Abstract
Insulin signaling in the liver blunts glucose production and stimulates triglyceride biosynthesis. FoxO1 is required for cAMP induction of hepatic glucose production and is permissive for the effect of insulin to suppress this process. Moreover, FoxO1 ablation increases lipogenesis. In this study, we investigated the pleiotropic actions of FoxO1 on glucose and lipid metabolism. To this end, we reconstituted FoxO1 function in mice with a liver-specific deletion of Foxo1 using targeted knock-in of an allele encoding a DNA binding–deficient FoxO1 mutant (L-DBD). Chow-reared L-DBD mice showed defects in hepatic glucose production but normal liver triglyceride content despite increased rates of de novo lipogenesis and impaired fatty acid oxidation in isolated hepatocytes. Gene expression studies indicated that FoxO1 regulates the expression of glucokinase via a cell-nonautonomous coregulatory mechanism, while its regulation of glucose-6-phosphatase proceeds via a cell-autonomous action as a direct transcriptional activator. These conclusions support a differential regulation of hepatic glucose and lipid metabolism by FoxO1 based on the mechanism by which it alters the expression of key target genes involved in each process.
Introduction
Hepatic insulin resistance is a hallmark of type 2 diabetes (1). In addition to causing an increase in the rate of glucose production, hepatic insulin resistance is also associated with multiple abnormalities of lipid metabolism, including increased triglyceride (TG) synthesis, accumulation, and secretion as VLDL (2). This association represents an unmet challenge to our basic understanding of the pathophysiology of diabetes, as well as a conundrum for the design of clinically useful insulin sensitizers (3). Thus, the identification of signaling nodes regulating these conjoined processes has widespread implications.
The forkhead transcription factor FoxO1 is a lynchpin of the control of hepatic glucose production (HGP) by insulin (4–6). Liver-specific deletion of FoxO1 (L-FoxO1) impairs cAMP induction of glucose-6-phosphatase (G6pc), resulting in increased insulin sensitivity and fasting hypoglycemia (5,7). Conversely, a constitutively active FoxO1 prevents the ability of insulin to curtail HGP (4,8). In addition, FoxO1 regulates hepatic lipid metabolism in multiple ways (9–11), including via its control of bile acid pool composition (12).
FoxO1 can regulate gene expression either by direct DNA binding or by acting as a transcriptional coregulator (13–15). However, it remains unclear whether FoxO1 regulation of hepatic glucose and lipid metabolism requires DNA binding. Understanding the mechanism by which FoxO1 regulates these processes may therefore allow for novel therapeutic approaches to this well-established mediator of diabetes pathophysiology. We have applied a genetic approach to address this question. We reintroduced an allele encoding a DNA binding–deficient (DBD) FoxO1 mutant in mice with a liver-specific FoxO1 knockout (L-FoxO1), and investigated the resulting phenotype. We show that the DBD mutant fails to restore glucose production in vivo, and is unable to suppress lipogenesis and activate lipid oxidation in primary hepatocytes. The data raise the possibility that FoxO1 controls glucose metabolism by functioning as a transcription factor, while regulating lipid metabolism both as a transcription factor and as a transcriptional coregulator.
Research Design and Methods
Mice and Diets
Male mice aged 12–20 weeks were used for all experiments. L-FoxO1 and L-FoxO1,3,4 mice have been previously described (5,7). Heterozygous DBD knock-in mice were generated through homologous recombination by recombinase-mediated cassette exchange (16,17). Targeting vector and genotyping primer sequences are available upon request. Mice were weaned at 3 weeks of age to a standard (chow) diet. A Western-type diet (WTD) (21% anhydrous milk fat, 34% sucrose, 0.2% cholesterol; Harlan) was fed to animals as indicated beginning at 6 weeks of age for 10 weeks. The Columbia University Institutional Animal Care and Use Committee approved all animal procedures.
Metabolic Testing
Body composition analysis of ad libitum–fed adult male mice was performed via MRI (Bruker Optics). Overnight fasts were conducted for 16 h, from 1700 to 0900 h. Mice to be refed were then given ad libitum access to chow from 0900 to 1300 h. Blood glucose measurements were made from tail vein blood using OneTouch glucose monitor and strips, immediately before mice were killed (LifeScan). Measurements of insulin and lipids were made by ELISA (Mercodia) and colorimetric assays (for nonesterified fatty acids and cholesterol; Wako; and for TG; Thermo Scientific), respectively, using blood collected by cardiac puncture immediately after killing of the mice. Intraperitoneal glucose and pyruvate tolerance tests (PTT) were performed in overnight-fasted mice using a dose of 2 g/kg dextrose aqueous (aq) or sodium pyruvate (aq); intraperitoneal insulin tolerance tests were performed in 5-h–fasted mice using a dose of 0.8 units/kg Novolog insulin (Novo Nordisk). Oral lipid tolerance tests (OLTTs) and TG secretion experiments were performed in mice that had been fasted for 5 h. An OLTT was performed using olive oil administered orally at 10 μL/g body wt. TG secretion was measured after intraperitoneal injection of Poloxamer 407 (aq) at 10 μL/g. In both cases, tail vein blood was collected at indicated time points, and TG content was measured by colorimetric assay. Hepatic lipids were extracted from ∼50 mg snap-frozen tissue samples using the method of Folch, as previously described (18). TG and cholesterol contents were assayed colorimetrically and normalized to sample weight (12).
Luciferase Assays
HEK293 cells were transiently transfected with plasmids encoding Foxo1wt, Foxo1dbd, or empty vector as well as 3× insulin-responsive element-luciferase reporter plasmid or empty vector using Lipofectamine 2000 (Invitrogen) in DMEM supplemented with 10% FBS. Thirty-six hours after the transfection of plasmids, media was changed to serum-free DMEM. Twelve hours after serum starvation, cells were lysed and luciferase assay was performed using the Dual Luciferase Reporter Assay System (Promega) in a Monolight 310 luminometer (PharMingen).
Primary Hepatocyte Studies
Primary hepatocytes were isolated from male mice via collagenase perfusion, as previously described (8). After attachment to collagen-coated cultureware, cells were washed with PBS and incubated in serum-free medium (Medium 199 plus 1% BSA, always weight for volume [w/v]) overnight except for β-oxidation experiments. For glucose production assay, serum-free medium was replaced with glucose production medium (glucose-free and phenol red–free DMEM supplemented with 1% BSA, 3.3 g/L NaHCO3, 20 mmol/L calcium lactate, and 2 mmol/L sodium pyruvate). Cells were incubated with 100 μmol/L 8-(4-chlorophenylthio) (CPT)-cAMP (Sigma-Aldrich) plus 1 μmol/L dexamethasone (dex) (Sigma-Aldrich) or vehicle for 6 h. At indicated time points, aliquots of medium were sampled and centrifuged, and the glucose content was measured via peroxidase-glucose oxidase assay (Sigma-Aldrich) and normalized to protein content. For gene expression data, after overnight serum starvation cells were incubated for 6 h in serum-free medium containing either vehicle or 100 μmol/L 8-CPT-cAMP plus 1 μmol/L dex with or without 100 nmol/L insulin (Novolog) and were lysed for RNA extraction. For de novo lipogenesis (DNL), after overnight serum starvation, medium was changed to serum-free medium with or without 10 nmol/L insulin. After 2 h, the medium was spiked with 0.6 µCi/mL [1,2-14C]-acetic acid (PerkinElmer Life Sciences) and incubated for an additional 3 h. Lipids were extracted using 3:2 hexane:isopropanol dried in glass scintillation vials under N2 gas and resuspended in 2:1 chloroform:methanol. For total DNL, resuspended lipids were analyzed by liquid scintillation counting. For measurement of TG, resuspended samples were transferred onto thin-layer chromatography (TLC) plates using a SpotOn TLC Sample Applicator (Analtech). TLC was performed using a mobile phase of 70:30:1 hexane:diethyl ether:acetic acid. Areas of silica containing TGs, as identified by staining with iodine vapor, were scraped into glass scintillation vials and radiolabeled TGs were then counted using a liquid scintillation counter (PerkinElmer). Counts were normalized to total cellular protein.
