Abstract
The blood brain barrier (BBB) symbolically represents the gateway to the central nervous system. It is a single layer of specialized endothelial cells that coats the central nervous system (CNS) vasculature and physically separates the brain environment from the blood constituents, to maintain the homeostasis of the CNS. However, this protective measure is a hindrance to the delivery of therapeutics to treat neurological diseases. Here, we show that activation of A2A adenosine receptor (AR) with an FDA-approved agonist potently permeabilizes an in vitro primary human brain endothelial barrier (hBBB) to the passage of chemotherapeutic drugs and T cells. T cell migration under AR signaling occurs primarily by paracellular transendothelial route. Permeabilization of the hBBB is rapid, time-dependent and reversible and is mediated by morphological changes in actin-cytoskeletal reorganization induced by RhoA signaling and a potent down-regulation of Claudin-5 and VE-Cadherin. Moreover, the kinetics of BBB permeability in mice closely overlaps with the permeability kinetics of the hBBB. These data suggest that activation of A2A AR is an endogenous mechanism that may be used for CNS drug delivery in human.
Introduction
The brain is the most vascularized organ in the body (Deeken and Loscher 2007). It is estimated that the brain has more than 100 billion capillaries (Deeken and Loscher 2007). If all the capillaries and blood vessels in the brain are strung together they will extend over 400 miles long (Deeken and Loscher 2007). The brain vasculature is lined by a single layer of endothelial cells that forms a tight barrier against unwanted substances from the blood circulation (Abbott et al. 2006). In addition, the gaps between adjacent endothelial cells are sealed with tight and adherens junction molecules to further increase the brain endothelial barrier resistance (Abbott et al. 2006; Deeken and Loscher 2007). Extracellular matrix proteins, pericytes and astrocytic endfoot processes (referred to as the neurovascular unit), insulate the endothelial lining, making this structure impermeable even to very small molecules (less than 450 Da) and polar and ionic substances (Deeken and Loscher 2007). In addition, transporters expressed on brain endothelial cells selectively regulate the influx of key molecules necessary for proper brain function, and thus, imposes additional restrictions on permeability (Abbott et al. 2006; Deeken and Loscher 2007; Giacomini et al. 2010; Pardridge 2005). The characteristic physico-chemical entity of the brain-blood vasculature is called the blood brain barrier (BBB) (Abbott et al. 2006; Ribatti et al. 2006). However, this inherent high-impermeability of the BBB impedes drug delivery to the brain that could treat myriad of neurodegenerative diseases such as brain cancers and multiple sclerosis (Deeken and Loscher 2007). There is a tremendous need to be able to modulate the permeability of the BBB to enhance the deliverability of therapeutics into the brain (Hossain et al. 2010).
Adenosine is a purine nucleoside that mediates its function through its 7-transmembrane G-protein coupled receptors. Adenosine is produced both extracellularly and intracellularly. Extracellular adenosine is produced from the conversion of extracellular adenosine triphosphate (ATP) into adenosine diphosphate (ADP) and adenosine monophosphate (AMP) by the extracellular enzyme CD39; and AMP is further converted to adenosine by the extracellular enzyme, CD73 (Blackburn et al. 2009; Hasko et al. 2008; Jacobson and Gao 2006). Adenosine receptors (ARs) are of four different subtypes, A1, A2A, A2B and A3 (Fredholm et al. 2011). ARs, and extracellular enzymes are expressed on brain endothelial cells in mice and human (Carman et al. 2011; Mills et al. 2011). Previously, we have shown that blockade of CD73 or inhibition of the A2A AR signaling inhibits the migration of leukocytes into the central nervous system (CNS) (Mills et al. 2008). Further, we showed that activation of ARs with a broad spectrum AR agonist increases BBB permeability and allow the entry of macro-molecules into the brain (Carman et al. 2011). These studies strongly indicate that signaling via the ARs represents a bone fide pathway that controls the entry of cells and molecules into the CNS. Because extracellular adenosine mediates many of its functions around inflammation or injury (Blackburn et al. 2009), we hypothesize that signaling via adenosine receptors on BBB endothelial cells signals the recruitment of cells and/or molecules into the CNS to sites of damage or inflammation. Based on these studies we now focus our attention on determining how we can exploit adenosine modulation of BBB permeability to deliver drugs into the CNS to treat neurological diseases ranging from Alzheimer’s to brain tumors. To do this we need to first determine whether AR signaling regulates human BBB permeability, and second, understand the mechanisms that regulate brain endothelial barrier permeability and determine where in the pathway AR signaling functions.
Recent studies demonstrated that AR activation regulates RhoA activity in various cell types mediated by second messenger signals including cyclic AMP (Sohail et al. 2009). RhoA is a small GTPase that is the master regulator of actin-cytoskeletal re-organization. The actin-cytoskeleton maintains the structure and morphology of cells (Pollard and Cooper 2009). Factors that modulate actin-cytoskeletal rearrangement have been shown to increase or decrease endothelial barrier permeability (Spindler et al. 2010).
In this study, we will determine a) whether AR modulates human brain endothelial barrier, b) determine its potential clinical application in drug delivery to the brain in treatment of neurological diseases and c) unravel/reveal the mechanism underlying the increased permeability imposed upon brain endothelial cells by activation of AR. To accomplish this, 1) we studied these processes in human primary brain endothelial cells and a well established human brain endothelial cell line as human BBB model as we cannot perform these studies in humans. 2) We used an FDA-approved A2A AR agonist, Lexiscan (used in humans for cardiac perfusion imaging), to determine its potential as a BBB permeabilizing (or brain)-drug delivery tool. Here, we report that activation of the A2A AR on primary human brain endothelial cells triggers a rapid increase in RhoA activity, re-organization of the actin cytoskeleton and consequently disruption of the endothelial cell-to-cell junctions, leading to the increased paracellular permeability. These processes promote transendothelial migration (TEM) of T cells through paracellular routes. These studies make use of an endogenous mechanism for BBB control. They demonstrate the potential for precise time dependent control of BBB permeability. Moreover, we would show that the process is reversible and there is the potential to tremendously improve the retention of therapeutics into the brain by targeting adenosine receptors on brain endothelial cells.
Results
Adenosine receptors and enzymes that produce it are abundantly expressed on primary human brain endothelial cells
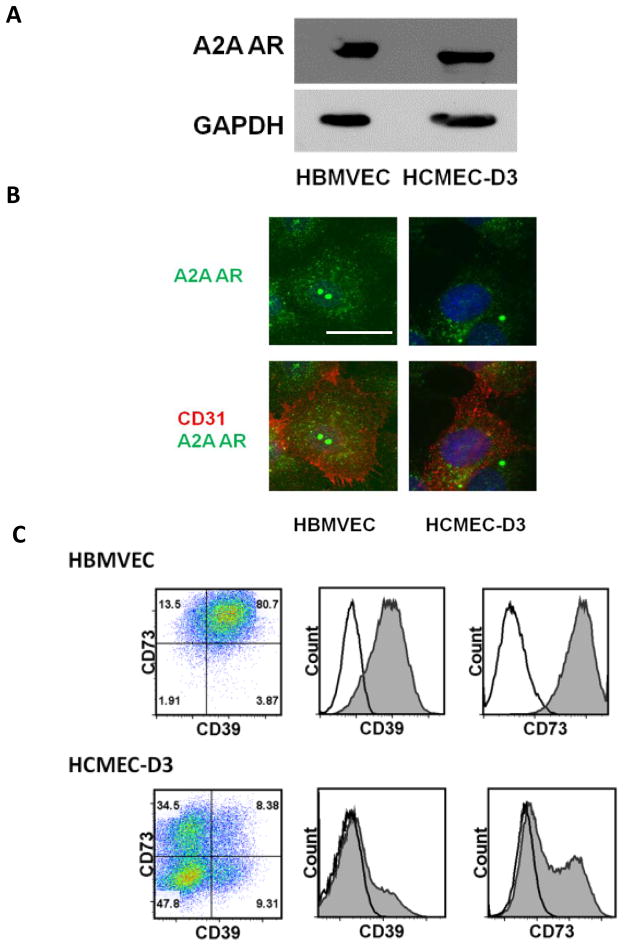
Adenosine mediates its function through its four G-protein coupled receptors (A1, A2A, A2B, and A3) (Hasko et al. 2008). Here, we showed that the A2A adenosine receptor (AR) that we have previously shown increases brain endothelial barrier permeability upon activation in mice is highly expressed on primary human brain endothelial cells (HBMVEC) and an established human brain endothelial cell line (HCMEC-D3) (Figure 1A and B). Extracellular adenosine acts locally due to its short half-life (approximately ten seconds) (Fredholm et al. 2011; Jacobson and Gao 2006). Hence, to mediate its function, adenosine receptors and the enzymes that generate it must also be present on the same cell or on adjacent cells. We confirmed that both CD39 and CD73 (ecto-enzymes responsible for generating extracellular adenosine), are highly expressed on both primary human brain endothelial cells and the human brain endothelial cell line HCMEC-D3 (Figure 1C). Taken together, these results suggest that human brain endothelial cells have the capacity to respond to AR-mediated signaling both in vivo and in vitro.
