Abstract
Objective(s):
Brucella spp. are facultative intracellular pathogens which can stay alive and multiply in professional and nonprofessional phagocytes. Immunity against Brucella melitensis involves antigen-specific CD4+ and CD8+ T-cells activation and humoral immune responses. Due to negative aspects of live attenuated vaccines, much attention has been focused on finding Brucella-protective antigens to introduce them as potential subunit vaccine candidates.
Materials and Methods:
A chimeric gene encoding trigger factor (TF), Omp3148-74 and BP2687-111 fragments (TOB) from B. melitensis was successfully cloned, expressed in Escherichia coli BL21-DE3 and purified by Ni-NTA agarose column. Antibodies to recombinant TOB (rTOB) have been investigated in Brucella-infected human sera and a pool serum prepared from B. melitensis-vaccinated rabbits.
Results:
Our results showed that the immunized rabbit pool serum strongly reacted with rTOB. In addition, antibodies against rTOB were detectable in 76.5% of sera obtained from infected patients.
Conclusion:
These findings suggest that rTOB may provide a potential immunogenic candidate which could be considered in future vaccine studies.
Keywords: Brucellamelitensis, ELISA, Fusion protein, Immune reactivity, Vaccine
Introduction
Brucella spp. are Gram-negative and facultative intracellular bacteria which are classified in the α-proteobacteria family causing brucellosis, a worldwide zoonotic disease which causes Malta fever in humans and abortion in domestic animals (1). Brucellosis is endemic in many developing countries causes economic burden for health services (2). Brucella can duplicate in infected macrophage and monocyte by hampering phagosome-lysosome fusion (3). Brucellamelitensis Rev.1 and Brucella abortus S19 are attenuated smooth strains broadly used to prevent Brucella infection in livestock. These strains are currently considered as the best vaccines for the prevention of animal brucellosis (4). However, due to different problems caused by administration of Brucella live attenuated vaccine, a safer vaccine such as subunit vaccine with a good protection against B. melitensis is desirable (5-8).
In this regard, different components of B. melitensis have been proposed as candidates for subunit vaccines (9-13). Trigger factor (TF) protein is an ATP independent chaperone that exhibits peptidylprolyl-cis-trans-isomerase activities in vitro (14). TF has also been reported as an immunogenic antigen (15) that gives a very good protection against B. melitensis infection (11). Omp31, an outer membrane protein from B. melitensis, reacts with some but not all serum samples from Brucella spp. infected human, dog, sheep, rabbit, and rat (16, 17). Vaccination with Omp3148-74 provided the protection in mice against B. melitensis infection (18). A periplasmic protein of Brucella, BP26 is another antigen introduced as an important diagnostic antigen in brucellosis (19). BP26 presents an adjuvant activity that induces humoral and cellular responses in immunized mice with a reasonable protection (11). In addition, it has been reported that two epitopes of BP26 localized between amino acids 87-111 have essential reactivity with Brucella-infected sheep sera (20).
We previously designed a chimeric gene encoding TF, Omp3148-74 and BP2687-111(TOB) for expression in prokaryotic system. Considering immunological importance of this kind of fusion proteins, we decided to clone, express, and purify prokaryotic recombinant TOB (rTOB) and then applied it for detection of Brucella rTOB specific antibody response in both B. melitensis infected patients and Brucella or B. melitensis-vaccinated rabbit serum.
Materials and Methods
Bacterial strains and immunization
Escherichia coli strainsBL21-DE3 and DH5α (Novagen, Madison, WI, USA) were provided by Avicenna Research Institute, Tehran, Iran. All E. coli strains were grown in Luria Bertani (LB) broth supplemented with kanamycin (50 μg/ml) (Sigma, St Louis, USA) while shaken at 250 rpm at 37°C.
