Abstract
This is the third in a series of Perspectives on intracellular signaling pathways coupled to proliferation in pancreatic β-cells. We contrast the large knowledge base in rodent β-cells with the more limited human database. With the increasing incidence of type 1 diabetes and the recognition that type 2 diabetes is also due in part to a deficiency of functioning β-cells, there is great urgency to identify therapeutic approaches to expand human β-cell numbers. Therapeutic approaches might include stem cell differentiation, transdifferentiation, or expansion of cadaver islets or residual endogenous β-cells. In these Perspectives, we focus on β-cell proliferation. Past Perspectives reviewed fundamental cell cycle regulation and its upstream regulation by insulin/IGF signaling via phosphatidylinositol-3 kinase/mammalian target of rapamycin signaling, glucose, glycogen synthase kinase-3 and liver kinase B1, protein kinase Cζ, calcium-calcineurin–nuclear factor of activated T cells, epidermal growth factor/platelet-derived growth factor family members, Wnt/β-catenin, leptin, and estrogen and progesterone. Here, we emphasize Janus kinase/signal transducers and activators of transcription, Ras/Raf/extracellular signal–related kinase, cadherins and integrins, G-protein–coupled receptors, and transforming growth factor β signaling. We hope these three Perspectives will serve to introduce these pathways to new researchers and will encourage additional investigators to focus on understanding how to harness key intracellular signaling pathways for therapeutic human β-cell regeneration for diabetes.
Introduction
This is the third in a series of Perspectives in Diabetes reviewing and emphasizing the importance of intracellular signaling pathways in rodent and human β-cells, with a specific focus on the links between β-cell proliferation and intracellular signaling pathways (1,2). We highlight what is known in rodent β-cells and compare and contrast that to the current knowledge base in human β-cells. Invariably, the human β-cell section is very brief compared with the rodent counterpart, reflecting the still primitive state of our understanding of mitogenic signaling in human β-cells. To emphasize this difference, each figure is divided into two panels, one summarizing rodent β-cell signaling and one for human β-cells. Our intended audience includes trainees in β-cell regeneration as well as experts in a given pathway who wish to refresh their knowledge regarding other pathways related to β-cell proliferation. We believe that knowledge of β-cell signaling lags significantly behind other areas in β-cell biology, that understanding why adult human β-cells are so recalcitrant to induction of proliferation is critically important, and that deepening knowledge in this area will reveal novel approaches and targets for the therapeutic induction of human β-cell expansion.
Readers are urged to refer to the prior two Perspectives for additional background and cross-correlation (1,2). These have covered the fundamentals of cell cycle control in the β-cell, and several key mitogenic β-cell signaling pathways: insulin/IGF/insulin receptor substrate (IRS)/phosphatidylinositol-3 kinase (PI3K)/Akt/glycogen synthase kinase-3β (GSK3β)/mammalian target of rapamycin (mTOR) signaling, protein kinase Cζ (PKCζ) signaling, glucose and nutrient signaling via AMPK/liver kinase B, carbohydrate response element–binding protein (ChREB) and cMyc, calcium-calcineurin–nuclear factor of activated T cells signaling, epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) signaling, Wnt/β-catenin signaling and leptin signaling, estrogen and progesterone signaling, and, a brief introduction to lactogenic signaling. Here, we focus in greater detail on cytokine/Janus kinase/signal transducers and activators of transcription (JAK-STAT) signaling, Ras/Raf/mitogen-activated protein kinase (MAPK) signaling, cell-cell signaling via cadherins and integrins, G-protein–coupled receptor (GPCR) signaling, and transforming growth factor β (TGFβ) superfamily signaling.
Cytokine and Hormone Signaling Through JAK-STAT Pathways
Canonical JAK-STAT Signaling
β-Cells are exposed to some 60 cytokines (e.g., interleukin [IL]-1, IL-2, and IL-6) and hormones (e.g., growth hormone [GH], prolactin [PRL], placental lactogens [PLs], leptin and erythropoietin [EPO]) that signal through JAK-STAT pathways. Connecting the dimeric or multimeric cell surface receptors for these molecules to downstream events is a family of intracellular signaling molecules that exert positive and negative feedback signals to activate signaling and then terminate it (reviewed in detail in references [3–9]).
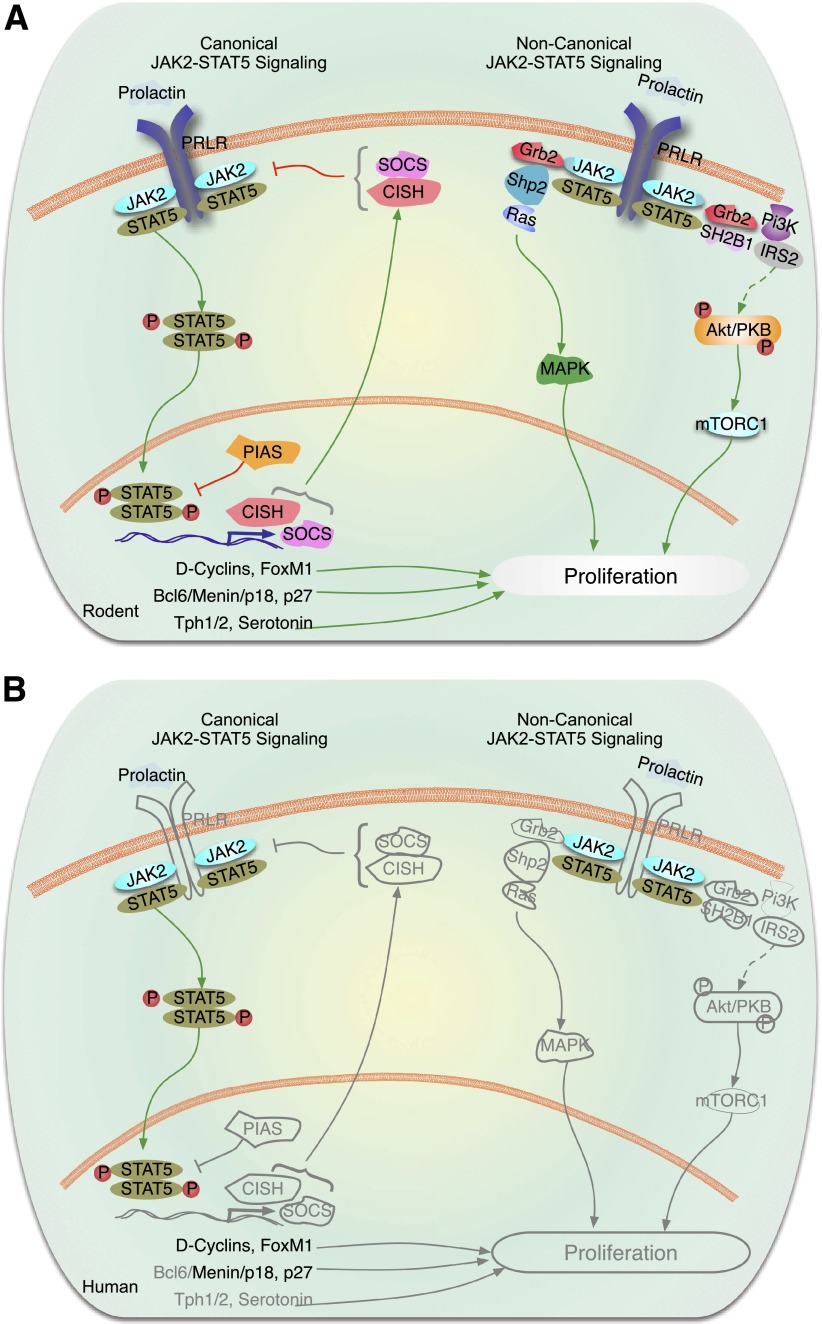
In a relevant example of JAK-STAT signaling (Fig. 1A) (3–7,10), under basal conditions, the PRL receptor (PRLR) or GH receptor (GHR) homodimers or heterodimers on the cell surface are coupled to two JAK2 molecules; cytoplasmic STAT5 homodimers are loosely bound to the receptor from a reservoir in the cytoplasm. GH binding to GHR or PRL to PRLR rearranges intracellular receptor alignment, activating the JAK2 pair that then cross-phosphorylate one another as well as the GHR or PRLR, which then strongly recruits STAT5 dimers to the GHR or PRLR. JAK2 then phosphorylates STAT5 homodimers, which causes their disassociation from the GHR or PRLR, permitting STAT5 translocation to the nucleus. Here, phospho-STAT5 homodimers bind to gamma-interferon–activated sequences (the canonical STAT response elements) in many promoters, thus initiating transcriptional activation of STAT5 target genes, exemplified by IGF-1 for GH signaling or cell cycle molecules for PRL signaling.
Figure 1.
Cytokine and hormone signaling through JAK-STAT pathways. In the canonical JAK-STAT signaling cascade, there are four JAKs, 1–3 and TYK2, seven STATs, 1, 2, 3, 4, 5a, 5b, and 6, as well as multiple inhibitors or SOCS1–7, CISH, as well as PIAS. In this complex multimember pathway, cell-type specificity is dictated by which family members are present, and which ones are preferred to others. A: Rodent β-cells. For example, as illustrated for prolactin in rodent β-cells on the left side of the figure showing canonical JAK-STAT signaling, prolactin or placental lactogens bind the dimeric PRLR. PRLR preferentially couples to JAK2, which phosphorylates/activates STAT5a/b. Phosphorylated STAT5a/b dimers then transit to the nucleus and bind to promoters of relevant genes such as cyclin D2 to drive proliferation. Rodent β-cells also contain the dimeric GHR and EPO receptor, which presumably acts via a similar mechanism(s). On the right half of the figure, examples of noncanonical signaling via the PRLR or GHR are illustrated. The major point here is that in addition to activating canonical pathways, cytokines and hormones can also engage MAPK and PI3K signaling pathways discussed in more detail in other figures. B: Human β-cells. Much less is known regarding human β-cell PRL and EPO signaling. PRL and PL fail to activate adult human β-cell proliferation, for reasons that remain unclear. JAK2 and STATs 5a and b are present, as is menin. Several SOCS members, CISH, and PIAS1 are present in human β-cells. Other components of the system present in rodent β-cells, including inhibitors of JAK-STAT signaling, such as SOCS, CISH, and PIAS1, have not been studied. Gray lines are molecules and pathways that are known to exist in rodents but are unknown in human β-cells.
