SUMMARY
Interleukin-2 (IL-2) is a pleiotropic cytokine that regulates immune cell homeostasis, and has been used to treat a range of disorders such as cancer and autoimmune disease. IL-2 signals via interleukin-2 receptor-β (IL-2Rβ):IL-2Rγ heterodimers on cells expressing high (regulatory T cells, Treg) or low (effector cells) amounts of IL-2Rα (CD25). When complexed with IL-2, certain anti-cytokine antibodies preferentially stimulate expansion of Treg (JES6-1) or effector (S4B6) cells, offering a strategy for targeted disease therapy. We found that JES6-1 sterically blocked the IL-2:IL-2Rβ and IL-2:IL-2Rγ interactions, but also allosterically lowered the IL-2:IL-2Rα affinity through a ‘triggered exchange’ mechanism favoring IL-2Rαhi Treg cells, creating a positive feedback loop for IL-2Rαhi cell activation. Conversely, S4B6 sterically blocked the IL-2:IL-2Rα interaction, while also conformationally stabilizing the IL-2:IL-2Rβ interaction, thus stimulating all IL-2 responsive immune cells, particularly IL-2Rβhi effector cells. Our insights provide a molecular blueprint for engineering selectively potentiating therapeutic antibodies.

INTRODUCTION
Interleukin (IL)-2 is a four-helix bundle cytokine that plays a critical role in immune cell differentiation, growth, and activity. IL-2 signals through formation of either a high-affinity quaternary complex with the interleukin-2 receptor-α (IL-2Rα, CD25), IL-2Rβ, and IL-2Rγ chains (Kd≈10 pM), or an intermediate-affinity ternary complex (Kd≈1 nM) with only the IL-2Rβ and IL-2Rγ chains (Boyman and Sprent, 2012; Liao et al., 2013). Consequently, expression of the non-signaling IL-2Rα subunit regulates cytokine sensitivity. IL-2Rα is robustly expressed on regulatory T (Treg) cells but is virtually absent from naïve effector cells such as memory-phenotype (MP) CD8+ T cells and natural killer (NK) cells, resulting in differential responsiveness of these immune cell subsets to IL-2 (Fontenot et al., 2005; Josefowicz et al., 2012; Malek and Bayer, 2004). Upon IL-2 complex formation, intracellular Janus kinase (JAK) proteins constitutively associated with IL-2Rβ and IL-2Rγ phosphorylate tyrosine residues in the receptor intracellular domains, which recruit and activate signal transducer and activator of transcription (STAT)-5 to coordinate immune-related gene expression programs (Malek, 2008). The IL-2 complex also signals secondarily through the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways (Malek, 2008; Taniguchi and Minami, 1993).
IL-2 exerts paradoxical effects on immune cell homeostasis, promoting activation and proliferation of both immunostimulatory effector cells and immunosuppressive Treg cells and its vital role in immune regulation has made IL-2 an attractive therapeutic target in a range of immune-linked diseases, both to promote the immune response, as in cancer and infectious disease, and to repress the immune response, as in autoimmune disorders and graft versus host disease (Boyman and Sprent, 2012; Brusko et al., 2008; Liao et al., 2013; Waldmann, 2006). However, the clinical performance of IL-2 has been limited by the multifarious nature of its activities, which can thwart efficacy and lead to toxicity or harmful off-target effects (Boyman et al., 2006b; Rosenberg, 2012; Shevach, 2012). It would thus be of tremendous therapeutic value to decouple the immunostimulatory and immunosuppressive activities of IL-2 to cater to particular disease applications.
One strategy for selectively modulating the effects of IL-2 is development of cytokine-directed antibodies that bias activity toward specific T cell subsets. Co-administration of antibodies with IL-2 offers important therapeutic advantages such as prolonged in vivo half-life due to Fc receptor interactions (Boyman et al., 2006b; Finkelman et al., 1993; Letourneau et al., 2010). Boyman and colleagues established that immunocomplexes formed by pre-association of two anti-mouse IL-2 (mIL-2) antibodies with the cytokine elicit contrasting effects: mIL-2:JES6-1 immunocomplexes actively induce proliferation of IL-2Rαhi cells, preferentially expanding Treg cells over effector cells, whereas mIL-2:S4B6 immunocomplexes stimulate proliferation of all immune cells, but particularly favor effector cells (Boyman et al., 2006a) (Figure 1A). Subsequent work has validated a vast array of therapeutic applications for these two antibodies: JES6-1 immunocomplexes promote graft tolerance (Park et al., 2010; Webster et al., 2009) and show efficacy in preclinical models of diabetes (Grinberg-Bleyer et al., 2010; Tang et al., 2008) and S4B6 immunocomplexes exhibit potent anti-tumor activity (Jin et al., 2008; Verdeil et al., 2008) without inducing toxicity (Krieg et al., 2010). Boyman and Sprent proposed that biased immunocomplex activity results from antibody obstruction of specific epitopes on the cytokine, namely that JES6-1 blocks only the IL-2Rβ binding site on mIL-2 to disrupt interaction with IL-2Rαlo effector cells whereas S4B6 blocks the mIL-2Rα binding site on mIL-2 to prevent high-affinity interactions with IL-2Rαhi Treg cells (Boyman and Sprent, 2012). However, in the absence of structural or molecular characterization, the mechanistic basis for selective cytokine potentiation remains speculative.
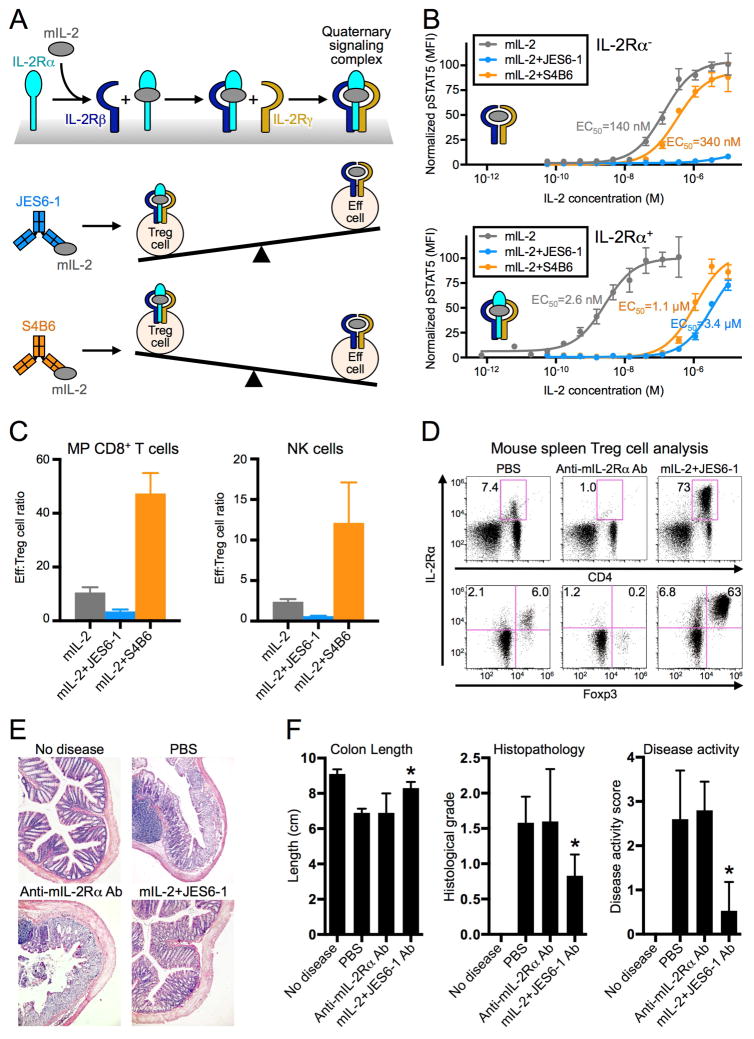
Figure 1. Anti-IL-2 antibodies bias cytokine signaling and functional outcomes.
