Abstract
There are currently no reports of the identification of stem cells in the human gallbladder. The differences between human gallbladder and intrahepatic bile duct (IHBD) cells have also not been explored. The goals of this study were to evaluate if human fetal gallbladder contains a candidate stem cell population and if fetal gallbladder cells are distinct from fetal IHBD cells. We found that EpCAM+CD44+CD13+ cells represent the cell population most enriched for clonal self-renewal from primary gallbladder. Primary EpCAM+CD44+CD13+ cells gave rise to EpCAM+CD44+CD13+ and EpCAM+CD44+CD13− cells in vitro, and gallbladder cells expanded in vitro exhibited short-term engraftment in vivo. Last, we found that CD13, CD227, CD66, CD26 and CD49b were differentially expressed between gallbladder and IHBD cells cultured in vitro indicating clear phenotypic differences between the two cell populations. Microarray analyses of expanded cultures confirmed that both cell types have unique transcriptional profiles with predicted functional differences in lipid, carbohydrate, nucleic acid and drug metabolism.
In conclusion, we have isolated a distinct clonogenic population of epithelial cells from primary human fetal gallbladder with stem cell characteristics and found it to be unique compared to IHBD cells.
Keywords: Gallbladder cells, intrahepatic bile duct cells, EpCAM, CD44, CD13, stem cells
Introduction
Understanding the resident stem cell populations of the bile duct system is important for both basic biology and developing therapeutic strategies to treat bile duct diseases. The bile duct system is divided into IHBD and extrahepatic bile duct (EHBD) systems. The EHBD system consists of the common hepatic duct, gallbladder, cystic duct and the common bile duct (CBD) (1, 2). The gallbladder stores bile while modifying its content and concentration (3, 4).
There is a paucity of data characterizing stem cells in both adult and fetal human gallbladders. In addition, the differences between IHBD and EHBD cells are not well understood. The EHBD system, liver and the ventral pancreas develop from the posterior ventral foregut endoderm (5–7). However, the cell-intrinsic factors responsible for IHBD and EHBD system specification are unclear. Recently, Spence et al. (8) by using a PDX1-Cre transgenic mouse showed that hepatocytes and IHBD cells descend from Pdx1− cells while the EHBD cells including the ventral pancreas derive from Pdx1+ cells. These data were corroborated by a study in our lab, where we found that adult mouse gallbladder stem cells have a distinct phenotypic and expression profile compared to adult mouse IHBD cells (9). However, the differences between human IHBD and EHBD cells have not yet been explored.
The goals of this study were to evaluate if human fetal gallbladder contains a clonogenic candidate stem cell population and compare its phenotypic and expression profile to those of fetal IHBD cells. The evaluation of human fetal gallbladder stem cells would have important ramifications for the study of congenital diseases such as biliary atresia (10) and gallbladder carcinoma, the most common malignancy of the bile duct system (11), because of the importance of stem cells in development, tissue regeneration and cancer (12). In addition, a comparison of human fetal gallbladder and IHBD cells would further elucidate the ontogeny of bile duct cells and represent the first time that the developmental differences between the human IHBD and EHBD cells have been explored.
Stem cells are defined by their ability for single cell self-renewal and lineage commitment (13). We have previously used colony forming assays along with single cell and morphogenesis assays to characterize a resident stem cell population in adult mouse gallbladder (9). In this report, we adapt these assays to the study of human cells. We identify an EpCAM+CD13+CD44+ epithelial subpopulation from primary human fetal gallbladder that can expand in vitro through seven passages, exhibits single-cell self-renewal and engrafts in the subcutaneous space of immunodeficient mice. Last, we found that expanded human IHBD cells and gallbladder cells had distinct phenotypic and expression profiles with many of the predicted functional differences between both cell types mirroring those from our previous report (9). To our knowledge, this is the first report to prospectively isolate a clonogenic epithelial population from human fetal gallbladder and evaluate its genealogy relative to IHBD cells.
Methods
Gallbladder and IHBD cell isolation and culture
Fetal liver and gallbladder tissues were obtained from the Tissue Bank at the Magee Women’s Hospital of UPMC. All samples were between 19–23 weeks of gestation and none of the fetal gallbladders were obtained from therapeutic abortions. (Supplementary Table 1). The research protocol was reviewed and approved by the Institutional Review Board for Human Research Studies at the University of Pittsburgh. Gallbladders were cut and opened along the middle in order to expose the mucosa and placed in HBSS. Bile was washed off by gently scraping the mucosal surface with blunt-ended forceps. Liver samples were minced into small pieces. Gallbladder and liver samples were incubated with EBSS/10mM EGTA/1% HEPES for 15min at 37C and treated with 1 mg/ml CollagenaseII (Invitrogen, CA) +1mg/ml Hyaluronidase (Sigma) + 100 μg/ml of DNaseI (Roche, IN) for 1–1.5 hrs followed by 0.25%Trypsin /0.1%EDTA (Fisher Scientific, MA) for 30 min to obtain a cell suspension. Cell suspensions were plated on irradiated rat feeder cells as described previously (9).
FACS Analysis
FACS analysis and sorting and subsequent data analysis was performed as previously described (9). LDAs were performed by sorting 1, 10, 25, 50, 100, 200, and 500 cells/well into respective (≥4) columns of 96-well plates (Corning, NY) seeded with irradiated feeders. Colonies were scored after 4–6 weeks post-plating and candidate stem cell frequencies of sorted sub-populations determined in L-Calc™ (StemCell Technologies, Vancouver). In experiments involving expanded cell populations, primary identification of sorted populations involved gating of human (HLA+) cells followed by epithelial (EpCAM+) cells.
Results
EpCAM is a human gallbladder epithelial cell marker
EpCAM is a cell surface marker that was first described in colorectal cancer (14). Its expression has since been found on a wide variety of epithelial cells such as keratinocytes, thymic epithelial cells and IHBD cells (15, 16). Previously, we have determined that mouse gallbladder epithelial cells were EpCAM+, and subsequently used EpCAM to label these cells by flow cytometry (9). EpCAM expression has also been observed on adult human gallbladder epithelial cells (17, 18) but no evidence exists for its expression in fetal gallbladder. We co-stained EpCAM and CK19, a pan biliary marker (19) on cross sections of fetal gallbaldders and found that most CK19+ cells were EpCAM+ (Figure 1A). We subsequently used EpCAM expression to separate fetal gallbladder epithelial cells from non-epithelial cells.