Terminating JAK-STAT Signaling
Activated STATs also trigger suppressors of cytokine signaling (SOCS), cytokine-inducible SH2 domain-containing proteins (CISH), and protein inhibitors of activated STAT (PIAS) transcription (Fig. 1A), which act in a classical negative feedback manner to terminate upstream signals (8,9). SOCS and CISH accomplish this by competing with JAK-STAT interactions, by blocking the kinase activity of JAK proteins, and by serving as E3 ligase complexes that target JAKs and their receptors for ubiquitination and proteosomal degradation. PIAS1 SUMOylates certain STATs, thereby inhibiting their actions.
Noncanonical JAK-STAT Signaling
Canonical JAK-STAT members also commonly act in noncanonical fashions. For example, JAKs and STATs can activate not only “their own pathway,” but also can cross talk with members of the Ras/Raf/MAPK and PI3K-Akt/protein kinase B (PKB)-mTOR pathways (Fig. 1A). This happens via adapter molecules such as Grb2, Shp2, SHC, SH2B1, IRS2, c-Src, SOS, and others (10–15). Thus, a response initiated by a cytokine or hormone receptor in the GHR/PRLR family may be driven or enhanced by a non–JAK-STAT signaling pathway. Further, unphosphorylated STATs can also access the nucleus and bind to promoters, in some cases in a competitive manner by inhibiting phospho-STAT DNA binding (3–5). For unphosphorylated STAT5, tetramers can form and recruit Ezh2 (3–5), a component of the polycomb repressive complex that regulates, for example, cell cycle inhibitors such as p16INK4. In yet another variation, some STATs can be methylated by lysine/histone methylases, such as SET9, and thereby serve to recruit other transcription complex members to select promoters (3–5). And in still another example, JAK2 has been reported to link the GHR to the IGF-1 receptor and thereby synergizes to enhance the effects of GH signaling (16).
Rodent β-Cells
As reviewed previously (1), PRL/PL have been reported to drive β-cell proliferation in pregnancy via a canonical JAK2-STAT5 pathway. This requires the PRLR, as the β-cell PRLR knockout (KO) mouse develops β-cell hypoplasia and gestational diabetes mellitus (17–19). JAK2 is present, and STATs 5a and 5b have been shown to be phosphorylated and to translocate to the nucleus in association with cell cycle activation via cyclin D2, forkhead box protein M1 (FoxM1), and other G1/S control molecules (20,21). In mice, conditional loss of both STAT5a/b using a pancreatic and duodenal homeobox 1 (Pdx1)-Cre (22) or transgenic dominant-negative STAT5 expression (23) results in mild reductions in β-cell proliferation and function. As also described previously (1), in two parallel cascades, PRLR-JAK2-STAT5 transcriptionally drives a Bcl6/menin–p18INK4/p27CIP pathway (24), as well as serotoninergic pathway via induction of tryptophan hydroxylases 1 and 2 (Tph1/2), with induction of proliferation via the serotonin receptor, 5HTR2b (25,26). As a footnote, rodent β-cells contain STAT3, but its conditional loss has no effect on β-cells (27).
Noncanonical JAK-STAT events occur in β-cells as well. For example, the cytosolic carboxy-terminal fragment of islet cell antigen 512 (ICA-512-CCF) is released from the β-cell membrane by insulin secretion and transits to the nucleus, where it enhances STAT5-mediated insulin secretion and d-cyclin transcription (28). As another noncanonical example, the adaptor protein SH2B1, which is known to interact with JAK2, enhances insulin, IGF1, and IRS1/2 signaling in β-cells (29).
EPO also has been shown to activate rodent β-cell proliferation and expansion, acting via the EPO receptor via JAK2-STAT5 signaling (30). Leptin, another member of the cytokine family of JAK-STAT ligands, as reviewed previously (2), also acts on β-cells but has not been associated with proliferation.
With respect to inhibitors of JAK-STAT signaling, in mouse islets SOCS2 and CISH are markedly induced during pregnancy, presumably in response to lactogenic JAK2-STAT5 signals, but other SOCS family members are unchanged (31). However, individual knockout of CISH (31) or SOCS2 (32) has no effect on β-cell mass or proliferation. Transgenic overexpression of SOCS3 reduces β-cell mass (33). Banks et al. (34) reported that global knockout of SOCS7, a large SOCS family member not traditionally associated with JAK-STAT signaling, results in multiple phenotypes including increased β-cell mass, but whether this is a direct effect or a result of SOCS7 deficiency in nonislet tissues remains unclear.
Human β-Cells
In contrast to rodents, little or no β-cell proliferation occurs in humans in pregnancy (35), and PRL is unable to drive adult human β-cells to replicate (36). JAK2, STAT5, and menin are present in human β-cells, as are relevant cell cycle molecules such as cyclins, cyclin-dependent kinases (cdks), menin, FoxM1, and others (Fig. 1B). Thus, the reasons for failure of lactogens to drive cell cycle activation are unclear. Menin loss or inactivation is associated with β-cell expansion in insulinoma (37), but whether the Bcl6, p18, p27, Tph1/2, serotonin, or 5HTR2b pathways are relevant to human β-cell proliferation is unknown. EPO has not been shown to drive human β-cell replication, and JAK2-STAT5 cross talk with other signaling pathways is unexplored in human β-cells.
In human islets, SOCS1, -2, and -3 are present at low levels. SOCS3 is induced by leptin signaling (38), and SOCS1, -2, and -3 are induced by cytokines (39). Little other information exists regarding SOCS/CISH/PIAS functions in human β-cells.
Ras/Raf/ERK Signaling
ERKs, also known as classical MAPKs, are widely expressed intracellular signaling molecules involved in the regulation of cell proliferation among other cellular functions (reviewed in 40,41). These are serine/threonine/tyrosine–specific protein kinases belonging to the CMGC (CDK/MAPK/GSK3/CLK) kinase group, with ERK1 (MAPK3) and its close relative ERK2 (MAPK1) being the members most studied in the pancreatic β-cell. Whether other members, such as classical ERK5 and atypical ERK3 and ERK4, have any effect in β-cell growth and function is unknown. Typically, binding of a ligand to the relevant tyrosine kinase or GPCR activates the small GTPase, Ras, leading to the formation of Ras-GTP (40,41) (Fig. 2A). Activated Ras then phosphorylates c-Raf, followed by activation of MAPK kinase (MKK/MEK) that then phosphorylates ERK1/2.
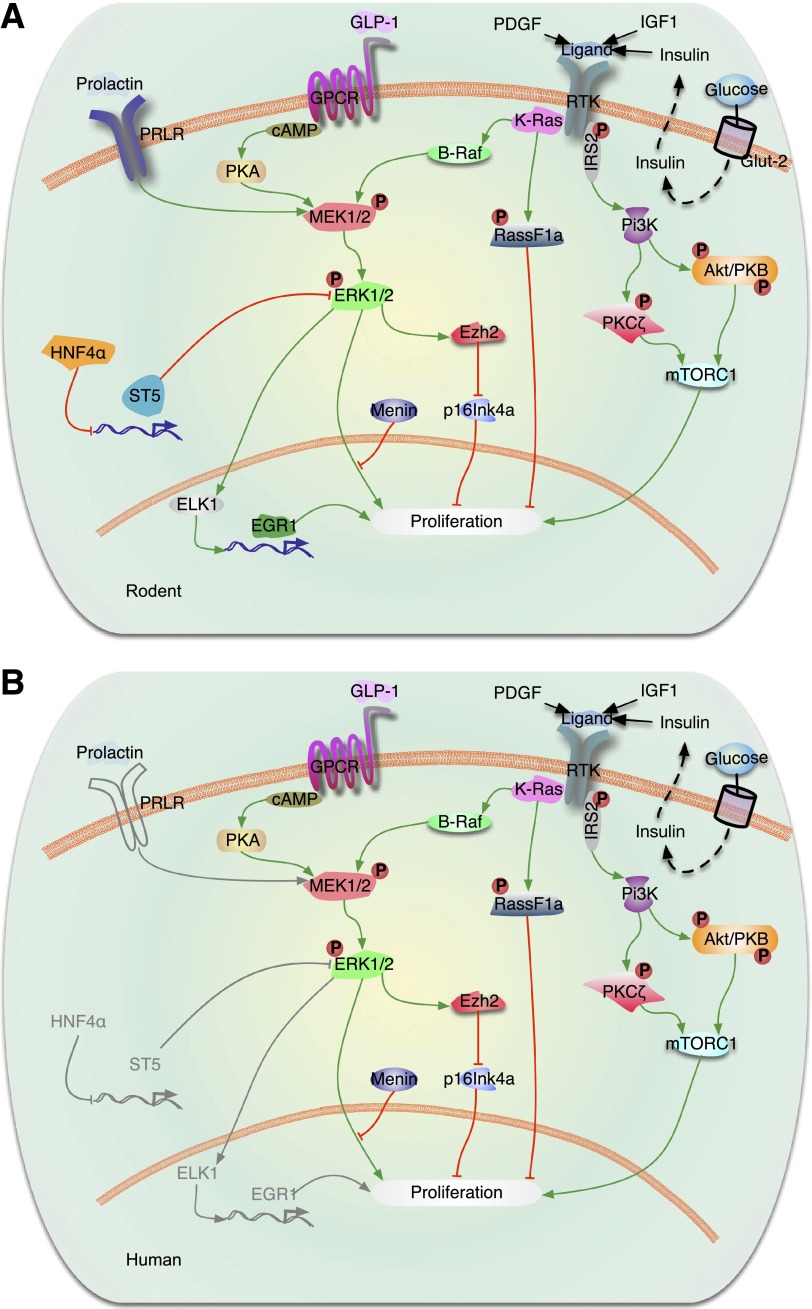
Figure 2.