(A) Schematic of IL-2 cytokine-receptor quaternary complex formation (top) and regulatory (Treg) versus effector (Eff) immune cell proliferation biases induced by mIL-2:JES6-1 (middle) and mIL-2:S4B6 (bottom) immunocomplexes. (B) STAT5 phosphorylation response to mIL-2 or mIL-2:antibody immunocomplex treatment in IL-2Ra− (top) or IL-2Ra+ (bottom) YT-1 human NK cells. Half-maximal effective concentrations (EC50s) for STAT5 activation are indicated. Data are representative of three independent experiments. (C) C57BL/6 mouse spleen effector cell:Treg cell ratios following mIL-2 or immunocomplex treatment. Data are representative of two independent experiments. (D) Treg cell expansion in BALB/c mouse spleens in response to anti-mIL-2Rα antibody (Ab) or mIL-2:JES6-1 treatment. Top plots display the percentage of CD4+ cells that are IL-2Rα+. Bottom plots show only CD4+ cells and the percentages of Treg (IL-2Rα+FoxP3+) cells and IL-2Rα+FoxP3− cells are shown for each treatment condition. (E) Colon histopathology of colitis-induced BALB/c mice pretreated with anti-mIL-2Rα Ab or mIL-2:JES6-1. (F) Evaluation of disease progression indicators for treated mice. *P<0.05 by Student’s t-test (colon length) or Mann-Whitney U test (histological grade and disease activity score). All error bars indicate SD. Also see Figure S1.
In this report, we have combined crystallographic, biophysical, and functional data to elucidate the molecular rationale for antibody-induced bias of cytokine activity. We show that JES6-1 and S4B6 exerted complex steric and allosteric effects on IL-2 to differentially activate immune cell subsets based on their IL-2 receptor surface expression profiles and that this biased activation was further propagated through transcriptional feedback. Our findings provide a direct link between the molecular interactions of cytokine-antibody complexes and their unique functional outcomes. These mechanistic insights into immune cell subset-specific potentiation establish a paradigm for antibody-mediated modulation of IL-2 behavior that will inform the design of enhanced cytokine-targeted therapeutics.
RESULTS
IL-2-targeted antibodies inhibit cytokine signaling to induce biased T cell subset proliferation
To elucidate the molecular properties of JES6-1 and S4B6 that contribute to their functional behavior, we probed antibody effects on mIL-2-induced signaling in IL-2Rα− and IL-2Rα+ human YT-1 NK cells as a surrogate for their effects on IL-2Rαlo versus IL-2Rαhi immune cell subsets. Cross-reactivity between mouse and human receptors (Figure S1A) enabled interrogation of murine cytokine signaling on human cells, although its potency was reduced compared to the human cytokine due to its weaker affinity for the human IL-2 receptor subunits. On IL-2Rα− cells, JES6-1 abolished IL-2-mediated STAT5 activation. S4B6 slightly inhibited activation, which was unexpected but is rationalized by structural and mechanistic experiments presented later in this report. On IL-2Rα+ cells, both JES6-1 and S4B6 greatly reduced IL-2 potency (Figure 1B). Importantly, IL-2 activity was greater on IL-2Rα+ cells in the presence of JES6-1 and greater on IL-2Rα− cells in the presence of S4B6, consistent with preferential activation of IL-2Rαhi Treg cells and IL-2Rαlo effector cells, respectively. STAT5 signaling modulation was independent of antibody bivalency, as the single-chain variable fragment (scFv) of JES6-1 and the Fab fragment of S4B6 induced the same qualitative effects as their corresponding antibodies, albeit with attenuated potency (Figure S1B), presumably a consequence of the >25-fold higher apparent affinity of the bivalent versus monovalent antibodies (Figure S1C).
In vitro IL-2Rα expression-based signaling blockade translated into in vivo skewing of the effector cell:Treg cell ratio. IL-2Rαhi Treg cells were enriched three-fold following mIL-2:JES6-1 immunocomplex injection into mice and, conversely, IL-2Rαlo MP CD8+ T cells and NK cells were enriched five-fold following mIL-2:S4B6 immunocomplex injection compared to unbound cytokine administration (Figure 1C).
JES6-1 induces potentiation of Treg cell growth and inhibits autoimmune disease progression in mice
As the effector cell bias induced by S4B6 has promising implications for cancer treatment (Jin et al., 2008; Krieg et al., 2010; Verdeil et al., 2008), we sought to explore the therapeutic potential of the Treg cell bias induced by JES6-1 in the context of autoimmunity using a mouse model of inflammatory bowel disease (IBD). To this end, we assessed the ability of mIL-2:JES6-1 complexes to prevent disease in the dextran sodium sulfate (DSS) colitis model. Prior to DSS exposure, mice were treated with a vehicle control, a Treg cell-depleting anti-mIL-2Rα antibody, or mIL-2:JES6-1 complexes, which enriched relative Treg cell (IL-2R α+Foxp3+ cell) numbers ten-fold (Figure 1D). We also observed three-fold enrichment of IL-2Rα+Foxp3− cells, consistent with previous reports that JES6-1-containing immunocomplexes induce expansion of IL-2Rα-expressing activated T cells (Castro et al., 2012; Tomala et al., 2009). One week after colitis induction, we observed that mIL-2:JES6-1 immunocomplex pretreatment yielded significant improvements in colon length and histopathology and drastically reduced disease activity, with treated mice exhibiting similar characteristics to those in which disease was not induced (Figure 1E,F). Notably, mice treated with PBS exhibited nearly identical disease severity to Treg cell-depleted mice (anti-mIL-2Rα antibody cohort), indicating that in the absence of expansion, background amounts of Treg cells in these mice provide no protective advantage. In combination with our signaling and proliferation studies, this mouse model demonstrates the therapeutic utility of selective immune cell subset stimulation.
