Abstract
Cellular senescence is a state of permanent growth arrest and is thought to play a pivotal role in tumor suppression. Cellular senescence may play an important role in tumor suppression, wound healing, and protection against tissue fibrosis in physiological conditions in vivo. However, accumulating evidence that senescent cells may have harmful effects in vivo and may contribute to tissue remodeling, organismal aging, and many age-related diseases also exists. Cellular senescence can be induced by various intrinsic and extrinsic factors. Both p53/p21 and p16/RB pathways are important for irreversible growth arrest in senescent cells. Senescent cells secret numerous biologically active factors. This specific secretion phenotype by senescent cells may largely contribute to physiological and pathological consequences in organisms. Here I review the molecular basis of cell cycle arrest and the specific secretion phenotype in cellular senescence. I also summarize the current knowledge of the role of cellular senescence in vivo in physiological and pathological settings.
Keywords: cellular senescence, cell proliferation, senescence-associated secretory phenotype, inflammation, immortalization, age-associated diseases
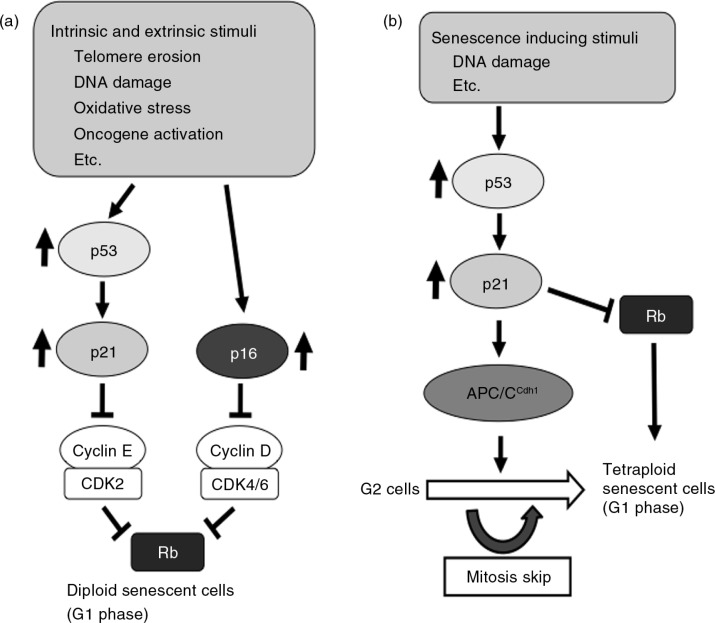
After undergoing a certain number of divisions, normal human diploid fibroblasts enter an irreversible non-dividing state, termed replicative senescence. Hayflick et al. reported that normal human diploid fibroblasts can divide 50–60 times but after that, they stop dividing irreversibly (1,2). The number of divisions that cells completely reach at the end of the replicative lifespan has been termed as the Hayflick limit. Senescent cells show enlarged and flattened morphology and the formation of a TOR-autophagy spatial coupling compartment (TASCC) in the cytoplasm (3,4) and senescence-associated heterochromatin foci (SAHF) in the nucleus (5–10). Active staining of senescence-associated β-galactosidase (SA-β-gal) is commonly used as a marker for cellular senescence (11). Senescence has been reported to occur in a number of other cell types such as keratinocytes (12), melanocytes (13), endothelial cells (14), epithelial cells (15), glial cells (16), adrenocortical cells (17), T lymphocytes (18), and even tissue stem cells (19). Replicative senescence is not dependent on chronological time in culture but rather depends on the number of divisions that cells undergo in culture (20–23). It is thought that telomere shortening, which occurs at each cell division because of incomplete replication, is the counting mechanism for the induction of replicative senescence (22,24). Telomeres become critically short after extensive division, and telomere ends are recognized as DNA double-strand breaks (25–27). This activates a DNA damage response (DDR), and cell division is then arrested by this activated DDR, mainly through p53 tumor suppressor activity. The expression of cyclin-dependent kinase (CDK) inhibitors, p21 and p16, is upregulated in senescent cells (28–35). p21 and p16 inhibit CDK2 (36–38) and CDK4/6 activities (39), respectively, and result in the activation of the tumor suppressor Rb, which is inactivated by CDK2 and CDK4/6 through phosphorylation. Activated Rb forms a complex with the E2F transcription factor, which is important for DNA synthesis and S phase progression of the cell cycle, and inhibits the E2F activity in senescent cells. Therefore, senescent cells cannot enter the S phase of the cell cycle and are basically maintained at the G1 phase of the cell cycle (40–42). p21 expression is transcriptionally regulated by p53, another important tumor suppressor (43). The senescence arrest is established and maintained through two major tumor suppressor pathways, p53/p21 and p16/RB (44–47), and it is now thought to be a barrier to malignant transformation (Fig. 1a).
Fig. 1.
Schematic diagram of cell cycle arrest in senescent cells. (a) Diploid senescent cells. In response to various intrinsic and extrinsic stimuli such as telomere erosion, DNA damage, oxidative stress, and activated oncogene overexpression, cells enter a senescent state. In senescent cells, CDK inhibitors, p21 and p16, are upregulated and the Rb protein is maintained in the active state. Active Rb inhibits the transition from the G1 to S phase of the cell cycle. (b) Tetraploid senescent cells. At the G2 phase of cell cycle, the p53/p21 pathway is activated in the cells exposed to senescence, inducing stimuli. APC/CCdh1 is prematurely activated via the accumulation of p21, and mitosis skip occurs in these cells. The Rb family of proteins is also important for the induction and maintenance of senescence.