Signaling by Ras/Raf/ERK in the regulation of β-cell proliferation. A: Rodent β-cells. Activation of tyrosine kinase receptors (RTKs) (IGF-1 and paracrine effects of insulin) induces β-cell proliferation by activation of the PI3K/Akt (Akt-protein kinase B)/mTOR and PKCζ and ERK1/2 (also called MAPK) signaling pathways. Recent studies have shown that K-Ras is a major regulator of β-cell proliferation. K-Ras activation regulates β-cell proliferation by modulating two major cascades, B-Raf/MEK/ERK and Rassf1a. Activation of K-Ras signaling activates both the ERK1/2 and the Rassf1a pathways, resulting in suppression of β-cell proliferation when menin is present. This indicates that menin ultimately determines the K-Ras effects on β-cell proliferation downstream of MAPK/ERK signaling. In the case of PDGF, PDGF receptor-α induces ERK1/2 phosphorylation, leading to upregulation of the polycomb group protein Ezh2 and repression of p16INK4a resulting in β-cell expansion. ERK activation also can regulate gene expression by induction of downstream transcription factors, such as ELK1 and Egr1, which in turn regulate expression of proproliferative genes in the nucleus. Other transcription factors, such as HNF-4α, indirectly regulate ERK signaling by downregulation of ST5 under some conditions. Ligand activation of the Gαs subunit of the GLP-1R stimulates adenyl cyclase (AC), which leads to an increase in cAMP and activation of PKA and MEK1/2. B: Human β-cells. Most of the components of the rodent β-cell system are also present in the human β-cell, as illustrated. Exceptions are that PDGF receptor-α is apparently absent in adult human β-cells, and PRLR has not been demonstrated in human β-cells. Gray lines are molecules and pathways that are known to exist in rodents but are unknown in human β-cells.
In rodent β-cells, multiple growth factors and hormones require the activation of ERK1/2 to induce proliferation (12,42–48). For example, activation of PDGF receptor-α in mouse β-cells stimulates ERK1/2 phosphorylation, leading to upregulation of the polycomb group protein Ezh2, p16INK4a repression, and expansion of adult β-cells (48). It has also been reported that other growth factors, nutrients, and hormones (such as IGF-1, incretins, glucose, triiodothyronine, prolactin, insulin, or serum) require activation of ERK1/2 and the PI3K/Akt/mTOR signaling pathways to fully induce rodent β-cell replication (12,42–47). However, the ERK1/2 pathway is not involved in rodent β-cell proliferation induced by other β-cell mitogens such as hepatocyte growth factor and parathyroid hormone–related protein (PTHrP) (49). In addition, TGFα and EGF have been reported to transiently activate ERK1/2, but this transient activation is not sufficient to induce β-cell proliferation (42). This is in contrast to the prolonged ERK1/2 activation and β-cell proliferation induced by IGF-1, suggesting that sustained activation of this pathway is required for β-cell replication (42). Thus, although ERK1/2 signaling seems to be induced by most β-cell mitogens, its role in the induction of β-cell replication seems to vary depending on the mitogen, the duration of its activation, and potentially the cross talk with other signaling targets induced by these mitogens. Activated ERKs are known to trigger downstream transcription factors, such as ELK1, that drive the expression of proproliferative genes in the nucleus (50). On the other hand, transcription factors, such as HNF-4α, have been shown to indirectly modulate ERK signaling, in part by the downregulation of a tumor suppressor called suppression of tumorigenicity 5 (ST5) in β-cells (51). In this study, activation of ERK1/2 during pregnancy was significantly reduced in HNF-4α–deficient islets, indicating that activation of ERK1/2 is required for HNF-4α to induce β-cell proliferation during pregnancy.
Guanine nucleotide–releasing factors (GRFs) and GTPase-activating proteins control Ras cycling between an inactive GDP-bound conformation and an active GTP-bound conformation, respectively. GRF1 is present in β-cells and its deletion causes decreased β-cell proliferation and neogenesis (52). GRF1-deficient β-cells do not activate PI3K/Akt or ERK signaling pathways, suggesting that normal cycling of Ras proteins is a requirement for induction of β-cell proliferation.
There are three Ras proteins, namely K-Ras, N-Ras, and H-Ras. Oncogenic mutations in these create constitutively active Ras proteins that result in uncontrolled proliferation of tumor cells (40,41). The importance of K-Ras expression during development is illustrated by the embryonic lethality of K-Ras KO mice, while H-Ras or N-Ras KO mice are viable without apparent phenotypes (53). Interestingly, K-Ras regulates signaling networks with opposing functions (54), suggesting that K-Ras may have cell context–dependent functions. Indeed, constitutively active K-RasG12D stimulates tumor formation in pancreatic ducts and acini but not in the pancreatic endocrine cells.
In an elegant study published recently, Chamberlain et al. (55) reported on the regulation of the Ras/Raf/ERK and Ras/Rassf1a (an antiproliferative effector of activated K-Ras signaling) in β-cells, arriving at several interesting and surprising conclusions. First, mice heterozygous for a null mutation in K-Ras had increased pancreatic β-cell mass, reflecting both increased neurogenin-3–expressing endocrine progenitors during embryogenesis as well as accelerated β-cell proliferation perinatally, suggesting K-Ras is a negative regulator of β-cell expansion. Indeed, expression of constitutively active K-RasG12D activated both the ERK1/2 and the Rassf1a pathways, and this resulted in suppressed β-cell proliferation. This dominance of the antiproliferative K-Ras effect in β-cells was diminished following menin silencing. This indicates that menin normally prevents the ERK1/2 pathway from driving β-cell proliferation, while leaving inhibitory effector pathways, such as Rassf1a, intact. Collectively, this generates a scenario in which K-Ras activates both the proproliferative ERK1/2 and the antiproliferative Rassf1a pathways, with the net result of reduced proliferation. However, in tissues susceptible to MEN1 gene inactivation, such as pancreatic endocrine cells, proliferation can result following MEN1 inactivation due to removal of the block in ERK-driven proliferation downstream of K-Ras. On the other hand, loss of K-Ras signaling increases β-cell proliferation by decreasing unopposed Rassf1a activity. These data provide some light on the absence of activating KRAS mutations and the high frequency of MEN1 and RASSF1A inactivation in pancreatic endocrine tumors (56,57).
In human β-cells, ERK1/2 are expressed, and PDGF enhances juvenile human β-cell proliferation via this pathway (Fig. 2B) (48). In addition, most of the knowledge on the role of ERK1/2 in human β-cell proliferation emerges from data obtained from studies in neuroendocrine tumors. Human pancreatic endocrine tumors have not been reported to carry activating KRAS mutations but more commonly harbor inactivating mutations in tumor-suppressor genes such as MEN1 or RASSF1A (56,57). Of high translational importance, inhibitors of K-Ras and menin signaling enhanced human β-cell proliferation in vitro (55). These studies clearly point to a need for detailed studies on this signaling pathway in human β-cells, as it has an important potential for significantly impacting human β-cell proproliferative therapies for diabetes.
Cadherin and Integrin Signaling
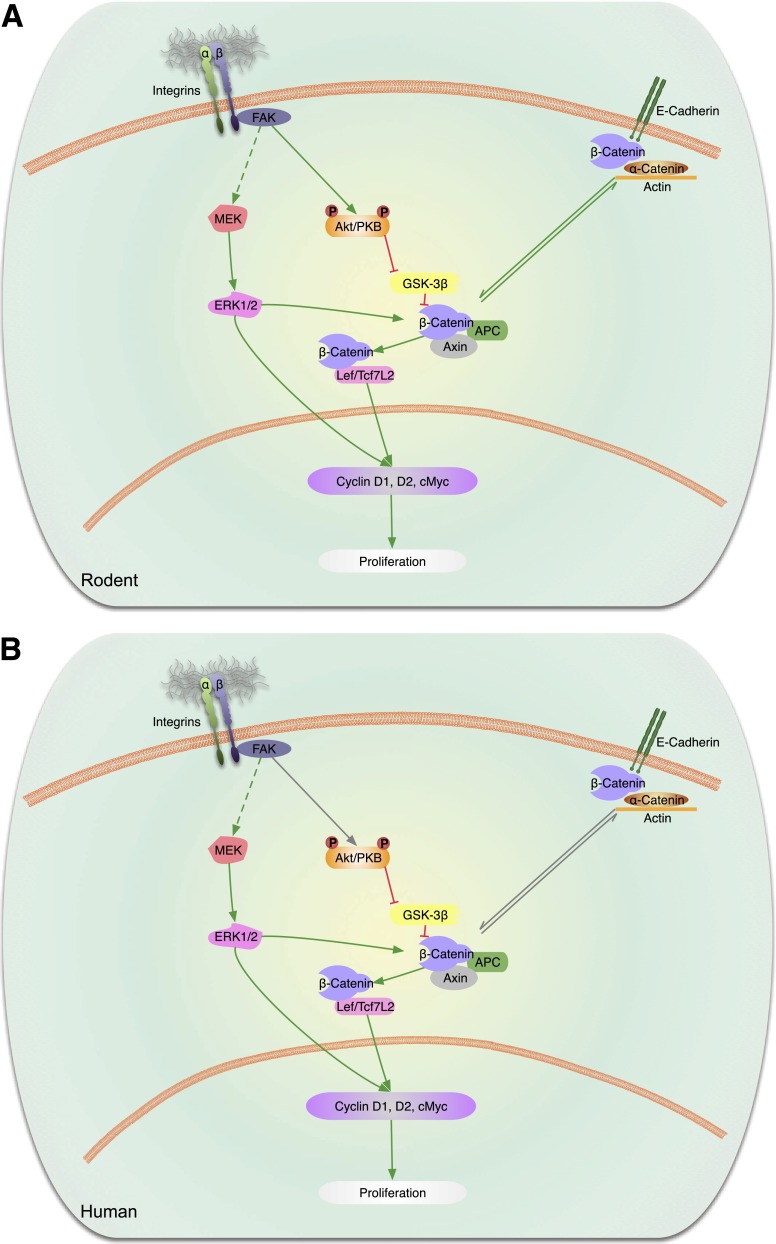
Cadherins are cell surface molecules that form clusters at sites of cell-cell contact. They are the principal cell membrane proteins responsible for cell adhesion between cells. In addition to providing structural stability, integrity, and cell-cell communication, members of the cadherin superfamily initiate signaling pathways to regulate diverse biological processes (Fig. 3A). The intracellular domain of classical cadherins forms a complex with β-catenin, γ-catenin, and p120-catenin, with several sites in the cytoplasmic tail, including the juxtamembrane region. β-Catenin and γ-catenin bind directly to α-catenin, which links the classical cadherin/β-catenin/α-catenin (CCC) complex to the actin cytoskeleton. Additional interacting partner molecules link the cadherins to the microtubule network and signaling molecules, such as STAT3. In the context of the endocrine pancreas, specific cadherins are expressed at different stages of development and contribute to the architecture and structure of the islet as well as to insulin secretion and β-cell survival. Conditional deletion of E-cadherin in mice negatively regulates β-cell proliferation by reducing expression of ccnd1 and ccnd2, and this was associated with a decrease in nuclear expression of β-catenin and cMyc (58).