For fatty acid oxidation (FAO), 24 h after plating, cells were washed three times with PBS, and incubated for 4 h with Medium 199 supplemented with 1.5% fatty acid–free BSA, 0.2 mmol/L unlabeled oleic acid, and 1 µCi/mL [1-14C]-oleic acid. Media from each well were transferred to glass Erlenmeyer flasks sealed with rubber plugs containing a suspended center well holding alkalinized filter paper. A 70% perchloric acid solution was injected into each flask. Flasks were then agitated at room temperature for 1 h. The radiolabeled CO2 content of each filter paper was then assessed by scintillation counting and normalized to total cellular protein after correcting for specific activity of the original labeling medium in each well.
mRNA Studies
Samples of frozen liver (∼10 mg) were homogenized in QIAzol (Qiagen) using a dounce homogenizer. Primary hepatocytes were lysed in QIAzol. Lysates were extracted with chloroform, and the aqueous phase was precipitated with 70% ethanol. Samples were processed using the RNeasy Lipid Tissue Mini Kit (Qiagen). One microgram of RNA was reverse transcribed using the GoScript Reverse Transcription System (Promega). cDNAs were diluted 1:10, and RT-PCR was performed using a DNA Engine Opticon 2 System (Bio-Rad) with SYBR Green (Promega). Primer sequences are available upon request. Gene expression levels were normalized by TATA-binding protein using the 2-∆∆Ct method (18).
Western Blotting
Frozen livers (∼50 mg) were homogenized in or primary hepatocytes were directly lysed in ice-cold lysis buffer (20 mmol/L Tris-HCl, 150 mmol/L NaCl, 10% glycerol, 2% NP-40, 1 mmol/L EDTA, 20 mmol/L NaF, 30 mmol/L Na4P2O7, 0.2% [w/v] SDS, and 0.5% [w/v] sodium deoxycholate) supplemented with protease/phosphatase inhibitors (Cell Signaling Technology). Protein concentration was assessed by bicinchoninic acid assay (Sigma-Aldrich). The following antibodies used in the study were all purchased from Cell Signaling Technology: FoxO1 C29H4, Akt, phosphorylated (p) Akt Thr308, glycogen synthase kinase 3β (GSK-3β), and pGSK-3β Ser9. Densitometric analysis was performed using ImageJ software (National Institutes of Health).
Results
Generation and Analysis of L-DBD Mice
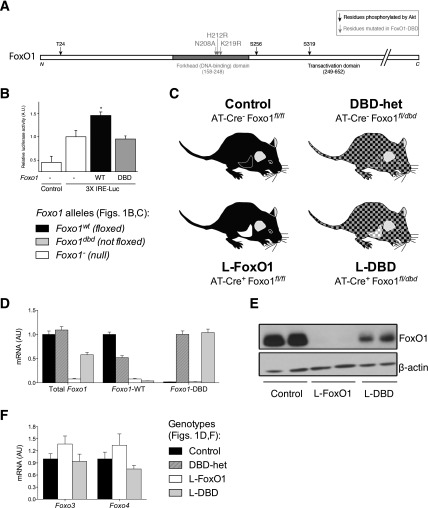
We generated a Foxo1 allele (Foxo1dbd) bearing mutations of residues necessary for DNA binding (N208A, H212R, K219R) (Fig. 1A) (19). These mutations abolish the binding of FoxO1 to target promoters, but do not affect Akt-mediated nucleocytoplasmic partitioning of the protein (20,21). We confirmed that FoxO1-DBD, unlike wild-type FoxO1, is incapable of driving luciferase activity from a reporter-gene construct containing canonical FoxO1 consensus binding sites (Fig. 1B).
Figure 1.
Generation and characterization of the Foxo1dbd allele. A: Schematic diagram of the FoxO1 primary sequence identifying the residues mutated in Foxo1dbd. B: Reporter gene assay in 293 cells transfected with FoxO1wt, or FoxO1dbd, or empty vector as well as with either 3× insulin-responsive element-luciferase reporter construct or control. Data represent mean ± SEM. *P < 0.05 relative to control by Tukey post hoc analysis after one-way ANOVA. C: Schematic diagram of mouse models used in this study. D: Liver RT-PCR using allele-specific primers for total Foxo1, Foxo1wt, or Foxo1dbd. Data represent the mean ± SEM. E: Western blot of liver extracts from fasted mice. F: mRNA levels of Foxo3 and -4 in mice of the described genotypes. AU, arbitrary units.
Homozygosity for alleles encoding FoxO1-DBD results in embryonic lethality, effectively phenocopying complete FoxO1 loss of function (22). To dissect the role of the transcriptional versus coregulatory functions of FoxO1 in the liver, we introduced the Foxo1dbd allele in mice bearing a liver-specific Foxo1 knockout. We obtained mice that are heterozygous for the Foxo1dbd allele throughout the body, but express only Foxo1dbd in the liver. Quantitative RT-PCR with allele-specific primers demonstrated the generation of the desired genotypes (Fig. 1D). Western blotting analysis verified the absence of FoxO1 protein in liver extracts from L-FoxO1, but not L-DBD mice (Fig. 1E), indicating that L-DBD mice express purely DBD FoxO1 in the liver. The expression of Foxo3 and Foxo4 was not significantly different from that in controls in either L-FoxO1 or L-DBD mouse livers (Fig. 1F), indicating that the loss of FoxO1 is not compensated for by upregulation of other FoxO isoforms (7).
Metabolic Features of Heterozygous Foxo1dbd Mice and Hepatocytes
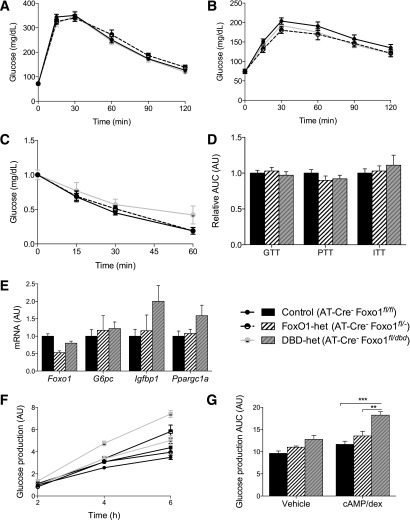
To rule out extrahepatic metabolic effects of Foxo1dbd heterozygosity per se, we compared adult male control mice (Foxo1fl/fl) and heterozygous Foxo1fl/dbd mice (henceforth, DBD-het) with mice heterozygous for a null allele of Foxo1 (FoxO1fl/−; henceforth, FoxO1-het) (Fig. 1C and D). We found no differences in fasting or refed glucose or insulin levels; glucose, pyruvate, or insulin tolerance test results; body weight; and composition (Fig. 2A–D and Table 1), or in the expression of known hepatic FoxO1 target genes after an overnight fast (Fig. 2E). These data are consistent with prior findings in FoxO1-het mice (4,23). Primary hepatocytes from control, FoxO1-het, and DBD-het mice showed no impairment of basal or cAMP-stimulated and dex-stimulated glucose production (Fig. 2F and G). Thus, we conclude that Foxo1dbd heterozygosity per se does not result in a metabolic phenotype that might confound the interpretation of data from the L-DBD mouse.
