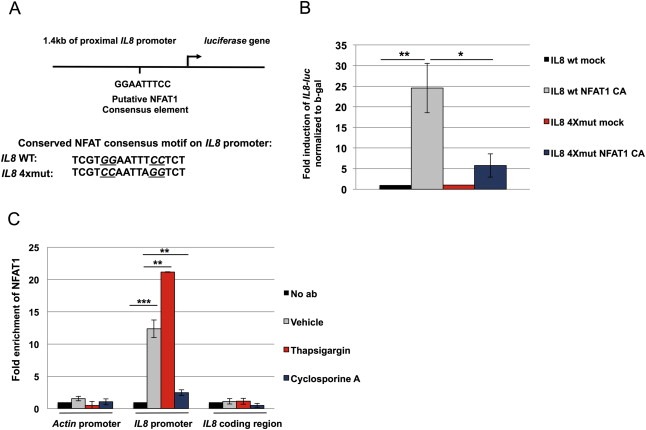
NFAT1 regulates the transcription of the IL8 gene by associating with the proximal promoter. A, Figure depicts a proximal segment of the endogenous IL8 promoter 1.4 kb upstream of translation start site, along with the sequence of the conserved NFAT consensus binding motif and the IL8‐luc 4Xmut reporter, in which crucial NFAT‐binding bases have been mutated. B, 293T cells were transfected with the IL8‐luc wt or IL8‐luc 4Xmut reporter along with either pcDNA3‐HA‐NFAT1 CA or pcDNA3 vector. Luciferase activity was normalized to beta‐galactosidase activity. C, MDA‐MB‐231 cells treated with vehicle, thapsigargin or cyclosporin A were lysed, cross‐linked and NFAT1 immunoprecipitated, followed by PCR using primers for IL8 promoter or actin coding region and IL8 coding region to control unspecific binding. Statistical significance was determined by Student's unpaired t‐test. *p < 0.05; **p < 0.01; ***p < 0.001. All results are representative of at least 2 independent experiments.
2.4. Cancer cell‐derived IL8 promotes the migration of neutrophils
Since MDA‐MB‐231 cells do not express the IL8 receptors CXCR1 and CXCR2 (Supplementary Figure S3), it is unlikely that IL8 functions in a cell‐autonomous manner to modulate autocrine phenotypes in this cell line. Neutrophils, in turn, express both CXCR1 and CXCR2 and thus may relay IL8‐stimulated responses (Knall et al., 1997). Neutrophils also facilitate angiogenesis, suppress antitumor immune responses and promote mutagenesis through the generation of reactive oxygen species (Dumitru et al., 2013). We therefore evaluated whether NFAT1‐stimulated IL8 expression modulates neutrophil signaling and migration.
Both recombinant human IL8 and MDA‐MB‐231‐secreted IL8 stimulate the activation of pS6K1, pAkt and pERK1/2 signaling pathways in isolated primary human neutrophils (Figure 4A). Pretreatment of neutrophils with an IL8‐neutralizing antibody significantly decreases the activation of pS6K1, whereas pAkt and pErk1/2 are less affected. Consistent with this, both recombinant IL8 and tumor cell‐derived IL8 promote neutrophil Transwell migration and this induction is blocked when cells are pre‐treated with the specific S6K1 inhibitor PF‐4708671 (Figure 4B). Next, conditioned media was collected from MDA‐MB‐231 cells engineered to express inducible active NFAT1 combined with constitutively expressed IL8 shRNA, revealing potent suppression of secreted IL8 protein (Figure 4C). Conditioned media collected from untreated breast cancer cells modestly increases migration of neutrophils (Figure 4D). Neutrophil migration is further increased, when cancer cells are induced to express active NFAT1, whereas concomitant silencing IL8 abrogates this effect. In addition, the production of extracellular reactive oxygen species (ROS) is more pronounced in neutrophils treated with conditioned media collected from MDA‐MB‐231 cells overexpressing active NFAT1 than in neutrophils exposed to conditioned media from wild‐type MDA‐MB‐231 cells or recombinant IL8 only (Figure 4E). Collectively, these data support a model whereby NFAT1 activity in cancer cells promotes neutrophil activation and where tumor cell‐derived IL8 is both necessary and sufficient to promote neutrophil migration.
Figure 4.
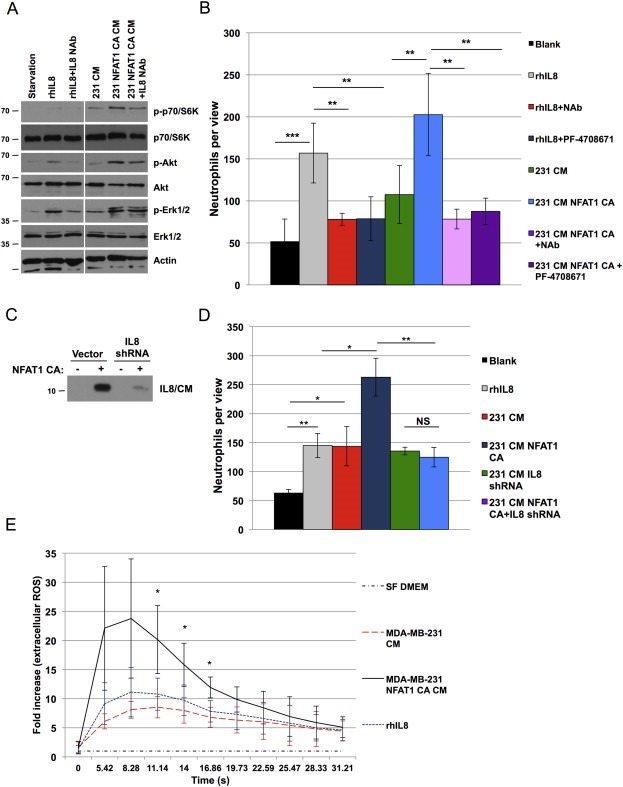
NFAT1‐induced IL8 expression promotes migration of human neutrophils. A, Primary human neutrophils were treated either with recombinant human IL8 (rhIL8; 10 ng/ml, 20 min) or with serum‐free conditioned media collected from either untreated MDA‐MB‐231 cells (231 CM) or cells in which the overexpression of NFAT1 was induced (231 NFAT1 CA CM; doxycycline 200 ng/ml, 48 h). Where indicated IL8‐neutralizing antibody was added (IL8 NAb; 50 μg/ml, 10 min). B, neutrophils were preactivated with fMLP (10 μM, 10 min), pretreated with IL8‐neutralizing antibody (NAb) or S6K1 inhibitor PF‐4708671 (5 μM), and added to transwell chambers in migration assays for 1 h towards serum‐free media (Blank), recombinant IL8 (rhIL8), or conditioned media collected from untreated MDA‐MB‐231 cells (231 CM), or cells in which the overexpression of NFAT1 had been induced (231 NFAT1 CA CM; doxycycline 200 ng/ml, 48 h). C, immunoblotting of IL8 in conditioned media (CM) collected from MDA‐MB‐231 cells transduced with NFAT1 CA with and without IL8 shRNA. D, neutrophils pre‐treated with fMLP as above were added to Transwell chambers and migrated towards recombinant human IL8 (rhIL8; 10 ng/ml), or conditioned media collected from the conditions in C. Graphs show mean numbers of neutrophils per 20× view ±SD. E, neutrophils were treated with serum‐free DMEM, rhIL8 (final concentration 1 ng/ml), or conditioned media collected after 2‐h incubation from MDA‐MB‐231 cells that were either untreated or treated with doxycycline (200 ng/ml, 48 h) to induce expression of active NFAT1 (final dilution of conditioned media 100×). Extracellular reactive oxygen species (ROS) was measured immediately afterwards and recorded over the indicated period of time and normalized to negative control. Statistical significance was determined by Student's unpaired t‐test. *p < 0.05, **p < 0.01; ***p < 0.001. All results are representative of at least 3 independent experiments.
