Abstract
Children with attention-deficit/hyperactivity disorder (ADHD) and traumatic brain injury (TBI) show deficient response inhibition. ADHD itself is a common consequence of TBI, known as secondary ADHD (S-ADHD). Similarity in inhibitory control in children with TBI, S-ADHD, and ADHD would implicate impaired frontostriatal systems; however, it is first necessary to delineate similarities and differences in inhibitory control in these conditions. We compared performance of children with ADHD and those with TBI without pre-injury ADHD on a stop signal, response inhibition task. Participants were 274 children aged 6-14 years. There were 92 children with ADHD, 103 children with TBI and 79 typically developing children who served as controls. Among the TBI participants, injury severity ranged from mild to severe. Children with ADHD and TBI showed deficient inhibition. The deficit in children with ADHD was as great as or greater than that in children with TBI, regardless of degree of TBI severity or the presence of S-ADHD. The finding indicates that TBI results in deficient inhibition regardless of the development of S-ADHD.
INTRODUCTION
Poor inhibitory control is a cardinal deficit in the developmental form of attention-deficit hyperactivity disorder (ADHD) (Oosterlann, Logan, & Sergent, 1998; Nigg, 1999; Schachar, Mota, Logan, Tannock, & Klim, 2000), but it has also been reported in children who have sustained traumatic brain injury (TBI) in childhood (Leblanc et al., 2005). ADHD is a common consequence of TBI in children and has recently come to be referred to as secondary ADHD or S-ADHD (Bloom et al., 2001; Gerring et al., 1998; Max, Arndt et al., 1998; Max, Koele et al., 1998).
The neurobiology of response inhibition involves frontal-striatal pathways. However, the origin of poor circuitry is different in the two groups. In children with ADHD, developmental processes have generated atypical brain structure and function in regions concerned with inhibitory control (Castellanos, 1997; Tamm, Menon, & Reiss, 2002). In children with TBI, a period of typical brain developmental has been interrupted by physical trauma that often includes frontal contusions (Mattson & Levin, 1990). Similarity in inhibitory control in children with TBI, S-ADHD, and ADHD would implicate impaired frontostriatal systems. Conversely, perhaps TBI and/or inhibitory control performance shapes the expression of ADHD, leading to an etiologically distinct form of ADHD. Better understanding of the commonalities as well as the differences with regard to inhibitory (and other) processes will lead to an appreciation of the pathophysiological mechanisms with possible implications for differing (or similar) treatment approaches. Furthermore, differences may be of relevance to the study of children with developmental ADHD in that some of the unexplained variance in inhibitory tasks reported in this sample may be elucidated. However, it is first necessary to delineate similarities and differences in inhibitory control in these conditions.
A number of unresolved questions arise from the phenotypic similarity of poor response inhibition in ADHD and TBI. One question is whether the magnitude of inhibitory control deficits is similar in the developmental and acquired forms of ADHD. Another is whether only children with TBI and diagnosed S-ADHD show inhibitory control deficits or whether, instead, TBI itself is associated with these deficits. A third is whether the magnitude of response inhibition deficits varies with TBI severity. The development of ADHD in children with TBI has been reported to be associated with injury severity (Max et al., 2004). Moreover, the magnitude of head injury severity is related to the extent of executive dysfunction (Levin et al., 1994; Max et al., 2004; cf. Gerring et al., 1998).
The present study attempts to address these questions. We compared children with ADHD without TBI and children with TBI, none of whom had pre-injury ADHD (which tends to persist (Max et al., 1997) after injury and would therefore confound study results) on an inhibitory control task involving inhibition of a speeded motor response. While inhibitory control has been extensively studied in ADHD (e.g., Schachar et al., 2000) there have been fewer studies of this function in TBI (Anderson, Fenwick, Manly, & Robertson, 1998; Leblanc et al., 2005; Ponsford & Kinsella, 1992; Slomine et al., 2005).