A similar irreversible, non-dividing state, called cellular senescence, can be induced by the exposure of cells to excessive extrinsic stressors. These stimuli include strong mitogenic signals such as overexpression of activated oncogenes, DNA-damaging agents, oxidative stress, disruption of epigenetic regulation, and ectopic expression of tumor suppressors (48–55). This is called stress-induced premature senescence (SIPS) (51). SIPS occurs independently of telomere shortening. Because there are many similarities when cells senesce in both cases, replicative senescence and SIPS, the term cellular senescence is commonly used to indicate both states of non-division.
In general, it has been thought that senescent cells were arrested in the G1 phase, although there was a report that replicatively senescent cells were arrested in both G1 and G2 phases (56). Recently, some groups have reported that p53 activation in the G2 phase in response to various senescence-inducing stimuli induces cellular senescence through mitosis skip (57–59). They elegantly used the FUCCI system and time-lapse live cell imaging and showed that these senescent cells are tetraploid (4N) but stay in the G1 phase of the cell cycle. This mitosis skip and senescence induction were mediated by p53-dependent premature activation of the anaphase-promoting complex/cyclosome and its coactivator Cdh1 (APC/CCdh1). Activation of p53 at the G2 phase in response to senescence-inducing stimuli resulted in the induction of p21 that inhibited CDK1 and CDK2 activities. This inhibition by p21 led to premature activation of APC/CCdh1 and degradation of various mitotic regulators to skip mitosis (Fig. 1b). p16 was required for the maintenance of the senescent state but not for the induction of the mitosis skip. These results suggest that the p53-dependent cell fate is determined by the cell cycle stage in which p53 is activated.
Senescence-associated secretory phenotype
Another important characteristic of senescent cells is that the expression of many genes largely changes during senescence. Senescent cells secrete numerous biologically active factors, including the proinflammatory cytokines interleukin (IL)-6 and IL-8, chemokines [monocyte chemoattractant proteins (MCPs), macrophage inflammatory proteins, growth-regulated protein alpha (GROα)], growth factors [vascular endothelial growth factor (VEGF), granulocyte/macrophage-colony stimulating factor, transforming growth factor-beta (TGF-β)], and proteases [matrix metalloproteinases (MMPs)] (60,61). Because these factors act in autocrine and paracrine manners and have pleiotropic effects for surrounding cells, they may affect the surrounding microenvironment as well as the senescent cell itself and may be involved in tissue remodeling in organisms. This is called senescence-associated secretory phenotype (SASP) (62) or senescence messaging secretome (SMS) (63). Because SASP has complex and divergent effects, this may explain the role of cellular senescence in organismal aging and the incidence of age-related diseases and pathologies. There are now many reports that indicate senescent cells accumulate in aged and disease-related tissues (64–67). This suggests that cellular senescence actively contributes to the aging process and progression of some diseases at the organismal level. It has been suggested that the low-level chronic inflammation often observed during aging in tissues without obvious infection is due to senescent cells and SASP (68–70).
It is now evident that senescence can be transmitted to normal cells by SASP in a paracrine or autocrine manner (71). Acosta et al. showed that SASP induced by oncogene-induced senescence (OIS) can induce paracrine senescence in normal cells using both coculture systems in vitro and human and mouse models of OIS. TGF-β, VEGF, CCL2, and CCL20 are among SASP components that were identified as the factors that mediate paracrine senescence. TGF-β was a major player for the induction of p21 and p15INK4b that contributed to cell growth arrest in paracrine senescent cells. The secretion of mature forms of IL-1 is increased during OIS, suggesting that the inflammasome is activated in oncogene-induced senescent cells (72). In fact, components of the inflammasome such as caspase-1, ASC, and NLRP3 were increased at the protein level during OIS. Finally, they showed that inhibitors for caspase-1 or IL-1 receptor were downregulated SASP components during OIS. This suggested that the activation of the inflammasome is directly involved in the expression of SASP. Inflammasome and IL-1 signaling are activated in senescent cells, and IL-1 that is induced from senescent cells by SASP is also involved in paracrine senescence.
SASP is mainly linked to DDR or epigenomic disruption (60,73, 74). SASP is not recognized in normal senescent cells that have ectopically overexpressed p21 or p16, although these cells undergo a senescence growth arrest and show several other features of senescent cells (75). It has been reported that glucocorticoid treatment of senescent cells suppresses the secretion of several SASP components, including some proinflammatory cytokines, without affecting tumor suppressive growth arrest (76). This finding indicates that growth suppression and SASP in senescent cells are segregated processes. ATM, Chk2, and NBS1, which are involved in DDR, are important for the initiation and maintenance of SASP. Importantly, these proteins contribute to SASP after the establishment of persistent DNA damage signaling (73). The rapid robust DDR that is activated immediately after DNA damage does not induce SASP. On the other hand, p53, which is located downstream of ATM and Chk2, suppresses SASP and knockdown of p53, resulting in augmented expression of IL-6.