Figure 1. A2A AR is expressed in primary human brain endothelial cells and human brain endothelial cell line.
Western blot (A) and immunofluoresecence assay (IFA) (B) show the presence of A2A adenosine receptor (AR) expression on human primary brain endothelial cells (HBMVEC) and human brain endothelial cell line (HCMEC-D3). For IFA, cells were stained with anti-A2A AR antibody (Green) and anti-human CD31 as endothelial cell marker (Red). Nucleus was counter stained with DAPI (Blue). Scale bar indicates 25 um. (C) FACS analysis (Dot-plot and histogram) shows expression of the extracellular enzymes, CD39 and CD73, in HBMVEC and HCMEC-D3.
A2A AR activation increases paracellular permeability in primary human brain endothelial cell monolayers
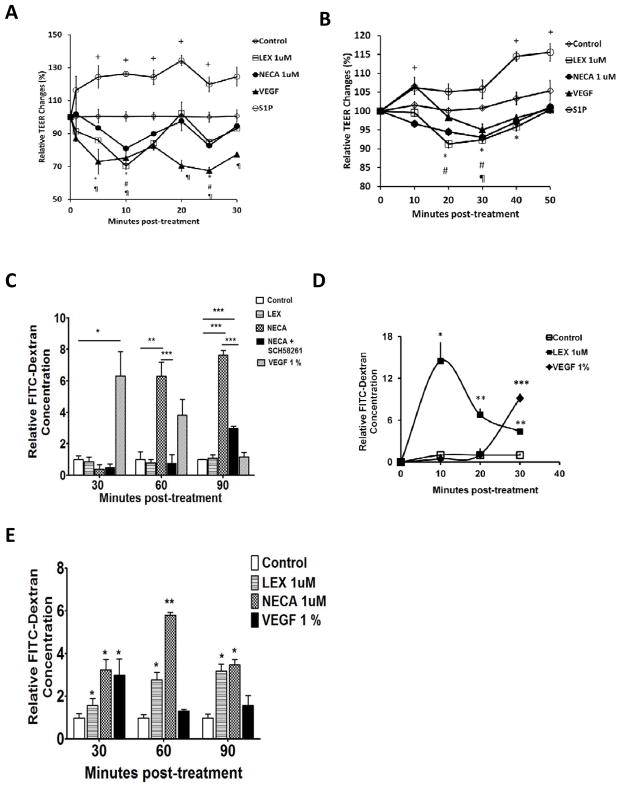
To begin to evaluate the role of the A2A AR in human brain endothelial cell permeability, HBMVEC cells were cultured on a porous membrane to form a monolayer to measure transendothelial resistance (TEER), which is a measure of endothelial cell monolayer permeability. Decrease in TEER correlates with increased paracellular space between adjacent endothelial cells and hence with increased permeability (Li et al. 2010). We next treated monolayers with an FDA-approved A2A AR agonist Lexiscan or a broad spectrum agonist NECA. Both Lexiscan and NECA induced rapid decrease in TEER by ten minutes after treatment with AR agonists, consistent with increase in paracellular permeability (Figure 2A). This was compared to vehicle, which showed no change, and S1P and VEGF controls which increased and decreased TEER, respectively(Spindler et al. 2010). Subsequently, TEER values gradually declined post-treatment in HBMVEC monolayers. A similar trend in TEER was observed in the mouse brain endothelial cell line, bEnd 3, although the kinetics was somewhat different (Figure 2B). These studies clearly indicate that human brain endothelial cells are capable of responding to AR modulation and that activation of the A2A receptor decreases brain endothelial paracellular permeability.
Figure 2. AR agonists increased trans-endothelial electrical resistance and endothelial cell permeability to passage of 10 kDa FITC-Dextran.
(A) Measurement of trans-endothelial electrical resistance (TEER) in primary human brain endothelial cells (HBMVEC) treated with AR agonists, Lexiscan or NECA or VEGF and SIP as positive and negative controls respectively, and vehicle (control). TEER measurement in primary human brain endothelial cell monolayer (HBMVEC) Data represents mean ± s.e.m. (n=3, + (S1P), ¶ (VEGF), * (Lexiscan), # (NECA) indicate p<0.05 by two-tailed student t-test). (B) TEER measurement in mouse brain endothelial cell monolayer (bEnd 3) after treatment with AR agonists. Data represents mean ± s.e.m. (n=3, + (S1P), ¶ (VEGF), * (Lexiscan), # (NECA) indicate p<0.05 by two-tailed student t-test). (C) FITC-Dextran extravasation through primary human brain endothelial cell monolayer in the presence of Lexiscan, NECA or NECA and SCH58261 at 30, 60, 90 minutes. Data represents mean ± s.e.m. (n=3, * p<0.05, ** p<0.01, *** p<0.001 two-way ANOVA with Bonferroni multiple comparison test). (D) Early time course measurement of FITC extravasation by Lexiscan. Data represents mean ± s.e.m. (n=3, * p<0.05, ** p<0.01, *** p<0.001 by two-way ANOVA with Bonferroni multiple comparison test). (E) 10 kDa FITC-Dextran extravasation in the presence of Lexiscan or NECA in mouse brain endothelial cell monolayer. Data represents mean ± s.e.m. (n=3, * p<0.05, ** p<0.01, two-way ANOVA with Bonferroni multiple comparison test).
To further evaluate the effects of AR activation on human brain endothelial cell permeability, we generated an in vitro human BBB (hBBB) model, to examine the passage of high molecular weight Dextrans. 10 kDa FITC-Dextran concentration in bottom chamber increased in a time dependent manner up to 90 minutes after NECA treatment in hBBB (Figure 2C). NECA’s effect on increased permeability was significantly abrogated at 60 and 90 minutes with concomitant treatment of SCH58261, a specific A2A AR antagonist. This suggests that the A2A AR activation has a specific role in increasing the permeability of hBBB. However, Lexiscan did not increase permeability to FITC-Dextran over the 30–90 minutes time course. We then examined earlier time points as we observed that Lexiscan decreased TEER within 5 minutes (Figure 2A). Indeed, by 10 minutes, Lexiscan induced a robust increase in FITC-Dextran extravasation across hBBB, which declined by 30 minutes (Figure 2D). These collective data indicate that A2A AR activation by Lexiscan causes a rapid and potent increase in hBBB permeability which is followed by a rapid reversal. NECA, by contrast, showed a more gradual reversal in hBBB permeability. These properties were similarly observed in mouse brain endothelial cells (Figure 2E). These data are the first to demonstrate the effects of an FDA-approved A2A AR agonist in primary human brain endothelial cell permeability which has strong translational potential for drug delivery.