We measured inhibitory control with the stop signal paradigm (SSP) (Logan, 1994), which requires the inhibition of a prepotent motor response. This task is sensitive to ADHD (e.g., Schachar et al., 2000), and a great deal of knowledge exists as to the underlying neurobiology of the kind of inhibition measured in this task (Chambers, Garavan, & Bellgrove, 2009; Crosbie, Perusse, Barr, & Schachar, 2008).
Based on the existing literature, we had three predictions about the pattern of results. We hypothesized that, compared to typically developing controls, children with TBI and children with ADHD would show an inhibition deficit; albeit, children with ADHD would show more marked dysfunction. We hypothesized that children with TBI and S-ADHD would exhibit a greater inhibition deficit than children with TBI without S-ADHD. We also hypothesized that deficient response inhibition in TBI would vary as a function of injury severity.
METHODS
Sample
Participants were 274 children aged 6-14 years. There were 92 children with ADHD, 103 children with TBI and 79 typically developing children who served as controls. TBI children were assessed in one of several large, urban pediatric hospitals in Toronto, Dallas or Houston. Children with ADHD were drawn from a clinical sample referred for assessment of disruptive behavior and learning disorders to an out-patient pediatric psychiatry department in a large urban hospital. Controls were recruited through newspaper advertisements. All participants were screened for eligibility and excluded from the study if they showed evidence of a neurological disorder, a chronic or serious medical condition, a history or evidence of psychosis or a clinically significant anxiety disorder. Control subjects were also excluded if they met research diagnostic criteria for ADHD (screening methods are reviewed below). Based on parental history, none of the children with ADHD or controls had a history of TBI. Children with ADHD currently treated with stimulant medication had their medication withdrawn a minimum of 48 hours preceding testing to rule out the effects of medication on cognitive performance. None of the S-ADHD cases were medicated at the time of the study. Parents of all participants gave written consent for their children to participate and all children gave verbal assent.
Assessment of ADHD children
The Parent Interview for Child Symptoms (PICS) (Ickowicz et al., 2006) covers the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) criteria for ADHD and other axis I diagnoses necessary to establish inclusion and exclusion criteria. A family history of psychopathology, developmental, medical and social history was also obtained. The Family and Household Form from the Ontario Child Health Survey Scales-Revised (OCHS-R) (Boyle et al., 1996) was used to provide information about psychosocial and environmental risk factors, and parent and teacher ratings of anxiety and depression. The children who were classified as having ADHD consisted of an inattentive subtype and a combined subtype. The children met DSM-IV criteria defined as the inattentive type if they presented with at least 6 of 9 inattentive symptoms, or the combined-type if they presented with at least 6 of 9 inattentive symptoms and 6 of 9 hyperactive/impulsive symptoms, and behavioral problems that emerged before the age of 7 years. To ensure pervasive impairment in two settings, each child was required to have met DSM-IV criteria based on either the parent or teacher interview, and to exhibit a minimum of 4 ADHD symptoms according to the other informant. The two subtypes did not differ for inhibitory control performance (data not shown); thus, the groups were pooled.
Assessment of TBI
The children with TBI were assessed retrospectively, a minimum of two years following the TBI. Based on parent and teacher descriptions of child behaviour prior to injury, the presence of short attention span, underachievement and overactivity was recorded. Children with TBI were excluded from the study if they presented with a pre-injury ADHD diagnosis, learning disabilities, or speech and language delay. The Glasgow Coma Scale (GCS) is a commonly used measure of brain injury severity. GCS scores were taken from each participant’s medical records; the scale ranges from 3 to 15 points with higher GCS scores reflecting better responsiveness. Injury severity was classified as follows: mild (13-15 points); moderate (9-12); and severe (3-8). GCS ratings were taken as the lowest, post resuscitation score assigned in the ambulance or on admission to the emergency room. Mild cases with evidence of brain damage, based on an initial computed tomography scan, were reclassified with TBI of moderate severity because of recent evidence that children with complicated mild TBI are at risk for poor long-term outcomes (Levin et al., 2008; Williams & Levin, 1990).
