Abstract
Problematic hypoglycemia, defined as two or more episodes per year of severe hypoglycemia or as one episode associated with impaired awareness of hypoglycemia, extreme glycemic lability, or major fear and maladaptive behavior, is a challenge, especially for patients with long-standing type 1 diabetes. Individualized therapy for such patients should include a composite target: optimal glucose control without problematic hypoglycemia. Therefore, we propose a tiered, four-stage algorithm based on evidence of efficacy given the limitations of educational, technological, and transplant interventions. All patients with problematic hypoglycemia should undergo structured or hypoglycemia-specific education programs (stage 1). Glycemic and hypoglycemia treatment targets should be individualized and reassessed every 3–6 months. If targets are not met, one diabetes technology—continuous subcutaneous insulin infusion or continuous glucose monitoring—should be added (stage 2). For patients with continued problematic hypoglycemia despite education (stage 1) and one diabetes technology (stage 2), sensor-augmented insulin pumps preferably with an automated low-glucose suspend feature and/or very frequent contact with a specialized hypoglycemia service can reduce hypoglycemia (stage 3). For patients whose problematic hypoglycemia persists, islet or pancreas transplant should be considered (stage 4). This algorithm provides an evidence-informed approach to resolving problematic hypoglycemia; it should be used as a guide, with individual patient circumstances directing suitability and acceptability to ensure the prudent use of technology and scarce transplant resources. Standardized reporting of hypoglycemia outcomes and inclusion of patients with problematic hypoglycemia in studies of new interventions may help to guide future therapeutic strategies.
Type 1 Diabetes and Problematic Hypoglycemia: Balancing the Effectiveness and Safety of Interventions
Hypoglycemia is a common and greatly feared complication of type 1 diabetes (T1D) (1–4). Severe hypoglycemia (SH), an event that because of profound neuroglycopenia requires the assistance of another person for recovery (5), is experienced by one-third of patients with T1D at least once a year (6–9). Many such events are single episodes caused by insulin dosing errors, exercise, and alcohol (Table 1). Conversely, problematic hypoglycemia is a condition in which episodes of SH are unpredictable, cannot be easily explained or prevented, and, therefore, have a significant negative impact on health and quality of life (QoL). The criteria of problematic hypoglycemia include two or more episodes of SH in the past 12 months or one episode of SH in the past 12 months associated with impaired awareness of hypoglycemia (IAH), extreme glycemic lability, or major fear and maladaptive behavior. Simple tools are available clinically to quantitate IAH (10,11), hypoglycemia severity (12), and glycemic lability (12).
Table 1.
Identification and initial assessment of people with problematic hypoglycemia
| Identification of patients with T1D and problematic hypoglycemia | Assessment of hypoglycemia risk should be performed annually for all patients with T1D. Frequently, episodes of hypoglycemia are not reported to physicians. Health care planners should consider whether appropriate referral pathways exist for patients experiencing SH (patients attended by emergency medical services or emergency department physicians or dispensed glucagon injections by their pharmacist). Number of calls for an ambulance or glucagon injections during the past month and past year should be considered as well as whether injuries may have been due to unidentified hypoglycemia. |
| History of hypoglycemia | Frequency of episodes |
| Nocturnal hypoglycemia | |
| Episodes of SH | |
| Ability to detect episodes | |
| Presence of adrenergic and neuroglycopenic symptoms of hypoglycemia | |
| Precipitating factors (e.g., insulin dosing errors, exercise, alcohol) | |
| Review diabetes self-care | Adequate frequency of SBGM? |
| Appropriate diet? | |
| Review for presence of risk factors for SH | Older age |
| Long duration of diabetes | |
| Renal impairment | |
| Low BMI | |
| IAH (Clarke or Gold scores ≥4) | |
| Erratic, unpredictable blood glucose levels | |
| Very low HbA1c | |
| Initial assessment of people identified with problematic hypoglycemia | |
| Insulin therapy | |
| Insulin preparations | Use of regular and NPH insulins have greater risk for hypoglycemia than insulin analogs |
| Premixed insulins are not recommended | |
| Insulin dosing | Inappropriate balance between basal and bolus doses |
| Excessive correction doses | |
| Inappropriate timing of insulin | |
| Lack of adjustment for (prior) exercise and/or heat | |
| Overestimation of meal size or carbohydrate content | |
| Insulin administration | Lipohypertrophy |
| Intramuscular injection | |
| Needle length | |
| Injection technique | |
| Physiologic/other causes | |
| Diabetes complications | HAAF |
| Gastroparesis | |
| Malabsorption | Celiac disease |
| Pancreatic exocrine insufficiency | |
| Endocrinopathies | Adrenal insufficiency |
| Hypopituitarism | |
| Factitious | Misuse of insulin |
| Alcohol excess | |
| Autoimmune | Insulin autoimmune syndrome |
| Metabolic | Renal failure |
| Hepatic failure | |
| Inborn errors of metabolism | |
| Psychological/psychosocial | Fear of hyperglycemia/diabetes complications |
| Fear of hypoglycemia | |
| Denial, not willing to attend educational programs or to use technology | |
| Depression or other psychiatric problems | |
| Cognitive impairment |
This approach may identify some reversible causes for hypoglycemia, which can be addressed relatively easily. It may also identify some individuals for whom some of the interventions described in this article are contraindicated or who require specific nonendocrine interventions.
Recurrent hypoglycemia impairs counterregulatory hormonal responses to and awareness of hypoglycemia, predisposing patients to more frequent hypoglycemia and SH (13). IAH, which increases in prevalence with diabetes duration, is found in 20–40% of patients with T1D (11,14–16) and increases the risk of SH by 6–20-fold (6,10,11). Recurrent SH (two or more episodes annually) is reported by 21% of patients with T1D (6) and by 66% of patients whose T1D is complicated by IAH (11). Recurrent hypoglycemia can cause significant morbidity (4,17) and mortality. Among individuals with T1D, 4–10% of all deaths are attributed to SH (18,19), and risk of death 5 years after an episode of SH is increased 3.4-fold in those who report SH (20).
The risk factors for SH depend mainly on residual C-peptide secretion, which reduces glycemic variability (21–23). Related to residual C-peptide secretion are the patient’s age at onset of T1D and disease duration (21). Other risk factors include autonomic failure, insulin sensitivity, BMI, genetics, and psychosocial factors (24) (Table 1). In the Diabetes Control and Complications Trial, residual endogenous insulin secretion was associated with a reduced risk of SH, regardless of treatment intensity (25). Unfortunately, most patients with T1D lose all measurable C-peptide within 10–15 years after diagnosis (26), making it more challenging for those with long-standing (>15 years) T1D to avoid hypoglycemia.
Besides a reduction of microangiopathic complications, long-term follow-up of the Diabetes Control and Complications Trial cohort demonstrated a reduction in cardiovascular morbidity (27) and all-cause mortality (28) in patients with an HbA1c <7.0% (53 mmol/mol), which concurs with Swedish and Austrian registries (29,30). However, even at that HbA1c level, the residual risk for cardiovascular and all-cause mortality remained twice as high in patients with T1D than in nondiabetic control subjects (29–31). A large U.S. registry of >20,000 patients demonstrated a U-shaped relationship between SH and HbA1c level, with the lowest risk of SH occurring when the HbA1c level is between 7.0% (53 mmol/mol) and 7.5% (58 mmol/mol) (9). Therefore, the selection of glycemic targets in each patient should be individualized to the lowest HbA1c level that does not cause SH, that preserves hypoglycemia awareness, and that avoids long-term micro- and macrovascular complications (32).
The objectives of this review article are to examine the evidence on educational, technological, and transplant interventions in patients with T1D complicated by problematic hypoglycemia (Table 2) and to propose clinical practice recommendations (Table 3) in a tiered, four-stage treatment algorithm (Fig. 1). To achieve these objectives, an international group of endocrinology clinician-investigators with expertise in evaluating all three treatment categories in this patient population was formed to critically appraise the available evidence and to formulate a consensus approach.
Table 2.