Figure 2.
Metabolic characterization of FoxO1-het and DBD-het Mice. Glucose (A) and PTT (B) in overnight-fasted mice (N ≥ 7 for all genotypes). C: Insulin tolerance test in 5-h–fasted mice (N = 5–6 for all genotypes). D: Quantification of the AUC for the results in A–C. E: Gene expression levels in fasted livers assessed by RT-PCR. Data represent the mean ± SEM. F: Glucose production assay performed in medium containing either vehicle (open circles) or cAMP/dex (closed circles). Data are normalized to vehicle-treated control at 2 h. G: AUC quantified from the data in panel F. Data in F and G represent the mean ± SEM of three representative experiments, each performed in triplicate. **P < 0.01, ***P < 0.001 by Tukey post hoc analysis after two-way ANOVA. AU, arbitrary units; GTT glucose tolerance test; ITT insulin tolerance test.
Table 1.
Metabolic features of mice analyzed in this study
| Feeding status |
Control mice
(n ≥ 9) |
DBD-het mice
(n ≥ 7) |
L-FoxO1 mice
(n ≥ 10) |
L-DBD mice
(n ≥ 7) |
|
|---|---|---|---|---|---|
| Chow | |||||
| Body weight (g) | Fed | 25.6 ± 0.6 | 25.2 ± 0.4 | 25.1 ± 0.8 | 24.2 ± 0.8 |
| Lean mass (%) | Fed | 80.2 ± 0.6 | 78.8 ± 1.2 | 80.3 ± 1.2 | 81.4 ± 0.2 |
| Fat mass (%) | Fed | 12.0 ± 0.6 | 13.2 ± 0.3 | 11.8 ± 1.2 | 10.5 ± 0.4 |
| Fluid mass (%) | Fed | 7.7 ± 0.3 | 8.0 ± 0.1 | 7.8 ± 0.2 | 8.1 ± 0.3 |
| FFAs (mEq/L) | Fasted | 1.09 ± 0.19 | 0.88 ± 0.08 | 1.23 ± 0.09 | 1.14 ± 0.13 |
| Refed | 0.19 ± 0.02 | 0.21 ± 0.03 | 0.20 ± 0.03 | 0.21 ± 0.03 | |
| TGs (mg/dL) | Fasted | 76 ± 6 | 67 ± 3 | 84 ± 4 | 72 ± 12 |
| Refed | 102 ± 11 | 106 ± 11 | 92 ± 7 | 121 ± 9 | |
| Cholesterol (mg/dL) | Fasted | 92 ± 4 | 98 ± 4 | 102 ± 5 | 96 ± 6 |
| Refed | 92 ± 3 | 84 ± 4 | 85 ± 4 | 90 ± 3 | |
| Liver cholesterol (mg/g liver) | Fasted | 1.66 ± 0.16 | 1.94 ± 0.17 | 1.56 ± 0.13 | 1.75 ± 0.11 |
| Refed | 1.17 ± 0.10 | ND | 1.10 ± 0.07 | 1.12 ± 0.13 | |
| WTD | |||||
| Body weight (g) | Fed | 33.2 ± 1.2 | 34.9 ± 1.8 | 38.0 ± 1.0 | 36.0 ± 2.8 |
| Glucose (mg/dL) | Fed | 218 ± 3 | 223 ± 2 | 190 ± 4 | 203 ± 7 |
| Fasted | 241 ± 14 | 242 ± 10 | 214 ± 7 | 220 ± 9 | |
| Insulin (ng/mL) | Fed | 2.87 ± 0.22 | 2.43 ± 0.55 | 4.22 ± 0.95 | 6.66 ± 3.15 |
| Fasted | 2.58 ± 0.30 | 2.11 ± 0.27 | 2.68 ± 0.32 | 2.25 ± 0.42 | |
| FFAs (mEq/L) | Fed | 0.76 ± 0.04 | 0.72 ± 0.08 | 0.71 ± 0.05 | 0.79 ± 0.06 |
| Fasted | 0.71 ± 0.06 | 0.70 ± 0.05 | 0.74 ± 0.05 | 0.71 ± 0.07 | |
| TGs (mg/dL) | Fed | 113 ± 11 | 118 ± 12 | 104 ± 7 | 118 ± 7 |
| Fasted | 59 ± 6 | 67 ± 6 | 53 ± 3 | 63 ± 9 | |
| Cholesterol (mg/dL) | Fed | 296 ± 21 | 344 ± 27 | 364 ± 28 | 428 ± 42 |
| Fasted | 337 ± 34 | 342 ± 27 | 398 ± 31 |
Data are reported as the mean ± SEM. None of the differences between genotypes reach statistical significance by Tukey post hoc test after one-way ANOVA. ND, not determined.
Metabolic Characterization of L-DBD Mice
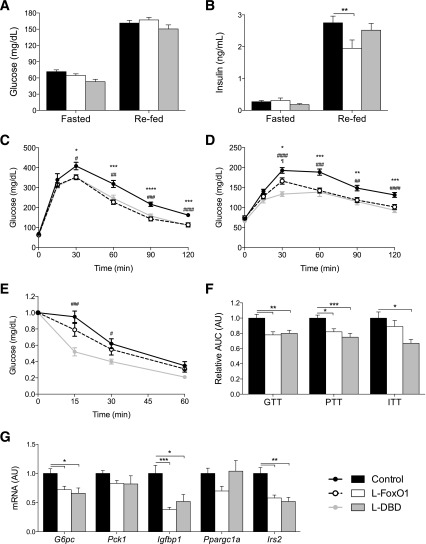
We analyzed the metabolic features of adult L-DBD male mice. They gained weight at the same rate as L-FoxO1 and control mice (Table 1 and data not shown), and showed no differences in body composition (Table 1). Likewise, there were no differences between L-DBD and control mice in glucose or insulin levels after an overnight fast or a 4-h refeed, whereas L-FoxO1 mice showed a modest decrease in refed insulin levels compared with controls (Fig. 3A and B).
Figure 3.
Glucose metabolism in L-FoxO1 and L-DBD Mice. Glucose (A) and insulin (B) levels in mice fasted overnight or refed for 4 h. **P < 0.01 by Tukey post hoc analysis after two-way ANOVA. Glucose (C) and pyruvate (D) tolerance tests in overnight-fasted mice. E: Insulin tolerance test in 5-h–fasted mice. For C–E, *P < 0.05, **P < 0.01, ***P < 0.001 for control vs. L-FoxO1; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 for control vs. L-DBD. F: Quantification of the AUC for the results in C–E. G: Gene expression levels in fasted livers assessed by RT-PCR. For F and G, *P < 0.05, **P < 0.01, ***P < 0.001 by Tukey post hoc analysis after one-way ANOVA. All mice were reared on a chow diet, and studies were performed at 16–20 weeks of age. N ≥ 9 for all genotypes in all experiments. Data represent the mean ± SEM. AU, arbitrary units; GTT glucose tolerance test; ITT insulin tolerance test.
