Abstract
Quantification of sickle hemoglobin (HbS) in patients with sickle cell disease (SCD) undergoing hydroxyurea or chronic transfusion therapy is essential to monitoring the effectiveness of these therapies. The clinical monitoring of %HbS using conventional laboratory methods is limited by high per-test costs and long turnaround times usually associated with these methods. Here we demonstrate a simple, rapid, inexpensive paper-based assay capable of quantifying %HbS in blood samples from patients with SCD. A 20 μL droplet of whole blood and hemoglobin solubility buffer was deposited on chromatography paper. The relative color intensities of regions of the resulting blood stain, determined by automated image analysis, are used to estimate %HbS. We compared the paper-based assay with hemoglobin electrophoresis (comparison method) using blood samples from 88 subjects. The test shows high correlation (R2 = 0.86) and strong agreement (standard deviation of difference = 7 %HbS) with conventional Hb electrophoresis measurement of %HbS, and closely approximates clinically predicted change in %HbS with transfusion therapy (mean difference 2.6 %HbS, n = 4). The paper-based assay can be completed in less than 35 minutes and has a per-test cost less than $0.25. The assay is accurate across a wide range of HbS levels (10–97%) and hemoglobin concentrations (5.6–12.9 g/dL) and is unaffected by high levels of HbF (up to 80.6%). This study demonstrates the feasibility of the paper-based %HbS assay. The paper-based test could improve clinical care for SCD, particularly in resource-limited settings, by enabling more rapid and less expensive %HbS monitoring.
Keywords: sickle, hemoglobin, paper, quantification, paper-fluidic
Introduction
Sickle cell disease (SCD) is a common recessively inherited blood disorder caused by a point mutation of the β-globin gene.(1) Unlike normal adult hemoglobin (HbA), sickle hemoglobin (HbS) polymerizes and becomes insoluble under hypoxic conditions, causing a number of structural and functional abnormalities in affected red blood cells (RBCs). Sickled RBCs have increased fragility and rigidity that render them prone to early breakdown and occlusion of blood vessels. Patients with SCD experience chronic hemolytic anemia, episodic pain crises and abnormal blood flow to critical organs that cumulatively result in significant illness and shortened lifespans.(2) Although, the severity of the disease varies greatly among patients, for each individual patient the rate of adverse events is strongly correlated with the intraerythrocytic concentration of HbS.(1)
The two main therapeutic options for SCD, hydroxyurea and chronic transfusion, both rely primarily on a reduction of the relative HbS level for their beneficial effect. Hydroxyurea induces the expression of hemoglobin F (HbF) in erythrocytes, reducing the intraerythrocytic HbS concentration and making the erythrocyte less likely to sickle in hypoxic conditions. Chronic transfusion therapy does not affect intraerythrocytic HbS concentration but reduces the overall proportion of sickle erythrocytes and partially corrects the anemia of SCD, improving organ perfusion and oxygenation.(3) The main goal of chronic RBC transfusion for SCD patients is to maintain a low level of HbS (<30%) in order to decrease the rate of adverse clinical outcomes, in particular the risk of primary or secondary stroke.(4–6) Generally, the transfusion of 2–3 RBC units or 10–15 mL/kg every 3–5 weeks is sufficient to keep the %HbS below 30% and the [Hb] at 9–10 g/dL and minimize the risk of progressive complications.(7)
Both hydroxyurea and chronic transfusion therapy require frequent monitoring of the relative HbS concentration to assess effectiveness of therapy. The monitoring of %HbS in patients on these therapies using conventional laboratory methods (e.g. Hb electrophoresis(8) and HPLC(9)) is impeded by the high cost and long processing time usually associated with these methods.(8) Typically, results are not available rapidly enough to guide clinical decision-making at the time of the clinic visit. Additionally, since HbS levels are not routinely measured during or after transfusions, relatively little is known about the evolution of HbS levels in SCD patients on chronic transfusion therapy. A rapid, inexpensive assay for measuring HbS concentration in patient blood samples could be useful in monitoring the effectiveness of hydroxyurea and transfusion therapy by enabling more frequent testing than currently possible with conventional laboratory methods, thereby providing additional information that could inform clinical decision making.