A2A AR signaling promotes paracellular trans-endothelial migration of T cells across an in vitro human primary BBB
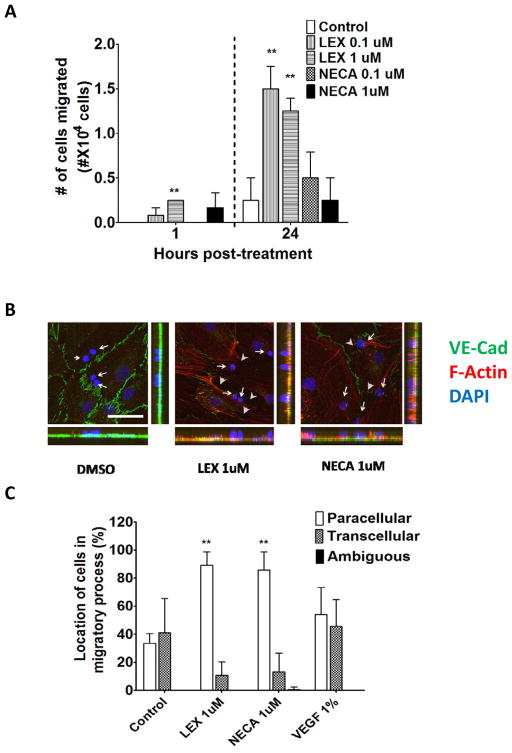
Transendothelial-migration (TEM) of leukocytes across the BBB occurs by transcellular or paracellular routes(Carman and Springer 2008; Wolburg et al. 2005). We investigated whether activation of A2A AR has any impact on paracellular versus transcellular TEM of leukocytes across hBBB. Lexiscan (1 uM) increased Jurkat T cell TEM up to 3-fold more than controls by 1 hr and up to 5-fold by 24 hrs (Figure 3A). NECA increased Jurkat T cell TEM by 24 hrs (Figure 3A). To determine whether TEM of these T cells occurred by transcellular and/or paracellular routes, we treated monolayers of primary human brain endothelial cells plated on coverslips with Lexiscan or NECA. Subsequently, Jurkat T cells were plated on endothelial cells monolayer and projections of Jurkat T cells undergoing paracellular diapedesis were determined by the disruption of junctional VE-Cadherin and the formation of paracellular gaps between adjacent endothelial cells (Figure 3B). Confocal microscopic analysis showed that treatment with Lexiscan or NECA promoted primarily paracellular diapedesis of Jurkat T cells (Figure 3B and C). This was in stark contrast to controls and to previous studies showing that leukocytes undergo TEM across the brain endothelium by both paracellular and transcellular TEM (Carman and Springer 2008; Wolburg et al. 2005). These data indicate that activation of A2A AR preferentially promotes paracellular TEM.
Figure 3. AR signaling increases transendothelial migration (TEM) of Jurkat T cells and promotes paracellular TEM across human primary brain endothelial cell barrier.
(A) Transendothelial migration (TEM) of Jurkat T cells across primary human in vitro BBB in the presence of varying concentrations (0.1 or 1 uM) of Lexiscan or NECA treatment. TEM of T cell migration was analyzed at 1 hr and 24 hours. Data represents mean ± s.e.m. (** indicates where p<0.01, two tailed student t-test). (B) IFA analysis of Jurkat T cells in the process of TEM across a human primary in vitro BBB after treatment with AR agonists, Lexiscan or NECA. After treatment with 1uM of Lexiscan or NECA for 2 hours on primary human brain enoothelial cells, cells were washed and 2×105 of Jurkat cells were plated on the endothelial cells for 5 minutes and samples were fixed with 4% paraformaldehyde (PFA). F-actin was stained with AF568 conjugated phalloidin (Red) and VE-Cadherin was stained with anti VE-Cadherin antibody (Green). Nucleus was counter stained with DAPI (Blue). Arrows indicate the nucleus of Jurkat T cells in the migratory process. Arrow heads indicate the disrupted junctional spaces between endothelial cells and dashed lines indicate out line of endothelial cells. Scale bar indicates 50 um. (C) Quantification of T Jurkat cells in the process of paracellular or transcellular TEM across on in vitro human primary BBB after treatment with Lexiscan or NECA. Data represents mean ± s.e.m. (** indicates where p<0.01, two tailed student t-test).
A2A AR activation induces rapid increase in RhoA activity and stress fiber formation in human brain endothelial cells
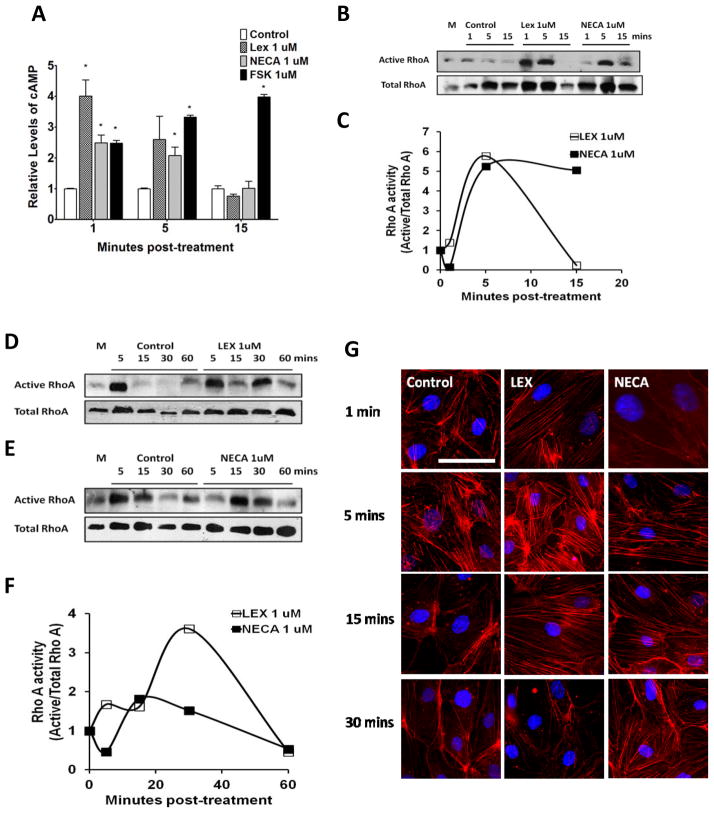
Actin-cytoskeletal reorganization is tightly regulated by RhoA(Adamson et al. 2002; Jaffe and Hall 2005; Riento and Ridley 2003; Tominaga et al. 2000), which is a family of small GTPases activated by G-protein coupled receptor signaling, including ARs(Rex et al. 2009; Sohail et al. 2009). Activation of A2A AR stimulates increase in cyclic adenosine monophosphate (cAMP)(Mills et al. 2011), which increases RhoA activity(Jeyaraj et al. 2012). We measured intracellular cAMP activity upon AR activation in primary human brain endothelial cells and observed that Lexiscan induced a rapid increase in cAMP within 1 minute which decreased within 5 minutes and was back to baseline levels by 15 minutes. By contrast, NECA induced a more gradual and modest increase in cAMP that declined by 15 minutes (Figure 4A). Thus, the kinetics of cAMP levels in primary brain endothelial cells demonstrates Lexiscan’s is rapid and robust, while NECA’s is gradual. This profile is consistent with TEER (Figure 2A), and BBB permeability induction (Figure 2C and D).