Key intervention studies in hypoglycemia unaware patients with T1D
| Baseline |
End of study |
|||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Intervention and duration of follow-up | Study design and duration | No. and duration of diabetes (years) | HbA1c (%) | SH rate and awareness score | HbA1c (%) | SH rate and awareness score | Comments |
| Fanelli et al. (42) | Change from twice daily to MDI regimen Daily contact Re-education | Prospective, single center Before and after 1-year follow-up | n = 21 (11.4 ± 1.8) | 5.8 ± 0.1 (normal 3.8–5.5) | 9 of 21 had SH in previous year All had IAH | 6.9 ± 0.1 (normal 3.8–5.5) | No SH in 12-month follow-up 10-fold reduction in mild hypoglycemic events | Restoration of symptom and counterregulatory hormone responses; sustained to 1 year |
| Cranston et al. (40) | Intensive insulin therapy Frequent contact Intensive education | Prospective, single center Before and after 4-month follow-up | n = 12 men (11–32) | Group A: 6.9 ± 0.3 Group B: 8.7 ± 0.3 | N/A At least three CBG <3 with no symptoms in 2 weeks | No significant deterioration (numbers not reported) | SH: N/A Restoration of symptoms and improvement in counterregulatory responses after 3 weeks of no CBG <3.0 mmol/L | Mean 4.1 ± 1.1 months of frequent contact required to achieve 3 weeks of hypoglycemia avoidance |
| Dagogo-Jack et al. (41) | Scrupulous avoidance of iatrogenic hypoglycemia | Prospective, single center Before and after 3-month follow-up | n = 6 (15.5 ± 4.4) Age <35 | 8.3 ± 1.0 | 10.6 ± 3.7 All IAH | 9.8 ± 1.1 | SH not reported 60% reduction in CBG <3.3 mmol/L | Restored symptom but not hormonal responses at 3 months |
| Hermanns et al. (44) | HyPOS vs. control intervention, with most patients also moving to CSII with more than one SH or IAH with >10 years of T1D | RCT 6 months (31-month follow-up reported separately) | n = 164, 85.3% with T1D (HyPOS: 20.2 ± 10.8 Control: 22.1 ± 10.9) | HyPOS: 7.2 ± 0.9 Control: 7.4 ± 1.1 | HyPOS: 3.5 ± 3.6/patient-year Clarke score 4.8 Control: 3.6 ± 36/patient-year Clarke score 5.0 | HyPOS: 7.1 ± 0.9 Control: 7.3 ± 1.1 | HyPOS: 0.9 ± 1.9/patient-year Clarke score 2.3 Control: 0.6 ± 1.2/patient-year Clarke score 3.0 | 30% reduction in mild hypoglycemia with HyPOS; further 31-month follow-up shows sustained benefit in HyPOS over standard education |
| Cox et al. (43) | HAAT vs. control Increased SMBG and monthly visits No previous education or SMBG | RCT 18-month follow-up | n = 60 (13) | HAATT: 8.1 ± 0.7 Control: 8.0 ± 0.7 | HAATT: 2.0 Control: 1.8 | HAATT: 8.0 Control: 8.1 | HAATT: 0.4 Control: 1.7 | Hypoglycemia-specific education program performed better than frequent contact |
| Little et al. (48) | HypoCOMPaSS MDI vs. CSII and SMBG vs. CGM in 2 × 2 design Both arms: education and monthly visits plus telephone support | RCT 24-month follow-up | n = 96 (28–30) | 8.2 ± 1.2 | 8.9 ± 13.4 77% had SH in prior year Gold score 5.1 ± 1.1 | 8.1 ± 1.2 | 0.8 ± 1.9 20% had SH in prior year Gold score 4.1 ± 1.6 | No differences among CSII, CGM, and SAP CGM had slightly greater reduction in mild and SH, but higher baseline than SMBG |
| Pedersen-Bjergaard et al. (56) | HypoAna study [Analog insulin (detemir and aspart) vs. human insulin (NPH and regular)] | Multicenter, open-label, crossover RCT (1:1) 2-year follow-up | n = 159 | 8.0 ± 1.0 | All had 2 or more episodes of SH in prior year | Decrease of 0.13% with analog treatment compared with human insulin (P = 0.02) | Insulin analogs: 105 episodes of SH Human insulin: 136 episodes of SH | Absolute rate reduction of 0.51 episodes of SH per patient-year with insulin analogs |
| Ly et al. (65) | CSII vs. SAP in young patients with IAH | RCT 6-month follow-up | n = 95 (11.0) | 7.4 ± 0.2 | SAP-LGS: 6 SH and 0.22/patient-year Clarke score 5.9 ± 1.5 CSII: 6 SH and 0.25/patient-year Clarke score 6.4 ± 1.5 | 7.4 ± 0.2 in both arms | SAP-LGS: 0 events = 0/patient-year Clarke score 4.7 (4.0–5.1) CSII: 6 events = 0.26/patient-year Clarke score 5.1 (5.5–6.4) | Young patients with short duration SAP-LGS reduced SH compared with CSII alone, although event rates were low Reduced time <70 and <60 mg/dL in LGS arm |
| de Zoysa et al. (47) | DAFNE-HART 6 sessions based on cognitive behavioral therapy and motivational interviewing 1-year follow-up | Small trial; 2 sites Uncontrolled pilot clinical trial | n = 24 (31 ± 12) Previous DAFNE 8 CSII | 7.8 ± 1.1 | Median 3.0 (0–103) Clarke score 5.4 ± 1.2 Ryan score 948 | 7.8 ± 1.2 | Median 0 (0–3) 70% reported no further SH Clarke score 3.8 ± 1.8 Ryan score 372 | 9 of 20 restored awareness Some accepted CSII or CGM, which they were resistant to previously |
| Choudhary et al. (66) | Observational clinical audit of CGM in patients with SH despite previous DAFNE and CSII 1-year follow-up | Retrospective audit | n = 35 (29.6 ± 13.6) | 8.1 ± 1.2 | SH 8.1 ± 13.6/patient-year Gold score 5.1 ± 1.5 | 7.6 ± 1.0 | 0.6 ± 1.2/patient-year Gold score 5.2 ± 1.9 54% reported subjective improvement in awareness | Reduction in SH with CGM 23 of 35 used SAP-LGS 3 with SH on SAP had no further episodes on SAP-LGS |
| Giménez et al. (58) | CSII and education program with 2–3- month follow-up | Small, uncontrolled pilot trial 2-year follow-up | n = 20 (16.2) | 6.7 ± 1.1 | 1.25 ± 0.4/patient-year Clarke score 5.5 ± 1.2 | 6.3 ± 0.9 | SH 0.05/patient-year at 2 years Clarke score 1.6 ± 2.03 | 16 of 19 restored awareness Reduced mild hypoglycemia Time <70 mg/dL reduced from 13.7 to 9.1% |
| Sämann et al. (35) | Structured education 5-day course (DTTP) 9,583 patients at 96 centers 1-year follow-up | Subgroup analysis of patients with 3 or more SH at baseline | n = 341 (18.7 ± 11.1) | 7.4 ± 1.9 | 6.1 ± 9.6/patient-year | 7.2 ± 1.5 | 1.4 ± 5.4/patient-year | 56% had 1, 20% had 2, and only 15% had more than 3 SH events Reduced time in hospital 8.6 ± 15.4 to 3.9 ± 10.7 days/patient-year |
| Hopkins et al. (16) | Structured education program in flexible insulin therapy (U.K. DAFNE) 5-day course 1-year follow-up IAH subgroup reported | Retrospective audit | n = 215 (Duration not reported) | N/A | 3.6 ± 13.6/patient-year | N/A | 1.3 ± 5.9/patient-year | Improved awareness of hypoglycemia at a blood glucose >3 mmol/L |
| O’Connell et al. (92) | Islet treatment (961 ± 445 kIEQ) Antithymocyte globulin followed by tacrolimus + mycophenolate mofetil 2 patients had 1 treatment, 7 had 2 treatments, and 8 had 3 islet grafts | 3-center observational study 1-year follow-up | n = 17 Duration not reported | 8.3 ± 2.0 | Hyposcore 2,976 ± 3,494 | 6.5 ± 1.3 | 2 of 17 patients with graft failure had SH | 82% achieved composite end point of HbA1c <7% and absence of SH at 1 year |
| Brooks et al. (93) | Islet treatment (535 kIEQ) | Observational multicenter trial 2-year follow-up | n = 20 30 (16.5–38.5) | 8.0 (7.0–9.6) | 20 (7–50) | 6.2 (5.7–8.4) | 0.3 (0–1.6) | 70% maintained composite end point of HbA1c <7% and no SH at 2 years |
CBG, capillary blood glucose; DTTP, diabetes teaching and treatment program; kIEQ, kilo islet equivalent; N/A, not available.
Table 3.
