Abstract
Astrocytes regulate multiple processes in the brain ranging from trophic support of developing neurons to modulation of synaptic neurotransmission and neuroinflammation in adulthood. It is, therefore, understandable that pathogenesis and pathophysiology of major psychiatric disorders involve astrocyte dysfunctions. Until recently, there has been the paucity of experimental approaches to studying the roles of astrocytes in behavioral disease. A new generation of in vivo models allows us to advance our understanding of the roles of astrocytes in psychiatric disorders. This review will evaluate the recent studies that focus on the contribution of astrocyte dysfunction to behavioral alterations pertinent to schizophrenia and will propose the possible solutions of the limitations of the existing approaches.
Keywords: neuron-astrocyte interaction, glutamate, tripartite synapse, neuroinflammation, psychiatric disorders
1. Introduction
Astrocytes are in the center of integration of homeostatic information to maintain neuronal functions, to coordinate immune responses, and to modulate metabolic exchange through the blood-brain barrier (Clarke and Barres, 2013; Hamilton and Attwell, 2010; Parpura et al., 2012; Araque et al., 2014). It is therefore not surprising that alterations in astrocytic functions produce behavioral abnormalities resembling aspects of schizophrenia and other major psychiatric disorders
Although the field of behavioral effects of astrocyte pathology is still growing, new reports are being regularly published (Pannasch et al., 2014). Thus, we sought to overview the recent studies that deal with behavioral abnormalities due to selective manipulations of astrocytes relevant to schizophrenia. Another goal of this review is to analyze the field from a perspective of animal models for psychiatric disease, which has been mainly advanced for neuronal animal preparations (Kannan et al, 2013).
2. Astrocyte pathology in schizophrenia
There is considerable evidence that pathological changes in astrocytes could contribute to the pathophysiological mechanisms of schizophrenia and related conditions. Furthermore, recent genome-wide association studies (GWAS) have directly implicated the astrocytic genes and/or gene sets in the etiology of schizophrenia (Goudriaan et al, 2014). We will briefly overview what is known about the pathology of astrocytes in schizophrenia to set a stage for our analysis of relevant animal models. For more comprehensive reviews of human data, we refer the readers to the recent publications on this topic (Cotter, 2001; Bernstein et al, 2009; 2014; Takahashi and Sakurai, 2013).
2.1 Postmortem histological studies
The main goal of postmortem histological studies has been to determine whether the number of astrocytes is altered in the brain of patients with schizophrenia. While some authors reported no changes (Casanova et al., 1990; Arnold et al., 1996; Falkai et al., 1999; Damadzic et al., 2001), others found both increased (Schnieder and Dwork, 2011) and reduced numbers of astrocytes (Rajkowska et al., 2002; Webster et al., 2001). Cotter and colleagues suggest that inconsistent findings could be explained, at least in part, by regional alterations and/or heterogeneity of schizophrenia symptoms. For example, schizophrenia patients with affective symptoms seem to exhibit more profound abnormalities (Cotter et al, 2001). Similarly, the increased density of S100β+ astrocytes was found in patients with paranoid but not residual schizophrenia (Steiner et al., 2008). In their review, Schnieder and Dwork also point to the limitations related to small samples, erroneous designs, and methodological biases (Schnieder and Dwork, 2011). Given considerable diversity of brain astrocytes (Clarke and Barres, 2013), a use of multiple markers for assessing the number of astrocytes could provide new and sometimes unexpected results. Notably, one study reports the altered number of chondroitin sulfate proteoglycan (CSPG) positive glial cells in the amygdala and entorhinal cortex without significant changes in the number of GFAP+ astrocytes (Pantazopoulos et al., 2010). Another approach includes an analysis of morphologically different astrocytes, e.g., fibrillary vs. gemistocytic (Williams et al, 2014) or an assessment of intracellular organelles, e.g., mitochondria (Uranova et al., 1996). With the advance of molecular tools, examination of subtle changes has become increasingly popular and may point to a more promising direction that would be consistent with mild pathology of astrocytes hardly detectable by histological methods.
2.2 Genes and proteins expression studies
There are several reports about altered expression of astrocytic genes in schizophrenia (Bernstein et al, 2014). Similar to postmortem studies, expression of glial fibrillary acidic protein (GFAP) at both mRNA or protein level has been extensively evaluated and the findings are also controversial. In addition to the absence of changes (Karson et al, 1993), both up-regulation (Webster et al., 2005; Bruneau et al., 2005; Fatemi et al, 2004) and down-regulation (Barley et al, 2009; Feresten et al, 2013; Catts et al, 2014) of GFAP levels have been observed.
Besides GFAP, altered expression of the factors involved in glutamate (GLU) metabolism (e.g., glial glutamate transporter (GLT-1), glutamine synthetase, glutaminase and serine racemase) was found (Matute et al, 2005; Burbaeva et al., 2003, 2007; Bruneau et al., 2005; Toro et al., 2006; Seffek et al., 2006; 2008). In addition, up-regulation of an inducible isoform of heme oxygenase (HMOX1), that is restricted to glial cells and oxidizes cellular heme to biliverdin, carbon monoxide (CO), and free ferrous iron (Schipper, 2004), was found in the prefrontal cortex (PFC) of patients with schizophrenia (Prabakaran et al., 2004).
In order to examine regional expression of the astrocytic markers, Katsel and associates (Katsel et al, 2011) used laser capture microdissection to study three distinct partitions of the anterior cingulate gyrus (layers I-III, IV-VI, and the underlying white matter) in the brains of 18 well-characterized persons with schizophrenia and 21 unaffected controls. While the expression of the astrocyte markers selected was not altered in the superficial layers or the underlying white matter of the cingulate cortex of subjects with schizophrenia, the expression of diodinase type II, aquaporin-4, S100β, glutaminase, excitatory amino-acid transporter 2 (EAAT2), and thrombospondin was significantly reduced in the deep layers of the anterior cingulate gyrus. These results suggest that a subset of astrocytes localized to specific cortical layers can be affected in schizophrenia. In addition to sub-regional differences, the inconsistent results may be due to variable aetiopathology, illness stage, or history of treatment (Catts et al, 2014).
2.3. Peripheral biomarkers
Astrocytes secrete soluble factors some of which have been evaluated as possible diagnostic and prognostic biomarkers for schizophrenia (Bernstein et al, 2009; 2014). A number of studies have reported increased serum and cerebrospinal fluid (CSF) levels of S100β in patients compared to control subjects (Pedersen et al., 2008; O'Connell et al., 2013; Qi et al, 2009). The association with schizophrenia has been found to be particularly strong in patients with negative symptoms (Rothermundt et al, 2004a; b). When two markers of astroglial activation (myo-inositol and S100β) were assessed by 1H-MRS or quantitative immunoassay, respectively, patients with increased S100β levels also had elevated concentrations of myoinositol, suggesting a general dysfunction of glial cells not restricted to the specific astrocytic protein (i.e., S100β) (Rothermundt et al, 2007). A recent meta-analysis has revealed elevated serum S100β in schizophrenia without any effects of antipsychotics and has proposed that this increase is related to active secretion of the protein by astrocytes in combination with blood-brain barrier dysfunction in schizophrenia (Schroeter et al, 2009). However, there have been negative studies as well (Uzbay et al, 2013; van der Leeuw et al., 2013). Steiner and colleagues have proposed that up-regulation of S100β in schizophrenia may be a result of alterations in glucose metabolism (Steiner et al., 2010). Besides astrocytes, adipocytes may contribute to serum levels of S100β, supporting the hypothesis that lifestyle choices and the illness itself may also be responsible for changing S100β levels (O'Connell et al, 2013). In this context, S100β should be considered as a “CRP-like” marker of general pathological changes (Sen and Belli, 2007). Similar to S100β, elevated CSF levels of neopterin thought to be secreted by astrocytes were found in patients with schizophrenia (Bechter et al, 2010).
Several studies have reported increased levels of kynurenic acid (KYNA) in CSF of schizophrenia patients (Schwarcz et al, 2001; Wonodi and Schwarcz, 2010; Linderholm et al., 2012; Steiner et al., 2012). In the brain, KYNA is produced by astrocytes and acts as an antagonist at N-Methyl-D-aspartate (NMDA) and α7 nicotinic acetylcholine receptors, providing the biological rationale for using this biomarker for diagnostic purposes and as a target of potential intervention (Bernstein et al, 2009; Schwarcz et al, 2012). Although the exact mechanisms of elevated KYNA levels in schizophrenia remain to be elucidated, both inflammation and genetic variants in the enzymes of the kynurenine pathway (e.g., kynurenine 3-monooxygenase) could be responsible for elevated levels of KYNA (Aoyama et al., 2006; Kapoor et al., 2006; Wonodi et al., 2011; Holtze et al., 2012). Decreased concentrations of a co-agonist of NMDA receptors, D-serine, were detected in the plasma and CSF of patients with schizophrenia. It has been proposed that lower levels of D-serine may be related to its decreased synthesis or enhanced degradation due to genetic variants of serine racemase (SRR) and/or D-amino acid oxidase, respectively (Morita et al., 2007; Caldinelli et al., 2013). Overall, it appears that there are several promising peripheral biomarkers that could be useful in the research and clinical settings but their specificity and reliability still need to be clearly demonstrated.
2. 4 Astrocytic genes and schizophrenia
It remains unclear whether astrocyte pathology results from primary genetic mutations in astrocytes or neuronal injury triggers activation of astrocytes and their dysfunctions observed in patients. Genetic association studies based on single nucleotide polymorphisms (SNPs) have been instrumental in identifying potential causative genetic variants (Goudriaan et al, 2014). Most recent publications focus on genes predominantly expressed in neurons. Few papers have reported association of astrocytic genes with schizophrenia. Schizophrenia-associated SNPs have been studied for the S100β gene (Bernstein, 2009; 2014; Liu et al, 2005; Hohoff et al, 2010; Zhai et al, 2011); thrombospondin 1 (THBS1), an astrocyte secreted glycoprotein that promotes synaptogenesis (Park et al, 2012); EAAT2, expression of which is decreased in the parahippocampal region and the dorsolateral prefrontal cortex (PFC) in schizophrenia (Shan et al, 2013; Spangaro et al, 2012); SRR (Morita et al, 2007); and the gene for the astrocytic enzyme that is involved in synthesis of glutathione, a key factor to guard the brain against oxidative stress (Tosic et al, 2006). However, single SNP associations provide limited insights in underlying molecular or cellular mechanisms. Pathway or functional gene sets analyses of the combined effect of multiple SNPs appear a more promising direction (Ramanan et al, 2012; Goudriaan et al, 2014; Duncan et al, 2014).