SASP is mainly mediated by transcription factors such as nuclear factor-kappa B (NF-kB) (77–79) and CCAAT/enhancer binding protein beta (C/EBPβ) (72). An initial step in SASP involves the transcriptional activation of IL-1α in response to senescence-inducing stimuli. A cell surface-bound isoform of this cytokine binds to its plasma membrane-associated IL-1 receptor, which in turn activates a downstream signaling cascade to stimulate NF-kB and C/EBPβ transcription factors (80). These transcription factors in turn activate the transcription of genes that encode various SASP proteins such as IL-6 and IL-8.
Epigenetic regulation of SASP induction has been described (81). This involves a decrease in the expression of DNA methyltransferase 1 (DNMT1), which is observed during senescence (82). The authors showed that IL-6 and IL8 expression related to SASP was induced by the knockdown of DNMT1 in normal human fibroblasts. The ubiquitination of G9a/GLP, H3K9 methyltransferases, by APC/CCdh1 ubiquitin ligase was induced in response to decreased expression of DNMT1, followed by proteasomal degradation of G9a/GLP. Consequently, H3K9me2 levels in transcriptional regulatory regions of IL-6 and IL-8 genes decreased and their expression was activated. It was also confirmed that the expression of G9a/GLP decreases and the expression of IL-6 and GROα mRNA is reduced in the lung, spleen, and intestine of aged mice. More recently, it has been reported that SIRT1, an NAD+-dependent histone deacetylase, is involved in suppressing the expression of SASP components such as IL-6 and IL-8 through deacetylating histones around the promoter regions of these genes (83).
Cellular senescence: beneficial effects
Tumor suppression
Although tumor-derived or virally transformed cells proliferate indefinitely in culture, normal cells enter senescence after reaching a typical number of divisions. Genetic studies using cell fusion technology by which normal human cells were fused with various immortal cell lines demonstrated that the resulting hybrids could not proliferate indefinitely (84–87). This result indicated that the senescent phenotype is dominant and suggested that immortal cells appear by defects in genes or pathways involved in growth arrest to escape cellular senescence. This was the first evidence for a role of cell senescence in tumor suppression (84).
In 1997, Serrano et al. reported that the overexpression of oncogenic H-Ras (H-RasG12V) in normal human, mouse, and rat fibroblasts induces growth arrest along with the accumulation of p53 and p16, similar to cellular senescence (48). They also showed that p53/p21 and Rb/p16 pathways are important for OIS because the inactivation of either p53 or p16 prevents Ras-induced growth arrest. Because OIS cannot be bypassed by the ectopic expression of hTERT (88), it is obvious that OIS is independent of telomere erosion. There is accumulating evidence that cellular senescence functions as a barrier against transformation and prevents the expansion of precancerous cells in vivo. Senescent cells can be identified in premalignant tumors in vivo because they are positive for SA-β-gal and express p16 (82,89–91). Braig et al. showed that the methylation of lysine 9 of histone H3 by Suv39h1 is important for the induction of cellular senescence in a T-cell lymphoma model using Eµ-N-Ras transgenic mice. The incidence of T-cell lymphomas strikingly increased by a defect in Suv39h1 in this model. Michaloglou et al. showed that mutant BRAFE600, which has an oncogenic mutation from valine to glutamic acid, induces cellular senescence in normal melanocytes along with an accumulation of SA-β-gal-positive senescent cells, which express mutant BRAFE600. Inactivation of senescence pathways by the deletion or mutation of tumor suppressor genes such as p53 or Rb as well as oncogene expression is required for the progression to malignant tumors (92,93). Although cells that have defects in the tumor suppressors PTEN and NF1 can still senesce, these cells easily transform into malignant tumors by the inactivation of other genes such as p53 (94,95). Interestingly, it has been reported that the reactivation of functional p53 in some mouse tumor models causes the induction of cellular senescence and tumor regression (96,97).
Wound healing
Cellular senescence is also important for wound healing in the skin. Fibroblasts are recruited into injury sites and differentiate into myofibroblasts, specialized contractile fibroblasts, which deposit extracellular matrix for repair. At the end of wound healing, the matricellular protein CCN1, which is highly expressed in affected areas, binds to its receptor, integrin α6β1, and activates the production of oxidative stress in myofibroblasts (98). Increased oxidative stress causes myofibroblast senescence during wound healing in the skin, which protects against progression to excessive fibrosis. Indeed, in mice expressing a mutant CCN1 that cannot bind to integrins, the wounds had fewer senescent cells and resulted in significantly more fibrosis.
Recently, Demaria et al. reported on a beneficial role of SASP by senescent cells in wound healing using a new transgenic mouse model (99). They generated a transgenic mouse line expressing the 3MR (trimodality reporter) fusion protein using a p16 promoter. This fusion protein contains functional domains of a synthetic Renilla luciferase (LUC) to identify senescent cells in vivo, monomeric red fluorescent protein (mRFP) to isolate senescent cells by fluorescence activated cell sorting, and truncated herpes simplex virus 1 thymidine kinase (HSV-TK) to selectively kill senescent cells by adding ganciclovir (GCV). Using this mouse model, they showed that senescent cells are transiently induced at the injury site during cutaneous wound healing and the effective elimination of senescent cells by GCV results in a significant delay in wound healing. This result indicated that senescent cells that are transiently induced at the injury site accelerate skin repair. They also found that platelet-derived growth factor AA (PDGF-AA), which is secreted as SASP from senescent cells at the injury site, is a key factor for wound closure. PDGF-AA secreted from senescent cells was involved in the differentiation of non-senescent fibroblasts into myofibroblasts, which plays a critical role in wound contraction during wound healing. Topically applied recombinant PDGF-AA to wounds on senescent cell-eliminated p16-3MR mice significantly increased the percentage of myofibroblasts and restored wound closure, although this was not significant for the reduction of collagen deposition (fibrosis). They speculated that other SASP factors such as proteases may contribute to the reduced fibrosis. This clearly indicates that SASP of senescent cells has a beneficial effect in physiological situations.