Proposed clinical practice recommendations for the treatment of patients with T1D complicated by problematic hypoglycemia
| 1 | Routinely provide to all MDI therapy patients with T1D using SMBG structured and curriculum-based educational programs that reduce the incidence of SH and restore awareness in a significant proportion of patients with IAH. |
| 2 | Add one diabetes technology, preferably CSII with SMBG or MDI with CGM, where available, with appropriate education, training, and support to patients with problematic hypoglycemia to reduce the incidence of SH and maintain or improve HbA1c. |
| 3 | Use SAPs, preferably with an automated threshold-suspend feature, where available, or very frequent contact with a specialized hypoglycemia service in patients whose problematic hypoglycemia persists despite the use of structured education and other diabetes technologies. |
| 4 | Consult with transplant program (and payer) about the eligibility of selected patients with persistent problematic hypoglycemia for an islet or a pancreas transplant when other interventions have not been effective and when the risk-benefit ratio is deemed favorable. |
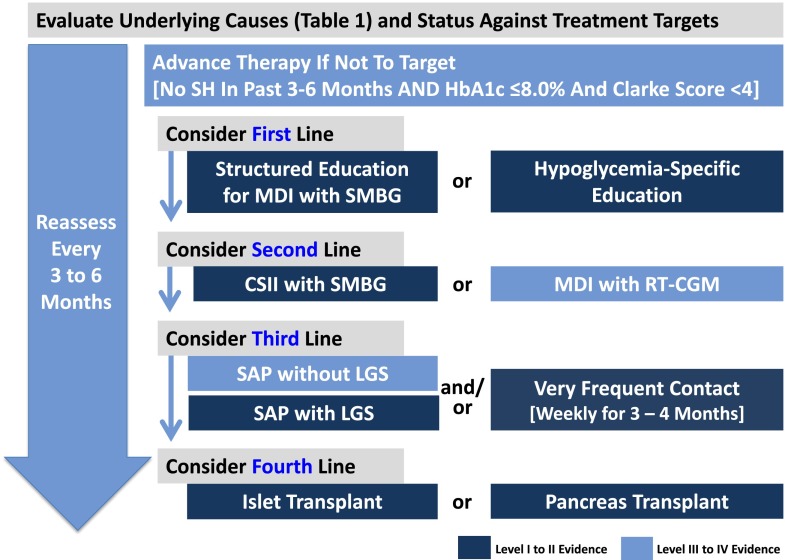
Figure 1.
Proposed treatment algorithm for patients with T1D and problematic hypoglycemia.
Educational Interventions
Mühlhauser et al. (33) demonstrated that intensified insulin therapy, when combined with a 5-day teaching program, improved glycemic control without increasing SH. A similar program was called Dose Adjustment For Normal Eating (DAFNE) (16). Such educational interventions typically consist of a 30- to 40-h group-learning curriculum based on adult learning principles around carbohydrate counting, frequent self-monitoring of blood glucose (SMBG), and adjustment of insulin doses in response to exercise, alcohol, and illness. To differentiate it from fixed-dose multiple daily injections (MDIs), active adjustment of insulin doses is often termed “functional insulin therapy.”
Large-scale audits of educational interventions in various countries found reliable and sustained (up to 6 years) reductions in the incidence of SH by 50–70% as well as an improvement in the mean HbA1c level to ∼7.6% (60 mmol/mol) in unselected patients with T1D (16,34). A subanalysis of 341 patients with three or more episodes of SH in the prior year demonstrated reductions in both the incidence of SH (from 6.1 to 1.4 per patient annually) and the mean length of hospitalization (from 8.6 to 3.9 days per patient annually) as well as improvements in the mean HbA1c level (34,35). The DAFNE program showed similar reductions in the incidence of SH, with restoration of hypoglycemia recognition in 43% of patients reporting IAH at baseline.
Blood glucose awareness training (BGAT) is a psychoeducational program developed by Cox et al. (36) to increase self-awareness of personal cues for detecting hypoglycemia. Originally developed as 8 weekly sessions, BGAT has undergone numerous iterations and has been successfully piloted as an online program. It has consistently resulted in improved detection of hypoglycemia, especially in patients with IAH and sustained reductions in SH and even in those with a high incidence of SH at baseline (37,38).
Few studies have examined educational interventions specifically in patients with IAH (Table 2). In the early 1990s, three small studies demonstrated improvement in symptom responses, with some (but variable) improvement in epinephrine responses after meticulous avoidance of hypoglycemia (39–42). Those improvements were achieved by extensive re-education, physiologic distribution of insulin with MDIs, and, most importantly, intensive contact and support (up to four telephone calls per day) from the study team.
Three large randomized clinical trials (RCTs) specifically recruited patients with problematic hypoglycemia. Cox et al. (43) randomized Bulgarian patients with very little access to SMBG and at least three episodes of SH annually to SMBG with and without hypoglycemia anticipation, awareness, and treatment training (HAATT) and found that the incidence of SH decreased with HAATT but not with increased SMBG alone.
Hermanns et al. (44) randomized 164 patients with more than one episode of SH or with IAH despite MDI and continuous subcutaneous insulin infusion (CSII) to either a hypoglycemia-specific education program (HyPOS) or a standard education program. They demonstrated greater improvement in awareness with HyPOS with a trend to a lower incidence of SH 1 year after completing HyPOS that became statistically significant at 31 months of follow-up.
Decision making is central to hypoglycemia avoidance, and qualitative research has identified fear of hypoglycemia and lack of concern regarding hypoglycemia as important factors beyond skills and education that predispose to IAH (45,46). These findings may explain the success of frequent contact and behavioral interventions such as BGAT and HAATT. The DAFNE-Hypoglycemia Awareness Restoration Training (HART) pilot study evaluated a different strategy built on behavioral changes identified through qualitative interviewing in patients with IAH (46), incorporating aspects of BGAT but delivered using cognitive behavioral therapy and motivational interviewing techniques. Of the 24 patients with SH despite previous treatments (including DAFNE), 17 experienced complete resolution of SH, and the remaining 7 experienced significant reductions in the incidence of SH (47).
The Comparison of Optimized MDI Versus Pumps With or Without Sensors in Severe Hypoglycemia (HypoCOMPaSS) trial randomized 96 patients with long-standing (mean duration 28 years) diabetes, IAH, and previous recurrent SH (mean 8.9 episodes per patient annually) in a 2 × 2 fashion to SMBG or real-time continuous glucose monitoring (RT-CGM) and MDI or CSII (48). All four arms underwent a 2-h standardized education program emphasizing rigorous avoidance of hypoglycemia, including advice on never delaying treatment, recognizing times of increased risk, detecting subtle symptoms, and confirming low readings through frequent SMBG. In addition, all patients had weekly telephone contact and monthly face-to-face visits with the study team. The educational intervention led to significant reductions in SH (from 8.9 to 0.8 episodes per patient annually) and to improvement in awareness scores irrespective of treatment allocation.
Thus, current data highlight the importance of structured education as an essential baseline strategy to reduce the proportion of patients with problematic hypoglycemia. Relatively inexpensive programs such as BGAT and DAFNE can reduce the incidence of SH by 50% and improve HbA1c levels and QoL in patients with long-standing (>15 years) T1D who continue to experience SH with associated IAH and excessive glycemic variability despite effective education and intensive insulin therapy. Intensive and frequent contact with health care providers appears to be the most effective therapy, yet such patients still have a higher incidence of SH than the general T1D population so may require more advanced therapies.
Technological Interventions
Before implementing diabetes technologies in treatment, insulin therapies should be optimized, with preference given to insulin analogs. Most studies of insulin analogs have excluded high-risk patients with prior SH or IAH (49) and used a noninferiority design and strict, treat-to-target dosing algorithms (50) so are likely to have underestimated benefits for patients with problematic hypoglycemia. Rapid-acting insulin analogs (aspart, glulisine, lispro) with faster onset and shorter duration than regular insulin are associated with a 20% reduction in the incidence of SH and a 45% reduction in the incidence of nocturnal hypoglycemia (51). Basal analogs, which have less intraindividual variability in bioavailability than neutral protamine Hagedorn (NPH) insulin (52), are also effective, providing a 27% reduction in the incidence of SH and a 31% reduction in the incidence of nocturnal hypoglycemia compared with NPH insulin (53,54). Newer basal insulin analogs (e.g., degludec and U300 glargine) may be even less variable and further reduce hypoglycemia risk. Studies of degludec found a 25% reduction in the incidence of symptomatic nocturnal hypoglycemia but no difference in the incidence of SH (55). In the single trial conducted in patients with problematic hypoglycemia, insulin analogs reduced the incidence of SH by 29% compared with regular and NPH insulin (56).
The effectiveness of CSII in patients with problematic hypoglycemia has not been tested in robust RCTs, but a meta-analysis found a fourfold reduction in the incidence of SH and a 0.6% improvement in HbA1c level with CSII (57). Older age and more frequent episodes of SH were independent predictors of a reduced incidence of SH with CSII. A small pilot study of CSII in 19 patients with IAH demonstrated restoration of awareness in 16 at 1 year (58).
