Abstract
Objective(s):
The cholinergic system plays an important role in learning and memory. This study investigated the effects of curcumin (turmeric extract) and the cholinergic system and their interaction on memory retention of passive avoidance learning in adult male Wistar rats.
Materials and Methods:
At first, an injection cannula was implanted in right ventricles of the animals. One week after the surgery, the animals were trained with a shuttle box set up. Post-training, injections were performed in all experiments. Administration of curcumin increased memory retention. Also administrations of nicotine and pilocarpine, the cholinergic receptor agonists, increased memory retention, while it is decreased by succinylcholine and scopolamine, the cholinergic receptor antagonists. Then co-administration of curcumin and cholinergic drugs were performed. Intraperitoneal and intracerebroventricular injections were applied for the curcumin and cholinergic drugs, respectively.
Results:
Co-administration of curcumin (45 mg/kg) with a low dose of nicotine (0.1 µg/rat) or pilocarpine (0.5 µg/rat) increased memory retention significantly. Effects of succinylcholine (0.01, 0.1 and 0.5 µg/rat) or scopolamine (0.1, 1 and 5 µg/rat) were attenuated by curcumin markedly (45 mg/kg).
Conclusion:
The results suggest that curcumin has a close interaction with cholinergic system in memory retention process.
Keywords: Cholinergic system, Curcumin, Memory retention, Step through latency
Introduction
The role of cholinergic system in learning and memory was suggested for the first time by Carew et al in 1973 (1). Numerous studies have shown that central cholinergic system plays an important role in cognitive functions, and drugs affecting this system have been shown to change performance in tests of learning and memory (2-6). Alzheimr’s disease (AD) is a progressive neurodegenerative dysfunction of central cholinergic system, which causes decrease in cognitive functions (7). Senile plaques, neurofibrillary tangles, and extensive neural loss are the main histological hallmarks observed in AD brains (8). It has been shown that the levels of acetylcholine (Ach) and acetylcholinetransferase (AChT) reduce in AD brain (9).
Curcumin longa is a medicinal plant with a wide distribution throughout South and Southeast Asia, especially in Iran and India; turmeric is derived from the rhizome of this plant. Curcumin, the main part of turmeric, has neuroprotective effects on a variety of nervous system damage (10). It has been shown to be non-toxic and non-mutagenic and exhibits a wide spectrum of biological activities, especially anti-oxidation, anti-inflammation, anti-cancer, anti-apoptotic and anti-coagulation (11-13). Many studies have shown that curcumin is potent scavenger; it also improves learning and memory (14). Curcumin may protect cells from the beta amyloid attack and subsequent oxidative stress-induced damage in the antioxidant pathway (15, 16). Antioxidants prevent the oxidative damage and improve learning and memory in animal models (17, 18), and exhibit a strong free radicals scavenging activity (19). Beta-amyloid (Aβ), the most important constituent of senile plagues, increases free radical in AD brain (20). Free radicals cause phospholipid peroxidation, oxidative DNA damage and protein denaturation (21). By increasing H2O2, these free radicals reduce synaptic plasticity and inhibit long term potentiation (LTP) (22, 23). Curcumin shows antioxidant activity equivalent to vitamins C and E (24). It has been reported that curcumin crosses the blood-brain barrier and has anti-oxidant effects on the brain (25, 26). Therefore, the present study was designed to investigate the interaction between curcumin and cholinergic system on memory retention by behavioral studies to arrive at a conclusion.
Materials and Methods
Drugs
Curcumin (Sigma, England), absolute ethanol (Hamoon, Iran), nicotin (Sigma, England), pilocarpine (Sigma, England), succinylcholine (Sigma, England), scopolamine (Sigma, England), chlorhydrate (Merk, Germany), and saline (Hamoon, Iran) were used in the present study.
Animals
Adult male Wistar rats (weighing 250 to 300 g) were housed at 24±2 °C in a controlled environment with a 12:12 hr light: dark cycle, and they had access to standard laboratory food and water ad libitum. All rats were treated humanely and in compliance with the recommendations of the animals care committee for Lorestan University of Medical Sciences (Khorramabad, Iran).