Figure 3.
Cadherin and integrin signaling pathways. A: Rodent β-cells. Activation of β-integrin receptors on β-cells leads to FAK-mediated phosphorylation of Akt/PKB that in turn blocks GSK3β to release the inhibition on β-catenin. The free β-catenin forms a complex with Lef/transcription factor 7–like 2 (Tcf7L2) to promote modulation of cell cycle genes and β-cell proliferation. It is uncertain whether integrins also act via MEK to promote ERK phosphorylation that might in turn activate the β-catenin pathway and/or directly regulate cell cycle genes. E-cadherins are proteins that have been shown to be present in intercellular complexes in islets. Activation of E-cadherins has been linked to the β- and α-catenin complex and promotion of proliferation via the β-catenin modulation of cell cycle genes. B: Human β-cells. Integrins, E-cadherins, and components of the signaling pathways detected in rodents have also been reported to be present in human β-cells. Integrins and E-cadherins have not been shown to be able to modulate proliferation in human β-cells. Gray lines are pathways that are known to exist in rodents but are unknown in human β-cells.
Integrins are heterodimeric cell surface glycoproteins that mediate interactions between the cell and extracellular matrix linking the extracellular environment to the actin cytoskeleton. These membrane-spanning proteins consist of two families of subunits, α and β, each with multiple isoforms. This provides a multitude of possible combinations with the potential to control a broad range of biological processes by binding to extracellular substrates. Several studies have demonstrated the role of β1-integrin in β-cell proliferation in rodent and human β-cells. The effects of integrins are mediated by activation of intracellular signaling pathways associated with focal adhesion kinase (FAK) (Fig. 3A). Conditional deletion of β1-integrin in mice demonstrated that this integrin is required for β-cell mass and proliferation via ERK and Akt signaling, pathways known to regulate β-cell expansion (1,2,59). In addition, β-cells lacking β1-integrin exhibited downregulation of Ccnd1, Ccnd2, Ccne1, E2f1, and E2f2 and increased expression of the inhibitor Cdkn1a (p21) among others. Similarly, disruption of β1-integrin in collagen-I–producing pancreatic cells resulted in reduced β-cell proliferation, mass, and function in vivo (60). This abnormality was associated with a reduction in β1-integrin/FAK/ERK signaling and Ccnd1 levels.
In human β-cells (Fig. 3B), perturbing β1-integrin function in fetal islet–epithelial cell cultures using β1-integrin–blocking antibody and β1-integrin siRNA and FAK expression vectors resulted in a decrease in FAK/ERK signaling activity and consequent alterations in differentiation and survival of fetal pancreatic islet cells (61). The E-, N-, and P-isoforms of cadherin are expressed in human islets and have been reported to modulate β-cell survival (62) and insulin secretion from single human β-cells (63). While E-cadherin disengagement has been suggested to be linked to tumor progression, the potential role(s) of cadherins and integrins in the maintenance of mature human β-cell proliferation has not been directly explored. Furthermore, to date, there are no reports of an association of polymorphisms in genes that code for cadherins or integrins with β-cell dysfunction and/or diabetes.
GPCR Signaling in the β-Cell
More than 500 GPCRs (also called GPRs) are encoded in the mammalian genome, and many of them are expressed on the surface of the β-cell (64,65). GPCRs signal through 1) heterotrimeric GTPases (also called heterotrimeric guanyl nucleotide binding proteins, or G-proteins) and 2) β-arrestins (66,67). The G-protein heterotrimers each consist of one α-, β-, and γ-subunit: the α-subunit determines which signaling pathway is activated after ligand binding to the respective GPCR. GDPα is complexed with β- and γ-subunits and is inactive in the basal state. Receptor binding catalyzes the exchange of GTP for GDP associated with the α-subunit, thereby releasing the “active” GTPα subunit from the β- and γ-subunits. The active GTPα subunit binds and regulates downstream signaling effectors. The Gαi/o family inhibits TMAC (transmembrane-bound adenylate cyclase) and inhibits cAMP synthesis, while the Gαs family stimulates TMAC and cAMP synthesis. cAMP predominantly signals via protein kinase A (PKA) activation. The Gαq/11 family activates phospholipase C, IP3, and DAG signaling. The Gα12/13 family activates the Rho family of GTPases (66).
Pertinent to rodent β-cell signaling (Fig. 4A) is that cAMP signaling activated by Gαs-coupled receptors (such as the GLP-1 receptor [GLP-1R]) signals through both PKA-dependent (cAMP-PKA branch) and PKA-independent (exchange protein activated by cAMP [EPAC] branch) pathways (68). EPAC1 and EPAC2 belong to the large class of “monomeric” G-proteins (also called Ras superfamily of GTPases). In β-cells, cAMP binds and activates EPAC2, which activates Rap1, which, in addition to mediating insulin vesicle exocytosis (69), is implicated in EPAC2-mediated β-cell proliferation via interface with the mTOR complex (70). Surprisingly, in vivo β-cell–selective upregulation of PKA signaling in mice markedly potentiates glucose-stimulated insulin secretion (GSIS) but does not stimulate β-cell proliferation (71,72). Further, intracellular A-kinase anchoring proteins (AKAPs) that target PKA catalytic subunit to distinct intracellular locations and modulate GLP-1 signaling and effects in β-cells may also regulate β-cell proliferation. AKAP inhibition in insulinoma cells (73) and AKAP150 KO in mice (74) result in impaired insulin secretion. Whether distinct AKAP(s) regulate β-cell proliferation is at present unclear.
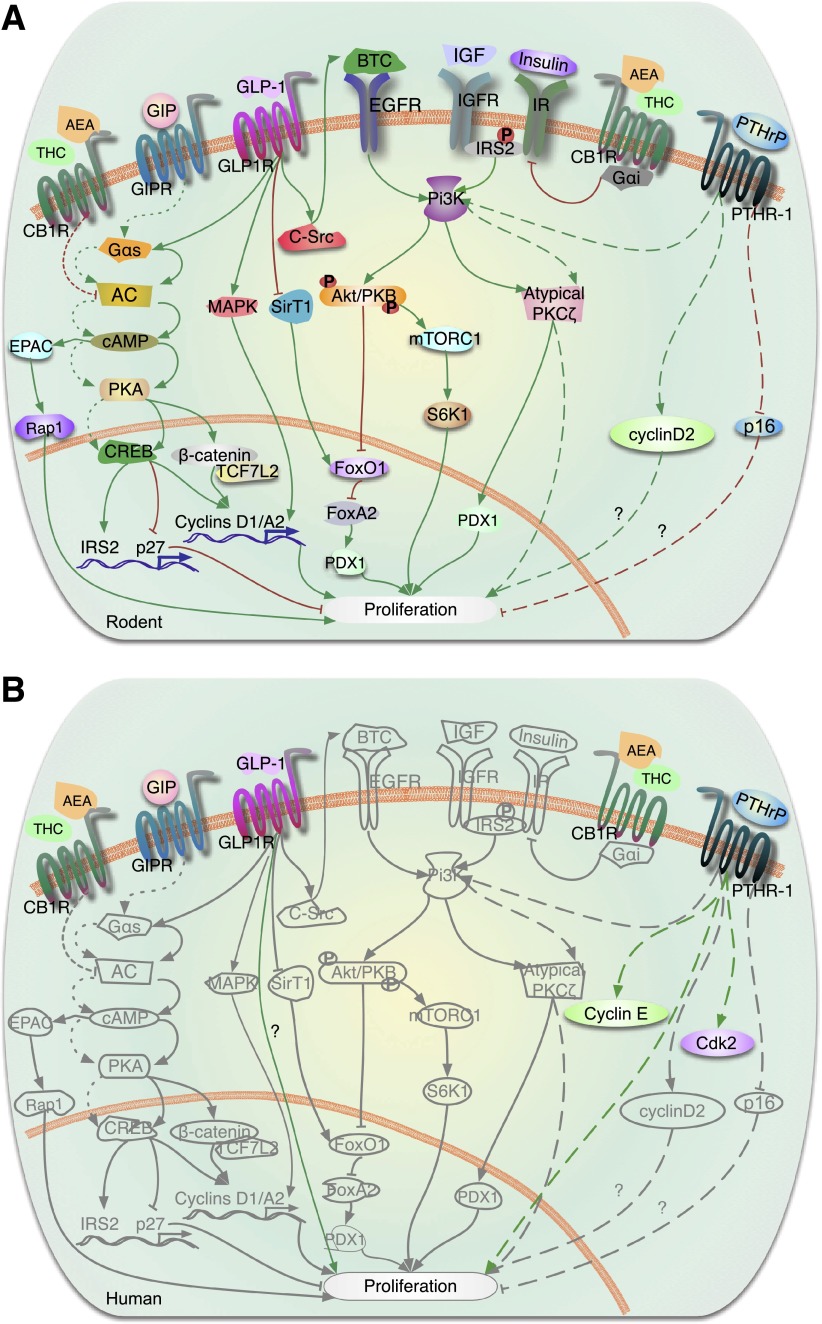
Figure 4.
