Abstract
OBJECTIVE
This study tested the hypothesis that intensive treatment in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial disproportionately produced adverse outcomes in patients with diabetes with a high hemoglobin glycation index (HGI = observed HbA1c − predicted HbA1c).
RESEARCH DESIGN AND METHODS
ACCORD was a randomized controlled trial of 10,251 patients with type 2 diabetes assigned to standard or intensive treatment with HbA1c goals of 7.0% to 7.9% (53 to 63 mmol/mol) and less than 6% (42 mmol/mol), respectively. In this ancillary study, a linear regression equation (HbA1c = 0.009 × fasting plasma glucose [FPG] [mg/dL] + 6.8) was derived from 1,000 randomly extracted participants at baseline. Baseline FPG values were used to calculate predicted HbA1c and HGI for the remaining 9,125 participants. Kaplan-Meier and Cox regression were used to assess the effects of intensive treatment on outcomes in patients with a low, moderate, or high HGI.
RESULTS
Intensive treatment was associated with improved primary outcomes (composite of cardiovascular events) in the low (hazard ratio [HR] 0.75 [95% CI 0.59–0.95]) and moderate (HR 0.77 [95% CI 0.61–0.97]) HGI subgroups but not in the high HGI subgroup (HR 1.14 [95% CI 0.93–1.40]). Higher total mortality in intensively treated patients was confined to the high HGI subgroup (HR 1.41 [95% CI 1.10–1.80]). A high HGI was associated with a greater risk for hypoglycemia in the standard and intensive treatment groups.
CONCLUSIONS
HGI calculated at baseline identified subpopulations in ACCORD with harms or benefits from intensive glycemic control. HbA1c is not a one-size-fits-all indicator of blood glucose control, and taking this into account when making management decisions could improve diabetes care.
Introduction
The purpose of this ancillary study was to determine if the risk for diabetes complications in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) glycemia trial differed among individuals with lower or higher HbA1c levels than predicted by fasting plasma glucose (FPG). ACCORD participants were middle-aged and older people with type 2 diabetes and established cardiovascular disease (CVD) or known cardiovascular risk factors (1). The trial tested whether intensive treatment targeting HbA1c levels of less than 6% (42 mmol/mol) would reduce the rate of cardiovascular events compared with a strategy targeting HbA1c levels between 7.0% and 7.9% (53 and 63 mmol/mol). This hypothesis was not supported: Intensive treatment failed to improve primary cardiovascular outcomes and was instead associated with 22% greater total mortality compared with standard treatment. The ACCORD trial thus demonstrated that increased mortality was a previously unrecognized harm of intensive glucose-lowering therapy in high-risk patients with type 2 diabetes. Although symptomatic severe hypoglycemia was associated with an increased risk of death in the intensive and standard treatment groups, differences in HbA1c or rates of hypoglycemia between the two groups did not explain the greater mortality observed in the intensive-treatment group (2).
Treating patients with diabetes with drugs that lower blood glucose levels inherently increases the risk for hypoglycemia. Intensively treating patients with diabetes to a low HbA1c target implicitly assumes that all patients will have roughly the same blood glucose level when they reach the target. Miller et al. (3) paradoxically reported that ACCORD participants with higher HbA1c levels had greater risk for hypoglycemia. If HbA1c were an unbiased estimate of blood glucose, this observation would incongruously suggest that participants with higher blood glucose levels had greater risk for hypoglycemia. Numerous studies have shown, however, that some patients with diabetes have HbA1c levels that are persistently lower or higher than predicted compared with other individuals with similar blood glucose levels (4–10).
We reasoned that intensive treatment to a one-size-fits-all HbA1c target of less than 6% (42 mmol/mol) may have inadvertently and disproportionately produced adverse outcomes in a subgroup of ACCORD patients with diabetes with lower blood glucose levels than their HbA1c would predict. To test this hypothesis, we used the hemoglobin glycation index (HGI) to identify ACCORD participants with incongruous HbA1c and FPG at baseline. HGI is the calculated difference between an individual’s observed HbA1c and a predicted HbA1c derived by inserting the individual’s blood glucose concentration into a population regression equation describing the linear relationship between HbA1c and blood glucose (HGI = observed HbA1c − predicted HbA1c) (4,9).
Assessment of HGI in the Diabetes Control and Complications Trial (DCCT) showed that patients with type 1 diabetes with a high HGI had a threefold greater risk for retinopathy and a sixfold greater risk for nephropathy (6). Most prior HGI research calculated predicted HbA1c based on mean blood glucose (self-monitored (4,9), timed profiles (6), or continuous glucose monitoring (11)). HGIs calculated using all glucose data downloaded from patient meters were highly correlated with HGIs calculated using only prebreakfast glucose data (4). The feasibility of using FPG to calculate the HGI in patients with type 2 diabetes was previously proposed (12). The glycation gap developed by Cohen et al. (5) is calculated in exactly the same way as HGI except fructosamine replaces directly measured glucose for obtaining a predicted HbA1c. Several studies have shown that patients with type 2 diabetes with a high glycation gap have greater risk for microvascular or macrovascular complications (5,10,13,14). HGI and the glycation gap are strongly positively correlated, which suggests they reflect the same biological phenomenon (15).
Patients with diabetes with low and high HGI have HbA1c levels that are lower or higher than predicted, respectively, compared with other patients with similar blood glucose levels. We hypothesized that intensive treatment produced disproportionately lower blood glucose levels and increased hypoglycemia in the subgroup of ACCORD participants with a high HGI. This could explain the otherwise paradoxical results reported by Miller et al. (3). Also, higher baseline HbA1c and higher average on-treatment HbA1c were both strong predictors of mortality associated with intensive treatment in ACCORD (16,17). Because HGI was associated with increased complications risk in patients with type 1 diabetes in the DCCT, we also hypothesized that patients with type 2 diabetes with high HGI in ACCORD might also have a greater risk for primary cardiovascular outcomes, total mortality, and microvascular disease.
