Abstract
Background:
The potential drawbacks of the existing antiplaque agents call for innovative strategies that are safe, effective, and easily available.
Objective:
The objective was to assess and compare antimicrobial efficacy of four plant extracts derived using hot and cold extraction methods against Streptococcus mutans, Streptococcus sanguis, and Streptococcus salivarius.
Materials and Methods:
The leaves of Acacia nilotica, P. guajava, Eucalyptus hybrid, and Murraya konigii L. Sprengel were collected from the surrounding areas, identified and authenticated by a taxonomist. The leaves were washed, shade-dried, and hand crushed to obtain coarse powder. This was subsequently ground into a fine powder and extracted using ethanol by cold infusion and hot extraction process. The antimicrobial efficacy testing was done on American Type Culture Collection strains of S. mutans, S. sanguis, and S. salivarius using agar well diffusion method. 0.2% chlorhexidine and dimethyl sulfoxide were used as positive and negative controls. The mean inhibition zone using 10% concentration of these extracts was compared using independent sample t-test and one-way analysis of variance.
Results:
All the four plant extracts inhibited the growth of S. mutans, S. sanguis, and S. salivarius irrespective of the method of extraction. The extracts of A. nilotica, P. guajava, and E. hybrid derived from both the methods of extraction exhibited a significantly higher inhibition zone against S. mutans in comparison with Murraya koenigii L. Sprengel and chlorhexidine. The cold extracts of A. nilotica and E. hybrid exhibited higher zone of inhibition against S. sanguis while the hot extracts of M. koenigii L. Sprengel exhibited a higher zone of inhibition against S. mutans.
Conclusion:
All the four plant extracts derived using either hot or cold extraction were effective against these bacteria and have the potential to be used as antiplaque agents.
Keywords: Acacia nilotica, antimicrobial efficacy, dental plaque, Eucalyptus hybrid, Murraya konigii L., sprengel, P. guajava, Streptococcus mutans
INTRODUCTION
Dental plaque is the community of microorganisms found on the tooth surface as a biofilm, embedded in a matrix of polymers of host and bacterial origin.[1] More than 500 bacterial strains may be found in dental plaque.[2] Bacteria are the primary etiologic agents in dental caries and periodontal disease, and these diseases have historically been considered the most important global oral health burdens. Dental caries is still a major health problem in most industrialized countries as it affects 60-90% of school-aged children and the vast majority of adults (nearly 100%).[3,4,5] Tooth loss due to severe periodontitis is found in 5-20% of most adult population worldwide.[3,6] Plaque-associated oral disease affects a considerable portion of the population and is considered one of the major causes of tooth loss.[7]
The cost of treating dental caries alone can overwhelm a country's health care expenditure.[8] At the same time, the cost of neglect of these diseases is also high due to the personal, financial, and social impact.[9] The approach suitable to the developing nations is to focus on prevention and identify alternative preventive strategies. The use of synthetic dentifrices with antimicrobial properties is known to produce harmful side effects on prolonged use. Chlorhexidine gluconate is the most commonly used antiplaque agent. However, some potential drawbacks such as altered taste sensation, staining of teeth, and development of resistant microorganisms incapacitate its long-term use.[10] This necessitates the development of innovative strategies that are safe, effective, and easily available. One such strategy would be to explore the enormous wealth of medicinal plants.
The standardization of extraction procedures contributes significantly to the final quality of the herbal drug. Among the various methods in vogue to extract soluble metabolites leaving behind insoluble cellular marc, Soxhlet extraction (37%) and infusion are the most frequently employed (22%).[11] The literature suggests Acacia nilotica,[12,13] Eucalyptus,[14] Psidium guajava,[15,16] and Murraya koenigii L[17,18] to exert an antimicrobial effect. However, the studies comparing the antimicrobial efficacy of these plant extracts derived using hot and cold extraction methods on dental plaque microorganisms was nonexistent. Hence, the present study was undertaken to assess and compare the antimicrobial efficacy of four plant extracts derived using hot and cold extraction methods against Streptococcus mutans, Streptococcus sanguis, and Streptococcus salivarius. We hypothesized that four plant extracts derived using hot and cold extraction methods have the antimicrobial efficacy against dental plaque microorganisms.
MATERIALS AND METHODS
Study design and setting
This was an in vitro study conducted over a period of 6 months from July to December 2013 at the Center for Scientific Research and Development, People's University, Bhopal.