Although a cornerstone of therapy in T1D, SMBG may only be effective in reducing the incidence of SH when combined with education (e.g., HAATT) (43). Although blinded CGM may be useful as a diagnostic tool to identify periods of hypoglycemia, a systematic review of blinded CGM versus SMBG did not demonstrate improved HbA1c levels and was unable to analyze hypoglycemia rates (because of heterogeneity in definitions and assessment of hypoglycemia) (59). In contrast, a meta-analysis showed that RT-CGM, which can alert patients to impending hypoglycemia, decreased HbA1c levels without increasing hypoglycemic episodes (60). Because patients with prior SH or IAH were excluded from this study, the proportion with major hypoglycemic episodes was numerically lower with RT-CGM but was not significantly different from the proportion with SMBG.
In the context of intensive education and frequent contact, no differences in reducing the incidence of SH or in restoring awareness were found between MDI versus CSII and SBGM versus RT-CGMS in the HypoCOMPaSS trial. CGM tended to show greater reductions in the incidence of SH possibly because of a higher incidence of SH at baseline (48). A substudy of the HypoCOMPaSS trial found improvements in symptom and catecholamine responses to experimental hypoglycemia, with trends to greater improvements with CSII and to a lesser degree with CGM (61).
Clinical trials of sensor-augmented pumps (SAPs), which are CSII devices with an integrated CGM system, have found superior glycemic control but no difference in the incidence of SH compared with MDI (and SMBG) (62). Many patients were noted to sleep through nocturnal alarms (63). One RCT of a device with an automated threshold-suspend feature (where insulin delivery is automatically suspended for up to 2 h if the sensor glucose falls below a prespecified threshold) demonstrated a 38% reduction in the duration of nocturnal hypoglycemia (64) compared with SAP. Another RCT in children and adolescents with IAH showed a reduction in the incidence of SH with SAP compared with CSII alone but no improvement in the epinephrine response to experimental hypoglycemia (65). A small observational study demonstrated significant reductions in the incidence of SH (from 8.1 to 0.6 episodes per patient annually) using SAP in patients with IAH but no improvement in awareness (66).
Clinical trials of fully automated integrated CGM/CSII technologies (artificial pancreas) have been reported (67,68). Some have used dual pumps to administer both insulin and glucagon, although glucagon was not required to protect from nocturnal hypoglycemia in a head-to-head study (69). Although potentially of great benefit, these technologies have not yet been evaluated in patients with problematic hypoglycemia and are not yet commercially available.
Transplant Interventions
Pancreas and, now, islet transplants can effectively prevent hypoglycemia and restore normoglycemia and may stabilize the complications of T1D (70–75). Patients with T1D who undergo an islet or a pancreas transplant exhibit recovery of physiologic islet cell hormonal responses to insulin-induced hypoglycemia whereby endogenous insulin secretion is suppressed and glucagon secretion restored (76,77), although in islet transplant recipients, the glucagon response remains partial likely due to lower islet mass being transplanted as evidenced from β-cell secretory capacity testing (78,79). Both islet and pancreas transplant recipients also have improved epinephrine and normalized autonomic symptom responses to hypoglycemia, providing evidence of amelioration of hypoglycemia-associated autonomic failure (HAAF) (76,77). These improved counterregulatory defense mechanisms may be sustained for more than a decade of pancreas graft function (80). Most importantly, islet and pancreas transplantation have been shown to normalize the endogenous (predominantly hepatic) glucose production response to insulin-induced hypoglycemia (77,81), thereby affording recipients protection and recovery from low blood glucose.
Pancreata procured from leaner and younger donors often are preferred for whole-organ pancreas transplants, whereas islets can be isolated from obese and older donors (82) unsuitable for whole-organ transplants, thereby increasing the proportion of donated organs that can contribute to the treatment of T1D. Thus, islet and pancreas transplants are evolving as complementary approaches to β-cell replacement for the elimination of problematic hypoglycemia in T1D.
Most pancreas transplants are performed simultaneously with a kidney transplant. Simultaneous pancreas-kidney (SPK) transplants confer superior long-term graft function compared with pancreas transplant alone or pancreas transplant after a kidney transplant. The 5-year pancreas graft survival rate for recipients of pancreas transplant alone and pancreas transplant after a kidney transplant is between 55 and 70%; for SPK recipients, it is >85% (83). With SPK transplants, most recipients can expect amelioration of problematic hypoglycemia for more than a decade (83–85).
Pancreas transplants are usually undertaken in patients who are relatively young (<50 years) and nonobese (<30 kg/m2) and who do not have coronary artery disease. These patient selection criteria minimize operative mortality (<1%) and reduce early technical pancreas graft loss (∼10%) (86,87). Removal of technically failed grafts and routine complications of abdominal surgery have led to a reoperation rate as high as 40% (85).
Islet transplantation, a minimally invasive procedure, allows for inclusion of older patients and patients with coronary artery disease who would be ineligible for a whole-pancreas transplant. In nonobese recipients, the target islet dose of ≥5,000 islet equivalents/kg can be isolated from a deceased donor pancreas (71). Most islet transplants are performed in nonuremic patients with T1D and problematic hypoglycemia and related excessive glycemic lability (12,71,88). Careful selection of patients and protocol optimization have led to substantial clinical improvements (71). Importantly, refined recipient treatment has improved long-term outcomes of islet transplants; insulin independence can now be maintained for 5 years in 50% of recipients (89,90).
Although restoring insulin independence remains an important objective, several multicenter clinical trials of islet transplants in patients with T1D and problematic hypoglycemia, including the phase 3 licensure trial of human islets conducted by the Clinical Islet Transplantation Consortium, have adopted a combination of near-normal glycemic control (HbA1c <7.0% [53 mmol/mol]) together with the elimination of SH as the primary end point and the clinically relevant dual goal of intervention (91–93). After having reported successful achievement of that goal in 82% of patients at 1 year (92) and in 70% at 2 years posttransplant (93), islet transplants are now approved and reimbursed in several countries for the treatment of problematic hypoglycemia in T1D. In the U.S., although a phase 3 trial of islet transplants in this patient population has been completed, a formal license application awaits submission, review, and approval.
Even with partial islet graft function, the endogenous glucose production response to insulin-induced hypoglycemia improves (94), so islet grafts protect against problematic hypoglycemia even when insulin may be required to maintain near-normoglycemia. This protection from hypoglycemia has been confirmed in CGM studies showing a near absence of time at glucose <70 mg/dL/<3.9 mmol/L (77). The reductions in mean glucose, glucose variability, and time spent hypoglycemic (<54 mg/dL/<3.0 mmol/L) relative to T1D were similar for both insulin-independent and insulin-requiring islet recipients (95), and those reductions were sustained for 18 months in one study (96). Importantly, Vantyghem et al. (23) showed that minimal islet graft function is sufficient to abrogate hypoglycemia (<54 mg/dL/<3.0 mmol/L), confirming that even suboptimal function (requiring insulin) significantly improves mean glucose and glucose variability. That the islet graft imparts these glycemic control benefits has been further supported by the demonstration of significant continuous associations with stimulated C-peptide levels in islet transplant recipients (97). This avoidance of hypoglycemia with islet or pancreas transplants as documented by CGM best explains the documented reversal of HAAF as well as the recovery of glucose counterregulation and hypoglycemia symptom recognition, thereby reversing the vicious hypoglycemia-begets-hypoglycemia cycle in T1D (98). Data from the Collaborative Islet Transplant Registry indicate that problematic hypoglycemia ameliorated for the duration of islet graft function is currently retained in 90% of recipients at 4 years posttransplant (71).
In addition to procedural risks and limited organ availability, the current need for lifelong immunosuppressive therapy represents a major limitation to widespread implementation of β-cell replacement therapies for patients with problematic hypoglycemia refractory to educational and technological interventions. Because kidney transplant recipients are already committed to immunosuppressive therapy, the addition of an islet or pancreas transplant may be considered to normalize glycemia, stabilize diabetes complications, and prevent recurrent diabetic nephropathy. In such T1D kidney transplant recipients, islet transplantation can be considered simultaneously with or after a kidney transplant for patients who are not surgical candidates for or willing to accept the risks of a pancreas transplant (85). At 5 years (99) and 13 years (85) posttransplant, the insulin independence rate was higher for pancreas than for islet recipients; however, the islet recipients experienced significantly fewer operative complications, and both the pancreas and islet recipients experienced significantly improved glycemic control and a reduction of >90% in the incidence of SH (85).