Research Design and Methods
This research was approved by the institutional review boards at the Louisiana State University Health Sciences Center and Children’s Hospital, New Orleans, LA.
ACCORD Study Design
ACCORD was a multicenter, randomized clinical trial that used a double 2 × 2 factorial design to incorporate intervention trials for glycemia, hyperlipidemia, and hypertension. The lipid intervention trial tested in 5,518 participants the hypothesis that a hypolipidemic agent would improve outcomes in subjects with good glycemic control. The blood pressure intervention trial tested in the remaining 4,733 participants the hypothesis that a therapeutic strategy that targets a systolic blood pressure of <120 mmHg will reduce the rate of CVD events compared with a strategy that targets a systolic blood pressure of <140 mmHg. All participants enrolled in the ACCORD trial had type 2 diabetes and had experienced a prior cardiovascular event or had other evidence of high risk for CVD. Median follow-up time was 5.0 years (mean 5.0; range 0.01–8.4). The rationale, study design, inclusion criteria, and other details of the ACCORD trial are described elsewhere (1,18–20).
The glycemia intervention trial included all 10,251 ACCORD participants, who were randomly assigned at baseline to a standard treatment group or an intensive treatment group. Any antihyperglycemic agent or combination of agents approved by regulatory authorities could be used as considered appropriate to achieve protocol-mandated target HbA1c levels of 7.0% to 7.9% (53 to 63 mmol/mol) in the standard treatment group, or less than 6.0% (42 mmol/mol) in the intensive treatment group. During the trial, excess mortality was demonstrated at 3.5 years of average follow-up, at which time all participants in the intensive treatment group were converted to the standard treatment regimen (20). Data for this ancillary study were obtained from the ACCORD coordinating center on all enrolled participants. Only data from participants with FPG and HbA1c recorded at baseline (n = 10,125) were used in these analyses.
Primary and Secondary Outcomes
The prespecified primary outcome for ACCORD was a composite of the first occurrence of nonfatal myocardial infarction (MI), nonfatal stroke, or death from cardiovascular causes (18). Causes of cardiovascular death included fatal MI, congestive heart failure, documented arrhythmia, death after invasive cardiovascular interventions, death after noncardiovascular surgery, fatal stroke, unexpected death due to ischemic CVD occurring less than 24 h after the onset of symptoms, and death due to other vascular diseases such as pulmonary emboli or abdominal aortic aneurysm rupture. Death from any cause was one of several prespecified secondary outcomes (1). Primary and secondary outcomes were assessed from baseline through the last set of scheduled study visits (March–June 2009) (20).
Definition of Severe Hypoglycemia
Symptomatic, severe hypoglycemia requiring any assistance was defined as an episode of hypoglycemia with a documented blood glucose concentration of less than 50 mg/dL (2.8 mmol/L) in which the participant reported receiving medical care or assistance from another individual or recovery with carbohydrate treatment. Symptomatic, severe hypoglycemia requiring medical assistance was defined as an episode of hypoglycemia in which the participant received care at a hospital or emergency department or from medical personnel. Hypoglycemia assessment included hypoglycemia events from baseline up to the transition period when intensive glycemic intervention was terminated (February 2008).
Deriving HGI from the HbA1c Versus FPG Regression Equation
Baseline FPG and HbA1c data from a random subsample of 1,000 ACCORD participants were used to estimate the linear relationship between FPG and HbA1c in the study population. A predicted HbA1c was calculated for the remaining 9,125 participants by inserting the baseline FPG into the subsample linear regression equation (HbA1c = 0.009 FPG [mg/dL] + 6.8). Baseline HGI was calculated by subtracting the predicted HbA1c from the observed HbA1c. The 9,125 participants were then assigned to low, moderate, or high HGI subgroups based on baseline HGI and HGI cut points that divided the population into three equally sized subgroups (low HGI ≤ −0.520 [n = 3,041], 33.3%; moderate HGI −0.520 to 0.202 [n = 3,042], 33.3%; high HGI >0.202 [n = 3,042], 33.3%). The use of a tertile classification system is by convention for consistency with previous HGI studies. We compared HGI classifications using simple linear regression with HGI classifications based on cubic spline regression and observed more than 90% identity. We chose the linear regression model because this approach is simpler and has precedent.
Statistical Analysis
Baseline characteristics and other variables were compared among low, moderate, and high HGI subgroups. Group comparisons used ANOVA or Kruskal-Wallis tests for normally distributed and nonnormally distributed continuous variables, respectively, and χ2 tests were used for categorical variables. Kaplan-Meier curves and log-rank tests were used to compare the distribution of time to first event. Post hoc analyses were performed to compare risk of primary outcomes, total mortality, and hypoglycemia between the intensive and standard glycemia treatment groups and among HGI subgroups with adjustment for covariates. Hazard ratios and 95% CI were determined by stratified Cox proportional hazards regression models. Tests of statistical significance were based on a two-tailed type 1 error at P < 0.05. The interaction term of HGI subgroup with glycemia treatment was added to each model, and a likelihood ratio test was applied. Whenever the interaction test did not meet the criteria for statistical significance, a Bonferroni correction for multiple comparisons was applied when evaluating the effect of treatment by subgroup. Proportional hazards assumptions were assessed by cumulative sums martingale residuals over follow-up times using Kolmogorov-type supremum tests and no violation was found.