mIL-2:JES6-1 and mIL-2:S4B6 complex structures reveal contrasting IL-2 receptor steric competition properties
To study the biochemical and structural mechanisms underlying JES6-1 and S4B6 effects, we isolated the variable region sequences of JES6-1 and S4B6 and expressed them in scFv and Fab formats. We separately determined the crystal structures of the mIL-2:scFv-JES6-1 and mIL-2:Fab-S4B6 complexes to resolutions of 2.8 and 2.2 Å, respectively (Figure S2A,B and Table S1), and compared them to the human IL-2 quaternary complex structure (Wang et al., 2005) (Figure 2A,B). We observed that JES6-1 binds ventrally, or ‘underneath’ mIL-2 as it would be disposed in the signaling complex, between the IL-2Rβ and IL-2Rγ interfaces on the cytokine, directly occluding both subunits. Conversely, S4B6 binds dorsally, or on ‘top’ of mIL-2, directly occluding the IL-2Rα subunit (Figure 2C). JES6-1 makes extensive contacts in the region between the A helix and the N-terminal end of the AB loop of mIL-2 through complementarity-determining regions (CDRs) 2 and 3 of the JES6-1 heavy chain (HC) and CDRs 1 and 3 of the JES6-1 light chain (LC) (Figure S2C). In particular, mIL-2 residues Q36 and E37, which correspond to hIL-2 residues (Q22 and M23) essential to the IL-2:IL-2Rβ and IL-2:IL-2Rγ interactions, respectively, engage in hydrogen bonds with JES6-1 LC CDRs 1 and 3, illustrating the basis for steric competition between JES6-1 and both the IL-2Rβ and IL-2Rγ subunits. In contrast, S4B6 primarily interacts with IL-2Rα-binding residues in the B and C helices of mIL-2, through contacts with S4B6 HC CDR3 and LC CDRs 1 and 2 (Figure S2D). Our crystallographic findings are consistent with recent epitope mapping studies performed for JES6-1 (Rojas et al., 2014) and S4B6 (Rojas et al., 2013).
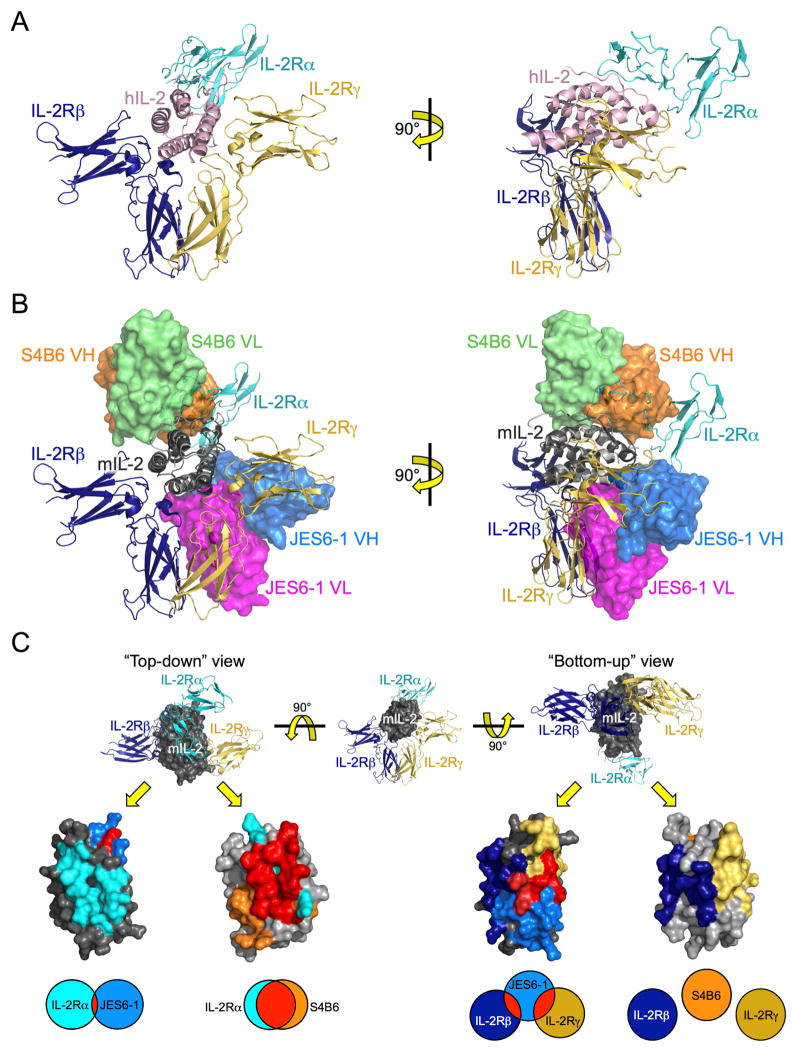
Figure 2. mIL-2:JES6-1 and mIL-2:S4B6 complex structures reveal basis for competitive binding between anti-IL-2 antibodies and IL-2 receptor.
(A) Orthosteric views of the hIL-2 cytokine-receptor quaternary complex (PDB ID 2B5I) comprised of hIL-2 (pink), IL-2Rα (cyan), IL-2Rβ (navy), and IL-2Rγ (gold). (B) Overlay of the mIL-2:JES6-1 and mIL-2:S4B6 complex structures on the hIL-2 quaternary complex. Both JES6-1-bound mIL-2 (dark gray) and S4B6-bound mIL-2 (light gray) are presented, with the JES6-1 variable heavy (VH, blue) and light (VL, magenta) and the S4B6 VH (orange) and VL (green) chains shown as surface representations. Crystallographic statistics for mIL-2:antibody complexes are provided in Table S1. (C) “Top-down” (left) and “bottom-up” (right) views of JES6-1-bound mIL-2 (dark gray) and S4B6-bound mIL-2 (light gray) with the predicted binding epitopes of IL-2Ra (cyan), IL-2Rβ (navy), IL-2R (gold), JES6-1 (blue), and S4B6 (orange) on the mIL-2 cytokine shaded. Residues shared between the mIL-2:antibody and the mIL-2:IL-2Ra, mIL-2:IL-2Rβ, or mIL-2:IL-2R interfaces are colored red. Venn diagrams at bottom indicate the relative extent of overlap between the antibody and receptor epitopes. Also see Figure S2.