Our research group has recently developed a low-cost paper-based test for diagnosing SCD in resource-limited settings.(10) This test utilizes the separation of HbS and non-sickle hemoglobin by differential wicking in a paper matrix to qualitatively diagnose SCD. We postulated that a similar test could be capable of quantifying the sickle component of total hemoglobin in patient blood samples. In this study, we demonstrate that this paper-based test can be used to rapidly and accurately quantify the HbS content in SCD patients on chronic transfusion and hydroxyurea therapy, as well as in infants with a relatively high endogenous level of Hb F.
Methods
Blood samples
Venous blood samples were collected from patients with SCD, after informed consent following an IRB-approved protocol, during clinic visits (January 2013 – September 2014) at the Texas Children’s Hematology Center (Houston, TX). Healthy, consenting volunteers donated normal venous blood samples. Blood samples were collected in Vacutainer tubes (K2EDTA, BD, Franklin Lakes, NJ), stored between 4–25°C and analyzed within 21 days after collection (mean storage time before analysis = 6.4 ± 3.6 days). Sample storage time up to 21 days was only weakly correlated with assay performance (R2 = 0.09, p < 0.01).
Hemoglobin Electrophoresis
Hemoglobin A, F, C and S for each sample were measured using conventional Hb electrophoresis performed on the semi-automated Sebia Hydrasys 2 Scan system (Sebia Inc., Norcross, GA). All materials were prepared and run according to manufacturer specifications. Phoresis curve editing software (Sebia Inc., Norcross, GA) was used to calculate hemoglobin composition. For post-transfusion samples, the expected %HbS was calculated using the following method: Post-transfusion %HbS = Total HbS (g) x 100 / (Total post-transfusion Hb (g)); Total HbS = Pre-transfusion [Hb] x pre-transfusion %HbS x Total blood volume (dl); Total post-transfusion Hb = (Pre-transfusion [Hb] x total blood volume (dl)) + (Total transfused volume (dl) x [Hb]Transfused blood).
Hemoglobin solubility buffer
The Hb solubility buffer was a mixture of a hemolytic agent (saponin), a reducing agent (sodium hydrosulfite) and a high-phosphate buffer. The high-phosphate buffer (2.49M) consisted of 1.24M (169 g/L) monobasic and 1.25M (217 g/L) dibasic potassium phosphate dissolved in deionized water. The saponin (4 g/L) permeabilizes red cell membranes, releasing Hb into the solution. The sodium hydrosulfite (30 g/L) converts the released Hb into deoxy-Hb. Converted HbA, HbE, HbF and HbC remain soluble in the high-phosphate buffer, while deoxy-HbS polymerizes and precipitates.(11, 12)
Quantification of the blood stain color intensities
The blood stains on patterned chromatography paper were digitized by scanning the paper with a portable flatbed scanner (CanoScan LiDE110, Canon USA Inc, Lake Success, NY). The digitized blood stains were analyzed with a custom image analysis algorithm (MATLAB, The Math Works Inc, Natick, MA). The algorithm automatically isolates features of the blood stain via contrast stretching and thresholding, then calculates the mean color intensity of each feature using RGB color data from the Blue (B) channel of each pixel within the feature (color intensity = 255 – B).(13)