2.5. NFAT1 promotes intratumoral neutrophil infiltration in an IL8‐dependent manner
To determine whether NFAT1‐induced IL8 stimulates neutrophil infiltration in vivo, we generated orthotopic breast cancer xenografts. A significant decrease in tumor mass and volume of MDA‐MB‐231 xenografts harboring tetracycline‐inducible active NFAT1 is observed when compared to control xenografts (Figure 5A and B). Similarly, MDA‐MB‐231 xenografts harboring IL8 shRNA also show a decrease in tumor mass and volume, albeit to a lesser extent than observed with NFAT1 expression. While NFAT1‐expressing xenografts remain small throughout the course of the experiment, combination of active NFAT1 and IL8 shRNA does not yield any resectable tumors at 12 weeks post‐inoculation (Figure 5B). To determine whether NFAT1 functions in a similar manner in established tumors, we treated established xenograft tumors with doxycycline to induce NFAT1 expression 9 weeks after inoculation. Remarkably, expression of active NFAT1 in established tumors results in regression in 15 days (Figure 5C), further supporting a tumor‐suppressive role for NFAT1.
Figure 5.
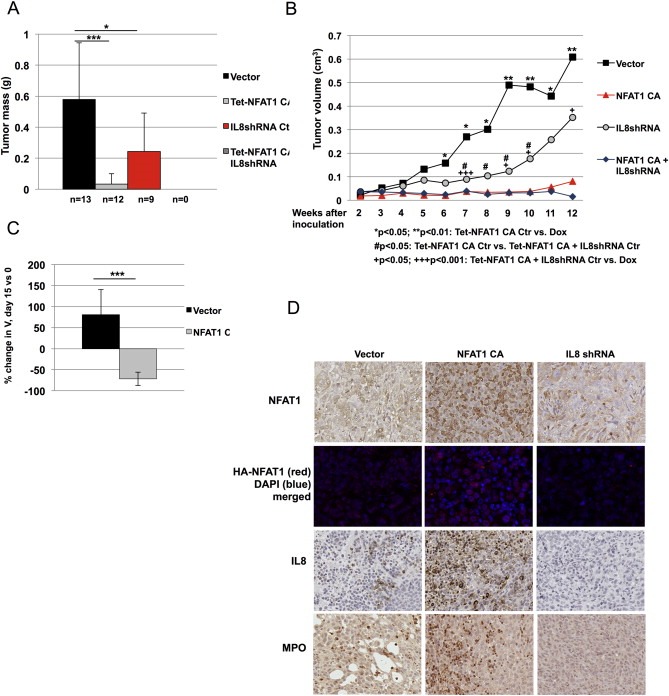
NFAT1 promotes IL8 production, neutrophil infiltration and suppresses xenograft tumor growth. A–B, 0.3 × 106 MDA‐MB‐231 cells containing inducible active NFAT1 plasmid either with or without constitutively expressed IL8 shRNA were inoculated into the mammary fat pads of 7‐week‐old female nude mice. Doxycycline was added to the drinking water of the NFAT1 CA Dox group. Tumor volume was measured weekly for 12 weeks starting two weeks after inoculation, and tumors weighed at end point. No tumors were detected at end point of the experiment in the group expressing active NFAT1 and IL8 shRNA. Statistical significance was determined by Student's unpaired t‐test. *,+,#p < 0.05; **p < 0.01; +++p < 0.001. C, NFAT1 expression was induced by doxycycline 9 weeks after tumor initiation, and tumor growth was monitored for 15 days every other day. Bar graph shows mean percent change in tumor volume on day 15 compared to day 0. Statistical significance was determined by Student's unpaired t‐test. ***p < 0.001. D, Xenograft tumor sections were stained against NFAT1, HA, IL8, and myeloperoxidase to monitor NFAT1 and IL8 expression and neutrophil infiltration, respectively.
Immunohistochemical and immunofluorescence analysis of the resulting xenografts grown for 12 weeks in Figure 5A confirms the expression of NFAT1 with a concomitant increase in IL8 staining in NFAT1‐overexpressing tumors (Figure 5D). We next stained paraffin sections of xenograft tumors against myeloperoxidase to detect intratumoral neutrophil infiltration. While a moderate number of neutrophils are present in control xenografts, tumors expressing active NFAT1 display an increase in neutrophil infiltration. By contrast, silencing of IL8 in tumor cells decreases the myeloperoxidase signal in these tumors. Because the silencing of NFAT1 does not affect tumor growth (Supplementary Figure S4), and overexpression of active NFAT1 does not affect the cell cycle profile of MDA‐MB‐231 cells (Supplementary Figure S5), we propose that NFAT1‐induced IL8 regulates tumor progression primarily by a non cell‐autonomous mechanism.
2.6. NFAT1 expression correlates with neutrophil infiltration in breast tumors
Finally, we evaluated epithelial and stromal NFAT1 expression in central tumor, tumor invasive front, and normal breast using tissue microarrays constructed from archival clinical breast cancer material. Examples of NFAT1 and MPO immunohistochemical staining in healthy tissue, tumor core and tumor periphery are shown (Figure 6A). Computational analyses of IHC staining shows overall increased expression of NFAT1 in the tumor invasive front compared to the tumor central region and normal breast (Figure 6B). The increased overall NFAT1 expression in the tumor invasive front is due to significantly increased NFAT1 levels in both the cancer epithelium and associated stroma (Figure 6C). We also analyzed neutrophil infiltration using myeloperoxidase staining and find that myeloperoxidase and NFAT1 levels correlate positively (Figure 6D). We do not see a significant difference in MPO levels in triple negative tumors compared to non‐triple negative tumors (p = 0.11; Supplementary Figure S6A) or Her2‐negative tumors compared to Her2‐positive tumors (p = 0.24; Supplementary Figure S6B). However, the tumors negative for estrogen receptor do express significantly higher levels of stromal MPO than tumors that are ER positive (Figure 6E). In summary, our results suggest that NFAT1 may promote intratumoral neutrophil recruitment in breast cancer.
Figure 6.