In summary, postmortem, genetic and biomarkers studies have implicated astrocytes in the etiology and pathophysiology of schizophrenia. These studies have guided the development of animal models to gain a deeper understanding of the mechanisms whereby genetic mutations and/or pathology of astrocytes result in behavioral disorders consistent with schizophrenia.
3. Animal models of astrocyte dysfunction
The field of animal models of astrocyte dysfunction in schizophrenia is rapidly expanding. We will review the existing animal models by grouping them in the categories related to the major functions of astrocytes found to be abnormal in schizophrenia. We will focus on genetic models but non-genetic preparations will be reviewed as well if they employ selective manipulations of astrocytes to induce schizophrenia-like behavioral phenotypes.
3.1 Models related to structural changes
In order to model decreased density of glia in cortical regions, an astrocyte specific toxin, L-alpha-aminoadipic acid (L-AAA), was injected in the PFC of adult rats. L-AAA induced anhedonia in sucrose preference test, anxiety, and helplessness in forced swim test (FST). Importantly, these effects were not seen after ibotenate-induced neurotoxic lesion of the PFC, suggesting specificity of the affective behaviors of astrocyte ablation (Banasr et al, 2008). This toxin was also found to affect attentional set-shifting, working memory and reversal learning (Lima et al., 2014). The effects of L-AAA appear to support the role of astrocytes in behavioral disorders due to dysfunction of the medial PFC. The limitation of using this toxin for neurobehavioral analysis is a progressive neuronal loss and dendritic atrophy in the surviving neurons in L-AAA-treated animals.
A recent paper describes a new transgenic mouse model with inducible expression of tetanus neurotoxin (TeNT) in astrocytes without globally affecting neuronal functions. TeTX expression led to a robust decrease in electroencephalographic (EEG) power in the gamma frequency range without affecting neuronal synaptic activity. This reduction in cortical gamma oscillations was accompanied by a selective deficit in novel object recognition, whereas working memory and fear conditioning were unaltered. Both EEG and behavioral phenotypes appeared to be transient as they were reversed by suppression of TeNT expression with doxycycline treatment. The results provide a further support for astrocytes as essential players in the mechanisms of information processing (Lee et al, 2014).
There have been several attempts to assess behavioral effects of genetic manipulation of the major astrocytic proteins (e.g., Shibuki et al., 1996). The effects of over-expression of S100β on exploratory behaviors were studied in transgenic mice. A significant difference in the spatial and temporal exploratory pattern was observed between control and S100β mutants (Roder et al., 1996). Mutant mice with deletion of S100β developed normally with the preserved cytoarchitecture of the brain but exhibited enhanced long-term potentiation (LTP) in the hippocampal CA1 region and a better performance in Morris water maze and contextual fear conditioning (Nishiyama et al., 2002). Both transgenic and knockout models have provided the initial characterization of behavioral outcomes of manipulations of the gene. However, these preparations do not mimic human SNPs and their relevance remains limited. New technologies (e.g, the CRISPR/Cas or TALERN systems) should facilitate the development of SNP-based animal models (Boch and Jens, 2011; Mali et al, 2013).
3. 2 Models related to glycogen metabolism
There is evidence of dysregulation of glucose metabolism and glycogen utilization in schizophrenia (Amar et al., 2011). Recent studies have shown that brain glycogen is contained in astrocytes that deliver energy substrates, e.g., lactate, to neurons (Gold et al., 2013). A study reports that training significantly increases levels of hippocampal astrocyte-derived extracellular lactate. Disruption of the astrocytic lactate transporters monocarboxylate transporter 4 (MCT4) or MCT1 produced amnesia and LTP impairment, which can be rescued by L-lactate but not equicaloric glucose. These data suggest that astrocyte-neuron lactate transport is critical for long-term memory (Suzuki et al., 2011). Consistently, another pharmacological study found that inhibition of astrocytic glycogenosis impaired memory and this impairment was rescued by lactate (Newman et al., 2011). Given that abnormal neuroplasticity and impaired cognitive functioning represent a major deficit of schizophrenia, new models targeting glycogen metabolism in astrocytes may help uncover new targets for therapeutic interventions to treat cognitive impairment in patients.
3.3 Models related to glutamate signaling
3.3.1 Glutamate uptake
GLU uptake is one of the major functions of astrocytes and is indispensable to influence GLU synaptic transmission (Danbolt, 2001). It has been suggested that GLT-1 function could be elevated in the PFC of patients with schizophrenia (Matute et al, 2005). In order to test this hypothesis in an animal model, pre-pulse inhibition (PPI) of the acoustic startle was evaluated in rats treated with ceftriaxone, an antibiotic that selectively enhances GLT-1 expression and activity. Ceftriaxone-induced GLT-1 up-regulation was associated with reduced PPI that was reversed by dihydrokainate (DHK), a GLT-1 antagonist (Bellesi et al, 2009). Curiously, the PPI inhibitory effects of ceftriaxone were additively enhanced by a single injection of phencyclidine (PCP) (Melone et al, 2009).
3.3.2 D-serine
D-serine is a gliotransmitter that acts as a co-agonist of the NMDAR glycine site. D-serine produced by SRR that converts L-serine in D-serine (Hashimoto and Oka, 1997; Kantrowitz and Javitt, 2010; Oliet and Mothet, 2009; Snyder and Kim, 2000). Although SRR appears to be predominantly expressed by neurons (at least in the normal conditions) (Balu et al., 2013), astrocytes can also express this enzyme and play a considerable role in secretion of D-serine. ASC-1 system is proposed to shuttle D-serine from neurons to astrocytes for subsequent secretion (Wolosker, 2011). A couple of studies have manipulated activity of SRR selectively in astrocytes and evaluated the behavioral consequences. Ma and colleagues have demonstrated that SRR binds to and is stabilized by Disrupted-In-Schizophrenia-1 (DISC1), a genetic risk factor (Kamiya et al, 2012). Mutant DISC1 selectively expressed in astrocytes fails to bind to SRR, facilitating ubiquitination and degradation of SRR and a decrease in D-serine production. Decreased production of D-serine was associated with greater responses to an NMDA antagonist, MK-801, in open field and PPI tests. The observed behavioral changes were rescued with D-serine treatment (Ma et al., 2013). Otte and associates have generated transgenic mice (SrrTg) to selectively over-express mouse SRR in astrocytes using the human GFAP promoter. As a result, these mutant mice have elevated brain levels of D-serine. Transgenic mice demonstrated decreased immobility in FST, shorter latency in novelty-suppression feeding test and blunted locomotor response to bulbectomy, suggesting diminished affective behaviors. The similar set of behavioral alterations was observed when control mice were given chronic D-serine supplementation (Otte et al., 2013). Although these studies confirmed the role of astrocytes in D-serine metabolism, given that SRR is a predominantly neuronal enzyme future models should try to alter D-serine metabolism in cell- and time-dependent manner in order to better appreciate the relative roles of astrocytes and neurons in D-serine metabolism.
3.3.3 Purines
Purines can be derived from many sources in the nervous system (Blutstein and Haydon, 2013). Adenosine triphosphate (ATP) is released in the extracellular space and metabolized to adenosine diphosphate (ADP), adenosine monophosphate (AMP) and ultimately to adenosine. Both neurons and astrocytes can release purines (Lopes et al, 2011). Adenosine is proposed to play a role of a homeostatic modulator of neural networks at different levels (receptors, bioenergetics, and epigenetics) and may be utilized to reverse the imbalance in glutamate and/or dopamine in schizophrenia (Boison et al, 2012; Hirota and Kishi, 2013).
Several publications report the behavioral effects of manipulation of adenosine signaling (Shen et al., 2012; Singer et al., 2013a; Singer et al., 2013b; Yee et al., 2007). The majority of those studies did not selectively modulate astrocytic sources of purines, making it difficult to infer cell-specific contributions to behavioral abnormalities. Recently, the behavioral effects of astrocyte-selective perturbation of adenosine's release have been published. In order to test the hypothesis that adenosine is released through soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) protein-dependent mechanisms, Haydon's lab has generated a mouse model of inducible and astrocyte-selective expression of dominant-negative form of the cytosolic portion of the SNARE domain of synaptobrevin 2 (Pascual et al, 2005). Mutant mice exhibit decreased release of adenosine, leading to “attenuated the accumulation of sleep pressure” that prevents cognitive deficits associated with sleep loss. It was suggested that astrocytes modulate sleep and its cognitive consequences through A1 receptors. (Halassa et al., 2009), providing a novel pathway for treatment of affective symptoms in schizophrenia and other major psychiatric disorders (Hines et al., 2013).
3.3.4 Models related to trophic factors and extracellular matrix proteins
Astrocytes secrete trophic factors and extracellular matrix (ECM) proteins (e.g., proteoglycans) to support neuronal growth and synaptic activity (Cohen-Cory et al., 2010; Faissner et al., 2010). Alterations in production of these factors could impact neurodevelopment and adult neuronal plasticity and have been implicated in schizophrenia (Autry and Monteggia, 2012; Martinotti et al., 2012; Pandya et al., 2013). Even if relevant variants of the genes for neurotrophic factors or ECM proteins remain scarce, animal models that selectively perturb the functioning of these genes in astrocytes can still help advance our understanding of the role of these systems to schizophrenia.
Brain-derived neurotrophic factor (BDNF) is a secreted protein that, in humans, is encoded by the BDNF gene (Barbacid, 1995). BDNF acts on neurons, supporting their survival, proliferation and differentiation. The recent findings have suggested that astrocytes produce BDNF (Girardet et al., 2013; Sun et al., 2014). Overexpressing BDNF in astrocytes, Quesseveur and colleagues examined behavioral outcomes of the altered neurons-to-astrocytes BDNF ratio. BDNF overexpression in hippocampal astrocytes was found to produce anxiolytic and antidepressant-like activity in the novelty suppressed feeding and to increase hippocampal neurogenesis. These observations implicate hippocampal astrocytes in the synthesis of BDNF, which can act through neurogenesis-dependent and independent mechanisms to regulate different facets of anxiolytic-like responses (Quesseveur et al., 2013). Unfortunately, behavioral changes related to schizophrenia-like phenotypes have not been evaluated in this animal model. Nevertheless, the data seem to be interesting in light of affective disorders associated with schizophrenia (Yasamy et al, 1987; Bartels et al, 1988; Tandon et al, 2013).