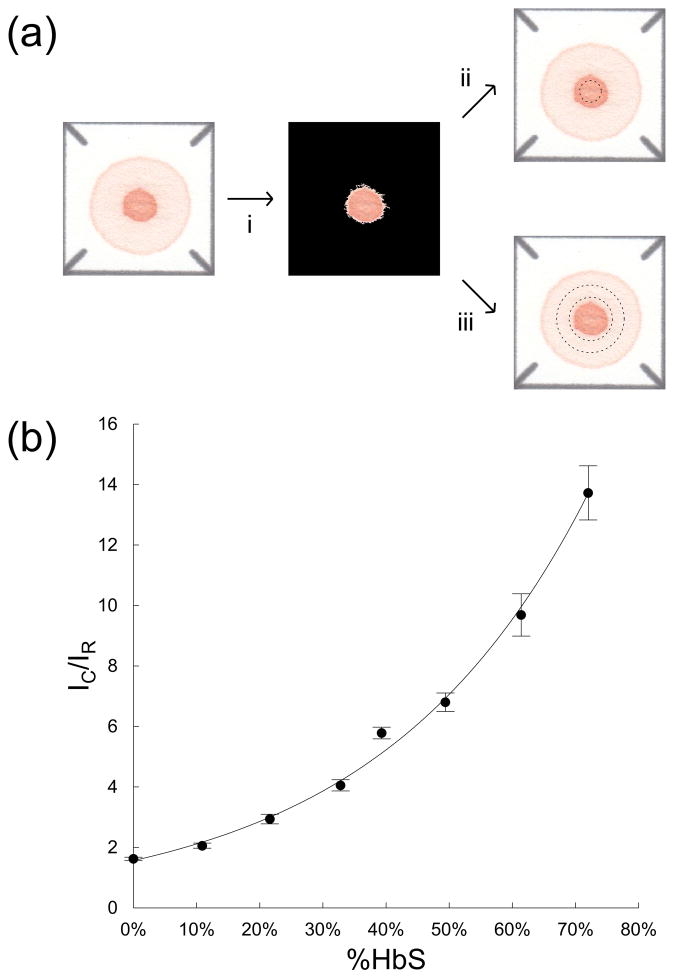
The relationship between the quotient of relative mean color intensities of the standardized blood stains and HbS content in patient samples was determined using a series of reconstituted blood samples with %HbS artificially adjusted from 0 – 75%. These calibration samples were created by mixing ABORh-matched, equal-hematocrit normal (HbAA) and SCD (HbSS) blood samples at various ratios to achieve the desired HbS concentrations. %HbS of artificially adjusted samples was verified by Hb electrophoresis. Figure 2b shows this calibration curve relating the quotient of mean color intensities to %HbS, an exponential curve was fit to the data, where estimated %HbS = 0.332 * ln(0.639 * IC/IR). This calibration curve was used throughout the rest of this study to convert IC/IR to estimated %HbS.
Figure 2.
Using a blood stain to estimate %HbS. (a) Schematic illustration of image analysis algorithm. (a-i) Centroid of the blood stain (marked by a cross) is automatically identified by the algorithm using contrast stretching and thresholding. (a-ii) The center spot (area inside dashed circle) and (a-iii) the peripheral ring (area between dashed circles) are extracted from the image through application of binary masks centered on the centroid of the stain. Mean color intensities of the center spot (IC) and the peripheral ring (IR) are extracted from the standardized blood stain. (b) Dependence of IC/IR on %HbS for a series of reconstituted blood (n = 5) samples with artificially adjusted %HbS varying from 0 to 75% (calibration curve, solid line). The values of the quotient of mean color intensities increased exponentially with increasing %HbS.
Results
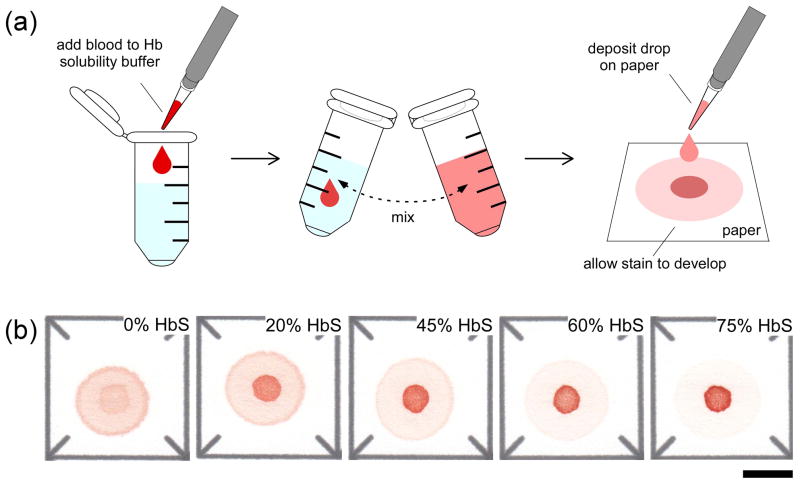
The design and operation of the paper-based assay for SCD has been previously described in detail.(10) Briefly, to perform the assay 20 μL of whole blood was added to 200 μL of solubility buffer, mixed by inversion, and allowed to sit for 10 minutes, to allow RBC lysis and hemoglobin deoxygenation. At the end of this period, 20 μL of the lysate was pipetted onto chromatography paper and allowed to wick laterally and dry for 25 minutes (Figure 1a). The entire assay was performed indoors, in ambient conditions (temperature 15°C to 25°C, 20% to 70% humidity, fluorescent lighting). The difference in transport of soluble and insoluble variants of hemoglobin (Hb) through the paper substrate produces a blood stain consisting of two parts: the area of the initial drop where polymerized deoxy-HbS is retained (center spot), and the area where all soluble forms of Hb are wicked laterally (peripheral ring). The color intensity of the center spot relative to that of the peripheral ring is indicative of the HbS level in the blood sample. Figure 1b shows representative blood stains for samples artificially reconstituted with %HbS varying from 0 to 75%. As expected, increasing HbS