Abstract
Context:
Interleukin-11 (IL-11) is a multifunctional cytokine with a probable regulatory role in the inflamed periodontal tissue. It has also been shown to inhibit the production of potent proinflammatory cytokines like tumor necrosis factor-alpha, IL-6 and IL-1β in vitro. Type 2 diabetes mellitus, which demonstrates an increase in proinflammatory cytokines, might hypothetically, display a decrease in the levels of IL-11, which down-regulates synthesis of the proinflammatory cytokines.
Aims:
This clinicobiochemical cross-sectional study was undertaken to try to interpret the link between IL-11, diabetes and periodontitis and to explore the probable protective role of IL-11.
Materials and Methods:
A total of 90 patients were included in the study and were divided into five groups based on community periodontal index scores and diabetes status. Probing pocket depth and clinical attachment level were measured in all the subjects. Gingival crevicular fluid (GCF) was collected from all the participants using micropipettes and blood samples were collected from subjects in Groups III, IV and V, for analysis of glycated hemoglobin. IL-11 levels were measured in GCF samples by enzyme-linked immunosorbent assay.
Statistical Analysis:
The data obtained were subjected to statistical analysis.
Results:
The GCF IL-11 levels decreased from periodontal health to disease and in periodontitis patients with type 2 diabetes with decreasing glycemic control.
Conclusions:
Interleukin-11 may play an important role in the modulation of immune response via the reduction of proinflammatory cytokine production and periodontal tissue damage. It was seen in this study that IL-11 could be detected in GCF and the levels of IL-11 in GCF decreased progressively from healthy to periodontitis sites. IL-11 levels were significantly lower in chronic periodontitis group when compared to gingivitis group. The decrease in the levels of IL-11 probably indicates that both diabetes and periodontitis may play a synergistic role in the suppression of protective host responses. The potential of IL-11 as a probable biomarker of inflammation in both periodontal disease and diabetes mellitus is indicated by the changeability of IL-11 levels with the change in periodontal disease status and glycemic control. Further longitudinal studies are needed to validate IL-11 as a “biomarker of inflammation” in periodontal disease and diabetes progression and to prove its role in the connecting link between periodontal disease and type 2 diabetes mellitus.
Keywords: Cytokines, gingival crevicular fluid, inflammation, interleukin-11
INTRODUCTION
Periodontitis is an inflammatory disease of the supporting tissues of the teeth and the majority of periodontal tissue destruction seen in periodontitis is caused by an inappropriate host response to the microorganisms and their products.[1] Our understanding of the complex interactions of the various immune cells and their products during the immune-inflammatory response to periodontal pathogens has been enhanced by the research conducted over the past two decades.[2,3]
Cytokines are proteins secreted by the cells of innate and adaptive immunity and are considered to play an important role in the initiation, progression, and the host modulation of periodontal disease,[4,5] and the complex cytokine network that mediates the immune response includes proinflammatory cytokines, anti-inflammatory cytokines and specific cytokine receptors. Even a minimal imbalance of pro- and anti-inflammatory cytokine production may affect induction of bone and collagen destruction in periodontal tissues.[6]
Interleukin-11 (IL-11) is a multifunctional cytokine that was originally isolated from the primate stromal cell line, PU-34, and later from the human MRC - 5 cell line. It has been recently described as a regulatory cytokine within inflamed periodontal tissues.[7] IL-11 has been shown to inhibit the production of potent proinflammatory cytokines like tumor necrosis factor-alpha (TNF-α), IL-6 and IL-1β in vitro.[7]
Periodontitis has been referred to as the sixth complication of diabetes.[8] Findings suggest a dose-response relationship between periodontal disease risk and glycemic control.[9] Evidence in the literature has shown an evident role for inflammation in periodontal diseases and in the pathogenesis of diabetes and diabetic complications.[10] It has been shown that the proinflammatory cytokines such as IL-6 and IL-1β are increased in type 2 diabetes.[11]
Research suggests that, as an infectious process with a prominent inflammatory component, periodontal disease can adversely affect the metabolic control of diabetes. Conversely, treatment of periodontal disease and reduction of oral inflammation may have a positive effect on the diabetic condition.