L-DBD mice exhibited an enhancement of glucose tolerance (on glucose tolerance test results) identical to that in L-FoxO1 mice (Fig. 3C) (5,7), suggesting that the FoxO1-DBD mouse is effectively a null mutant with respect to glucose tolerance. These results were borne out by the results of PTT, which showed similar curves in L-FoxO1 and L-DBD mice (Fig. 3D) (5). Intraperitoneal insulin tolerance tests conducted in fasted animals failed to reveal differences between control and L-FoxO1 mice, but showed a modest enhancement in L-DBD mice (Fig. 3E). Quantitative analyses of the areas under the curve (AUCs) from experiments on multiple cohorts confirmed these conclusions (Fig. 3F). Moreover, RT-PCR analysis of RNA extracted from livers of overnight-fasted L-FoxO1 and L-DBD mice showed equally decreased G6pc, Igfbp1, and Irs2 relative to controls (Fig. 3G). Consistent with our previous reports (5), we did not detect a significant decrease in Pck1 in either L-FoxO1 or L-DBD livers. These results indicate that deletion of hepatocellular FoxO1 results in decreased HGP.
Impaired Glucose Production in Hepatocytes From L-DBD Mice
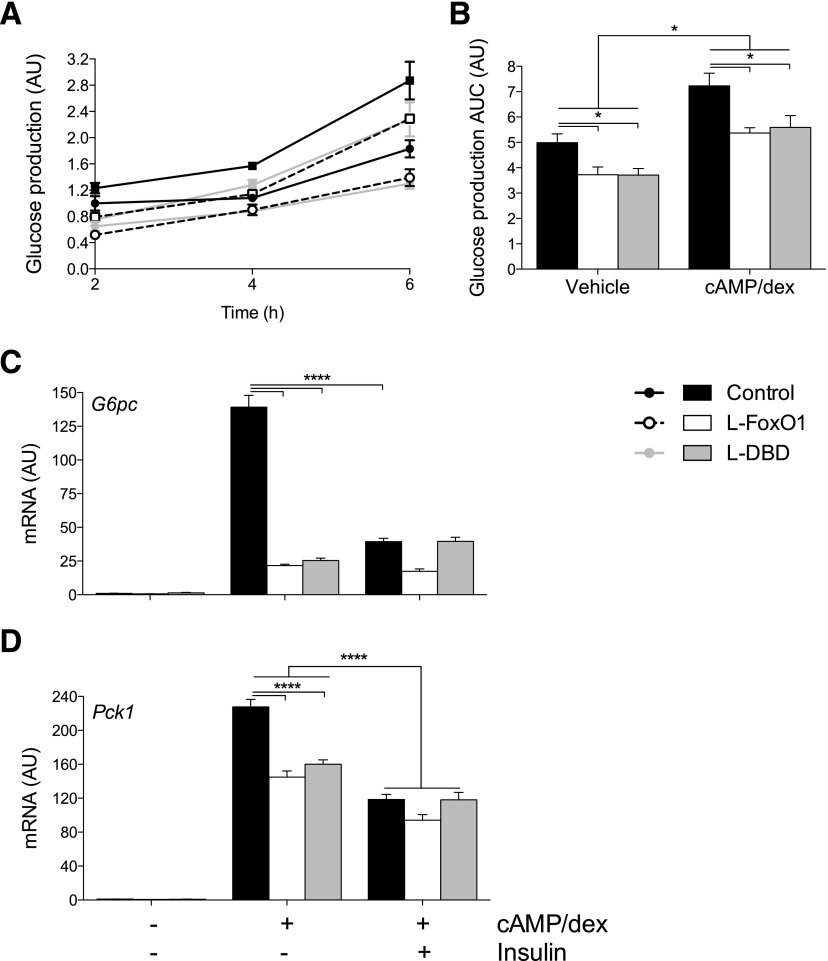
Next, we isolated primary hepatocytes from control, L-FoxO1, or L-DBD mice and assessed their ability to generate glucose from pyruvate and lactate either basally or in the presence of CPT-cAMP and dex (cAMP/dex). Glucose production nearly doubled in control hepatocytes in a time-dependent manner after the addition of cAMP/dex (Fig. 4A and B). In contrast, primary hepatocytes from L-DBD mice showed a nearly 30% decrease in basal and cAMP/dex-stimulated glucose production, similar to L-FoxO1 hepatocytes (Fig. 4A and B). Consistent with these findings, L-FoxO1 and L-DBD primary hepatocytes showed a >80% decrease in the effect of cAMP/dex on G6pc and a ∼40% decrease of Pck1, as a result of which the suppressive effect of insulin on both genes was virtually abolished (Fig. 4C and D) (5).
Figure 4.
Glucose production in primary hepatocytes. Glucose production assays in cells incubated with glucose production medium supplemented with vehicle (circles) (A) or with cAMP/dex (squares) for 6 h (B). Quantification of the AUC from the data in panel A. C and D: RT-PCR of G6pc and Pck1 levels in the presence or absence of cAMP/dex and insulin. *P < 0.05 and ****P < 0.0001 by Tukey post hoc analysis after two-way ANOVA. All data are presented as the mean ± SEM of three representative experiments, each performed in triplicate. AU, arbitrary units.
Hepatic Lipid Metabolism in L-DBD Mice
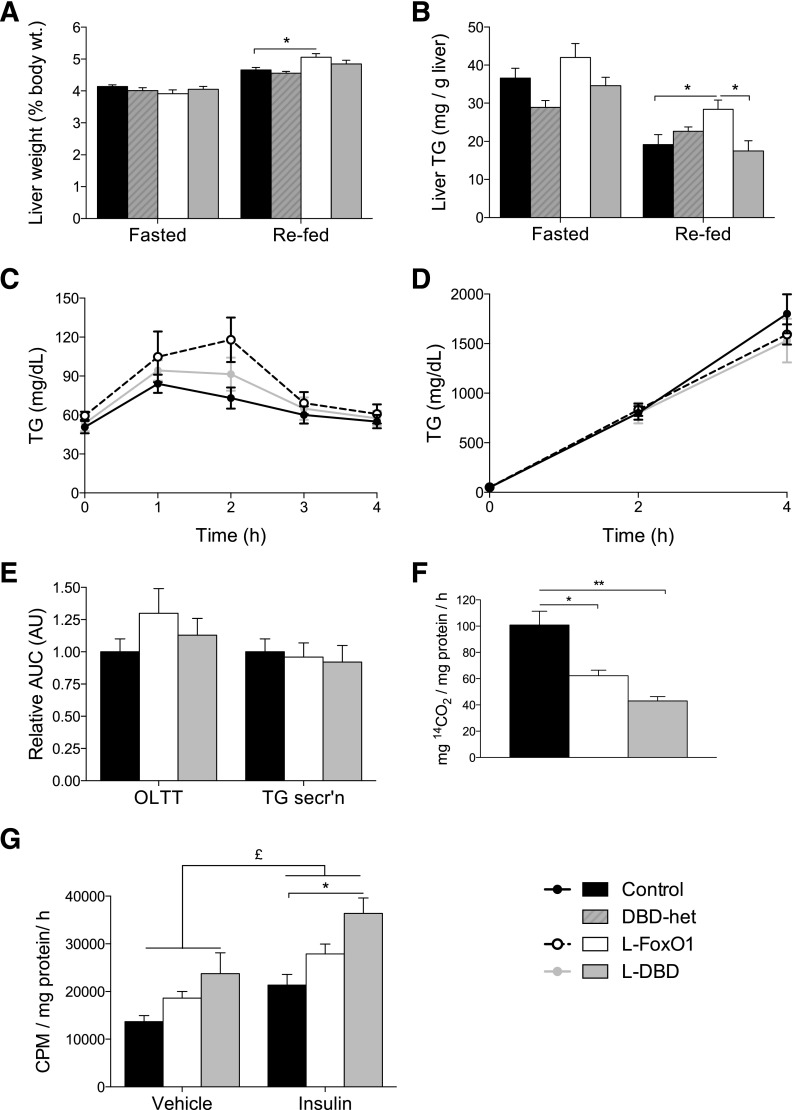
Next, we examined features of hepatic lipid metabolism in L-DBD mice. We found no differences in circulating levels of nonesterified fatty acids, TGs, or cholesterol among mice of different genotypes (Table 1) (5,12). Liver weight was modestly increased in refed, but not in overnight-fasted L-FoxO1 mice (Fig. 5A). This difference was due at least in part to increased TG content (Fig. 5B) and was not observed in L-DBD mice. There was no difference in liver cholesterol content among genotypes in the fasted or refed states (Table 1).