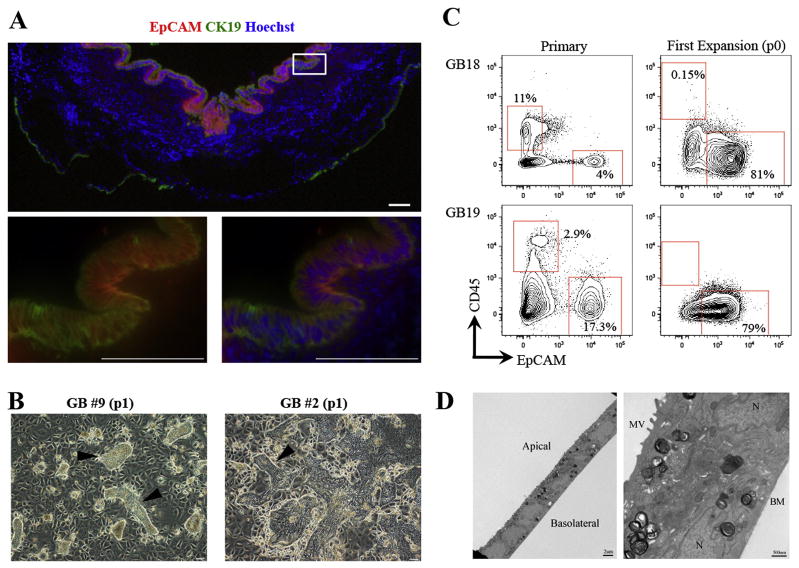
Figure 1. Human fetal gallbladder cells expand in vitro on rat feeder cells.
(A) Sections of human fetal gallbladder were stained with EpCAM (Red) and CK19 (Green), and counterstained for nuclear staining with Hoechst (Blue). Bottom two panels, the magnified images of the white box (top panel). Most CK19+ cells were EpCAM+. (B) Representative pictures of two human gallbladder samples indicating epithelial expansion (arrowheads) on lethally irradiated LA7 rat feeder cells; “p” indicates passage. (C) In vitro growth conditions select for gallbladder epithelial (CD45−EpCAM+) cells. Flow cytometric analyses at primary and first expansion (p0) of two gallbladder samples indicating strong enrichment of CD45−EpCAM+ cells after a single expansion in vitro (p0). The number values assigned to the gates (red boxes) represent the percentage of total live gallbladder cells within that gate. Plots display 5% probability contours. In all plots dead cells were gated out by propidium iodide staining (not shown). For the expanded cells, rat feeder cells were gated out by HLA−ABC staining (not shown). (D) Expanded gallbladder cells exhibit hallmark ultrastructure of bile duct epithelial cells, consisting of small cuboidal cells with defined apical-basolateral polarity and interdigitating lateral membranes. MV: Microvilli, N: Nucleus, BM: Basement membrane. Unless specified otherwise, scale bars: 100μm.
Fetal gallbladder epithelial cells expand in vitro
Similar to our previous study on mouse gallbladder cells (9), human gallbladder cells were cultured in vitro on lethally irradiated rat feeder cells that select for epithelial growth (20). In total 28 fetal gallbladder samples were processed (Supplementary Table 1). All samples placed in culture (n=21) exhibited in vitro expansion (Figure 1B). The hallmarks of these cultures were either cobblestone-like epithelial colonies or colonies comprising small cells with large nuclear to cytoplasmic ratios. Flow cytometry analyses of primary and expanded gallbladder cells at first expansion (p0) showed that feeder cells select for epithelial (EpCAM+) cell expansion (Figure 1C). EpCAM− cells that were sorted from primary gallbladder did not proliferate on rat feeder cells (data not shown). Expanded human gallbladder cells had an ultrastructure typical of bile duct epithelial cells expanding in vitro (Figure 1D). They were small cuboidal cells with defined apical-basolateral polarity, apical microvilli and junctional apparatus. Importantly, feeder cells were ultrastructurally distinct from human gallbladder cells indicating that fusion was not taking place (Supplementary Figure 1). Last, we observed expansion of gallbladder cells for up to seven passages. These data are similar to previous studies with human gallbladder cells (18, 21).
CD13 and CD44 as candidate gallbladder stem cell markers
Since there is a paucity of cell surface markers that select for clonogenic human gallbladder cells, we screened primary and expanded cells with a panel of 46 commercially available cell surface markers of stem and progenitor cells (Supplementary Table 2). As stem cells have the capacity for self-renewal or clonogenicity, we predicted that expansion in vitro would select for cells capable of self-renewal, thereby enriching for potential stem cells. We have successfully used this approach to identify mouse gallbladder stem cell markers (9). Therefore, we looked for cell surface markers that were heterogeneously expressed on primary gallbladder epithelial cells and enriched on expanded gallbladder cells. In this manner, we hoped to identify clonogenic cell surface markers.
Three primary and five expanded gallbladder samples were screened with the foregoing panel of cell surface markers (Supplementary Table 3). We found that the phenotypic profiles of primary gallbladder epithelial cells were variable, with expression of CD54, CD133.2, CD166 and CD104 differing between samples, indicating the potential difference between separate biological samples. Interestingly, expression of CD13, CD44, CD49f and CD81 was consistently heterogeneous on primary gallbladder epithelial cells (Figure 2A, Supplementary Table 3). CD13 (aminopeptidase N) has previously been described as a stem cell marker in the developing mouse liver (22, 23) as well in human hepatocellular carcinoma (24). In addition, recent studies have indicated that CD44 (the receptor for hyaluronic acid) is a human gallbladder cancer stem cell marker (25, 26). CD49f (integrin α-6) has been shown to be a stem cell marker in fetal and adult liver (27–29), the breast (30, 31) and more recently in the adult mouse gallbladder (9). We found that CD44, CD49f, and CD81 co-stained almost completely (Supplementary Figure 2). Therefore, primary gallbladder epithelial cells were divided into CD44+CD49f+CD81+ and CD44−CD49f−CD81− fractions. We chose to focus on CD44 as we observed the cleanest separation of positive and negative populations with this marker.
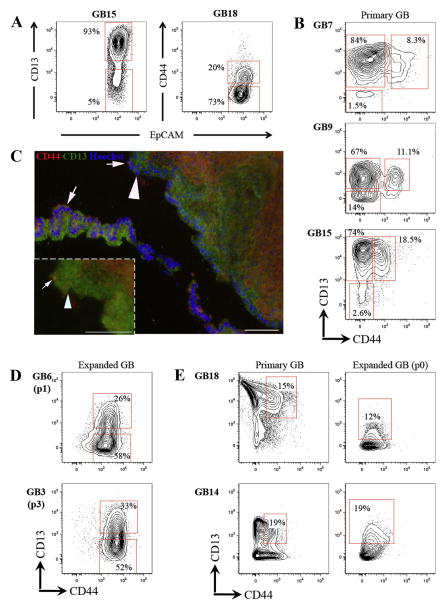
Figure 2. CD13 and CD44 highlight distinct heterogeneity on primary gallbladder epithelial cells and EpCAM+CD44+CD13+ cells expand in vitro.