Type 2 diabetes mellitus, which demonstrates an increase in proinflammatory cytokines, might hypothetically, display a decrease in the levels of IL-11, which down-regulates synthesis of the proinflammatory cytokines. However, there are very limited studies reporting IL-11 levels in gingival crevicular fluid (GCF) of patients with periodontitis and periodontitis with type 2 diabetes with different glycemic controls. The link between IL-11 levels in both diabetes and periodontitis remains an unexplored territory, which, if properly mapped would lead to a better understanding of the two-way relationship between diabetes and periodontitis.
This study was thus undertaken to assess the levels of IL-11 in periodontal health and disease and its association with type 2 diabetes.
MATERIALS AND METHODS
The study population consisted of a total of 90 subjects in the age group of 35–75 years attending the outpatient section, Department of Periodontics, D. A. Pandu Memorial R. V. Dental College, Bengaluru, India, from December 2011 to May 2012. Ethical clearance for the study was received from the Institutional Ethical Committee and Review Board, D. A. Pandu Memorial R. V. Dental College, Bengaluru, India. Written informed consent was obtained from all patients. Exclusion criteria were patients with systemic diseases such as type 1 diabetes mellitus, cardiovascular disorder, immunologic disorders, hepatitis, and human immunodeficiency virus infections, smokers, pregnant and lactating women and those taking oral contraceptive drugs or any anti-inflammatory or corticosteroid drugs. Subjects who had received antibiotics or treatment for periodontal disease in 6 months preceding the study were also excluded.
The selected 90 subjects were divided into five groups on the basis of their glycemic control (as indicated by glycated hemoglobin level) and based on their periodontal status assessed using the community periodontal index (CPI) recorded using a CPITN-C probe.
Criteria for subject grouping:
Group I: Consisted of 15 subjects with clinically healthy periodontium with no evidence of disease. CPI score 0
Group II: Consisted of 15 subjects with a CPI score of 3 or more
Group III: Consisted of 20 diabetic subjects, who showed CPI score of 3 or more. The hemoglobin A1c (HbA1c) value was 6–7%
Group IV: Consisted of 20 diabetic subjects, who showed CPI score of 3 or more. The HbA1c value was 7–8%
Group V: Consisted of 20 diabetic subjects, who showed CPI score of 3 or more. The HbA1c value was >8%.
A total of 60 diabetic subjects were included as against the 30 nondiabetic individuals so as to make a relevant analysis of the hypothetical relationship between IL-11, diabetes and periodontitis.
Clinical evaluation of subjects
All the selected participants underwent a detailed periodontal examination for the measurement of probing pocket depth (PPD) and clinical attachment level (CAL) using a University of North Carolina-15 Probe. In subjects with periodontitis, the site with the highest CPI score was chosen for GCF collection. In the healthy group, to standardize site selection and obtain adequate fluid volume, sampling was predetermined to be from the mesio-buccal region of the maxillary right first molar, in the absence of which the left first molar was sampled.
Procedure for sample collection
Method of collection of blood
After seating them comfortably, the procedure was once again described to the patients. The left antecubital fossa was swabbed with an alcohol swab, and a cuff was used to apply pressure above the fossa. A 5 ml syringe was used to draw blood, and the blood was immediately transferred to a vacutainer. HbA1c was estimated by the turbidimetric inhibition assay method.
Method of collection of gingival crevicular fluid
The site for sample collection was freed of any debris or calculus and was then dried and isolated with cotton rolls. Calibrated, volumetric, micro capillary pipettes with 0–5 μl range were placed at the entrance of the gingival crevice and 2–3 μl of GCF was collected from each subject. The GCF was transferred into vials containing 100 μl phosphate buffer saline, and the samples were frozen at − 70°C till they were assayed for IL-11.
Measurement of interleukin-11 levels
The colorimetric assay procedure was done at the Department of Microbiology, Maratha Mandal's Nathajirao G Halgekar Dental College, Belgaum. The IL-11 levels were measured using a commercially available colorimetric assay kit for human IL-11 (RayBiotech Inc., USA).