Design and construction of chimeric TOB
GenBank was the main reference for retrieval of the sequences of the gene encoding TF, Omp3148-74, and BP87-111. The genes were translated to protein and then the obtained protein sequence was back–translated to the corresponding nucleotide sequence based on E. coli codon usage. After that bioinformatic analyses were performed (21, 22). These fragments were used to code trivalent protein by linkers, the restriction sites for EcoRI, and HindIII enzymes at the 5’ and the 3’ ends, respectively. Linkers consisted of EAAAK repeats expected to form a monomeric hydrophobic α-helix were designed and used to separate the different domains. The coding sequence for target protein (552 amino acids) was verified by GenScript (Piscataway, NJ, USA) and synthesized by Biomatik (Ontario, Canada) into pUC57 cloning vector.
Expression of chimeric rTOB
The synthetic gene was subcloned into pET28a (+) with the 6X His-tag at the N-terminal and C-terminal expressed under the control of the T7 promoter. The pET-tob construct was transformed into competent E. coli strain BL21-DE3 and cells were inoculated into 5 ml of LB medium as starter. This culture was used to inoculate LB medium containing 50 μg/ml kanamycin. Then, the culture was grown at 37°C to an optical density (600 nm) of 0.5-0.7. Expression of the chimeric sequence was induced by the addition of 1mM (isopropyl-β-d-galactopyranoside) IPTG (Sigma). Bacterial pellet were collected by centrifugation (15,000 ×g, 20 min, 4°C) and kept at-70°C.
Determination of solubility of recombinant proteins
To determine solubility of the rTOB protein in phosphate buffered saline (PBS), pellets of 5 ml culture samples were re-suspended in 5 ml PBS and bacteria were broken by sonication 3 times with 100% power at 4°C. Lysates were centrifuged and supernatants and pellets were used in Western blot. Bacterial lysates were fractionated by electrophoresis on 12% SDS-PAGE gel and transferred to nitrocellulose membrane (Millipore Corporation, MA, USA). The blot was blocked overnight at 4°C with PBS containing 0.1% Tween-20 (PBS-T) and 5% nonfat dry milk and subsequently incubated with horseradish peroxidase (HRP) conjugated anti-his-tag polyclonal antibody (Roche,Mannheim, Germany) (1/40000) for 1 hr. The membrane was washed 3 times with PBS-T and the bound conjugates were then detected using 3, 3’diaminobenzidine (DAB) (Sigma) as the chromogen.
Purification of recombinant fusion protein
The rTOB protein was purified using Ni-NTA agarose (Qiagen,Hilden, Germany) under native condition. Purity was assessed by SDS-PAGE and coomassie blue staining. Endotoxin was removed from purified rTOB protein by a phase separation with Triton X-114 (21, 23, 24). This preparation had an endotoxin content of less than 0.05 endotoxin units per mg of protein assessed by a Limulusamebocyte lysate analysis kit (Lonza,Basel, Switzerland). Concentration of purified rTOB protein was obtained by Bradford method (25, 26).
Serum samples
Pool sera before and after immunization of 10 B. melitensis-immunized rabbit (gifted by Dr. Hojat Ahmadi, Pasteur Institute of Iran) were used in both Western blot and ELISA experiments. The study also included 34 serum samples from patients with brucellosis who referred to medical diagnostic laboratories in Tehran and were diagnosed on the basis of clinical, serological, and/or bacteriological findings. Blood cultures were performed for patients and 16 out of 22 patients were positive for Brucella infection (11 patients with B. melitensis and 5 with B. abortus). Standard tube agglutination was positive in all samples. The total IgG to Brucella smooth lipopolysaccharide (LPS) was identified in all patients by ELISA. In addition, the total IgG produced against B. abortus cytosolic proteins (CP) was detected in 28 patients by ELISA. Sera from 31 healthy volunteers with no record of brucellosis were used to obtain the cutoff value of the assay (17).
Western blot
The reactivity of the immunized rabbits’ pool serum with rTOB protein was investigated by Western blot. After transferring rTOB on nitrocellulose membrane, the membrane was incubated with immunized serum (1/2000) followed by HRP-conjugated goat anti-rabbit immunoglobulin (Avicenna Research Institute). The bands were visualized using enhanced chemilu-minescence (ECL), detection system (GE Healthcare) and exposed by ECL Hyperfilm.