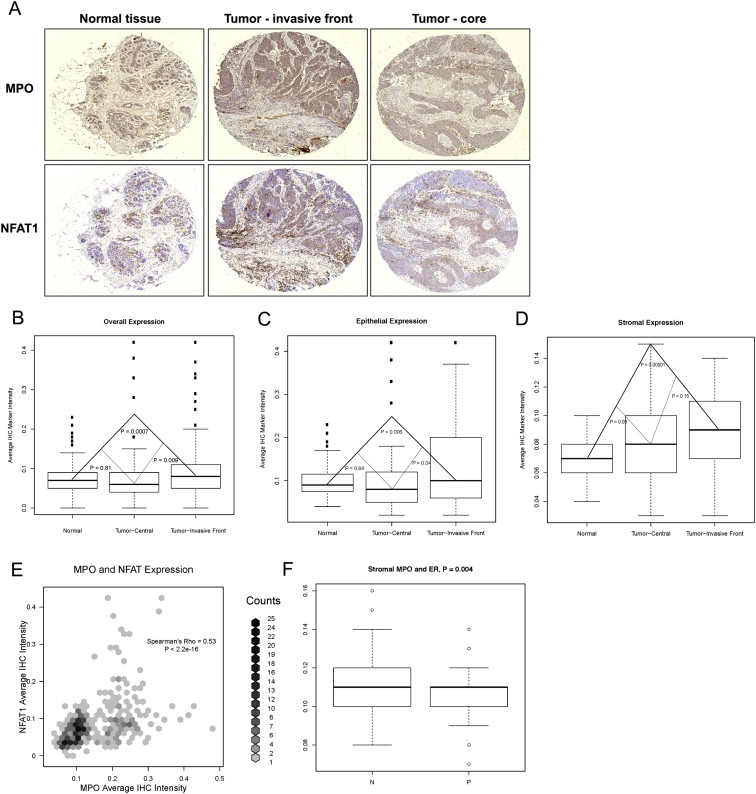
NFAT1 expression correlates with neutrophil infiltration in breast tumor tissue microarrays. Tissue microarrays of matched invasive breast cancer and normal breast tissue of 49 patients were stained with antibodies against NFAT1 and myeloperoxidase (MPO), and relative expression levels were quantified computationally and reported as average IHC marker pixel intensity per cell. A, Representative examples of average NFAT1 and MPO marker intensity are shown for normal tissue, tumor core and tumor periphery. B, Tumor invasive front displays higher overall levels of NFAT1 than the central parts of the tumor or the normal breast. In contrast, there is no difference in overall NFAT1 expression between central tumor region and normal breast. C, NFAT1 is expressed at higher levels in tumor invasive front epithelium than in the central epithelium or normal breast epithelium. Stromal NFAT1 levels are higher both in the central and invasive front tumor stroma than in normal breast stroma. Statistical significance was determined by Student's T test. D, Intensity of NFAT1 staining correlates with strong myeloperoxidase expression, indicating that NFAT1 expression may promote neutrophil infiltration into the breast tumors. Correlation was determined by Spearman's rank correlation coefficient (Spearman's rho). E, Stroma in tumors that are negative for estrogen receptor (ER) displays higher levels of MPO than stroma in tumors that are positive for estrogen receptor. Statistical significance was determined by Student's T test. ER: estrogen receptor; N: negative; P: positive.
3. Materials and methods
3.1. Cell culture
HEK293T, MDA‐MB‐231, MDA‐MB‐435, and MDA‐MB‐468 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Cellgro, Manassas, VA) and HCC70 cells in RPMI‐1640 (Cellgro) supplemented with 10% of fetal bovine serum (HyClone, Waltham, MA) or tet‐tested fetal bovine serum (Clontech, Mountain View, CA).
3.2. Lentiviral silencing and cDNA overexpression
HA‐NFAT1 CA was a gift from Anjana Rao (Addgene plasmid #11792, pENTR11 CA NFAT1) and cloned into pcDNA3 using HindIII and XbaI. For shRNA, oligonucleotides for NFAT1 and IL8 shRNA were annealed and cloned into the pLKO lentiviral expression system (Chin and Toker, 2010). For NFAT1 lentiviral overexpression, constitutively active HA‐NFAT1 was cloned into the pTRIPZ vector using AgeI and ClaI. To produce lentiviral particles, 293T cells were transfected with the above vectors, VSVG, and psPAX2 for 48 h, after which supernatants were collected and passed through 0.45 μm filters.
shRNA oligo sequences were as follows:
NFAT1 shRNA1: sense 5′‐CCGGtccttaagccgcacgccttctCTCGAGagaaggcgtgcggcttaagga TTTTTG‐3′; anti‐sense 5′‐AATTCAAAAAtccttaagccgcacgccttctCTCGAGagaaggcgtgcggcttaagga‐3′.
NFAT1 shRNA2: sense 5′‐CCGGccgagtccaaagttgtgtttaCTCGAGtaaacacaactttggactcgg TTTTTG‐3′; anti‐sense 5′–AATTCAAAAAccgagtccaaagttgtgtttaCTCGAGtaaacacaactttggactcgg‐3′.
IL8 shRNA: sense 5′‐CCGGccgaactttaatttcaggaatCTCGAGattcctgaaattaaagttcggTTTTTG‐3′; anti‐sense 5′‐AATTCAAAAAccgaactttaatttcaggaatCTCGAGattcctgaaattaaagttcgg‐3′.
3.3. Cell treatments
Cells were treated with 200 nM thapsigargin (Santa Cruz Biotechnologies, Dallas, TX) for 20 h. Where indicated, cells were pretreated with 1 μM cyclosporine A (Sigma–Aldrich, St. Louis, MO) for 1 h. After treatments, cells were harvested, and conditioned media was collected and filtered through 0.45 μm filters.
Primary human neutrophils were isolated as described (Zhu et al., 2006). Isolated cells were resuspended in serum‐free RPMI and, where indicated, pretreated with IL8‐neutralizing antibody (0.3 μg/ml, R&D Systems, Minneapolis, MN) or pS6K1 inhibitor PF‐4708671 (5 μM; Calbiochem Millipore, Billerica, MA) for 10 min prior to treatment with recombinant human IL8 (10 ng/ml; R&D Systems) or conditioned serum‐free media for 20 min before harvesting.
3.4. Quantitative real‐time RT‐PCR and multi‐gene transcriptional profiling (MGTP)
Total RNA was isolated using the RNeasy kit (Qiagen, Hilden, Germany). Reverse transcription was performed using random hexamers and SuperScript III (Life Technologies, Carlsbad, CA) or Multiscribe reverse transcriptase (Applied Biosystems, Carlsbad, CA). Quantitative real‐time RT‐PCR was performed using SYBR Green PCR Master Mix (BioRad, Hercules, CA) and the 7500 Fast Real‐Time PCR Systems (Life Technologies) or ABI Prism 7900 sequence detector (Applied Biosystems). Quantification of mRNA expression was calculated by the ΔCT method with GAPDH or 18S as reference. MGTP was used to determine mRNA copy numbers per cell as previously described (Shih and Smith, 2005; Wada et al., 2011). The number of mRNA copies per cell was calculated by normalizing to 18S rRNA assuming an average of 106 18S rRNA copies per cell. Mean values from cDNA samples from separate experiments were calculated to determine final mean and SD.