Liver fibrosis
Liver fibrosis occurs as a result of excessive accumulation of extracellular matrix proteins, including collagen. Advanced liver fibrosis can result in cirrhosis and liver failure. The role of the senescence program in acute liver injury induced by a liver-damaging agent (CCl4) in vivo has been reported (100). Hepatic stellate cells are activated by damage and begin to produce the components of the extracellular matrix for repairing the damage. The stellate cells subsequently become senescent and secrete SASP factors, including MMPs, to repair the fibrotic scar. SASP associated with stellate cell senescence attracts immune cells and the senescent stellate cells are cleared by attracted natural killer cells. The clearance of senescent cells by immune cells attracted by SASP factors, which are secreted by senescent cells, also seems to be an important step in halting tissue repair when the process is completed. In mice deficient in the p53/p21 or p16/Rb pathways, stellate cells continue to proliferate and do not enter senescence, and fibrosis in the liver is markedly increased. Therefore, it is thought that cellular senescence is important for controlling tissue repair and the maintenance of the integrity of the organ.
Cardiac fibrosis
Cellular senescence also plays a pivotal role in the regulation of cardiac fibrosis after myocardial infarction (MI) in a mouse model (101). Senescent cardiac fibroblasts accumulated in infarcted hearts 1 week after MI in wild-type mice. This was accompanied by upregulation of the senescence markers p53, p16, and p21 as well as SA-β-gal activity. Importantly, in p53-deficient mice, the accumulation of senescent fibroblasts, macrophage infiltration, and MMPs such as MMP2 and MMP9 were significantly reduced; however, collagen deposition was enhanced after MI. This result indicated that p53-mediated cellular senescence is important for limiting cardiac collagen deposition and cardiac fibrosis.
Developmentally programmed senescence
More recently, an interesting finding that cellular senescence occurs during development, and senescent cells are most likely to be involved in promoting tissue remodeling during development has been reported (102,103). The senescent cells were identified throughout the mouse embryo, including the mesonephros, apical ectodermal ridge, neural roof plate, and endolymphatic sac of the inner ear. The authors suggested that embryonic senescent cells are important for tissue growth and organ formation during development. Embryonic senescent cells were highly dependent on p21 but not p53 and DNA damage. Indeed, the expression of p21 was positively regulated by TGF-β/SMAD and PI3K/FOXO pathways. p21-deficient mice had defects in embryonic senescence, although apoptosis partially compensated outcomes by loss of senescence because of p21 deficiency. Developmental senescent cells also shared an expression profile with OIS, including SASP factors. Because developmental senescent cells were cleared by infiltrating macrophages during tissue remodeling, SASP in these senescent cells plays a pivotal role in tissue remodeling during development.
Apart from embryonic development, it is known that cellular senescence occurs in a physiologically programmed manner. Physiological senescence is induced in normal megakaryocytes (104) and placental syncytiotrophoblasts (105) during their maturation.
Cellular senescence: detrimental effects
Tumor promotion
Although cellular senescence suppresses malignant transformation, secreted factors by SASP such as inflammatory cytokines (IL-1, IL-6, IL-8, MCP2, and others), proteases (MMPs and others), and growth factors (VEGF and others) (60,72) may facilitate the progression of surrounding tumor cells and accelerate metastasis by affecting the tissue microenvironment (106–109). Coinjection of senescent fibroblasts with mouse or human epithelial tumor cells into immunocompromised mice significantly stimulated tumor growth (106,107,110). SASP factors secreted from senescent cells can also stimulate precancerous cells to obtain more malignant phenotypes, including epithelial to mesenchymal transition (EMT), which accelerates the invasion and migration of tumor cells into tissues (111). There are reports that IL-6 and IL-8 secreted by senescent cells contribute to EMT in premalignant epithelial cells (60,111,112). Some proteases such as MMPs, which are secreted by senescent cells by SASP, may also contribute to tissue remodeling and tumor cell migration. Similar upregulation of proteases is also observed in some tumor cells. Secreted proteases from senescent cells in the microenvironment of cancer tissues may accelerate tumor migration in a coordinated manner with tumor-derived proteases.
Age-related degenerative phenotypes
It is thought that senescent cells are implicated in many age-associated degenerative diseases in both normal and pathological situations. Senescent cells in tissues are most likely to affect the normal tissue structure and tissue integrity through SASP. To elucidate whether senescent cells can drive age-associated degenerative pathology, Baker et al. (113) have produced a transgenic mouse line in which senescent cells during the progression of age-related disorders could be eliminated by the administration of a drug. In this model, termed INK-ATTC (apoptosis through targeted activation of caspase), a transgene expresses the p16 promoter-driving ATTC fusion protein (caspase 8 fused to the FK506-binding protein). Upon the administration of the inducer AP20187, the fusion protein dimerizes, thereby activating caspase 8 activity, and p16-positive senescent cells are specifically killed by apoptosis. INK-ATTC mice were crossed with the hypomorphic BubR1 (BubR1H/H) progeroid mice because these mice have a markedly shortened lifespan in comparison with most mice. Although the elimination of p16-positive senescent cells by the administration of the drug did not cause extension of lifespan in this progeroid model, it significantly delayed the onset of age-related phenotypes such as sarcopenia, cataracts, and loss of subcutaneous fat. More importantly, this improvement was able to be achieved even in late-life clearance of the p16-positive senescent cells. This study provided the first direct evidence that senescent cells contribute to the progression of tissue pathology during aging.