ELISA
ELISA 96-well plate (Greinerbio-one, Fricken-hausen, Germany) was coated with 100 µl of 2.5 µg/ml rTOB re-suspended in 0.1 M PBS and then incubated overnight at room temperature. Additional wells were coated with 100 µl B. melitensis lysate at 1 µg/ml in PBS as positive controls. The plates were then washed 5 times with PBS plus 0.05% Tween 20 for 3 min each time. Three hundred µl of 10% fetal bovine serum (FBS) in PBS were plated and incubated for 2 hr at room temperature. ELISA was then performed using 1:1000 dilutions of either normal or immunized rabbit pool sera and 1:200 dilutions of either normal or infected human sera. The plates were again washed with PBST as described earlier. One hundred µl of HRP-conjugated goat anti-rabbit immunoglobulin G (Avicenna Research Institute) (diluted 1/1000) or anti-human immunoglobulin G (Abcam, Cambridge, USA) (diluted 1/10000) were added to each well. The plates were again incubated for 1 hr at room temperature. TMB (Pishtaz Teb, Tehran, Iran) was added to produce a color change. The reaction was stopped after 10 min by the addition of 30 µl of 20% H2SO4. An ELISA plate reader (Bio-Tek Instruments, Winooski, Vt, USA) was used to read the absorbance at 492 nm. All samples were tested in duplicates, with average absorbance values being reported.
Statistical analysis
The statistical difference between two groups was analyzed by t-test and the data among several groups was analyzed by one factor analysis of variance (ANOVA) in SPSS 13.). P-values <0.05 were considered as statistically significant.
Results
Production of Recombinant Proteins
The rTOB was successfully expressed in pET-tob transformed E. coli BL21-DE3. Produced rTOB was purified by Ni-NTA and was analyzed by SDS-PAGE and Western blot (Figure 1). The rTOB specific band was located at correct size (62 kDa) as theoretically expected (Figure 1A and 1B). Moreover, purified rTOB had high purity without any detectable impurity. Twenty six mg of the recombinant protein was obtained from one liter cultivation. Representative result obtained for rTOB purification is shown in Figure 1.
Figure 1.
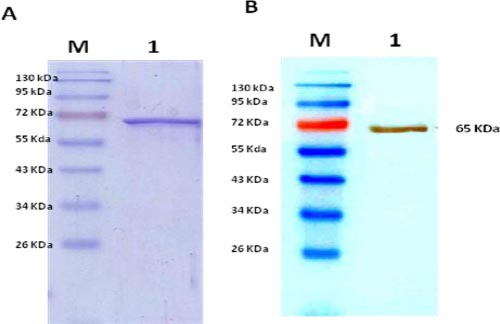
SDS- PAGE (A) and Western blot (B) analyses of purified rTOB protein
1; Purified rTOB protein, M; protein marker, HRP-conjugated anti-His-tag polyclonal antibody was applied in Western blot.
Screening of rTOB protein with immunized rabbits pool serum
Immunized rabbits but not pre-immunized pool sera strongly reacted with B. melitensis lysate and at a lower extent with the rTOB in ELISA (Figure 2A). The rTOB protein reacted strongly with the immunized rabbit serum in Western blot (Figure 2A).
Figure 2.
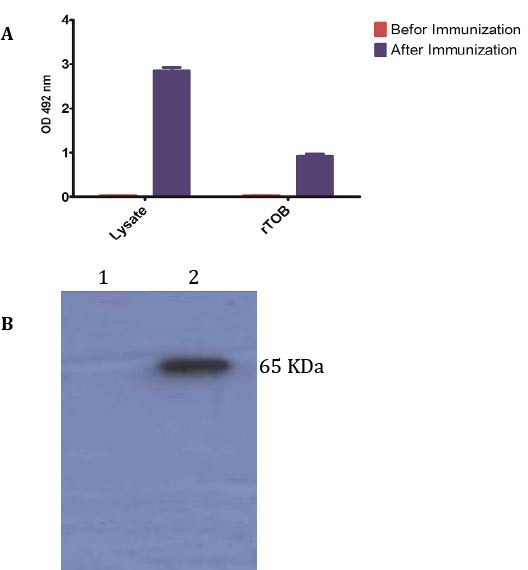
Immune reactivity analysis of vaccinated-rabbits pool serum to rTOB and Brucella melitensis lysate by ELISA (A) and Western blot (B).