Statistical analyses
All data were analyzed using a software program (SPSS, version 14.0, SPSS, Chicago, IL). Analysis of variance (ANOVA) was carried out to test the hypothesis of equality among the five groups for IL-11. Multiple comparisons for IL-11 levels using Bonferroni test was carried out to find out which pair or pairs differed significantly. Pearson's correlation coefficient test was used to observe any correlation between the parameters recorded, that is, PPD, CAL, HbA1c and GCF IL-11 levels.
RESULTS
Probing pocket depth and clinical attachment level
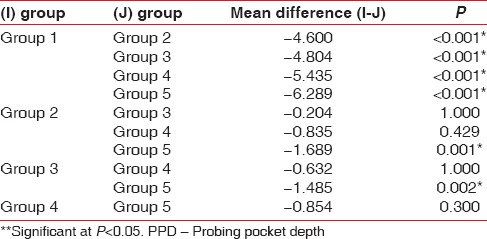
The mean PPD and CAL for the groups are given in Tables 1 and 2 respectively. The difference in mean PPD and CAL between all the groups was found to be statistically significant (P < 0.001). Higher mean PPD and CAL were recorded in Group V, followed by Group IV, Group III, Group II and Group I. Bonferroni test was used to find out among which pair of groups, there existed a significant difference. The difference in mean PPD was found to be statistically significant (P < 0.001) between Group I and all the other groups, Group II and Group V, and Group III and Group V [Table 3].
Table 1.
Comparison of PPD in all the groups
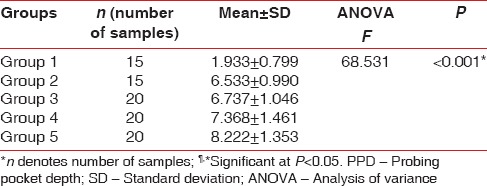
Table 2.
Comparison of CAL in all the groups
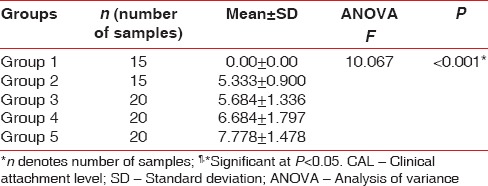
Table 3.
Group-wise comparison for PPD in mm using Bonferroni test
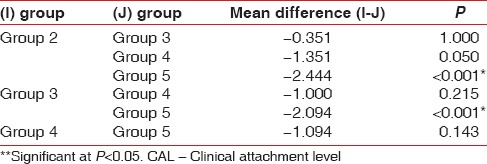
The difference in mean CAL was found to be statistically significant (P < 0.001) between Group II and Group V as well as Group III and Group V [Table 4].
Table 4.
Group-wise comparison for CAL in mm using Bonferroni test
Interleukin-11 concentrations
Table 5 shows the distribution of mean IL-11 levels in all the groups. It was seen that the mean IL-11 levels decreased with a decline in glycemic control. Furthermore, higher levels of IL-11 were observed in periodontally healthy sites when compared to sites with periodontitis.
Table 5.
Descriptive statistics: Showing mean values, SD, minimum and maximum values for IL-11 levels (μmol/l)
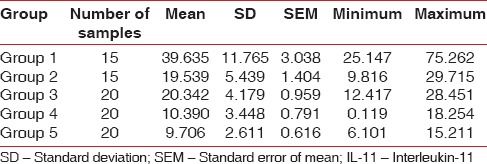
To test the hypothesis of equality among the five groups ANOVA test was carried out [Table 6]. The decision criterion was to reject the null hypothesis if the P < 0.001. Since there was a significant difference between the groups, multiple comparisons (post-hoc test) using Bonferroni test was carried out [Table 7]. The results obtained showed that the mean levels of IL-11 were highest in systemically and periodontally healthy subjects and lowest in periodontitis patients with poorly controlled diabetes.
Table 6.
Results of ANOVA test comparing the mean IL-11 levels (μmol/l) between all the groups
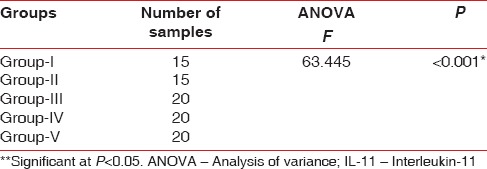
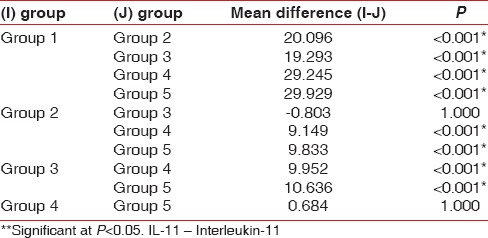
Table 7.