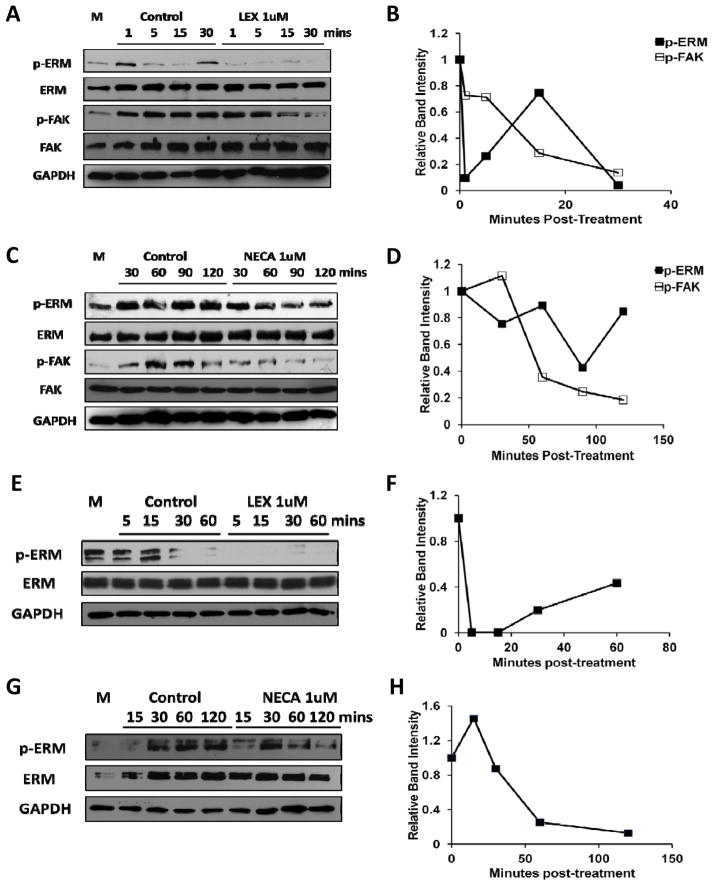
Figure 4. A2A AR signaling activates RhoA in human brain endothelial cells and induces stress fiber formation.
(A) Changes in the level of cyclic AMP (cAMP) by Lexiscan or NECA were determined in human brain endothelial cells. Intracellular cAMP levels in primary human brain endothelial cells were measured using a cAMP screening kit (Applied biosystems) after treatment with 1 uM of Lexiscan or NECA. 1 uM of Forskolin (FSK) was used as a positive control. (B) RhoA pull down assay performed in HBMVEC, or primary human brain endothelial cells with Lexiscan and NECA stimulation up to 15 minutes. M indicates media only control. (C) Densitometric analysis of western blot data from (B). The band intensity from each treatment group was divided by that of control group from each time point. (D and E) Western blot analysis of active RhoA levels using a pull down assay performed in the human brain endothelial cell line. HCMEC D3 cells lysates were activated with Lexiscan (D), or NECA (E). M indicates media only control. (F) Densitometric analysis of western blot data from (D) and (E). Intensity of band from treated group was divided by that of control group from each time point. (G) IFA analysis of stress fiber formation by Lexiscan and NECA treatment in HBMVEC which was visualized with AF568 conjugated phalloidin (Red). Nucleus was counterstained with DAPI (Blue). Induction of stress fiber formation was determined up to 30 minutes. Scale bar indicates 50 um.
To determine the effect of AR activation on RhoA activity, we performed a RhoA pull down assay using primary human brain endothelial cells and human brain endothelial cell line (HCMEC-D3). In primary human brain endothelial cells, RhoA activity increased rapidly by both Lexiscan and NECA compared to DMSO control (Figure 4B and C). Lexiscan increased RhoA activity within 1 minute, which began to decline after 5 minutes, whereas NECA treatment induced RhoA activity by 5 minutes and it was maintained up to 15 minutes post-NECA treatment (Figure 4B and C). This suggests that the kinetics of cAMP levels in primary brain endothelial cells by Lexiscan and NECA activation follows a similar trend in RhoA activity. Similarly, in HCMEC-D3 cells, Lexiscan induced a rapid increase in RhoA activity within 5 minutes and it peaked at 30 minutes, and declined by 60 minutes in HCMEC-D3 cells (Figure 4D and F). By contrast, NECA induced a modest increase in RhoA activity within 15 minutes and it began to decrease thereafter up to 60 minutes (Figure 4E and F). As the activity of RhoA is directly correlated to stress fiber formation that is coupled with BBB permeability(Jaffe and Hall 2005), we performed immunofluorescence assay (IFA) to visualize F-actin formation using AF568-conjugated phalloidin (Figure 4G). Lexiscan induced abrupt and rapid stress fiber formation that was maintained up to 15 minutes. In contrast, NECA’s formation of stress fibers was maintained up to 30 minutes. Also, more rapid incereases of RhoA activity and F-actin by Lexiscan may explain the rapid increase in the permeability to FITC-Dextran (Figure 2D). These data suggest that activation of A2A AR, either by Lexiscan, or NECA, induced changes in human brain endothelial cells that is consistent with changes in hBBB permeability. Importantly, this permeability in hBBBis reversible. It suggests that the kinetics of AR activation/deactivation window on BBB endothelial cells can be exploited for drug delivery to the brain.
Signaling through A2A AR down-modulates phosphorylation of focal adhesion in primary human brain endothelial cells
Focal adhesion is critical in maintaining brain endothelial cells monolayer resistance (Bretscher et al. 2002; Wu 2005). We next determined the effect of A2A AR activation in phosphorylation of focal adhesion proteins. In primary human brain endothelial cells, the level of phosphorylated Ezrin-Radixin-Moesin (ERM) was transiently decreased by Lexiscan, increased by 15 minutes and decreased again at 30 minutes. In contrast, phosphorylation of focal adhesion kinase (FAK), which is another representative focal adhesion molecule, gradually decreased up to 30 minutes (Figure 5A and B). This indicates that Lexiscan treatment decreased focal adhesion over a short time frame followed by rapid recovery. However, NECA increased phosphorylated ERM, which was maintained for up to 60 minutes after which it decreased by 120 min. Meanwhile, phosphorylated FAK began to decrease at 60 minutes (Figure 5C and D). These results indicate that the phosphorylation of ERM and FAK kinetics correlates with the increased permeability window of Lexiscan and NECA observed in primary human brain endothelial cells (Figure 2C and D). Similar kinetics was observed in HCMEC-D3 cells, which showed that phosphorylated ERM decreased very rapidly after Lexiscan treatment (within 5 minutes), and this decrease was maintained up to 60 minutes (Figure 5E and F). By contrast, NECA increased rapid phosphorylation of ERM that was maintained up to 60 minutes, and began to decrease thereafter (up to 120 minutes) (Figure 5G and H). These data suggest that stimulation of A2A AR caused suppression of micro-adhesion between human brain endothelial cells and their matrix.
Figure 5. Activation of A2A AR decreases the focal adhesion activity of human brain endothelial cell mediated by decrease in phosphorylation of ERM and focal adhesion kinase.
(A) Western blot on phosphorylated ERM (p-ERM) and phosphorylated focal adhesion kinase (p-FAK) were performed in Lexiscan treated primary human brain endothelial cells (HBMVEC) upto 30 minutes. (B) Intensity of the band of phosphorylated form was divided by that of total protein to obtain the ratio. Ratio from treated group was normalized by GAPDH and was divided by the value of control group at each time point (from A) and plotted as graph. (C) Western blot analysis of p-ERM, p-FAK were performed in NECA treated HBMVEC upto 120 minutes. (D) Intensity of the band of phosphorylated form was divided by that of total protein to obtain the ratio. Ratio from treated group was normalized by GAPDH and was divided by the value of control group at each time point (from C) and plotted as graph. (E) Western blot analysis of p-ERM in Lexiscan treated HCMEC-D3 cells up to 60 minutes. (F) Intensity of the band of phosphorylated form was divided by that of total protein to obtain the ratio. Normalized ratio by GAPDH from treated group was divided by that of control group at each time point (from E) and plotted as graph. (G) Western blot analysis of p-ERM in NECA treated HCMEC D3 cells up to 120 minutes. (H) Intensity of the band of phosphorylated form was divided by that of total protein to obtain the ratio. Normalized ratio by GAPDH from treated group was divided by that of control group at each time point (from G) and plotted as graph. In all images M indicates media only control.