Plant material
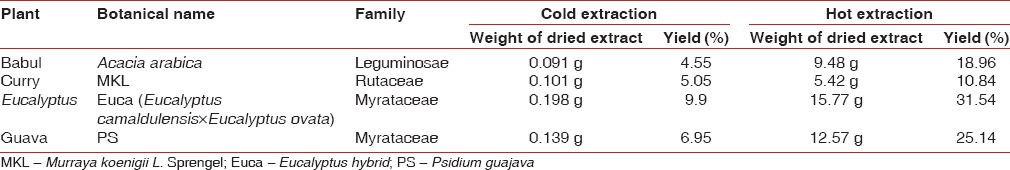
The details of the four plants and their yield following cold and hot extraction methods are denoted in Table 1. The leaves of four plants were collected from the surrounding areas, identified and authenticated by a taxonomist. The leaves were thoroughly rinsed using water treated with reverse osmosis and shade-dried over a period of 3-4 weeks at room temperature. The dried leaves were hand crushed separately to obtain coarse powder. Subsequently, the fine powder was prepared using a mixer grinder and stored in airtight plastic bottles. The bottles were labeled and stored in the refrigerator at 4°C till further use.
Table 1.
The plant profile and yield of the four herbal extracts assessed in the present study
Bacteria
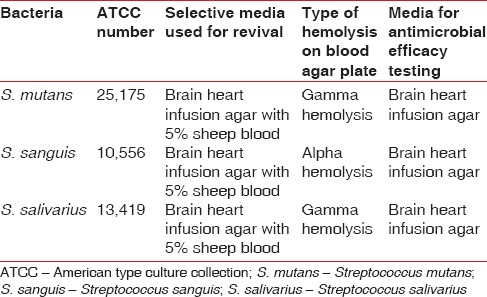
The American Type Culture Collection strains of S. mutans, S. sanguis, and S. salivarius were imported from USA and revived for further microbiological assay. The three bacterial cultures were maintained on Brain Heart Infusion agar slants with periodic subculturing and stored at 4° C [Table 2].
Table 2.
Details of the bacteria used for antimicrobial efficacy testing
Plant extraction
The extraction procedure was carried out using hot and cold extraction methods.
Cold infusion method
An amount of 2 g of fine powder was weighed into a bottle and mixed with 10 ml of ethanol. The mixture was subjected to gentle and intermittent stirring for 15 min every 2 h for 48 h on a rotary shaker. The mixture was then filtered using Whatman filter paper. The filtrate was allowed to dry completely. The dried leaf extract was mixed with the required quantity of dimethyl sulfoxide (DMSO) to obtain the working concentration of the extract (100 mg/ml).
Hot extraction method
The extraction process was carried out using Soxhlet apparatus. The finely ground crude powder (50 g) from the plant was placed in a porous bag or “thimble” made of strong filter paper and loaded into the main chamber of the Soxhlet extractor. This extractor was placed onto a distillation flask containing the solvent (ethanol). The Soxhlet was then equipped with a condenser, and the solvent was heated to reflux. The warm solvent vapor travelled up the distillation arm and flooded into the chamber housing the thimble. Then, it was automatically emptied by a siphon side arm back down to the distillation flask once the chamber was almost full. This cycle was allowed to repeat many times so that the desired compound gets concentrated in the distillation flask. The solvent extracts were filtered, concentrated under reduced pressure (30 ± 10 mbar) in a rotary evaporator at 30°C–60°C to a syrupy consistency and finally dried at room temperature. The weight of the dried mass was recorded and used for experimental studies.[11]
Antimicrobial efficacy testing
The antimicrobial efficacy of the plant extracts at 10% concentration (50 μl volume) was assessed using agar well diffusion method. The diameter of the inhibition zone was measured at three different planes on the undersurface of the agar plate using a transparent scale. 0.2% chlorhexidine and DMSO were used as positive and negative controls, respectively. The experiment was done in duplicate and mean inhibition zone was computed using the six readings after accounting for the diameter of the well (7 mm).
Photochemical constituents assay
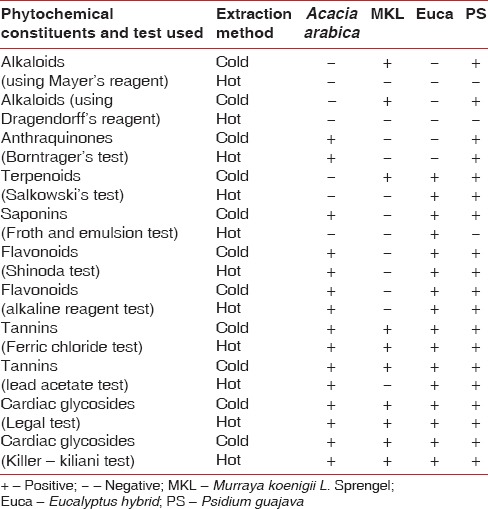
A qualitative assay was carried out to identify the presence of alkaloids (using Mayer's reagent and Dragendorff's reagent), anthraquinones (Borntrager's test), terpenoids (Salkowski's test), saponins (Froth and emulsion test), flavonoids (Shinoda and alkaline reagent tests), tannins (Ferric chloride and lead acetate tests), and cardiac glycosides (Legal test and Keller–Killani test).