Group-wise comparison for IL-11 levels (μmol/l) using Bonferroni test
Correlations
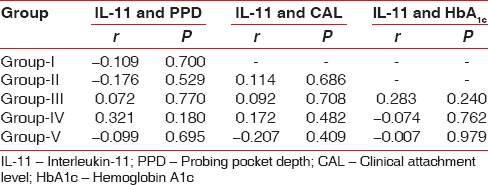
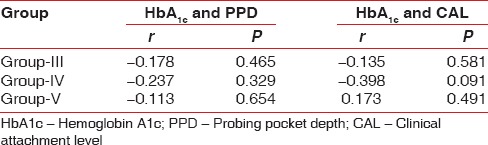
Pearson's correlation coefficient test was used to observe any correlation between the parameters recorded, that is, PPD, CAL, HbA1c and GCF IL-11 levels. IL-11 was negatively correlated with CAL and PPD in Group V. IL-11 and HbA1c were also correlated, and a negative correlation was obtained in Groups IV and V [Table 8].
Table 8.
Pearson correlation coefficient test comparing IL-11 levels (μmol/l) with PPD, CAL and HbA1c
A negative correlation was obtained when Pearson's correlation coefficient test was used to observe any correlation between HbA1c and PPD, CAL [Table 9].
Table 9.
Pearson correlation coefficient test comparing HbA1c levels with PPD and CAL
DISCUSSION
Periodontitis is a multifactorial disease with the presence of pathogenic bacteria being necessary for the initiation of inflammation; but the progression of periodontal disease depends equally on the host response to various pathogenic bacterial products and components. The bacterial products initiate a local host response in gingiva that involves recruitment of inflammatory cells, generation of prostanoids and cytokines, elaboration of lytic enzymes and activation of osteoclasts.[12]
In diabetes a subclinical inflammatory reaction has been shown to precede the onset of type 2 diabetes. TNF-α, IL-6 and IL-1β are potent proinflammatory cytokines whose levels are found to be elevated in case of diabetes, thus contributing to increased severity of the disease and the complications seen.[11] Research has revealed that combined elevation of IL-6 and IL-1β increased the risk of type 2 diabetes by 3-fold.[11,13]
Diabetes mellitus and periodontal disease share a two-way relationship.[14] Hyperglycemia has been thought to play a role in periodontal disease incidence and prevalence in diabetic patients. Patients with relatively good metabolic control are less prone to periodontal destruction as proven by a number of longitudinal studies.[8] IL-11 has been known to inhibit the production of the cytokines IL-6 and IL-1β[7] and has been found to act as a regulatory cytokine within the periodontal tissues.
Studies have evaluated the level of IL-11 in GCF and gingival biopsies of gingivitis and periodontitis patients.[7,15,16,17] However, to the best of our knowledge, there are no studies reporting and relating levels of GCF IL-11 in periodontal disease and type 2 diabetes mellitus with different glycemic controls.
Totally, 90 patients with age ranging from 35 to 75 years were selected for the study. As the prevalence of periodontitis increases with age and also, since most people with type 2 diabetes belong to this age group, this age group was chosen for the study.[18,19]
Patients were divided into five groups, Group I to Group V, based on their periodontal disease status and glycated hemoglobin levels. Glycated hemoglobin levels estimate the glycemic control of patients over the preceding 3 months.[20]
Community periodontal index score, which is a practical method for routine screening and recording of periodontal disease indicators, has been used for the screening of periodontal disease in this study.[21]
Gingival crevicular fluid sample collected using microcapillary pipettes using the extracrevicular method has been used to assess the cytokine levels. Microcapillary pipettes facilitated the collection of a standardized GCF volume of 3 μl for all the subjects as required for the biochemical analysis of GCF and avoided the drawback of nonspecific attachment of the analyte to filter paper fibers.