Screening of rTOB protein with human sera
A cut-off value of 0.483 was calculated from healthy controls that yielded absorbance between 0.025 and 0.406 (mean, 0.101; SD, 0.105) by ELISA (Figure 3). Samples from patients with brucellosis yielded ODs between 0.22 and 1.16 (mean, 0.729; SD, 0.308). Totally, 26 patients (76.5%) were positive for antibodies against rTOB including 10 cases of B. melitensis infection and3 cases of B. abortus infection. According to the duration of the disease patients were divided into two groups as the latest brucellosis patients (up to 1 year since diagnosis) or the earlier period brucellosis patients (more than 1 year since diagnosis). Group 1 consisted of 15 patients as latest brucellosis patients, of which 13 patients had clinical manifestations. Of 19 patients placed in group 2 as earlier period brucellosis patients, 10 patients showed clinical manifestations. In group 1, antibodies against rTOB were detected in 11 patients compared to 15 patients in another group. Our results showed that there was no significant difference between these two groups for anti-rTOB reactivity (Figure 3).
Figure 3.
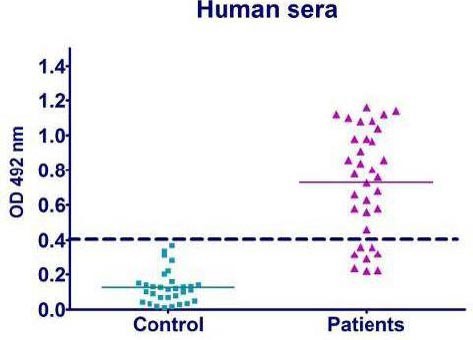
Reactivity of antibodies against rTOB in sera obtained from patients with brucellosis and control subjects
Antibodies were determined by an indirect ELISA. Each symbol represents a serum sample from one healthy person or patient. The cutoff value of the assay is indicated by the broken line.
Discussion
B. melitensis is the most pathogenic species in humans and causes abortions in sheep, goats, and cows. The live attenuated B. melitensis Rev.1 and B. abortus S19 have been mostly used for vaccination of sheep and goats (27, 28). However, the antibody responses elicited by those two live vaccines are difficult to differentiate from naturally Brucella infected animals using the conventional serological tests. This makes it difficult to discriminate between infected and vaccinated animals by standard serological tests. In addition, due to irregular problems caused by attenuated vaccine, its application is forbidden in countries free of B. melitensis. These problems comprise occasional induction of abortion when administered during pregnancy, pathogenicity for humans, and resistance to streptomycin which is one of the preferred antibiotics for treatment of brucellosis (29). In order to avoid these drawbacks, alternative vaccination approaches like subunit vaccines are desired. To find a new subunit vaccine, new immunodominant proteins and epitopes are needed to be identified. In this regards, much works were done on different components of Brucella (9-11, 13, 30, 31) and fortunately, there is valuable information on some antigens of Brucella spp. TF is a cytoplasmic protein of B. melitensis that can give an acceptable protection in mice against B. melitensis (11). It has been also reported that the two linear epitopes of BP26 located between position 87 and 111 have 65–70% reactivity with Brucella infected sheep sera (20). In addition, the presence of BP26 enhanced antibody response to the chaperone protein TF, suggests that BP26 can be incorporated with other relevant antigens as adjuvant in the vaccination protection against Brucella infection (11). A monoclonal antibody (mAb) against Omp31 provided an acceptable passive protection when administered alone (28). Further studies showed that mAb could recognize epitope located in a hydrophilic loop situated between amino acids 43 and 83 (46) and is conserved among strains of different geographic origins (47). Serological assay of candidate antigens by vaccinated animals or infected human was used as an additional tool to choose candidate selection for subunit vaccines (15-17). Specifically reactive antibodies showed that the proteins were immunogenic and thus it may be possible that play an important role to the outcome of infection (32).