Adipocyte senescence, obesity, and diabetes
Obesity is a condition in which excess fat is accumulated in the body and has a negative effect on health. Obesity is most commonly caused by excessive food intake and/or low physical activity, leading to the accumulation of white adipose tissues. Excess accumulation of fat in adipocytes triggers an inflammatory response in adipose tissue and results in the initiation of systemic pathological processes (114). Undesired adipokines such as TNF-α are produced by this inflammatory response and lead to insulin resistance and type 2 diabetes (115–117). SA-β-gal-positive senescent cells are accumulated in the adipose tissues obtained from obese mice or humans, and this is accompanied by the accumulation of p53 and p21, upregulation of SASP factors, and infiltration of inflammatory cells (118). Interestingly, the deletion of the p53 gene in mice reduced senescent cells in adipose tissue, reduced the production of inflammatory cytokines, and improved insulin resistance induced by a high-fat diet (HFD). These results suggested that p53 is activated in adipose tissues in obesity, induces senescence in adipocytes, and these adipocytes produce inflammatory cytokines because of SASP. Therefore, adipocyte senescence is associated with obesity and is tightly linked to pathological consequence led by obesity.
It is known that insulin resistance initially causes compensatory proliferation of β cells during the pathogenesis of type 2 diabetes. It is generally thought that this compensatory proliferation eventually leads to proliferative exhaustion of β cells and diabetes. The number of β cells and their proliferating rate were significantly increased in C57BL/6J mice 4 months after feeding HFD. However, at 12 months after feeding HFD, proliferative β cells were reduced, oxidative stress was increased, and SA-β-gal-positive senescent β-cells were significantly increased in β-cell islets (119). This strongly supports the idea that the cellular senescence of β cells contributes to the pathogenesis of diet-induced diabetes.
Atherosclerosis
Cellular senescence has been implicated in the development of vascular pathologies, particularly atherosclerosis. SA-β-gal-positive senescent endothelial cells are recognized in advanced atherogenic plaques on human coronary arteries (120). It is also recognized that the expression of endothelial nitric oxide synthase (eNOS) decreases and the expression of proinflammatory factors as well as p53, p21, and p16 increases in these pathological blood vessels. The induction of premature senescence through the p53/p21-dependent pathway has been observed in human vascular smooth muscle cells (VSMCs) by angiotensin II (Ang II) treatment in a mouse model of atherosclerosis (121). This was accompanied by the production of proinflammatory factors from senescent VSMCs via SASP by Ang II treatment. The deficiency of p21 markedly reduced the production of proinflammatory factors by Ang II treatment and prevented the development of atherosclerosis in this mouse model.
However, the beneficial effect of cellular senescence on atherosclerosis has also been reported. Mouse models deficient in cell cycle regulators such as p53 (122), p21 (123), p27 (124), and ARF (125) show augmented susceptibility to the development of atherosclerosis, whereas the overexpression of p53 in mice protects them from mechanically induced neointimal thickening in femoral arteries but not native atherosclerosis (126). Further investigation is required to resolve this discrepancy.
Other diseases associated with cellular senescence
It is thought that other human diseases such as sarcopenia (127–130), osteoarthritis (131,132), and pulmonary fibrosis (133,134) are associated with cellular senescence. Astrocyte senescence has also been proposed to be involved in the pathogenesis of Alzheimer's disease (135) and Parkinson's disease (136). Reports that indicate cellular senescence associated with human diseases has been increasing. Cellular senescence may contribute to more pathologies in many age-associated diseases under both beneficial and detrimental circumstances.
Concluding remarks
Cellular senescence is a state of essentially irreversible growth arrest, and it has been proposed that this has developed as an antitumor mechanism. In addition to growth arrest, senescent cells secret numerous proinflammatory factors through SASP. There is now much evidence that SASP of senescent cells contributes to the pathogenesis of age-associated diseases. It has been speculated that the accumulation of senescent cells in tissues accelerates tissue remodeling triggered by SASP factors, reduces tissue integrity and function, and contributes to organismal aging. In fact, senescent cells are found in many tissues under pathological conditions or advanced aging. It has also been shown that senescent cells have a positive impact in vivo. For example, cellular senescence coordinates the process of tissue remodeling in some physiological situations. In the case of embryonic development, cellular senescence occurs throughout the embryo and functions to promote tissue remodeling. There is some evidence that transiently induced senescent cells protect the progression of pathogenesis in some diseases and, in fact, may have more beneficial functions in vivo than what is thought.
Further investigation of the molecular mechanism of cellular senescence, particularly in vivo, promises to open a new avenue for achieving healthy aging and establishing a new strategy to prevent age-associated diseases.
Conflict of interest and funding
The author has no conflicts of interest. This work was supported by Grants-in-Aid for Scientific Research (KAKENHI Grant Number 24613006).