Astrocytes express aquaporin 4, (AQP4), a water channel protein, involved in water homeostasis, edema formation and neuroinflammation that is frequently associated with blood brain barrier (BBB) dysfunction (Badaut et al., 2002; Badaut et al., 2014). AQP4 is the primary AQP in the CNS, and is primarily expressed in astrocytic endfeet at the BBB (Nagelhus and Ottersen, 2013; Scharfman and Binder, 2013). A dysfunction of AQP4 could induce pathological conditions in neuronal activity. Several genome scan studies found a suggestive linkage on 18q, where human AQP4 (18q11.2-12.1) is located nearby, although negative findings were also reported (Muratake et al, 2005; Chung and Lung, 2012; Katsel et al, 2011). Skucas et al evaluated LTP and long-term depression (LTD) in AQP4 knock-out (KO) and wild-type mice. KO mice had deficient LTP and LTD and unaffected basal transmission or short-term plasticity. Notably, it was BDNF-dependent not BDNF-independent LTP that was impaired in KO mice. LTD was also impaired in KO mice, which was rescued by a scavenger of BDNF or blockade of tropomyosin-receptor-kinase (Trk) receptors. The KO mice also had cognitive impairment as was observed in location-specific object memory test but not MWM or contextual fear conditioning. The results suggest that AQP4 channels in astrocytes may be involved in neurotrophin-dependent plasticity and some forms of learning and memory (Skucas et al., 2011).
Among astrocytes-produced factors, ephrin-A3 is the major ligand for EphA, which is a member of the Eph family of receptor tyrosine kinases that express in forebrain neurons to regulate synaptic function and plasticity (Klein, 2009; Pasquale, 2008). Notably, significant association between SNP rs9520087 of the ephrin-B2 (EFNB2) gene and schizophrenia was reported (Zhang et al, 2010). Further, ephrin signaling is an essential component of the Reelin pathway (Sentürk et al, 2011), and reelin signaling is involved in neuronal migration and is found to be abnormal in schizophrenia (Folsom and Fatemi, 2013). Carmona and associates report that the ephrin-A3 ligand, which is located in the perisynaptic processes of astrocytes, is essential for maintaining EphA4 activation and normal spine morphology. Ephrin-A3-KO mice have dendritic spine irregularities similar to those observed in EphA4-KO mice. There were found no alterations in locomotor activity, sensorimotor responses, motor coordination, anxiety, or depression-related behaviors in ephrin-A3-KO mice. In order to assess hippocampus-dependent learning, cue- and context-dependent fear conditioning was used. While cue-dependent amygdala-supported conditional fear was unaffected, the freezing responses to the contextual cues were significantly reduced in KO mice, indicating impaired hippocampus-dependent contextual memory. In the object placement test, compared to WT mice, the ephrin-A3-KO mice spent more time exploring the object during the training phase but less preference for a new location of the same object during the test phase, suggesting poorer recognition of a new place. Notably, this deficit was no longer observed if the training phase was prolonged to 30 minutes. The performance of KO mice in the Barnes maze was unaltered. The findings suggest that interplay between neuronal EphA4 and glial ephrin-A3 regulates synapse morphology and hippocampal function (Carmona et al., 2009).
3.4 Models related to inflammatory molecules
Astrocytes coordinate immune response to invading pathogens and peripheral inflammatory factors (Ovanesov et al., 2008; Ransohoff and Brown, 2012). A growing body of evidence suggests the involvement of inflammatory processes in the pathophysiology of psychiatric disorders, and a leading role of astrocytes in mediating neuroinflammation (Meyer, 2011; Meyer et al., 2011; Muller et al., 2013; Shastri et al., 2013). Given the absence of gliosis in postmortem brain samples, it is subtle activation of astrocytes that likely plays a role in the pathophysiology of schizophrenia (Sofroniew and Vinters, 2010; Burda and Sofroniew, 2014). A number of studies have convincingly demonstrated that neuroinflammation is associated with activation of the enzyme, indoleamine 2,3-dioxygenase (IDO), resulting in increased production of KYNA (Muller et al, 2011; Schwarcz et al., 2012). KYNA is a tryptophan metabolite that is synthesized and released by astrocytes and acts as a competitive antagonist of the glycine site of NMDAR and as a noncompetitive antagonist of the α7-nicotinic acetylcholine receptor. The discovery of increased cortical KYNA levels in schizophrenia prompted the hypothesis that elevated KYNA concentration may underlie working memory impairment observed in patients (Schwarcz et al., 2001). In order to experimentally test this hypothesis, Chess et al treated rats with ip administration of kynurenine (100 mg/kg) that led to its 37-fold increase in the brain. They found that treated animals demonstrated increased errors in the radial arm maze without changes in locomotor activity or the latency to retrieve food reward (Chess et al., 2007). In a follow-up study, rats were given kynurenine on postnatal days 7-10 and were tested as adults. Kynurenine-treated animals exhibited decreased social behavior and locomotor activity; however no effects on attentional function and fear conditioning were observed (Iaccarino et al., 2013). Although the doses of kynurenine used in these studies appear very high, the results suggest a possible mechanism whereby neuroinflammation can influence cognitive function via glia-secreted molecular factors in a time-dependent manner.
Toxoplasma gondii (T. gondii) infection is a risk factor for schizophrenia (Yolken et al., 2009; Yolken and Torrey, 2008). T. gondii infection has been shown to stimulate the synthesis of KYNA, presumably in astrocytes (Schwarcz and Hunter, 2007; Notarangelo et al, 2014). It is plausible that T. gondii infection could contribute to the disorder by increasing KYNA production by astrocytes. Although there have been several reports on schizophrenia-like behaviors in chronically T. gondii-infected rodents, the contribution of KYNA or other astrocyte-produced factors has to be yet determined given a considerable range of direct and indirect effects of the parasite on the brain (Kannan and Pletnikov. 2012).
Using an immune dysfunction model of schizophrenia, it has been found that a short-term one-week cuprizone exposure produces increased responses to methamphetamine and phencyclidine, as well as impaired working memory. The study reports that in contrast to long-term cuprizone exposures, this short-term treatment led to perturbation of astrocytes and microglia and significant up-regulation of interleukin-6 (IL-6) in GFAP+ cells consistent with some inflammatory markers observed in schizophrenia (Tezuka et al., 2013).
Expression of many pro-inflammatory factors is regulated by the transcription nuclear factor-kappa B (NF-κB) (Bales et al., 2000; Petegnief et al., 2001). There are several genetic (Liou et al, 2012; Chen at al, 2014), biomarker (Song et al, 2009) and postmortem studies (Sun et al, 2001; Roussos et al, 2013) that implicate NF-κB in schizophrenia. Brambilla and colleagues generated transgenic mice where NF-κB function was selectively suppressed in astrocytes by over-expressing a dominant-negative N-terminal truncated form of the inhibitor of nuclear factor kappa B α NF-κB (IκBα), under the control of the GFAP promoter (Brambilla et al., 2005). No differences between control and mutant mice were observed in general health measures, locomotor activity, sensorimotor function or anxiety. Female GFAP-IκBα-dn mice exhibited mild deficiency in the last stage of the non-cued version of the Barnes maze as evidenced by the greater latency to the first correct nose poke and less time spent in the quadrant of the maze previously containing the goal box. These data point to a hippocampal deficit in learning and memory in female mice. Curiously, GFAP-IκBα-dn female mice were even more impaired in the cued version of the Barnes maze, consistent with a deficit in extra-hippocampal components of learning and memory; however, one cannot completely rule out an abnormality in vision. Sex-related alterations were also observed in fear conditioning, highlighting importance of evaluating consequences of genetic manipulations in males and females. The cognitive deficits were associated with reduced LTP and lower expression of metabotropic glutamate receptor 5 and post-synaptic density protein 95 (PSD95) in female transgenic mice. Taken together, these findings indicate that astroglial NF-κB is involved in synaptic plasticity underlying learning and memory (Bracchi-Ricard et al., 2008).
Cytokines are polypeptides that mediate the communication between cells during inflammation (Heinrich et al., 2003). Interleukin-6 (IL-6), identified as B-cell differentiation factor (Hirano et al., 1985), is a major cytokine involved in interplay between the immune system and brain (Deverman and Patterson, 2009). IL-6 has been implicated in stress response, synaptic plasticity, learning, sleep and neurodevelopment (Bauer et al., 2007; Deverman and Patterson, 2009). Altered levels of IL-6 have been found in patients with schizophrenia (Smith et al., 2007).
Several studies assessed the behavioral changes in conventional il-6 KO mice and reported inconsistent results (Quintana et al., 2013). To overcome the limitations of that KO model, floxed mouse lines were generated to study the role of IL-6 in a cell-specific manner. Floxed mice either for IL-6 or its receptor were crossed with GFAP-Cre mice to delete either gene in astrocytes. The data indicate the astrocyte IL-6 system mediates locomotor activity, anxiety and exploratory behaviors. The authors conclude that both similar and distinct phenotypes were observed in il-6 vs. il6r conditional KO models. Notably, some alterations in conditional KO models were totally unexpected from previous results with conventional il-6 KO model (Quintana et al., 2013). These findings implicate astrocytic IL-6 in normal brain physiology and behavior.
Heme oxygenases 1(HO-1) expression found to be elevated in schizophrenia can be induced by oxidative and inflammatory stimuli (Prabakaran et al., 2004). Selective overexpression of HO-1 in astrocytes in transgenic mice results in oxidative stress, lower neuronal reelin content, elevated dopamine and serotonin concentrations in the basal ganglia; reduced D1 receptor binding in the nucleus accumbens and altered hippocampal cytoarchitectonics. These pathological signs were associated with increased locomotor activity and decreased PPI, without affecting anxiety or motor balance in transgenic mice. The data shed light on the possible role of glial HO-1 in the development of monoaminergic circuitry and suggest that abnormal expression of HO-1 in astrocytes can be responsible for schizophrenia-like phenotypes in transgenic mice (Song et al., 2012).
3. 5 Models for emerging astrocytic targets
Cannabinoid receptors (i.e., CB1) are expressed on glia cells and regulate both development and adult functions of glia cells (Navarrete and Araque, 2008; Stella, 2010). Importantly, CB1 receptors are the targets of marihuana, abuse of which during adolescence has been linked to the increased risk for schizophrenia and/or cognitive impairment (Arseneault et al., 2004; Burns, 2013; Stone, 1973; van Os and Murray, 2008). That's why recent studies of the role of CB1 receptors on astrocytes in adverse effects of marihuana on learning and memory have drawn much attention.