Anti-cytokine mAbs elicit allosteric effects on cytokine-receptor interactions
Our structural analysis also revealed allosteric effects of antibody binding on IL-2 complex formation that are key to function. No structure of the mouse IL-2:IL-2Rα complex has yet been determined to compare with the mIL-2:JES6-1 complex; however, the human IL-2:IL-2Rα complex structure serves as an accurate model for comparison since human and mouse IL-2 show a high degree of sequence and structural conservation in residues that engage their receptors. Although there is no steric clash between JES6-1 and IL-2Rα (Figure 2B), key residues that contact IL-2Rα ‘switch’ conformations upon binding to JES6-1. Superposition of the hIL-2:hIL-2Rα and mIL-2:JES6-1 interfaces reveals a large-scale remodeling of the AB loop in the respective complexes, with drastic changes in both side chain and main chain positioning of residues important for both JES6-1 and receptor binding (Figure 3). More specifically, two mIL-2 residues (K49 and R52) at the N-terminal end of the AB loop that contact JES6-1 (Figure S2C) also mediate contacts between hIL-2 (residues K35 and R38) and hIL-2Rα. JES6-1 binding distorts the entire AB loop of mIL-2, repositioning K49 and K52 as well as K57 (hIL-2 residue K43) and Y59 (hIL-2 residue Y45) from their presumed receptor-bound states as seen in the human complex. Potentially, IL-2Rα engagement is initiated by interaction with residues K49 and R52 at the N-terminal end of the AB loop that could, then, progressively ‘peel off’ the cytokine from JES6-1 in a zipper-like mechanism ultimately leading to dissociation of JES6-1 (Figure 3). Once JES6-1 dissociates, the IL-2Rα-bound cytokine is liberated to recruit IL-2Rβ and IL-2Rγ to form the functional signaling complex. These allosteric changes cannot be explained by differences in structure between the cytokine species, as the AB loop region of mIL-2 from the S4B6-bound structure overlays closely (RMSD=0.771 Å for S4B6 versus 3.15 Å for JES6-1) with receptor-bound hIL-2 (Figure S3). Moreover, the conformational flexibility in the AB loop region contrasts with the close correlation between JES6-1-bound mIL-2 and receptor-bound hIL-2 in other regions of the structures (RMSD=3.15 Å for AB loops versus 0.729 Å for remainder of structures). Thus JES6-1 capitalizes on the previously noted malleability of the IL-2Rα binding site on IL-2 (Thanos et al., 2006; Thanos et al., 2003), and conformational changes are confined locally to this region of the cytokine. We suggest that JES6-1 binding to one site allosterically impairs IL-2Rα binding to a non-overlapping site, and this impaired binding (i.e. lowered affinity for IL-2Rα) renders JES6-1-bound IL-2 selective for IL-2Rαhi cells, which express sufficient IL-2Rα to displace the antibody by mass action and initiate the exchange mechanism.
Figure 3. JES6-1 allosterically obstructs IL-2Rα binding to IL-2 via epitope distortion.
Overlay of the hIL-2 quaternary complex (PDB ID 2B5I) with the mIL-2:JES6-1 complex. Surface depictions of the IL-2Rα (cyan), IL-2Rβ (navy), and IL-2Rγ (gold) subunits and the JES6-1 antibody (blue) and cartoon representations of receptor-bound hIL-2 (pink) and JES6-1-bound mIL-2 (red) are shown. An enlargement of the IL-2Rα binding site is provided at right with key IL-2Ra-interacting residues in the hIL-2 AB loop and the corresponding JES6-1-bound mIL-2 residues labeled. Arrows highlight differences between the structures. Also see Figure S3.
Overlaying the IL-2 receptor subunits with S4B6-bound mIL-2 clearly illustrates direct occlusion of IL-2Rα binding by the antibody (Figure 2B), to the extent that S4B6 appears to be a structural mimic of IL-2Rα. Comparison of the hIL-2:hIL-2Rα and mIL-2:S4B6 interfaces portrays the striking similarity between these two interactions (Figure 4A). Both the side chain and main chain positioning of the AB loop and B helix are highly conserved, particularly for interacting residues that are shared between the structures. Here again, close examination of the structural effects of S4B6 binding illuminates an unanticipated conformational consequence of the antibody on IL-2 engagement of IL-2Rβ. We previously noted that human IL-2 undergoes a slight repositioning of the C helix following IL-2Rα engagement (Wang et al., 2005) and showed that this structural change in the cytokine is recapitulated by a variant of IL-2 (denoted super-2) that exhibits 200-fold higher affinity for the IL-2Rβ subunit than the wild-type cytokine (Levin et al., 2012). S4B6 engages IL-2 in a similar manner to IL-2Rα, and we find that the antibody does indeed induce a shift in the C helix compared to the unbound cytokine, coinciding with the structures of both IL-2Rα-bound hIL-2 and super-2 (Figure 4B and Figure S4A). Thus S4B6 mediates steric blockade but also, to a lesser extent, allosteric effects, eliciting structural changes distal from its binding site that could impact IL-2Rβ binding. Overlay of the IL-2Rβ subunit from the hIL-2 quaternary complex with the S4B6-bound mIL-2 also reveals that the antibody may slightly obstruct accessibility of IL-2Rβ through a tangential steric clash between S4B6 LC CDR1 and domain 1 of IL-2Rβ (Figure S4B) which could partially mitigate the affinity-enhancing effect of S4B6 binding on the IL-2:IL-2Rβ interaction. S4B6-mediated blockade of IL-2Rα binding together with stabilization of the IL-2Rβ-binding conformation of IL-2 would be expected to promote proliferation of all T and NK cells bearing IL-2Rβ and IL-2Rγ, particularly favoring IL-2Rβhi effector cells, consistent with the functional activity of this antibody.
Figure 4. S4B6 mimics the IL-2Ra subunit, allosterically enhancing binding of IL-2Rβ.
(A) Overlay of hIL-2 (pink) bound to IL-2Rα (cyan), IL-2Rβ (navy), and IL-2Rγ and mIL-2 (light gray) bound to S4B6 (orange) with detailed views of the hIL-2:hIL-2Ra (left) and mIL-2:S4B6 (right) interfaces in the AB loops and B helices of the cytokines juxtaposed at right. Analogous human and mouse IL-2 residues implicated in both interactions are labeled. (B) Superposition of cytokine orientations proximal to the IL-2Rβ binding site for unbound super-2 (PDB ID 3QB1, salmon), receptor-bound hIL-2 from the quaternary complex (PDB ID 2B5I, pink), S4B6-bound mIL-2 (red), and unbound hIL-2 (PDB ID 3INK, green). Also see Figure S4.
Anti-IL-2 antibodies exert distinct effects on IL-2 cytokine:receptor interactions
To rationalize our structural analysis in the context of biophysical measurements, we displayed mIL-2 on the surface of yeast and characterized antibody-receptor competition. Both JES6-1 and S4B6 potently blocked the mIL-2:mIL-2Rα interaction and JES6-1 also blocked the mIL-2:mIL-2Rβ interaction. S4B6 partially impaired the mIL-2:mIL-2Rβ interaction (Figure 5A,B), accounting for its mild inhibition of STAT5 signaling (Figure 1B, top), which presumably results resulted from the steric clash between the antibody and IL-2Rβ (Figure S4B). Consistent with our structural findings, these competition studies demonstrate that both antibodies impact cytokine engagement of the IL-2Rα and IL-2Rβ subunits.
Figure 5. Anti-IL-2 antibodies and IL-2 receptor compete for cytokine binding.
Yeast surface mIL-2 competition studies between anti-mIL-2 antibodies (Abs) and saturating concentrations of mIL-2Rα (A) or mIL-2Rβ (B) are shown (Error bars, SD). Data are representative of three independent experiments. Half maximal inhibitory concentrations (IC50s) of the antibodies are indicated. Equilibrium surface plasmon resonance titrations of mIL-2:antibody immunocomplex interactions with immobilized mIL-2Rα (C) or mIL-2Rβ (D) and mIL-2Rα (E) or mIL-2Rβ (F) interactions with unbound mIL-2 compared to interactions with immobilized mIL-2:antibody immunocomplexes are presented. Also see Figure S5.