Real‐time quantitative PCR primer sequences were as follows:
18S Forward 5′‐TCGAGGCCCTGTAATTGGAA‐3′
18S Reverse 5′‐CCCTCCAATGGATCCTCGTT‐3′
GAPDH Forward 5′‐GCAAATTCCATGGCACCGT‐3′
GAPDH Reverse 5′‐TCGCCCCACTTGATTTTGGAGG‐3′
NFAT1 Forward 5′‐CAGCTTCATTTCTGACACCTTCTC‐3′
NFAT1 Reverse 5′‐GGGAATAATGAGCAGGGATGTTT‐3′
IL8 Forward 5′‐CTCTGTGGTATCCAAGAATCAGTGA‐3′
IL8 Reverse 5′‐TATTGCATCTGGCAACCCTACA‐3′
CHOP Forward 5′‐CTGCTTCTCTGGCTTGGCTG‐3′
CHOP Reverse 5′‐GCTCTGGGAGGTGCTTGTGA‐3′
GRP78 Forward 5′‐GTTCTTGCCGTTCAAGGTGG‐3′
GRP78 Reverse 5′‐TGGTACAGTAACAACTGCATG‐3′
3.5. Chromatin immunoprecipitation
Chromatin immunoprecipitation was according to a modified version of a previously published protocol (Takahashi et al., 2000). Briefly, 20 × 106 MDA‐MB‐231 cells were cross‐linked with formaldehyde and the reaction quenched with glycine. Cells were washed and lysed with lysis buffer (1% SDS, 50 mM Tris–HCl pH8, 10 mM EDTA, Proteinase inhibitor cocktail, 50 nM calyculin), and the chromatin was fragmented by sonication. For immunoprecipitation of DNA‐bound protein complexes, sonicated lysate was diluted in IP buffer (150 mM NaCl, 20 mM Tris–HCl pH 8, 1% Triton X‐100, Proteinase inhibitor cocktail, 50 nM calyculin). Sonicated lysate was precleared and incubated overnight at 4 °C with and without antibody (anti‐NFAT1 XP, Cell Signaling Technologies, Danvers, MA). Blocked protein A/G beads (Amersham Biosciences, Pittsburgh, PA) were added to the lysate and the samples incubated for 1 h at 4 °C. Beads were washed and Tris–EDTA buffer and 0.5% SDS were added, and proteins were digested by proteinase K for 2 h at 37 °C. Subsequently, RNase A was added to digest RNA at 37 °C for 2 h. Cross‐links were reversed by overnight incubation at 65 °C. DNA was phenol:chloroform extracted and ethanol‐precipitated. DNA samples were used as templates in quantitative real‐time RT‐PCR. Mean values of technical triplicates from separate experiments were calculated to determine final means and SD.
ChIP RT‐qPCR primer sequences were as follows:
Actin promoter Forward 5′‐CAGTGCCTAGGTCACCCACT‐3′; Actin promoter Reverse 5′‐AGAAGTCGCAGGACCACACT‐3′.
IL8 promoter Forward 5′‐AAGTGTGATGACTCAGGTTTGC‐3′; IL8 promoter Reverse 5′‐GAAGCTTGTGTGCTCTGCTG‐3′.
IL8 reading frame Forward 5′‐CAGCCAAAACTCCACAGTCA‐3′; IL8 reading frame Reverse 5′‐TTTCCATACAATCAGAAACAGTAAAAAA‐3′.
3.6. Immunoblotting
Immunoblotting was carried out as described (Chin and Toker, 2010). Cells were lysed in lysis buffer (150 mM NaCl, 50 mM Tris–HCl [pH 7.4], 2 mM EDTA, 1% Triton X‐100, Proteinase inhibitor cocktail, 50 nM calyculin) and centrifuged (15,000 × g, 10 min, 4 °C). Proteins were resolved by SDS‐PAGE and transferred electrophoretically to nitrocellulose membranes (Bio‐Rad). Membranes were blocked with 5% milk and incubated with primary antibodies against NFAT1, p‐pS6K1, p‐S6K1, p‐Erk1/2, Erk1/2, p‐AktSer473, and pan‐Akt (Cell Signaling Technologies); IL8 (Santa Cruz Biotechnologies); actin (Sigma); p85 (produced in‐house (Kapeller et al., 1995)); and HA (purified from 12CA5 hybridoma). Membranes were washed, incubated with horseradish peroxidase‐conjugated secondary antibody, and developed with enhanced chemiluminescence substrate (Millipore, Billerica, MA).
3.7. ELISA
Equal numbers of cells were plated and media were collected 48–72 h after plating. IL8 in the media was determined using the Duoset ELISA kit (BD Biosciences, San Jose, CA), and the results were analyzed according to manufacturer's instructions.
3.8. Luciferase reporter assay
1.4‐kb fragment of the IL8 proximal promoter was amplified from genomic DNA and ligated into the pGL2 basic vector. To generate pGL2‐IL84Xmut‐luc mutant construct, four point mutations were introduced to the putative NFAT1 binding site. pGL2‐IL8wt‐luc or pGL2‐IL84Xmut‐luc were transfected together with beta‐galactosidase and NFAT1 plasmids. 48 h after transfection cells were harvested and lysed, after which luciferase and beta‐galactosidase activities were detected by the Luciferase Assay system (Promega, Madison, WI) and the Tropix® Galacto‐Star assay system (Applied Biosystems, Foster City, CA). Luminescence was detected by BioTek Synergy 2 luminometer (BioTek, Winooski, VT).
3.9. Transwell migration
Primary human neutrophils were isolated as previously described (Zhu et al., 2006) from buffy coats prepared from whole blood of healthy volunteers (protocol IBC‐P00000090). Starved cells were pre‐activated by N‐formyl‐Met‐Leu‐Phe (Nova Biochem, Darmstadt, Germany) for 10 min, washed, and treated with inhibitors or IL8‐neutralizing antibody for 10 min where indicated. 1.5 × 105 cells resuspended in serum‐free medium containing 0.1% BSA were added to 3 μm‐pore Transwell migration chambers (BD Biosciences, Franklin Lakes, NJ). Conditioned medium collected from MDA‐MB‐231 cells or serum‐free DMEM with indicated treatments was added to the lower chambers. After a 1hr incubation at 37 °C, non‐migrated cells were removed from the filters and the migrated cells were fixed, stained using the Hema‐3 staining set (Fisher Scientific, Pittsburgh, PA), and counted.
3.10. Detection of extracellular ROS production
Primary human neutrophils were isolated from buffy coats and red blood cells were lysed. Isolated polymorphonuclear cells were diluted to 106 cells/ml into Hank's balanced salt solution containing calcium, magnesium and 0.2% BSA. Extracellular ROS production was measured using isoluminol (VWR Scientific, Radnor, PA) and type XII horseradish peroxidase (Sigma). Kinetic measurement of luminescent signal was performed using TriStar spectrometer (Berthold Technologies, Bad Wildbad, Germany). Before measurements, neutrophils were subjected to serum‐free DMEM, recombinant human IL8 in serum‐free DMEM (final concentration 1 ng/ml), or conditioned media collected from MDA‐MB‐231 cells that overexpress active NFAT1 in a doxycycline‐inducible fashion (final dilution 100×). Results were recorded as arbitrary units and the readings were normalized to negative control.
Conditioned media for neutrophil stimulations were collected from MDA‐MB‐231 cells 24 h after culture in serum‐free DMEM, either with or without doxycycline (200 ng/ml, 48 h), and filtered through 0.45 μm filters.
3.11. Mouse xenografts
For orthotopic injection of tumor cells into 7‐week‐old female athymic nude mice (Taconic, Hudson, NY), 3 × 105 cells from stably infected MDA‐MB‐231 pools were resuspended 1:1 in Matrigel (BD Biosciences) and injected into the fourth mouse mammary fat pad. 0.8 mg/ml doxycycline was provided in the drinking water where indicated. Tumors were measured weekly or as indicated, and tumor volume was assessed using the formula 0.5(length × width2). At the end of the experiment, mice were euthanized by CO2. All studies were conducted following the Institutional Animal Care and Use Committee guidelines of Beth Israel Deaconess Medical Center.