Drugs-preparation and administrations
The powder of curcumin was dissolved in absolute ethanol and stored at -20 °C. For injection, it was administrated intraperitonealy (IP) at low, middle and high doses (5, 15 and 45 mg/kg). An insulin syringe was used to inject 0.2 ml of curcumin solution or vehicle (ethanol) IP. The cholinergic system drugs were dissolved in normal saline and applied (1 µl/rat) by intracerebroventricular injection (ICV). In the sham group, vehicle of cholinergic system drugs (%0.9 Saline) and vehicle of curcumin (ethanol) were injected ICV and IP, respectively.
Intracerebroventricular injection
The rats were anaesthetized with chlorhydrate (400 mg/kg) and placed in a stereotaxic frame. The rat skull was orientated according to Paxinos and Watson stereotaxic atlas (27). The Bregma suture was located after a sagittal incision, and holes were drilled with an electrical drill at the following co-ordination; 0.8 mm posterior to Bregma, 1.5 mm lateral to the sagittal suture and 3.7 mm ventral. A Hamilton syringe with a cannula of diameter of 0.3 mm was used to inject 1 µl of solutions of cholinergic system drugs or vehicle (saline). The injection was carried out in the brain right ventricle at a rate of 1 µl per min.
Behavioral study
The effects of cholinergic drugs alone or in combination with curcumin on the rat behavior were studied by passive avoidance learning test. Studies were begun 24 hr after the injections.
Passive avoidance learning apparatus
The apparatus (shuttle box) consisted of equal sized light and dark compartment (30×20×30 cm), separated by a guillotine door (7×9 cm) that could be raised to 10 cm. The floor was made from stainless steel (2.5 mm in diameter) and connected to a shock stimulator. A single electrical shock (50 Hz, 2 sec, 1mA intensity) was delivered to the grid floor of the dark compartment by a stimulator.
Training
A week after the surgery during which cannulas were implanted in the right ventricle, the animals were trained. Each animal was placed in the light compartment, and after 10 sec, the door was opened, and animals were allowed to enter a dark place. The habituation trial was repeated after 30 min. It followed by the acquisition trial after an additional 30 min. Immediately after entering the rat to the dark chamber with all the four feet inside, the door was closed and an electrical foot shock (1 mA, 50 Hz, 2 sec) was delivered. After 20 sec, the rats were removed from the dark chamber and returned to their own cages. Two minute later, the rats were re-tested in the same way. If the rats did not enter the dark compartment during 120 sec, successful acquisition of passive avoidance response would be recorded (3). The drugs were administrated immediately after training.
Retention test
Twenty-four hr after training, the retention test was performed. Each rat was placed in the light compartment for 10 sec, then the door was opened and the latency to enter to the dark compartment was measured and termed “step through latency” time (STL). The test was concluded when the animal entered the dark compartment or remained in the light site for 600 sec as an upper cut of time (3).
Histological study
After the step-through avoidance test, 1 µl of methylene blue solution (1%) was injected (ICV) to right ventricle. Then, the brains of rats were removed and were immersed in 4% neutral buffered paraformaldehyde for 24 hr. Then removed brains were cut to verify the site of injection.
Statistical analysis
Data were expressed as mean±standard error mean (SEM). The data of behavioral studies were analysed using one-way and two-way analysis of variance (ANOVA). If the F values were significant, the Tukey test was used to compare the experimental and control groups. P-values less than 0.05 were considered to be statistically significant. All statistical analysis was performed using SPSS statistical software package version 19.
Results
Effect of cholinergic drugs on memory retention in trained rats
Performance of the rats in the step through latency is shown in Table 1. We found that administrating of nicotine at doses 0.5 and 1 µg/rat (F3, 20=7.83, P<0.001) and pilocarpine at dose 1 µg/rat (F3, 20=5.84, P<0.01) improved memory compared to the control and sham groups, significantly.
Table 1.