The primary behavioral measure used to determine S-ADHD was the OCHS-R questionnaires, completed by both parents and teachers. Ratings represented behaviors exhibited during the past six months. A research diagnosis of ADHD was given if the parent or teacher scores exceeded the threshold for ADHD (mean plus one standard deviation) using age and gender norms derived from a general childhood population (Boyle et al., 1996).
PROCEDURE
The SSP provides a direct measure of the speed for executing and voluntarily inhibiting a motor response and involves two concurrent tasks, a ‘go’ task and a ‘stop’ task. The go task involves a simple choice reaction time task to be performed as quickly and as accurately as possible. The stop task involves a tone emitted from the computer. This tone follows the presentation of the go task stimulus and instructs participants to withhold their response on that particular trial (see Figure 1). The tone occurs randomly on 25% of trials and the later the tone is presented, the more difficult it is to stop the response to the go stimulus. Inhibitory control depends on the latency of two independent processes – the response to the go signal (go reaction time, goRT) and the response to the SSRT. The outcome of the race between go and stop processes depends on the interval between onset of the go signal and the onset of the stop signal, referred to as stop signal delay. Stop signal delay was set initially at 250 msec. This ‘tracking’ algorithm converges on the stop signal delay at which individuals are able to inhibit 50% of the time. The mean latency of the goRT is observable from the 75% of trials in which no stop signal is presented. The latency of the stop process is unobservable. If the individual stops, no response is evident. If the go process finishes before the stop process, the individual responds as if no stop had been presented. However, the latency of the unobserved stop process can be computed by subtracting the mean delay (at which the individual inhibits 50% of the time) from the mean goRT. Slower speed of this stopping process (i.e., a larger latency) reflects deficient inhibition.
Figure 1.
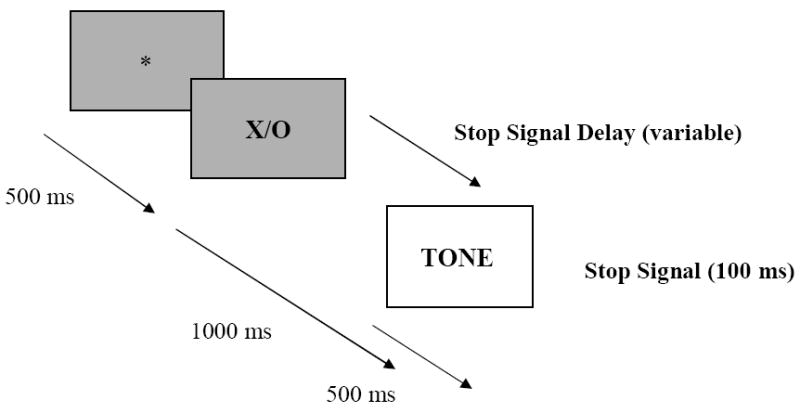
The Stop-Signal Paradigm.
The go task stimuli were upper case letters X and O presented in the centre of the screen for 1000 msec. Each trial was preceded by a 500 msec fixation point that was presented in the middle of the screen, and then followed by a 2000 msec blank screen. The stop signal was a tone of 1000 Hz. The task was made up of 8 blocks each of 32 trials. Twenty-four of these trials are go trials without a stop signal while 8 trials include the stop signal. Children held a push button box and were instructed to use the left index finger on the X button and their right index finger on the O button. The X or O stimuli appeared equally often in each block.
For each participant the following measures were recorded: SSRT, the probability of inhibiting, goRT, and the probability of correct go trials.