3.12. Tissue microarray construction
Paraffin tissue blocks of invasive ductal carcinoma resection specimens from 49 patients were obtained from the archives of the Department of Pathology at the Beth Israel Deaconess Medical Center under an institutionally approved IRB protocol for discarded de‐identified tissues. Paraffin blocks containing representative tumor areas and separate blocks containing normal breast tissue were identified on the corresponding hematoxylin/eosin‐stained sections, and digital images of the selected blocks were created. Areas of interest were identified and marked on the digital image using the Pannoramic Viewer, 3D‐Histech (Budapest, Hungary). The source block was cored, and 1.5‐mm cores were transferred to the recipient TMA master block, 3D‐Histech. Two representative cores of tumor (central and invasive front) and two cores of the normal breast tissue (normal terminal duct lobular unit and normal stroma) were arrayed for each of 49 specimens, except for one specimen that had no available normal breast tissue.
3.13. Immunohistochemistry and immunofluorescence
IHC staining was performed on tissue microarray sections and formalin‐fixed, paraffin‐embedded xenografts. Sections were blocked and incubated with anti‐NFAT1 (Cell Signaling Technology), anti‐IL8 (R&D) and anti‐Myeloperoxidase (Abcam) antibodies. Slides were washed and incubated with biotinylated donkey anti‐rabbit secondary antibody or biotinylated donkey anti‐goat secondary antibody (Jackson Immuno Research), and enhanced with Elite Vectashield ABC kit (Vector Lab). The slides were developed in diaminobenzidine metal enhanced kit (Vector lab) and counterstained with hematoxylin. After dehydration steps, the slides were mounted with Permount. IHC images were taken using Zeiss AxioImager M1 microscope, AxioCamHR camera, 20×/0.8 Plan‐Apochromat objective, and AxioVision software.
For immunofluorescence, xenograft tumor sections were treated with sodium borohydride to reduce autofluorescence, washed, blocked and incubated with Rabbit anti‐HA (Rockland). Slides were then washed and incubated with Alexa 549 conjugated donkey anti‐Rabbit secondary antibody for 90 min at room temperature. Samples were then washed and mounted with Prolong Gold anti‐fade mounting media with DAPI. Confocal images were taken using a Zeiss LSM510 Meta confocal system using Zeiss LSM510 image acquisition software. The images were taken using a 20×/0.8 Plan‐Apochromat objective and a 40×/1.3 Oil Plan‐Apochromat objective.
3.14. Tissue microarray image analysis
Computational image analysis of NFAT1 and myeloperoxidase staining of the tissue microarray samples were performed with Definiens Tissue Studio image analysis software (Definiens AG, Munich, Germany). For both stains, epithelial and stromal classifiers were trained to provide tissue‐region specific expression measurements. Expression was measured as average IHC marker pixel intensity per cell. To analyze the statistical significance of differences in marker expression between tissue regions, Student's T‐test was used. Correlation between NFAT1 and myeloperoxidase expression was determined by calculating Spearman's rank correlation coefficient (Spearman's rho).
4. Discussion
Although originally identified as key modulators of the immune response in humans, the NFAT transcription factors have also been recognized as regulators of cancer progression (Mancini and Toker, 2009). In this context, the calcium‐responsive NFAT isoforms have been shown to modulate multiple phenotypes associated with malignancy both in vitro and in mouse models. NFATs enhance the invasive migration of breast cancer cells through the induction of genes whose products catalyze the synthesis of soluble secreted motogenic factors, such as lysophosphatidic acid and prostaglandins (Chen and O'Connor, 2005; Yiu and Toker, 2006). Similarly, NFATs may promote or inhibit the proliferation of cancer cells through multiple redundant mechanisms (Baumgart et al., 2012; Perotti et al., 2012; Robbs et al., 2008). Moreover, endothelial calcineurin/NFAT signaling controls tumor angiogenesis through the expression of VEGF (Baek et al., 2009; Ryeom et al., 2008). Thus, NFAT‐induced genes function in both cell‐autonomous and paracrine mechanisms to modulate cancer progression at multiple levels.
The present study adds to our understanding of the complex mechanisms by which NFATs regulate cancer phenotypes by demonstrating that IL8 is an NFAT‐induced gene in breast cancer. We provide evidence for a model of NFAT‐mediated, endoplasmic reticulum stress‐induced expression of IL8 in breast cancer cells, particularly in triple negative breast cancer cell lines. Cancer cell‐derived IL8 promotes the migration and intratumoral infiltration of neutrophils in a non cell‐autonomous manner, which counteracts the overall tumor‐suppressing effect of active NFAT1. In addition, cancer cell‐expressed NFAT1 generates soluble factors that potently induce ROS production and thus neutrophil activation. Analyses of tissue microarrays from primary breast tumors and matched normal tissue reveals that while the overall levels of NFAT1 are similar in malignant and normal tissue, tumor‐associated stromal cells express elevated levels of NFAT1 compared to normal stroma. In tissue microarrays collected from 49 patients, stromal myeloperoxidase expression is higher in tumors negative for estrogen receptor compared to tumors that are estrogen receptor‐positive. Moreover, NFAT1 levels in these patient samples correlate with myeloperoxidase expression, pointing to a link between NFAT1 activity and neutrophil infiltration in breast cancer.
Several studies have elucidated the transcriptional control of IL8 induction in cancer, demonstrating the involvement of the NF‐κB pathway, nuclear hormone receptor activity and the JAK2/STAT5 pathway (Bobrovnikova‐Marjon et al., 2004; Britschgi et al., 2012; Kanda and Watanabe, 2001; Karashima et al., 2003; Patel et al., 2000). The finding that IL8 is an NFAT1 target gene is also consistent with previous reports that have revealed upregulation of IL8 in response to PGE2α stimulation mediated by calcineurin/NFAT in endometrial adenocarcinoma (Sales et al., 2009) and the binding of transcriptional enhancers close to a putative AP1‐NFAT binding motif in the IL8 promoter in colonic epithelial cells in response to PGE2α (Srivastava et al., 2012). Our data show that NFAT1 binds to the IL8 promoter and drives the transcription of IL8 constitutively and during ER stress. Thapsigargin‐induced IL8 secretion is not restricted to MDA‐MB‐231 cells, but occurs also in other triple‐negative breast cancer cell lines, while the levels of IL8 remain significantly lower in non‐triple negative cell lines despite similar activation of NFAT1. In agreement with these findings, ER stress as well as hypoxia, low pH, oxidative stress, and nutrient deprivation all promote the expression of IL8 (Bobrovnikova‐Marjon et al., 2004; Lakshminarayanan et al., 1998; Marjon et al., 2004; Shi et al., 1999; Yu et al., 2001). We find that while thapsigargin treatment activates NFAT1 and upregulates ER stress markers in all the studied cell lines, IL8 is only efficiently induced in triple‐negative cell lines. This suggests that the differential induction of IL8 is not due to disparate stress responses or NFAT regulation, but more likely results from distinct epigenetic landscapes, pre‐existing cofactors and favorable chromatin architecture on the IL8 gene. The NFAT binding site on the IL8 promoter overlaps with a putative NF‐κB site and has been shown to bind recombinant NFAT1 (Jin et al., 2003). This raises the intriguing possibility of crosstalk between the NFAT1 and NF‐κB transcriptional networks in the regulation of the IL8 gene.