(A) Flow cytometric profile showing that CD13 and CD44 are heterogeneous in primary human fetal gallbladder epithelial cells. Flow plots represent only EpCAM+CD45− live cells from primary gallbladder (see Fig. 1C). (B) Co-staining of CD13 and CD44 identified three subpopulations in primary human fetal gallbladder epithelial cells: CD13+CD44+, CD44-CD13+ & CD13−CD44− cells. (C) Immunofluoresence analysis of primary gallbladder sections stained with CD44 (Red), CD13 (Green) and counterstained with Hoechst (blue). Images were taken of luminal cells that correspond to epithelial cells. We observed distinct CD44+CD13+ (white arrows) and CD44−CD13+ populations (white arrowheads). CD44−CD13− cells were not noted probably because of the low frequency of the population (see Fig. 1). (D) Flow cytometric profiles showing that expanded gallbladder cells are CD44+ and heterogeneous for CD13. Rat feeder cells and any HLA+EpCAM− cells have been gated out of these plots. (E) Analyses on the same gallbladder sample from primary tissue (d0) and first expansion (p0) suggest that the frequency CD44+CD13+ cells remains constant at early passage. Plots display 5% probability contours and the number values assigned to the gates (red boxes) represent the percentage of live gallbladder epithelial (EpCAM+CD45−) cells within that gate. Scale bars: 100μm.
Co-staining of CD13 and CD44 on primary gallbladder epithelial cells revealed additional heterogeneity (Figure 2B). We noted the presence of three distinct subpopulations of EpCAM+ cells: CD44+CD13+, CD44−CD13+ and CD44−CD13− cells. All three populations did not express the hematopoietic or endothelial markers CD45, CD34 and CD36 (not shown). The CD44−CD13+ subpopulation was the largest in most samples (13 out of 15 samples analyzed), followed by the CD44+CD13+ subpopulation. Heterogeneous expression of CD13 and CD44 was confirmed by immunofluorescence analysis (Figure 2C). We then examined their expression on expanded gallbladder cells to determine if these markers were linked to self-renewal.
Interestingly, expanded EpCAM+ cells exhibited a remarkably conserved phenotypic profile in culture suggesting expansion of a homogeneous subpopulation of cells (Supplementary Table 3). Expanded EpCAM+ cells were CD44+ and heterogeneous for CD13 expression (Figure 2D). Similar to CD44 expression, CD81 and CD49f were expressed on all expanded cells (data not shown). Therefore, we observed two distinct subpopulations of expanded cells: CD44+CD49f+CD81+CD13+ and CD44+CD49f+CD81+CD13− cells. For the sake of simplicity, these subpopulations will be referred to as CD44+CD13+ and CD44+CD13− subpopulations.
Analysis of the same gallbladder samples from primary tissue and at first expansion (p0) revealed that the frequency of CD44+CD13+ cells remained relatively constant (Figure 2E). Based on these data, we hypothesized that the EpCAM+CD44+CD13+ cells in primary gallbladder represented those capable of clonogenic self-renewal.
CD44 and CD13 enrich for clonogenic human gallbladder cells from primary cells
In order to evaluate CD13 and CD44 as clonogenic gallbladder cell markers, we performed Limiting Dilution Analyses (LDAs). The LDA assay quantifies the frequency of the subpopulation of cells capable of forming colonies (32) and was key to the isolation of hematopoietic (33) and neural stem cells (34). More recently, our group has used LDAs to identify mouse gallbladder stem cells (9).
We first determined if CD44 and CD13 could independently enrich for clonogenicity. We separated EpCAM+CD44+ and EpCAM+CD44− cells from primary gallbladders and performed LDAs with the sorted cells. EpCAM+CD44+ cells exhibited a significantly higher colony forming unit (CFU) frequency (1/17) than EpCAM+CD44− cells (1/66) (Table 1A). In a separate experiment, EpCAM+CD13+ cells exhibited a significantly higher CFU frequency (1/28) than EpCAM+CD13− cells (1/195) (Table 1A). Therefore, both CD13 and CD44 independently enrich for colony forming cells.
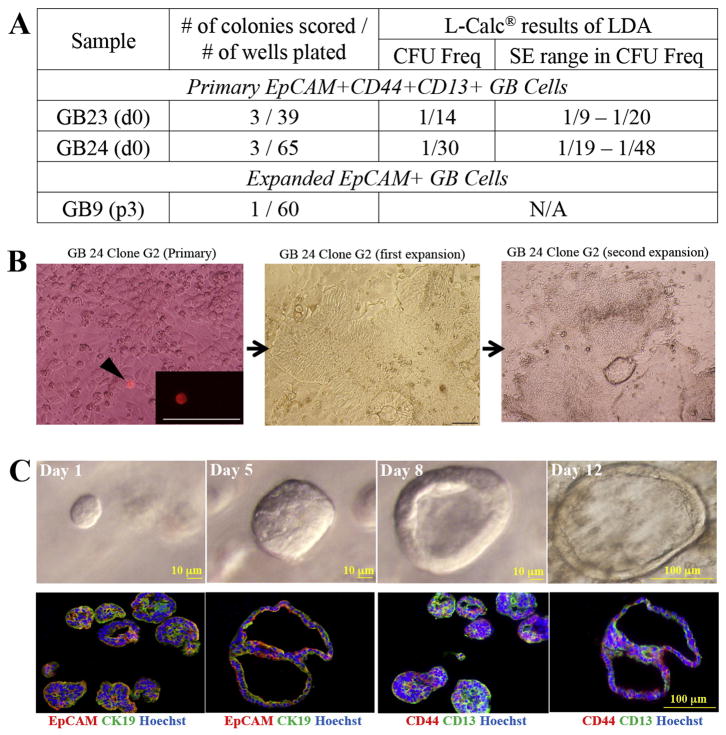
Table 1. Primary EpCAM+CD44+CD13+ gallbladder cells are the most enriched in colony forming ability.
(A) In two biologically separate experiments, LDAs were carried out on EpCAM+CD44+ and EpCAM+CD44− or EpCAM+CD13+ and EpCAM+CD13− cells. LDAs were performed on total EpCAM+ cells as controls. CFU frequency±SE (L-Calc®) indicates CD13 and CD44 each independently enrich for colony forming ability. (B) Data from four biologically separate experiments indicating that EpCAM+CD44+CD13+ cells have the highest colony forming ability. “N/A” not performed because of insufficient cell number.