GPCR (or GPR) signaling in the regulation of β-cell proliferation. A: Rodent β-cells. The incretin hormones, GLP-1 and GIP, bind to their respective GPCRs, GLP-1R and GIPR, releasing the “active” Gαs subunit, which stimulates adenylate cyclase (AC) and cAMP synthesis. cAMP signals though either PKA or EPAC pathways. PKA phosphorylates CREB, which upregulates transcription of key proliferative β-cell genes (e.g., cyclin D1, cyclin A2, Pdx1, Irs2) and downregulates transcription of the cell cycle inhibitor p27. IRS2 supports autocrine/paracrine stimulation of IGF/IGF receptor (IGFR)/PI3K-Akt (Akt-PKB) signaling. Akt activation enhances proliferation through a dual effect: activation of mTORC1 and its target p70 ribosomal S6K1 and phosphorylation and inactivation of FOXO1, which allows FOXA activation and PDX1 expression. GLP-1 also inactivates FOXO1 by inhibiting SirT1-mediated deacetylation of FOXO1. Through a variety of cross-talk mechanisms, GLP-1 activates multiple other signaling pathways: PI3K/atypical PKCζ, transactivation of EGFR through c-Src tyrosine kinase activation of its ligand betacellulin (BTC), ERK1/2, and Wnt signaling pathway molecules β-catenin and transcription factor 7–like 2 (TCF7L2). Another GPCR member, PTHR-1, is activated by PTHrP, leading to increased β-cell proliferation through PI3K-atypical PKCζ phosphorylation. Overexpression of PTHrP in β-cells induces cyclin D2 expression and reduces levels of the cell cycle inhibitor p16. Activation of the cannabinoid receptor, CB1R, by its ligands anandamide (AEA) and Δ-9-tetrahydrocannabinol (THC), reduces β-cell proliferation via inhibition of the insulin/IGF-1/IRS2/AKT signaling pathway through the heterotrimeric G-protein, Gαi. The potential effect of CB1R on proliferation by modulating AC is not fully understood. Green arrows indicate activation of pathways and red lines denote inhibition of pathways. Signaling pathways are denoted by solid lines downstream of GLP-1R, dashed lines downstream of PTHR-1, and dotted lines downstream of GIPR. B: Human β-cells. The GPCRs, GLP-1R, GIPR, PTHR-1, and CB1R are all present on human β-cells. Whether GLP-1 enhances human β-cell proliferation is unclear, as one report reveals a modest increase in proliferation (85) and another indicates no increase in replication with GLP-1 analogs (36). PTHrP induces human β-cell proliferation in vitro and increases expression of the cell cycle activators cyclin E and cdk2 in human islets. In gray are molecules and pathways that are known to be activated by GPCRs in rodents but are not known to be downstream of GPCRs in human β-cells.
β-Arrestins uncouple GPCRs from their cytoplasmic heterotrimeric G-proteins to terminate signaling but also form scaffolds that enable ligand-bound GPCRs to cross-signal through transduction pathways of the tyrosine kinase, ERK1/2, and JNK pathways (67). Proliferation and antiapoptotic effects of GLP-1 (see glp-1 and gip family gpcr signaling) on β-cells involve GLP-1–induced intracellular β-arrestin-1/c-Src interaction (75) and GLP-1–induced β-arrestin-1/ERK1/2/phospho-Bad signaling cascade (76), respectively. Further, based on genetic mouse models, there appears to be a role for β-arrestin-2 in modulating β-cell mass and proliferation (77); however, some studies allocate a role for β-arrestin-2 in modulating GSIS but not in β-cell mass expansion in response to diet-induced insulin resistance (78). Thus, both β-arrestin-1 and -2 participate in distinct β-cell functions related to G-protein signaling.
Little is known about the role of β-arrestin–mediated signaling in β-cells except that β-arrestin KO mice exhibit β-cell dysfunction and lack β-cell mass expansion in response to high fat–diet feeding (77).
GLP-1 and GIP Family GPCR Signaling
In β-cells, among the GPCR ligands, the actions mediated by the incretin hormones, GLP-1, and glucose-dependent insulinotropic polypeptide (GIP) are best understood (Fig. 4A). GLP-1(7–36) (and its analogs such as the more stable exendin-4 and liraglutide) and GIP bind to their respective GPCRs—GLP-1R and GIPR—to support juvenile rodent β-cell replication (promitotic) as well as prosurvival (antiapoptotic) mechanisms, resulting in β-cell mass expansion in in vitro and in vivo model systems (79,80).
In β-cells, GLP-1 directly or indirectly activates multiple signaling pathways, including cAMP-PKA-CREB, PI3K-Akt and atypical PKCζ, transactivation of EGFR through c-Src tyrosine kinase, ERK1/2, and Wnt pathways (79,80). Through a variety of cross-talk mechanisms, PKA-CREB signaling facilitates ERK1/2, Wnt, and PI3K activation. cAMP-PKA phosphorylates and upregulates CREB-dependent transcription of key β-cell genes (e.g., Ins1, Ins2, Pdx1, Irs2). IRS2 in turn supports autocrine/paracrine stimulation of prosurvival IGF-2/IGF-1R signaling (81,82). Conversely, mTOR complex 1/S6 kinase 1 (mTORC1/S6K1) activation by IGF-1R/PI3K-Akt is required for GLP-1–induced β-cell proliferation (83). A permissive role for CREB in rodent β-cell proliferation is derived from observations of transgenic overexpression of the endogenous CREB transcriptional repressor ICER (inducible cAMP element repressor) (84). In addition, FoxO1, a molecular brake for β-cell replication, is inactivated by GLP-1 through two distinct mechanisms: Akt-mediated phosphorylation of FoxO1, resulting in increased Pdx1 expression (79,80,85), and reduced SirT1-mediated FoxO1 deacetylation (86). Finally, downstream of these events, GLP-1 induces β-cell proliferation by increasing expression of cell cycle activators, cyclin D1 and cyclin A2, in rat and mouse β-cells, respectively (45,79,80), and by degrading the cell cycle inhibitor, p27, via the SCF ubiquitin ligase complex containing Skp2 (87).
GIP enhances replication in rodent β-cell lines and primary cells in vitro (Fig. 4A) (70,80). Dominant-negative GIPR expression in transgenic pigs reduces β-cell proliferation and mass (88), suggesting a role for GIP in β-cell proliferation in vivo. As with GLP-1, cAMP-PKA-CREB, ERK1/2, and PI3K-Akt all contribute to GIP-induced rodent β-cell proliferation; however, the downstream targets are unknown. Additional details on GIP and GLP-1 are contained in excellent reviews (79,80).
In contrast to the large literature on GPCR ligands in rodent islets, little is known in human islets, aside from effects mediated by GLP-1 and GIP receptors on β-cell proliferation (Fig. 4B). In one report, liraglutide treatment of sorted or unsorted human β-cells from adult human islet donors, cultured on several different matrices, showed no replication (36), while in another report, liraglutide induced a fivefold increase over the low basal rate of replication in unsorted human β-cells on a bovine corneal endothelial cell matrix (89). Exendin-4 treatment of immunodeficient diabetic mice transplanted with human islets under the kidney capsule produced a twofold increase in β-cell replication in islets obtained from young donors (average age 18 years) but not from older donors (average age 47 years) (90). No information on signaling mechanisms is available in human islets.
Fatty Acid GPCR Signaling
GPCRs for short-chain (GRP-41 and -43, also called FFA2 and FFA3, respectively) and long-chain fatty acids (GRP-40, also called FFA1, and GRP-120) and fatty acid metabolites (GPR-119) have gained increasing attention for their potential as pharmacologic targets on α-cells (91) to suppress glucagon secretion and on β-cells to potentiate GSIS (92). The mechanisms underlying these events may reflect direct action on β-cells and/or indirect effects via incretin hormone (i.e., GLP-1, GIP) release from intestinal epithelial cells (91–94). Among these receptors, activated GRP-119 stimulates intracellular cAMP synthesis and small molecular GRP-119 activators potentiate GSIS and also stimulate rodent β-cell proliferation (93,94). FFA2 and -3 receptors signal through Gαi/o and inhibit GSIS. Islets of rodents exposed to a diabetogenic high-fat diet produce FFA2 and -3 ligands, which suppress GSIS, and ablation of these receptors results in increased GSIS and improved glucose homeostasis (95). Very little is known about the function of these novel receptors in human β-cells. There is little evidence for induction of β-cell proliferation by fatty acid receptor ligands.