STATISTICAL ANALYSIS
Demographic characteristics were compared using analyses of variance (ANOVAs) or X2 tests. ANOVAs were carried out to compare mean performance differences among the ADHD, TBI and control groups on the SSP. When an overall group difference was significant, post hoc Tukey Honestly Significant Difference tests were applied. To determine whether the pattern of stop signal performance in the TBI group was related to TBI severity or post-injury ADHD, supplementary within-group comparisons were carried out. For these contrasts, the children with TBI were divided by severity and the presence or absence of S-ADHD. It is possible that some of the variation of the differences in test performance among the groups was due to difference in age and/ or gender. In order to account for these variables, regression analyses were conducted and where significant, it is noted in the text. Additionally, a logistic regression analysis was carried out in order to determine if age at injury, age at time of assessment, time since injury, gender, and GCS predict S-ADHD in the TBI group. The goodness of fit of the resulting models was evaluated with the R-squared coefficient (R2). The index of variability shown in all cases was the standard deviation. Significance levels for all analyses were two-tailed and set at p≤0.05.
RESULTS
Demographics
ANOVA revealed a significant difference among the groups for age (F= 20.74, df= 2, 273, P<0.001), such that the ADHD group was younger than the TBI group and controls (P’s<0.01). There was no age difference between the controls and children with TBI. There was also a significant difference for gender among the groups (X2=16.96, P<0.001), such that the control group has more females while the ADHD group has more males compared to the TBI group (P’s<0.001). Age at injury or time since injury was similar in the mild, moderate, and severe TBI children, and between the children with TBI with and without S-ADHD. Demographic information is provided in Table 1.
Table 1.
Demographic characteristics
| ADHD (N = 92) | TBI (N = 103) | Controls (N = 79) | |
|---|---|---|---|
| Gender (M: F) | 66: 26 | 66: 37 | 33: 46 |
| Age at testing (years) | 8.84 (1.66) | 10.93 (2.75) | 9.72 (2.19) |
| Age at injury (years) | 6.02 (2.76) | ||
| Time since injury (years) | 4.91 (1.82) |
Means and standard deviations in parentheses are presented.
Stop signal task performance among ADHD, TBI and Controls
The mean probability of inhibiting a response given a stop signal was non-significantly different among the children with ADHD, TBI and controls. There was a significant difference among the three groups for SSRT (F =7.53, df=2,212, P<0.001). On average, ADHD children had the poorest inhibitory control, followed by the TBI group. As shown in Table 2, the ADHD children were slower in stopping than the controls (P<0.01), and the children with TBI were slower in stopping than controls (P<0.05). Regression analysis revealed that the difference remained after controlling for age and gender. Children with ADHD and TBI, and controls all performed with acceptably high levels of accuracy (90%; 94%; 94%). However, there was an overall difference in the accuracy of go-task responding among the groups (F=10.84, df=2,227, P<0.001). The control subjects and children with TBI were more accurate than the children with ADHD (P<0.01 and P<0.01, respectively). There were no significant differences in goRT among the three groups.
Table 2.
Mean (SD) for the Stop Signal Paradigm.
| N | SSRT (ms) | % of Trials Inhibited | Go Trial Reaction Times | % correct Go Trials | |
|---|---|---|---|---|---|
| ADHD | 92 | 294 (115) | 50 (5.4) | 602 (138) | 90 (9.4) |
| TBI | 103 | 263 (94) | 51 (7.4) | 579 (166) | 94 (6.4) |
| Controls | 79 | 229 (74) | 50 (5.4) | 554 (113) | 94 (4.6) |
|
| |||||
| TBI Subgroups: | |||||
| With S-ADHD | 30 | 296 (117) | 50 (8.7) | 587 (196) | 93(9.0) |
| Without S-ADHD | 73 | 247 (78) | 51 (6.6) | 574 (152) | 95(4.6) |
|
| |||||
| Mild | 57 | 265 (94) | 51 (7.3) | 578 (175) | 95 (5.0) |
| Moderate | 18 | 281 (79) | 53 (7.3) | 604 (225) | 95 (6.0) |
| Severe | 28 | 247 (106) | 51 (7.7) | 568 (113) | 94 (9.0) |
Stop signal task performance among ADHD and TBI children with and without S-ADHD
Based on mean scores, inhibitory control performance in the TBI children with S-ADHD appears to be similar to that of the children with ADHD, while that of the children with TBI without S-ADHD seems like that of controls (see Table 2). Statistically, there were no differences in performance between the children with ADHD and those with TBI and S-ADHD. There was also no significant difference between the children with TBI without S-ADHD and controls.