| Sample | Sorted Population | CFU | SE in CFU |
|---|---|---|---|
| A | |||
| GB15 (d0) | EpCAM+ | 1/38 | 1/27 – 1/54 |
| EpCAM+CD44+ | 1/17 | 1/12 – 1/25 | |
| EpCAM+CD44− | 1/66 | 1/47 – 1/94 | |
| GB18 (d0) | EpCAM+ | 1/24 | 1/17 – 1/33 |
| EpCAM+CD13+ | 1/28 | 1/21 – 1/39 | |
| EpCAM+CD13− | 1/195 | 1/125 – | |
| B | |||
| GB10 (d0) | EpCAM+ | 1/309 | 1/247 – |
| EpCAM+CD44+CD13+ | 1/39 | 1/28 – 1/56 | |
| EpCAM+CD44−CD13− | 1/664 | 1/428 – | |
| EpCAM+CD44−CD13+ | 1/418 | 1/285 – | |
| GB19 (d0) | EpCAM+ | 1/22 | 1/16 – 1/30 |
| EpCAM+CD44+CD13+ | 1/8 | 1/5 – 1/13 | |
| EpCAM+CD44−CD13− | 1/134 | 1/65 – 1/274 | |
| EpCAM+CD44−CD13+ | 1/23 | 1/16 – 1/33 | |
| GB26 (d0) | EpCAM+ | 1/37 | 1/27 – 1/51 |
| EpCAM+CD44+CD13+ | 1/12 | 1/8 – 1/18 | |
| EpCAM+CD44−CD13− | N/A | ||
| EpCAM+CD44−CD13+ | 1/44 | 1/31 – 1/62 | |
| GB27 (d0) | EpCAM+ | 1/47 | 1/35 – 1/65 |
| EpCAM+CD44+CD13+ | 1/17 | 1/12 – 1/24 | |
| EpCAM+CD44−CD13− | N/A | ||
| EpCAM+CD44−CD13+ | 1/67 | 1/49 – 1/97 | |
We then determined if combined expression of CD13 and CD44 further enriched for clonogenicity. In four independent experiments representing four different human gallbladder samples, EpCAM+CD44+CD13+, EpCAM+CD44−CD13+ and EpCAM+CD44−CD13− subpopulations of cells were sorted from primary gallbladders (Table 1B). In all experiments, we noted that the EpCAM+CD44+CD13+ cells exhibited the highest CFU frequency (ranging from 1/8 to 1/39) and the EpCAM+CD44−CD13− cells the lowest (1/134 to 1/664). The CFU frequency of EpCAM+CD44−CD13+ cells was statistically the same as that of total epithelial or EpCAM+ cells. Of note, EpCAM+CD44+CD13+ cells had a significantly higher CFU frequency than the EpCAM+ cells in all experiments. These data indicate that combined expression of EpCAM, CD44 and CD13 significantly enriches for clonogenic gallbladder epithelial cells, and EpCAM+CD44+CD13+ cells represent the cells that expand in vitro.
CD44 and CD13 enrich for human gallbladder stem cells from expanded cells
We then tested if we could further also for clonogenic cells from expanded gallbladder by performing LDAs with expanded EpCAM+CD44+CD13+ and EpCAM+CD44+CD13− cells. The rationale was that as primary EpCAM+CD44+CD13+ cells represent the colony-forming cells and expand in vitro, it might be possible to further enrich for clonogenicity by separating expanded EpCAM+CD44+CD13+ cells and demonstrating their colony-forming ability.
Expanded EpCAM+CD44+CD13+ cells were separated from EpCAM+CD44+CD13− cells in four different human gallbladder samples. In all samples analyzed, we observed that EpCAM+CD44+CD13+ cells had a significantly higher CFU frequency (ranging from 1/18 to 1/39) than EpCAM+CD44+CD13− cells (1/77 to 1/208) but not significantly higher than total EpCAM+ cells (Supplementary Table 4), mostly due to the low number of CD13− cells present during the sorting experiments. These data confirm that CD44 and CD13 enrich for clonogencity, but also indicate that in vitro culture selects for a more homogeneous expansion of primitive cells.
EpCAM+CD44+CD13+ cells grow from single cells
Stem cells are usually defined by their ability to expand from single cells. We tested the ability of primary EpCAM+CD44+CD13+ cells to grow from single cells. In two separate experiments, primary gallbladder cells were labeled with the lipophilic membrane labeling red fluorescent dye PKH26 (35) to allow for single-cell visualization. Single EpCAM+CD44+CD13+ cells were sorted into 96-well plates and imaged (Figure 3). To account for inter-sample variability in CFU frequency (Table 1), we ran LDAs with the EpCAM+CD44+CD13+ cells to have an accurate measure of clonal frequency relative to each specific sample.
Figure 3. Primary and expanded gallbladder cells exhibit single cell self-renewal and morphogenesis.
(A) Single cell clonal assays were performed on primary and expanded GB. Primary cells (EpCAM+CD44+CD13+ and EpCAM+CD44−CD13+) and expanded cells (EpCAM+) were pre-labeled with the red fluorescent dye PKH26 and single cell sorted into 96-well plates and imaged. LDA sorts for primary cells were performed on the same populations in order to determine their CFU frequencies. Clonal colonies were calculated 6–10 weeks post plating. Both primary EpCAM+CD44+CD13+ and expanded EpCAM+ cells exhibited clonal self-renewal, however primary EpCAM+CD44−CD13+ cells did not (data not shown). (C) Top panel: single cell EpCAM+CD44+CD13+ (Day 1 to 12) cultured on matrigel in media containing AdDMEM/F12 (Invitrogen) supplemented with B27 and N2 (Invitrogen), 1.25mM N-acetylcysteine (Sigma-Aldrich), 10nM gastrin (Sigma-Aldrich) and the following growth factors: 50ng/ml, EGF (Peprotech), 1mg/ml, Rspo2 (Peprotech), 10mM nicotinamide (Sigma-Aldrich), Noggin (100ng/ml), Wnt3a (100ng/ml) . Bottom Panel, Day 12 cultures co-stained with EpCAM/CK19 or CD44/CD13 indicate that organoids were EpCAM+CK19+ and heterogeneous for CD44 and CD13 expression.
In both experiments, EpCAM+CD44+CD13+ cells exhibited robust single cell self-renewal at a CFU frequency similar to that obtained by LDA (Figure 3A). All clones generated in this manner (n=6) were capable of serial passage on the LA7 feeder cells (Figure 3B) indicating that these cells are capable of single cell self-renewal and long-term expansion. Importantly, when similar experiments were performed with EpCAM+CD44−CD13+ cells, no clones were generated (data not shown). We did not attempt clonal assays with EpCAM+CD44−CD13− cells as they have the lowest CFU frequency amongst the three candidate subpopulations (Table 1) and insufficient cell numbers made these experiments technically challenging.
Clonal analyses were also carried out with expanded gallbladder cells to confirm that they retain the ability for single cell self-renewal. In this experiment, single EpCAM+ cells were sorted onto 96-well plates and imaged. As the CFU frequency of total EpCAM+ cells from expanded gallbladder was not significantly different from that of expanded EpCAM+CD44+CD13+ cells (Table 2), we reasoned that sorting total EpCAM+ cells would be an appropriate assay to determine clonal self-renewal. Similar to the primary gallbladder cells, expanded gallbladder cells exhibited single cell self-renewal (Figure 3B), and the clones generated were capable of serial passage (data not shown).
Finally, we evaluated if single EpCAM+CD44+CD13+ cells could exhibit gallbladder differentiation or morphogenesis. Single primary EpCAM+CD44+CD13+ cells were sorted into Matrigel supplemented with Epidermal Growth Factor (EGF) (50ng/ml), Noggin (100ng/ml) and R-spondin2 (1mg/ml) and Wnt3a (100ng/ml). We were particularly interested to determine if R-spondin2 and Wnt3a, two Wnt pathway effectors, would support in vitro expansion and morphogenesis of gallbladder cells, similar to Lgr5+ liver stem cells (36). Given the importance of the Wnt-dependent pathway for stem cell expansion, we reasoned that clonogenic gallbladder cells could possibly be expanded under the previously defined organoid culture conditions (36–38). Every EpCAM+CD44+CD13+ single sorted cell formed cysts that grew into large organoids that expressed CD44 and CD13. Without the presence of the Wnt factors, the cultures deteriorated within a few passages (not shown).