Effect of different doses of cholinergic drugs on step through latency (mean±SEM)
| Control | 60.83±3.52 | ||
|---|---|---|---|
| Sham (saline 1 µ/rat) | 60.66±5.58 | ||
| Cholinergic drug (µg/rat) | Low dose | Middle dose | High dose |
| Nicotine (0.1, 0.5, 1) | 75.66±3.83 | 85.50±5.95* | 95.33±6.88*** |
| Pilocarpine (0.1, 0.5, 1) | 62.33±5.07 | 69±6.20 | 92.33±7.30** |
| Succinylcholine (0.01, 0.1, 0.5) | 66.83±4.51 | 19±3.68*** | 12.83±1.81*** |
| Scopolamine (0.1, 1, 5) | 47.66±4.38 | 41.16±4.40 | 7.33±2.01*** |
P<0.05,
P<0.01 and
P<0.001 significantly different from control and sham group (n=6)
Administration of succinylcholine as the antagonist of the cholinergic system in different doses (0.01, 0.1 and 0.5 µg/rat) showed that only low dose (0.01µg/rat) had no significant effect, but the other doses had significant effect on memory retention (F3, 20=45.27, P<0.001). Administration of scopolamine in 3 doses (0.1, 1 and 5 µg/rat) and its significant effect in high dose (5 µg/rat) on STL in comparison to the control and sham groups has been shown in Table 1 (F3, 20=23.39, P<0.001).
Effects of co-administration of curcumin and cholinergic agonist on memory retention in trained rats
We showed that administration of cholinergic agonist including nicotine at doses 0.5 and 1 µg/rat (P<0.05, P<0.001 respectively) and pilocarpine at dose 1 µg/rat (P<0.01) significantly improved memory in comparison to the control and sham groups (Table 1).
Figure 1 shows the effect of curcumin and its interaction with nicotine on memory retention. Analysis of data by one-way ANOVA indicated that high dose of curcumin (45 mg/kg) improved the memory, and the difference in STL was significant between ethanol group, curcumin (5 and 15 mg/kg) groups and curcumin (45 mg/kg) group (F4, 25=8.67, P<0.01). Co-administration of curcumin (15 and 45 mg/kg) and nicotine (0.1 µg/rat) had significant effect on memory retention (F7, 40=13.80, P<0.001).
Figure 1.
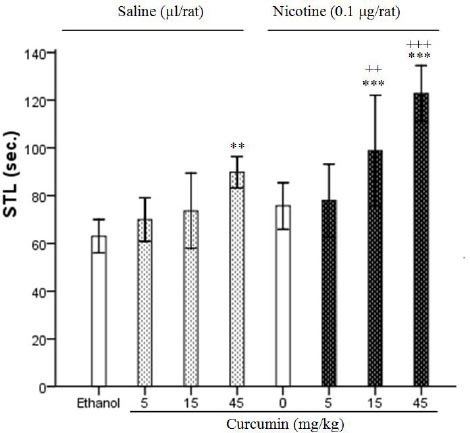
Effect of curcumin (5, 15 and 45 mg/kg) in the presence or absence a low dose of nicotine (0.1 µg/rat) on memory retention. Post-training injections of nicotine (ICV) at volume 1 µl/rat and curcumin (IP) at volume 0.2 ml/rat were done and step-through latencies were tested 24 hr later. Each column represents the mean±SEM for six rats. ** P<0.01, *** P<0.001 different from ethanol group. ++ P<0.01, +++ P<0.001 different from nicotine control group
Analysis of data by two-way ANOVA showed the effect of curcumin (5, 15 and 45 mg/kg) in the presence or absence of low dose of nicotine (0.1 µg/rat) on memory retention. Two-way ANOVA showed significant effects of administration of curcumin (5, 15 and 45 mg/kg), nicotine (0.1 µg/rat) and curcumin-nicotine within groups in Figure 1 (F1, 48=28.74, P<0.001; F3, 48=20.2, P<0.001; F3, 48=2.42, P<0.05, respectively).
When curcumin (5, 15 and 45 mg/kg) was injected with a low dose of pilocarpine (0.5 µg/rat), the STL was increased significantly (F7, 40=31.29, P<0.001). Analysis of data by two-way ANOVA showed the significant effects of administration of curcumin (5, 15 and 45 mg/kg), pilocarpine (0.5 µg/rat) and curcumin-pilocarpine within groups in Figure 2 (F1, 48=80.42, P<0.001; F3, 48=35.89, P<0.001; F3, 48=10.31, P<0.05, respectively).
Figure 2.