References
- 1.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 3.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–70. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young AR, Narita M, Narita M. Spatio-temporal association between mTOR and autophagy during cellular senescence. Autophagy. 2011;7:1387–8. doi: 10.4161/auto.7.11.17348. [DOI] [PubMed] [Google Scholar]
- 5.Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–16. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell. 2005;8:19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Narita M, Krizhanovsky V, Nunez S, Chicas A, Hearn SA, Myers MP, et al. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126:503–14. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 8.Adams PD. Remodeling of chromatin structure in senescent cells and its potential impact on tumor suppression and aging. Gene. 2007;397:84–93. doi: 10.1016/j.gene.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funayama R, Ishikawa F. Cellular senescence and chromatin structure. Chromosoma. 2007;116:431–40. doi: 10.1007/s00412-007-0115-7. [DOI] [PubMed] [Google Scholar]
- 10.Kosar M, Bartkova J, Hubackova S, Hodny Z, Lukas J, Bartek J. Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16(ink4a) Cell Cycle. 2011;10:457–68. doi: 10.4161/cc.10.3.14707. [DOI] [PubMed] [Google Scholar]
- 11.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–43. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 13.Bandyopadhyay D, Timchenko N, Suwa T, Hornsby PJ, Campisi J, Medrano EE. The human melanocyte: a model system to study the complexity of cellular aging and transformation in non-fibroblastic cells. Exp Gerontol. 2001;36:1265–75. doi: 10.1016/s0531-5565(01)00098-5. [DOI] [PubMed] [Google Scholar]
- 14.Thornton SC, Mueller SN, Levine EM. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983;222:623–5. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- 15.Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr Biol. 1999;9:939–45. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 16.Blomquist E, Westermark B, Ponten J. Ageing of human glial cells in culture: increase in the fraction of non-dividers as demonstrated by a minicloning technique. Mech Ageing Dev. 1980;12:173–82. doi: 10.1016/0047-6374(80)90093-7. [DOI] [PubMed] [Google Scholar]
- 17.McAllister JM, Hornsby PJ. Improved clonal and nonclonal growth of human, rat and bovine adrenocortical cells in culture. In Vitro Cell Dev Biol. 1987;23:677–85. doi: 10.1007/BF02620980. [DOI] [PubMed] [Google Scholar]
- 18.Effros RB, Walford RL. T cell cultures and the Hayflick limit. Hum Immunol. 1984;9:49–65. doi: 10.1016/0198-8859(84)90006-5. [DOI] [PubMed] [Google Scholar]
- 19.Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20:870–80. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dell'Orco RT, Mertens JG, Kruse PF., Jr. Doubling potential, calendar time, and senescence of human diploid cells in culture. Exp Cell Res. 1973;77:356–60. doi: 10.1016/0014-4827(73)90588-0. [DOI] [PubMed] [Google Scholar]
- 21.Roberts TW, Smith JR. The proliferative potential of chick embryo fibroblasts: population doublings vs. time in culture. Cell Biol Int Rep. 1980;4:1057–63. doi: 10.1016/0309-1651(80)90042-9. [DOI] [PubMed] [Google Scholar]
- 22.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 23.Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay JW, et al. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220:194–200. doi: 10.1006/excr.1995.1306. [DOI] [PubMed] [Google Scholar]
- 24.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 25.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–8. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 26.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–56. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 27.d'Adda di Fagagna F, Teo SH, Jackson SP. Functional links between telomeres and proteins of the DNA-damage response. Genes Dev. 2004;18:1781–99. doi: 10.1101/gad.1214504. [DOI] [PubMed] [Google Scholar]
- 28.Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–8. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 29.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–67. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–4. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs JJL, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–8. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 32.Stein GH, Drullinger LF, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–17. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour C, Jacobs JJ, et al. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 2003;23:389–401. doi: 10.1128/MCB.23.1.389-401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobs JJ, de Lange T. Significant role for p16INK4a in p53-independent telomere-directed senescence. Curr Biol. 2004;14:2302–8. doi: 10.1016/j.cub.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs JJ, de Lange T. p16INK4a as a second effector of the telomere damage pathway. Cell Cycle. 2005;4:1364–8. doi: 10.4161/cc.4.10.2104. [DOI] [PubMed] [Google Scholar]
- 36.Gu Y, Turck CW, Morgan DO. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366:707–10. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- 37.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 38.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–4. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 39.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–7. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 40.Seshadri T, Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990;247:205–9. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- 41.Stein GH, Beeson M, Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990;249:666–9. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- 42.Stein GH, Drullinger LF, Robetorye RS, Pereira-Smith OM, Smith JR. Senescent cells fail to express cdc2, cycA, and cycB in response to mitogen stimulation. Proc Natl Acad Sci USA. 1991;88:11012–16. doi: 10.1073/pnas.88.24.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 44.Gorman SD, Cristofalo VJ. Reinitiation of cellular DNA synthesis in BrdU-selected nondividing senescent WI-38 cells by simian virus 40 infection. J Cell Physiol. 1985;125:122–6. doi: 10.1002/jcp.1041250116. [DOI] [PubMed] [Google Scholar]
- 45.Hara E, Tsurui H, Shinozaki A, Nakada S, Oda K. Cooperative effect of antisense-Rb and antisense-p53 oligomers on the extension of life span in human diploid fibroblasts, TIG-1. Biochem Biophys Res Commun. 1991;179:528–34. doi: 10.1016/0006-291x(91)91403-y. [DOI] [PubMed] [Google Scholar]