Probing pocket depth and CAL were measured in all the groups, and it showed a progressive increase from healthy to periodontitis site and an increase with worsening of glycemic control. Serrano et al., in a study, reported that the degree of metabolic control had a relation to periodontal parameters, that is, PPD and CAL, and that the diabetic subjects with periodontitis demonstrated poorer periodontal status when compared to nondiabetic individuals with periodontitis.[22]
In this study, the levels of IL-11 in GCF decreased progressively from healthy to periodontitis sites. Yücel et al. compared the GCF level of IL-11 in gingivitis and periodontitis group. They found that IL-11 levels were significantly lower in chronic periodontitis group when compared to gingivitis group.[23] A number of other studies have reported increased GCF and gingival biopsy IL-11 levels in periodontal health when compared to periodontitis subject.[7,15,16,17] Further, as the glycemic control worsened, the levels of IL-11 decreased gradually from well-controlled diabetics to moderately-controlled and poorly controlled diabetics, suggesting that IL-11 may play an important role in the modulation of immune response via the reduction of proinflammatory cytokine production and periodontal tissue damage. This claim is supported by findings from the study conducted by Trepicchio et al., who, in an animal study, showed that, twice weekly administration of recombinant IL-11 in developing periodontal disease model acted by blocking the proinflammatory cytokines associated with inflammation, leading to reduction in attachment loss.[24]
It was observed in the study that IL-11 and HbA1c were negatively correlated in Groups IV and V. To the best of our knowledge, no other study in the literature has assessed the levels of IL-11 in type 2 diabetes patients with different levels of glycemic control, and hence the results of this study could not be compared to any other study.
It is plausible that IL-11, which down-regulates the synthesis of proinflammatory cytokines like TNF-α, IL-6 and IL-1β in vitro, could be decreased in case of type 2 diabetes mellitus as well, since it is preceded by a subclinical inflammatory reaction wherein the synthesis of above mentioned proinflammatory cytokines are increased.
The decrease in the levels of IL-11 perhaps indicates that both diabetes and periodontitis may play a synergistic role in the suppression of protective host responses. This in turn, could result in the worsening of both diabetes and periodontitis disease status and thereby, perpetuate the vicious cycle comprising of suppression of the immune response, periodontitis, and impaired glycemic control.
The variability of IL-11 concentrations with variation in periodontal disease status and glycemic control implies that IL-11 may be a potential biomarker of inflammation in both periodontal disease and diabetes mellitus.
The study was limited by its small sample size. Longitudinal interventional studies on a larger population are required to arrive at a more definitive conclusion.
CONCLUSION
Interleukin-11 may play an important role in the modulation of the immune response via the reduction of proinflammatory cytokine production and periodontal tissue damage. It was seen in this study that IL-11 could be detected in GCF and the levels of IL-11 in GCF decreased progressively from healthy to periodontitis sites. IL-11 levels were significantly lower in chronic periodontitis group when compared to gingivitis group. The decrease in the levels of IL-11 probably indicates that both diabetes and periodontitis may play a synergistic role in the suppression of protective host responses. The potential of IL-11 as a probable biomarker of inflammation in both periodontal disease and diabetes mellitus is indicated by the changeability of IL-11 levels with the change in periodontal disease status and glycemic control.