Acute exposure to Δ(9)-tetrahydrocannabinol (Δ(9)THC) was reported to affect spatial working memory and LTD at hippocampal CA3-CA1 synapses, and these effects were completely blocked in mice lacking CB1 receptors in astroglial but not in glutamatergic or GABAergic neurons (Han et al., 2012). A different group extended this finding by demonstrating that that synaptic and cognitive impairments produced by repeated THC injections were dependent on the activation of cyclooxygenase-2 (COX-2), an inducible enzyme that converts arachidonic acid to prostanoids in the brain. Importantly, the study presents the pharmacological and genetic findings that COX-2 induction is also mediated via CB1 receptor-coupled G protein βγ subunits. These studies have directly implicated astrocytic CB1 receptors in cognitive functions in the brain (Chen et al., 2013). Future research should address time-dependent aspects of CB1 involvement in neuron-astrocyte interplay in cognition and evaluate if genetic risk factors expressed by astrocytes could potentiate adverse effects of adolescent exposure to marihuana. In addition, since marihuana exposure has been associated with neuroinflammation and ensuing behavioral and cognitive deficits, this putative link needs to be explored in greater details as it could offer a new avenue for treating marihuana abuse-related cognitive impairments (James et al, 2013; Burns, 2013).
The animal models reviewed here have provided important insights in how astrocyte dysfunction could contribute to behavioral responses relevant to schizophrenia. Table 1 summarizes the strengths and weaknesses of each animal model reviewed. Figure 1 depicts the astrocytic functions found to be abnormal in schizophrenia and animal models that recapitulate aspects of pathological changes in patients.
Table 1. Summary of animal models related to astrocyte dysfunctions in schizophrenia.
| Gene or factor | Animal model | Phenotypes | Mechanisms | Strengths | Caveats | Citations |
|---|---|---|---|---|---|---|
| Models related to structural changes | ||||||
| L-α-aminoadipate L-AA | Injection into mPFC in rats | Abnormal set-shifting, working memory and reversal learning; anhedonia | Loss of astrocytes and a progressive loss of neurons | Cell selectivity Clinical relevance | Limited etiological value |
Banasr, 2008 Lima, 2014 |
| TeNT model | Expression of tetanus neurotoxin | Deficit in novel object recognition test | Decreased EEG power in the gamma frequency | Cell selectivity Inducible system | Limited etiological value | Lee, 2014 |
| S100β | Tg model | Abnormal exploration | No studied | Cell selectivity | No time or region specificity | Roder, 1996. |
| S100β | KO model | Better MWM and contextual conditioning | Increased LTP in the CA1 area | Cell selectivity | No time or region specificity | Nishiyama, 2002 |
| Models related to glycogen metabolism | ||||||
| MCT4 | Conditional KO model | Impaired learning | L-lactate transport | Cell selectivity | No time or region specificity |
Suzuki, 2011 Newman, 2011 |
| Models related to glutamate signaling | ||||||
| GLT-1 up-regulation | Injection of centriaxone | Impaired PPI Increased responses to PCP | Induced up-regulation of GLT-1 in the brain | Cell selectivity Clinical relevance | Limited etiological relevance |
Bellesi, 2009 Melone, 2009 |
| Mutant DISC1 | Inducible Tg model | Increased response to amphetamine rescued by D-serine | Decreased D-serine production | Time- and cell-specific | Over-expression No regional specificity | Ma, 2013 |
| Serine racemase, SRR | Tg model | Decreased depression and anxiety | Elevated D-serine levels | Cell selectivity | Over-expression No regional specificity | Otte, 2013 |
| Mutant SNARE | Inducible Tg model | Abnormal sleep Cognitive deficits Anti-depressant effects | Decreased ATP/adenosinerelease | Time- and cell-specificity | Over-expression No regional specificity |
Halassa, 2009 Hines, 2013 |
| Models related to trophic support and ECM proteins | ||||||
| BDNF | Viral vector expression | Anxiolytic and antidepressant-like activity | Increased hippocampal neurogenesis | Cell specific factor | Non-germline model | Quesseveur, 2013 |
| AQP4 | KO mice | Cognitive deficits | Impaired LTP and LTD | Cell specific factor | No time or region specificity | Skucas, 2004 |
| Ephrin-A3 | KO mice | Deficient memory | Spines deficit | Cell specific factor | No time or region specificity | Carmona, 2009 |
| Models related to inflammatory molecules | ||||||
| Kynurenic acids | Injection of kynurenine in rats | Poor learning Decreased social behavior | Effects on GLU and/or Ach receptors | Clinical relevance Specific targets | No regional specificity |
Chess, 2007 Iaccarino, 2013 |
| Cuprizone | 1-week diet with 0.2% cuprizone | Increased responses to MA and PCP Impaired working memory | Perturbation of astrocytes and microglia | Some clinical relevance | Limited etiological value | Tezuka T, 2013 |
| NF-κB | Tg model | Poor learning and memory in females | Deficient LTP Altered levels of mGluR5 and PSD95 | Cell specific model | No time or region specificity |
Brambilla, 2005 Bracchi-Ricard, 2008 |
| IL-6 and IL-6R | Conditional KO model | Altered activity and exploration | Not studied | Cell specific deletion | No time or region specificity | Quintana, 2013 |
| Heme Oxygenases HO-1 | Tg model | Increased activity Decreased PPI | Oxidative stress | Cell specific model | No time or Region specificity | Song, 2012 |
| Models related to emerging targets | ||||||
| CB1 receptors | THC treatments | Impaired learning and memory | CB1 receptors,COX2 signaling | Cell specificity, Identified pathway | Limited relevance to human conditions |
Han, 2012 Chen, 2013 |
Figure 1. Functions found abnormal in schizophrenia and recapitulated in animal models.
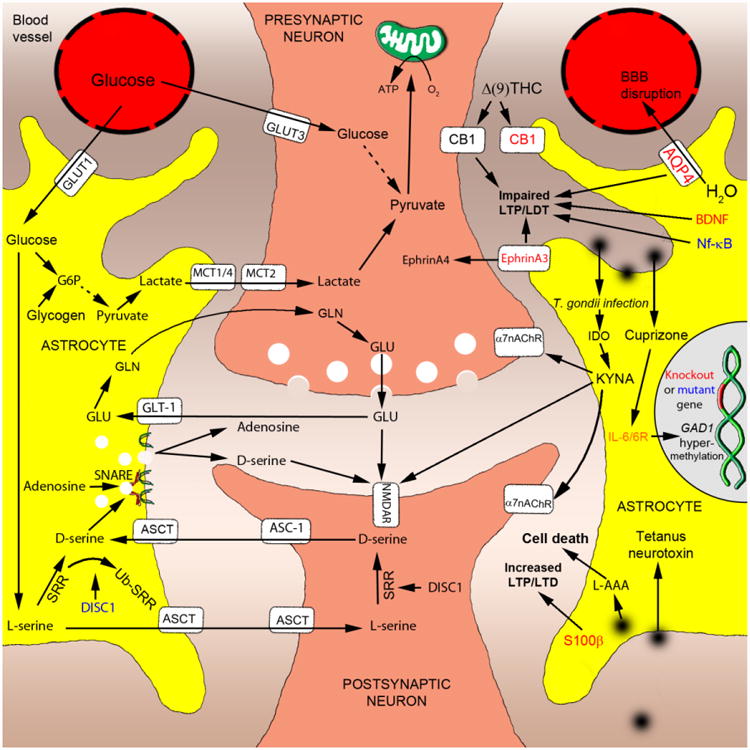
- Conditional knockout of monocarboxylate transporter 1 or 4 (MCT1/4) alters a release of lactate from astrocytes and impairs long-term potentiation (LTP);
- Over-expression of glial glutamate transporter 1 (GLT-1) or serine racemase (SRR) increases glutamate uptake or levels of D-serine, respectively;
- Over-expression of dominant-negative soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) domain selectively blocks a release of adenosine, leading to suppression of synaptic transmission;
- Over-expression of mutant Disrupted-In-Schizophrenia-1 (DISC1) facilitates ubiquitination of SRR, resulting in decreased production of D-serine and associated schizophrenia-like behaviors.
- Knockout of aquaporin 4 (AQP4), brain-derived neurotrophic factor (BDNF) or EphrinA3 impaired LTP and/or long-term depression (LDT);
- Astrocytic (cannabinoid receptor type 1) CB1 receptors are indispensable for the detrimental effects of Δ(9)-tetrahydrocannabinol (Δ(9)-THC) on learning and memory;
- T.gondii infection- or direct administration-produced increase in levels of kynurenic acid (KYNA) results in antagonism at NMDA and α7 nicotinic acetylcholine receptors (α7nACR) and associated cognitive impairment;
- Administration of cuprizone or conditional knockout of interleukin-6 or IL-6 receptor (IL-6/IL-6R) result in abnormal methylation of the GAD1 gene (Kundakovic et al, 2009; Lee et al, 2011);
- Over-expression of dominant-negative nuclear factor-kappa B (NF-κB) affects LTP in transgenic mice.
- Administration of the astrocyte specific toxin, L-alpha-aminoadipic acid (L-AAA), produces a loss of astrocytes and impaired working memory;
- Inducible, transient and astrocyte-restricted expression of tetanus neurotoxin leads to altered gamma oscillations and impaired novel object recognition;
- Over-expression or knockout of S100β affects LTP and exploratory behaviors.
Abbreviations: Glucose transporter 1 (GLUT1) and 3 (GLUT3), Glycine (GLN), Glucose 6- phosphate (G6P), monocarboxylate transporter 2 (MCT2), alanine-serine-cysteine transporter (ASCT) and alanine-serine-cysteine transporter 1 (ASC-1); indoleamine 2,3-dioxygenase (IDO), blood–brain barrier (BBB)
4. Future directions
The field of behavioral models of astrocytes dysfunction is constantly expanding. As it continues to make inroads, it needs to be cognizant of the pitfalls that other behavioral models have been plagued with. Refining methodological designs and implementing new technological tools will be important to overcome the limitations of the existing preparations.
4.1. Design issues
One obvious but often-neglected way of addressing variability among different studies is to utilize standard measures and approaches of behavioral neuroscience, including the same protocols for the same behavioral tests, strains of mice, and even the equipment whenever possible. Another issue is lack of systematic analyses of sex-dependent effects. Future research in behavioral models of astrocyte dysfunction should attempt to better characterize sex-specific abnormalities resulting from the astrocytic effects on the gonads or from modulatory actions of sex hormones on astrocytes themselves. This line of research may help uncover the mechanisms of gender differences in psychiatric disorders and could help identify risk/protective factors.
As with any behavioral studies, an ever-present issue is underpowered statistical analyses. Reporting “mild but significant alterations” may be misleading if the effect size is not presented. As numbers of animals is limited by cost-prohibitive housing, it is still important effect sizes and confidence intervals be reported together with significance.