Figure 5.
Lipid metabolism in mice and primary hepatocytes. A: Liver weight relative to body weight. Mice of each genotype were fasted overnight or fasted overnight and refed for 4 h (N ≥ 10 for each genotype). B: Liver TG content in fasted or 4-h–refed mice, normalized to total liver weight (N ≥ 6 for each genotype). C: An OLTT conducted in 5-h–fasted mice. D: TG secretion assay in 5-h–fasted mice (N ≥ 5 for each genotype). E: AUC of the OLTT and TG secretion data in C and D. Data are normalized to a control sample for each procedure. All mice used in A–D were reared on chow diet, and studies were performed at 16–20 weeks of age. F: FAO in primary hepatocytes from control, L-FoxO1, and L-DBD mice. Data are shown as the average of three independent experiments, each performed in triplicate. For A–F, *P < 0.05 by Tukey post hoc analysis after one-way ANOVA. G: DNL of TGs in primary hepatocytes isolated from control, L-FoxO1, and L-DBD mice, and treated with vehicle or 10 nmol/L insulin. Data shown are the mean ± Satterthwaite-corrected SEM of three independent experiments performed in triplicate. £P < 0.05 for main effect as assessed by two-way ANOVA; *P < 0.05, **P < 0.01 using Bonferroni post hoc analysis. AU, arbitrary units; CPM, counts per minute; secr’n, secretion.
We analyzed different aspects of hepatic lipid handling in order to parse out the mechanism underlying differential liver TG content. OLTT results and hepatic TG secretion were normal (Fig. 5C–E). In contrast, β-oxidation of radiolabeled oleic acid decreased by ∼40% in L-FoxO1 hepatocytes and by ∼60% L-DBD hepatocytes (Fig. 5F). Analysis of DNL demonstrated a ∼35% increase in TG synthesis in primary hepatocytes from L-FoxO1 mice under basal as well as insulin-stimulated conditions. Hepatocytes of L-DBD mice showed an even greater increase of ∼75% (Fig. 5G). The inability of L-DBD hepatocytes to restore lipid oxidation and lipogenesis to their control levels indicates that these effects require direct FoxO1 DNA binding.
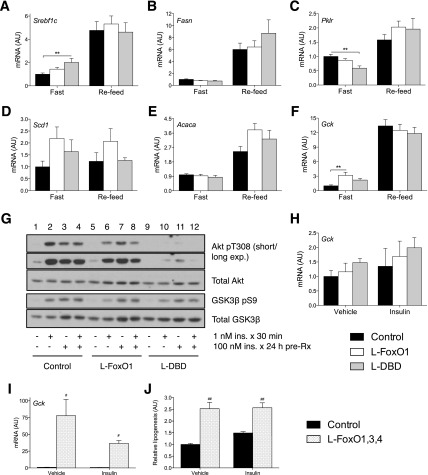
To determine the mechanism of the alteration in DNL, we measured levels of several regulators of lipogenesis (Fig. 6A–F). We observed significant elevations in fasting levels of stearoyl-CoA desaturase-1 (Scd1) in L-FoxO1 mice, but not in L-DBD mice, compared with controls; Srebf1c expression was significantly higher in L-DBD mice than in control mice, whereas there was no significant difference in L-FoxO1. On the other hand, fasting levels of pyruvate kinase (Pklr), a target of the lipogenic transcription factor carbohydrate binding element binding protein (ChREBP) (24), were significantly lower, while those of acetyl-CoA carboxylase-1 (Acaca) were unchanged in L-FoxO1 and L-DBD mouse livers compared with controls. We also sought to determine whether the significant increase of DNL in insulin-treated L-DBD hepatocytes was due to enhanced insulin signaling. However, phosphorylation of Akt (T308) and GSK-3β (S9) in response to insulin was rather decreased in primary hepatocytes from L-FoxO1 and L-DBD mice (Fig. 6G).
Figure 6.
Factors affecting lipogenesis in mice and primary hepatocytes. A–F: Expression of lipogenic genes in livers from either fasted or refed mice. N ≥ 7 for all conditions tested. **P < 0.01 by Tukey post hoc analysis after one-way ANOVA. G: Insulin signaling in primary hepatocytes treated with saline solution or with 1 nmol/L insulin for 30 min after treatment for 24 h with either saline solution or 100 nmol/L insulin. H and I: Expression of Gck in primary hepatocytes from mice of the indicated genotypes after 6 h of treatment either with 10 nmol/L insulin or vehicle. Data in H are the mean of two (insulin) or three (vehicle) independent experiments performed in triplicate ± Satterthwaite-corrected SEM and analyzed by Tukey post hoc test after one-way ANOVA. J: Total DNL in primary hepatocytes isolated from L-FoxO1,3,4 or Cre control mice and treated with vehicle or 10 nmol/L insulin. Data shown are normalized to vehicle-treated control cells and are representative of two independent experiments performed in triplicate. Data in I and J are the mean ± SEM of two representative experiments, each performed in triplicate. #P < 0.05, ##P < 0.01 vs. corresponding control by unpaired, two-tailed Student t test. AU, arbitrary units.