Thus, regardless of β-cell replacement approach (pancreas or islets), the majority of recipients can anticipate amelioration from problematic hypoglycemia for at least 5 years together with near-normal glycemic control. In fact, islet and pancreas transplants are the only approaches to date that confer both sustained recovery from HAAF and restoration of glucose counterregulation (by endogenous glucose production) and, thereby, reliable protection from SH in patients with long-standing (>15 years) T1D.
Treatment Algorithm
The treatment algorithm has to take into account the number of patients with T1D who have problematic or recurrent SH, existing educational tools and technologies to reduce hypoglycemia, and the available resources for various treatment strategies, including transplants. Individual countries or reimbursement plans might need to adapt the algorithm in accordance with their resources.
Individualized treatment targets must balance the risk of complications with that of hypoglycemia in an effort to achieve the lowest attainable HbA1c level without problematic hypoglycemia (32). Published studies have indicated that educational and technological interventions can prevent SH while maintaining HbA1c levels between 7.2 and 8.0% (55 and 64 mmol/mol) (Table 2).
Many patients rarely or never inform their physician about episodes of hypoglycemia (100); therefore, health care practitioners must routinely inquire about a given patient’s frequency of and risk factors for hypoglycemia and glycemic lability (12) and must screen for impaired awareness. Commonly used validated scores are those by Clarke et al. (10) and Gold et al. (11) where a score ≥4 indicates hypoglycemia unawareness, but increasingly, other measures are used, such as glucose SD (>40 mg/dL/>2.8 mmol/L) (23), low blood glucose index (101), or average daily risk range (102).
The Four Stages of the Proposed Tiered Algorithm
Stage 1
All MDI therapy patients using SMBG should have routine evaluation of hypoglycemia awareness status using validated scores along with assessment of glycemic control. Patients with problematic hypoglycemia should be regularly evaluated for possible underlying causes of hypoglycemia (Table 1), for the presence of hypoglycemia unawareness, and for meeting their own individual HbA1c level and other treatment targets (32). If the patient is not at target, first-line therapy is a structured education or hypoglycemia-specific education program (Fig. 1). A robust evidence base supports functional insulin therapy (33) through educational programs such as DAFNE (16) and behavioral interventions such as BGAT (36). These programs reduce the incidence of SH by 50–70% and restore hypoglycemia awareness in up to 40% of patients (Table 2). Some data suggest additional benefits with programs focused on hypoglycemia avoidance, such as HyPOS, over standard education. The choice of insulin in patients with SH has been tested in only one trial, which demonstrated a 29% reduction in SH using analogs compared with regular or NPH insulin (56). But no data exist so far regarding the impact on IAH of newer insulins, such as degludec, U300 glargine, or pegylated insulin (103).
Stage 2
Robust evidence exists for the use of CSII as second-line therapy to reduce SH (57). Although a paucity of randomized evidence supports the use of RT-CGM and less evidence supports the use of RT-CGM in patients with T1D for the purpose of reducing the incidence of SH, this technology offers a logical step forward for individual patients. Limitations to the use of technology must be considered, and data suggest that CGM must be used continuously for sustained benefit (104).
Stage 3
If the composite treatment target of no SH, Clarke score <4, and HbA1c <8.0% (64 mmol/mol) is still not met (Fig. 1), the use of SAP, preferably with low-glucose suspension (LGS), and/or very frequent contact with a specialized hypoglycemia service should be considered for third-line therapy (Fig. 1). Small-scale nonrandomized trials of psychoeducational therapies such as DAFNE-HART and BGAT suggested that they offer further benefit, especially in patients with behavioral contributors to recurrent SH (39–41).
Because most trials of SAP in T1D excluded patients with problematic hypoglycemia, only anecdotal evidence supports the use of SAP alone for reducing the incidence of SH in this patient population. Studies using threshold suspension of insulin were successful in reducing hypoglycemia over and above SAP alone, suggesting that this may be the preferred evidence-based option in patients with T1D and problematic hypoglycemia (64,65). In patients with ongoing problematic hypoglycemia, very frequent and intensive contact with an expert diabetes team can restore their awareness of symptoms and, to some extent, counterregulatory responses, but such contact may not always be feasible in the clinical scenario. Weekly to monthly contact as offered in the HypoCOMPaSS trial seem to be effective in reducing the incidence of SH, but restoration of awareness is modest (Table 2).
The algorithm proposed in Fig. 1 shows the level of evidence supporting each intervention. The system should be flexible, and clinicians will make individual decisions based on specific circumstances, taking into account the preference of the patient as well as the patient’s involvement and possibilities. For certain patients with behavioral issues, further behavioral programs such as BGAT, HyPOS, or DAFNE-HART may be beneficial. For others, more expensive treatment such as CSII with and without RT-CGM may be more appropriate.
Stage 4
Pancreas and islet transplants, the fourth-line therapy, are very effective in achieving the composite target of eliminating SH with near-normal HbA1c levels, but lifelong immunosuppressive therapy and its possible complications represent a major limiting factor. Because islet and pancreas transplants are both effective in preventing SH and achieving near-normoglycemia, the optimal treatment option will require individualized discussion of multiple factors, including the procedural risks (which are higher for a pancreas transplant), importance of insulin independence, waiting time, and sensitization. Some contraindications to a pancreas transplant (age >50 years, high cardiac risk) are common in patients with problematic hypoglycemia; they may only be eligible for an islet transplant. Yet, a small proportion of patients may be ineligible for an islet transplant because of their weight or insulin requirements. The transplant team should consider each patient’s preferences and perceptions of risks and benefits.
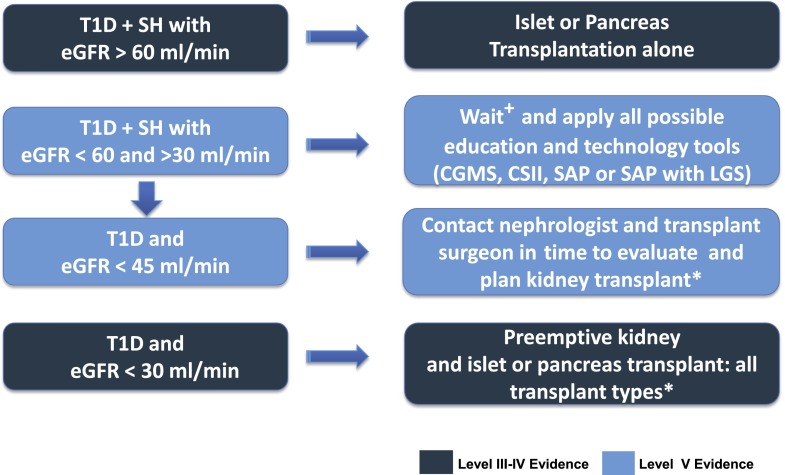
In the absence of contraindications, the main determinant of which type of transplant to choose is the patient’s kidney function. A living donor kidney transplant might be the best option for a given patient with chronic kidney disease followed by either an islet or a pancreas transplant (105). If a living donor is not available, then a simultaneous deceased donor kidney transplant and islet or pancreas transplant may be indicated (Fig. 2).
Figure 2.
Transplant options for patients with T1D (expert consensus). CGMS, continuous glucose monitoring system. +eGFR >30 and <60 mL/min/1.73 m2: islet or pancreas transplantation alone, high risk for developing end-stage renal disease under calcineurin-based immunosuppression. *All transplant types: living kidney and deceased donor islet or pancreas after kidney transplantation, simultaneous pancreas or islet kidney transplantation, or deceased donor kidney alone and islet or pancreas after kidney transplantation.
For patients requiring a kidney transplant, it is clear that both pancreas and islet transplants, either simultaneously or sequentially, are effective in preventing SH and protecting the kidney graft from hyperglycemia (85). However, how best to treat problematic hypoglycemia in patients with an intermediate estimated glomerular filtration rate (eGFR) (30–60 mL/min) (Fig. 2) is unclear because the immunosuppressive therapy required after a pancreas or islet transplant can increase the risk of end-stage renal failure in these patients (106,107).
Currently, organ donation rates in the U.S. and Europe are stalled at between 10 and 35 donors per million population (108,109), yet potentially 1,000 patients with T1D per million population are affected by recurrent SH (6–9). The organ shortage is the second major factor (in addition to lifelong immunosuppression) limiting β-cell replacement. Until new sources of β-cells are available, β-cell replacement by means of a whole-pancreas transplant or an isolated islet transplant will be limited to a carefully selected group of patients with problematic hypoglycemia refractory to medical and technological interventions.