Studies of NFAT1 in tumor progression have yielded contradictory results, likely reflecting the context dependency of the NFAT1 transcriptome. Although the silencing of NFAT1 retards the growth of melanoma xenografts (Braeuer et al., 2012), we do not detect significant reduction in tumor growth upon NFAT1 silencing in MDA‐MB‐231 breast cancer xenografts. Instead, we find that overexpressed active NFAT1 suppresses tumor growth, consistent with previous reports showing a similar phenotype in fibroblast‐derived xenografts (Robbs et al., 2008). However, since active NFAT1 does not alter the cell cycle profile of MDA‐MB‐231 cells, but its expression still effectively suppresses established tumors, we propose a model in which tumor suppression occurs in a non‐cell‐autonomous manner.
MDA‐MB‐231 cells do not express the IL8 receptors CXCR1 and CXCR2, yet the silencing of IL8 reduces tumor growth, suggesting that this reduction occurs in a non cell‐autonomous manner. Although high levels of IL8 may counteract the tumor‐suppressing activity of NFAT1, this effect is insufficient to negate the overall effect of active NFAT1, suggesting that NFAT1‐mediated tumor suppression is most likely due to secreted factors other than IL8. In addition, silencing of NFAT1 likely affects the expression of numerous target genes whose products may overcome the net effect of IL8 reduction, thereby masking any potential IL8‐dependent phenotypes. We propose that tumor cells harboring active NFAT1 initiate a potent immunological antitumor response. Additional studies are needed to characterize the spectrum of target genes responsible for NFAT1‐mediated tumor suppression.
Few studies have addressed the causal role of NFAT transcription factors in mediating chemokine signaling. NFAT4 recruits neutrophils through CXCL2 transcription during acute pancreatitis (Awla et al., 2012). In addition, NFAT2 contributes to a mitogenic tumor microenvironment by inducing multiple chemokine genes (Tripathi et al., 2013). Because MDA‐MB‐231 cells do not express IL8 receptors, we assessed the recruitment of CXCR1/2‐expressing neutrophils. In agreement with previous findings (Gomez‐Cambronero et al., 2003; Henkels et al., 2011), we show that IL8 enhances neutrophil migration and that tumor cell‐expressed IL8 promotes intratumoral neutrophil infiltration. Interestingly, studies have revealed a connection between NFAT activation, neutrophil recruitment and chemokine expression during sepsis (Zhang et al., 2014), supporting the biological basis of our model. Combined expression of active NFAT1 and silencing of IL8 has an additive effect in tumor suppression, indicative of a tumor‐promoting role for IL8. In this context, similar to tumor‐associated macrophages, neutrophils may display either N1‐like anti‐tumor or N2‐like pro‐tumor properties, depending on the nature and strength of environmental cues (Fridlender and Albelda, 2012; Houghton, 2010). Our results point to NFAT1‐IL8‐mediated neutrophil function favoring the N2 over the N1 type, although more studies are needed to reaffirm this hypothesis. In this context, a fully immunocompetent model organism whose genome includes an IL8 ortholog could provide additional insight. Although human IL8 binds to murine CXCR1 and CXCR2 (Sparmann and Bar‐Sagi, 2004), neither mouse nor rat express IL8 (van der Aa et al., 2010; Zlotnik et al., 2006), and thus do not represent ideal models for testing these hypotheses.
Thus far, suppression of the calcineurin/NFAT pathway has been proposed as a potential therapeutic intervention in colorectal cancer and metastatic melanoma (Flockhart et al., 2009; Spreafico et al., 2013). Moreover, neutralizing antibodies against IL8 or CXCR1/2 suppress tumor growth, angiogenesis, and metastasis in melanoma and bladder cancer (Huang et al., 2002; Mian et al., 2003; Sparmann and Bar‐Sagi, 2004), and in breast cancer, circulating neutrophils provide a first line of defense against metastatic seeding (Granot et al., 2011). Although expression of active NFAT1, in turn, promotes the migration of breast cancer cells in vitro (Jauliac et al., 2002) potentially increasing their metastatic potential, it is possible that pronounced expression of IL8 might counteract metastatic progression by attracting neutrophils in vivo.
Analysis of normal and malignant breast patient tissue samples reveals similar expression levels of NFAT1 in tumor cells and normal epithelium. However, total NFAT1 levels are not necessarily indicative of NFAT1 activity. In contrast to tumor epithelium, expression levels of NFAT1 are several‐fold higher in tumor‐associated stromal cells than in normal stroma. This supports previous observations of immune cell‐expressed NFAT1 as a potential regulator of tumorigenesis (Abe et al., 2012). Moreover, NFAT1 expression correlates with myeloperoxidase expression, supporting the model that NFAT1‐mediated signaling promotes neutrophil infiltration in breast cancer. This may have implications on disease progression, as high neutrophil‐to‐lymphocyte ratio is associated with high mortality in breast cancer patients (Azab et al., 2012). While NFAT1 levels do not correlate with triple‐negativity, estrogen receptor or Her2 status in patient samples (data not shown), tumors negative for estrogen receptor display higher expression of myeloperoxidase and therefore neutrophil infiltration than tumors positive for estrogen receptor. Future experiments on large data sets are warranted to further elucidate the connection between hormone dependency, NFAT pathway and tumor–stromal interactions in breast cancer. Taken together, our study highlights a role for NFAT1 in tumor progression through modulation of the tumor microenvironment.
Author contributions
A.K.and A.T. planned the experiments; A.K, W.S.H., S‐C.J., L.M‐K., E‐Y.H. and L.Z. performed experiments; A.K., S‐C.J, L.M‐.K, H.R.L., A.H.B. and A.T. analyzed the data; A.K. and A.T. wrote the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Supporting information
Supplementary data
Acknowledgments
The authors thank Drs. Joan Brugge, Wolfgang Junger, Tanya Mayadas, Anjana Rao, and Wenyi Wei for providing research materials, Lay‐Hong Ang, Dan Li and Casey Stottrup for expert technical assistance, and the former and present members of the Toker laboratory and Julius Anckar for helpful discussions. This work was supported in part by grants from the Emil Aaltonen Foundation, Osk. Huttunen Foundation, Orion‐Farmos Research Foundation, K. Albin Johansson Foundation (A.K.); the Klarman Family Foundation (A.H.B.); National Institutes of Health (H.R.L., R01 HL092020, P01 HL095489; A.T., R01 CA096710, P01 CA092644); and a Howard Hughes Medical Institute International Student pre‐doctoral fellowship (W.S.H.).
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2015.02.004.
Kaunisto Aura, Henry Whitney S., Montaser-Kouhsari Laleh, Jaminet Shou-Ching, Oh Eun-Yeong, Zhao Li, Luo Hongbo R., Beck Andrew H., Toker Alex, (2015), NFAT1 promotes intratumoral neutrophil infiltration by regulating IL8 expression in breast cancer, Molecular Oncology, 9, doi: 10.1016/j.molonc.2015.02.004.