Major mental disorders are disorders of brain development (Insel, 2011; Jaaro-Peled et al., 2009). Astrocyte models with manipulation in astrocytic genes at different stages of neurodevelopment (including postnatal period) are likely to be most relevant to psychiatric disorders. Thus, developmental aspects should become a focus of the future studies, including models allowing for studying time-dependent effects (Pletnikov, 2009).
4.2. Technological advances
Simple knockout and transgenic models are artificial systems inconsistent with the molecular pathology of schizophrenia or related diseases. New models with mutations in regulatory elements of risk genes with subtle and time-dependent expression or “humanized” models would seem to better approximate the genetic complexity of mental illnesses (Papaleo et al., 2012). Additionally, given heterogeneity of astrocytes in the brain, circuitry-specific and astrocyte type-specific manipulations will be needed. This approach may prove to be particularly important for a better understanding of circuitry-specific pathology observed in schizophrenia (Arnsten 2011; Lewis, 2012). For example, increases in Ca2+ are induced in cortical astrocytes by GLU and norepinephrine, while hippocampal astrocytes show calcium responses to ATP, GABA, acetylcholine, and endocannabinoids (Oberheim et al, 2012), suggesting that various neurotransmitter systems will be differentially affected be the same pathology of astrocytes in the cortex vs. hippocampus. In addition, different developmental and regional profiles of expression of GLAST and GLT-1 in the brain (cortex vs. cerebellum) might contribute to differential vulnerability to environmental stressors implicated in schizophrenia.
A recent analysis of genome-wide association studies (GWAS) database of glial gene sets has provided a list of promising astrocytic candidates for the future studies (Goudriaan et al., 2013). In this context, it would be also helpful to expand the use of non-rodent genetic models to identify the common molecular processes of neuron-glia interaction across species (e.g., worms, fruit flies, zebrafish) (Freeman and Rowitch, 2013; Han et al., 2013).
Our understanding of neuron-astrocyte interaction in health and disease is unlikely to be facilitated without tools that allow us to monitor and manipulate astrocytes in behaving animals. Recent studies utilizing optogenetics and two-photon microscopy in combination with behavioral tests have demonstrated the possibility of developing and characterizing such models (Sasaki et al., 2012; Paukert et al, 2014).
There is a growing acceptance of significance of gene-environment models for schizophrenia (Kannan et al., 2013). Until recently, most gene-environment studies have focused on neuronal functions of genetic risk factors. Given the leading role of astrocytes in mediating adverse effects of environmental stressors, it is tempting to predict that future models will include glial cell-specific genes to help sort out their contribution to abnormal brain and behavior development.
Although studying complex neuron-glia communication in vitro will always be limited, cell models offer an excellent tool for identifying the disease-related molecular pathways in a cell-specific manner (Jiang et al, 2010; Lavoie et al., 2011). Future studies are anticipated to utilize astrocytes differentiated from induced pluripotent stem cells derived from patients. This line of investigation will likely inform our knowledge of disease-specific abnormalities in gene expression, molecular signatures and, hopefully, novel treatments (McCarroll and Hyman, 2013).
In conclusion, animal models of astrocyte dysfunction in schizophrenia have already improved our understanding of how astrocytes regulate brain circuitries to influence behavior and will continue to provide new insights into the etiological complexity and heterogeneity of schizophrenia.
Acknowledgments
Role of funding source: This review was prepared with support by the following grants: MH-083728, MH-094268 Silvo O. Conte center, the Brain and Behavior Research Foundation, the Stanley Medical Research Institute, Tabakman Trust Gift Grant (MVP).
Footnotes
Conflict of Interest: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amar S, Belmaker RH, Agam G. The possible involvement of glycogen synthase kinase-3 (GSK-3) in diabetes, cancer and central nervous system diseases. Curr Pharm. 2011;(22):2264–77. doi: 10.2174/138161211797052484. [DOI] [PubMed] [Google Scholar]
- Aoyama N, Takahashi N, Saito S, Maeno N, Ishihara R, Ji X, Miura H, Ikeda M, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, Yoshida K, Iwata N, Inada T, Ozaki N. Association study between kynurenine 3-monooxygenase gene and schizophrenia in the Japanese population. Genes, brain, and behavior. 2006;5(4):364–368. doi: 10.1111/j.1601-183X.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81(4):728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Franz BR, Trojanowski JQ, Moberg PJ, Gur RE. Glial fibrillary acidic protein-immunoreactive astrocytosis in elderly patients with schizophrenia and dementia. Acta neuropathologica. 1996;91(3):269–277. doi: 10.1007/s004010050425. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci. 2011;29(3):215–23. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. The British journal of psychiatry: the journal of mental science. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacological reviews. 2012;64(2):238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22(4):367–378. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Badaut J, Fukuda AM, Jullienne A, Petry KG. Aquaporin and brain diseases. Biochim Biophys Acta. 2014;1840(5):1554–65. doi: 10.1016/j.bbagen.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Du Y, Holtzman D, Cordell B, Paul SM. Neuroinflammation and Alzheimer's disease: critical roles for cytokine/Abeta-induced glial activation, NF-kappaB, and apolipoprotein E. Neurobiology of aging. 2000;21(3):427–432. doi: 10.1016/s0197-4580(00)00143-3. [DOI] [PubMed] [Google Scholar]
- Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, Bolshakov VY, Coyle JT. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(26):E2400–2409. doi: 10.1073/pnas.1304308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64(10):863–70. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. Neurotrophic factors and their receptors. Current opinion in cell biology. 1995;7(2):148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophrenia research. 2009;112(1-3):54–64. doi: 10.1016/j.schres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Bartels SJ, Drake RE. Depressive symptoms in schizophrenia: comprehensive differential diagnosis. Compr Psychiatry. 1988;29(5):467–83. doi: 10.1016/0010-440x(88)90062-4. [DOI] [PubMed] [Google Scholar]
- Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nature reviews Neuroscience. 2007;8(3):221–232. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. Journal of psychiatric research. 2010;44(5):321–330. doi: 10.1016/j.jpsychires.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Bellesi M, Melone M, Gubbini A, Battistacci S, Conti F. GLT-1 upregulation impairs prepulse inhibition of the startle reflex in adult rats. Glia. 2009;57(7):703–13. doi: 10.1002/glia.20798. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Steiner J, Bogerts B. Glial cells in schizophrenia: pathophysiological significance and possible consequences for therapy. Expert review of neurotherapeutics. 2009;9(7):1059–1071. doi: 10.1586/ern.09.59. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophrenia research. 2014 doi: 10.1016/j.schres.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Blutstein T, Haydon PG. The Importance of astrocyte-derived purines in the modulation of sleep. Glia. 2013;61(2):129–139. doi: 10.1002/glia.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch J. TALEs of genome targeting. Nature biotechnology. 2011;29(2):135–136. doi: 10.1038/nbt.1767. [DOI] [PubMed] [Google Scholar]
- Boison D, Singer P, Shen HY, Feldon J, Yee BK. Adenosine hypothesis of schizophrenia--opportunities for pharmacotherapy. Neuropharmacology. 2012;62(3):1527–1543. doi: 10.1016/j.neuropharm.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracchi-Ricard V, Brambilla R, Levenson J, Hu WH, Bramwell A, Sweatt JD, Green EJ, Bethea JR. Astroglial nuclear factor-kappaB regulates learning and memory and synaptic plasticity in female mice. Journal of neurochemistry. 2008;104(3):611–623. doi: 10.1111/j.1471-4159.2007.04993.x. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. The Journal of experimental medicine. 2005;202(1):145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau EG, McCullumsmith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Increased expression of glutaminase and glutamine synthetase mRNA in the thalamus in schizophrenia. Schizophrenia research. 2005;75(1):27–34. doi: 10.1016/j.schres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Burbaeva G, Boksha IS, Tereshkina EB, Savushkina OK, Starodubtseva LI, Turishcheva MS, Mukaetova-Ladinska E. Systemic neurochemical alterations in schizophrenic brain: glutamate metabolism in focus. Neurochemical research. 2007;32(9):1434–1444. doi: 10.1007/s11064-007-9328-7. [DOI] [PubMed] [Google Scholar]
- Burbaeva G, Boksha IS, Turishcheva MS, Vorobyeva EA, Savushkina OK, Tereshkina EB. Glutamine synthetase and glutamate dehydrogenase in the prefrontal cortex of patients with schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2003;27(4):675–680. doi: 10.1016/s0278-5846(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–48. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JK. Pathways from Cannabis to Psychosis: A Review of the Evidence. Frontiers in psychiatry/Frontiers Research Foundation. 2013;4:128. doi: 10.3389/fpsyt.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldinelli L, Sacchi S, Molla G, Nardini M, Pollegioni L. Characterization of human DAAO variants potentially related to an increased risk of schizophrenia. Biochimica et biophysica acta. 2013;1832(3):400–410. doi: 10.1016/j.bbadis.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Carmona MA, Murai KK, Wang L, Roberts AJ, Pasquale EB. Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(30):12524–12529. doi: 10.1073/pnas.0903328106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Stevens JR, Kleinman JE. Astrocytosis in the molecular layer of the dentate gyrus: a study in Alzheimer's disease and schizophrenia. Psychiatry research. 1990;35(2):149–166. doi: 10.1016/0925-4927(90)90017-z. [DOI] [PubMed] [Google Scholar]