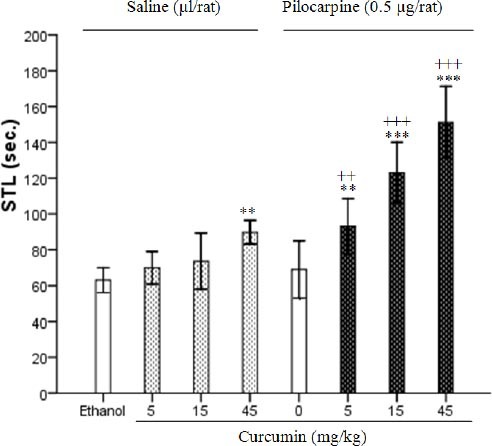
Effect of curcumin (5, 15 and 45 mg/kg) in the presence or absence a low dose of pilocarpine (0.5 µg/rat) on memory retention. Post-training injections of pilocarpine (ICV) at volume 1 µl/rat and curcumin (IP) at volume 0.2 ml/rat were done and step-through latencies were tested 24 hr later. Each column represents the mean±SEM for six rats. ** P<0.01, *** P<0.001 different from ethanol group. ++ P<0.01, +++ P<0.001 different from pilocarpine control group
Effects of co-administration of curcumin and cholinergic antagonist on memory retention in trained rats
We found that administration of cholinergic antagonist, including succinylcholine at doses 0.1 and 0.5 µg/rat (P<0.001) and scopolamine at dose 5 µg/rat (P<0.001) decreased memory retention in comparison to the control and sham groups, significantly (Table 1).
Results showed that effects of succinylcholine (0.01, 0.1 and 0.5 µg/rat) were attenuated by high doses of curcumin, and STL was increased markedly as compared to the succinylcholine sham group (F7, 40=67.24, P<0.001) (Figure 3). Analysis of data by two-way ANOVA showed the effects of administration of succinylcholine (0.01, 0.1 and 0.5 µg/rat), curcumin (45 mg/kg) and curcumin-succinylcholine within groups in Figure 3 (F1, 48=23.29, P<0.001; F3, 48=17.16, P<0.001; F3, 48=4.58, P<0.01, respectively).
Figure 3.
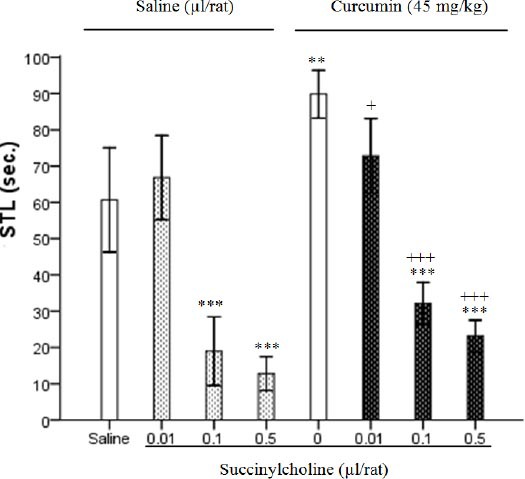
Effect of curcumin (45 mg/kg) in the presence or absence of succinylcholine (0.01, 0.1 and 0.5 µg/rat) on memory retention. Post-training injections of saline or succinylcholine (ICV) at volume 1 µl/rat were done. Ethanol or curcumin (IP) was injected 5 min after them at volume 0.2 ml/rat and step-through latencies (STL) were tested 24 hrs later. Each column represents the mean±SEM for six rats. **P<0.01, ***P<0.001 different from saline group. +P< 0.05, +++P<0.001 different from succinylcholine control group
Figure 4 shows effect of scopolamine alone or in combination with curcumin on STL. Result indicated that co-administration of curcumin (45 mg/kg) and scopolamine (5 µg/rat) has a significant effect and increased memory in comparison to the sham group (F7, 40=55.17, P<0.001) (Figure 4). Two-way ANOVA showed the effects of administration of scopolamine (0. 1, 1 and 5 µg/rat), curcumin (45 mg/kg) and curcumin-scopolamine within groups in Figure 4 (F1, 48=86.57, P<0.001; F3, 48=95.46, P<0.001; F3, 48=4.41, P<0.01 respectively).
Figure 4.