We probed the kinetics of mIL-2 interactions with its receptor chains in the absence or presence of the JES6-1 and S4B6 antibodies using surface plasmon resonance (SPR). We immobilized mIL-2Rα and then flowed over pre-incubated mIL-2:antibody complexes containing a fixed mIL-2 concentration and variable concentrations of competitor antibody. The two antibodies exhibited qualitatively different effects on binding: Whereas S4B6 completely abrogated binding of mIL-2, residual cytokine binding occurred in the presence of JES6-1, even when it was in 40-fold excess over mIL-2 (Figure 5C), implying that mIL-2 can simultaneously, but transiently, engage both JES6-1 and mIL-2Rα in an exchanging intermediate state wherein JES6-1 ‘releases’ as mIL-2Rα engages a low-affinity binding site. Upon JES6-1 dissociation, the mIL-2Rα binding site on mIL-2 remodels into the high-affinity state. Accordingly, kinetic profiles of JES6-1-modulated mIL-2:mIL-2Rα interactions indicated that the antibody concurrently decreased the association rate (Kon) and increases the dissociation rate (Koff) of the mIL-2:mIL-2Rα complex (Figure S5A). In contrast, S4B6 competes with IL-2Rα for binding to mIL-2 in a classical manner, in that IL-2α has to disengage before S4B6 can access mIL-2: dissociation of IL-2Rα from mIL-2 is unaffected by S4B6 (Figure S5C). These distinct modes of IL-2Rα displacement by JES6-1 and S4B6 reflect their unique structural properties and are essential to their biological activities.
We also analyzed the mIL-2:mIL-2Rβ interaction through SPR studies by immobilizing mIL-2Rβ and flowing over immunocomplexes containing variable ratios of competitor antibody to mIL-2. JES6-1 fully blocked mIL-2:mIL-2Rβ interaction whereas S4B6-conjugated mIL-2 bound to mIL-2Rβ (Figure 5D). Kinetic profiles showed that JES6-1 eliminated binding without impacting koff, consistent with direct competitive inhibition (Figure S5B), and S4B6 led to an increase in the molecular mass of bound analyte reflecting mIL-2Rβ engagement of both the cytokine and antibody (Figure S5D).
We modified the topology of our binding studies, immobilizing either JES6-1 or S4B6, saturating the antibody-coated surface with mIL-2, and subsequently measuring mIL-2Rα or mIL-2Rβ binding to the immobilized mIL-2:antibody complexes. mIL-2Rα did not bind to the immobilized mIL-2:S4B6 immunocomplex (Figure 5E and Figure S5I) but bound to the mIL-2:JES6-1 immunocomplex with 200-fold weaker affinity compared to its interaction with mIL-2 alone (Figure 5E). The kinetic profile revealed a dramatic increase in the mIL-2Rα Koff in the presence of JES6-1 (Figure S5E,G), reiterating the exchange between receptor and antibody binding to the cytokine. Steric inhibition precluded binding of mIL-2Rβ to the immobilized mIL-2:JES6-1 immunocomplex (Figure 5F and Figure S5H) but, interestingly, we observed 6-fold enhancement in the binding of mIL-2Rβ to the mIL-2:S4B6 immunocomplex compared to its binding to free mIL-2 (Figure 5F). This affinity improvement was a net consequence of simultaneous decreases in Kon and Koff for the mIL-2:mIL-2Rβ interaction in the presence of S4B6 (Figure S5F,J). The kinetics of this immunocomplex:mIL-2Rβ interaction were rationalized by structural observations, as the predicted clash between S4B6 and mIL-2Rβ (Figure S4B) would presumably impede accessibility of the receptor binding site on mIL-2, hampering Kon. However, this effect was counteracted by the conformational change in the mIL-2 C helix induced by S4B6 binding (Figure 4B), which improved complex stability and would be predicted to lower Koff. Indeed, it was found that super-2 primarily achieves its IL-2Rβ affinity enhancement relative to wild-type IL-2 by decreasing Koff (Levin et al., 2012). Taken together, our competitive binding studies validate the steric and allosteric effects of anti-IL-2 antibodies seen in the crystal structures, which manifest as biased functional activities in vivo.
IL-2:JES6-1 immunocomplex signaling feeds back onto IL-2Rα expression to perpetuate Treg cell proliferation
To further explore the mechanistic basis of the JES6-1-mediated Treg cell proliferation bias, we directly compared proliferation and IL-2Rα expression in five immune cell subsets following immunocomplex treatment. As anticipated, per cell IL-2Rα expression and the proportion of IL-2Rα+ cells were substantially higher on Treg cells compared to effector cells and other subsets (Figure 6A,B). Both IL-2Rα surface density and the percentage of IL-2Rα+ cells in the Treg cell subset (Figure 6C) and all other subsets (Figure 6A,B) increased in response to IL-2:JES6-1 immunocomplex treatment compared to mIL-2:S4B6 immunocomplex treatment or unbound mIL-2 treatment. However, the JES6-1-induced rise in receptor expression only corresponded with increased cell proliferation in the Treg cell subset, implying that a threshold amount of surface IL-2Rα is required to induce proliferation in the presence of JES6-1 (Figure 6D,E). This finding resonates with our proposed triggered release mechanism, wherein sufficient amounts of surface IL-2Rα are required to displace JES6-1 by mass action. Elevation of IL-2Rα expression following mIL-2:JES6-1 treatment in turn heightens sensitivity to the immunocomplex, creating a positive feedback loop that further biases immune cell homeostasis to favor IL-2Rαhi Treg cells (Figure S6A).
Figure 6. mIL-2:JES6-1 immunocomplex induces increased IL-2Rα expression to create a positive feedback loop favoring IL-2Rαhi cell signaling.
(A) IL-2Rα expression quantification (mean fluorescence intensity, MFI) for five immune cell subsets (Treg, MP CD8+ T, NK, NKT, and γd TCR) in BALB/c mouse spleens following treatment with mIL-2 or mIL-2:antibody immunocomplex. (B) Percentage of IL-2Rα+ cells within each immune cell subset in treated mouse spleens. (C) IL-2Rα expression histograms for Treg cells isolated from treated mouse spleens. Representative plots from one mouse per cohort are presented. (D) Relative cell expansion (transparent thick bars) and IL-2Rα expression quantification (solid thin bars) in treated mouse spleens. Responses for each treatment cohort are normalized to those of untreated control mice for each cell subset. (E) Relative cell expansion (transparent thick bars) and IL-2Rα+ cell percentage (solid thin bars) in treated mouse spleens, normalized as in (D). Error bars indicate SD. Data are representative of two independent experiments.