- Catts VS, Wong J, Fillman SG, Fung SJ, Shannon Weickert C. Increased expression of astrocyte markers in schizophrenia: Association with neuroinflammation. The Australian and New Zealand journal of psychiatry. 2014;48(8):722–734. doi: 10.1177/0004867414531078. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhang J, Fan N, Teng ZQ, Wu Y, Yang H, Tang YP, Sun H, Song Y, Chen C. Delta9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell. 2013;155(5):1154–1165. doi: 10.1016/j.cell.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SF, Chao YL, Shen YC, Chen CH, Weng CF. Resequencing and association study of the NFKB activating protein-like gene (NKAPL) in schizophrenia. Schizophr Res. 2014;157(1-3):169–74. doi: 10.1016/j.schres.2014.05.038. [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophrenia bulletin. 2007;33(3):797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung TS, Lung FW. Different impacts of aquaporin 4 and MAOA allele variation among olanzapine, risperidone, and paliperidone in schizophrenia. Journal of clinical psychopharmacology. 2012;32(3):394–397. doi: 10.1097/JCP.0b013e31825370f4. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nature reviews Neuroscience. 2013;14(5):311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70(5):271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain research bulletin. 2001;55(5):585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Damadzic R, Bigelow LB, Krimer LS, Goldenson DA, Saunders RC, Kleinman JE, Herman MM. A quantitative immunohistochemical study of astrocytes in the entorhinal cortex in schizophrenia, bipolar disorder and major depression: absence of significant astrocytosis. Brain research bulletin. 2001;55(5):611–618. doi: 10.1016/s0361-9230(01)00529-9. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, O'Tuathaigh C, Clarke G, O'Leary C, Petit E, Clarke N, Tighe O, Lai D, Harvey R, Cryan JF, Dinan TG, Waddington JL. Phenotypic effects of repeated psychosocial stress during adolescence in mice mutant for the schizophrenia risk gene neuregulin-1: a putative model of gene × environment interaction. Brain Behav Immun. 2012;26(4):660–671. doi: 10.1016/j.bbi.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64(1):61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Holmans PA, Lee PH, O'Dushlaine CT, Kirby AW, Smoller JW, Öngür D, Cohen BM. Pathway analyses implicate glial cells in schizophrenia. PLoS One. 2014;9(2):e89441. doi: 10.1371/journal.pone.0089441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faissner A, Pyka M, Geissler M, Sobik T, Frischknecht R, Gundelfinger ED, Seidenbecher C. Contributions of astrocytes to synapse formation and maturation - Potential functions of the perisynaptic extracellular matrix. Brain research reviews. 2010;63(1-2):26–38. doi: 10.1016/j.brainresrev.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Falkai P, Honer WG, David S, Bogerts B, Majtenyi C, Bayer TA. No evidence for astrogliosis in brains of schizophrenic patients. A post-mortem study. Neuropathology and applied neurobiology. 1999;25(1):48–53. doi: 10.1046/j.1365-2990.1999.00162.x. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Araghi-Niknam M, Laurence JA, Stary JM, Sidwell RW, Lee S. Glial fibrillary acidic protein and glutamic acid decarboxylase 65 and 67 kDa proteins are increased in brains of neonatal BALB/c mice following viral infection in utero. Schizophrenia research. 2004;69(1):121–123. doi: 10.1016/S0920-9964(03)00175-0. [DOI] [PubMed] [Google Scholar]
- Feresten AH, Barakauskas V, Ypsilanti A, Barr AM, Beasley CL. Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophrenia research. 2013;150(1):252–257. doi: 10.1016/j.schres.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Folsom TD, Fatemi SH. The involvement of Reelin in neurodevelopmental disorders. Neuropharmacology. 2013;68:122–35. doi: 10.1016/j.neuropharm.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR, Rowitch DH. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron. 2013;80(3):613–623. doi: 10.1016/j.neuron.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardet C, Lebrun B, Cabirol-Pol MJ, Tardivel C, Francois-Bellan AM, Becquet D, Bosler O. Brain-derived neurotrophic factor/TrkB signaling regulates daily astroglial plasticity in the suprachiasmatic nucleus: electron-microscopic evidence in mouse. Glia. 2013;61(7):1172–1177. doi: 10.1002/glia.22509. [DOI] [PubMed] [Google Scholar]
- Gold PE, Newman LA, Scavuzzo CJ, Korol DL. Modulation of multiple memory systems: from neurotransmitters to metabolic substrates. Hippocampus. 2013;23(11):1053–1065. doi: 10.1002/hipo.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan A, de Leeuw C, Ripke S, Hultman CM, Sklar P, Sullivan PF, Smit AB, Posthuma D, Verheijen MH. Specific glial functions contribute to schizophrenia susceptibility. Schizophrenia bulletin. 2014;40(4):925–935. doi: 10.1093/schbul/sbt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61(2):213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nature reviews Neuroscience. 2010;11(4):227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous DN, Mendizabal-Zubiaga J, Grandes P, Liu Q, Bai G, Wang W, Xiong L, Ren W, Marsicano G, Zhang X. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148(5):1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Han L, Wang Y, Sangaletti R, D'Urso G, Lu Y, Shaham S, Bianchi L. Two novel DEG/ENaC channel subunits expressed in glia are needed for nose-touch sensitivity in Caenorhabditis elegans. The Journal of neuroscience. 2013;33(3):936–949. doi: 10.1523/JNEUROSCI.2749-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A, Oka T. Free D-aspartate and D-serine in the mammalian brain and periphery. Progress in neurobiology. 1997;52(4):325–353. doi: 10.1016/s0301-0082(97)00019-1. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. The Biochemical journal. 2003;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines DJ, Schmitt LI, Hines RM, Moss SJ, Haydon PG. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Translational psychiatry. 2013;3:e212. doi: 10.1038/tp.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Taga T, Nakano N, Yasukawa K, Kashiwamura S, Shimizu K, Nakajima K, Pyun KH, Kishimoto T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2) Proceedings of the National Academy of Sciences of the United States of America. 1985;82(16):5490–5494. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Kishi T. Adenosine hypothesis in schizophrenia and bipolar disorder: a systematic review and meta-analysis of randomized controlled trial of adjuvant purinergic modulators. Schizophrenia research. 2013;149(1-3):88–95. doi: 10.1016/j.schres.2013.06.038. [DOI] [PubMed] [Google Scholar]
- Hohoff C, Ponath G, Freitag CM, Kastner F, Krakowitzky P, Domschke K, Koelkebeck K, Kipp F, von Eiff C, Deckert J, Rothermundt M. Risk variants in the S100B gene predict elevated S100B serum concentrations in healthy individuals. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2010;153B(1):291–297. doi: 10.1002/ajmg.b.30950. [DOI] [PubMed] [Google Scholar]
- Holtze M, Saetre P, Engberg G, Schwieler L, Werge T, Andreassen OA, Hall H, Terenius L, Agartz I, Jonsson EG, Schalling M, Erhardt S. Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. Journal of psychiatry & neuroscience : JPN. 2012;37(1):53–57. doi: 10.1503/jpn.100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino HF, Suckow RF, Xie S, Bucci DJ. The effect of transient increases in kynurenic acid and quinolinic acid levels early in life on behavior in adulthood: Implications for schizophrenia. Schizophrenia research. 2013;150(2-3):392–397. doi: 10.1016/j.schres.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2011;468(7321):187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- James A, James C, Thwaites T. The brain effects of cannabis in healthy adolescents and in adolescents with schizophrenia: a systematic review. Psychiatry Res. 2013;214(3):181–9. doi: 10.1016/j.pscychresns.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends in neurosciences. 2009;32(9):485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Saetre P, Radomska KJ, Jazin E, Lindholm Carlstrom E. QKI-7 regulates expression of interferon-related genes in human astrocyte glioma cells. PloS one. 2010;5(9) doi: 10.1371/journal.pone.0013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Sedlak TW, Pletnikov MV. DISC1 Pathway in Brain Development: Exploring Therapeutic Targets for Major Psychiatric Disorders. Frontiers in psychiatry / Frontiers Research Foundation. 2012;3:25. doi: 10.3389/fpsyt.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan G, Pletnikov MV. Toxoplasma gondii and cognitive deficits in schizophrenia: an animal model perspective. Schizophrenia bulletin. 2012;38(6):1155–1161. doi: 10.1093/schbul/sbs079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan G, Sawa A, Pletnikov MV. Mouse models of gene-environment interactions in schizophrenia. Neurobiology of disease. 2013;57:5–11. doi: 10.1016/j.nbd.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain research bulletin. 2010;83(3-4):108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R, Lim KS, Cheng A, Garrick T, Kapoor V. Preliminary evidence for a link between schizophrenia and NMDA-glycine site receptor ligand metabolic enzymes, d-amino acid oxidase (DAAO) and kynurenine aminotransferase-1 (KAT-1) Brain research. 2006;1106(1):205–210. doi: 10.1016/j.brainres.2006.05.082. [DOI] [PubMed] [Google Scholar]
- Karson CN, Casanova MF, Kleinman JE, Griffin WS. Choline acetyltransferase in schizophrenia. The American journal of psychiatry. 1993;150(3):454–459. doi: 10.1176/ajp.150.3.454. [DOI] [PubMed] [Google Scholar]
- Katsel P, Byne W, Roussos P, Tan W, Siever L, Haroutunian V. Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharmacology. 2011;36(6):1171–1177. doi: 10.1038/npp.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nature neuroscience. 2009;12(1):15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Guidotti A, Grayson DR. The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Molecular pharmacology. 2009;75:342–354. doi: 10.1124/mol.108.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie S, Allaman I, Petit JM, Do KQ, Magistretti PJ. Altered glycogen metabolism in cultured astrocytes from mice with chronic glutathione deficit; relevance for neuroenergetics in schizophrenia. PloS one. 2011;6(7):e22875. doi: 10.1371/journal.pone.0022875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Ghetti A, Pinto-Duarte A, Wang X, Dziewczapolski G, Galimi F, Huitron-Resendiz S, Piña-Crespo JC, Roberts AJ, Verma IM, Sejnowski TJ, Heinemann SF. Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci U S A. 2014;111(32):E3343–52. doi: 10.1073/pnas.1410893111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Schwab C, McGeer PL. Astrocytes are GABAergic cells that modulate microglial activity. Glia. 2011;59:152–165. doi: 10.1002/glia.21087. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Cortical circuit dysfunction and cognitive deficits in schizophrenia-implications for preemptive interventions. Eur J Neurosci. 2012;35(12):1871–8. doi: 10.1111/j.1460-9568.2012.08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima A, Sardinha VM, Oliveira AF, Reis M, Mota C, Silva MA, Marques F, Cerqueira JJ, Pinto L, Sousa N, Oliveira JF. Astrocyte pathology in the prefrontal cortex impairs the cognitive function of rats. Molecular psychiatry. 2014;19(7):834–41. doi: 10.1038/mp.2013.182. [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophrenia bulletin. 2012;38(3):426–432. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou YJ, Wang HH, Lee MT, Wang SC, Chiang HL, Chen CC, Lin CH, Chung MS, Kuo CC, Liao DL, Wu CK, Liu CM, Liu YL, Hwu HG, Lai IC, Tsai SJ, Chen CH, Liu HF, Chou YC, Chen CH, Chen YT, Hong CJ, Wu JY. Genome-wide association study of treatment refractory schizophrenia in Han Chinese. PLoS One. 2012;7(3):e33598. doi: 10.1371/journal.pone.0033598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Shi Y, Tang J, Guo T, Li X, Yang Y, Chen Q, Zhao X, He G, Feng G, Gu N, Zhu S, Liu H, He L. SNPs and haplotypes in the S100B gene reveal association with schizophrenia. Biochemical and biophysical research communications. 2005;328(1):335–341. doi: 10.1016/j.bbrc.2004.12.175. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Sebastião AM, Ribeiro JA. Adenosine and related drugs in brain diseases: present and future in clinical trials. Curr Top Med Chem. 2011;11(8):1087–101. doi: 10.2174/156802611795347591. [DOI] [PubMed] [Google Scholar]
- Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(24):7743–7752. doi: 10.1523/JNEUROSCI.0341-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TM, Abazyan S, Abazyan B, Nomura J, Yang C, Seshadri S, Sawa A, Snyder SH, Pletnikov MV. Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Molecular psychiatry. 2013;18(5):557–567. doi: 10.1038/mp.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nature methods. 2013;10(10):957–963. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G, Di Iorio G, Marini S, Ricci V, De Berardis D, Di Giannantonio M. Nerve growth factor and brain-derived neurotrophic factor concentrations in schizophrenia: a review. Journal of biological regulators and homeostatic agents. 2012;26(3):347–356. [PubMed] [Google Scholar]
- Matute C, Melone M, Vallejo-Illarramendi A, Conti F. Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia. 2005;49(3):451–455. doi: 10.1002/glia.20119. [DOI] [PubMed] [Google Scholar]
- McCarroll SA, Hyman SE. Progress in the genetics of polygenic brain disorders: significant new challenges for neurobiology. Neuron. 2013;80(3):578–587. doi: 10.1016/j.neuron.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melone M, Bellesi M, Conti F. Synaptic localization of GLT-1a in the rat somatic sensory cortex. Glia. 2009;57(1):108–17. doi: 10.1002/glia.20744. [DOI] [PubMed] [Google Scholar]
- Meyer U. Anti-inflammatory signaling in schizophrenia. Brain, behavior, and immunity. 2011;25(8):1507–1518. doi: 10.1016/j.bbi.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatric research. 2011;69(5 Pt 2):26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Ujike H, Tanaka Y, Otani K, Kishimoto M, Morio A, Kotaka T, Okahisa Y, Matsushita M, Morikawa A, Hamase K, Zaitsu K, Kuroda S. A genetic variant of the serine racemase gene is associated with schizophrenia. Biological psychiatry. 2007;61(10):1200–1203. doi: 10.1016/j.biopsych.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Müller N, Myint AM, Schwarz MJ. Kynurenine pathway in schizophrenia: pathophysiological and therapeutic aspects. Curr Pharm Des. 2011;17(2):130–6. doi: 10.2174/138161211795049552. [DOI] [PubMed] [Google Scholar]
- Muller N, Myint AM, Krause D, Weidinger E, Schwarz MJ. Anti-inflammatory treatment in schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2013;42:146–153. doi: 10.1016/j.pnpbp.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Muratake T, Fukui N, Kaneko N, Amagane H, Someya T. Linkage disequilibrium in aquaporin 4 gene and association study with schizophrenia. Psychiatry and clinical neurosciences. 2005;59(5):595–598. doi: 10.1111/j.1440-1819.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiological reviews. 2013;93(4):1543–1562. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57(6):883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PloS one. 2011;6(12):e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama H, Knopfel T, Endo S, Itohara S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):4037–4042. doi: 10.1073/pnas.052020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo FM, Wilson EH, Horning KJ, Thomas MA, Harris TH, Fang Q, Hunter CA, Schwarcz R. Evaluation of kynurenine pathway metabolism in Toxoplasma gondii-infected mice: implications for schizophrenia. Schizophrenia research. 2014;152(1):261–267. doi: 10.1016/j.schres.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell K, Thakore J, Dev KK. Levels of S100B are raised in female patients with schizophrenia. BMC psychiatry. 2013;13:146. doi: 10.1186/1471-244X-13-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Mothet JP. Regulation of N-methyl-D-aspartate receptors by astrocytic D-serine. Neuroscience. 2009;158(1):275–283. doi: 10.1016/j.neuroscience.2008.01.071. [DOI] [PubMed] [Google Scholar]
- Otte DM, Barcena de Arellano ML, Bilkei-Gorzo A, Albayram O, Imbeault S, Jeung H, Alferink J, Zimmer A. Effects of Chronic D-Serine Elevation on Animal Models of Depression and Anxiety-Related Behavior. PloS one. 2013;8(6):e67131. doi: 10.1371/journal.pone.0067131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovanesov MV, Ayhan Y, Wolbert C, Moldovan K, Sauder C, Pletnikov MV. Astrocytes play a key role in activation of microglia by persistent Borna disease virus infection. Journal of neuroinflammation. 2008;5:50. doi: 10.1186/1742-2094-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya CD, Kutiyanawalla A, Pillai A. BDNF-TrkB signaling and neuroprotection in schizophrenia. Asian journal of psychiatry. 2013;6(1):22–28. doi: 10.1016/j.ajp.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Archives of general psychiatry. 2010;67(2):155–166. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Lipska BK, Weinberger DR. Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology. 2012;62(3):1204–1220. doi: 10.1016/j.neuropharm.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim SK, Kim JW, Kang WS, Chung JH. Association of thrombospondin 1 gene with schizophrenia in Korean population. Molecular biology reports. 2012;39(6):6875–6880. doi: 10.1007/s11033-012-1513-3. [DOI] [PubMed] [Google Scholar]
- Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF, Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. Journal of neurochemistry. 2012;121(1):4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310(5745):113–6. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133(1):38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Diedrich M, Kaestner F, Koelkebeck K, Ohrmann P, Ponath G, Kipp F, Abel S, Siegmund A, Suslow T, von Eiff C, Arolt V, Rothermundt M. Memory impairment correlates with increased S100B serum concentrations in patients with chronic schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32(8):1789–1792. doi: 10.1016/j.pnpbp.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Petegnief V, Saura J, de Gregorio-Rocasolano N, Paul SM. Neuronal injury-induced expression and release of apolipoprotein E in mixed neuron/glia co-cultures: nuclear factor kappaB inhibitors reduce basal and lesion-induced secretion of apolipoprotein E. Neuroscience. 2001;104(1):223–234. doi: 10.1016/s0306-4522(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Pletnikov MV. Inducible and conditional transgenic mouse models of schizophrenia. Progress in brain research. 2009;179:35–47. doi: 10.1016/S0079-6123(09)17905-0. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, Karp NA, Hester S, Tkachev D, Mimmack ML, Yolken RH, Webster MJ, Torrey EF, Bahn S. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Molecular psychiatry. 2004;9(7):684–697. 643. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. 2014;82(6):1263–70. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LY, Xiu MH, Chen da C, Wang F, Kosten TA, Kosten TR, Zhang XY. Increased serum S100B levels in chronic schizophrenic patients on long-term clozapine or typical antipsychotics. Neuroscience letters. 2009;462(2):113–117. doi: 10.1016/j.neulet.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Quesseveur G, David DJ, Gaillard MC, Pla P, Wu MV, Nguyen HT, Nicolas V, Auregan G, David I, Dranovsky A, Hantraye P, Hen R, Gardier AM, Deglon N, Guiard BP. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Translational psychiatry. 2013;3:e253. doi: 10.1038/tp.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Erta M, Ferrer B, Comes G, Giralt M, Hidalgo J. Astrocyte-specific deficiency of interleukin-6 and its receptor reveal specific roles in survival, body weight and behavior. Brain, behavior, and immunity. 2013;27(1):162–173. doi: 10.1016/j.bbi.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, Meltzer H, Overholser J, Stockmeier C. Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophrenia research. 2002;57(2-3):127–138. doi: 10.1016/s0920-9964(02)00339-0. [DOI] [PubMed] [Google Scholar]
- Ramanan VK, Shen L, Moore JH, Saykin AJ. Pathway analysis of genomic data: concepts, methods, and prospects for future development. Trends in genetics : TIG. 2012;28(7):323–332. doi: 10.1016/j.tig.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. The Journal of clinical investigation. 2012;122(4):1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder JK, Roder JC, Gerlai R. Conspecific exploration in the T-maze: abnormalities in S100 beta transgenic mice. Physiology & behavior. 1996;60(1):31–36. doi: 10.1016/0031-9384(95)02247-3. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Falkai P, Ponath G, Abel S, Burkle H, Diedrich M, Hetzel G, Peters M, Siegmund A, Pedersen A, Maier W, Schramm J, Suslow T, Ohrmann P, Arolt V. Glial cell dysfunction in schizophrenia indicated by increased S100B in the CSF. Molecular psychiatry. 2004a;9(10):897–899. doi: 10.1038/sj.mp.4001548. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Ponath G, Glaser T, Hetzel G, Arolt V. S100B serum levels and long-term improvement of negative symptoms in patients with schizophrenia. Neuropsychopharmacology. 2004b;29(5):1004–1011. doi: 10.1038/sj.npp.1300403. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Ohrmann P, Abel S, Siegmund A, Pedersen A, Ponath G, Suslow T, Peters M, Kaestner F, Heindel W, Arolt V, Pfleiderer B. Glial cell activation in a subgroup of patients with schizophrenia indicated by increased S100B serum concentrations and elevated myo-inositol. Progress in neuro-psychopharmacology & biological psychiatry. 2007;31(2):361–364. doi: 10.1016/j.pnpbp.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Roussos P, Katsel P, Davis KL, Giakoumaki SG, Lencz T, Malhotra AK, Siever LJ, Bitsios P, Haroutunian V. Convergent findings for abnormalities of the NF-κB signaling pathway in schizophrenia. Neuropsychopharmacology. 2013;38(3):533–9. doi: 10.1038/npp.2012.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Beppu K, Tanaka KF, Fukazawa Y, Shigemoto R, Matsui K. Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(50):20720–20725. doi: 10.1073/pnas.1213458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Binder DK. Aquaporin-4 water channels and synaptic plasticity in the hippocampus. Neurochemistry international. 2013;63(7):702–711. doi: 10.1016/j.neuint.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper HM. Heme oxygenase expression in human central nervous system disorders. Free radical biology & medicine. 2004;37(12):1995–2011. doi: 10.1016/j.freeradbiomed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Schnieder TP, Dwork AJ. Searching for neuropathology: gliosis in schizophrenia. Biological psychiatry. 2011;69(2):134–139. doi: 10.1016/j.biopsych.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Abdul-Khaliq H, Krebs M, Diefenbacher A, Blasig IE. Neuron-specific enolase is unaltered whereas S100B is elevated in serum of patients with schizophrenia--original research and meta-analysis. Psychiatry research. 2009;167(1-2):66–72. doi: 10.1016/j.psychres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nature reviews Neuroscience. 2012;13(7):465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Hunter CA. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophrenia bulletin. 2007;33(3):652–653. doi: 10.1093/schbul/sbm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biological psychiatry. 2001;50(7):521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Sen J, Belli A. S100B in neuropathologic states: the CRP of the brain? Journal of neuroscience research. 2007;85(7):1373–1380. doi: 10.1002/jnr.21211. [DOI] [PubMed] [Google Scholar]