content results in increased color intensity of the center spot and decreased intensity of the peripheral ring. The absolute color intensity of the stain is dependent on the Hb concentration of the sample, but the relative color intensities of the center spot and peripheral ring are independent of Hb concentration. The resulting blood stain is digitized using a portable flatbed scanner (in reflected light) and analyzed with a custom image analysis algorithm. The entire assay can be performed for up to 20 samples in less than 35 minutes.
Figure 1.
Schematic illustration of the paper-based HbS assay and the characteristic blood stains. (a) To perform the assay a 20 μL droplet of blood mixed with Hb solubility buffer (phosphate buffer containing saponin and sodium hydrosulfite) is deposited on chromatography paper, a blood stain is allowed to form (polymerized deoxy-HbS is trapped in the paper mesh and soluble forms of Hb are wicked laterally), and the stain is scanned and analyzed automatically to estimate %HbS in the sample. (b) Characteristic blood stains produced on paper by samples with various %HbS. Scale bar is 1 cm.
Figure 2 describes the process of estimating %HbS based on the relative color intensity of the center spot and the peripheral ring areas of the blood stain. First, the custom image analysis algorithm applies a standard contrast stretching and thresholding routine to the digitized image of the blood stain to determine the center of the stain (Figure 2a-i). A standardized binary mask(14) is then used to isolate the appropriate parts of the blood stain image to calculate, IC, the mean color intensity of the center spot (Figure 2a-ii) and, IR, the mean color intensity of the peripheral ring (Figure 2a-iii). For each sample, the quotient of these two values (IC/IR) is then converted to estimated %HbS using the equation (estimated %HbS = 0.332 * ln(0.639 * IC/IR)) derived from the calibration curve (Figure 2b).
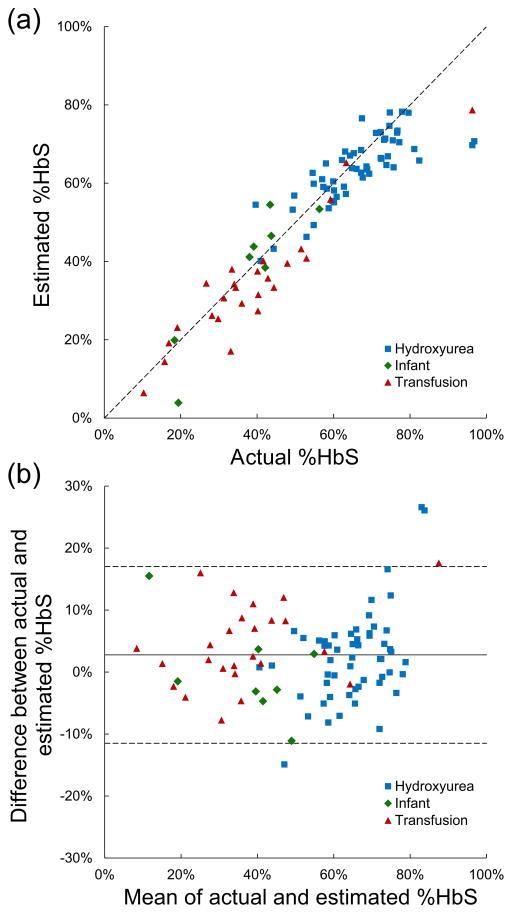
To test the accuracy of our paper-based quantification method, we then compared the value of %HbS in individual patient samples measured by conventional Hb electrophoresis (Sebia Hydrasys 2 Scan, Sebia Inc., Norcross, GA) and by the paper-based assay. Of the 88 samples used in this analysis, 55 were from patients on hydroxyurea therapy, 25 were from patients on chronic transfusion therapy, and 8 were from infants. The ages of the patients ranged from one month to 18.8 years, hemoglobin concentrations ranged from 5.6 to 12.9 g/dL, and %HbS ranged from 10 to 97%. Five patients had the SC genotype, 3 patients had the Sβ0 genotype, and 2 patients had the Sβ+ genotype. As shown in Figure 3a, the values of %HbS estimated using the paper-based assay (Estimated %HbS) and measured using conventional hemoglobin electrophoresis (Actual %HbS) were highly correlated (R2 = 0.86). When broken down by sample type, the correlation between %HbS measured by electrophoresis and that measured by the paper-based assay remained high, although the correlation was weaker for patients undergoing hydroxyurea therapy (R2hydroxyurea = 0.61, R2transfusion = 0.87, R2infant = 0.83), indicating that the accuracy of the paper-based assay was not affected by significant variations in %HbS and %HbA, and %HbF or by the relative distribution of these hemoglobin complexes within erythrocytes. The lower correlation for patients undergoing hydroxyurea therapy resulted from decreased accuracy of the test at very high %HbS levels (the samples from patients undergoing hydroxyurea therapy tended to have the highest HbS concentrations) rather than from inherent differences between patient groups.
Figure 3.
Comparison of paper-based %HbS measurements with a reference method (hemoglobin electrophoresis). (a) %HbS for a group of patient samples (n = 88) was measured using the paper-based assay (Estimated %HbS) and conventional Hb electrophoresis (Actual %HbS). Estimated %HbS is highly correlated (R2 = 0.86) with actual %HbS (solid line = data trend line; dashed line = perfect correlation). Patient characteristics are indicated by dot color. (b) Bland–Altman plot shows strong agreement (SD difference = 7 %HbS) between estimated and actual %HbS (solid line = mean difference; dashed lines = ± 2 SD difference). The majority of the differences between actual and estimated %HbS (95.5%) are within 2 standard deviations of the mean of the differences. Patient characteristics are indicated by dot color.
Figure 3b shows a Bland-Altman plot(15) of the %HbS values obtained using both methods. The values of %HbS for patient samples estimated using the paper-based assay and measured using conventional hemoglobin electrophoresis show strong agreement. The standard deviation of the differences between different %HbS values obtained using both methods was 7 %HbS. The majority (95.5%) of the differences between actual and estimated %HbS are within 2 standard deviations of the mean of the differences. The limits of agreement between the paper-based HbS assay and conventional hemoglobin electrophoresis were −11.5 %HbS and 17.0 %HbS. The paper-based HbS assay agreed with the hemoglobin electrophoresis system within 5 %HbS 58.0% of the time, overestimating %HbS by >5 %HbS in 10.2% of subjects and underestimating %HbS by >5 %HbS in 31.8% of subjects.
We tested the repeatability of the paper-based method for quantifying HbS by repeatedly measuring (n = 5) the %HbS for a series of blood samples with HbS content of approximately 10, 20, 30, 40, 50, 60, 70 and 80 %HbS. The standard deviation for the %HbS measurements performed with different droplets of the same blood sample was consistently < 1.5 %HbS for all values of %HbS measured: 0.9 %HbS (CV 6.3%) for the sample with 10 %HbS; 1.0 %HbS (CV 4.8%) for 20 %HbS; 1.3 %HbS (CV 4.1%) for 30 %HbS; 1.1 %HbS (CV 2.6%) for 40 %HbS; 0.7 %HbS (CV 1.3%) for 50 %HbS; 1.3 %HbS (CV 2.1%) for 60 %HbS; 1.1 %HbS (CV 1.6%) for 70 %HbS; and 1.5 %HbS (CV 1.9%) for 80 %HbS. For comparison, the standard deviations for the %HbS measurements performed by the manufacturer of the Sebia Hydrasys 2 Scan system for blood samples with %HbS ranging from 8.8 – 83.6 %HbS was 0.2 – 0.6 %HbS (CV 0.7 – 2.1%).