References
- Abe, B.T. , Shin, D.S. , Mocholi, E. , Macian, F. , 2012. NFAT1 supports tumor-induced energy of CD4(+) T cells. Cancer Res. 72, 4642–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armesilla, A.L. , Lorenzo, E. , Gomez del Arco, P. , Martinez-Martinez, S. , Alfranca, A. , Redondo, J.M. , 1999. Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression. Mol. Cell. Biol. 19, 2032–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awla, D. , Zetterqvist, A.V. , Abdulla, A. , Camello, C. , Berglund, L.M. , Spegel, P. , Pozo, M.J. , Camello, P.J. , Regner, S. , Gomez, M.F. , Thorlacius, H. , 2012. NFATc3 regulates trypsinogen activation, neutrophil recruitment, and tissue damage in acute pancreatitis in mice. Gastroenterology 143, 1352–1360. e1351–1357 [DOI] [PubMed] [Google Scholar]
- Azab, B. , Bhatt, V.R. , Phookan, J. , Murukutla, S. , Kohn, N. , Terjanian, T. , Widmann, W.D. , 2012. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann. Surg. Oncol. 19, 217–224. [DOI] [PubMed] [Google Scholar]
- Baek, K.H. , Zaslavsky, A. , Lynch, R.C. , Britt, C. , Okada, Y. , Siarey, R.J. , Lensch, M.W. , Park, I.H. , Yoon, S.S. , Minami, T. , Korenberg, J.R. , Folkman, J. , Daley, G.Q. , Aird, W.C. , Galdzicki, Z. , Ryeom, S. , 2009. Down's syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature 459, (7250) 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart, S. , Glesel, E. , Singh, G. , Chen, N.M. , Reutlinger, K. , Zhang, J. , Billadeau, D.D. , Fernandez-Zapico, M.E. , Gress, T.M. , Singh, S.K. , Ellenrieder, V. , 2012. Restricted heterochromatin formation links NFATc2 repressor activity with growth promotion in pancreatic cancer. Gastroenterology 142, 388–398. e381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovnikova-Marjon, E.V. , Marjon, P.L. , Barbash, O. , Vander Jagt, D.L. , Abcouwer, S.F. , 2004. Expression of angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 is highly responsive to ambient glutamine availability: role of nuclear factor-kappaB and activating protein-1. Cancer Res. 64, 4858–4869. [DOI] [PubMed] [Google Scholar]
- Braeuer, R.R. , Zigler, M. , Kamiya, T. , Dobroff, A.S. , Huang, L. , Choi, W. , McConkey, D.J. , Shoshan, E. , Mobley, A.K. , Song, R. , Raz, A. , Bar-Eli, M. , 2012. Galectin-3 contributes to melanoma growth and metastasis via regulation of NFAT1 and autotaxin. Cancer Res. 72, 5757–5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britschgi, A. , Andraos, R. , Brinkhaus, H. , Klebba, I. , Romanet, V. , Muller, U. , Murakami, M. , Radimerski, T. , Bentires-Alj, M. , 2012. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell 22, 796–811. [DOI] [PubMed] [Google Scholar]
- Buchholz, M. , Schatz, A. , Wagner, M. , Michl, P. , Linhart, T. , Adler, G. , Gress, T.M. , Ellenrieder, V. , 2006. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 25, 3714–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M. , O'Connor, K.L. , 2005. Integrin alpha6beta4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene 24, 5125–5130. [DOI] [PubMed] [Google Scholar]
- Chin, Y.R. , Toker, A. , 2010. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol. Cell 38, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru, C.A. , Lang, S. , Brandau, S. , 2013. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin. Cancer Biol. 23, 141–148. [DOI] [PubMed] [Google Scholar]
- Flockhart, R.J. , Armstrong, J.L. , Reynolds, N.J. , Lovat, P.E. , 2009. NFAT signalling is a novel target of oncogenic BRAF in metastatic melanoma. Br. J. Cancer 101, 1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender, Z.G. , Albelda, S.M. , 2012. Tumor-associated neutrophils: friend or foe?. Carcinogenesis 33, 949–955. [DOI] [PubMed] [Google Scholar]
- Gomez-Cambronero, J. , Horn, J. , Paul, C.C. , Baumann, M.A. , 2003. Granulocyte-macrophage colony-stimulating factor is a chemoattractant cytokine for human neutrophils: involvement of the ribosomal p70 S6 kinase signaling pathway. J. Immunol. 171, 6846–6855. [DOI] [PubMed] [Google Scholar]
- Granot, Z. , Henke, E. , Comen, E.A. , King, T.A. , Norton, L. , Benezra, R. , 2011. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20, 300–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkels, K.M. , Frondorf, K. , Gonzalez-Mejia, M.E. , Doseff, A.L. , Gomez-Cambronero, J. , 2011. IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3). FEBS Lett. 585, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton, A.M. , 2010. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle 9, 1732–1737. [DOI] [PubMed] [Google Scholar]
- Huang, S. , Mills, L. , Mian, B. , Tellez, C. , McCarty, M. , Yang, X.D. , Gudas, J.M. , Bar-Eli, M. , 2002. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am. J. Pathol. 161, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauliac, S. , Lopez-Rodriguez, C. , Shaw, L.M. , Brown, L.F. , Rao, A. , Toker, A. , 2002. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat. Cell Biol. 4, 540–544. [DOI] [PubMed] [Google Scholar]
- Jin, L. , Sliz, P. , Chen, L. , Macian, F. , Rao, A. , Hogan, P.G. , Harrison, S.C. , 2003. An asymmetric NFAT1 dimer on a pseudo-palindromic kappa B-like DNA site. Nat. Struct. Biol. 10, 807–811. [DOI] [PubMed] [Google Scholar]
- Jinnin, M. , Medici, D. , Park, L. , Limaye, N. , Liu, Y. , Boscolo, E. , Bischoff, J. , Vikkula, M. , Boye, E. , Olsen, B.R. , 2008. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat. Med. 14, 1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda, N. , Watanabe, S. , 2001. 17beta-estradiol, progesterone, and dihydrotestosterone suppress the growth of human melanoma by inhibiting interleukin-8 production. J. Invest. Dermatol. 117, 274–283. [DOI] [PubMed] [Google Scholar]
- Kapeller, R. , Toker, A. , Cantley, L.C. , Carpenter, C.L. , 1995. Phosphoinositide 3-kinase binds constitutively to alpha/beta-tubulin and binds to gamma-tubulin in response to insulin. J. Biol. Chem. 270, 25985–25991. [DOI] [PubMed] [Google Scholar]
- Karashima, T. , Sweeney, P. , Kamat, A. , Huang, S. , Kim, S.J. , Bar-Eli, M. , McConkey, D.J. , Dinney, C.P. , 2003. Nuclear factor-kappaB mediates angiogenesis and metastasis of human bladder cancer through the regulation of interleukin-8. Clin. Cancer Res. 9, 2786–2797. [PubMed] [Google Scholar]
- Knall, C. , Worthen, G.S. , Johnson, G.L. , 1997. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc. Natl. Acad. Sci. USA 94, 3052–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig, A. , Linhart, T. , Schlengemann, K. , Reutlinger, K. , Wegele, J. , Adler, G. , Singh, G. , Hofmann, L. , Kunsch, S. , Buch, T. , Schafer, E. , Gress, T.M. , Fernandez-Zapico, M.E. , Ellenrieder, V. , 2010. NFAT-induced histone acetylation relay switch promotes c-Myc-dependent growth in pancreatic cancer cells. Gastroenterology 138, 1189–1199. e1181–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayanan, V. , Drab-Weiss, E.A. , Roebuck, K.A. , 1998. H2O2 and tumor necrosis factor-alpha induce differential binding of the redox-responsive transcription factors AP-1 and NF-kappaB to the interleukin-8 promoter in endothelial and epithelial cells. J. Biol. Chem. 273, 32670–32678. [DOI] [PubMed] [Google Scholar]