Activation of A2A AR disrupts tight and adherens junctional molecules, increases the permeability of chemotherapeutic agents and induces glioblastoma cell death in human brain endothelial cell monolayer
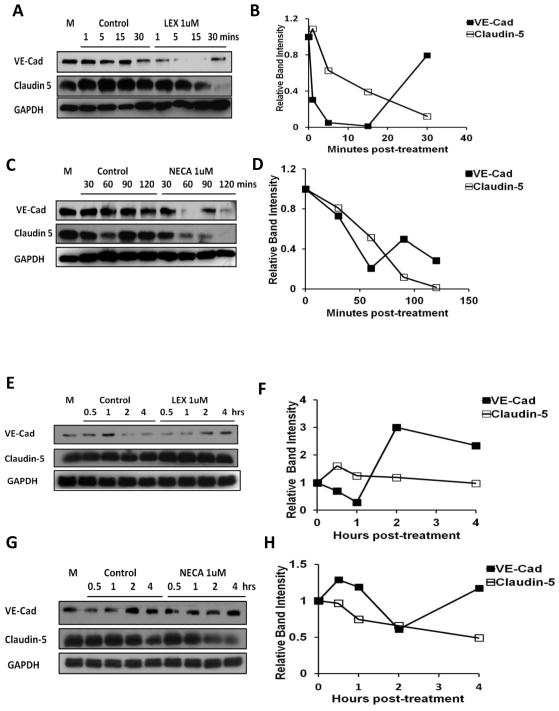
Claudin-5(Stevenson and Keon 1998) and vascular endothelial (VE)-Cadherin(Hordijk et al. 1999; Millan et al. 2010), play a major role in formation of vascular network and is necessary for endothelial barrier integrity. We examined the effect of AR signaling on the expression level and/or distribution of VE-Cadherin and Claudin-5 in our human and mouse brain endothelial cell monolayers. In primary human brain endothelial cells, Lexiscan decreased VE-Cadherin expression within 1 minute. It was maintained up to 15 minutes, recovered rapidly by 30 minutes, and lasted up to 90 minutes. The expression level of Claudin-5 gradually decreased up to 30 minutes after Lexiscan treatment (Figure 6A and B). Similar kinetics of VE-Cadherin expression was observed with NECA treatment (Figure 6C and D). In fact, NECA caused a dramatic decline in VE-Cadherin that lasted for 60 minutes and was recovered by 90 minutes. Claudin-5 expression also gradually decreased over 2 hrs with NECA.
Figure 6. Activation of A2A AR decreases expression level of tight and adherens junction molecules in human and mouse brain endothelial cell.
(A–D) Western blot result of VE-Cadherin and Claudin 5 in primary human brain endothelial cells with Lexiscan and NECA activation. (A and B) Western blot analysis of Claudin-5 and VE-Cadherin was performed on the HBMVEC cells treated with Lexiscan upto 30 minutes (A). Normalized intensity of band by GAPDH from treated group was divided by that of control group at each time point and plotted as graph (B). (C and D) Western blot on Claudin-5 and VE-Cadherin was performed on the HBMVEC cells treated with NECA upto 120 minutes (C). Normalized intensity of band by GAPDH from treated group was divided by that of control group at each time point and plotted as graph (D). (E–H) Western blot analysis of Claudin-5 and VE-Cadherin levels in mouse brain endothelial cell line (bEnd3). (E and F) Western blot analysis of Claudin-5 and VE-Cadherin was performed on the bEnd 3 cells treated with Lexiscan upto 4 hours (E). Normalized intensity of band by GAPDH from treated group was divided by that of control group at each time point and plotted as graph (F). (G and H) Western blot on Claudin-5 and VE-Cadherin was performed on the bEnd 3 cells treated with NECA upto 4 hours (G). Normalized intensity of band by GAPDH from treated group was divided by that of control group at each time point and plotted as graph (H). In all western blot images M indicates media only control.
In mouse brain endothelial monolayer (bEnd 3 cells), Lexiscan down-regulated VE-cadherin expression in a time dependent manner, from 30 minutes to 1 hr, after which it increased to baseline levels and was maintained steadily up to 4 hrs (Figure 6E and F). In contrast to VE-cadherin, Lexiscan induced a transient increase in Claudin-5, which returned to baseline and remained at steady state up to 4 hours (Figure 6E and F). NECA treatment decreased VE-Cadherin levels after 2 hrs, which is much later than Lexiscan, with a gradual return to baseline levels by 4 hrs. Claudin-5 expression was gradually decreased after 1 hr with significant decrease by 4 hrs after NECA treatment (Figure 6G and H). This is in striking contrast to Lexiscan which induced a rapid and robust decrease in VE-cadherin levels but had minimal effect on Claudin-5. This indicates that the expression level of adherens junction molecules, is decreased by both Lexiscan and NECA, but Lexiscan’s effect is more potent and occurs much earlier than NECA’s. Thus in mouse brain endothelial cells, A2A AR specific agonist Lexiscan, exerts its effect specifically through down-regulation of VE-Cadherin.
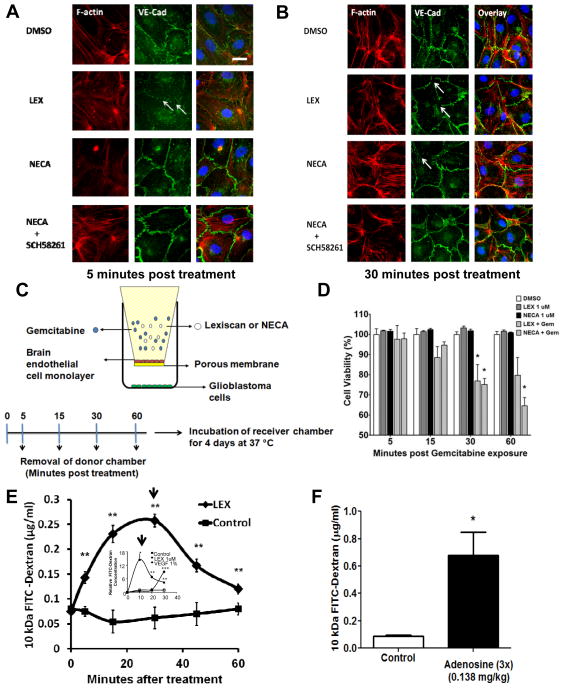
IFA analysis showed that treatment with Lexiscan or NECA caused junctional disruption of VE-Cadherin (Figure 7A and B). We also confirmed that VE-Cadherin disruption was mediated by A2A AR, as a specific A2A AR antagonist, SCH58261, concomitant with NECA treatment inhibited NECA’s effects (Fig 7A and B, 5 and 30 minutes post-treatment, respectively). This indicates that the window of increased permeability to polymerized Dextran observed in primary brain endothelial cells by Lexiscan (up to 30 minutes) and NECA (30–90 minutes) (Figure 2C and D) matches the window of VE-Cadherin and phosphorylated focal adhesion molecules down-regulation (Figure 6F and H).
Figure 7. Activation of A2A AR disrupts adherens junction molecules in primary human brain endothelial cell and increases the permeability to chemotherapeutics, Gemcitabine.