- 46.Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33–9. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- 47.Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–23. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- 48.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 49.Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J. 1998;332(Pt 1):43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen QM. Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints. Ann N Y Acad Sci. 2000;908:111–25. doi: 10.1111/j.1749-6632.2000.tb06640.x. [DOI] [PubMed] [Google Scholar]
- 51.Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35:927–45. doi: 10.1016/s0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 52.Ramirez RD, Morales CP, Herbert BS, Rohde JM, Passons C, Shay JW, et al. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 2001;15:398–403. doi: 10.1101/gad.859201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright WE, Shay JW. Historical claims and current interpretations of replicative aging. Nat Biotechnol. 2002;20:682–8. doi: 10.1038/nbt0702-682. [DOI] [PubMed] [Google Scholar]
- 54.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 55.Sedivy JM, Banumathy G, Adams PD. Aging by epigenetics – a consequence of chromatin damage? Exp Cell Res. 2008;314:1909–17. doi: 10.1016/j.yexcr.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mao Z, Ke Z, Gorbunova V, Seluanov A. Replicatively senescent cells are arrested in G1 and G2 phases. Aging (Albany NY) 2012;4:431–5. doi: 10.18632/aging.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johmura Y, Shimada M, Misaki T, Naiki-Ito A, Miyoshi H, Motoyama N, et al. Necessary and sufficient role for a mitosis skip in senescence induction. Mol Cell. 2014;55:73–84. doi: 10.1016/j.molcel.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Krenning L, Feringa FM, Shaltiel IA, van den Berg J, Medema RH. Transient activation of p53 in G2 phase is sufficient to induce senescence. Mol Cell. 2014;55:59–72. doi: 10.1016/j.molcel.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 59.Panopoulos A, Pacios-Bras C, Choi J, Yenjerla M, Sussman MA, Fotedar R, et al. Failure of cell cleavage induces senescence in tetraploid primary cells. Mol Biol Cell. 2014;25:3105–18. doi: 10.1091/mbc.E14-03-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: a link between cancer and age-related degenerative disease? Semin Cancer Biol. 2011;21:354–9. doi: 10.1016/j.semcancer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campisi J. Aging, cellular senescence, and cancer. Ann Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 64.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 65.Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Cecco M, Jeyapalan J, Zhao X, Tamamori-Adachi M, Sedivy JM. Nuclear protein accumulation in cellular senescence and organismal aging revealed with a novel single-cell resolution fluorescence microscopy assay. Aging (Albany NY) 2011;3:955–67. doi: 10.18632/aging.100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kreiling JA, Tamamori-Adachi M, Sexton AN, Jeyapalan JC, Munoz-Najar U, Peterson AL, et al. Age-associated increase in heterochromatic marks in murine and primate tissues. Aging Cell. 2011;10:292–304. doi: 10.1111/j.1474-9726.2010.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 70.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–90. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 73.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–9. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodier F, Munoz DP, Teachenor R, Chu V, Le O, Bhaumik D, et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coppe JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem. 2011;286:36396–403. doi: 10.1074/jbc.M111.257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laberge RM, Zhou L, Sarantos MR, Rodier F, Freund A, de Keizer PL, et al. Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell. 2012;11:569–78. doi: 10.1111/j.1474-9726.2012.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 78.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–48. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pazolli E, Alspach E, Milczarek A, Prior J, Piwnica-Worms D, Stewart SA. Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Res. 2012;72:2251–61. doi: 10.1158/0008-5472.CAN-11-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA. 2009;106:17031–6. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi A, Imai Y, Yamakoshi K, Kuninaka S, Ohtani N, Yoshimoto S, et al. DNA damage signaling triggers degradation of histone methyltransferases through APC/C(Cdh1) in senescent cells. Mol Cell. 2012;45:123–31. doi: 10.1016/j.molcel.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 82.Yamakoshi K, Takahashi A, Hirota F, Nakayama R, Ishimaru N, Kubo Y, et al. Real-time in vivo imaging of p16Ink4a reveals cross talk with p53. J Cell Biol. 2009;186:393–407. doi: 10.1083/jcb.200904105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayakawa T, Iwai M, Aoki S, Takimoto K, Maruyama M, Maruyama W, et al. SIRT1 suppresses the senescence-associated secretory phenotype through epigenetic gene regulation. PLoS One. 2015;10:e0116480. doi: 10.1371/journal.pone.0116480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pereira-Smith OM, Smith JR. Evidence for the recessive nature of cellular immortality. Science. 1983;221:964–6. doi: 10.1126/science.6879195. [DOI] [PubMed] [Google Scholar]
- 85.Pereira-Smith OM, Smith JR. Genetic analysis of indefinite division in human cells: identification of four complementation groups. Proc Natl Acad Sci USA. 1988;85:6042–6. doi: 10.1073/pnas.85.16.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith JR, Pereira-Smith OM. Replicative senescence: implications for in vivo aging and tumor suppression. Science. 1996;273:63–7. doi: 10.1126/science.273.5271.63. [DOI] [PubMed] [Google Scholar]
- 87.Tominaga K, Olgun A, Smith JR, Pereira-Smith OM. Genetics of cellular senescence. Mech Ageing Dev. 2002;123:927–36. doi: 10.1016/s0047-6374(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 88.Wei S, Sedivy JM. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 1999;59:1539–43. [PubMed] [Google Scholar]