In the case of mIL-2:S4B6 treatment, we found that induced proliferation did not correlate with surface IL-2Rα quantities, as the immunocomplex promotes proliferation of all IL-2 responsive immune subsets, particularly IL-2Rαlo but IL-2Rβhi effector cell subsets (Figure 6A–E). This corroborates our proposed S4B6 mechanism of action, wherein steric blockade renders IL-2 insensitive to IL-2Rα expression and allosteric enhancement of IL-2Rβ binding directs IL-2’s effects toward IL-2Rβhi effector cells (Figure S6B). Collectively, these subset expansion and IL-2Rα profiling studies reveal an additional layer through which IL-2-directed antibodies regulate immune homeostasis by altering transcriptional behavior.
DISCUSSION
Since the discovery that certain anti-IL-2 antibodies evoke selective immune cell proliferation, there has been a great deal of interest in exploiting these molecules to treat immune diseases. However, in order to harness their therapeutic potential, it is critical to understand the mechanistic basis for their activities. Half-life extension alone cannot explain their effects since different high-affinity IL-2 antibodies exhibit distinct potentiation profiles. Therefore, we explored the atomic basis for their IL-2 interactions as a possible explanation. It was previously thought that antibody selectivity was achieved by strict steric blockade of specific epitopes on the cytokine (Boyman and Sprent, 2012), but we found the mechanism to be more complex. Based on our collective structural and functional studies, we propose that the mechanisms for selective immune cell subset potentiation by JES6-1 and S4B6 are multi-layered, but ultimately rooted in the unique structural properties of each antibody.
JES6-1 acted through a three-tiered mechanism that induced IL-2 to potently and exclusively activate IL-2Rαhi cells, skewing the immune cell balance to favor Treg cells. The first layer of IL-2 modulation involved extension of the cytokine’s in vivo half-life. Complexing cytokines with antibodies has long been pursued as a strategy for enhancing activity through prolonged persistence in the bloodstream (Finkelman et al., 1993) and Fc receptor-mediated half-life extension has been previously shown to be necessary though not sufficient for maximal activity of anti-IL-2 antibody immunocomplexes (Letourneau et al., 2010). The second layer of the JES6-1 mechanism of action comprised the combined steric and allosteric effects of antibody binding on the IL-2 structure. JES6-1 sterically blocked binding of IL-2Rβ and IL-2Rγ and also allosterically impeded binding of IL-2Rα to the cytokine. Allosteric disruption of the mIL-2:mIL-2Rα interaction conferred exquisite sensitivity for Treg cells, as high amounts of IL-2Rα expression were required to overcome this disruption to allow for IL-2 complex formation and signal activation. IL-2Rαlo effector cells were unresponsive to mIL-2:JES6-1 immunocomplex treatment since IL-2Rβ and IL-2Rγ interactions with the cytokine were occluded and there were not sufficient quantities of IL-2Rα to displace the antibody. The third layer of JES6-1 action consisted of IL-2Rα expression upregulation to create a positive feedback loop that perpetuated proliferation of IL-2Rαhi cells. Increased IL-2Rα expression following mIL-2:JES6-1 treatment presumably resulted from a coalescence of two effects: (1) Increased IL-2Rα transcription, which has been shown to occur in response to IL-2 signaling (Depper et al., 1985), and (2) selective proliferation of cells with particularly high surface densities of IL-2Rα. Elevated IL-2Rα expression resulted in enhanced sensitivity to the mIL-2:JES6-1 immunocomplex, further heightening selectivity for IL-2Rαhi Treg cell expansion.
An important component to the action of JES6-1 is its monovalent affinity for IL-2. The mIL-2:JES6-1 affinity is closely matched to that of the mIL-2:mIL-2Rα complex, allowing the antibody to directly compete with the receptor for access to mIL-2. One can imagine that a higher affinity antibody might bind too tightly to the cytokine and fail to let go and enable IL-2 complex formation. Conversely, a lower affinity antibody might dissociate more readily from the cytokine to allow interaction with IL-2Rβ and IL-2Rγ on all cell types, diminishing the advantage JES6-1 affords Treg cells. The mIL-2:mIL-2Rβ affinity is two orders of magnitude weaker than that of the mIL-2:JES6-1 interaction, positioning the antibody in an affinity ‘sweet spot’ that primes it for IL-2Rαhi Treg cell bias.
S4B6 also acted through a multiple-tiered mechanism to preferentially direct IL-2 toward potentiation of effector cells. As with JES6-1, the first layer of S4B6-mediated IL-2 modification occurred through extension of in vivo half-life (Letourneau et al., 2010). However, as the functional consequences of S4B6 are unique to this antibody, increased half-life alone cannot account for the biased potentiation it orchestrates. The second layer of S4B6 action included the collection of steric and allosteric structural effects it exerts on mIL-2. S4B6 sterically occluded IL-2Rα binding and mildly obstructed IL-2Rβ binding but it also allosterically strengthened the mIL-2:mIL-2Rβ interaction by inducing an affinity-enhancing conformational change in mIL-2. The net result was that sensitivity to IL-2Rα expression was lost as S4B6-bound mIL-2 signals equivalently through the IL-2 ternary complex on all IL-2 responsive immune cells, and susceptibility to IL-2 signaling was governed by IL-2Rβ expression. Allosteric enhancement of IL-2:IL-2Rβ interaction by S4B6 led to increased stimulatory activity that particularly favored IL-2Rβhi effector cells. As it has been established that IL-2 signaling induces IL-2Rβ upregulation (Siegel et al., 1987), it is enticing to speculate that, analogous to the JES6-1 immunocomplex, S4B6 exerts a third layer of regulation on mIL-2 activity, initiating a positive feedback cycle for IL-2Rβ expression to further bias stimulation in favor of IL-2Rβhi effector cells.
The complementary JES6-1 and S4B6 pair of IL-2-targeted antibodies serves as an archetypal system for exploring the molecular mechanisms underlying selective immune cell subset potentiation. Future work could expand upon our findings to design antibody variants with altered affinities and receptor subunit competition propensities that accentuate immune cell biasing effects. One could also envision applying our mechanistic insights to other systems by evolving cytokine- or growth factor-directed antibodies with desired receptor competitive properties. While JES6-1 and S4B6 have limited cross-reactivity with hIL-2 and IL-2Rα expression in activated effector T cells is more pervasive in human versus mouse tissues (Malek, 2008), the structural and biophysical studies we present illuminate a clear engineering-based path to modify these antibodies so that they may exert analogous effects on hIL-2 and serve as biased T cell subset-selective potentiating agents for a wide range of immunotherapeutic objectives.
EXPERIMENTAL PROCEDURES
Protein expression and purification
mIL-2 was expressed in the periplasm of Escherichia coli cells. hIL-2 and the mouse and human IL-2 receptor subunits were secreted from a baculovirus expression system. Sequences for the JES6-1 and S4B6 antibodies were isolated from their respective hybridoma cell lines and used to recombinantly express scFv and Fab fragments in a baculovirus expression system. Details are provided in the Supplemental Experimental Procedures.