(A and B) IFA of VE-cadherin (Green) and F-actin (Red) in HBMVEC cells 5 and 30 minutes post-treatment with Lexiscan and NECA. Additionally, NECA was treated concomitantly with SCH58261 which is A2A specific antagonist. Arrows indicate the disrupted junction formation. Nucleus was counterstained with DAPI (Blue). Scale bar indicates 25 um. (C and D) AR agonists increase the permeability to chemotherapeutics, Gemcitabine, in primary human brain endothelial monolayer. Changes in permeability to the chemotherapeutic drug, Gemcitabine, after AR activation was determined using primary human brain endothelial cell monolayer. Donor chamber was treated with Gemcitabine (Gem) (10 ug/ml) for 5, 15, 30, 60 minutes with or without 1uM of Lexiscan or NECA and donor chambers were removed. Receiver chambers on which the YFP-transfected human glioblastoma cells (U251) were cultured and further incubated for 96 hrs and cell viability was measured by the relative intensity of YFP signal compared to untreated YFP-U251 (n=3). Lexiscan or NECA only treatment group was also set as control to test its effect on glioma cell viability (n=3). Data represents mean ± s.e.m. (* indicates where p<0.05, two tailed student t-test). (E) Selective A2A AR agonist Lexiscan increases the permeability of the BBB to 10 kDa FITC-Dextran in mice. Lexiscan (0.05 mg/kg) was intravenously administered concomitantly with 10 kDa FITC-Dextran and perfused with ice-cold PBS at different time point (n=10). Brain was collected and processed for analysis of FITC-Dextran concentration using fluometry (** indicates where p<0.01, two tailed student t-test). Graph from Figure 2D showing the effect of Lexiscan on hBBB permeability to 10 kDa FITC-Dextran was juxtaposed as inset for comparison. Arrows indicate the time point with maximal FITC-Dextran concentration from two different graphs. (F) Adenosine increases the peremeability of the BBB to 10 kDa FITC-Dextran in mice. Adenosine was intravenously administered three times (0.138 mg/kg, 20 seconds apart) concomitantly with 10 kDa Dextran and perfused with ice cold PBS at 1 minute after treatment (n=2). Brain was collected and processed for analysis of FITC-Dextran concentration using fluometry (* indicates where p<0.05, two tailed student t-test).
Glioblastoma multiforme (GBM) is an aggressive type of brain tumors whose treatment is limited by the BBB that blocks the entry of chemotherapeutic agents into the brain(Adamson et al. 2009; Deeken and Loscher 2007). We next determined whether AR activation could increase the permeability of hBBB to the chemotherapeutic drug, Gemcitabine which is effective in killing GBM (in vitro) but does not cross the BBB. Both Lexiscan and NECA decreased glioblastoma cell viability from 30 to 60 minutes. This is compared to DMSO that did not induce any changes in glioblastoma cell viability. Further, we tested the effect of Lexiscan or NECA without Gemcitabine on the glioblastoma viability and we did not observe any significant effect. (Figure 7C and D). Based on this result, we speculate that the decrease in glioblastoma cell viability observed by Lexiscan and NECA is attributed to increased hBBB permeability to Gemcitabine at the indicated time points.
To determine whether the kinetics of Lexiscan modulation of the human BBB coincides with in vivo BBB permeability, we treated mice with 10 kDa FITC dextran in the presence or absence of Lexiscan (Figure 7E). We observed that Lexiscan treated mice has significantly more FITC-Dextran in their brains compared to vehicle treated controls. Moreover, the kinetics of dextran entry in to the brain closely correlates with the kinetics of Lexiscan modulation of the in vitro human BBB permeability. We next tested the hypothesis that adenosine (with a half-life of 8–10 sec) is an endogenous modulator of BBB permeability that signals the recruitment of substances to sites of damage or inflammation (Figure 7F). We treated mice with FITC-Dextran with or without adenosine for 1 minute in a triple-injection manner (20 seconds apart). Adenosine treatment increased FITC-Dextran in the brain up to 4 times more than vehicle treated animals. Because adenosine, like other purines, are danger signaling molecules, these findings suggest that adenosine signaling at the BBB may signal the recruitment of substances (cells and or molecules) into the brain under conditions of inflammation or injury.
Discussion
Drug delivery to the brain is one of the most challenging factors facing drug development for CNS diseases (Deeken and Loscher 2007; Hossain et al. 2010; Pardridge 2005). Many efficacious drug candidates are developed against neurological diseases but most are dropped from the pipeline because they cannot cross the BBB to effectively treat diseases of the brain. To circumvent this hurdle, a variety of approaches are used to make access to the brain easier. These range from modification of drug properties to invasive delivery methods (Pardridge 2005). Our goal in this study was to determine whether human brain endothelial cells have the capacity to be permeabilized upon AR activation and to reveal the molecular mechanism behind the increased BBB permeability. Also, we hoped to evaluate the potential applicability of Lexiscan, which is an FDA-approved A2A AR agonist to permeabilize human brain endothelial barrier cells and increase drug delivery to the brain for treatment of neurological diseases. We used primary human brain endothelial cells and a human brain endothelial cell line (HCMEC-D3) as in vitro models for human BBB. We established that human brain endothelial cells highly and abundantly express CD73, which produces extracellular adenosine and express the A2A AR. Our findings indicate that not only do human brain endothelial cells have the capability to produce and respond to adenosine, but that activation of the A2A AR, Lexiscan or NECA potently permeabilize the in vitro human BBB. Importantly, the increase in BBB permeability upon A2A AR activation is rapid and reversible, two key components that are critical for patients’ safety.
Activation of A2A AR induces a rapid increase in RhoA activity and actin stress fiber formation (Spindler et al. 2010; Wojciak-Stothard and Ridley 2002). Also, upon AR activation, focal adhesion, which enhances micro attachment between endothelial cells and their extracellular matrix, decreases as the phosphorylation of ERM decreases (Bretscher et al. 2002). Although we did not directly test the relationship between changes in the phosphorylation level of focal adhesion molecules and endothelial monolayer permeability, we speculate that these changes might be important for endothelial permeability, as the kinetics of phosphorylation levels matches with kinetics of endothelial permeability. Also, we observed rapid decrease of VE-Cadherin and Claudin-5 after Lexiscan treatment, while NECA induced a more gradual decrease in their expression. Thus, increased RhoA activity, and induction of stress fiber formation, is consistent with decreased TEER, increased extravazation of polymerized Dextran and increased T cell TEM across our in vitro human brain endothelial barriers in the presence of both Lexiscan and NECA treatment. Most striking was A2A AR signaling promoted exclusively paracellular TEM. This is notable as most studies show that leukocyte migration into the CNS or in vitro BBB models occur by both paracellular and transcellular pathways (Carman and Springer 2008; Wolburg et al. 2005). Thus, in vivo paracellular TEM under physiological conditions may be mediated by AR signaling. As proof of principle that AR stimulation permeabilizes human brain endothelial barrier cells, we determined the effect of BBB permeability to Gemcitabine extravasation on glioblastoma viability. Gemcitabine is one of few chemotherapeutic drugs that kill glioblastomas but it does not cross the BBB. Consistent with the kinetics of BBB permeability induced by AR activation, we observed time-dependent cell death of glioblastoma cells. We attributed this to be due to the increased permeability window, increasing Gemcitabine concentration in the lower transwell chamber with glioblastoma cells. We further determined that Lexiscan permeabilized the BBB in mice with very similar kinetics observed in the human in vitro BBB model. Finally, we showed that despite the extremely short half life of adenosine which lasts about 10 seconds, also significantly permeabilized the BBB to FITC-Dextran in 1 minute. Taken together, we propose that adenosine modulation of the BBB is an endogenous mechanism developed in response to stress, to recruit substances to sites of inflammation or injury. This suggests that modulation of human BBB by AR signaling may be a viable tool for the delivery of therapeutics to the brain.