In all, these data confirm that primary EpCAM+CD44+CD13+ cells are capable of single cell expansion and in vitro morphogenesis,
Expanded gallbladder cells can engraft in vivo
We have shown that gallbladder cells can expand long-term (>passage 3) in vitro. We then determined if they could survive and engraft in vivo. An ideal site for engraftment is the native gallbladder. As there are currently no protocols that allow for the native engraftment of gallbladder cells, we chose an ectopic site. The engraftment of in vitro explants of human gallbladder cells has been noted in the subcutaneous space of athymic nude mice (39). In addition, we have demonstrated the short-term engraftment of adult mouse gallbladder stem cells in the subcutaneous neck space (9).
Obtaining sufficient numbers of primary EpCAM+CD44+CD13+ cells proved technically challenging for injections. As the CFU frequency of expanded EpCAM+ cells was statistically the same as expanded EpCAM+CD44+CD13+ cells (Supplementary Table 4), we considered total EpCAM+ cells expanded in vitro to be a relatively homogeneous population of clonogenic gallbladder cells, and consequently used these cells in downstream analyses. We injected expanded EpCAM+ gallbladder cells premixed with collagen supplemented with epidermal growth factor (EGF) in the subcutaneous neck space of immunodeficient mice. We observed survival and engraftment of CK19+ gallbladder epithelial cells at one week post-injection (Figure 4A). However, there did not appear to be any apparent structure formation. Engraftment was observed in 3 out of 3 mice (100%) at one week, 2 out of 3 (67%) at two weeks. No engraftment was observed at three weeks.
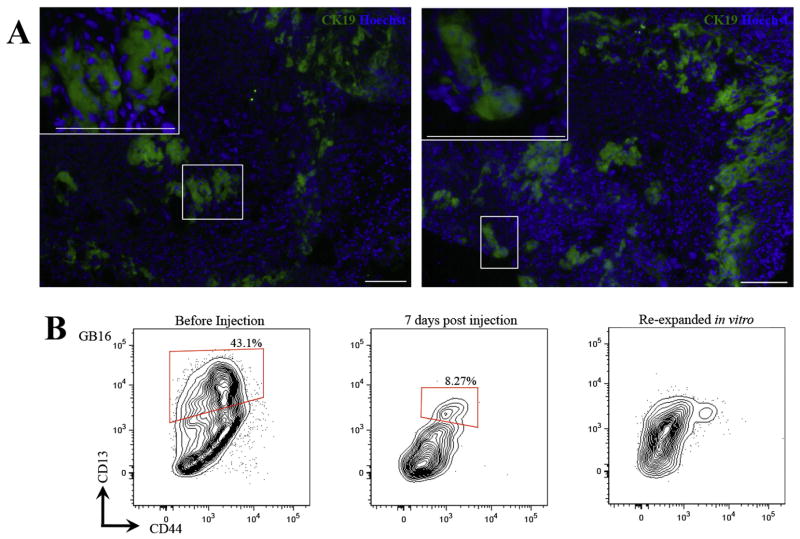
Figure 4. Expanded gallbladder cells engraft in vivo.
(A) Expanded gallbladder cultures were mixed with collagen supplemented with rhEGF (50ng/ml) and injected into the subcutaneous space of Rag2−/−γC−/− mice. Candidate engrafted areas were dissected out and stained with an anti-human CK19 to test presence of human cells. Engraftment was observed at 1 week in 3 out of 3 mice and at 2 weeks in 2 out of 3 mice in two separate human gallbladder cultures. No engraftment was observed at 3 weeks (0 out of 2 mice). Representative images of engrafted areas at 1 week post injection. (B) Phenotypic profile of gallbladder cells does not change in vivo. Flow cytometric analyses of gallbladder cells isolated at 1 week post injection show that the cells are CD44+ and exhibit clear heterogeneity for CD13. These cells re-expanded in vitro but eventually lost CD13 heterogeneity. Debris and cell aggregates were gated out in each plot. The middle plot represents HLA+EpCAM+CD45/TER119− cells from engrafted areas. All plots display 5% probability contours and the number values assigned to the gates (red boxes) represent the percentage of live gallbladder epithelial (EpCAM+CD45−) cells within that gate. Scale bars: 100μm.
Cells isolated from engrafted areas at one week post injection and expanded in vitro successfully reinitiated primitive gallbladder cultures that were CD44+ and heterogeneous for CD13 (Figure 4B).
Expanded human gallbladder cells are unique compared to human IHBD cells
A recent study has shown that IHBD and EHBD systems develop from separate progenitors during mouse development (8). In addition, we have previously shown that adult mouse gallbladder cells have a distinct phenotypic and expression profile compared to IHBD cells (9). However, there are no reports of the molecular differences between human gallbladder and IHBD cells.
Our goal here was to compare candidate human fetal gallbladder cells to human fetal IHBD cells. However, we reasoned that as the phenotypic profiles of primary human gallbladder cells were variable, similar variability would be obtained with primary IHBD cells making evaluation of phenotypic differences difficult. Moreover, separating sufficient numbers of either primary gallbladder epithelial- or IHBD epithelial cells would be challenging for microarray analyses. Therefore, we chose to compare expanded total EpCAM+ gallbladder and IHBD cells to each other.
Briefly, fetal livers from age-matched donors (Supplementary Table 1) were digested and cell suspensions were grown on lethally irradiated LA7 feeder cells. We observed that at least two serial passages allowed for expansion of cobblestone-like bile duct colonies comprised of small cells with large nuclear to cytoplasmic ratios (Figure 5A). Expanded fetal IHBD cells were EpCAM+, CD227+ and CK19+ and did not express hepatocyte markers such as alpha-1-antitrypsin (AAT) (Figure 5B&C). CD227 (Mucin 1) is known to be expressed on human fetal IHBD cells (40) confirming that EpCAM+CD227+CK19+ cells are IHBD epithelial cells and not hepatoblasts or hepatocytes. Therefore, human fetal IHBD cells can be successfully expanded on LA7 feeder cells.
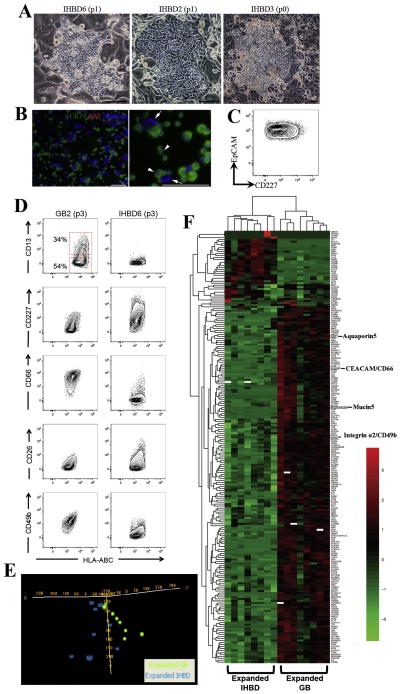
Figure 5. Expanded gallbladder and IHBD cells have unique phenotypic and expression profiles.