- Shan D, Lucas EK, Drummond JB, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporters in temporal lobe areas in elderly patients with schizophrenia. Schizophr Res. 2013;144(1-3):1–8. doi: 10.1016/j.schres.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri A, Bonifati DM, Kishore U. Innate immunity and neuroinflammation. Mediators of inflammation. 2013;2013:342931. doi: 10.1155/2013/342931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Singer P, Lytle N, Wei CJ, Lan JQ, Williams-Karnesky RL, Chen JF, Yee BK, Boison D. Adenosine augmentation ameliorates psychotic and cognitive endophenotypes of schizophrenia. The Journal of clinical investigation. 2012;122(7):2567–2577. doi: 10.1172/JCI62378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K, Gomi H, Chen L, Bao S, Kim JJ, Wakatsuki H, Fujisaki T, Fujimoto K, Katoh A, Ikeda T, Chen C, Thompson RF, Itohara S. Deficient cerebellar long-term depression, impaired eyeblink conditioning, and normal motor coordination in GFAP mutant mice. Neuron. 1996;16(3):587–599. doi: 10.1016/s0896-6273(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Singer P, Wei CJ, Chen JF, Boison D, Yee BK. Deletion of striatal adenosine A(2A) receptor spares latent inhibition and prepulse inhibition but impairs active avoidance learning. Behavioural brain research. 2013a;242:54–61. doi: 10.1016/j.bbr.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P, Zhang C, Boison D, Yee BK. Dysregulation of brain adenosine is detrimental to the expression of conditioned freezing but not general Pavlovian learning. Pharmacology, biochemistry, and behavior. 2013b;104:80–89. doi: 10.1016/j.pbb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skucas VA, Mathews IB, Yang J, Cheng Q, Treister A, Duffy AM, Verkman AS, Hempstead BL, Wood MA, Binder DK, Scharfman HE. Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(17):6392–6397. doi: 10.1523/JNEUROSCI.6249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH, Kim PM. D-amino acids as putative neurotransmitters: focus on D-serine. Neurochemical research. 2000;25(5):553–560. doi: 10.1023/a:1007586314648. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Zukor H, Lin SH, Hascalovici J, Liberman A, Tavitian A, Mui J, Vali H, Tong XK, Bhardwaj SK, Srivastava LK, Hamel E, Schipper HM. Schizophrenia-like features in transgenic mice overexpressing human HO-1 in the astrocytic compartment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(32):10841–10853. doi: 10.1523/JNEUROSCI.6469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry. 2009;65(6):481–8. doi: 10.1016/j.biopsych.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Spangaro M, Bosia M, Zanoletti A, Bechi M, Cocchi F, Pirovano A, Lorenzi C, Bramanti P, Benedetti F, Smeraldi E, Cavallaro R. Cognitive dysfunction and glutamate reuptake: effect of EAAT2 polymorphism in schizophrenia. Neuroscience letters. 2012;522(2):151–155. doi: 10.1016/j.neulet.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Steffek AE, Haroutunian V, Meador-Woodruff JH. Serine racemase protein expression in cortex and hippocampus in schizophrenia. Neuroreport. 2006;17(11):1181–1185. doi: 10.1097/01.wnr.0000230512.01339.72. [DOI] [PubMed] [Google Scholar]
- Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophrenia research. 2008;103(1-3):71–82. doi: 10.1016/j.schres.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bernstein HG, Bielau H, Farkas N, Winter J, Dobrowolny H, Brisch R, Gos T, Mawrin C, Myint AM, Bogerts B. S100B-immunopositive glia is elevated in paranoid as compared to residual schizophrenia: a morphometric study. Journal of psychiatric research. 2008;42(10):868–876. doi: 10.1016/j.jpsychires.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Steiner J, Schroeter ML, Schiltz K, Bernstein HG, Müller UJ, Richter-Landsberg C, Müller WE, Walter M, Gos T, Bogerts B, Keilhoff G. Haloperidol and clozapine decrease S100B release from glial cells. Neuroscience. 2010;167(4):1025–1031. doi: 10.1016/j.neuroscience.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bogerts B, Sarnyai Z, Walter M, Gos T, Bernstein HG, Myint AM. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2012;13(7):482–492. doi: 10.3109/15622975.2011.583941. [DOI] [PubMed] [Google Scholar]
- Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58(9):1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MH. Drug-related schizophrenic syndromes. International journal of psychiatry. 1973;11(4):391–437. [PubMed] [Google Scholar]
- Sun XL, Chen BY, Duan L, Xia Y, Luo ZJ, Wang JJ, Rao ZR, Chen LW. The proform of glia cell line-derived neurotrophic factor: a potentially biologically active protein. Molecular neurobiology. 2014;49(1):234–250. doi: 10.1007/s12035-013-8515-6. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang L, Johnston NL, Torrey EF, Yolken RH. Serial analysis of gene expression in the frontal cortex of patients with bipolar disorder. Br J Psychiatry Suppl. 2001;41s:137–41. doi: 10.1192/bjp.178.41.s137. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Sakurai T. Roles of glial cells in schizophrenia: possible targets for therapeutic approaches. Neurobiology of disease. 2013;53:49–60. doi: 10.1016/j.nbd.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, Malaspina D, Owen MJ, Schultz S, Tsuang M, Van Os J, Carpenter W. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3–10. doi: 10.1016/j.schres.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Tezuka T, Tamura M, Kondo MA, Sakaue M, Okada K, Takemoto K, Fukunari A, Miwa K, Ohzeki H, Kano S, Yasumatsu H, Sawa A, Kajii Y. Cuprizone short-term exposure: astrocytic IL-6 activation and behavioral changes relevant to psychosis. Neurobiology of disease. 2013;59:63–68. doi: 10.1016/j.nbd.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro CT, Hallak JE, Dunham JS, Deakin JF. Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neuroscience letters. 2006;404(3):276–281. doi: 10.1016/j.neulet.2006.05.067. [DOI] [PubMed] [Google Scholar]
- Tosic M, Ott J, Barral S, Bovet P, Deppen P, Gheorghita F, Matthey ML, Parnas J, Preisig M, Saraga M, Solida A, Timm S, Wang AG, Werge T, Cuenod M, Do KQ. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. American journal of human genetics. 2006;79(3):586–592. doi: 10.1086/507566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova NA, Casanova MF, DeVaughn NM, Orlovskaya DD, Denisov DV. Ultrastructural alterations of synaptic contacts and astrocytes in postmortem caudate nucleus of schizophrenic patients. Schizophrenia research. 1996;22(1):81–83. doi: 10.1016/0920-9964(96)00059-x. [DOI] [PubMed] [Google Scholar]
- Uzbay T, Goktalay G, Kayir H, Eker SS, Sarandol A, Oral S, Buyukuysal L, Ulusoy G, Kirli S. Increased plasma agmatine levels in patients with schizophrenia. Journal of psychiatric research. 2013;47(8):1054–1060. doi: 10.1016/j.jpsychires.2013.04.004. [DOI] [PubMed] [Google Scholar]
- van der Leeuw C, Marcelis M, Peeters SC, Verbeek MM, Menheere PP, de Haan L, van Os J, van Beveren NJ, Genetic R, Outcome in P. Replicated evidence of absence of association between serum S100B and (risk of) psychotic disorder. PloS one. 2013;8(12):e82535. doi: 10.1371/journal.pone.0082535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Murray R. Gene-environment interactions in schizophrenia. Introduction. Schizophrenia bulletin. 2008;34(6):1064–1065. doi: 10.1093/schbul/sbn116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, Johnston-Wilson N, Nagata K, Inagaki M, Yolken RH. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain, behavior, and immunity. 2001;15(4):388–400. doi: 10.1006/brbi.2001.0646. [DOI] [PubMed] [Google Scholar]
- Webster MJ, O'Grady J, Kleinman JE, Weickert CS. Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience. 2005;133(2):453–461. doi: 10.1016/j.neuroscience.2005.02.037. [DOI] [PubMed] [Google Scholar]
- Williams M, Pearce RK, Hirsch SR, Ansorge O, Thom M, Maier M. Fibrillary astrocytes are decreased in the subgenual cingulate in schizophrenia. European archives of psychiatry and clinical neuroscience. 2014;264(4):357–362. doi: 10.1007/s00406-013-0482-4. [DOI] [PubMed] [Google Scholar]
- Wolosker H. Serine racemase and the serine shuttle between neurons and astrocytes. Biochimica et biophysica acta. 2011;1814(11):1558–1566. doi: 10.1016/j.bbapap.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. Schizophrenia bulletin. 2010;36(2):211–218. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, Kajii Y, Thaker GK, Schwarcz R. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Archives of general psychiatry. 2011;68(7):665–674. doi: 10.1001/archgenpsychiatry.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasamy MT. Schizoaffective disorder: a dimensional approach. Acta Psychiatr Scand. 1987;76(6):609–18. doi: 10.1111/j.1600-0447.1987.tb02931.x. [DOI] [PubMed] [Google Scholar]
- Yee BK, Singer P, Chen JF, Feldon J, Boison D. Transgenic overexpression of adenosine kinase in brain leads to multiple learning impairments and altered sensitivity to psychomimetic drugs. The European journal of neuroscience. 2007;26(11):3237–3252. doi: 10.1111/j.1460-9568.2007.05897.x. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Dickerson FB, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite immunology. 2009;31(11):706–715. doi: 10.1111/j.1365-3024.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Molecular psychiatry. 2008;13(5):470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- Zhai J, Zhang Q, Cheng L, Chen M, Wang K, Liu Y, Deng X, Chen X, Shen Q, Xu Z, Ji F, Liu C, Dong Q, Chen C, Li J. Risk variants in the S100B gene, associated with elevated S100B levels, are also associated with visuospatial disability of schizophrenia. Behavioural brain research. 2011;217(2):363–368. doi: 10.1016/j.bbr.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhong NN, Liu XG, Yan H, Qiu C, Han Y, Wang W, Hou WK, Liu Y, Gao CG, Guo TW, Lu SM, Deng HW, Ma J. Is the EFNB2 locus associated with schizophrenia? Single nucleotide polymorphisms and haplotypes analysis. Psychiatry research. 2010;180(1):5–9. doi: 10.1016/j.psychres.2010.04.037. [DOI] [PubMed] [Google Scholar]