Byrne et al. (110) demonstrated that specialized clinics with expertise in hypoglycemia management are essential to concentrating limited transplant resources for patients who need them most. Of 36 patients with recurrent SH referred to a specialized hypoglycemia service, 47.2% experienced resolution of their problematic hypoglycemia with optimal medical therapy, and another 25% achieved clinically relevant improvement. Of those highly selected patients, however, 27.8% required a transplant despite having elevated HbA1c levels of 8.0% (64 mmol/mol) and despite having access to all educational and technological interventions.
Another crucial factor is the reimbursement policy of each country or insurance plan. If an expensive technology option does not deliver the expected results within a 6-month period, it may be discontinued. In many countries, CSII is reimbursed by health insurance, but RT-CGM is not. The same is true for β-cell replacement; in many countries, pancreas transplants are reimbursed, but only in a much smaller number of countries are islet transplants reimbursed. The cost-effectiveness of these technologies has not been tested in larger populations; it will take many years to prove the cost savings or at least the cost-effectiveness, although QoL improves in most trials.
Clinical Research Needs
As new insulin analogs and devices become available, we recommend including in future trials and studies patients with recent SH or IAH and then separately reporting changes in HbA1c level, SH, and IAH in those with prior problematic hypoglycemia. Doing so would allow data on the impact of new therapies in this complex group of patients to become available sooner. Also crucial in evaluating new therapies are robust studies of changes in symptoms, counterregulatory hormone responses, and endogenous glucose production responses to experimental hypoglycemia.
Educational interventions have the evidence base to be recommended as first-line therapy. Further investigation into appropriate baseline factors that may aid personalization of treatment is desirable. In the home studies using single- or dual-hormone closed-loop systems now taking place, the role of such systems in patients with problematic hypoglycemia remains to be tested.
The effectiveness of CSII with SMBG and CGM with MDI in preventing SH in patients with IAH remains to be compared in larger trials. Although HypoCOMPaSS suggests equivalence, the technology selection may depend on the availability of systems, reimbursement policies, and patient choice. Also remaining to be studied is which patients with persistent problematic hypoglycemia despite structured education should use SAP as the next intervention.
In the field of transplantation, areas that require further clinical research include improvement of islet engraftment to prolong graft survival and minimization of immunosuppression through antigen-specific immunotherapy, adoptive transfer of immunoregulatory cells, and use of biocompatible immune-isolating devices. Moving forward, preclinical work is already showing potential for expanding the role of transplantation through the use of alternate tissue sources, such as porcine islet xenografts or human stem cell–derived insulin-secreting cells.
Conclusions
Problematic hypoglycemia is another complication of long-term T1D, causing morbidity and mortality in a significant proportion of patients. It is important to screen patients for problematic hypoglycemia using validated tools and to implement individualized therapeutic targets based on the balance between glycemic control and hypoglycemia risk. Problematic hypoglycemia can be resolved with appropriate educational and technological interventions in most patients with acceptable glycemic control; however, in a subset of patients, transplants offer the only solution. Especially for that subset, existing interventions need to be more thoroughly evaluated and new therapies developed.
Article Information
Acknowledgments. The authors thank Mary E. Knatterud, University of Minnesota, for expert editorial assistance.
Funding. P.C. is supported in part by Diabetes UK grants 14/0004865 and 13/0005643. M.R.R. is supported in part by U.S. Public Health Service research grants R01-DK-091331 and U01-DK-070430 from the National Institutes of Health. P.A.S. is supported by the Academic Alternate Relationship Plan and in part by Alberta Innovates Health Solutions and JDRF. M.-C.V. is supported by the French Ministry of Health (PHRC 2001, 2008, and 2009), European Community (Fond Européen de Développement Régional), Conseil Régional Nord Pas de Calais (IFR 114), European FP7, JDRF, Société Francophone du Diabète, Association de Recherche pour le Diabète, and Agence de Biomédecine. T.W.K. is supported by the National Health and Medical Research Council (APP1037321), JDRF, Diabetes Australia Research Trust, Operational Infrastructure Support scheme of the Victorian Government, and Nationally Funded Centres scheme. F.S. is supported in part by Ministry of Health of the Czech Republic grant NT14020-3/2013. B.J.H. is supported in part by U.S. Public Health Service research grants U01-AI-065193 and U01-AI-102463 from the National Institutes of Health and by research grants 17-2012-527 and 17-2013-495 from JDRF.
Duality of Interest. P.C. has participated on advisory boards for Medtronic, Johnson and Johnson, and Roche regarding insulin pumps and CGM and has participated in commercial research funded by Medtronic. P.A.S. has received research support from Novo Nordisk and Eli Lilly and consulting fees from Eli Lilly, Medtronic, and Novo Nordisk and is on the speakers bureau for Animas, Eli Lilly, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. P.C., M.R.R., P.A.S., and M.-C.V. researched data, conducted the systematic review, identified studies for inclusion in the review and rated their quality, and contributed to the writing, review, editing, and final approval of the manuscript. P.M., T.W.K., B.K., N.I., and F.S. contributed to the design of the review and writing, editing, and final approval of the manuscript. R.L. researched data, conducted the systematic review, identified studies for inclusion in the review and rated their quality, led the development of the comprehensive treatment algorithm, and contributed to the writing, review, editing, and final approval of the manuscript. B.J.H. developed the concept of a comprehensive treatment algorithm for patients with T1D and problematic hypoglycemia in collaboration with councilors of the International Pancreas and Islet Transplant Association, assembled the writing team, researched data, conducted the systematic review, identified studies for inclusion in the review and rated their quality, led the writing of the manuscript, and contributed to the review, editing, and final approval of the manuscript.
Footnotes
References
- 1.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008;57:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes 2010;59:2333–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol 2014;10:711–722 [DOI] [PubMed] [Google Scholar]
- 5.Workgroup on Hypoglycemia, American Diabetes Association . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245–1249 [DOI] [PubMed] [Google Scholar]
- 6.Pedersen-Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev 2004;20:479–486 [DOI] [PubMed] [Google Scholar]
- 7.UK Hypoglycaemia Study Group . Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007;50:1140–1147 [DOI] [PubMed] [Google Scholar]
- 8.Gruden G, Barutta F, Chaturvedi N, et al. Severe hypoglycemia and cardiovascular disease incidence in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 2012;35:1598–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstock RS, Xing D, Maahs DM, et al.; T1D Exchange Clinic Network . Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013;98:3411–3419 [DOI] [PubMed] [Google Scholar]
- 10.Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995;18:517–522 [DOI] [PubMed] [Google Scholar]
- 11.Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697–703 [DOI] [PubMed] [Google Scholar]
- 12.Ryan EA, Shandro T, Green K, et al. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes 2004;53:955–962 [DOI] [PubMed] [Google Scholar]
- 13.Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 2013;369:362–372 [DOI] [PubMed] [Google Scholar]
- 14.Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med 2008;25:501–504 [DOI] [PubMed] [Google Scholar]
- 15.Choudhary P, Geddes J, Freeman JV, Emery CJ, Heller SR, Frier BM. Frequency of biochemical hypoglycaemia in adults with type 1 diabetes with and without impaired awareness of hypoglycaemia: no identifiable differences using continuous glucose monitoring. Diabet Med 2010;27:666–672 [DOI] [PubMed] [Google Scholar]
- 16.Hopkins D, Lawrence I, Mansell P, et al. Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes: the U.K. DAFNE experience. Diabetes Care 2012;35:1638–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frier BM. Morbidity of hypoglycemia in type 1 diabetes. Diabetes Res Clin Pract 2004;65(Suppl. 1):S47–S52 [DOI] [PubMed]