(A) Livers were digested and total cells were plated on rat feeder cells. Usually following two passages, we observed a robust expansion of bile duct colonies. Occasionally bile duct colonies were observed at the first expansion (p0). Representative pictures of 3 fetal liver samples at early passage indicating IHBD cell expansion. (B) Immunofluorescence analysis of an IHBD culture showing that all cells were CK19+AAT− (arrowheads). Arrows indicate rat feeder cells. (C) Flow cytometric profile of IHBD6 (passage 1) showing that all cells are EpCAM+CD227+. The plot display 5% probability contours of live IHBD cells. Scale bars: 100μm. (D) Expanded IHBD cells (n=5) were screened with the same panel of cell surface markers screened on gallbladder cells (n=5). We found expression of CD13, CD227, CD66, CD26, and CD49b were different between expanded gallbladder and IHBD cells. All plots display 5% probability contours and the number values assigned to the gates (red boxes) represent the percentage of live gallbladder cells within that gate. (E) Oligonucleotide microarrays were performed on expanded IHBD cells (n=8) and gallbladder (GB) cells (n=8) cells. PCA was performed by Euclidean correlation. The data indicate that IHBD cells (6 out of 8 samples) and gallbladder cells (6 out of 8 samples) self-segregate into two independent groups. (F) Heatmap of 193 genes differentially regulated (p<0.001, q<0.05, fold change ≥2) between IHBD and gallbladder cells defined in BRB Analysis Toolpack. This list comprises 171 genes upregulated and 22 genes downregulated in gallbladder cells relative to IHBD cells. Heatmaps were generated in R (package- pheatmap) by using Euclidean distance measure and complete linkage. Black represents genes whose expression was at the mean intensity; red represents intensities that are greater than the mean; green represents intensities that are less than the mean. White boxes indicate genes whose expression was adjusted to 0, when differentially regulated transcripts were initially defined in BRB Analysis Toolpack. Scale bars: 100μm.
We screened expanded IHBD cells with the same panel of cell surface markers used on gallbladder cells. Similar to the gallbladder cells, we found that five independant IHBD cell samples had a conserved phenotypic profile in vitro (Supplementary Table 5). Interestingly, we found that expression of six cell surface markers CD13, CD227, CD66 (Carcinoembryonic antigen-related cell adhesion molecule (CEACAM), CD26, and CD49b (Integrin α2) was different between expanded gallbladder and IHBD cells (Figure 5D) suggesting that these cells are distinct from each other. We then ran oligonucleotide microarrays on expanded EpCAM+ IHBD and gallbladder cells to evaluate differences in their gene expression profiles.
EpCAM+ fetal IHBD and EpCAM+ fetal gallbladder cells (n=8 for each group) at early passage (<passage 5) were separated from rat feeder cells by Magnetic Activated Cell Sorting (MACS) as previously described (9) (Supplementary Figure 3). Principal component analysis (PCA) revealed significant level of clustering between IHBD (6 out of 8 samples) and gallbladder cells (6 out of 8 samples) (Figure 5E). In addition, unpaired t-tests (BRB Analysis Toolpack) revealed 715 transcripts that were significantly different (p<0.001, q<0.05, fold change ≥2) between the IHBD and gallbladder cells, of which 479 mapped to known genes (Supplementary Table 6). We manually curated this list and identified 193 genes that we considered important for bile duct function (Figure 5F). Interestingly, the microarray analysis indicated that that CD49b and CD66 were upregulated in cultured gallbladder cells compared to IHBD cells, confirming our phenotypic comparisons between the two cell types (Figure 5A) In addition, Mucin5 (MUC5) was upregulated in gallbladder cells, an observation that has been shown previously (41). Aquaporin 5 (AQP5) was also found to be upregulated in gallbladder cells adding to the current list of aquaporins (AQP8 and AQP4) known to be differentially expressed between both cell types (42). Other notable groups of genes that were differentially expressed were the cytochrome (CYP) P450 family, solute carrier (SLC) family as well as other mucin family members (MUC13, 16 and 20) and Indian hedgehog (Ihh). CYP and SLC family members and Ihh are especially relevant as we have previously identified that they are differentially expressed between adult mouse IHBD and gallbladder stem cells (9). Pathways analysis suggested that lipid metabolism (50 genes), vitamin and mineral metabolism (20 genes), drug metabolism and carbohydrate metabolism functions are different between gallbladder and IHBD cells (Supplementary Table 7). These data corroborate our previous work that found that lipid metabolism and drug metabolism are different between adult mouse IHBD and gallbladder stem cells (9). In all, the phenotypic and transcriptional differences between human expanded EpCAM+ gallbladder and IHBD cells confirm that these cells are unique or distinct from each other and suggest functional differences as well.
Discussion
Here we reported a novel culture system that selects for human gallbladder epithelial cells and supports single cell self-renewal. In a previous study, Kobayashi et al. (43) used human gallbladder myofibroblasts as feeders to culture gallbladder cells. However, their culture system does not select for epithelial cells and clonal expansion was not reported. Our use of the LA7 feeder cell culture system and an epithelial cell marker (EpCAM) to definitively separate epithelial from mesenchymal and hematopoietic cells, allows for the identification of resident clonogenic cells from gallbladder epithelium. This is especially important as we identified EpCAM−CD13+ and EpCAM−CD44+ cells in primary gallbladder (data not shown).
A key characteristic of stem cells is their ability to grow from single cells and differentiate into lineage-committed progeny. And for some stem cell populations clonogenicity in vitro can be used as a surrogate assay for the identification of their in vivo counterparts. Combined expression of EpCAM, CD13 and CD44 highlighted specific heterogeneity on primary gallbladder epithelial cells, allowing for their fractionation into three distinct populations. We found that EpCAM+CD44+CD13+ cells are the most enriched in colony forming ability, exhibit single cell self-renewal and morphogenesis and give rise to cultures in vitro that survived and engrafted in the subcutaneous space of immunodeficient mice. These cells are candidates to represent bona fide gall bladder stem cells in vivo. As there are no specific markers of gallbladder differentiation, we could only evaluate morphogenesis, but not specific differentiation of gallbladder cells. Our in vitro morphogenesis experiments were supported by in vivo experiments, where we observed short-term (≤ 2 weeks) engraftment of expanded gallbladder cells. Importantly, in our previous work with adult mouse gallbladder cells, we also only observed short-term engraftment of the cells (9). This could be for lack of growth stimulus from the recipient animal. Moreover, the rate of self-renewal of gallbladder cells in vivo is known to be low (44). We concluded that as the primary EpCAM+CD44+CD13+ cells exhibited clonal self-renewal, expansion in vitro and engraftment in vivo, they represent the most clonogenic cell population from fetal gallbladder identified to date.