Covariates included baseline characteristics, study location among the seven clinical-center networks, and for other ACCORD intervention assignments (the blood pressure trial, assignment to the intensive blood pressure intervention group, or the lipid trial, assignment to receive fibrate in the lipid trial). Sensitivity analyses were performed to determine hazard ratio stability after including intervention assignments in the Cox model as stratifying factors rather than as covariates. Baseline characteristics were age, sex, ethnicity, education level, medical history (smoking history, duration of diabetes, retinopathy detected at baseline, history of CVD, high risk of congestive heart failure, evidence of significant atherosclerosis, albuminuria), and laboratory and clinical measures at baseline (FPG, diastolic blood pressure, estimated glomerular filtration rate, LDL-cholesterol, HDL-cholesterol, and triglycerides). Outcomes based on Cox regression models were similar to those obtained using logistic regression. We used Cox regression in reporting our results to account for the fact that ACCORD participants were monitored for different lengths of time, leading to a changing denominator over time that is not adequately modeled by the constant average denominator imposed by the logistic model. Statistical analyses were performed using SAS 9.3 or STATA 13 software.
Results
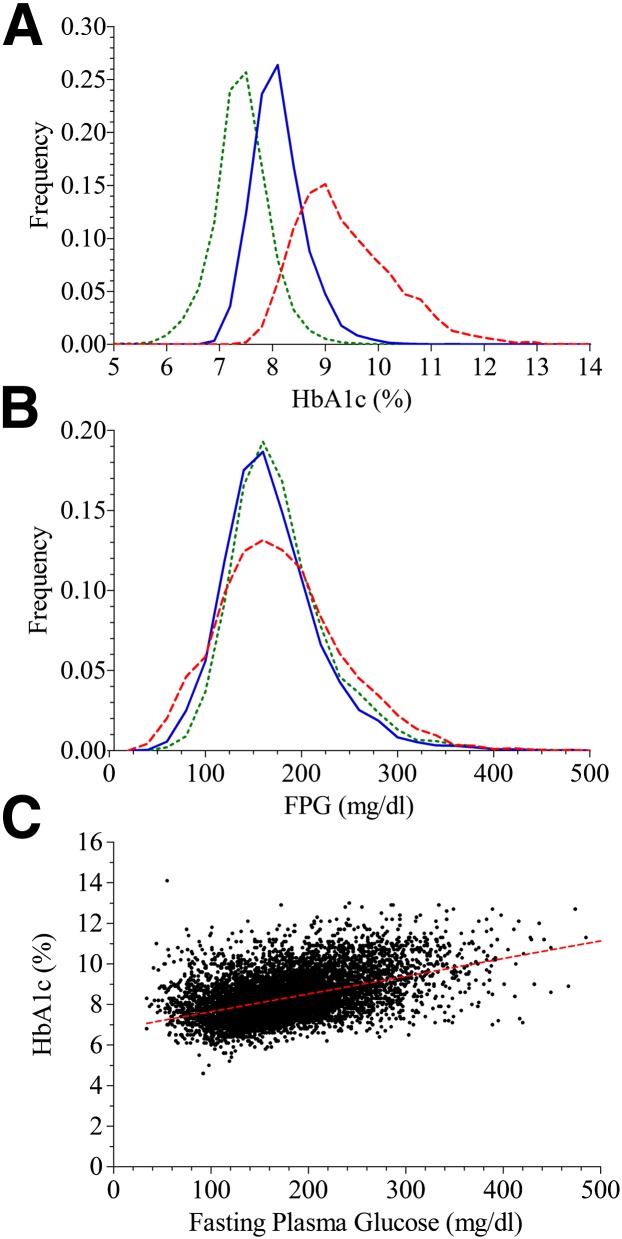
Figure 1C shows the linear relationship between HbA1c and FPG in the ACCORD population at baseline. Figure 1A and B shows that the frequency distribution of HbA1c was markedly different in the low, moderate, and high HGI subgroups. In contrast, FPG distribution was similar among the subgroups. Selected baseline demographic, biochemical, and clinical characteristics are compared among the HGI subgroups in Table 1. Of particular note, there were disproportionately more black and Hispanic participants and fewer white participants in the high HGI subgroup. Furthermore, high HGI participants were younger, had longer duration of diabetes, were more likely to already have retinopathy and a history of albuminuria at baseline, and were more likely to have used insulin before the start of the study.
Figure 1.
Assessment of HbA1c and FPG at baseline. The low, moderate, and high HGI subgroups have green, blue, or red lines, respectively. A: Distribution of HbA1c by HGI subgroup. B: Distribution of FPG by HGI subgroup. C: The red dotted line is the simple linear population regression line.
Table 1.