- 18.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006;49:298–305 [DOI] [PubMed] [Google Scholar]
- 19.Feltbower RG, Bodansky HJ, Patterson CC, et al. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: results from the Yorkshire Register of diabetes in children and young adults. Diabetes Care 2008;31:922–926 [DOI] [PubMed] [Google Scholar]
- 20.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherr J, Tamborlane WV, Xing D, et al.; Diabetes Research in Children Network (DirecNet) Study Group . Achievement of target A1C levels with negligible hypoglycemia and low glucose variability in youth with short-term type 1 diabetes and residual β-cell function. Diabetes Care 2012;35:817–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter? Endocr Rev 2010;31:171–182 [DOI] [PubMed] [Google Scholar]
- 23.Vantyghem MC, Raverdy V, Balavoine AS, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (β-score greater than 7) Is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (β-score greater than 3). J Clin Endocrinol Metab 2012;97:E2078–E2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuckey HL, Mullan-Jensen CB, Reach G, et al. Personal accounts of the negative and adaptive psychosocial experiences of people with diabetes in the second Diabetes Attitudes, Wishes and Needs (DAWN2) study. Diabetes Care 2014;37:2466–2474 [DOI] [PubMed] [Google Scholar]
- 25.The Diabetes Control and Complications Trial Research Group . Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 26.Tsai EB, Sherry NA, Palmer JP, Herold KC. The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia 2006;49:261–270 [DOI] [PubMed] [Google Scholar]
- 27.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orchard TJ, Nathan DM, Zinman B, et al.; Writing Group for the DCCT/EDIC Research Group . Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stadler M, Peric S, Strohner-Kaestenbauer H, et al. Mortality and incidence of renal replacement therapy in people with type 1 diabetes mellitus—a three decade long prospective observational study in the Lainz T1DM cohort. J Clin Endocrinol Metab 2014;99:4523–4530 [DOI] [PubMed] [Google Scholar]
- 30.Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014;371:1972–1982 [DOI] [PubMed] [Google Scholar]
- 31.Schoenaker DA, Simon D, Chaturvedi N, Fuller JH, Soedamah-Muthu SS; EURODIAB Prospective Complications Study Group . Glycemic control and all-cause mortality risk in type 1 diabetes patients: the EURODIAB prospective complications study. J Clin Endocrinol Metab 2014;99:800–807 [DOI] [PubMed] [Google Scholar]
- 32.Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 2014;63:2188–2195 [DOI] [PubMed] [Google Scholar]
- 33.Mühlhauser I, Bruckner I, Berger M, et al. Evaluation of an intensified insulin treatment and teaching programme as routine management of type 1 (insulin-dependent) diabetes. The Bucharest-Düsseldorf Study. Diabetologia 1987;30:681–690 [DOI] [PubMed] [Google Scholar]
- 34.Sämann A, Mühlhauser I, Bender R, Kloos Ch, Müller UA. Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation study. Diabetologia 2005;48:1965–1970 [DOI] [PubMed] [Google Scholar]
- 35.Sämann A, Mühlhauser I, Bender R, Hunger-Dathe W, Kloos C, Müller UA. Flexible intensive insulin therapy in adults with type 1 diabetes and high risk for severe hypoglycemia and diabetic ketoacidosis. Diabetes Care 2006;29:2196–2199 [DOI] [PubMed] [Google Scholar]
- 36.Cox D, Gonder-Frederick L, Polonsky W, Schlundt D, Julian D, Clarke W. A multicenter evaluation of blood glucose awareness training-II. Diabetes Care 1995;18:523–528 [DOI] [PubMed] [Google Scholar]
- 37.Broers S, van Vliet KP, le Cessie S, Spinhoven P, van der Ven NC, Radder JK. Blood glucose awareness training in Dutch type 1 diabetes patients: one-year follow-up. Neth J Med 2005;63:164–169 [PubMed] [Google Scholar]
- 38.Schachinger H, Hegar K, Hermanns N, et al. Randomized controlled clinical trial of Blood Glucose Awareness Training (BGAT III) in Switzerland and Germany. J Behav Med 2005;28:587–594 [DOI] [PubMed] [Google Scholar]
- 39.Fanelli CG, Epifano L, Rambotti AM, et al. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes 1993;42:1683–1689 [DOI] [PubMed] [Google Scholar]
- 40.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 1994;344:283–287 [DOI] [PubMed] [Google Scholar]
- 41.Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes 1994;43:1426–1434 [DOI] [PubMed] [Google Scholar]
- 42.Fanelli C, Pampanelli S, Epifano L, et al. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of rational, intensive insulin therapy in IDDM (published correction appears in Diabetolgia 1995;38:254). Diabetologia 1994;37:1265–1276 [DOI] [PubMed] [Google Scholar]
- 43.Cox DJ, Kovatchev B, Koev D, et al. Hypoglycemia anticipation, awareness and treatment training (HAATT) reduces occurrence of severe hypoglycemia among adults with type 1 diabetes mellitus. Int J Behav Med 2004;11:212–218 [DOI] [PubMed] [Google Scholar]
- 44.Hermanns N, Kulzer B, Krichbaum M, Kubiak T, Haak T. Long-term effect of an education program (HyPOS) on the incidence of severe hypoglycemia in patients with type 1 diabetes. Diabetes Care 2010;33:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rankin D, Elliott J, Heller S, et al. Experiences of hypoglycaemia unawareness amongst people with Type 1 diabetes: a qualitative investigation. Chronic Illn 2013;10:180–191 [DOI] [PubMed] [Google Scholar]
- 46.Rogers HA, de Zoysa N, Amiel SA. Patient experience of hypoglycaemia unawareness in type 1 diabetes: are patients appropriately concerned? Diabet Med 2012;29:321–327 [DOI] [PubMed] [Google Scholar]
- 47.de Zoysa N, Rogers H, Stadler M, et al. A psychoeducational program to restore hypoglycemia awareness: the DAFNE-HART pilot study. Diabetes Care 2014;37:863–866 [DOI] [PubMed] [Google Scholar]
- 48.Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014;37:2114–2122 [DOI] [PubMed] [Google Scholar]
- 49.Little S, Shaw J, Home P. Hypoglycemia rates with basal insulin analogs. Diabetes Technol Ther 2011;13(Suppl. 1):S53–S64 [DOI] [PubMed] [Google Scholar]
- 50.Holleman F, Gale EA. Nice insulins, pity about the evidence. Diabetologia 2007;50:1783–1790 [DOI] [PubMed] [Google Scholar]
- 51.Singh SR, Ahmad F, Lal A, Yu C, Bai Z, Bennett H. Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis. CMAJ 2009;180:385–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004;53:1614–1620 [DOI] [PubMed] [Google Scholar]
- 53.Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues vs. NPH human insulin in type 1 diabetes. A meta-analysis. Diabetes Obes Metab 2009;11:372–378 [DOI] [PubMed] [Google Scholar]
- 54.Tricco AC, Antony J, Khan PA, et al. Safety and effectiveness of dipeptidyl peptidase-4 inhibitors versus intermediate-acting insulin or placebo for patients with type 2 diabetes failing two oral antihyperglycaemic agents: a systematic review and network meta-analysis. BMJ Open 2014;4:e005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heller S, Buse J, Fisher M, et al.; BEGIN Basal-Bolus Type 1 Trial Investigators . Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012;379:1489–1497 [DOI] [PubMed] [Google Scholar]
- 56.Pedersen-Bjergaard U, Kristensen PL, Beck-Nielsen H, et al. Effect of insulin analogues on risk of severe hypoglycaemia in patients with type 1 diabetes prone to recurrent severe hypoglycaemia (HypoAna trial): a prospective, randomised, open-label, blinded-endpoint crossover trial. Lancet Diabetes Endocrinol 2014;2:553–561 [DOI] [PubMed] [Google Scholar]
- 57.Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008;25:765–774 [DOI] [PubMed] [Google Scholar]
- 58.Giménez M, Lara M, Conget I. Sustained efficacy of continuous subcutaneous insulin infusion in type 1 diabetes subjects with recurrent non-severe and severe hypoglycemia and hypoglycemia unawareness: a pilot study. Diabetes Technol Ther 2010;12:517–521 [DOI] [PubMed] [Google Scholar]
- 59.Chetty VT, Almulla A, Odueyungbo A, Thabane L. The effect of continuous subcutaneous glucose monitoring (CGMS) versus intermittent whole blood finger-stick glucose monitoring (SBGM) on hemoglobin A1c (HbA1c) levels in type I diabetic patients: a systematic review. Diabetes Res Clin Pract 2008;81:79–87 [DOI] [PubMed] [Google Scholar]
- 60.Szypowska A, Ramotowska A, Dzygalo K, Golicki D. Beneficial effect of real-time continuous glucose monitoring system on glycemic control in type 1 diabetic patients: systematic review and meta-analysis of randomized trials. Eur J Endocrinol 2012;166:567–574 [DOI] [PubMed] [Google Scholar]
- 61.Leelarathna L, Little SA, Walkinshaw E, et al. Restoration of self-awareness of hypoglycemia in adults with long-standing type 1 diabetes: hyperinsulinemic-hypoglycemic clamp substudy results from the HypoCOMPaSS trial. Diabetes Care 2013;36:4063–4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bergenstal RM, Tamborlane WV, Ahmann A, et al.; STAR 3 Study Group . Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 63.Buckingham B, Block J, Burdick J, et al.; Diabetes Research in Children Network . Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther 2005;7:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergenstal RM, Klonoff DC, Garg SK, et al.; ASPIRE In-Home Study Group . Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–232 [DOI] [PubMed] [Google Scholar]