Cell Lines
Procedures for culturing YT-1 cells and sorting for IL-2Rα expression are described in the Supplemental Experimental Procedures.
YT-1 cell STAT5 phosphorylation studies
YT-1 or IL-2Rα+ YT-1 cells were stimulated with mIL-2, mIL-2:antibody immunocomplexes, or mIL-2:antibody fragment complexes (2:1 molar ratio of antibody or antibody fragment to mIL-2). STAT5 activation was analyzed via flow cytometry as described previously (Ring et al., 2012). Details are provided in the Supplemental Experimental Procedures.
Immune cell subset proliferation and receptor expression studies
For relative effector cell:Treg cell proliferation studies (Figure 1C), C57BL/6 mice were injected i.p. with mIL-2 immunocomplexes (2:1 cytokine:antibody molar ratio) or free mIL-2 on days 1, 2, 3, and 4. For immune cell subset expansion and IL-2Rα profiling studies (Figures 6A–E), C57BL/6 mice were injected i.p. with mIL-2 immunocomplexes or free mIL-2 on days 1, 2, and 3. In both studies, mice were sacrificed on day 5 and spleens were harvested, homogenized, and analyzed for surface and intracellular markers via flow cytometry. Cells were profiled and average relative expansion of various immune cell subsets compared to untreated control mice was determined for each cohort. Details are described in the Supplemental Experimental Procedures.
Mouse dextran sodium sulfate (DSS)-induced colitis model
BALB/c mice were injected i.p. either daily for seven days with PBS, once on day 6 with anti-mIL-2Ra antibody (EXBIO, clone PC61.5), or daily for seven days with mIL-2:JES6-1 immunocomplexes (2:1 cytokine:antibody molar ratio). On day 8, two mice per condition were sacrificed to assess spleen Treg cell counts by flow cytometry. The remaining pretreated mice from each cohort were administered 3% DSS (MP Biomedicals Inc.) in their drinking water beginning on day 8 to induce colitis. On day 15, disease severity was assessed by a clinical disease activity index (CDAI) and on day 16, mice were sacrificed for colon measurement and histological analysis. See Supplemental Experimental Procedures for details.
Yeast surface and SPR affinity titrations
hIL-2 and mIL-2 were displayed on the surface of yeast as described previously (Boder and Wittrup, 1997; Rao et al., 2004). For SPR studies, biotinylated human and mouse IL-2 receptors or biotinylated anti-IL-2 antibodies were immobilized to streptavidin-coated chips and binding of hIL-2 and mIL-2 was analyzed on a Biacore T100 instrument (GE Healthcare). Protocol details are provided in the Supplemental Experimental Procedures.
Crystallization and data collection
Purified mIL-2 and JES6-1 scFv or mIL-2 and S4B6 Fab were complexed overnight at 4° C in the presence of carboxypeptidases-A and B (Sigma), co-eluted over a Superdex-200 size-exclusion chromatography column, and concentrated to >10 mg/mL. mIL-2:JES6-1 scFv crystals were grown in sitting drops at 22° C from 0.1 M Bis-tris propane pH 6.4, 0.2 M sodium citrate, and 19% PEG 3350 and flash frozen in liquid nitrogen, cryoprotected with the addition of 25% 2-Methyl-2,4-pentanediol (Sigma). A 2.8 Å dataset was collected at beamline 8-2 at the Advanced Light Source. mIL-2:S4B6 Fab crystals were grown in sitting drops at 22° C from 0.1 M Bis-tris propane pH 6.5, 0.2 M sodium fluoride, and 20% PEG 3350 and flash frozen in liquid nitrogen, cryoprotected with the addition of 25% glycerol (Sigma). A 2.2 Å dataset was collected at beamline 11-1 at the Stanford Synchrotron Radiation Laboratory. Diffraction data were processed using HKL2000. Crystallographic data collection and refinement statistics are reported in Table S1.
Structure determination and refinement
The mIL-2:JES6-1 scFv and mIL-2:S4B6 Fab complex structures were solved by molecular replacement using PHASER (McCoy, 2007). For mIL-2, a one-to-one threaded model of mIL-2 to a human IL-2 structure (PDB ID 2B5I) generated in Phyre (Kelley and Sternberg, 2009) was used, and for the JES6-1 and S4B6 VH and VL domains, multiple-threaded models obtained from the I-TASSER server (Roy et al., 2010; Zhang, 2008) were used. Models of the S4B6 CH1 and CL domains were obtained from the previously solved structure of a rat Fab (PDB ID 1LK3). Iterative model rebuilding and refinement were performed using the Phenix software (Adams et al., 2002) and COOT (Crystallographic Object-Oriented Toolkit) (Emsley and Cowtan, 2004). For initial refinement, rigid body, coordinate, and real-space refinement were used with individual atomic displacement parameter refinement. Translation, libration, and screw-rotation refinement was added in later iterations. Ramachandran and rotamer analysis were performed using MolProbity (Davis et al., 2007). Electron density connected to N31 of the JES6-1 VH was modeled as an N-linked GlcNAc2 with α-1,6 and α-1,3 difucosylation of the proximal GlcNAc. Interacting residues were identified using the protein interfaces, surfaces, and assemblies (PISA) service at the European Bioinformatics Institute (Krissinel and Henrick, 2007). Structural figures were created using PyMOL (DeLano, The PyMOL Molecular Graphics System, 2002).
Yeast surface and SPR antibody-receptor competitive IL-2 binding assays
For yeast surface competition studies, mIL-2-displaying yeast were incubated concurrently with saturating concentrations of mIL-2Ra or mIL-2Rβ and serial dilutions of unlabeled competitor antibody and subsequently analyzed for receptor binding via flow cytometry.
SPR-based competition studies were performed on a Biacore T100 instrument in two different topologies. In one orientation, biotinylated mIL-2Ra or mIL-2Rβ was immobilized to a streptavidin-coated chip and binding of pre-incubated complexes containing saturating amounts of mIL-2 and serial dilutions of competitor antibody was evaluated. In the other orientation, biotinylated antibodies JES6-1 or S4B6 were immobilized to a streptavidin-coated chip, a saturating amount of mIL-2 was then captured on the immobilized antibodies, and binding of soluble mIL-2Rα or mIL-2Rβ was evaluated. See Supplemental Experimental Procedures for details.
Supplementary Material
HIGHLIGHTS.