Baseline characteristics of ACCORD participants by HGI subgroup
| Low HGI | Moderate HGI | High HGI | ||
|---|---|---|---|---|
| Variablea | n = 3,041 | n = 3,042 | n = 3,042 | P valueb |
| HbA1c (%) | 7.4 ± 0.5 | 8.1 ± 0.5 | 9.4 ± 0.9 | <0.001 |
| Median HbA1c (%) | 7.4 | 8.1 | 9.2 | |
| HbA1c (mmol/mol) | 57.4 ± 18.0 | 65.0 ± 18.0 | 79.2 ± 13.7 | |
| FPG (mg/dL) | 178.5 ± 52.3 | 169.0 ± 51 | 178.3 ± 64.2 | <0.001 |
| FPG (mmol/L) | 9.9 ± 2.9 | 9.4 ± 2.8 | 9.9 ± 3.6 | |
| HGI (%) | −0.961 ± 0.4 | −0.183 ± 0.2 | 0.996 ± 0.7 | <0.001 |
| Age (years) | 62.6 ± 6.8 | 62.7 ± 6.9 | 61.5 ± 6.8 | <0.001 |
| Median [IQR] duration of diabetes (years) | 8 [10] | 10 [10] | 10 [11] | <0.001 |
| Female sex | 1,072 (35.3) | 1,196 (39.3) | 1,235 (40.6) | <0.001 |
| Race or ethnic group (group %) | <0.001 | |||
| White | 2,148 (37.6) | 1,957 (34.3) | 1,606 (28.1) | |
| Black | 375 (21.7) | 551 (31.9) | 799 (46.3) | |
| Hispanic | 162 (24.9) | 211 (32.5) | 277 (42.6) | |
| Other | 356 (34.3) | 323 (31.1) | 360 (34.6) | |
| Education | <0.001 | |||
| Less than high school | 377 (12.4) | 438 (14.4) | 523 (17.2) | |
| High school graduate | 773 (25.4) | 800 (26.3) | 825 (27.1) | |
| Some college | 1,017 (33.5) | 1,035 (34.1) | 950 (31.3) | |
| College degree or higher | 872 (28.7) | 767 (25.2) | 742 (24.4) | |
| Cigarette smoking status | <0.01 | |||
| Current | 399 (13.1) | 437 (14.4) | 452 (14.9) | |
| Former | 1,415 (46.6) | 1,348 (44.3) | 1,271 (41.9) | |
| Never | 1,223 (40.3) | 1,255 (41.3) | 1,314 (43.3) | |
| Alcohol use | 798 (26.3) | 755 (24.8) | 640 (21.1) | <0.001 |
| Insulin use | 835 (27.5) | 1,036 (34.1) | 1,321 (43.4) | <0.001 |
| Metformin use | 1,887 (62.1) | 1,877 (61.7) | 1,681 (55.3) | <0.001 |
| Sulfonylurea use | 1,576 (51.8) | 1,584 (52.1) | 1,433 (47.1) | <0.001 |
| Thiazolidinedione use | 595 (19.6) | 645 (21.2) | 538 (17.7) | 0.025 |
| History of hypertension | 2,856 (93.9) | 2,864 (94.2) | 2,837 (93.3) | 0.33 |
| Previous CVD | 1,014 (33.3) | 1,114 (36.6) | 1,085 (35.7) | 0.02 |
| High risk of heart failure | 75 (2.5) | 70 (2.3) | 69 (2.3) | 0.86 |
| History of albuminuria | 944 (31.0) | 990 (32.5) | 1,133 (37.3) | <0.001 |
| History of left ventricular hypertrophy | 125 (4.1) | 107 (3.5) | 151 (5) | 0.02 |
| History of arterial stenosis | 141 (4.6) | 158 (5.2) | 157 (5.2) | 0.53 |
| Retinopathy at baseline | 697 (22.9) | 819 (26.9) | 883 (29.0) | <0.001 |
| Neuropathy at baseline | 1,889 (62.2) | 1,951 (64.3) | 1,920 (63.1) | 0.25 |
| Serum creatinine (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.12 |
| Estimated GFR (mL/min/1.73 m2) | 89.7 ± 27.8 | 90.8 ± 24.8 | 92.8 ± 29 | <0.001 |
| Cholesterol (mg/dL) | ||||
| Total | 180.3 ± 39.5 | 182.3 ± 41.6 | 187 ± 44 | <0.001 |
| LDL | 101.1 ± 32 | 104.4 ± 33.7 | 108.6 ± 35.3 | <0.001 |
| HDL | ||||
| Men | 38.6 ± 9.8 | 38.2 ± 8.9 | 39.0 ± 10.0 | <0.001 |
| Women | 46.7 ± 12.0 | 46.7 ± 12.7 | 47.9 ± 13.1 | <0.001 |
| Median [IQR] triglycerides (mg/dL) | 160 [126] | 155 [120] | 149 [122] | <0.001 |
| Blood pressure (mmHg) | ||||
| Diastolic | 74.8 ± 10.7 | 74.3 ± 10.6 | 75.4 ± 10.7 | <0.001 |
| Systolic | 136 ± 16.6 | 136.2 ± 17.1 | 136.8 ± 17.6 | 0.21 |
| BMI (kg/m2) | 32.2 ± 5.4 | 32.3 ± 5.4 | 32.2 ± 5.6 | 0.56 |
| Waist circumference (cm) | 107 ± 13.8 | 107.1 ± 13.9 | 106.4 ± 14.1 | 0.15 |
GFR, glomerular filtration rate; IQR, interquartile range.
aUnless otherwise noted, values are means ± SD for continuous variables or number (%) for categorical variables. bOverall differences between HGI groups using ANOVA, Kruskal-Wallis tests, or χ2 tests.
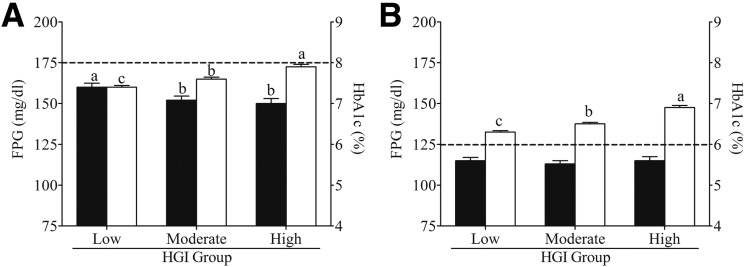
After 1 year of standard (Fig. 2A) or intensive (Fig. 2B) treatment, mean HbA1c remained significantly different (P < 0.001) between HGI subgroups and was highest in the high HGI subgroup. In contrast, the mean FPG was significantly lower (P < 0.001) in the moderate and high HGI subgroups after 1 year of standard treatment (Fig. 2A) and was not different (P > 0.05) between HGI subgroups after 1 year of intensive treatment (Fig. 2B). The number of participants above or below the HbA1c intensification thresholds varied after 1 year in the standard treatment arm: 43.0% of high HGI participants remained above the 8% (64 mmol/mol) intensification threshold compared with only 30.6% of moderate and 21.9% of low HGI participants. After 1 year in the intensive treatment arm, 88.7% of high HGI participants remained above the 6% (42 mmol/mol) intensification threshold compared with 83.9% of moderate and 72.7% of low HGI participants. Barring mitigating circumstances, such as a recent hypoglycemic event, having disproportionately more participants above the intensification threshold should have resulted in greater treatment intensification at the 1-year visit.