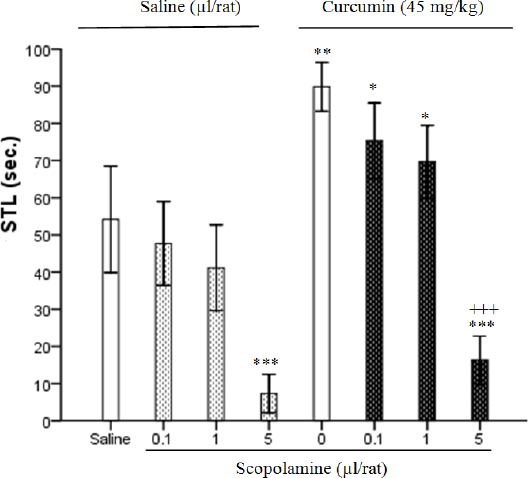
Effect of curcumin (45 mg/kg) in the presence or absence of scopolamine (0.1, 1 and 5 µg/rat) on memory retention. Post-training injections of saline or scopolamine (ICV) were done at volume 1 µl/rat. Ethanol or curcumin (IP) was injected 5 min after them at volume 0.2 ml/rat and step-through latencies (STL) were tested 24 hr later. Each column represents the mean±SEM for six rats. *P<0.05, **P<0.01, ***P<0.001 different from saline group. +++P<0.001 different from scopolamine control group
Discussion
This study showed that curcumin has a close interaction with cholinergic system and improves memory retention process in rats. In our studies, administration of curcumin at low, middle and high doses (5, 15 and 45 mg/kg) showed that low and middle doses (5 and 15 mg/kg) had no effects on the STL, but 45 mg/kg increases it, which indicates a significant increase in memory (Figure 1 and 2). In a previous study, Pan et al showed that curcumin at a dose of 200 mg/kg in mice and 20 to 40 µmol/L in cells was effective in blocking apoptosis, indicating that a low dose of curcumin may be effective in preventing Alzheimer’s disease. They investigated the neuroprotective effect of curcumin on the memory of AD mice (14). Kumar et al showed that chronic treatment with curcumin (10, 20 and 50 mg/kg) once daily for a period of 8 days beginning 4 days prior to 3-Nitropropionic acid (3-NP) administration, dose-dependently improved the 3-NP-induced motor and cognitive impairment (28). Several biological activities have been reported for curcumin (29)including, anti-inflammatory activity via down regulation of cyclo-oxygenase 2 and nitric oxide synthase through suppression of NF-kappa B activation (30). These reports suggested that curcumin, as a natural antioxidant and a neuroprotectant against the neurodegenerative disorders, is a potential compound for the prevention and treatment of AD. There are reports on the central nervous system penetration of curcumin (31, 32). Curcumin also attenuated the neuropathological changes in the hippocampus and inhibited apoptosis (14).
There is evidence indicating that the central cholinergic system play central roles in learning and memory processes mediated by both muscarinic and nicotinic receptors (33-36). The results of this study suggest that administration of acetylcholine agonist (pilocarpine and nicotine) increased learning and memory levels, but acetylcholine antagonist (scopolamine and succinylcholine) decreased them. Some studies have shown that during learning, the level of acetylcholine is increased in the amygdala, which plays an important role in memory consolidation (37). It appears that the cholinergic system is involved in mediating this process (37). The perfect performance of central cholinergic systems (nicotinic and muscarinic systems) is important for consolidation with shuttle box. Administration of acetylcholine agonist and antagonist via ICV affects the consolidation, in a dose-dependent manner (3). Our results confirm their reports.
The present study showed that co-administration of different doses of curcumin (5, 15 and 45 mg/kg) and a low dose of nicotine (0.1 µg/rat) or pilocarpine (0.5 µg/rat) increased learning and memory levels (Figure 1, 2). As shown in the result section, the effects of succinylcholine or scopolamine on memory were attenuated by curcumin (Figure 3, 4). It has been shown that curcumin could affect the AChE activation and increases the acetylcholine level in the hippocampus and frontal cortex (38). On the other hand, it has been shown that curcumin increases the expression of N-Methyl-D-Aspartate (NMDA) receptor gene, and decreases time to reach the platform in the Morris water maze test. Perhaps reinforcing effects of curcumin on cognitive function in animals is related to the regulating expression of NMDA receptor genes (39). Therefore, multiple mechanisms may be involved in the improvement of the memory retention, such as, antioxidant activity of curcumin and its effects on acetylcholinesterase and expression of NMDA receptor genes.