- Mancini, M. , Toker, A. , 2009. NFAT proteins: emerging roles in cancer progression. Nat. Rev. Cancer 9, 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjon, P.L. , Bobrovnikova-Marjon, E.V. , Abcouwer, S.F. , 2004. Expression of the pro-angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 by human breast carcinomas is responsive to nutrient deprivation and endoplasmic reticulum stress. Mol. Cancer 3, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian, B.M. , Dinney, C.P. , Bermejo, C.E. , Sweeney, P. , Tellez, C. , Yang, X.D. , Gudas, J.M. , McConkey, D.J. , Bar-Eli, M. , 2003. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin. Cancer Res. 9, 3167–3175. [PubMed] [Google Scholar]
- Muller, M.R. , Rao, A. , 2010. NFAT, immunity and cancer: a transcription factor comes of age. Nat. Rev. Immunol. 10, 645–656. [DOI] [PubMed] [Google Scholar]
- Oikawa, T. , Nakamura, A. , Onishi, N. , Yamada, T. , Matsuo, K. , Saya, H. , 2013. Acquired expression of NFATc1 Downregulates E-Cadherin and promotes Cancer cell invasion. Cancer Res. 73, 5100–5109. [DOI] [PubMed] [Google Scholar]
- Patel, B.J. , Pantuck, A.J. , Zisman, A. , Tsui, K.H. , Paik, S.H. , Caliliw, R. , Sheriff, S. , Wu, L. , deKernion, J.B. , Tso, C.L. , Belldegrun, A.S. , 2000. CL1-GFP: an androgen independent metastatic tumor model for prostate cancer. J. Urol. 164, 1420–1425. [PubMed] [Google Scholar]
- Perotti, V. , Baldassari, P. , Bersani, I. , Molla, A. , Vegetti, C. , Tassi, E. , Dal Col, J. , Dolcetti, R. , Anichini, A. , Mortarini, R. , 2012. NFATc2 is a potential therapeutic target in human melanoma. J. Invest. Dermatol. 132, 2652–2660. [DOI] [PubMed] [Google Scholar]
- Qin, L. , Zhao, D. , Liu, X. , Nagy, J.A. , Hoang, M.V. , Brown, L.F. , Dvorak, H.F. , Zeng, H. , 2006. Down syndrome candidate region 1 isoform 1 mediates angiogenesis through the calcineurin-NFAT pathway. Mol. Cancer Res. 4, 811–820. [DOI] [PubMed] [Google Scholar]
- Robbs, B.K. , Cruz, A.L. , Werneck, M.B. , Mognol, G.P. , Viola, J.P. , 2008. Dual roles for NFAT transcription factor genes as oncogenes and tumor suppressors. Mol. Cell. Biol. 28, 7168–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryeom, S. , Baek, K.H. , Rioth, M.J. , Lynch, R.C. , Zaslavsky, A. , Birsner, A. , Yoon, S.S. , McKeon, F. , 2008. Targeted deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer Cell 13, 420–431. [DOI] [PubMed] [Google Scholar]
- Sales, K.J. , Maldonado-Perez, D. , Grant, V. , Catalano, R.D. , Wilson, M.R. , Brown, P. , Williams, A.R. , Anderson, R.A. , Thompson, E.A. , Jabbour, H.N. , 2009. Prostaglandin F(2alpha)-F-prostanoid receptor regulates CXCL8 expression in endometrial adenocarcinoma cells via the calcium-calcineurin-NFAT pathway. Biochim. Biophys. Acta 1793, 1917–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Q. , Abbruzzese, J.L. , Huang, S. , Fidler, I.J. , Xiong, Q. , Xie, K. , 1999. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorogenic and metastatic. Clin. Cancer Res. 5, 3711–3721. [PubMed] [Google Scholar]
- Shih, S.C. , Smith, L.E. , 2005. Quantitative multi-gene transcriptional profiling using real-time PCR with a master template. Exp. Mol. Pathol. 79, 14–22. [DOI] [PubMed] [Google Scholar]
- Sparmann, A. , Bar-Sagi, D. , 2004. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 6, 447–458. [DOI] [PubMed] [Google Scholar]
- Spreafico, A. , Tentler, J.J. , Pitts, T.M. , Tan, A.C. , Gregory, M.A. , Arcaroli, J.J. , Klauck, P.J. , McManus, M.C. , Hansen, R.J. , Kim, J. , Micel, L.N. , Selby, H.M. , Newton, T.P. , McPhillips, K. , Gustafson, D.L. , Degregori, J.V. , Messersmith, W.A. , Winn, R.A. , Eckhardt, S.G. , 2013. Rational combination of a MEK inhibitor, Selumetinib, and the Wnt/Calcium pathway modulator, cyclosporin a, in Preclinical models of colorectal Cancer. Clin. Cancer Res. 19, (15) 4149–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, V. , Dey, I. , Leung, P. , Chadee, K. , 2012. Prostaglandin E2 modulates IL-8 expression through formation of a multiprotein enhanceosome in human colonic epithelial cells. Eur. J. Immunol. 42, 912–923. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y. , Rayman, J.B. , Dynlacht, B.D. , 2000. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 14, 804–816. [PMC free article] [PubMed] [Google Scholar]
- Tripathi, P. , Wang, Y. , Coussens, M. , Manda, K.R. , Casey, A.M. , Lin, C. , Poyo, E. , Pfeifer, J.D. , Basappa, N. , Bates, C.M. , Ma, L. , Zhang, H. , Pan, M. , Ding, L. , Chen, F. , 2013. Activation of NFAT signaling establishes a tumorigenic microenvironment through cell autonomous and non-cell autonomous mechanisms. Oncogene 33, (14) 1840–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Aa, L.M. , Chadzinska, M. , Tijhaar, E. , Boudinot, P. , Verburg-van Kemenade, B.M. , 2010. CXCL8 chemokines in teleost fish: two lineages with distinct expression profiles during early phases of inflammation. PloS One 5, e12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, Y. , Li, D. , Merley, A. , Zukauskas, A. , Aird, W.C. , Dvorak, H.F. , Shih, S.C. , 2011. A multi-gene transcriptional profiling approach to the discovery of cell signature markers. Cytotechnology 63, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu, G.K. , Kaunisto, A. , Chin, Y.R. , Toker, A. , 2011. NFAT promotes carcinoma invasive migration through glypican-6. Biochem. J. 440, 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu, G.K. , Toker, A. , 2006. NFAT induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2. J. Biol. Chem. 281, 12210–12217. [DOI] [PubMed] [Google Scholar]
- Yoeli-Lerner, M. , Yiu, G.K. , Rabinovitz, I. , Erhardt, P. , Jauliac, S. , Toker, A. , 2005. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol. Cell 20, 539–550. [DOI] [PubMed] [Google Scholar]
- Yu, Y. , De Waele, C. , Chadee, K. , 2001. Calcium-dependent interleukin-8 gene expression in T84 human colonic epithelial cells. Inflamm. Res. 50, 220–226. [et al.] [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Luo, L. , Wang, Y. , Gomez, M.F. , Thorlacius, H. , 2014. Nuclear factor of activated T cells regulates neutrophil recruitment, systemic inflammation, and T-cell dysfunction in abdominal sepsis. Infect. Immun. 82, 3275–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, D. , Hattori, H. , Jo, H. , Jia, Y. , Subramanian, K.K. , Loison, F. , You, J. , Le, Y. , Honczarenko, M. , Silberstein, L. , Luo, H.R. , 2006. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc. Natl. Acad. Sci. USA 103, 14836–14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik, A. , Yoshie, O. , Nomiyama, H. , 2006. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 7, 243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