Stop signal task performance between the TBI Children With and Without S-ADHD
Although the mean SSRT appears longer in children with TBI and S-ADHD as compared to those with TBI and without S-ADHD, no significant difference emerged between the groups. Similarly, there were no there was no significant differences between the groups for accuracy of go-task responding or goRT.
TBI Severity
There were no differences in performance among children with mild, moderate and severe head injury. Moreover, there was no significant difference in the proportion of mild, moderate and severe TBI children with TBI and S-ADHD. Logistic regression showed that age at injury, age at time of assessment, time since injury, GCS, and gender were not significant predictors of S-ADHD.
DISCUSSION
The aim of this study was to compare the performance of children with ADHD and children with TBI on a measure of response inhibition. We predicted that both children with TBI and ADHD would share an inhibition deficit compared to normal controls albeit children with ADHD would show more marked dysfunction. We anticipated that TBI would be associated with deficient inhibition and that such impairment likely varies with injury severity. We also expected that children with TBI and S-ADHD would show a greater inhibition deficit than children with TBI without S-ADHD.
Both children with ADHD and children with TBI demonstrated impaired ability to inhibit prepotent motor responses. This finding confirms previous studies that have reported deficient inhibitory control in ADHD (Barkley, 1997; Schachar et al., 2000; Schachar et al., 2007), and adds to the few published studies that suggest an inhibitory control deficit in TBI (Konrad, Gauggel, Manz, & Scholl, 2000; Schachar, Levin, Max, & Purvis, 2004; Stewart & Tannock, 1999). The new information we add is that an inhibitory control deficit may be demonstrated in these groups with direct comparisons on the same test paradigms. Similar patterns of inhibitory control in childhood ADHD and TBI may be related to structural brain changes and neurochemical alterations that involve networks that affect, among other areas, the frontal lobes (e.g., Casey, Tottenham, & Fossella, 2002). Poor voluntary response suppression in childhood may be related to perturbed neurodevelopment of, or to damage to functional connectivity in frontal subcortical circuits. Alternatively, it may be the case that more widespread substrates subserve executive control while frontal regions continue to develop into the teenage years. (For a more thorough description of anatomy, refer to Dennis, Sinopoli, Fletcher, & Schachar, 2008). Nevertheless, how frontal-striatal circuits are similar and different in ADHD and TBI is important, but not fully understood.
The magnitude of inhibitory control deficits was not equal in the two clinical groups. Children with ADHD tended to show poorer response inhibition than those with TBI. We speculated however that the magnitude of the inhibition deficit in children with TBI would vary with TBI severity. Attentional difficulties, including response inhibition, has been found to be impaired in children with moderate and/or severe TBI as compared to controls (Konrad et al., 2000; Power, Catroppa, Coleman, Ditchfield, & Anderson, 2007) while limited comparisons have been made among mild, moderate, and severely injured children. Nevertheless, and unexpectedly, we found that children with TBI had poor inhibitory performance irrespective of injury severity. It may be the case that the presence of cognitive impairment is perhaps a function of lesion location rather than severity per se (Power et al., 2007). Power et al. support other published studies that suggest that injury severity involving cerebral pathology among frontal and extrafrontal brain areas contributes to problems with executive control processes, including response inhibition (Aron & Poldrack, 2005).
Another explanation for this finding may due to the employment of GCS as a measure of injury severity. Leblanc et al. (2005) found greater initial deficit in more severely injured children when severity was indexed by duration of coma, and not GCS score in their sample of children with TBI. It is becoming clear that GCS, while a good measure of acute impairment of consciousness, is a limited measure of cognitive morbidity. One reason may be, as Bullock et al. (2008) points out, there are multiple routes to a poor GCS score, and each route involves a different pattern of brain injury.