It has recently been shown that murine IHBD and EHBD systems develop from distinct stem cells (8). In addition, we have found adult mouse gallbladder stem cells to be unique or distinct compared to IHBD cells (9). We therefore chose to evaluate the differences between fetal human IHBD cells and gallbladder cells. IHBD epithelial cells expanded on LA7 feeder cells over several passages while hepatoblasts and non-epithelial cells did not. IHBD cells are thought to develop around seven or eight weeks post gestation (45). Hepatoblasts that are not involved in ductal plate formation, are CK19− by around 14 weeks post gestation (46). By 20 weeks of gestation, IHBD epithelial cells are CK19+ and CD227+ (46). All the fetal livers that we processed were between 19–23 weeks of gestation. Accordingly, we observed that expanded IHBD cells were CK19+, CD227+, AAT− and EpCAM+, confirming expansion of IHBD epithelial cells. It is possible that in vitro expansion may select for a primitive population of IHBD cells, similar to what we observe with gallbladder cells. However, the evaluation of IHBD epithelial stem cells is beyond the scope of this study and we accordingly referred to them simply as IHBD cells.
Phenotypic comparison of cultured IHBD and gallbladder cells indicated differential expression of CD13, CD227, CD66, CD26 and CD49b. These data are especially interesting, as we have identified CD13 as enriching for clonogenic cells. Foregoing phenotypic differences were corroborated by transcriptional profiling of expanded gallbladder and IHBD cells. PCA analysis showed that gallbladder and IHBD cells clustered into two independent groups. In addition, assessment of differentially regulated transcripts revealed a substantial dataset (715 transcripts including 479 known genes) replete with genes suggesting functional differences in gallbladder and IHBD cells. We noted that CYP and SLC families of genes, along with various mucins including MUC5, were differentially expressed between gallbladder and IHBD cells. Predictive functional analysis of the differentially regulated genes indicated that lipid, vitamin and mineral, carbohydrate, drug, and nucleic acid metabolisms could be different between gallbladder and IHBD cells. In addition, we found Interleukin 8, Interferon (IFN) receptor 1 and Interleukin receptor 10 were upregulated in gallbladder cells. The immunologic properties of bile duct cells have been long considered and various inflammatory diseases such as primary biliary cirrhosis (47) and biliary atresia (10) directly affect bile duct cells. They also play a causal role in liver allograft rejection (48). In addition, we have previously identified differences in immunologic genes such as IFN-inducible protein 27 between adult mouse IHBD cells and gallbladder stem cells (9), as well as differences in drug and lipid metabolism and CYP and SLC families of genes. This remarkable symmetry between our human and mouse data indicate that differences between gallbladder and IHBD cells persist from development through to adulthood as well as across species. Although all of the comparative studies were performed on cultured cells, we believe that their in vivo counterparts are likely to show similar differences.
In conclusion, the identification of fetal gallbladder stem cells could have important ramifications for study of biliary atresia and shed light on adult human gallbladder stem cells and gallbladder cancer. These cells could also potentially be reprogrammed into hepatocytes and endocrine cells as demonstrated recently (49–51). In addition, since laparoscopic cholecystectomy became clinically available in 1989, the removal of the gallbladder has become routine with 500,000 – 600,000 surgeries every year (52). This sheer volume of tissue along with candidate plasticity of gallbladder stem cells make them attractive candidates for cell-based therapy.
Supplementary Material
Highlights.
EpCAM+CD44+CD13+ cells represent a clonogenic cell population in epithelial cells of primary human fetal gallbladder.
EpCAM+CD44+CD13+ gallbladder cells can self-renew and expand in vitro.
Gallbladder cells and intrahepatic bile duct cells expanded in vitro have unique gene expression signatures.
Acknowledgments
We would like to thank Mara Sullivan and Ming Sun for tissue processing for Electron Microscopy, Michael Burger and Christin Sciulli at the Clinical Genomics Facility for sample processing and initial data analysis, and Soumya Luthra at the Department of Bioinformatics and Drs. Michalopoulos, Fox, Orwig, Demetris and Strom for their valuable advice and input. This work was supported by the NIH grant R01 DK085711.
Abbreviations
- IHBD
Intrahepatic bile duct
- EHBD
Extrahepatic bile duct
- CBD
Common bile duct
- LDA
Limiting Dilution Analysis
- CFU
Colony Forming Unit
- EGF
epidermal growth factor
- MACS
magnetic activated cell sorting
- PCA
Principal component analysis
- CEACAM
Carcinoembryonic antigen-related cell adhesion molecule
- AQP
Aquaporin
- CYP
Cytochrome
- SLC
Solute carrier
- Ihh
Indian hedgehog
- IFN
Interferon
Footnotes
The authors have no additional financial interests.
Contributor Information
Rohan Manohar, Email: rohanraoul@Gmail.com.
Yaming Li, Email: liy6@upmc.edu.
Helene Fohrer, Email: fohrerh@gmail.com.
Lynda Guzik, Email: GuziLJ@ccm.upmc.edu.
Donna Beer Stolz, Email: dstolz@pitt.edu.
Uma R. Chandran, Email: chandranur@upmc.edu.
William A. LaFramboise, Email: laframboisewa@upmc.edu.
References
- 1.Hand BH. Anatomy and function of the extrahepatic biliary system. Clin Gastroenterol. 1973;2:3–29. [PubMed] [Google Scholar]
- 2.Cardinale V, Wang Y, Carpino G, Alvaro D, Reid L, Gaudio E. Multipotent stem cells in the biliary tree. Ital J Anat Embryol. 2010;115:85–90. [PubMed] [Google Scholar]
- 3.Nakanuma Y, Katayanagi K, Kawamura Y, Yoshida K. Monolayer and three-dimensional cell culture and living tissue culture of gallbladder epithelium. Microsc Res Tech. 1997;39:71–84. doi: 10.1002/(SICI)1097-0029(19971001)39:1<71::AID-JEMT6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Frizzell RA, Heintze K. Transport functions of the gallbladder. Int Rev Physiol. 1980;21:221–247. [PubMed] [Google Scholar]
- 5.Shiojiri N. Development and differentiation of bile ducts in the mammalian liver. Microsc Res Tech. 1997;39:328–335. doi: 10.1002/(SICI)1097-0029(19971115)39:4<328::AID-JEMT3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 6.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Spence JR, Lange AW, Lin SC, Kaestner KH, Lowy AM, Kim I, Whitsett JA, et al. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manohar R, Komori J, Guzik L, Stolz DB, Chandran UR, Laframboise WA, Lagasse E. Identification and expansion of a unique stem cell population from adult mouse gallbladder. Hepatology. 2011;54:1830–1841. doi: 10.1002/hep.24568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassett MD, Murray KF. Biliary atresia: recent progress. J Clin Gastroenterol. 2008;42:720–729. doi: 10.1097/MCG.0b013e3181646730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol. 2008;34:306–312. doi: 10.1016/j.ejso.2007.07.206. [DOI] [PubMed] [Google Scholar]
- 12.Lagasse E. Cancer stem cells with genetic instability: the best vehicle with the best engine for cancer. Gene Ther. 2008;15:136–142. doi: 10.1038/sj.gt.3303068. [DOI] [PubMed] [Google Scholar]