These studies are the first to investigate A2A AR signaling in human primary brain endothelial cells. Moreover, they are the first to utilize an FDA-approved AR agonist and demonstrate its function in human primary brain endothelial cell function. They make use of an endogenous mechanism for BBB control and they demonstrate the potential for precise, time dependent modulation of BBB permeability. These results strongly suggest that modulation of A2A AR, is a potential target for delivery of therapeutic drugs to the brain, or the delivery of stem cells in treatment of a wide range of neurological diseases including brain tumors, Alzheimer’s or HIV-AIDS.
Materials and Methods
Cells and reagents
The hCMEC-D3 cell was a kind gift from Dr. Babette Weksler (Weill Cornell Medical Center) and bEnd3 cell was purchased from ATCC. Primary human brain microvascular endothelial cell (ACBRI 376 V), attachment factor, and growth media were purchased from Cell Systems (Kirkland, WA). RhoA specific antibody and RhoA pulldown assay kit were purchased from Cytoskeleton (Denver, CO). Anti ROCK-1 antibody, Phycoerythrin (PE) conjugated anti-human CD73 antibody, human Collagen IV were purchased from BD bioscience (Carlsbad, CA). Rabbit anti Ezrin-Radixin-Moesin (ERM), phospo-ERM, VE-Cadherin, GAPDH were purchased from Cell Signaling (Danvers, MA). Alexa-Fluor 568 conjugated-Phalloidin, rabbit-anti-Claudin-5 antibody, anti rabbit and mouse Alexa Fluor 488, Texas Red conjugated secondary antibody, Fluorescein isothiocyanate (FITC) conjugated 10 kDa Dextran, Prolong Gold with DAPI, and cAMP-screening-kit were purchased from Life Technologies (Carlsbad, CA). Allophycocyanin (APC) conjugated anti-human CD39 antibody was purchased from ebioscience (San Diego, CA). Anti-phospo-focal adhesion kinase (FAK) was purhcased from Millipore (Billerica, MA). Mouse human CD31 antibody was purchased from R&D systems (Minneapolis, MN). Rabbit anti-A2A adenosine receptor antibody was purchased from Alomone labs (Jerusalem, Israel). EBM-2 media and supplementary bullet kit were purchased from Lonza (Allendale, NJ). 5′-N-(Ethylcarboxamido)adenosine (NECA) was purchased from Tocris (Bristol, UK) and 2-[4-[(Methylamino)carbonyl]-1H-pyrazol-1-yl]adenosine (Lexiscan) was purchased from Toronto Research Chemicals (Ontario, Canada). Adenosine was purchased from Sigma Aldrich (St. Louis, MO).
Flow Cytometry
HCMEC-D3 and HBMVEC cells were stained with PE anti-human CD73 and APC anti-human CD39 and the frequencies of positive cells were analyzed with BD Canto flow cytometer (BD Bioscience, Carlsbad, CA).
Immunofluorescence Assay (IFA)
Cells were treated with adenosine receptor agonists which was grown on coverslips, fixed with 4% Paraformaldehyde and permeablized with 0.2% Triton X and washed twice. Subsequently, it was blocked with 5% goat serum in 0.5 % BSA-PBS solution for 45 minutes. Primary antibodies (1:200) were incubated at room-temperature for 2 hrs and washed twice. Fluorchrome conjugated secondary antibody (1:1000) was incubated at room-temperature for 1 hr followed by two washes. For stress fiber staining, cells were additionally counter stained with AF568 conjugated phalloidin. Coverslip was washed with double distilled water and placed on the slide-glass with anti-fade mounting medium, Prolong Gold-DAPI. Samples were analyzed with Axiovision flurorescent microscope and images were recorded and analyzed with Axiovision (Carl Zeiss, Thornwood, NY).
Rho-GTPase pull down assay
HCMEC-D3 and HBMVEC were plated on collagen IV or attachment factor coated 10 cm petri dish and grown to 100 % confluency then treated with Lexiscan or NECA (1uM) over multiple time points: the reaction was halted by placing them on ice. Plates were washed with ice cold PBS and lysed with 250 ul of lysis buffer. The lysate was preserved at −80 °C until further analysis. Rho-GTPase pull down assay was performed according to the manufacturers’ protocol (Cytoskeleton, Denver, CO). The intensity of Active-RhoA was divided by that of total RhoA for densitometric analysis and to measure its activity at different time point.
Measurement of intracellular cyclic AMP (cAMP)
Primary human brain endothelial cells were plated on the 48 well plates until it reached confluency and treated with 1 uM of Lexiscan and NECA for 1, 5, 15 minutes (n=3). 1 uM of Forsklon (FSK) was used as positive control. Cells were lysed with lysis buffer at 37 °C for 30 minutes. Samples were processed following the protocols provided from manufacturer (cAMP-Screen System, Life Technologies) and the levels of cAMP were analyzed using luminometer function in Synergy 4 (Biotek, Winooski, VT).
Western Blot Analysis
Adenosine receptor agonist treated cells were lysed with lysis buffer containing protease inhibitor and mixed with 5X laemaeli buffer. Samples were loaded and separated by the 10 % SDS-PAGE gel and transferred to nitrocellulose paper. Subsequently, membranes were blocked by 1 % bovine serum albumin (BSA) in TBST buffer for 30 minutes at 4 °C. Primary antibody (1:2000) was incubated in 1% or 5 % BSA (p-ERM) for overnight at 4 °C and blot was washed three times with TBST. Subsequently, membranes were incubated with secondary mouse or rabbit antibody in non-fat dry milk (1:2000) for 1 hr at room temperature and washed three times with TBST. Membrane was visualized by West Pico enhanced chemiluminescence (ECL) solution which was exposed to X-ray film.
Transendothelial electrical resistance (TEER) assay
Mouse brain endothelial cells (bEnd 3) or primary human brain endothelial cells (HBMVEC) were plated (1×105) on the collagen IV (BD bioscience, Car) coated 8.0 um porous membrane insert (BD bioscience). When the confluency reached 100%, growth media was replaced with serum deprived media for overnight. Subsequently, 1 uM of Lexiscan, NECA which are A2A specific and broad spectrum adenosine receptor agonists, respectively, were applied into the insert along with DMSO control. The changes in the resistance were measured using Ohm voltmetry (World Precision Instrument, Sarasota, FL) at different time point. The TEER of different time points were subtracted by that of vacant porous membrane and the value was normalized by that of 0 time point.
FITC Dextran Permeability assay
Mouse and human brain endothelial cells were plated (1×105) on the collagen IV or attachment factor coated 3.0 um porous membrane insert (BD bioscience, San Jose, CA). Experiments initiated when its confluency reached 100%. Subsequently, media in insert and bottom well was replaced by pre-warmed HBSS and incubated at 37 °C for 2 hrs as acclimatization. 100 ug/ml of 10 kDa FITC-Dextran with or without 1 uM of Lexiscan and 1uM of NECA was applied into the insert. 50 ul of HBSS at the bottom well was collected at each time point (n=3). The concentration of FITC-Dextran was measured using fluometry (BioTek) with excitation at 488 nm, and emission at 523 nm. Acquired values were normalized by that of DMSO control.
Mice
C57BL/6 mice (Jackson Laboratories) aged 7–9 weeks and weighed between 20 and 25 g were used for experiments. Animals were bred and housed under specific pathogen-free conditions at Cornell University, Ithaca, NY. All procedures were done in accordance with approved Institutional Animal Care and Use Committee protocols.
Administration of drugs and tissue collection
Lexiscan was dissolved in DMSO and further diluted in the PBS. For vehicle controls, DMSO was diluted in PBS to the same concentration. Dextrans labeled with FITC were suspended in PBS to 10 mg/ml. Experiments involving dextran injection used 1.0 mg of dextran in PBS. When drug and dextran were injected concomitantly, 1.0 mg of dextran was mixed with the drug to the desired concentration in a final volume of 200 μl. Lexiscan was administered retro-orbitally and at indicated times mice were anesthetized and perfused with cold PBS through the left ventricle of the heart. Brains were weighed and frozen for later analysis.