Figure 2.
HbA1c and FPG disparity among HGI subgroups. Mean (± 95% CI) HbA1c (□) and FPG levels (■) for low, moderate, and high HGI subgroups after 1 year of standard (A) or intensive (B) glycemia treatment. For each panel, HbA1c or FPG values with different superscripts (a, b, c) are significantly different (P < 0.05). The dotted lines denote protocol-mandated HbA1c treatment-intensification thresholds.
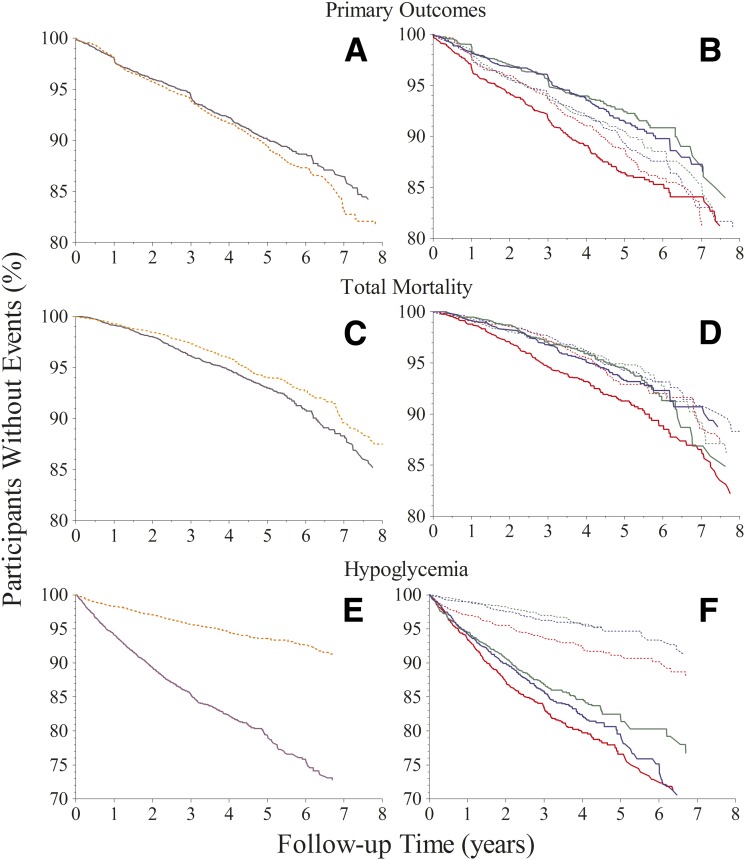
Kaplan-Meier (Fig. 3) and Cox regression analyses (Table 2) show that the effect of intensive treatment differed markedly among the HGI subgroups. For example, if we ignore HGI, the event rate for primary outcomes was not significantly different (P = 0.09) between the intensive (9.8%) and standard (10.7%) treatment groups (Table 2 and Fig. 3A). A statistically significant interaction was detected between treatment and HGI (P = 0.0091). Subsequent HGI subgroup analysis showed that intensive treatment reduced primary outcomes by 25% (P = 0.02) in the low HGI subgroup and by 23% (P = 0.02) in the moderate HGI subgroup (Table 2 and Fig. 3B). In contrast, primary outcomes in the high HGI subgroup were not significantly different between the standard and intensive glycemia treatment groups (P = 0.20).
Figure 3.
Kaplan-Meier curves for primary outcomes, total mortality, and hypoglycemia requiring any assistance. Proportions of participants free of the specified outcome over time are compared between standard and intensive treatment groups (panels A, C, D) and among the HGI subgroups (panels B, D, F). Standard treatment is depicted by orange dashed lines and intensive treatment by solid purple lines. Low, moderate, and high HGI subgroups have green, blue, or red lines, respectively.
Table 2.
Risk and adjusted hazard ratios of composite primary outcomes, total mortality, and hypoglycemia by glycemia treatment group and HGI subgroup
| Intensive treatment (I) |
Standard treatment (S) |
Adjusted hazard ratio (I/S) |
Interaction between treatment and HGI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HGI subgroup | At risk | Events | % | At risk | Events | % | Estimate | 95% CI | P value | P value | |
| Primary outcomesa | Overall | 4,570 | 446 | 9.8 | 4,555 | 487 | 10.7 | 0.89 | 0.97–1.02 | 0.09 | |
| Low | 1,532 | 119 | 7.8 | 1,509 | 147 | 9.7 | 0.75 | 0.59–0.95 | 0.02 | 0.0091 | |
| Moderate | 1,515 | 128 | 8.4 | 1,527 | 161 | 10.5 | 0.77 | 0.61–0.97 | 0.02 | ||
| High | 1,523 | 199 | 13.1 | 1,519 | 179 | 11.8 | 1.14 | 0.93–1.40 | 0.20 | ||
| Total mortalityb | Overall | 4,570 | 350 | 7.7 | 4,555 | 287 | 6.3 | 1.21 | 1.04–1.42 | 0.02 | |
| Low | 1,532 | 97 | 6.3 | 1,509 | 86 | 5.7 | 1.08 | 0.81–1.44 | 1.00d | 0.2240 | |
| Moderate | 1,515 | 102 | 6.7 | 1,527 | 92 | 6.0 | 1.06 | 0.80–1.41 | 1.00d | ||
| High | 1,523 | 151 | 9.9 | 1,519 | 109 | 7.2 | 1.41 | 1.10–1.81 | 0.02d | ||
| Hypoglycemiac | Overall | 4,570 | 763 | 16.7 | 4,555 | 238 | 5.2 | 3.64 | 3.14–4.21 | <0.001 | |
| Low | 1,532 | 222 | 14.5 | 1,509 | 56 | 3.7 | 4.27 | 3.21–5.78 | <0.001 | 0.0350 | |
| Moderate | 1,515 | 254 | 16.8 | 1,527 | 68 | 4.5 | 4.32 | 3.32–5.68 | <0.001 | ||
| High | 1,523 | 287 | 18.8 | 1,519 | 114 | 7.5 | 2.91 | 2.35–3.63 | <0.001 | ||
aFirst occurrence of nonfatal MI, nonfatal stroke, or death from cardiovascular events. Covariates include age, sex, race, education, diabetes duration, history of smoking, previous CVD, high risk of heart failure, history of arterial stenosis, history of albuminuria, retinopathy at baseline, estimated glomerular filtration rate, HDL-cholesterol, LDL-cholesterol, and intervention trial (hypertension treatment, intensive vs. standard; hypertension trial vs. lipid trial; hyperlipidemia treatment, fibrate vs. no fibrate).