In this study, the chimeric gene encoding TF, Omp3148-74, and BP2687-111 was cloned into an expression vector. In silico analysis was applied to find the best condition for expressing of fusion protein in E. coli. The fusion protein was expressed in E. coli and successfully purified from soluble fraction. Then, the presence of antibodies against rTOB was evaluated in sera from patients with brucellosis and B. melitensis-vaccinated rabbit pool serum. Analysis of rTOB interaction with immunized rabbit pool serum showed that rTOB could react with antibodies in the serum much more strongly than pre-immunized rabbit pool serum as determined by ELISA. As shown in Figure 3, antibodies to rTOB were detected in approximately 76.5% of infected patients regardless of the infecting species (B. melitensis or B. abortus). The frequency of detection was similar for patients with latest or earlier period brucellosis (73% and 79%, respectively). Notably, the frequency of antibodies against rTOB in human was similar and higher than amounts obtained from assessment of two linear epitopes of BP26 P11-KLH and P129-KLH, respectively, with Brucella infected sheep sera (20). In another study, it was shown that rOmp31 only reacted with 47.3% of infected human sera with Brucella spp. (17) that was lower than our results. In this regard immunoreactive soluble proteins from the B. melitensis would provide new antigen candidates for the development of subunit vaccines (33, 34). Thus, our results suggest that the rTOB fusion protein could be considered as a candidate vaccine in further vaccine experiments in animal models.
Conclusion
In summary, our results showed that rTOB was expressed in soluble form in E. coli and could be purified by Ni-NTA agarose. This fusion protein reacted strongly with sera from patients with brucellosis and B. mlitensis-vaccinated rabbits pool serum as judged by ELISA and Western blot. Thus, we think this new fusion protein could be considered as a candidate for using in vaccine experiments. Further studies are needed to establish this notion which is the theme of our future research.
Acknowledgment
This work was supported by Molecular Biology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
Footnotes
Conflict of interest
The authors declare that they have no competing interest.
References
- 1.Liang L, Leng D, Burk C, Nakajima-Sasaki R, Kayala MA, Atluri VL, et al. Large scale immune profiling of infected humans and goats reveals differential recognition of Brucella melitensis antigens. PLoS Negl Trop Dis. 2010;4:e673. doi: 10.1371/journal.pntd.0000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain-Gupta N, Contreras-Rodriguez A, Smith GP, Garg VK, Witonsky SG, Isloor S, et al. Immuno-therapeutics to prevent the replication of Brucella in a treatment failure mouse model. Vaccine. 2014;32:918–923. doi: 10.1016/j.vaccine.2013.12.058. [DOI] [PubMed] [Google Scholar]
- 3.Sanakkayala N, Sokolovska A, Gulani J, Hogenesch H, Sriranganathan N, Boyle SM, et al. Induction of antigen-specific Th1-type immune responses by gamma-irradiated recombinant Brucella abortus RB51. Clin Diagn Lab Immunol. 2005;12:1429–1436. doi: 10.1128/CDLI.12.12.1429-1436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avila-Calderon ED, Lopez-Merino A, Sriranganathan N, Boyle SM, Contreras-Rodriguez A. A history of the development of Brucella vaccines. Biomed Res Int. 2013;2013:743509. doi: 10.1155/2013/743509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez G, Adams LG, Rice-Ficht A, Ficht TA. Host-Brucella interactions and the Brucella genome as tools for subunit antigen discovery and immunization against brucellosis. Front Cell Infect Microbiol. 2013;3:17. doi: 10.3389/fcimb.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durward MA, Harms J, Magnani DM, Eskra L, Splitter GA. Discordant Brucella melitensis antigens yield cognate CD8+T cells in vivo. Infect Immun. 