Fluorimetric analysis of FITC-Dextran in the brain
Tris-Cl, 50mM, pH7.6, was added to brains (100 μl per 100 mg brain). Brains were homogenized with a Dounce homogenizer and centrifuged at 16.1 × g for 30 min. Supernatants were transferred to new tubes and an equal volume absolute methanol was added. Samples were centrifuged at 16.1 × g for 30 min. Supernatant (200 μl) was transferred to a Corning Costar 96 well black polystyrene assay plate (clear bottom). Fluorimetric analysis was performed on a BioTek Synergy 4.
Chemotherapeutics extravasation assay
Primary human brain endothelial cells were plated (1×105) on the attachment factor coated 0.4 um porous membrane insert (BD bioscience, San Jose, CA). Experiments initiated when its confluency reached 100%. Subsequently, media in insert and bottom well was replaced the day before experiment. At the receiver well, 2.5×105 of YFP transfected human gliobliostoma cells (U251, NCI cell repository, Frederick, MD) were plated. On donor well, 10 ug/ml of Gemzar (Eli Lilly, Indianapolis, IN) with or without 1 uM of Lexiscan or NECA (n=3) was administered. Also, 1 uM of Lexiscan or NECA without Gemzar was administered to further test the effect of these molecules on glioma viability. At 5, 15, 30, 60 minutes post treatment, donor well was removed and glioblastoma cells were further incubated for 4 days at 37 °C with 5 % CO2. Viability of glioblastoma compared to untreated group was measured using fluometry (BioTek) with excitation at 488 nm, and emission at 523 nm.
Jurkat Cell migration assay
To test the effect of adenosine receptor signaling in promoting the migration of immune cells, we performed migration assay of Jurkat Cell through in vitro blood brain barrier model using primary brain endothelial monolayer. Primary brain endothelial cell was cultured on the porous membrane insert (BD bioscience, San Jose, CA) which was pre-coated with attachment factor. When the confluency reached 100 %, media in both donor and reciever chamber was replaced with fresh media and acclimatized for overnight. 2.5 × 105 of Jurkat cells with or without 0.1 and 1 uM Lexiscan or NECA were placed on the upper chamber and the number of Jurkat cells migrated to the bottom well was counted at 1 and 24 hrs by hemocytometer.
To test if the effect of adenosine receptor signaling on migratory process of Jurkat cell is mediated by paracellular or transcellular pathway, primary brain endothelial cells were plated on the cover slip until it reached 100 % confluency. Cells were treated with 1 uM of Lexiscan and NECA for 2 hrs and washed with PBS. Jurkat cells were added and incubated for 5 minutes. Cells were fixed with 4% PFA and permeabilized with 0.1 % Triton X and blocked with 5 % Goat serum. Endothelial cell was stained with anti VE-Cadherin antibody which was subsequently stained with AF 647 conjugated 2ndary anti-Rabbit antibody. F-Actin was counterstained with AF568 conjugated phalloidin. Cells were visualized with Leica Confocal Microscope (Leica Microsystems, Buffalo Grove, IL) and images were recorded and analyzed by Leica Application Suite software. The route of transmigration of cells were classified as paracellular (cells on the borderline of endothelial cell to cell junction), transcellular (cells on the top of cytoplasmic area of endothelial cells), ambiguous depending on the localization of migrating Jurkat cells on the endothelial monolayer and quantified.
Statistical analysis
All statistical analysis was carried out using GraphPad 5.0 software. Statistical significances were assessed using either unpaired two tailed Student’s t-test or two-way analysis of variance (ANOVA) with Bonferroni multiple comparison test. P values less than 0.05 were considered to be statistically significant.
Acknowledgments
This study was supported by National Institute of Health (NIH) Grant R01 NS063011 (to M.S.B)
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. nrn1824 [pii] [DOI] [PubMed] [Google Scholar]
- Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, Bigner DD. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–1083. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- Adamson RH, et al. Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice. J Physiol. 2002;539:295–308. doi: 10.1113/jphysiol.2001.013117. PHY_13117 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn MR, Vance CO, Morschl E, Wilson CN. Adenosine receptors and inflammation. Handb Exp Pharmacol. 2009:215–269. doi: 10.1007/978-3-540-89615-9_8. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. nrm882 [pii] [DOI] [PubMed] [Google Scholar]
- Carman AJ, Mills JH, Krenz A, Kim DG, Bynoe MS. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J Neurosci. 2011;31:13272–13280. doi: 10.1523/JNEUROSCI.3337-11.2011. 31/37/13272 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman CV, Springer TA. Trans-cellular migration: cell-cell contacts get intimate. Curr Opin Cell Biol. 2008;20:533–540. doi: 10.1016/j.ceb.2008.05.007. S0955-0674(08)00088-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. 13/6/1663 [pii] [DOI] [PubMed] [Google Scholar]
- Fredholm BB, APIJ, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. pr.110.003285 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini KM, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. nrd3028 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. nrd2638 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci. 1999;112(Pt 12):1915–1923. doi: 10.1242/jcs.112.12.1915. [DOI] [PubMed] [Google Scholar]
- Hossain S, Akaike T, Chowdhury EH. Current approaches for drug delivery to central nervous system. Curr Drug Deliv. 2010;7:389–397. doi: 10.2174/156720110793566245. BSP/CDD/E-Pub/00032 [pii] [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. nrd1983 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jeyaraj SC, et al. Cyclic AMP-Rap1A signaling activates RhoA to induce alpha(2c)-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells. Am J Physiol Cell Physiol. 2012;303:C499–511. doi: 10.1152/ajpcell.00461.2011. ajpcell.00461.2011 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, et al. Permeability of endothelial and astrocyte cocultures: in vitro blood-brain barrier models for drug delivery studies. Ann Biomed Eng. 2010;38:2499–2511. doi: 10.1007/s10439-010-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan J, et al. Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol. 2010;8:11. doi: 10.1186/1741-7007-8-11. 1741-7007-8-11 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JH, Alabanza L, Weksler BB, Couraud PO, Romero IA, Bynoe MS. Human brain endothelial cells are responsive to adenosine receptor activation. Purinergic Signal. 2011;7:265–273. doi: 10.1007/s11302-011-9222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JH, et al. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105:9325–9330. doi: 10.1073/pnas.0711175105. 0711175105 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. 326/5957/1208 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. jcb.200901084 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Crivellato E, Artico M. Development of the blood-brain barrier: a historical point of view. Anat Rec B New Anat. 2006;289:3–8. doi: 10.1002/ar.b.20087. [DOI] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. nrm1128 [pii] [DOI] [PubMed] [Google Scholar]
- Sohail MA, et al. Adenosine induces loss of actin stress fibers and inhibits contraction in hepatic stellate cells via Rho inhibition. Hepatology. 2009;49:185–194. doi: 10.1002/hep.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. cvq086 [pii] [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Keon BH. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol. 1998;14:89–109. doi: 10.1146/annurev.cellbio.14.1.89. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Sahai E, Chardin P, McCormick F, Courtneidge SA, Alberts AS. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000;5:13–25. doi: 10.1016/s1097-2765(00)80399-8. S1097-2765(00)80399-8 [pii] [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:187–199. doi: 10.1016/s1537-1891(03)00008-9. S1537189103000089 [pii] [DOI] [PubMed] [Google Scholar]
- Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109:181–190. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- Wu MH. Endothelial focal adhesions and barrier function. J Physiol. 2005;569:359–366. doi: 10.1113/jphysiol.2005.096537. jphysiol.2005.096537 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]