PTHrP GPCR Signaling
PTHrP is a broadly expressed precursor protein that undergoes posttranslational processing to produce multiple mature peptides, including an amino-terminal peptide, PTHrP(1–36). This binds to and activates its GPCR, the parathyroid hormone receptor, called PTH receptor-1 (PTHR-1). PTHrP and PTHR-1 are expressed in rodent islets and β-cells. PTHrP(1–36) stimulates proliferation of rodent β-cell lines and primary β-cells in culture (96). Transgenic expression of full-length PTHrP(1–139) in β-cells (49) or systemic administration of PTHrP(1–36) in mice (97) stimulates β-cell replication in vivo, with an increase in β-cell mass. Pharmacological, small interfering RNA, and dominant-negative approaches have identified atypical PKCζ as the mediator of PTHrP-induced proliferation in rodent β-cells (49). PTHrP increases cyclin D2 and decreases p16 in transgenic islets (97) (Fig. 4A).
In humans, PTHrP and PTHR-1 are present in β-cells. PTHrP is overexpressed in human insulinomas, suggesting a role in human β-cell replication (96). Adenoviral transduction of full-length PTHrP(1–139) in adult human islets significantly enhances β-cell proliferation (98). The principal PTHrP peptide required for human β-cell proliferation appears to be PTHrP(1–36). This peptide does not diminish differentiated function; indeed, it appears to enhance GSIS in human islets, analogous to other GPCR incretins, the GLP/GIP family. PTHrP increases the cell cycle activators, cyclin E and cdk2, in human islets (Fig. 4B) (98). The specific pathways through which this occurs are unknown.
Cannabinoid Receptor Signaling
The cannabinoid receptors, especially CB1, and its endogenous ligands, the endocannabinoids, originally associated with energy intake, have additionally been recognized to be involved in multiple additional aspects of energy homeostasis in mammals (99). Both peripheral and central components of endocannabinoid regulation of energy balance may become dysregulated and lead to lipid disorders, obesity, or type 2 diabetes. While these findings indicate a potential positive therapeutic role for CB1 receptor antagonists, emerging data suggest that some cannabinoids also may have negative effects on β-cells.
Endocannabinoids have been shown to be generated within β-cells, and CB1 receptors have been reported to be expressed and to exert functional effects when activated by ligands in both mouse and human β-cells (100–102) (Fig. 4A). Studies focused mostly in mouse islets and β-cells indicate that blockade of CB1 receptors leads to enhanced signaling via the insulin/IGF-1 signaling pathway via the IRS2/Akt cascade to promote increased β-cell proliferation and mass. In vivo antagonism of CB1 receptors improved glycemia and enhanced β-cell proliferation in the db/db mouse model of diabetes (101). While some studies report that CB1 receptors mediate their effects on β-cells indirectly by modulating effects via macrophages (103), other studies provide direct evidence that CB1 receptors in mouse β-cells form a complex with insulin receptors and the heterotrimeric G-protein, Gαi (104). Gαi inhibited the kinase activity of the insulin receptor in β-cells by directly binding to the activation loop in the tyrosine kinase domain of the insulin receptor. This leads to attenuated phosphorylation of the proapoptotic protein, Bad, with resultant β-cell death (104). However, it is unclear whether CB1 receptors can influence activation of PKA by modulating adenylate cyclase. These findings suggest that CB1 antagonists acting peripherally (i.e., outside the central nervous system) may have direct beneficial effects on β-cells, with the potential to improve β-cell proliferation and function.
While the presence of CB1 receptors and the endocannabinoid system have been reported in human islets (Fig. 4B) (100,101), little information is available on cannabinoid signaling and the regulation of human β-cell proliferation. Additional work in this area is warranted, as centrally acting cannabinoid agonists are in development.
TGFβ Superfamily
TGFβ family member signals are transduced by cell surface serine/threonine kinase receptors that activate downstream signaling effectors called SMAD proteins that translocate to the nucleus to induce changes in gene expression (105–108). In addition, TGFβ family members can signal through noncanonical signaling pathways via activation of ERK, p38, JNK, and Akt (107).
A central theme from the work from several laboratories is emerging that inhibition of TGFβ signaling in adult murine β-cells can promote β-cell replication (109–113). Mice lacking Smad2/3 in islets display islet hyperplasia and show increased replicative response after pancreatectomy. Interestingly, replicating β-cells after pancreatectomy expressed Smad7, a TGFβ inhibitory Smad, supporting the idea that inhibition of TGFβ signaling results in islet expansion. Mice with β-cell–specific ablation of Smad7 provided more direct evidence, as β-cell replication was prevented after pancreatectomy (111). In addition, conditional expression of Smad7, an inhibitor of TGFβ signaling, resulted in islet hyperplasia, although β-cells had reduced expression of β-cell–specific transcription factors and impaired function (110). Recent insightful work showed that Smad7 was upregulated in β-cells after pancreatic duct ligation. Furthermore, Xiao et al. (111) show that macrophages release TGFβ1 to stimulate Smad7 expression in β-cells to promote islet cell expansion.
While inhibition of TGFβ signaling in adult β-cells can be a useful strategy for β-cell regeneration, there are several challenges. First, the mechanism by which TGFβ inhibition can lead to increased β-cell replication is not clear. Second, the effect of TGFβ inhibition on the function of β-cells is not clear. In vivo studies in mice suggest that increased β-cell replication also results in de-differentiation and impaired function (109,110). Other studies that inhibit TGFβ signaling in vitro show enhanced insulin transcription and protection from de-differentiation of islets from diabetic mice (112,113). These seemly opposing effects on β-cell–specific gene regulation, differentiation, and replication may lie in the fact that TGFβ has pleiotropic effects that depend on the cellular history and context of the responding cells (108). This context is determined by the repertoire of factors present in the responding β-cell that cooperate with the Smad proteins to guide the gene response as well as the epigenetic landscape of the β-cells that will regulate the response of the β-cell to TGFβ signaling.
Other TGFβ superfamily members include activins, inhibins, follistatins, and bone morphogenetic proteins. Space precludes a full discussion of these, but there is clear evidence that manipulation of these TGFβ superfamily members influences downstream SMAD signaling in β-cells, in most instances appearing to inhibit proliferation (114–118).
Finally, for human β-cells, it is not known whether inhibition of TGFβ family members can promote replication. Experiments that inhibit TGFβ signaling in human islets in vitro and in vivo using humanized mice are needed. Recent data suggest that bone morphogenetic proteins may inhibit human β-cell proliferation (118).
Summary and Conclusions
In these three Perspectives, we review key intracellular signaling pathways involved in rodent and human β-cell proliferation and how they impact on downstream cell cycle machinery, highlighting what is known and what remains to be studied. We have not covered every signaling pathway: additional ligands and pathways (including adrenergic, muscarinic, purinergic and adenosine kinase, ephrin, hypoxia-inducible factor-1α/von Hippel-Lindau, VGF/TLQP-21, and osteocalcin pathways) have all been reported to drive rodent β-cell replication. We apologize to authors whose work in these areas has not been reviewed because of space limitations.
Two surmountable challenges remain. First, there currently is no cell surface molecule that is entirely β-cell specific. This means that drugs delivered systemically for β-cells may have off-target mitogenic effects. Thus, β-cell–specific targeting strategies must be developed. Second is the risk of activating oncogenic pathways. Here, one must recall that β-cell proliferation is a normal physiologic event in childhood that leads to physiologic and safe islet expansion. It is also important to be clear that essentially every mitogenic pathway, if overactivated or activated for too long, can be oncogenic. Thus, it is critical to develop dosing protocols and therapeutic durations that mimic physiology to drive therapeutic—but not oncogenic—β-cell proliferation.
Finally, it is important to emphasize that therapeutic opportunities beyond β-cell proliferation exist. For example, many of the pathways that activate proliferation also activate survival pathways, another important therapeutic objective. Further, β-cell expansion might be achieved through other approaches, notably stem cell differentiation and transdifferentiation from α- or δ-cells to β-cells. And of course, creating new β-cells requires effective addressing of autoimmunity as well. All are critical, fertile, and active areas of β-cell therapeutic research.
Article Information
Acknowledgments. The authors recognize the many authors whose important work in these and other receptor signaling–mediated pathways that were unable to be cited because of space limitations.
Funding. This work was supported by NIH grants R01 DK55023 (A.F.S.), U01 DK089538 (A.F.S.), R01 DK67536 (R.N.K.), R01 DK103215 (R.N.K.), R01 DK101591 (M.A.H.), R01 DK081472 (M.A.H.), P60 DK079637 (M.A.H.), R01 DK073716 (E.B.-M.), R01 DK084236 (E.B.-M.), R01 DK078060 (R.C.V.), R01 DK06735 (A.G.-O.), and DK077096 (A.G.-O.); JDRF grants 1-2011-603 (A.F.S.), 17-2013-416 (E.B.-M.), 17-2012-37 (R.C.V.), 47-2012-750 (R.C.V.) and 17-2011-595 (A.G.-O.); and American Diabetes Association grant 1-14-BS-069.