bDeath from any cause. Covariates include age, sex, race, education, history of smoking, statin uses, insulin uses, previous CVD, history of albuminuria, history of arterial stenosis, retinopathy at baseline, and intervention trial (hypertension treatment, intensive vs. standard; hypertension trial vs. lipid trial; hyperlipidemia treatment, fibrate vs. no fibrate).
cSymptomatic, severe hypoglycemia requiring any assistance. Covariates include age, sex, race, education, diabetes duration, living alone or not, insulin uses, previous CVD, retinopathy at baseline, neuropathy at baseline, and estimated glomerular filtration rate.
dBonferroni correction for multiple comparisons.
Ignoring HGI again, total mortality (Table 2 and Fig. 3C) was significantly greater (P = 0.02) in the intensive treatment group (7.7%) compared with the standard treatment group (6.3%). There was no interaction between treatment and HGI (P = 0.22). HGI subgroup analysis showed that although intensive treatment significantly increased total mortality by 41% (P = 0.02) in the high HGI subgroup, it had no effect on mortality in the moderate or low HGI subgroups compared with standard treatment (Table 2 and Fig. 3D).
Ignoring HGI once more, hypoglycemia requiring any assistance was more than threefold greater (P < 0.001) in the intensive treatment group compared with the standard treatment group (Table 2 and Fig. 3E). HGI subgroup analysis showed that the incidence of hypoglycemia was progressively higher in the low, moderate, and high HGI subgroups in the intensive (14.5, 16.8, and 18.8%, respectively) and standard (3.7, 4.5, and 7.5%, respectively) glycemia treatment groups (Table 2 and Fig. 3F). Results were similar for hypoglycemia requiring medical assistance. Sensitivity analyses that included other intervention assignments as stratifying factors in the Cox models, rather than as covariates, showed that the estimated HRs for primary outcomes, total mortality, and hypoglycemia remained stable.
Conclusions
Interindividual variation in HbA1c caused by factors other than blood glucose concentration appears to be partly hereditary (21,22) and has been reported in patients with type 1 diabetes (4–9,23,24), with type 2 diabetes (12–14,25–27), and without diabetes (28–32). Pediatric patients with type 1 diabetes with higher HGI had higher levels of skin advanced glycation end products (33). HbA1c levels that are persistently lower or higher than expected among individuals with similar blood glucose levels have been detected in studies where blood glucose was estimated based on FPG (12), self-monitored mean blood glucose (4,8,9,34), 7-point mean blood glucose profile sets (6,35), continuous glucose monitoring (7,11), or fructosamine (5,26,27). Unlike other methods for estimating blood glucose, continuous glucose monitoring and fructosamine are relatively free of sampling bias yet produce similar results when assessing interindividual variation in HbA1c.
The present studies indicate that subjects in ACCORD with high HGIs had more retinopathy and nephropathy at baseline, as previously reported in the DCCT (6). The differences in drug use observed between HGI subgroups at baseline could be related to the fact that insulin is more likely to be prescribed for individuals with persistently higher HbA1c (high HGI). Alternatively, different drugs have been shown to influence the quantitative relationship between HbA1c and blood glucose concentration (36), which could in turn influence the HGI. Higher HGI among black ACCORD participants supports our previous observation of racial variation in HGI in children with type 1 diabetes (8). Evidence of clinically significant interindividual variation in the quantitative relationship between blood glucose concentration and HbA1c markedly complicates the use of HbA1c for the diagnosis and management of diabetes, especially in mixed-race populations (37–42).
Heterogeneity of Treatment Outcomes Among HGI Subgroups in ACCORD
Our present analyses examine the ACCORD glycemia trial through a new lens, namely, the HGI calculated using FPG and HbA1c measured at baseline. As previously reported by the ACCORD investigators, we observed no difference in primary outcomes between the standard and intensive treatment groups despite our use of a slightly different data set (we omitted patients without HbA1c or FPG at baseline) and different statistical methods. HGI subgroup analysis indicated, however, that intensive treatment actually improved primary outcomes in low and moderate HGI participants, a beneficial effect that was offset and masked by the apparently detrimental effects of intensive treatment in the high HGI subgroup. Although total mortality was significantly higher in the intensive treatment group, HGI subgroup analysis showed that the higher mortality associated with intensive treatment was restricted to the high HGI subgroup. Collectively, these observations show that HGI identifies two subpopulations in ACCORD, one that experiences benefits and one that experiences harms from the same intensive glucose-lowering strategy.