- 89.Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–5. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 90.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 91.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–4. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 92.Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, et al. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997;11:2822–34. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bardeesy N, Morgan J, Sinha M, Signoretti S, Srivastava S, Loda M, et al. Obligate roles for p16(Ink4a) and p19(Arf)-p53 in the suppression of murine pancreatic neoplasia. Mol Cell Biol. 2002;22:635–43. doi: 10.1128/MCB.22.2.635-643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Courtois-Cox S, Genther Williams SM, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–72. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–5. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 97.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–85. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–33. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–67. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu F, Li Y, Zhang J, Piao C, Liu T, Li HH, et al. Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS One. 2013;8:e74535. doi: 10.1371/journal.pone.0074535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–30. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 103.Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–18. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 104.Besancenot R, Chaligne R, Tonetti C, Pasquier F, Marty C, Lecluse Y, et al. A senescence-like cell-cycle arrest occurs during megakaryocytic maturation: implications for physiological and pathological megakaryocytic proliferation. PLoS Biol. 2010;8:e1000476. doi: 10.1371/journal.pbio.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chuprin A, Gal H, Biron-Shental T, Biran A, Amiel A, Rozenblatt S, et al. Cell fusion induced by ERVWE1 or measles virus causes cellular senescence. Genes Dev. 2013;27:2356–66. doi: 10.1101/gad.227512.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–7. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–26. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 108.Bartholomew JN, Volonte D, Galbiati F. Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res. 2009;69:2878–86. doi: 10.1158/0008-5472.CAN-08-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perrigue PM, Silva ME, Warden CD, Feng NL, Reid MA, Mota DJ, et al. The histone demethylase jumonji coordinates cellular senescence including secretion of neural stem cell-attracting cytokines. Mol Canc Res. 2015;13:636–50. doi: 10.1158/1541-7786.MCR-13-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–74. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 111.Laberge RM, Awad P, Campisi J, Desprez PY. Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. 2012;5:39–44. doi: 10.1007/s12307-011-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–96. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–6. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Ann Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 115.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 116.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15:1082–7. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 119.Sone H, Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia. 2005;48:58–67. doi: 10.1007/s00125-004-1605-2. [DOI] [PubMed] [Google Scholar]
- 120.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–4. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 121.Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–60. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 122.Mercer J, Figg N, Stoneman V, Braganza D, Bennett MR. Endogenous p53 protects vascular smooth muscle cells from apoptosis and reduces atherosclerosis in ApoE knockout mice. Circ Res. 2005;96:667–74. doi: 10.1161/01.RES.0000161069.15577.ca. [DOI] [PubMed] [Google Scholar]
- 123.Khanna AK. Enhanced susceptibility of cyclin kinase inhibitor p21 knockout mice to high fat diet induced atherosclerosis. J Biomed Sci. 2009;16:66. doi: 10.1186/1423-0127-16-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Diez-Juan A, Andres V. The growth suppressor p27(Kip1) protects against diet-induced atherosclerosis. FASEB J. 2001;15:1989–95. doi: 10.1096/fj.01-0130com. [DOI] [PubMed] [Google Scholar]
- 125.Gonzalez-Navarro H, Abu Nabah YN, Vinue A, Andres-Manzano MJ, Collado M, Serrano M, et al. p19(ARF) deficiency reduces macrophage and vascular smooth muscle cell apoptosis and aggravates atherosclerosis. J Am Coll Cardiol. 2010;55:2258–68. doi: 10.1016/j.jacc.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 126.Sanz-Gonzalez SM, Barquin L, Garcia-Cao I, Roque M, Gonzalez JM, Fuster JJ, et al. Increased p53 gene dosage reduces neointimal thickening induced by mechanical injury but has no effect on native atherosclerosis. Cardiovasc Res. 2007;75:803–12. doi: 10.1016/j.cardiores.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 127.Kudryashova E, Kramerova I, Spencer MJ. Satellite cell senescence underlies myopathy in a mouse model of limb-girdle muscular dystrophy 2H. J Clin Invest. 2012;122:1764–76. doi: 10.1172/JCI59581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, Olwin BB. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 2014;20:265–71. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–64. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–21. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 131.Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 132.Martin JA, Brown TD, Heiner AD, Buckwalter JA. Chondrocyte senescence, joint loading and osteoarthritis. Clin Orthop Relat Res. 2004;(427 Suppl):S96–103. doi: 10.1097/01.blo.0000143818.74887.b1. [DOI] [PubMed] [Google Scholar]
- 133.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration. 2010;80:59–70. doi: 10.1159/000268287. [DOI] [PubMed] [Google Scholar]
- 134.Salazar LM, Herrera AM. Fibrotic response of tissue remodeling in COPD. Lung. 2011;189:101–9. doi: 10.1007/s00408-011-9279-2. [DOI] [PubMed] [Google Scholar]
- 135.Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, et al. Astrocyte senescence as a component of Alzheimer's disease. PLoS One. 2012;7:e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chinta SJ, Lieu CA, Demaria M, Laberge RM, Campisi J, Andersen JK. Environmental stress, ageing and glial cell senescence: a novel mechanistic link to Parkinson's disease? J Intern Med. 2013;273:429–36. doi: 10.1111/joim.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]