Data entry and statistical analysis
The data analysis was done using SPSS version 20 (IBM, Chicago USA). The mean diameter of inhibition zone between different categories was compared using one-way analysis of variance and Tukey's post hoc test. The mean value between hot and cold extraction methods was compared using independent sample t-test. The statistical significance was fixed at 0.05.
RESULTS
Antimicrobial efficacy of extracts obtained by hot extraction process
All the four plant extracts inhibited the growth of S. mutans, S. sanguis, and S. salivarius. The maximum mean diameter of inhibition zone against S. mutans was noted with A. nilotica (22.00 ± 0.63) (mean ± standard deviation). The difference in the zone of inhibition between the various extracts and chlorhexidine (14.5 ± 2.07) was statistically significant (P = 0.001). The post hoc results revealed a significantly higher zone of inhibition against S. mutans with A. nilotica, P. guajava, and Eucalyptus hybrid in comparison with M. koenigii L. Sprengel and chlorhexidine. However, no significant difference was observed between M. koenigii L. Sprengel and chlorhexidine as well as between other combinations [Table 3].
Table 3.
Antimicrobial efficacy of four plant extracts against S. mutans, S. sanguis, and S. salivarius using hot extraction process
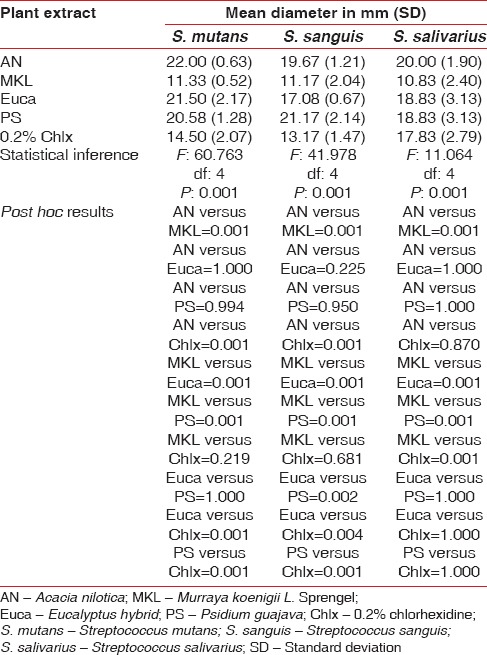
Among the various extracts, P. guajava (21.17 ± 2.14) was the most effective against S. sanguis. The mean diameter of inhibition zone by P. guajava, A. nilotica, E. hybrid against S. sanguis was significantly higher compared to one produced by M. koenigii L. Sprengel (11.17 ± 2.04) and chlorhexidine (13.17 ± 1.47) (P = 0.001). The difference between M. koenigii L. Sprengel and chlorhexidine was not statistically significant [Table 3].
A. nilotica (20.0 ± 1.9) produced the highest inhibition zone against S. salivarius. M. koenigii L. Sprengel (10.83 ± 2.4) was the least effective. The difference in the mean diameter of inhibition zone between M. koenigii L. Sprengel and other plant extracts, as well as chlorhexidine (17.83 ± 2.79), was statistically significant (P = 0.001). However, no significant difference was observed between other categories [Table 3]. DMSO failed to inhibit the growth of all the three bacteria and hence, was not considered for comparison.
Antimicrobial efficacy of extracts obtained by cold extraction process
All the four plant extracts derived by cold infusion method inhibited the growth of S. mutans, S. sanguis, and S. salivarius. The maximum inhibition zone against S. mutans was observed with A. nilotica (21.83 ± 0.41). The difference in the zone of inhibition between the various extracts and chlorhexidine was statistically significant (P = 0.001). The post hoc results revealed a significantly higher zone of inhibition against S. mutans with A. nilotica, P. guajava, and E. hybrid in comparison with M. koenigii L. Sprengel and chlorhexidine. M. koenigii L. Sprengel was less effective compared to chlorhexidine. The difference between other categories was not statistically significant [Table 4].
Table 4.