The relation of post-injury S-ADHD to inhibitory control deficits is somewhat complex. Schachar et al. (2004) showed that children who exhibited both severe TBI and post injury S-ADHD had longer SSRTs on the stop signal task. Neither mild or moderate head injury, nor the presence of S-ADHD in the absence of severe head injury conferred a risk for poor inhibition. In this study, we did not find that the children with severe TBI combined with S-ADHD predicted poor inhibition. In this instance, there may have been too few cases of more severely impaired children with TBI, and a larger sample of children may help establish severity as a significant predictor of performance on response inhibition.
Several limitations must be acknowledged. In this study, co-morbidity was not formally investigated. Several disorders are commonly co-morbid with ADHD, including conduct disorder (Biederman, 2005), which itself is characterized by inhibitory deficits (e.g., Nigg, 1999). However, several studies have found that deficient response inhibition in ADHD is not due to the effect of such co-morbidity, per se (e.g., Crosbie & Schachar, 2001; Slaats-Willemse, Swaab-Barneveld, de Sonneville, van der Meulen, & Buitellar, 2003). Nevertheless, future studies should take co-morbidity into account.
Another limitation in the approach of this study is that the association of neuropsychological sequelae in children with TBI and S-ADHD may be affected by the way S-ADHD was diagnosed. In the only published study examining the nature of inhibitory control in children with ADHD and closed head injury with or without S-ADHD, no performance differences emerged between the children with or without S-ADHD (Konrad et al., 2004). Konrad et al. used a less rigorous method for diagnosing S-ADHD in children who had sustained TBI. They made use of hospital staff informants’ observations to classify children as hyperactive or not. In the present study, both parent and teacher ratings of S-ADHD were employed, although we recognize there are inherent limitations related to the sole use of questionnaires (Power et al., 2007) in order to identify S-ADHD.
Age at head injury may also be significantly related to whether or not a diagnosis of S-ADHD can be determined. The mean age of head injury in the present TBI sample was 6 years (SD = 2.76). Consequently, it may be difficult to accurately determine whether some of the children would have developed ADHD even without TBI. It is possible that mildly impaired children with S-ADHD may actually have primary ADHD that was obscured by retrospective assessment or early age of injury.
Our key finding is that behavioral inhibition is impaired in both children with TBI and children with ADHD. Interestingly, the development of secondary ADHD symptomatology, conjectured to produce a greater inhibition deficit in TBI, need not necessarily be related to poorer inhibitory control. Even though the two disorders appear to share a common cognitive deficit, the underlying mechanism of this impairment remains to be determined, although it is in keeping with prefrontal dysfunction due to injury or aberrant maturation associated with primary ADHD or alternatively, related to a more distributed network of cerebral brain areas that are dependent on the normal development of frontal lobe regions.
It is entirely possible that the development of S-ADHD reflects the same developmental processes as that seen in ADHD and is not just a consequence of brain injury per se. Alternatively, the ADHD evident in TBI may be cognitively distinct from that found when ADHD occurs in the absence of TBI. Further study is needed to examine inhibitory control differences in childhood TBI with and without S-ADHD of varying severity and age.
How similar ADHD and S-ADHD are in terms of a broader range of neuropsychological correlates remains to be determined. The cognitive commonality of these two disorders contributes to learning about the disorders, and can potentially lead to more targeted treatment interventions for children with TBI. Accordingly, future research and treatment programs may be developed following an increased understanding of the neuropsychological mechanisms involved in TBI and the effects of treatment.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research (No. MOP44070), the National Institute of Mental Health (No. K-08-MH01800), and the National Institute of Health, NINDS division (No. NS-21889).