The risk for hypoglycemia was greatest in the high HGI subgroup in the standard and intensive treatment groups. More high HGI participants remained above the protocol-mandated HbA1c treatment thresholds after 1 year of standard or intensive treatment. Despite higher mean HbA1c, high HGI participants in both glycemia treatment groups had mean FPG values after 1 year that were as low as or lower than those observed in low HGI participants. Collectively, these observations are consistent with our hypothesis that intensive treatment may have inadvertently caused high HGI participants to receive more intensive treatment and could explain the otherwise paradoxical results reported by Miller et al. (3).
Clinical Implications
The twin goals of diabetes management are to keep blood glucose levels low enough to limit the development of long-term diabetes complications but high enough to avoid hypoglycemia. After the ACCORD trial reported that intensive treatment increased mortality and hypoglycemia, the American Diabetes Association (ADA) recommended that HbA1c treatment goals for individual patients should be personalized according to characteristics such as age and frequency of hypoglycemia, while also reiterating that lowering HbA1c generally helps prevent or delay long-term complications (43). Our observations of markedly different outcomes in the different HGI subgroups in response to intensive treatment strongly supports the ADA recommendation for more personalized diabetes management and suggests that HGI could be used to help individualize treatment goals.
Exactly how the results of this study might be used to reinterpret other clinical trials or how the results might be applied in future trial designs or in clinical practice remains to be determined. One reason is that ACCORD participants were older than the general public and selected for elevated risk for CVD. This could explain why the baseline regression equation in ACCORD is markedly different from the linear regression equations reported by studies such as the A1C-Derived Average Glucose (ADAG) study (44). As such, the results may not be generalizable to other clinical trials or to the general population with diabetes and we cannot recommend the use of the ACCORD regression equation in other populations. Furthermore, only one other study has used FPG to assess the HGI in patients with type 2 diabetes, and these results were only reported in a meeting abstract (12). All other prior HGI and glycation gap studies in patients with diabetes used some estimate of mean blood glucose or fructosamine. Additional studies of HGI in other clinical trials could help determine how best to use the HGI in clinical practice.
Conclusion
Intensive treatment to a low HbA1c target of less than 6% (42 mmol/mol) cannot be recommended for all patients with type 2 diabetes because primary cardiovascular outcomes, total mortality, and hypoglycemia were all adversely affected in high HGI participants in ACCORD. Further studies should determine if the observed beneficial effects of intensive treatment on primary outcomes in low and moderate HGI participants outweigh any detrimental effects that might be caused by the increase in hypoglycemia associated with intensive treatment. Our results confirm that HbA1c is not a one-size-fits-all indicator of blood glucose concentration and suggest that failure to take this into account can result in suboptimal diabetes care.
Article Information
Acknowledgments. The authors express their gratitude to the investigators, staff, and patients in the ACCORD study for their efforts that made this resource available and especially thank Greg Evans of the ACCORD coordinating center for his invaluable assistance. The authors also thank the ACCORD steering committee for their comments in review of the manuscript.
Funding. The research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Healthhttp://dx.doi.org/10.13039/100000002 under award number R01-HL-110395 and in part by 1-U54-GM-104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.M.H. was supported in part by intramural funding from Children's Hospital of New Orleans and Louisiana State University Health Sciences Center. J.B.B. received additional support from the National Center for Research Resources (grant UL1-RR-025747) and the National Center for Advancing Translational Sciences (grants UL1-TR-000083 and UL1-TR-001111).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M.H. wrote the manuscript and researched data. S.L. researched data, performed statistical analyses, and reviewed and edited the manuscript. L.M. and R.J.M. performed statistical analyses and reviewed and edited the manuscript. J.B.B. and V.F. contributed to discussion and reviewed and edited the manuscript. J.M.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
Clinical trial reg. no. NCT00000620, clinicaltrials.gov.
References
- 1.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller ME, Bonds DE, Gerstein HC, et al.; ACCORD Investigators . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hempe JM, Gomez R, McCarter RJ Jr, Chalew SA. High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control. J Diabetes Complications 2002;16:313–320 [DOI] [PubMed] [Google Scholar]
- 5.Cohen RM, Holmes YR, Chenier TC, Joiner CH. Discordance between HbA1c and fructosamine: evidence for a glycosylation gap and its relation to diabetic nephropathy. Diabetes Care 2003;26:163–167 [DOI] [PubMed] [Google Scholar]
- 6.McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care 2004;27:1259–1264 [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Research in Children Network (DirecNet) Study Group; Wilson DM, Kollman C.. Relationship of A1C to glucose concentrations in children with type 1 diabetes: assessments by high-frequency glucose determinations by sensors. Diabetes Care 2008;31:381–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamps JL, Hempe JM, Chalew SA. Racial disparity in A1C independent of mean blood glucose in children with type 1 diabetes. Diabetes Care 2010;33:1025–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soros AA, Chalew SA, McCarter RJ, Shepard R, Hempe JM. Hemoglobin glycation index: a robust measure of hemoglobin A1c bias in pediatric type 1 diabetes patients. Pediatr Diabetes 2010;11:455–461 [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Segade S, Rodríguez J, Cabezas-Agricola JM, Casanueva FF, Camiña F. Progression of nephropathy in type 2 diabetes: the glycation gap is a significant predictor after adjustment for glycohemoglobin (Hb A1c). Clin Chem 2011;57:264–271 [DOI] [PubMed] [Google Scholar]