Further longitudinal studies are needed to validate IL-11 as a “biomarker of inflammation” in periodontal disease and diabetes progression and to prove its role in the connecting link between periodontal disease and type 2 diabetes mellitus.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lamster IB, Novak MJ. Host mediators in gingival crevicular fluid: Implications for the pathogenesis of periodontal disease. Crit Rev Oral Biol Med. 1992;3:31–60. doi: 10.1177/10454411920030010501. [DOI] [PubMed] [Google Scholar]
- 2.Tatakis DN. Interleukin-1 and bone metabolism: A review. J Periodontol. 1993;64:416–31. [PubMed] [Google Scholar]
- 3.Stashenko P, Dewhirst FE, Peros WJ, Kent RL, Ago JM. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987;138:1464–8. [PubMed] [Google Scholar]
- 4.Bascones A, Noronha S, Gómez M, Mota P, Gónzalez Moles MA, Villarroel Dorrego M. Tissue destruction in periodontitis: Bacteria or cytokines fault? Quintessence Int. 2005;36:299–306. [PubMed] [Google Scholar]
- 5.Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol. 2005;32(Suppl 6):108–29. doi: 10.1111/j.1600-051X.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 6.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RB, Wood N, Serio FG. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J Periodontol. 2004;75:37–43. doi: 10.1902/jop.2004.75.1.37. [DOI] [PubMed] [Google Scholar]
- 8.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 9.Papapanou PN. Epidemiology of periodontal diseases: An update. J Int Acad Periodontol. 1999;1:110–6. [PubMed] [Google Scholar]
- 10.Iacopino AM. Periodontitis and diabetes interrelationships: Role of inflammation. Ann Periodontol. 2001;6:125–37. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 11.Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–7. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 12.Champagne CM, Buchanan W, Reddy MS, Preisser JS, Beck JD, Offenbacher S. Potential for gingival crevice fluid measures as predictors of risk for periodontal diseases. Periodontol 2000. 2003;31:167–80. doi: 10.1034/j.1600-0757.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 13.Lgssiar A, Hassan M, Schott-Ohly P, Friesen N, Nicoletti F, Trepicchio WL, et al. Interleukin-11 inhibits NF-kappaB and AP-1 activation in islets and prevents diabetes induced with streptozotocin in mice. Exp Biol Med (Maywood) 2004;229:425–36. doi: 10.1177/153537020422900511. [DOI] [PubMed] [Google Scholar]
- 14.Taylor GW, Borgnakke WS. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 15.Martuscelli G, Fiorellini JP, Crohin CC, Howell TH. The effect of interleukin-11 on the progression of ligature-induced periodontal disease in the beagle dog. J Periodontol. 2000;71:573–8. doi: 10.1902/jop.2000.71.4.573. [DOI] [PubMed] [Google Scholar]
- 16.Ay ZY, Yilmaz G, Ozdem M, Koçak H, Sütçü R, Uskun E, et al. The gingival crevicular fluid levels of interleukin-11 and interleukin-17 in patients with aggressive periodontitis. J Periodontol. 2012;83:1425–31. doi: 10.1902/jop.2012.110585. [DOI] [PubMed] [Google Scholar]
- 17.He L, Nagasawa T, Ishikawa I. Production of interleukin-11 in human gingival fibroblasts stimulated with IL-1alpha and tumor necrosis factor-alpha. Zhonghua Kou Qiang Yi Xue Za Zhi. 2004;39:488–91. [PubMed] [Google Scholar]
- 18.Mealey B. Diabetes mellitus. In: Rose LF, Genco RJ, Mealy BL, Cohen WD, editors. Periodontal Medicine. Hamilton, Ontario: B. C. Decker Inc; 2000. pp. 121–51. [Google Scholar]
- 19.Powers AC. Diabetes mellitus. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's Principles of Internal Medicine. 16th ed. Vol. 2. New York: McGraw Hill Companies; 2005. pp. 2137–52. [Google Scholar]
- 20.Faria-Almeida R, Navarro A, Bascones A. Clinical and metabolic changes after conventional treatment of type 2 diabetic patients with chronic periodontitis. J Periodontol. 2006;77:591–8. doi: 10.1902/jop.2006.050084. [DOI] [PubMed] [Google Scholar]
- 21.Nomura Y, Tamaki Y, Tanaka T, Arakawa H, Tsurumoto A, Kirimura K, et al. Screening of periodontitis with salivary enzyme tests. J Oral Sci. 2006;48:177–83. doi: 10.2334/josnusd.48.177. [DOI] [PubMed] [Google Scholar]
- 22.Serrano C, Perez C, Rodríguez M. Periodontal conditions in a group of Colombian type 2 diabetic patients with different degrees of metabolic control. Acta Odontol Latinoam. 2012;25:132–9. [PubMed] [Google Scholar]
- 23.Yücel OO, Berker E, Gariboglu S, Otlu H. Interleukin-11, interleukin-1beta, interleukin-12 and the pathogenesis of inflammatory periodontal diseases. J Clin Periodontol. 2008;35:365–70. doi: 10.1111/j.1600-051X.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- 24.Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol. 1996;15(157):3627–34. [PubMed] [Google Scholar]