References
- Anderson V, Fenwick T, Manly T, Robertson I. Attentional skills following traumatic brain injury in childhood: a componential analysis. Brain Injury. 1998;12:937–949. doi: 10.1080/026990598121990. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in ADHD. Biological Psychiatry. 2005;57:1285–92. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unify theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity Disorder: A selective overview. Biological Psychiatry. 2005;57:1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Bloom DR, Levin HS, Ewing-Cobbs L, Saunders AE, Song J, Fletcher JM, Kovatch RA, et al. Lifetime and novel psychiatric disorders after pediatric traumatic brain injury. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:572–579. doi: 10.1097/00004583-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Boyle MH, Offord DR, Racine Y, Szatmari P, Fleming JE, Sanford M. Identifying thresholds for classifying childhood psychiatric disorder: Issues and prospects. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35:1440–1448. doi: 10.1097/00004583-199611000-00012. [DOI] [PubMed] [Google Scholar]
- Bullock R, Duhame AC, Maas AIR, Manley GT, Saatman KE, Valadka A. Classification of traumatic brain injury for targeted therapies. Journal of Neurotrauma. 2008;25:718–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Fossella A. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Developmental Psychobiology. 2002;40:237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- Castellanos, F X. Neuroimaging of attention–deficit hyperactivity Disorder. Child & Adolescent Psychiatric Clinics of North America. 1997;6:383–411. doi: 10.1016/j.chc.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neuroscience & Biobehavioral Reviews. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Crosbie J, Perusse D, Barr CL, Schachar RJ. Validating psychiatric endophenotypes: Inhibitory control and attention deficit hyperactivity disorder. Neuroscience & Biobehavioral Reviews. 2008;32:40–55. doi: 10.1016/j.neubiorev.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Crosbie J, Schachar R. Deficient inhibition as a marker for familial ADHD. American Journal of Psychiatry. 2001;158:1884–1890. doi: 10.1176/appi.ajp.158.11.1884. [DOI] [PubMed] [Google Scholar]
- Dennis M, Sinopoli KJ, Fletcher JM, Schachar R. Puppets, robots, critics, and actors within a taxonomy of attention for developmental disorders. Journal of the International Neuropsychological Society. 2008;14:673–690. doi: 10.1017/S1355617708080983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerring JP, Brady KD, Chen A, Vasa R, Grados M, Bandeen-Roche KJ, Denckla B, et al. Premorbid prevalence of ADHD and development of secondary ADHD after closed head injury. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37:647–654. doi: 10.1097/00004583-199806000-00015. [DOI] [PubMed] [Google Scholar]
- Ickowicz A, Schachar R, Sugarman R, Chen SX, Milette C, Cook L. The Parent Interview for Child Symptoms (PICS): A situation-specific clinical research interview for attention deficit hyperactivity and related disorders. Canadian Journal of Psychiatry. 2006;50:325–328. doi: 10.1177/070674370605100508. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Scholl M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD) Brain Injury. 2000;14:859–875. doi: 10.1080/026990500445691. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Chen S, Swank PR, Ewing-Cobbs L, Barnes M, Dennis M, Schachar R, et al. Response inhibition after traumatic brain injury (TBI) in children: Impairment and recovery. Developmental Neuropsychology. 2005;28(3):829–848. doi: 10.1207/s15326942dn2803_5. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Robertson G, Li X, Ewing-Cobbs L, Dennis M, Swank P, et al. Abnormal CT after mild traumatic brain injury predicts cognitive sequelae in school aged children. Journal of Neurosurgery: Pediatrics. 2008;1:461–470. doi: 10.3171/PED/2008/1/6/461. [DOI] [PubMed] [Google Scholar]