Conclusion
In summary, our study suggests that there is a close interaction between curcumin and cholinergic system, and curcumin may have protective effects on cholinergic system. Varieties of mechanisms are suggested for its effect, such as increasing the release of acetylcholine and its antioxidant property. Further studies are required to clarify its mechanism.
Acknowledgment
This study is part of MSc dissertation in Biology Department of Science and Research branch of Islamic Azad University of Tehran, Tehran, Iran.
References
- 1.Carew TJ, Hawkins RD, Kandel ER. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science. 1983;219:397–400. doi: 10.1126/science.6681571. [DOI] [PubMed] [Google Scholar]
- 2.Atri A, Sherman S, Norman KA, Kirchhoff BA, Hasselmo ME, Stern CE. Blockade of central cholinergic receptors impairs new learning and increases proactive interference in a word paired-associated memory task. Behav Neurosci. 2004;118:223–236. doi: 10.1037/0735-7044.118.1.223. [DOI] [PubMed] [Google Scholar]
- 3.Eidi A, Eidi M, Mahmoodi G, Oryan SH. Effect of vitamin E on memory retention in rats: Possible involvement of cholinergic system. Eur Neuropharmacol. 2006;16:101–106. doi: 10.1016/j.euroneuro.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Gupta L, Mediratta P, Bhattacharya SK. Effect of resveratrol on scopolamine-induced cognitive impairments in mice. Pharmacol Rep. 2012;64:438–444. doi: 10.1016/s1734-1140(12)70785-5. [DOI] [PubMed] [Google Scholar]
- 5.Kruk M, Tendera K, Bioela G. Memory-related effect of cholinergic receptor ligands in mice as measured by elevated plus maze test. Pharmacol Rep. 2011;63:1372–1382. doi: 10.1016/s1734-1140(11)70701-0. [DOI] [PubMed] [Google Scholar]
- 6.Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem. 2003;80:178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 7.Hardy J. Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 8.Hamaguchi T, Ono K, Yamada M. Curcumin and Alzheimer’s disease. CNS Neurosci & Therapeutics. 2010;16:285–297. doi: 10.1111/j.1755-5949.2010.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blokland A. Acetylcholine: a neurotransmitter for learning and memory? Brain Res Rev. 1995;21:285–300. doi: 10.1016/0165-0173(95)00016-x. [DOI] [PubMed] [Google Scholar]
- 10.Noorafshan A, Asadi-Golshan R, Karbalay-Doust S, Abdollahifar MA, Rashidiani-Rashidabadi A. Curcumin, the main part of turmeric, prevents learning and memory changes induced by sodium metabisulfite, a preservative agent, in rats. Exp Neurobiol. 2013;22:23–30. doi: 10.5607/en.2013.22.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal BB, Sandaram C, Malani N, Ichikawa H. Curcumin: The Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 12.Eptein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 13.Noorafshan A, Ashani-Esfahani S. A review of therapeutic effects of curcomin. Curr Pharm. 2013;19:2032–2046. [PubMed] [Google Scholar]
- 14.Pan R, Qiu S, Lu DX, Dong J. Curcumin improves learning and memory ability and its neuroprotective mechanicm in mice. Chin Med J. 2008;121:832–839. [PubMed] [Google Scholar]
- 15.Kim DS, Park SY, Kim JY. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from Aβ (1-42) insult. Neurosci Lett. 2001;303:57–61. doi: 10.1016/s0304-3940(01)01677-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhu YG, Chen XC, Chen ZZ, Zeng YQ, Shi GB, Su YH, et al. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol Sin. 2004;25:1606–1612. [PubMed] [Google Scholar]
- 17.Tamaddonfard E, Farshid AA, Asri-Rezaee S, Javadi Sh, Khosravi V, et al. Croin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2013;16:91–100. [PMC free article] [PubMed] [Google Scholar]
- 18.Sheibani V, Afarinesh M, Hajializadeh Z, Abbasnejad M, Haghpanah T, et al. Evaluation of Origanum vulgar L. ssp. Viridis leaves extract effect on discrimination learning and LTP induction in the CA1 region of the rat hippocampus. Iran J Basic Med Sci. 2010;14:177–184. [Google Scholar]
- 19.Qiao S, Li W, Tsubouchi R, Haneda M, Murakami K, Takeuchi F, et al. Rosmarinic acid inhibits the formation of reactive oxygen and nitrogen species in RAW264.7 macrophages. Free Radic Res. 2005;39:995–1003. doi: 10.1080/10715760500231836. [DOI] [PubMed] [Google Scholar]