JES6-1 blocks IL-2/IL-2Rβ interaction and allosterically disrupts IL-2/IL-2Rα binding
JES6-1 selectivity for IL-2Rαhi cells perpetuated by transcriptional feedback loop
S4B6 sterically blocks IL-2/IL-2Rα interaction but enhances IL-2/IL-2Rβ interaction
JES6-1-mediated IL-2Rαhi cell growth bias inhibits colitis pathogenesis in mice
Acknowledgments
We thank members of the Garcia and Kovar laboratories for helpful advice and discussions, Miloslav Kverka for help with the mouse DSS colitis model, and A. Velasco, D. Waghray, and S. Fischer for technical assistance. We thank Onur Boyman for providing the JES6-1 hybridoma cell line and for helpful discussions. This work was supported by the US National Institutes of Health (R01 AI51321 to K.C.G., R01 AI108626 to M.P., and National Research Service Award NIH-F30DK094541 to A.M.R.), the Mathers Fund, the Ludwig Foundation, the Czech Science Foundation (Grant 13-12885S to M.K.), the Institute of Microbiology of the Academy of Sciences of the Czech Republic the Institutional Research Concept (Grant RVO 61388971 to M.K.), and Project BIOCEV of the European Regional Development Fund (Grant CZ.1.05/1.1.00/02.0109 to M.K.). K.C.G. is an investigator of the Howard Hughes Medical Institute, J.B.S. is the recipient of a Leukemia & Lymphoma Society Career Development Program fellowship, and V.C.L. is the recipient of a Cancer Research Institute Irvington postdoctoral fellowship. M.K. is listed as a co-inventor on the patent entitled “Methods for improving immune function and methods for prevention or treatment of disease in a mammalian subject”, which was filed on February 16, 2007 and now bears International Application Number PCT/US2007/0623631.
Footnotes
ACCESSION NUMBERS
Coordinates and structure factors for the mIL-2:JES6-1 and mIL-2:S4B6 complexes have been deposited into the Protein Data Bank under accession codes 4YQX and 4YUE, respectively.
AUTHOR CONTRIBUTIONS
J.B.S., V.C.L., and K.M.J. performed crystallographic studies of the mIL-2:antibody complexes and determined and refined these structures; J.B.S. performed binding characterization studies; J.B.S and S.D. carried out cell signaling studies; J.T. and P.V. performed in vivo signaling and therapeutic studies; J.B.S. and A.M.R. designed and prepared the cytokine proteins and recombinant antibody fragments used in this work; J.B.S., J.T., M.P., M.K., and K.C.G. designed experiments; J.B.S. and J.T. prepared the figures; J.B.S. and K.C.G. wrote the paper; and M.P, M.K., and K.C.G. supervised the research.
The authors have no other competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- Boder ET, Wittrup KD. Yeast surface display for screening combinatorial polypeptide libraries. Nature biotechnology. 1997;15:553–557. doi: 10.1038/nbt0697-553. [DOI] [PubMed] [Google Scholar]
- Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006a;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- Boyman O, Surh CD, Sprent J. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin Biol Ther. 2006b;6:1323–1331. doi: 10.1517/14712598.6.12.1323. [DOI] [PubMed] [Google Scholar]
- Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunological reviews. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- Castro I, Dee MJ, Malek TR. Transient enhanced IL-2R signaling early during priming rapidly amplifies development of functional CD8+ T effector-memory cells. Journal of immunology. 2012;189:4321–4330. doi: 10.4049/jimmunol.1202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, 3rd, Snoeyink J, Richardson JS, Richardson DC. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic acids research. 2007;35:W375–383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific; San Carlos, CA, USA: 2002. [Google Scholar]
- Depper JM, Leonard WJ, Drogula C, Kronke M, Waldmann TA, Greene WC. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM, Maliszewski CR. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. Journal of immunology. 1993;151:1235–1244. [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. The Journal of experimental medicine. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin GH, Hirano T, Murakami M. Combination treatment with IL-2 and anti-IL-2 mAbs reduces tumor metastasis via NK cell activation. International immunology. 2008;20:783–789. doi: 10.1093/intimm/dxn036. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nature protocols. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Krieg C, Letourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11906–11911. doi: 10.1073/pnas.1002569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. Journal of molecular biology. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Letourneau S, van Leeuwen EM, Krieg C, Martin C, Pantaleo G, Sprent J, Surh CD, Boyman O. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2171–2176. doi: 10.1073/pnas.0909384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, Moraga I, Raeber ME, Bowman GR, Novick P, et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature. 2012;484:529–533. doi: 10.1038/nature10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek TR. The biology of interleukin-2. Annual review of immunology. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- McCoy AJ. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr D Biol Crystallogr. 2007;63:32–41. doi: 10.1107/S0907444906045975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Koo SK, Kim Y, Kim HM, Joe IY, Park CS, Kim SC, Han DJ, Lim DG. Effect of in vitroexpanded CD4(+)CD25(+)Foxp3(+) regulatory T cell therapy combined with lymphodepletion in murine skin allotransplantation. Clinical immunology. 2010;135:43–54. doi: 10.1016/j.clim.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Rao BM, Driver I, Lauffenburger DA, Wittrup KD. Interleukin 2 (IL-2) variants engineered for increased IL-2 receptor alpha-subunit affinity exhibit increased potency arising from a cell surface ligand reservoir effect. Mol Pharmacol. 2004;66:864–869. doi: 10.1124/mol.66.4.. [DOI] [PubMed] [Google Scholar]
- Ring AM, Lin JX, Feng D, Mitra S, Rickert M, Bowman GR, Pande VS, Li P, Moraga I, Spolski R, et al. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nature immunology. 2012;13:1187–1195. doi: 10.1038/ni.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas G, Cabrera Infante Y, Pupo A, Carmenate T. Fine epitope specificity of antibodies against interleukin-2 explains their paradoxical immunomodulatory effects. mAbs. 2014;6:273–285. doi: 10.4161/mabs.27224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas G, Pupo A, Leon K, Avellanet J, Carmenate T, Sidhu S. Deciphering the molecular bases of the biological effects of antibodies against Interleukin-2: a versatile platform for fine epitope mapping. Immunobiology. 2013;218:105–113. doi: 10.1016/j.imbio.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Science translational medicine. 2012;4:127ps128. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nature protocols. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM. Application of IL-2 therapy to target T regulatory cell function. Trends in immunology. 2012;33:626–632. doi: 10.1016/j.it.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JP, Sharon M, Smith PL, Leonard WJ. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987;238:75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993;73:5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- Thanos CD, DeLano WL, Wells JA. Hot-spot mimicry of a cytokine receptor by a small molecule. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15422–15427. doi: 10.1073/pnas.0607058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos CD, Randal M, Wells JA. Potent small-molecule binding to a dynamic hot spot on IL-2. Journal of the American Chemical Society. 2003;125:15280–15281. doi: 10.1021/ja0382617. [DOI] [PubMed] [Google Scholar]
- Tomala J, Chmelova H, Mrkvan T, Rihova B, Kovar M. In vivo expansion of activated naive CD8+ T cells and NK cells driven by complexes of IL-2 and anti-IL-2 monoclonal antibody as novel approach of cancer immunotherapy. Journal of immunology. 2009;183:4904–4912. doi: 10.4049/jimmunol.0900284. [DOI] [PubMed] [Google Scholar]
- Verdeil G, Marquardt K, Surh CD, Sherman LA. Adjuvants targeting innate and adaptive immunity synergize to enhance tumor immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16683–16688. doi: 10.1073/pnas.0805054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 2005;310:1159–1163. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. The Journal of experimental medicine. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.