References
- 1.Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 2012;61:2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocaña A. Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes 2014;63:819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiu H, Nicholson SE. Biology and significance of the JAK/STAT signalling pathways. Growth Factors 2012;30:88–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stark GR, Darnell JE Jr. The JAK-STAT pathway at twenty. Immunity 2012;36:503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 2012;36:542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhuang S. Regulation of STAT signaling by acetylation. Cell Signal 2013;25:1924–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks AJ, Dai W, O’Mara M, et al. . Mechanism of action of protein kinase JAK2 by the growth hormone receptor. Science 2014;344:710. [DOI] [PubMed] [Google Scholar]
- 8.Linossi EM, Babon JJ, Hilton DJ, Nicholson SE. Suppression of cytokine signaling: the SOCS perspective. Cytokine Growth Factor Rev 2013;24:241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shuai K, Liu B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat Rev Immunol 2005;5:593–605 [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Zhang Y, Berry PA, et al. Growth hormone signaling in human T47D breast cancer cells: potential role for a growth hormone receptor-prolactin receptor complex. Mol Endocrinol 2011;25:597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyga R, Pecquet C, Harir N, et al. Activated STAT5 proteins induce activation of the PI 3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter. Biochem J 2005;390:359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amaral MEC, Cunha DA, Anhê GF, et al. Participation of prolactin receptors and phosphatidylinositol 3-kinase and MAP kinase pathways in the increase in pancreatic islet mass and sensitivity to glucose during pregnancy. J Endocrinol 2004;183:469–476 [DOI] [PubMed] [Google Scholar]
- 13.Doche ME, Bochukova EG, Su HW, et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Invest 2012;122:4732–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamauchi T, Kaburagi Y, Ueki K, et al. Growth hormone and prolactin stimulate tyrosine phosphorylation of insulin receptor substrate-1, -2, and -3, their association with p85 phosphatidylinositol 3-kinase (PI3-kinase), and concomitantly PI3-kinase activation via JAK2 kinase. J Biol Chem 1998;273:15719–15726 [DOI] [PubMed] [Google Scholar]
- 15.Binart N, Bachelot A, Bouilly J. Impact of prolactin receptor isoforms on reproduction. Trends Endocrinol Metab 2010;21:362–368 [DOI] [PubMed] [Google Scholar]
- 16.Ma F, Wei Z, Shi C, et al. Signaling cross talk between growth hormone (GH) and insulin-like growth factor-I (IGF-I) in pancreatic islet β-cells. Mol Endocrinol 2011;25:2119–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasavada RC, Garcia-Ocaña A, Zawalich WS, et al. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 2000;275:15399–15406 [DOI] [PubMed] [Google Scholar]
- 18.Freemark M, Avril I, Fleenor D, et al. Targeted deletion of the PLR: effects on islet development, insulin production and glucose tolerance. Endocrinology 2002;143:1378–1385 [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 2009;150:1618–1626 [DOI] [PubMed] [Google Scholar]
- 20.Stout LE, Svensson AM, Sorenson RL. Prolactin regulation of islet-derived INS-1 cells: characteristics and immunocytochemical analysis of STAT5 translocation. Endocrinology 1997;138:1592–1603 [DOI] [PubMed] [Google Scholar]
- 21.Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab 2010;21:151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J-Y, Gavrilova O, Davani B, et al. The transcription factors Stat5a/b are not required for islet development but modulate pancreatic beta-cell physiology upon aging. Biochim Biophys Acta 2007;1773:1455–1461 [DOI] [PMC free article] [PubMed]
- 23.Jackerott M, Møldrup A, Thams P, et al. STAT5 activity in pancreatic beta-cells influences the severity of diabetes in animal models of type 1 and 2 diabetes. Diabetes 2006;55:2705–2712 [DOI] [PubMed] [Google Scholar]
- 24.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science 2007;318:806–809 [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 2010;16:804–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schraenen A, Lemaire K, de Faudeur G, et al. Placental lactogens induce serotonin biosynthesis in a subset of mouse beta cells during pregnancy. Diabetologia 2010;53:2589–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J-Y, Hennighausen L. The transcription factor Stat3 is dispensable for pancreatic beta-cell development and function. Biochem Biophys Res Commun 2005;334:764–768 [DOI] [PubMed] [Google Scholar]
- 28.Mziaut H, Kersting S, Knoch K-P, et al. ICA512 signaling enhances pancreatic beta-cell proliferation by regulating cyclins D through STATs. Proc Natl Acad Sci USA 2008;105:674–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris DL, Cho KW, Zhou Y, Rui L. SH2B1 enhances insulin sensitivity by both stimulating the insulin receptor and inhibiting tyrosine dephosphorylation of insulin receptor substrate proteins. Diabetes 2009;58:2039–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi D, Schroer SA, Lu SY, et al. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J Exp Med 2010;207:2831–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao Y, Rieck S, Le Lay J, Kaestner KH. CISH has no non-redundant functions in glucose homeostasis or beta cell proliferation during pregnancy in mice. Diabetologia 2013;56:2435–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puff R, Dames P, Weise M, Göke B, Parhofer K, Lechner A. No non-redundant function of suppressor of cytokine signaling 2 in insulin producing β-cells. Islets 2010;2:252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindberg K, Rønn SG, Tornehave D, et al. Regulation of pancreatic beta-cell mass and proliferation by SOCS-3. J Mol Endocrinol 2005;35:231–243 [DOI] [PubMed] [Google Scholar]
- 34.Banks AS, Li J, McKeag L, et al. Deletion of SOCS7 leads to enhanced insulin action and enlarged islets of Langerhans. J Clin Invest 2005;115:2462–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler AE, Cao-Minh L, Galasso R, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010;53:2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parnaud G, Bosco D, Berney T, et al. Proliferation of sorted human and rat beta cells. Diabetologia 2008;51:91–100 [DOI] [PubMed] [Google Scholar]
- 37.Thakker R. Multiple endocrine neoplasia type 1. In Endocrinology. 6th ed. DeGroot L, Jameson L, Eds. Philadelphia, Saunders Elsevier, 2010, p. 2719–2741 [Google Scholar]
- 38.Laubner K, Kieffer TJ, Lam NT, Niu X, Jakob F, Seufert J. Inhibition of preproinsulin gene expression by leptin induction of suppressor of cytokine signaling 3 in pancreatic beta-cells. Diabetes 2005;54:3410–3417 [DOI] [PubMed] [Google Scholar]
- 39.Santangelo C, Scipioni A, Marselli L, Marchetti P, Dotta F. Suppressor of cytokine signaling gene expression in human pancreatic islets: modulation by cytokines. Eur J Endocrinol 2005;152:485–489 [DOI] [PubMed] [Google Scholar]
- 40.Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 2001;22:153–183 [DOI] [PubMed] [Google Scholar]
- 41.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer 2003;3:459–465 [DOI] [PubMed] [Google Scholar]
- 42.Lingohr MK, Dickson LM, McCuaig JF, Hugl SR, Twardzik DR, Rhodes CJ. Activation of IRS-2-mediated signal transduction by IGF-1, but not TGF-alpha or EGF, augments pancreatic beta-cell proliferation. Diabetes 2002;51:966–976 [DOI] [PubMed] [Google Scholar]
- 43.Briaud I, Lingohr MK, Dickson LM, Wrede CE, Rhodes CJ. Differential activation mechanisms of Erk-1/2 and p70(S6K) by glucose in pancreatic beta-cells. Diabetes 2003;52:974–983 [DOI] [PubMed] [Google Scholar]
- 44.Kim TK, Lee JS, Jung HS, et al. Triiodothyronine induces proliferation of pancreatic β-cells through the MAPK/ERK pathway. Exp Clin Endocrinol Diabetes 2014;122:240–245 [DOI] [PubMed] [Google Scholar]
- 45.Friedrichsen BN, Neubauer N, Lee YC, et al. Stimulation of pancreatic beta-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J Endocrinol 2006;188:481–492 [DOI] [PubMed] [Google Scholar]
- 46.Schuppin GT, Pons S, Hügl S, et al. A specific increased expression of insulin receptor substrate 2 in pancreatic beta-cell lines is involved in mediating serum-stimulated beta-cell growth. Diabetes 1998;47:1074–1085 [DOI] [PubMed] [Google Scholar]
- 47.Beith JL, Alejandro EU, Johnson JD. Insulin stimulates primary beta-cell proliferation via Raf-1 kinase. Endocrinology 2008;149:2251–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Gu X, Liu Y, et al. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature 2011;478:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasavada RC, Wang L, Fujinaka Y, et al. Protein kinase C-zeta activation markedly enhances beta-cell proliferation: an essential role in growth factor mediated beta-cell mitogenesis. Diabetes 2007;56:2732–2743 [DOI] [PubMed] [Google Scholar]
- 50.Bernal-Mizrachi E, Wen W, Srinivasan S, Klenk A, Cohen D, Permutt MA. Activation of Elk-1, an Ets transcription factor, by glucose and EGF treatment of insulinoma cells. Am J Physiol Endocrinol Metab 2001;281:E1286–E1299 [DOI] [PubMed] [Google Scholar]
- 51.Gupta RK, Gao N, Gorski RK, et al. Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev 2007;21:756–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Font de Mora J, Esteban LM, Burks DJ, et al. Ras-GRF1 signaling is required for normal beta-cell development and glucose homeostasis. EMBO J 2003;22:3039–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura K, Ichise H, Nakao K, et al. Partial functional overlap of the three ras genes in mouse embryonic development. Oncogene 2008;27:2961–2968 [DOI] [PubMed] [Google Scholar]
- 54.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol 2008;9:517–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chamberlain CE, Scheel DW, McGlynn K, et al. Menin determines K-RAS proliferative outputs in endocrine cells. J Clin Invest 2014;124:4093–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.House MG, Herman JG, Guo MZ, et al. Aberrant hypermethylation of tumor suppressor genes in pancreatic endocrine neoplasms. Ann Surg 2003;238:423–431; discussion 431–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wakae-Takada N, Xuan S, Watanabe K, Meda P, Leibel RL. Molecular basis for the regulation of islet beta cell mass in mice: the role of E-cadherin. Diabetologia 2013;56:856–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diaferia GR, Jimenez-Caliani AJ, Ranjitkar P, et al. β1 integrin is a crucial regulator of pancreatic β-cell expansion. Development 2013;140:3360–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riopel M, Krishnamurthy M, Li J, Liu S, Leask A, Wang R. Conditional β1-integrin-deficient mice display impaired pancreatic β cell function. J Pathol 2011;224:45–55 [DOI] [PubMed] [Google Scholar]