- 20.Tran MH, Yamada K, Nakajima A, Mizuno M, Kamei H, et al. Tyrosine nitration of a synaptic protein synaptophysin contributes to amyloid beta-induced cholinergic dysfunction. Mol Psychiaty. 2003;8:407–412. doi: 10.1038/sj.mp.4001240. [DOI] [PubMed] [Google Scholar]
- 21.Blomhoff R. Antioxidants and oxidative stress. Tiddssr Nor Laegoforen. 2004;124:1643–1645. [PubMed] [Google Scholar]
- 22.Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26:3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson JB, Arnold MM, Ho YS, O’Dell TJ. Age-dependent modulation of hippocampal long-term potentiation by antioxidant enzymes. J Neurosci Res. 2006;84:1564–1574. doi: 10.1002/jnr.21040. [DOI] [PubMed] [Google Scholar]
- 24.Blumenthal M, Goldberg A, Brinkman J. Herbal Medicine: the expended commission E monographs. Newton. Integra Med Com. 2000 [Google Scholar]
- 25.Garcia–Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzhimer mouse model. J Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 26.Akram M, Shahab-uddin, Ahmad A, Khan U, Hanna A, et al. Curcuma longa and curcumin: a review article. Rom J Biol – Plant Biol. 2010;55:65–70. [Google Scholar]
- 27.Paxinos G, Watson C. 2nd ed. San Diego: Acadamic Press; 1986. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- 28.Kumar P, Padi SS, Nadiu PS, Kumar A. Possible neuroprotective machanisms of curcumin in attenuating 3-nitropropionic acid-induced neurotoxicity. Methods Find Exp Clin Pharmacol. 2007;29:19–26. doi: 10.1358/mf.2007.29.1.1063492. [DOI] [PubMed] [Google Scholar]
- 29.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin, a short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Bengmark S. Curcumin, a toxic antioxidant and natural NFkappaB, cyclooxygenase-2, lippoxygenase, and inducible nitric oxide synthase inhibitor: A shield against acute and chronic disease. JPEN J Parenter Enteral Nutr. 2006;30:45–51. doi: 10.1177/014860710603000145. [DOI] [PubMed] [Google Scholar]
- 31.Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr Alzheimer Res. 2005;2:131–136. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadav Rs, Sankwar ML, Shula PK, Chandra R, Pant AB, Islam F, Khanna V. Attenuation of arsenic neurotoxicity by curcumin in rats. Toxical Appl Pharmacol. 2009;240:367–376. doi: 10.1016/j.taap.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Caril M, Balducci C, Samanin R. Low doses of 8-OH-DPAT prevent the impairment of spatial learning caused by intra-hippocampal scopolamine through 5-HT (1A) receptors in the dorsal raphe. Br J Pharmacol. 2000;131:375–381. doi: 10.1038/sj.bjp.0703567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khajepour L, Rezayof A, Zarrindast MR. Involvement of dorsal hippocampal nicotinic receptors ion the effect of morphine on memory retrieval in passive avoidance task. Eur J Pharmacol. 2008;584:343–351. doi: 10.1016/j.ejphar.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Parfitt G, Campos RC, Barbosa AK, Koth AP, Barros DM. Paticipation of hippocampal cholinergic system in memory persistence for inhibitory avoidance in rats. Neurobiol Learn Mem. 2012;97:183–188. doi: 10.1016/j.nlm.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Ramkrez MJ, Honer WG, Minger SL, Francis PT. Changes in hippocampal SNAP-25 expression following afferent lessions. Brain Res. 2004;997:133–135. doi: 10.1016/j.brainres.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 37.McGaugh JL. The amygdala modulates the consoledation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 38.Ali EH, Arafa NM. Comparative protective action of curcumin, memantine and diclofenac against scopolamine-induced memory dysfunction. Fitoterapia. 2011;82:601–608. doi: 10.1016/j.fitote.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 39.Dong J, Lu DX, Pan R, Tang HM. Effect and mechanism of curcumin on learning and memory dysfunction induced by gp120 in rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008;24:328–331. [PubMed] [Google Scholar]