Finally, to assess the utility of the paper-based assay in measuring the change in %HbS with transfusion therapy, we compared the change in %HbS in five patients based on pre- and post-transfusion measurements made using Hb electrophoresis to the same measurements made using the paper-based assay and to an estimate of post-transfusion %HbS made using a formula currently used to guide clinical transfusion practices (based on patient weight, Hb concentration and initial %HbS). As presented in Table 1, the change in %HbS with transfusion measured by Hb electrophoresis was very close to both that estimated by the clinical formula (mean difference 3.3 %HbS) and that measured with the rapid assay (mean difference 3.9 %HbS) for all five patients, indicating that our paper-based assay is capable of accurately determining changes in %HbS following transfusion therapy.
Table 1.
%HbS information of patients (n = 5) receiving transfusion therapy. Paired blood samples were drawn from each patient before (Initial) and after (Final) transfusion. Initial and final %HbS was measured by Hb electrophoresis and by the paper-based assay. A clinical estimation of final %HbS (based on patient weight, Hb concentration and initial %HbS measured by Hb electrophoresis), used to guide transfusion practices, is shown for comparison. The mean difference of change in %HbS measured by Hb electrophoresis and by the paper-based assay is 3.9 %HbS. The mean difference of change in %HbS measured by Hb electrophoresis and with the clinical estimation is 3.3 %HbS. The clinical estimation overestimated the change in %HbS with respect to Hb electrophoresis in 1 of 5 patients. The decrease in %HbS measured by the paper-based assay was smaller than the decrease in %HbS measured by Hb electrophoresis in 5 of 5 patients.
| Hb electrophoresis | Clinical estimation | Paper-based assay | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Initial %HbS | Final %HbS | Change in %HbS | Initial %HbS | Final %HbS | Change in %HbS | Initial %HbS | Final %HbS | Change in %HbS |
| 1 | 41.2 | 27.8 | −13.4 | 41.2 | 32.1 | −9.1 | 34.3 | 22.8 | −11.5 |
| 2 | 30.5 | 20.2 | −10.3 | 30.5 | 22.4 | −8.1 | 26.3 | 20.4 | −5.9 |
| 3 | 43.5 | 30.4 | −13.1 | 43.5 | 34.4 | −9.1 | 30.4 | 24.7 | −5.7 |
| 4 | 33.7 | 23.2 | −10.5 | 33.7 | 21 | −12.7 | 32.3 | 22.1 | −10.2 |
| 5 | 33.3 | 19.7 | −13.6 | 33.3 | 23.4 | −9.9 | 21.8 | 13.6 | −8.2 |
Discussion
In this report, we present a novel paper-based method of quantifying HbS levels in blood samples from patients with sickle cell disease. Our method is based on the previously described qualitative diagnostic test for sickle cell disease that relies on the differential wicking of insoluble HbS and soluble non-sickle hemoglobins on a paper substrate to produce characteristic blood stain patterns. We now extend this method to permit the quantification of sickle hemoglobin using the patterns produced by this diagnostic test. This quantification is effected by digitization of the rapid test paper containing the blood stain and measurement of the relative color intensity of the center spot, composed of trapped, precipitated sickle hemoglobin, and the peripheral ring, composed of free, soluble hemoglobins. As indicated by the data presented above, quantification of %HbS by this method is highly accurate and reproducible relative to conventional hemoglobin electrophoresis. This method is also accurate across a wide range of HbS levels and hemoglobin concentrations and is unaffected by high levels of endogenous HbF or exogenous HbA. As such, it is potentially applicable to monitor any therapeutic modality that alters the relative level of HbS, including acute or chronic transfusion therapy and hydroxyurea therapy.