- Levin HS, Mendelsohn D, Lilly MA, Fletcher JM, Culhane KA, Chapman SB, Eisenberg HM, et al. Tower of London performance in relation to magnetic resonance imaging following closed head injury in children. Neuropsychology. 1994;8:171–179. [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: a users’ guide to the stop signal paradigm, in Inhibitory processes in attention, memory and language. In: Dagenback D, Carr TH, editors. Inhibitory processes in attention, memory and language. New York: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Mattson AJ, Levin HS. Frontal lobe dysfunction following closed head injury. A review of the literature Journal of Nervous and Mental Disease. 1990;178(5):279–341. doi: 10.1097/00005053-199005000-00002. [DOI] [PubMed] [Google Scholar]
- Max JE, Arndt S, Castillo CS, Bokwa H, Robin DA, Lindgren SD, Matheis PJ, et al. Attention Deficit Hyperactivity symptomatology after traumatic brain injury: a prospective study. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37:841–847. doi: 10.1097/00004583-199808000-00014. [DOI] [PubMed] [Google Scholar]
- Max JE, Koele SL, Smith WL, Sato Y, Lindgren SD, Robin DA, Arndt S, et al. Psychiatric disorders in children and adolescents after severe traumatic brain injury: a controlled study. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37:832–840. doi: 10.1097/00004583-199808000-00013. [DOI] [PubMed] [Google Scholar]
- Max JE, Lansing AE, Koele KL, Castillo CS, Hirokazu B, Schachar R. Attention deficit hyperactivity disorder in children and adolescents following traumatic brain injury. Developmental Neuropsychology. 2004;25(1&2):159–177. doi: 10.1080/87565641.2004.9651926. [DOI] [PubMed] [Google Scholar]
- Max JE, Lindgren SD, Knutson C, Pearson CS, Ihring D, Welborn A. Child and adolescent traumatic brain injury: psychiatric findings from a paediatric outpatient specialty clinic. Brain Injury. 1997;11:699–711. doi: 10.1080/026990597123070. [DOI] [PubMed] [Google Scholar]
- Nigg JT. The ADHD response-inhibition deficit as measured by the stop task: replication with DSM-IV combined type, extension, and qualification. Journal of Abnormal Child Psychology. 1999;27:393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- Oosterlann J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD+CD, anxious, and control children: a meta-analysis of studies with the stop task. Journal of Child Psychology and Psychiatry. 1998;39:411–425. [PubMed] [Google Scholar]
- Ponsford J, Kinsella G. Attentional deficits following closed-head injury. Journal of Clinical and Experimental Neuropsychology. 1992;14:822–838. doi: 10.1080/01688639208402865. [DOI] [PubMed] [Google Scholar]
- Power T, Catroppa C, Coleman L, Ditchfield M, Anderson V. Do lesion site and severity predict deficits in attentional control after preschool traumatic brain injury (TBI)? Brain Injury. 2007;21(3):279–292. doi: 10.1080/02699050701253095. [DOI] [PubMed] [Google Scholar]
- Schachar R, Gordon LD, Robaey P, Chen S, Ickowicz A, Barr C. Restraint and Cancellation: Multiple inhibition deficits in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2007;35(2):229–238. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- Schachar R, Mota VL, Logan GD, Tannock R, Klim P. Confirmation of an inhibition control deficit in attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2000;28:227–235. doi: 10.1023/a:1005140103162. [DOI] [PubMed] [Google Scholar]
- Schachar R, Levin HS, Max JE, Purvis K. Attention deficit hyperactivity disorder symptoms and response inhibition deficit after closed head injury in children: do preinjury behavior and injury severity predict outcome? Developmental Neuropsychology. 2004;25:179–198. doi: 10.1080/87565641.2004.9651927. [DOI] [PubMed] [Google Scholar]
- Slaats-Willemse D, Swaab-Barneveld H, de Sonneville L, van der Meulen E, Buitellar J. Deficient Response Inhibition as a Cognitive Endophenotype of ADHD. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(10):1242–1248. doi: 10.1097/00004583-200310000-00016. [DOI] [PubMed] [Google Scholar]
- Slomine BS, Salorio CF, Grados MA, Vasa RA, Christensen JR, Gerring JP. Differences in attention, executive functioning, and memory in children with and without ADHD after severe traumatic brain injury. Journal of the International Neuropsychological Society. 2005;11:645–653. doi: 10.1017/S1355617705050769. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Tannock R. Inhibitory control differences following mild head injury. Brain Cognition. 1999;41:411–416. doi: 10.1006/brcg.1999.1141. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Williams DH, Levin HS. Mild head injury classification. Neurosurgery. 1990;27:422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]