- 11.Wilson DM, Xing D, Cheng J, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Persistence of individual variations in glycated hemoglobin: analysis of data from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Randomized Trial. Diabetes Care 2011;34:1315–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JI, Stevens RJ, Holman RR. The haemoglobin glycation index is an independent risk factor for microvascular complications in UKPDS patients with newly diagnosed type 2 diabetes (Abstract) Diabetes 2005;54:A244–A245 [Google Scholar]
- 13.Cosson E, Banu I, Cussac-Pillegand C, et al. Glycation gap is associated with macroproteinuria but not with other complications in patients with type 2 diabetes. Diabetes Care 2013;36:2070–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nayak AU, Nevill AM, Bassett P, Singh BM. Association of glycation gap with mortality and vascular complications in diabetes. Diabetes Care 2013;36:3247–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalew SA, McCarter RJ, Thomas J, Thomson JL, Hempe JM. A comparison of the glycosylation gap and hemoglobin glycation index in patients with diabetes. J Diabetes Complications 2005;19:218–222 [DOI] [PubMed] [Google Scholar]
- 16.Calles-Escandón J, Lovato LC, Simons-Morton DG, et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riddle MC, Ambrosius WT, Brillon DJ, et al.; Action to Control Cardiovascular Risk in Diabetes Investigators . Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010;33:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buse JB, Bigger JT, Byington RP, et al.; ACCORD Study Group . Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007;99:21i–33i [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC, Riddle MC, Kendall DM, et al.; ACCORD Study Group . Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99:34i–43i [DOI] [PubMed] [Google Scholar]
- 20.Gerstein HC, Miller ME, Genuth S, et al.; ACCORD Study Group . Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen RM, Snieder H, Lindsell CJ, et al. Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care 2006;29:1739–1743 [DOI] [PubMed] [Google Scholar]
- 22.Snieder H, Sawtell PA, Ross L, Walker J, Spector TD, Leslie RD. HbA(1c) levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins. Diabetes 2001;50:2858–2863 [DOI] [PubMed] [Google Scholar]
- 23.Madsen H, Kjaergaard JJ, Ditzel J. Relationship between glycosylation of haemoglobin and the duration of diabetes: a study during the third trimester of pregnancy. Diabetologia 1982;22:37–40 [DOI] [PubMed] [Google Scholar]
- 24.Hudson PR, Child DF, Jones H, Williams CP. Differences in rates of glycation (glycation index) may significantly affect individual HbA1c results in type 1 diabetes. Ann Clin Biochem 1999;36:451–459 [DOI] [PubMed] [Google Scholar]
- 25.Fukudome M, Nakazaki M, Fukushige E, et al. Interindividual divergence in the relationship between the values of plasma glucose and hemoglobin A1c in type 2 diabetes. Intern Med 2009;48:273–279 [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Segade S, Rodríguez J, García Lopez JM, Casanueva FF, Camiña F. Estimation of the glycation gap in diabetic patients with stable glycemic control. Diabetes Care 2012;35:2447–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafon C, Ciudin A, Valladares S, Mesa J, Simó R. Variables involved in the discordance between HbA1c and fructosamine: the glycation gap revisited. PLoS One 2013;8:e66696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yudkin JS, Forrest RD, Jackson CA, Ryle AJ, Davie S, Gould BJ. Unexplained variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Diabetologia 1990;33:208–215 [DOI] [PubMed] [Google Scholar]
- 29.Gould BJ, Davie SJ, Yudkin JS. Investigation of the mechanism underlying the variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Clin Chim Acta 1997;260:49–64 [DOI] [PubMed] [Google Scholar]
- 30.Kilpatrick ES, Maylor PW, Keevil BG. Biological variation of glycated hemoglobin. Implications for diabetes screening and monitoring. Diabetes Care 1998;21:261–264 [DOI] [PubMed] [Google Scholar]
- 31.Rohlfing C, Wiedmeyer HM, Little R, et al. Biological variation of glycohemoglobin. Clin Chem 2002;48:1116–1118 [PubMed] [Google Scholar]
- 32.Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ 2001;322:15–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felipe DL, Hempe JM, Liu S, et al. Skin intrinsic fluorescence is associated with hemoglobin A1c and hemoglobin glycation index but not mean blood glucose in children with type 1 diabetes. Diabetes Care 2011;34:1816–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hempe JM, Soros AA, Chalew SA. Estimated average glucose and self-monitored mean blood glucose are discordant estimates of glycemic control. Diabetes Care 2010;33:1449–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarter RJ, Hempe JM, Chalew SA. Mean blood glucose and biological variation have greater influence on HbA1c levels than glucose instability: an analysis of data from the Diabetes Control and Complications Trial. Diabetes Care 2006;29:352–355 [DOI] [PubMed] [Google Scholar]
- 36.Ramachandran A, Riddle MC, Kabali C, Gerstein HC; ORIGIN Investigators . Relationship between A1C and fasting plasma glucose in dysglycemia or type 2 diabetes: an analysis of baseline data from the ORIGIN trial. Diabetes Care 2012;35:749–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen RM. A1C: does one size fit all? Diabetes Care 2007;30:2756–2758 [DOI] [PubMed] [Google Scholar]
- 38.Dagogo-Jack S. Pitfalls in the use of HbA1c as a diagnostic test: the ethnic conundrum. Nat Rev Endocrinol 2010;6:589–593 [DOI] [PubMed] [Google Scholar]
- 39.Davidson MB, Schriger DL. Effect of age and race/ethnicity on HbA1c levels in people without known diabetes mellitus: implications for the diagnosis of diabetes. Diabetes Res Clin Pract 2010;87:415–421 [DOI] [PubMed] [Google Scholar]
- 40.Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab 2012;97:1067–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalew SA, McCarter RJ, Hempe JM. Biological variation and hemoglobin A1c: relevance to diabetes management and complications. Pediatr Diabetes 2013;14:391–398 [DOI] [PubMed] [Google Scholar]
- 42.Wolffenbuttel BHR, Herman WH, Gross JL, Dharmalingam M, Jiang HH, Hardin DS. Ethnic differences in glycemic markers in patients with type 2 diabetes. Diabetes Care 2013;36:2931–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Diabetes Association . Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group . Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]