We recently showed that FoxO regulation of DNL in the transition to refeeding is partly based on modulation of carbon flux through coordinated activation of G6pc and inhibition of Gck expression during fasting (25). Consistent with these data, we found Gck expression to be significantly increased by over threefold in L-FoxO1 hepatocytes compared with controls, while in L-DBD hepatocytes Gck expression was intermediate and not significantly different from controls (Fig. 6F). FoxO1 inhibition of Gck in vivo therefore likely proceeds in part by a coregulatory mechanism, as has previously been suggested by reporter-gene studies (26,27). On the other hand, we found no significant differences in Gck expression between genotypes in isolated hepatocytes (Fig. 6H). Thus, it appears that FoxO1 regulation of Gck expression is not cell autonomous. On the other hand, the measurement of DNL in primary hepatocytes can necessarily reflect only processes that are cell autonomous; for example, the regulation of G6pc expression or of glucose production generally. This may, therefore, help us to reconcile the apparent discrepancy between measured in vitro DNL and liver TG levels. Indeed, in primary hepatocytes isolated from L-FoxO1,3,4 mice, which also lack the other two major FoxO isoforms FoxO3 and FoxO4 in the liver, the expression of Gck was increased by up to nearly 80-fold versus controls (Fig. 6I) (25). In keeping with the expectation of increased glycolytic flux in the presence of higher Gck expression, the rate of total DNL was increased by more than 2.5-fold in L-FoxO1,3,4 hepatocytes (Fig. 6J), which is consistent with previous studies (10).
These experiments indicate that the loss of FoxO1 function increases lipogenesis and decreases free fatty acid (FFA) oxidation, and that FoxO1-DBD fails to restore these functions. We conclude that FoxO1 physiologically inhibits these processes in a DNA binding–dependent manner (Fig. 7I).
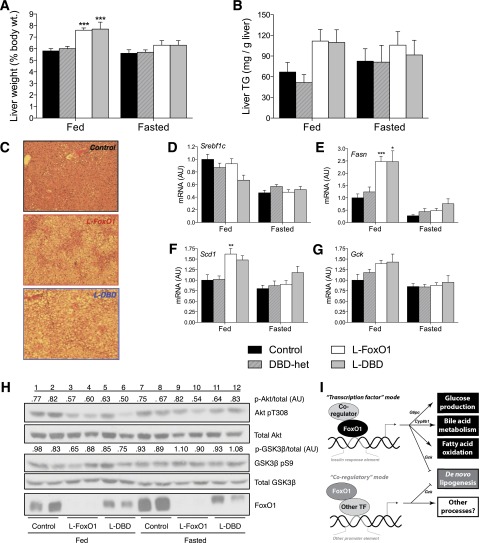
Figure 7.
Metabolic characterization of mice on WTD. Liver weight relative to body weight (A) and liver TG content (B) in 5-h–fasted or ad libitum–fed mice. C: Hematoxylin-eosin staining of liver sections from WTD-fed mice. D–G: RT-PCR measurements of lipogenic genes in livers from ad libitum–fed or 5-h–fasted mice. Data represent the mean ± SEM (N ≥ 7 for each genotype). *P < 0.05, **P < 0.01, ***P < 0.001 vs. corresponding control by Tukey post hoc analysis after one-way ANOVA. H: Western blots of livers from ad libitum–fed or 5-h–fasted mice. Each lane represents pooled liver homogenate from three mice of the same cohort. Relative phosphorylation of Akt and GSK-3β are depicted above the respective blot and are calculated densitometrically as the ratio of phosphorylated to total protein. I: Schematic diagram depicting the mechanism of the metabolic actions of FoxO1 in the liver. AU, arbitrary units; TF, transcription factor.
Lipid Metabolism in WTD-Fed L-DBD Mice
We have previously demonstrated that FoxO1 ablation increases hepatic TG deposition in mice fed a WTD (12). We therefore placed L-DBD, L-FoxO1, and control mice on a WTD for 10 weeks and analyzed them in either the ad libitum-fed or 5-h–fasted state. At the completion of the diet, there were no significant differences among genotypes in body weight or circulating levels of glucose, insulin, FFA, TG, and cholesterol in either state (Table 1). Liver weight increased by ∼25% in fed L-FoxO1 and L-DBD mice (Fig. 7A), and was accompanied by a near doubling of liver TG levels, although this difference did not reach statistical significance, owing to large individual variations (Fig. 7B). Histologic examination of liver sections taken from these mice confirmed the presence of hepatic steatosis in L-FoxO1 and L-DBD mice (Fig. 7C). These findings were complemented by coordinate increases in levels of mRNA encoding Fasn, Gck, and Scd1 (Fig. 7D–G).
Finally, we analyzed whether FoxO1-DBD modified the effects of WTD feeding on insulin signaling in liver and primary hepatocytes. Fasting levels of pAkt and pGSK-3β were uniformly increased in WTD-fed mice of all genotypes, blunting the increase in response to feeding (Fig. 7H). This is probably due to hyperinsulinemia (28). We investigated this process by preincubating primary hepatocytes with insulin as a surrogate of in vivo hyperinsulinemia (Fig. 6H) (29–31). After this treatment, basal (i.e., “fasted”) phosphorylation levels of Akt and GSK-3β increased relative to nonexposed cells, but were not further augmented by short-term insulin treatment (“fed” state). As in fed livers, L-FoxO1 and L-DBD hepatocytes exhibited a trend toward lower levels of Akt and GSK-3β phosphorylation after short-term insulin challenge. Thus, it appears that FoxO1-DBD does not exert independent effects on insulin signaling.
Discussion
This study demonstrates a mechanistic dissociation of the pleiotropic effects of FoxO1 on hormone- and nutrient-dependent gene expression on the basis of DNA binding (Fig. 7I). FoxO1 regulation of gene expression via binding to conserved cis acting elements in target promoters is well characterized, and this study demonstrates that this action of FoxO1 is required for its regulation of HGP. Another, less recognized, mode of action exists whereby FoxO1 engages in non-DNA–based interactions with components of the transcriptional complex to regulate gene expression (14). The current study indicates that a coregulatory mode of action is at least partly responsible for FoxO1 regulation of net hepatic TG content. Surprisingly, however, we show that reconstitution of a FoxO1 DBD allele in mice that lack endogenous FoxO1 fails to restore lipogenesis in isolated hepatocytes. While the conclusion that FoxO1 controls HGP by binding to consensus sites on target promoters was predicted by previous work (8,32), the finding of increased lipogenesis in L-DBD hepatocytes is surprising in the face of normal hepatic TG levels, especially as the inhibition of this process by FoxO1 is more easily reconciled with a corepressor function (15). Another important finding of the current study is the heretofore unrecognized effect of FoxO1 ablation, which is mimicked by the DBD mutant, to reduce FFA oxidation (6,10,11,33).
The segregation of different functional outputs of a transcription factor on the basis of DNA binding–dependent versus DNA binding–independent actions has been observed in other contexts. For instance, it appears to be a feature of basic helix-loop-helix transcription factors, including Hand2 and Scl (34,35). With regard to FoxO1, a DBD mutant can suppress myogenic differentiation of C2C12 myoblasts as efficiently as wild-type FoxO1 (14). Likewise, constitutively nuclear FoxO1-DBD retains the ability to enhance basal phosphorylation of Akt in the liver (9). DNA binding–defective FoxO1 does not merely represent a hypomorphic variant; expression profiling of cultured cells shows that DBD mutant FoxO1 induces a distinct class of genes compared with the native protein (13). Our study provides a critical in vivo extension of these results.