- 65.Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA 2013;310:1240–1247 [DOI] [PubMed] [Google Scholar]
- 66.Choudhary P, Ramasamy S, Green L, et al. Real-time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia-unaware patients with type 1 diabetes. Diabetes Care 2013;36:4160–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014;371:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011;342:d1855 [DOI] [PMC free article] [PubMed]
- 69.Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol 2015;3:17–26 [DOI] [PubMed] [Google Scholar]
- 70.Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol 2013;9:555–562 [DOI] [PubMed] [Google Scholar]
- 71.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care 2012;35:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niclauss N, Morel P, Berney T. Has the gap between pancreas and islet transplantation closed? Transplantation 2014;98:593–599 [DOI] [PubMed] [Google Scholar]
- 73.Bassi R, Fiorina P. Impact of islet transplantation on diabetes complications and quality of life. Curr Diab Rep 2011;11:355–363 [DOI] [PubMed] [Google Scholar]
- 74.Thompson DM, Meloche M, Ao Z, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation 2011;91:373–378 [DOI] [PubMed] [Google Scholar]
- 75.Robertson RP. Islet transplantation for type 1 diabetes, 2015: what have we learned from alloislet and autoislet successes? Diabetes Care 2015;38:1030–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kendall DM, Rooney DP, Smets YF, Salazar Bolding L, Robertson RP. Pancreas transplantation restores epinephrine response and symptom recognition during hypoglycemia in patients with long-standing type I diabetes and autonomic neuropathy. Diabetes 1997;46:249–257 [DOI] [PubMed] [Google Scholar]
- 77.Rickels MR, Fuller C, Dalton-Bakes C, et al. Restoration of glucose counterregulation by islet transplantation in long-standing type 1 diabetes. Diabetes 2015;64:1713–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rickels MR, Mueller R, Teff KL, Naji A. β-Cell secretory capacity and demand in recipients of islet, pancreas, and kidney transplants. J Clin Endocrinol Metab 2010;95:1238–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rickels MR, Liu C, Shlansky-Goldberg RD, et al. Improvement in β-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes 2013;62:2890–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paty BW, Lanz K, Kendall DM, Sutherland DE, Robertson RP. Restored hypoglycemic counterregulation is stable in successful pancreas transplant recipients for up to 19 years after transplantation. Transplantation 2001;72:1103–1107 [DOI] [PubMed] [Google Scholar]
- 81.Barrou Z, Seaquist ER, Robertson RP. Pancreas transplantation in diabetic humans normalizes hepatic glucose production during hypoglycemia. Diabetes 1994;43:661–666 [DOI] [PubMed] [Google Scholar]
- 82.Matsumoto I, Sawada T, Nakano M, et al. Improvement in islet yield from obese donors for human islet transplants. Transplantation 2004;78:880–885 [DOI] [PubMed] [Google Scholar]
- 83.Gruessner AC, Sutherland DE, Gruessner RW. Long-term outcome after pancreas transplantation. Curr Opin Organ Transplant 2012;17:100–105 [DOI] [PubMed] [Google Scholar]
- 84.Sollinger HW, Odorico JS, Becker YT, D’Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg 2009;250:618–630 [DOI] [PubMed] [Google Scholar]
- 85.Lehmann R, Graziano J, Brockmann J, et al. Glycemic control in simultaneous islet-kidney versus pancreas-kidney transplantation in type 1 diabetes: a prospective 13-year follow-up. Diabetes Care 2015;38:752–759 [DOI] [PubMed] [Google Scholar]
- 86.Gruessner RW, Dunn DL, Gruessner AC, Matas AJ, Najarian JS, Sutherland DE. Recipient risk factors have an impact on technical failure and patient and graft survival rates in bladder-drained pancreas transplants. Transplantation 1994;57:1598–1606 [PubMed] [Google Scholar]
- 87.Kandaswamy R, Sutherland DE. Pancreas versus islet transplantation in diabetes mellitus: how to allocate deceased donor pancreata? Transplant Proc 2006;38:365–367 [DOI] [PubMed] [Google Scholar]
- 88.Senior PA, Bellin MD, Alejandro R, et al. Consistency of quantitative scores of hypoglycemia severity and glycemic lability and comparision with continuous glucose monitoring system measures in long-standing type 1 diabetes. Diabetes Technol Ther 2015;17:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 2012;12:1576–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qi M, Kinzer K, Danielson KK, et al. Five-year follow-up of patients with type 1 diabetes transplanted with allogeneic islets: the UIC experience. Acta Diabetol 2014;51:833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clinical Islet Transplantation Consortium. Available from http://www.isletstudy.org. Accessed 2 February 2015
- 92.O’Connell PJ, Holmes-Walker DJ, Goodman D, et al.; Australian Islet Transplant Consortium . Multicenter Australian trial of islet transplantation: improving accessibility and outcomes. Am J Transplant 2013;13:1850–1858 [DOI] [PubMed] [Google Scholar]
- 93.Brooks AM, Walker N, Aldibbiat A, et al. Attainment of metabolic goals in the integrated UK islet transplant program with locally isolated and transported preparations. Am J Transplant 2013;13:3236–3243 [DOI] [PubMed] [Google Scholar]
- 94.Ang M, Meyer C, Brendel MD, Bretzel RG, Linn T. Magnitude and mechanisms of glucose counterregulation following islet transplantation in patients with type 1 diabetes suffering from severe hypoglycaemic episodes. Diabetologia 2014;57:623–632 [DOI] [PubMed] [Google Scholar]
- 95.Paty BW, Senior PA, Lakey JR, Shapiro AM, Ryan EA. Assessment of glycemic control after islet transplantation using the continuous glucose monitor in insulin-independent versus insulin-requiring type 1 diabetes subjects. Diabetes Technol Ther 2006;8:165–173 [DOI] [PubMed] [Google Scholar]
- 96.Gorn L, Faradji RN, Messinger S, et al. Impact of islet transplantation on glycemic control as evidenced by a continuous glucose monitoring system. J Diabetes Sci Tech 2008;2:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brooks AM, Oram R, Home P, Steen N, Shaw JA. Demonstration of an intrinsic relationship between endogenous C-peptide concentration and determinants of glycemic control in type 1 diabetes following islet transplantation. Diabetes Care 2015;38:105–112 [DOI] [PubMed] [Google Scholar]
- 98.Cryer PE. Hypoglycemia begets hypoglycemia in IDDM. Diabetes 1993;42:1691–1693 [DOI] [PubMed] [Google Scholar]
- 99.Gerber PA, Pavlicek V, Demartines N, et al. Simultaneous islet-kidney vs pancreas-kidney transplantation in type 1 diabetes mellitus: a 5 year single centre follow-up. Diabetologia 2008;51:110–119 [DOI] [PubMed] [Google Scholar]
- 100.Östenson CG, Geelhoed-Duijvestijn P, Lahtela J, Weitgasser R, Markert Jensen M, Pedersen-Bjergaard U. Self-reported non-severe hypoglycaemic events in Europe. Diabet Med 2014;31:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998;21:1870–1875 [DOI] [PubMed] [Google Scholar]
- 102.Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care 2006;29:2433–2438 [DOI] [PubMed] [Google Scholar]
- 103.Bode BW, Buse JB, Fisher M, et al.; BEGIN® Basal-Bolus Type 1 Trial Investigators . Insulin degludec improves glycaemic control with lower nocturnal hypoglycaemia risk than insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN(®) Basal-Bolus Type 1): 2-year results of a randomized clinical trial. Diabet Med 2013;30:1293–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wiseman AC. Pancreas transplant options for patients with type 1 diabetes mellitus and chronic kidney disease: simultaneous pancreas kidney or pancreas after kidney? Curr Opin Organ Transplant 2012;17:80–86 [DOI] [PubMed] [Google Scholar]
- 106.Maffi P, Bertuzzi F, De Taddeo F, et al. Kidney function after islet transplant alone in type 1 diabetes: impact of immunosuppressive therapy on progression of diabetic nephropathy. Diabetes Care 2007;30:1150–1155 [DOI] [PubMed] [Google Scholar]
- 107.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003;349:931–940 [DOI] [PubMed] [Google Scholar]
- 108.Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the United States, 1999-2008. Am J Transplant 2010;10:973–986 [DOI] [PubMed] [Google Scholar]
- 109.Matesanz R, Domínguez-Gil B, Coll E, de la Rosa G, Marazuela R. Spanish experience as a leading country: what kind of measures were taken? Transpl Int 2011;24:333–343 [DOI] [PubMed] [Google Scholar]
- 110.Byrne ML, Hopkins D, Littlejohn W, et al. Outcomes for adults with type 1 diabetes referred with severe hypoglycaemia and/or referred for islet transplantation to a specialist hypoglycaemia service. Horm Metab Res 2015;47:9–15 [DOI] [PubMed] [Google Scholar]