- 13.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 14.Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 15.Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM) J Mol Med. 1999;77:699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- 16.de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol. 1999;188:201–206. doi: 10.1002/(SICI)1096-9896(199906)188:2<201::AID-PATH339>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Momburg F, Moldenhauer G, Hammerling GJ, Moller P. Immunohistochemical study of the expression of a Mr 34,000 human epithelium-specific surface glycoprotein in normal and malignant tissues. Cancer Res. 1987;47:2883–2891. [PubMed] [Google Scholar]
- 18.Auth MK, Keitzer RA, Scholz M, Blaheta RA, Hottenrott EC, Herrmann G, Encke A, et al. Establishment and immunological characterization of cultured human gallbladder epithelial cells. Hepatology. 1993;18:546–555. [PubMed] [Google Scholar]
- 19.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 20.Ehmann UK, Peterson WD, Jr, Misfeldt DS. To grow mouse mammary epithelial cells in culture. J Cell Biol. 1984;98:1026–1032. doi: 10.1083/jcb.98.3.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoerl BJ, Vroman BT, Kasperbauer JL, LaRusso NF, Scott RE. Biological characteristics of primary cultures of human gallbladder epithelial cells. Lab Invest. 1992;66:243–250. [PubMed] [Google Scholar]
- 22.Kakinuma S, Ohta H, Kamiya A, Yamazaki Y, Oikawa T, Okada K, Nakauchi H. Analyses of cell surface molecules on hepatic stem/progenitor cells in mouse fetal liver. J Hepatol. 2009;51:127–138. doi: 10.1016/j.jhep.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Okada K, Kamiya A, Ito K, Yanagida A, Ito H, Kondou H, Nishina H, et al. Prospective Isolation and Characterization of Bipotent Progenitor Cells in Early Mouse Liver Development. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0229. [DOI] [PubMed] [Google Scholar]
- 24.Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, Akita H, et al. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120:3326–3339. doi: 10.1172/JCI42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi C, Tian R, Wang M, Wang X, Jiang J, Zhang Z, Li X, et al. CD44+ CD133+ population exhibits cancer stem cell-like characteristics in human gallbladder carcinoma. Cancer Biol Ther. 2010;10:1182–1190. doi: 10.4161/cbt.10.11.13664. [DOI] [PubMed] [Google Scholar]
- 26.Shi CJ, Gao J, Wang M, Wang X, Tian R, Zhu F, Shen M, et al. CD133(+) gallbladder carcinoma cells exhibit self-renewal ability and tumorigenicity. World J Gastroenterol. 2011;17:2965–2971. doi: 10.3748/wjg.v17.i24.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng YW, Taniguchi H. Diversity of hepatic stem cells in the fetal and adult liver. Semin Liver Dis. 2003;23:337–348. doi: 10.1055/s-2004-815557. [DOI] [PubMed] [Google Scholar]
- 28.Tsuchiya A, Heike T, Baba S, Fujino H, Umeda K, Matsuda Y, Nomoto M, et al. Long-term culture of postnatal mouse hepatic stem/progenitor cells and their relative developmental hierarchy. Stem Cells. 2007;25:895–902. doi: 10.1634/stemcells.2006-0558. [DOI] [PubMed] [Google Scholar]
- 29.Kamiya A, Kakinuma S, Yamazaki Y, Nakauchi H. Enrichment and clonal culture of progenitor cells during mouse postnatal liver development in mice. Gastroenterology. 2009;137:1114–1126. 1126 e1111–1114. doi: 10.1053/j.gastro.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 31.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 32.Fazekas de St G. The evaluation of limiting dilution assays. J Immunol Methods. 1982;49:R11–23. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- 33.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci U S A. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B, Brisken C, et al. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 36.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 38.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009 doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 39.Okumura H, Sakamoto K, Nakano G, Ikeda H, Nagashima K, Nagamachi Y, Yuasa Y. Long-term maintenance of human gall-bladder epithelia as xenografts following explant organ culture. Nippon Geka Gakkai Zasshi. 1988;89:388–393. [PubMed] [Google Scholar]
- 40.Sasaki M, Nakanuma Y, Terada T, Kim YS. Biliary epithelial expression of MUC1, MUC2, MUC3 and MUC5/6 apomucins during intrahepatic bile duct development and maturation. An immunohistochemical study. Am J Pathol. 1995;147:574–579. [PMC free article] [PubMed] [Google Scholar]
- 41.Buisine MP, Devisme L, Degand P, Dieu MC, Gosselin B, Copin MC, Aubert JP, et al. Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. II. Duodenum and liver, gallbladder, and pancreas. J Histochem Cytochem. 2000;48:1667–1676. doi: 10.1177/002215540004801210. [DOI] [PubMed] [Google Scholar]
- 42.Portincasa P, Palasciano G, Svelto M, Calamita G. Aquaporins in the hepatobiliary tract. Which, where and what they do in health and disease. Eur J Clin Invest. 2008;38:1–10. doi: 10.1111/j.1365-2362.2007.01897.x. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi K, Kan M, Yamane I, Ishii M, Toyota T. Primary culture of human gallbladder epithelial cells. Gastroenterol Jpn. 1991;26:363–369. doi: 10.1007/BF02781926. [DOI] [PubMed] [Google Scholar]
- 44.Lamote J, Willems G. DNA synthesis, cell proliferation index in normal and abnormal gallbladder epithelium. Microsc Res Tech. 1997;38:609–615. doi: 10.1002/(SICI)1097-0029(19970915)38:6<609::AID-JEMT5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Roskams T, Desmet V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec (Hoboken) 2008;291:628–635. doi: 10.1002/ar.20710. [DOI] [PubMed] [Google Scholar]
- 46.Blankenberg TA, Lund JK, Ruebner BH. Normal and abnormal development of human intrahepatic bile ducts. An immunohistochemical perspective. Perspect Pediatr Pathol. 1991;14:143–167. [PubMed] [Google Scholar]
- 47.Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481–1496. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Nakanuma Y, Tsuneyama K, Harada K. Pathology and pathogenesis of intrahepatic bile duct loss. J Hepatobiliary Pancreat Surg. 2001;8:303–315. doi: 10.1007/s005340170002. [DOI] [PubMed] [Google Scholar]
- 49.Kuver R, Savard CE, Lee SK, Haigh WG, Lee SP. Murine gallbladder epithelial cells can differentiate into hepatocyte-like cells in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;293:G944–955. doi: 10.1152/ajpgi.00263.2006. [DOI] [PubMed] [Google Scholar]
- 50.Lee SP, Savard CE, Kuver R. Gallbladder epithelial cells that engraft in mouse liver can differentiate into hepatocyte-like cells. Am J Pathol. 2009;174:842–853. doi: 10.2353/ajpath.2009.080262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hickey RD, Galivo F, Schug J, Brehm MA, Haft A, Wang Y, Benedetti E, et al. Generation of islet-like cells from mouse gall bladder by direct ex vivo reprogramming. Stem Cell Res. 2013;11:503–515. doi: 10.1016/j.scr.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacFadyen BV, Jr, Vecchio R, Ricardo AE, Mathis CR. Bile duct injury after laparoscopic cholecystectomy. The United States experience. Surg Endosc. 1998;12:315–321. doi: 10.1007/s004649900661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.