Under what circumstances does this dual regulatory mechanism spring into action? At this point, we can only speculate. One possibility is that the multiple post-translational modifications of FoxO1 modulate its ability to bind to DNA without affecting its nuclear localization. In this regard, we and others (36,37) have shown that, even when FoxO1 is restricted to the nucleus, it is still subject to regulation, either by targeting to subnuclear bodies or by modification of its stability. Supportive of this view is the little remarked upon observation that nuclear exclusion of FoxO1 is a heterogeneous process (38). In response to insulin or growth factor treatment, it is not uncommon to see cells with cytoplasmic FoxO1 juxtaposed with cells with nuclear FoxO1, indicating that, aside from cellular heterogeneity, factors other than nuclear exclusion modulate FoxO1 function.
Moreover, the interaction of FoxO1 with any given promoter could entail transcriptional and coregulatory functions. For example, chromatin immunoprecipitation studies reveal that FoxO1-DBD can be recruited to the G6pc promoter without activating the expression of the gene (data not shown), likely through interactions with hepatocyte nuclear factor-4α and peroxisome proliferator–activated receptor γ coactivator-1α (27,32). Ergo, the mechanism of FoxO1 regulation of gene expression must be assessed on a case-by-case basis by coupling promoter occupancy with gene expression data.
Mechanism of FoxO1 Regulation of Lipogenesis and Liver TG Content
Perhaps the most striking finding of our study is the ability of FoxO1 to regulate liver TG content as a transcriptional coregulator. Our data suggest a model in which FoxO1 alters lipid metabolism at multiple levels. First, in a cell-autonomous fashion, FoxO1 represses DNL (and activates FAO) via methods requiring direct binding to DNA. Work from our laboratory has shown that the ratio of G6pc to Gck expression is a reliable indicator of the direction of glucose flux (i.e., of gluconeogenesis/glycogenolysis → HGP vs. glycolysis → DNL) (25). FoxO1 inhibition of Gck, unlike its activation of G6pc, is non–cell autonomous, which is in keeping with previous reports (39) on neural modulation of insulin-induced Gck expression in the liver. Thus, in both L-FoxO1 and L-DBD primary hepatocytes, a defect in G6pc expression in the absence of a significant change in Gck would decrease the G6pc:Gck ratio. This, in turn, would impede gluconeogenesis, as observed in this study, while increasing the availability of acetyl-CoA for use in DNL, especially in the presence of insulin (25,40). In L-FoxO1,3,4 primary hepatocytes, Gck expression is frankly increased and G6pc is decreased, which is consistent with the dramatic elevation in DNL compared with controls (10,25). Even if altered expression of G6pc per se is not directly responsible, gluconeogenesis is decreased in L-FoxO1 and L-DBD hepatocytes and thus, as in the case of decreased G6Pase action, would be expected to promote lipogenesis (40).
Unlike in primary hepatocytes, fasting and feeding regulation of Gck expression via FoxO1 can proceed as normal in the whole liver. Thus, by the end of an overnight fast, L-FoxO1 livers have accumulated significantly more Gck mRNA than controls. At the onset of refeeding, these livers are better primed for efficient TG synthesis (41–43), hence the increase in refed liver TGs in L-FoxO1 mice but not in L-DBD mice relative to controls. On the other hand, L-DBD livers retain a partial ability to suppress Gck expression, thus not allowing them as much of a “head start” on DNL after refeeding. That the expression of Gck is elevated to the same extent in both L-FoxO1 and L-DBD livers in the WTD-fed state may explain the lack of difference in liver TG levels between these mice, especially given the heightened contribution of DNL to hepatic TG in the steatotic liver (44,45). Evidently, the ability of haplosufficient FoxO1-DBD to regulate Gck expression in the chow-fed state is lost in the WTD-fed state, thus altering the G6pc:Gck ratio similarly in these mice.
This model is not mutually exclusive with other hypotheses regarding FoxO1 control of hepatic TGs, such as via modulation of bile acid metabolism (12). Indeed, this model alone is not sufficient to explain the augmentation in DNL observed in L-DBD primary hepatocytes even relative to those in L-FoxO1. Thus, it is likely that other mechanisms also come into play. One possibility is a partial dominant-negative effect of FoxO1-DBD on FoxO3a and FoxO4 through sequestration of coregulatory proteins (46), especially as Gck expression trends slightly higher in L-DBD cells. In light of the decrease in Akt signaling in L-FoxO1 and L-DBD primary hepatocytes, another possible pathway through which FoxO1 affects lipogenesis is p38, which may mediate a feedback loop between FoxO1 and Akt (47), and thereby regulate DNL (48).
The decrease in Akt phosphorylation that we observe in primary hepatocytes from L-FoxO1 mice and especially L-DBD mice appears to be at odds with our observation of increased DNL ex vivo and preserved or even increased liver TG content in vivo. We therefore performed intravenous insulin injections in mice of each genotype but did not detect any difference in the phosphorylation of Akt or GSK-3β between genotypes (data not shown). We also did not detect any differences in the phosphorylation of these signaling intermediaries in the more physiologic context of fasting/refeeding of chow-reared mice (data not shown). Thus, the difference we see in the phosphorylation of Akt in Fig. 6G appears to be restricted to the setting of primary hepatocytes and may reflect a greater contribution of a FoxO1 → IRS2 homeostatic loop to the regulation of insulin responsiveness ex vivo than in vivo (9). In support of this hypothesis, we detected decreased levels of IRS2 at both the mRNA and protein levels in primary hepatocytes lacking FoxO1 with no difference in phosphorylation or total levels of insulin receptor (data not shown).
Similarly, our finding of decreased Akt phosphorylation in the livers of WTD-fed L-FoxO1 and L-DBD mice appears inconsistent with the increased liver TG content and lipogenic gene expression even relative to WTD-fed controls. Again, DNL—a process, again, stimulated by insulin—has been shown to be increased in hepatic steatosis, while we would expect a relative impairment in the face of decreased Akt activation (11,44,45). However, even though Akt and GSK-3β phosphorylation are somewhat lower in L-FoxO1 and L-DBD livers, this may not translate into a functional impairment of lipogenic gene expression and lipid biosynthesis. For example, mRNA and protein levels of the lipogenic transcription factor SREBP-1c, the expression of which is stimulated by insulin (29), are not significantly decreased in L-FoxO1 or L-DBD livers, although it does trend lower in the latter. Thus, the decrement in Akt activation is not sufficient to significantly impair its action in this context.
Furthermore, insulin signaling is not absolutely necessary to drive lipogenesis, as carbohydrates per se can induce lipogenic gene expression and ramp up DNL through activation of the ChREBP pathway (24). Well-established ChREBP targets include Fasn and Scd1, both of which are increased in WTD-fed L-FoxO1 and L-DBD livers compared with controls (Fig. 7E and G). Another classic ChREBP target, Pklr, is expressed at equivalent levels in each genotype (data not shown), again consistent with intact ChREBP activity. In conclusion, this study provides new insight into the coordinated regulation of hepatic glucose and lipid metabolism by FoxO1.
Article Information
Acknowledgments. The authors thank the members of the Accili laboratory for insightful data discussions. The authors also thank Mr. Thomas Kolar, Ms. Ana Flete-Castro, Dr. Utpal Pajvani, Ms. Elizabeth Millings, and Dr. Donna Conlon (Columbia University) for outstanding technical support.