2010;78:168–176. doi: 10.1128/IAI.00994-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitry MA, De Trez C, Goriely S, Dumoutier L, Akira S, Ryffel B, et al. Crucial role of gamma interferon-producing CD4+Th1 cells but dispensable function of CD8+T cell, B cell, Th2, and Th17 responses in the control of Brucella melitensis infection in mice. Infect Immun. 2012;80:4271–4280. doi: 10.1128/IAI.00761-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannella AP, Tsolis RM, Liang L, Felgner PL, Saito M, Sette A, et al. Antigen-specific acquired immunity in human brucellosis: implications for diagnosis, prognosis, and vaccine development. Front Cell Infect Microbiol. 2012;2:1. doi: 10.3389/fcimb.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Mariri A. Protection of BALB/c mice against Brucella melitensis 16 M infection induced by vaccination with live Escherchia coli expression Brucella P39 protein. Vaccine. 2010;28:1766–1770. doi: 10.1016/j.vaccine.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Cassataro J, Pasquevich KA, Estein SM, Laplagne DA, Velikovsky CA, de la Barrera S, et al. A recombinant subunit vaccine based on the insertion of 27 amino acids from Omp31 to the N-terminus of BLS induced a similar degree of protection against B. ovis than Rev.1 vaccination. Vaccine. 2007;25:4437–4446. doi: 10.1016/j.vaccine.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Hudson M, Walters N, Bargatze RF, Pascual DW. Selection of protective epitopes for Brucella melitensis by DNA vaccination. Infect Immun. 2005;73:7297–7303. doi: 10.1128/IAI.73.11.7297-7303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoover DL, Crawford RM, Van De Verg LL, Izadjoo MJ, Bhattacharjee AK, Paranavitana CM, et al. Protection of mice against brucellosis by vaccination with Brucella melitensis WR201(16MDeltapurEK) Infect Immun. 1999;67:5877–5884. doi: 10.1128/iai.67.11.5877-5884.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Letesson JJ, Tibor A, van Eynde G, Wansard V, Weynants V, Denoel P, et al. Humoral immune responses of Brucella-infected cattle, sheep, and goats to eight purified recombinant Brucella proteins in an indirect enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1997;4:556–564. doi: 10.1128/cdli.4.5.556-564.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferbitz L, Maier T, Patzelt H, Bukau B, Deuerling E, Ban N. Trigger factor in complex with the ribosome forms a molecular cradle for nascent proteins. Nature. 2004;431:590–596. doi: 10.1038/nature02899. [DOI] [PubMed] [Google Scholar]
- 15.Ghasemi A, Salari MH, Zarnani AH, Pourmand MR, Ahmadi H, Shirazi MH, et al. Immunogenicity assessment of Brucella mellitensis HSP and TF proteins by immunized rabbit serum. Iran J Allergy Asthma Immunol. 2013;12:192–194. [PubMed] [Google Scholar]
- 16.Ghasemi A, Salari MH, Zarnani AH, Pourmand MR, Ahmadi H, Mirshafiey A, et al. Immune reactivity of Brucella melitensis-vaccinated rabbit serum with recombinant Omp31 and DnaK proteins. Iran J Microbiol. 2013;5:19–23. [PMC free article] [PubMed] [Google Scholar]
- 17.Cassataro J, Pasquevich K, Bruno L, Wallach JC, Fossati CA, Baldi PC. Antibody reactivity to Omp31 from Brucella melitensis in human and animal infections by smooth and rough Brucellae. Clin Diagn Lab Immunol. 2004;11:111–114. doi: 10.1128/CDLI.11.1.111-114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassataro J, Estein SM, Pasquevich KA, Velikovsky CA, de la Barrera S, Bowden R, et al. Vaccination with the recombinant Brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4+T helper 1 response that protects against Brucella melitensis infection. Infect Immun. 2005;73:8079–8088. doi: 10.1128/IAI.73.12.8079-8088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu WX, Hu S, Qiao ZJ, Chen WY, Liu LT, Wang FK, et al. Expression, purification, and improved antigenic specificity of a truncated recombinant bp26 protein of Brucella melitensis M5-90: a potential antigen for differential serodiagnosis of brucellosis in sheep and goats. Biotechnol Appl Biochem. 2011;58:32–38. doi: 10.1002/bab.11. [DOI] [PubMed] [Google Scholar]