Our paper-based assay offers several advantages over conventional methods for the quantification of HbS. The first and most prominent is the low cost of our assay. The cost of consumable materials and reagents for our test is less than $0.25 per sample, and the initial investment for a computer and scanner for image digitization is less than $400. By contrast, the cost of required supplies for the conventional hemoglobin electrophoresis system used as a reference standard in our laboratory is $1,800 annually for system solutions and controls, plus an additional $5.77 to $12.07 per sample for consumable materials and reagents, and the initial investment for the system is more than $20,000. The estimated cost per sample of both IEF and HPLC at our collaborating center is approximately $60. Our rapid assay thus offers significant cost savings compared to other methods of HbS quantitation.
A second advantage of our paper-based assay is the ease of use of the system. Processing of blood samples to develop the blood stain is a simple operation requiring only two steps (mixing and sample deposition). Subsequent digitization and quantification of the blood stain itself is also straightforward and is largely accomplished by the automated image analysis algorithm. The overall process of blood stain development, image scanning, and HbS quantification can therefore be performed with minimal technical training by any operator with basic laboratory skills.
A final advantage of the paper-based assay is the rapidity of the assay. Conventional laboratory methods of hemoglobin quantification can be completed rapidly, but due to the limited availability of clinical technicians and costs associated with sample processing, samples are often grouped to be run in large batches, delaying the availability of results. In contrast, our paper-based assay does not require batching and has an extremely low per-test cost, even for single samples. A sample obtained from a patient can be processed, digitized, and quantified within less than 35 minutes, permitting rapid clinical assessment of a patient’s HbS burden and the adjustment of therapeutic interventions. This capability could be especially useful for patients on chronic transfusion therapy since, unlike conventional measures of %HbS, the paper-based test could determine the current %HbS level in time to assist the clinician in making decisions about current transfusion volume and subsequent transfusion frequency to maintain a target HbS level.
The paper-based assay for HbS quantification offers obvious advantages for resource-limited settings. The low cost of the assay and its technical simplicity are major advantages in environments in which clinical resources, a reliable stream of specialized supplies for complex medical testing, and training for clinical and laboratory personnel are deficient. This assay is highly portable and can be set up quickly in any clinical setting, including those in which the supply of electricity may be uncertain. A major barrier to the implementation of transfusion and hydroxyurea therapies in resource-limited settings is the inability to monitor their efficacy in a timely manner that could influence patient care. The use of this assay could therefore make chronic transfusion therapy targeting a specific %HbS level, such as for primary or secondary stroke prophylaxis, more feasible in such settings. It can also be used in conjunction with other simple measurements (e.g. hemoglobin concentration and patient clinical status) to indirectly monitor the efficacy of HbF induction with hydroxyurea therapy since the reduction of %HbS in patients on hydroxyurea correlates with the degree of HbF induction.
There are a few technical limitations to the paper-based assay. The most prominent of these is that conditions resulting in clotting of blood samples can affect the quantification of HbS in the sample. In our validation cohort of 88 patient samples, the presence of clots in the samples resulted in greater deviation of the value for %HbS determined by the paper-based assay from that measured by hemoglobin electrophoresis. Secondly, the paper-based assay is not as accurate as more technically complex methods of HbS quantitation, especially at very high HbS levels, though this inaccuracy is relatively minor. Thirdly, very high levels of HbF (such as those found in newborns) may interfere with the polymerization of HbS and therefore decrease the accuracy of the assay. Finally, the assay in its current form cannot distinguish between non-pathological soluble hemoglobins, such as HbA, HbA2, and HbF, and those that can cause SCD in association with HbS, such as HbC or HbD. As such, the utility of this assay is currently limited to patients with homozygous sickle cell disease and Sβ0 thalassemia. Of note, however, patients with homozygous sickle cell disease and Sβ0 thalassemia constitute the majority of all sickle cell patients (>70% in the United States and over 95% in many sub-Saharan African countries(16)) and are also the two most severe forms of the disorder with the most pressing need for clinical intervention.