- 61.Saleem S, Li J, Yee SP, Fellows GF, Goodyer CG, Wang R. beta1 integrin/FAK/ERK signalling pathway is essential for human fetal islet cell differentiation and survival. J Pathol 2009;219:182–192 [DOI] [PubMed] [Google Scholar]
- 62.Parnaud G, Gonelle-Gispert C, Morel P, et al. Cadherin engagement protects human β-cells from apoptosis. Endocrinology 2011;152:4601–4609 [DOI] [PubMed] [Google Scholar]
- 63.Parnaud G, Lavallard V, Bedat B, et al. Cadherin engagement improves insulin secretion of single human β-cells. Diabetes 2015;64:887–896 [DOI] [PubMed]
- 64.Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol Ther 2013;139:359–391 [DOI] [PubMed] [Google Scholar]
- 65.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 2009;8:369–385 [DOI] [PubMed] [Google Scholar]
- 66.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 2002;3:639–650 [DOI] [PubMed] [Google Scholar]
- 67.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 2002;115:455–465 [DOI] [PubMed] [Google Scholar]
- 68.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 2005;85:1303–1342 [DOI] [PubMed] [Google Scholar]
- 69.Shibasaki T, Takahashi H, Miki T, et al. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA 2007;104:19333–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelly P, Bailey CL, Fueger PT, Newgard CB, Casey PJ, Kimple ME. Rap1 promotes multiple pancreatic islet cell functions and signals through mammalian target of rapamycin complex 1 to enhance proliferation. J Biol Chem 2010;285:15777–15785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song WJ, Seshadri M, Ashraf U, et al. Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab 2011;13:308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaihara KA, Dickson LM, Jacobson DA, et al. β-Cell-specific protein kinase A activation enhances the efficiency of glucose control by increasing acute-phase insulin secretion. Diabetes 2013;62:1527–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lester LB, Langeberg LK, Scott JD. Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc Natl Acad Sci USA 1997;94:14942–14947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hinke SA, Navedo MF, Ulman A, et al. Anchored phosphatases modulate glucose homeostasis. EMBO J 2012;31:3991–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Talbot J, Joly E, Prentki M, Buteau J. β-Arrestin1-mediated recruitment of c-Src underlies the proliferative action of glucagon-like peptide-1 in pancreatic β INS832/13 cells. Mol Cell Endocrinol 2012;364:65–70 [DOI] [PubMed] [Google Scholar]
- 76.Quoyer J, Longuet C, Broca C, et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J Biol Chem 2010;285:1989–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ravier MA, Leduc M, Richard J, et al. β-Arrestin2 plays a key role in the modulation of the pancreatic beta cell mass in mice. Diabetologia 2014;57:532–541 [DOI] [PubMed] [Google Scholar]
- 78.Zhang M, Zhu Y, Mu K, et al. Loss of β-arrestin2 mediates pancreatic-islet dysfunction in mice. Biochem Biophys Res Commun 2013;435:345–349 [DOI] [PubMed] [Google Scholar]
- 79.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013;17:819–837 [DOI] [PubMed] [Google Scholar]
- 80.Lavine JA, Attie AD. Gastrointestinal hormones and the regulation of β-cell mass. Ann N Y Acad Sci 2010;1212:41–58 [DOI] [PubMed] [Google Scholar]
- 81.Jhala US, Canettieri G, Screaton RA, et al. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev 2003;17:1575–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cornu M, Modi H, Kawamori D, Kulkarni RN, Joffraud M, Thorens B. Glucagon-like peptide-1 increases β-cell glucose competence and proliferation by translational induction of insulin-like growth factor-1 receptor expression. J Biol Chem 2010;285:10538–10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie J, El Sayed NM, Qi C, Zhao X, Moore CE, Herbert TP. Exendin-4 stimulates islet cell replication via the IGF1 receptor activation of mTORC1/S6K1. J Mol Endocrinol 2014;53:105–115 [DOI] [PubMed] [Google Scholar]
- 84.Inada A, Hamamoto Y, Tsuura Y, et al. Overexpression of inducible cyclic AMP early repressor inhibits transactivation of genes and cell proliferation in pancreatic beta cells. Mol Cell Biol 2004;24:2831–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buteau J, Spatz ML, Accili D. Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic β-cell mass. Diabetes 2006;55:1190–1196 [DOI] [PubMed] [Google Scholar]
- 86.Bastien-Dionne PO, Valenti L, Kon N, Gu W, Buteau J. Glucagon-like peptide 1 inhibits the sirtuin deacetylase SirT1 to stimulate pancreatic β-cell mass expansion. Diabetes 2011;60:3217–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tschen SI, Georgia S, Dhawan S, Bhushan A. Skp2 is required for incretin hormone-mediated β-cell proliferation. Mol Endocrinol 2011;25:2134–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Renner S, Fehlings C, Herbach N, et al. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes 2010;59:1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rutti S, Sauter NS, Bouzakri K, Prazak R, Halban PA, Donath MY. In vitro proliferation of adult human beta-cells. PLoS One 2012;7:e35801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tian L, Gao J, Weng G, et al. Comparison of exendin-4 on beta-cell replication in mouse and human islet grafts. Transpl Int 2011;24:856–864 [DOI] [PubMed] [Google Scholar]
- 91.Odori S, Hosoda K, Tomita T, et al. GPR119 expression in normal human tissues and islet cell tumors: evidence for its islet-gastrointestinal distribution, expression in pancreatic beta and alpha cells, and involvement in islet function. Metabolism 2013;62:70–78 [DOI] [PubMed] [Google Scholar]
- 92.Zhang M, Feng Y, Wang J, et al. High-throughput screening for GPR119 modulators identifies a novel compound with anti-diabetic efficacy in db/db mice. PLoS One 2013;8:e63861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahlkvist L, Brown K, Ahrén B. Upregulated insulin secretion in insulin-resistant mice: evidence of increased islet GLP1 receptor levels and GPR119-activated GLP1 secretion. Endocr Connect 2013;2:69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao J, Tian L, Weng G, et al. Stimulating beta cell replication and improving islet graft function by GPR119 agonists. Transpl Int 2011;24:1124–1134 [DOI] [PubMed] [Google Scholar]
- 95.Tang C, Ahmed K, Gille A, et al. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med 2015;21:173–177 [DOI] [PubMed] [Google Scholar]
- 96.Mozar A, Guthalu NK, Pollack I, Fenutria R, Vasavada RC. The role of PTHrP in pancreatic beta cells and implications for diabetes pathophysiology and treatment. Clin Rev Bone Miner Metab 2014;12:165–177 [Google Scholar]
- 97.Williams K, Abanquah D, Joshi-Gokhale S, et al. Systemic and acute administration of parathyroid hormone-related peptide(1-36) stimulates endogenous beta cell proliferation while preserving function in adult mice. Diabetologia 2011;54:2867–2877 [DOI] [PubMed] [Google Scholar]
- 98.Guthalu NK, Joshi-Gokhale S, Harb G, et al. Parathyroid hormone-related protein enhances human β-cell proliferation and function with simultaneous induction of cyclin-dependent kinase 2 and cyclin E expression. Diabetes 2010;59:3131–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Marzo V, Piscitelli F, Mechoulam R. Cannabinoids and endocannabinoids in metabolic disorders with focus on diabetes. Handbook Exp Pharmacol 2011;203:75–104 [DOI] [PubMed] [Google Scholar]
- 100.Bermúdez-Silva FJ, Suárez J, Baixeras E, et al. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia 2008;51:476–487 [DOI] [PubMed] [Google Scholar]
- 101.Kim W, Doyle ME, Liu Z, et al. Cannabinoids inhibit insulin receptor signaling in pancreatic β-cells. Diabetes 2011;60:1198–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li C, Vilches-Flores A, Zhao M, Amiel SA, Jones PM, Persaud SJ. Expression and function of monoacylglycerol lipase in mouse β-cells and human islets of Langerhans. Cell Physiol Biochem 2012;30:347–358 [DOI] [PubMed] [Google Scholar]
- 103.Jourdan T, Godlewski G, Cinar R, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med 2013;19:1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim W, Lao Q, Shin YK, et al. Cannabinoids induce pancreatic β-cell death by directly inhibiting insulin receptor activation. Sci Signal 2012;5:ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development 2009;136:3699–3714 [DOI] [PubMed] [Google Scholar]
- 106.Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol 2009;19:385–394 [DOI] [PubMed] [Google Scholar]
- 107.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003;425:577–584 [DOI] [PubMed] [Google Scholar]
- 108.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol 2012;13:616–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smart NG, Apelqvist AA, Gu X, et al. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS Biol 2006;4:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.El-Gohary Y, Tulachan S, Wiersch J, et al. A smad signaling network regulates islet cell proliferation. Diabetes 2014;63:224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiao X, Gaffar I, Guo P, et al. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc Natl Acad Sci USA 2014;111:E1211–E1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin HM, Lee JH, Yadav H, et al. Transforming growth factor-beta/Smad3 signaling regulates insulin gene transcription and pancreatic islet beta-cell function. J Biol Chem 2009;284:12246–12257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blum B, Roose AN, Barrandon O, et al. Reversal of β cell de-differentiation by a small molecule inhibitor of the TGFβ pathway. eLife 2014;3:e02809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown ML, Schneyer AL. Emerging roles for the TGFbeta family in pancreatic beta-cell homeostasis. Trends Endocrinol Metab 2010;21:441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown ML, Kimura F, Bonomi LM, Ungerleider NA, Schneyer AL. Differential synthesis and action of TGFß superfamily ligands in mouse and rat islets. Islets 2011;3:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mukherjee A, Sidis Y, Mahan A, et al. FSTL3 deletion reveals roles for TGF-β family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci USA 2007;104:1348–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu H, Mezghenna K, Marmol P, et al. Differential regulation of mouse pancreatic islet insulin secretion and Smad proteins by activin ligands. Diabetologia 2014;57:148–156 [DOI] [PubMed]
- 118.Bruun C, Christensen GL, Jacobsen MLB, et al. Inhibition of beta cell growth and function by bone morphogenetic proteins. Diabetologia 2014;57:2546–2554 [DOI] [PubMed] [Google Scholar]