- 20.Qiu J, Wang W, Wu J, Zhang H, Wang Y, Qiao J, et al. Characterization of periplasmic protein BP26 epitopes of Brucella melitensis reacting with murine monoclonal and sheep antibodies. PLoS One. 2012;7:34246. doi: 10.1371/journal.pone.0034246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghasemi A, Ranjbar R, Amani J. In silico analysis of chimeric TF, Omp31 and BP26 fragments of Brucella melitensis for development of a multisubunit vaccine candidate. Iran J Basic Med Sci. 2014;17:172–180. [PMC free article] [PubMed] [Google Scholar]
- 22.Amani J, Mousavi SL, Rafati S, Salmanian AH. In silico analysis of chimeric espA, eae and tir fragments of Escherichia coli O157: H7 for oral immunogenic applications. Theor Biol Med Model. 2009;6:28. doi: 10.1186/1742-4682-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–195. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- 24.Petsch D, Anspach FB. Endotoxin removal from protein solutions. J Biotechnol. 2000;76:97–119. doi: 10.1016/s0168-1656(99)00185-6. [DOI] [PubMed] [Google Scholar]
- 25.Stoscheck CM. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- 26.Ghasemi A, Salari MH, Pourmand MR, Zarnani AH, Ahmadi H, Shirazi MH, et al. Optimization and Efficient Purification in Production of Brucella melitensis Recombinant HSP A and TF Proteins With Low Endotoxin Contents. Jundishapur J Microbiol. 2013;6:e6875. [Google Scholar]
- 27.Ghasemi A, Zarnani AH, Ghoodjani A, Rezania S, Salari MH, Jeddi-Tehrani M. Identification of a new immunogenic candidate conferring protection against Brucella melitensis infection in Mice. Mol Immunol. 2014;62:142–9. doi: 10.1016/j.molimm.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Niu J, Wang S, Lv Y, Wu Q. In vivo differences in the virulence, pathogenicity, and induced protective immunity of wboA mutants from genetically different parent Brucella spp. Clin Vaccine Immunol. 2013;20:174–180. doi: 10.1128/CVI.00573-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Skyberg JA, Cao L, Clapp B, Thornburg T, Pascual DW. Progress in vaccine development. Front Biol. 2013;8:60–77. doi: 10.1007/s11515-012-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Wang L, Yin J, Wang X, Cheng S, Lang X, et al. Immunoproteomic analysis of Brucella melitensis and identification of a new immunogenic candidate protein for the development of brucellosis subunit vaccine. Mol Immunol. 2011;49:175–184. doi: 10.1016/j.molimm.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Wu J, Qiao J, Weng Y, Zhang H, Liao Q, et al. Evaluation of humoral and cellular immune responses to BP26 and OMP31 epitopes in the attenuated Brucella melitensis vaccinated sheep. Vaccine. 2014;32:825–833. doi: 10.1016/j.vaccine.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 32.Commander NJ, Spencer SA, Wren BW, MacMillan AP. The identification of two protective DNA vaccines from a panel of five plasmid constructs encoding Brucella melitensis 16M genes. Vaccine. 2007;25:43–54. doi: 10.1016/j.vaccine.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Yin J, Guo D, Lang X, Wang X. Immunization of mice with recombinant S-adenosyl-L-homocysteine hydrolase protein confers protection against Brucella melitensis infection. FEMS Immunol Med Microbiol. 2011;61:159–167. doi: 10.1111/j.1574-695X.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 34.Ghasemi A, Jeddi-Tehrani M, Mautner J, Salari MH, Zarnani AH. Immunization of mice with a novel recombinant molecular chaperon confers protection against Brucella melitensis infection. Vaccine. 2014;32:6659–6666. doi: 10.1016/j.vaccine.2014.09.013. [DOI] [PubMed] [Google Scholar]