In summary, we have developed a new, rapid, inexpensive paper-based assay for the quantification of %HbS in blood samples from patients with sickle cell disease. The assay determines %HbS across a wide range of values with a high degree of accuracy relative to hemoglobin electrophoresis and is unaffected by variations in overall hemoglobin concentration or the presence of HbA or HbF in the patient sample. Our assay has obvious applications in resource-limited settings and could be an important step towards making chronic transfusion therapy and hydroxyurea therapy feasible in such settings. The assay could also be useful in resource-rich settings by permitting more rapid and less expensive monitoring of response to these therapies, thus improving clinical care and reducing costs associated with the management of sickle cell disease.
Acknowledgments
The authors would like to thank Ms. Norma Estrada and Dr. Bogdan Dinu for coordinating sample collection, and Dr. Tariq Elghetany for critical review of the manuscript. This work was supported in part by a 2012 NIH Director’s Transformative Research Award (NHLBI R01HL117329, PI: Shevkoplyas). SSS, NZP and XY are inventors on a utility PCT application “Paper based diagnostic test” (PCT/US2012/064856, 11/13/2012) claiming priority benefit of US 61/692,994 (8/24/2012) and US 61/558,009 (11/10/ 2011). SSS is a part-owner of Halcyon Biomedical Incorporated, a company which may benefit from commercialization of the paper-based SCD test. All data and described custom-written scripts are available from the authors upon reasonable request.
Footnotes
All other authors report no potential conflicts of interest.
References
- 1.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364:1343–60. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 2.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–31. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 3.Simon TL, Snyder EL, Stowell CP, Strauss RG, Solheim BG, Petrides M. Rossi’s principles of transfusion medicine. Wiley; 2011. [Google Scholar]
- 4.Josephson CD, Su LL, Hillyer KL, Hillyer CD. Transfusion in the patient with sickle cell disease: A critical review of the literature and transfusion guidelines. Transfusion medicine reviews. 2007;21:118–33. doi: 10.1016/j.tmrv.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Roback JD, Banks AAoB. Technical manual. American Association of Blood Banks; 2008. [Google Scholar]
- 6.Hillery CA, Panepinto JA. Pathophysiology of stroke in sickle cell disease. Microcirculation. 2004;11:195–208. doi: 10.1080/10739680490278600. [DOI] [PubMed] [Google Scholar]
- 7.Wanko SO, Telen MJ. Transfusion management in sickle cell disease. Hematology/oncology clinics of North America. 2005;19:803–26. v–vi. doi: 10.1016/j.hoc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Clarke GM, Higgins TN. Laboratory investigation of hemoglobinopathies and thalassemias: Review and update. Clinical chemistry. 2000;46:1284–90. [PubMed] [Google Scholar]
- 9.Head CE, Conroy M, Jarvis M, Phelan L, Bain BJ. Some observations on the measurement of haemoglobin a2 and s percentages by high performance liquid chromatography in the presence and absence of alpha thalassaemia. J Clin Pathol. 2004;57:276–80. doi: 10.1136/jcp.2003.008037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Kanter J, Piety NZ, Benton MS, Vignes SM, Shevkoplyas SS. A simple, rapid, low-cost diagnostic test for sickle cell disease. Lab Chip. 2013;13:1464–7. doi: 10.1039/c3lc41302k. [DOI] [PubMed] [Google Scholar]
- 11.Itano HA. Solubilities of naturally occurring mixtures of human hemoglobin. Archives of biochemistry and biophysics. 1953;47:148–59. doi: 10.1016/0003-9861(53)90444-5. [DOI] [PubMed] [Google Scholar]
- 12.Nalbandian RM, Camp FR, Jr, Conte NF, Prothro WB. Automated mass screening for hemoglobin s: A rational method. Health services reports. 1973;88:165–73. [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Piety NZ, Vignes SM, Benton MS, Kanter J, Shevkoplyas SS. Simple paper-based test for measuring blood hemoglobin concentration in resource-limited settings. Clinical chemistry. 2013;59:1506–13. doi: 10.1373/clinchem.2013.204701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neal FB, Russ JC. Measuring shape. Taylor & Francis; 2012. [Google Scholar]
- 15.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 16.Serjeant GR. The natural history of sickle cell disease. Cold Spring Harbor perspectives in medicine. 2013;3:a011783. doi: 10.1101/cshperspect.a011783. [DOI] [PMC free article] [PubMed] [Google Scholar]