Funding. This work was supported by National Institutes of Health grants DK-100038, DK-57539, and DK-63608 (to Columbia University Diabetes Research Center).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.R.C. designed and performed the experiments, analyzed the data, and wrote the manuscript. M.M. designed and performed the experiments. A.S.B. and T.K. designed and generated Foxo1dbd transgenic mice. K.T. performed original breeding, established the transgenic mouse colony, and provided technical guidance. D.A. designed the experiments, oversaw the research, and wrote the manuscript. D.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab 2011;14:9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 2011;22:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim-Muller JY, Accili D. Cell biology. Selective insulin sensitizers. Science 2011;331:1529–1531 [DOI] [PubMed] [Google Scholar]
- 4.Nakae J, Biggs WH 3rd, Kitamura T, et al. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet 2002;32:245–253 [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab 2007;6:208–216 [DOI] [PubMed] [Google Scholar]
- 6.Dong XC, Copps KD, Guo S, et al. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab 2008;8:65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haeusler RA, Kaestner KH, Accili D. FoxOs function synergistically to promote glucose production. J Biol Chem 2010;285:35245–35248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 2001;108:1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest 2006;116:2464–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tao R, Wei D, Gao H, Liu Y, DePinho RA, Dong XC. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J Biol Chem 2011;286:14681–14690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan M, Leavens KF, Saleh D, et al. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab 2011;14:516–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeusler RA, Pratt-Hyatt M, Welch CL, Klaassen CD, Accili D. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab 2012;15:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell 2002;2:81–91 [DOI] [PubMed] [Google Scholar]
- 14.Kitamura T, Kitamura YI, Funahashi Y, et al. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest 2007;117:2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan W, Imamura T, Sonoda N, et al. FOXO1 transrepresses peroxisome proliferator-activated receptor gamma transactivation, coordinating an insulin-induced feed-forward response in adipocytes. J Biol Chem 2009;284:12188–12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turan S, Zehe C, Kuehle J, Qiao J, Bode J. Recombinase-mediated cassette exchange (RMCE) - a rapidly-expanding toolbox for targeted genomic modifications. Gene 2013;515:1–27 [DOI] [PubMed] [Google Scholar]
- 17.Banks AS, Kim-Muller JY, Mastracci TL, et al. Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knockin of acetylation-defective alleles in mice. Cell Metab 2011;14:587–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med 2013;19:1054–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brent MM, Anand R, Marmorstein R. Structural basis for DNA recognition by FoxO1 and its regulation by posttranslational modification. Structure 2008;16:1407–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang ED, Nuñez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem 1999;274:16741–16746 [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Gan L, Pan H, et al. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J 2004;378:839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosaka T, Biggs WH 3rd, Tieu D, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA 2004;101:2975–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pajvani UB, Shawber CJ, Samuel VT, et al. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat Med 2011;17:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Postic C, Dentin R, Denechaud PD, Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr 2007;27:179–192 [DOI] [PubMed] [Google Scholar]
- 25.Haeusler RA, Hartil K, Vaitheesvaran B, et al. Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors. Nat Commun 2014;5:5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganjam GK, Dimova EY, Unterman TG, Kietzmann T. FoxO1 and HNF-4 are involved in regulation of hepatic glucokinase gene expression by resveratrol. J Biol Chem 2009;284:30783–30797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirota K, Sakamaki J, Ishida J, et al. A combination of HNF-4 and Foxo1 is required for reciprocal transcriptional regulation of glucokinase and glucose-6-phosphatase genes in response to fasting and feeding. J Biol Chem 2008;283:32432–32441 [DOI] [PubMed] [Google Scholar]
- 28.Han S, Liang CP, Westerterp M, et al. Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J Clin Invest 2009;119:1029–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell 2000;6:77–86 [PubMed] [Google Scholar]
- 30.Gonzalez E, Flier E, Molle D, Accili D, McGraw TE. Hyperinsulinemia leads to uncoupled insulin regulation of the GLUT4 glucose transporter and the FoxO1 transcription factor. Proc Natl Acad Sci U S A 2011;108:10162–10167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegarty BD, Bobard A, Hainault I, Ferré P, Bossard P, Foufelle F. Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory element-binding protein-1c. Proc Natl Acad Sci U S A 2005;102:791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puigserver P, Rhee J, Donovan J, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 2003;423:550–555 [DOI] [PubMed] [Google Scholar]
- 33.Lu M, Wan M, Leavens KF, et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med 2012;18:388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porcher C, Liao EC, Fujiwara Y, Zon LI, Orkin SH. Specification of hematopoietic and vascular development by the bHLH transcription factor SCL without direct DNA binding. Development 1999;126:4603–4615 [DOI] [PubMed] [Google Scholar]
- 35.Liu N, Barbosa AC, Chapman SL, et al. DNA binding-dependent and -independent functions of the Hand2 transcription factor during mouse embryogenesis. Development 2009;136:933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomizawa M, Kumar A, Perrot V, Nakae J, Accili D, Rechler MM. Insulin inhibits the activation of transcription by a C-terminal fragment of the forkhead transcription factor FKHR. A mechanism for insulin inhibition of insulin-like growth factor-binding protein-1 transcription. J Biol Chem 2000;275:7289–7295 [DOI] [PubMed] [Google Scholar]
- 37.Buteau J, Shlien A, Foisy S, Accili D. Metabolic diapause in pancreatic beta-cells expressing a gain-of-function mutant of the forkhead protein Foxo1. J Biol Chem 2007;282:287–293 [DOI] [PubMed] [Google Scholar]
- 38.Kitamura T, Nakae J, Kitamura Y, et al. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest 2002;110:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramnanan CJ, Kraft G, Smith MS, et al. Interaction between the central and peripheral effects of insulin in controlling hepatic glucose metabolism in the conscious dog. Diabetes 2013;62:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bandsma RH, Wiegman CH, Herling AW, et al. Acute inhibition of glucose-6-phosphate translocator activity leads to increased de novo lipogenesis and development of hepatic steatosis without affecting VLDL production in rats. Diabetes 2001;50:2591–2597 [DOI] [PubMed] [Google Scholar]
- 41.Dentin R, Pégorier JP, Benhamed F, et al. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem 2004;279:20314–20326 [DOI] [PubMed] [Google Scholar]
- 42.Ferre T, Riu E, Franckhauser S, Agudo J, Bosch F. Long-term overexpression of glucokinase in the liver of transgenic mice leads to insulin resistance. Diabetologia 2003;46:1662–1668 [DOI] [PubMed] [Google Scholar]
- 43.Peter A, Stefan N, Cegan A, et al. Hepatic glucokinase expression is associated with lipogenesis and fatty liver in humans. J Clin Endocrinol Metab 2011;96:E1126–E1130 [DOI] [PubMed] [Google Scholar]
- 44.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab 2003;29:478–485 [DOI] [PubMed] [Google Scholar]
- 45.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol 2013;14:83–97 [DOI] [PubMed] [Google Scholar]
- 47.Naïmi M, Gautier N, Chaussade C, Valverde AM, Accili D, Van Obberghen E. Nuclear forkhead box O1 controls and integrates key signaling pathways in hepatocytes. Endocrinology 2007;148:2424–2434 [DOI] [PubMed] [Google Scholar]
- 48.Xiong Y, Collins QF, An J, et al. p38 mitogen-activated protein kinase plays an inhibitory role in hepatic lipogenesis. J Biol Chem 2007;282:4975–4982 [DOI] [PubMed] [Google Scholar]