Antimicrobial efficacy of four plant extracts against S. mutans, S. sanguis, and S. salivarius using cold extraction process
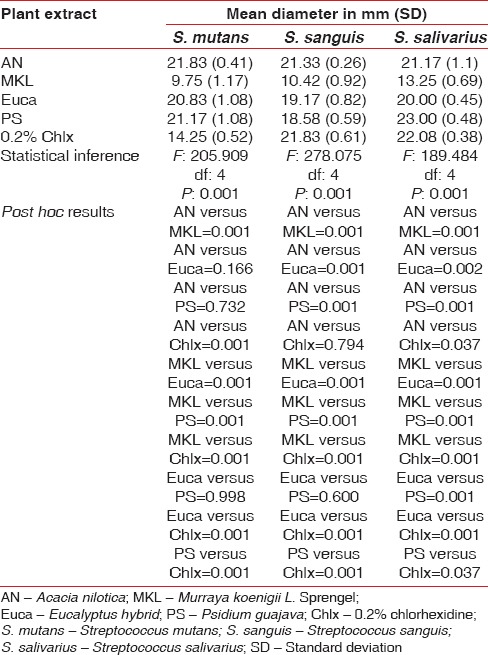
A. nilotica (21.33 ± 0.26) and chlorhexidine (21.83 ± 0.61) were the most effective in inhibiting S. sanguis. M. koenigii L. Sprengel was the least effective (10.42 ± 0.92). The difference in the mean inhibition zone between A. nilotica and other plant extracts was statistically significant (P = 0.001). Similarly, the difference between chlorhexidine and other plant extracts was also significant (P = 0.001). A. nilotica, P. guajava (18.58 ± 0.59) and E. hybrid (19.17 ± 0.82) exhibited a significantly higher inhibition zone compared to M. koenigii L. Sprengel with no difference between E. hybrid and P. guajava [Table 4].
Chlorhexidine (22.08 ± 0.38) exhibited a significantly higher mean inhibition zone against S. salivarius compared to all other plant extracts (P = 0.001). M. koenigii L. Sprengel (13.25 ± 0.69) exhibited the least inhibition zone compared to other plant extracts with no significant difference between other categories [Table 4].
Comparison of antimicrobial efficacy between hot and cold extraction methods
Acacia nilotica
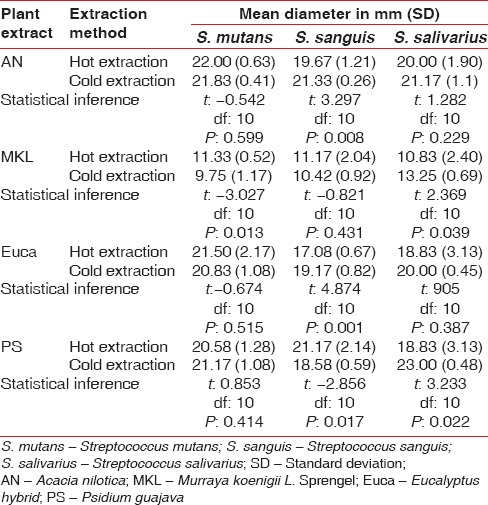
The extract derived from cold extraction produced a significantly higher inhibition zone compared to one using hot extraction against S. sanguis (P = 0.008). However, there was no significant difference in the efficacy between the extracts of hot and cold methods against S. mutans (P = 0.599) and S. salivarius (P = 0.229) [Table 5].
Table 5.
Comparison of antimicrobial efficacy on S. mutans, S. sanguis, and S. salivarius using plant extracts derived using hot and cold extraction methods
Murraya koenigii L. Sprengel
The extract derived from cold extraction process showed a significantly higher inhibition zone compared one using hot extraction on S. salivarius (P = 0.039). The extract from Soxhlet extraction showed higher inhibition zone against S. mutans (P = 0.013) with no difference between the two categories against S. sanguis (P = 0.431) [Table 5].
Eucalyptus hybrid
The extract obtained using cold extraction showed higher inhibition zone against S. sanguis (P = 0.001) with no difference between the two categories against S. mutans (P = 0.515) and S. salivarius (P = 0.387) [Table 5].
Psidium guajava
The extract derived from cold extraction exhibited higher inhibition zone against S. salivarius (P = 0.022) while hot extraction method was better against S. sanguis (P = 0.017) with no difference between the categories against S. mutans [Table 5].
The details of the qualitative assay of various phytochemical constituents in the plant extracts derived using hot and cold extraction process are summarized in Table 6.
Table 6.
Summary of qualitative assay of phytochemical constituents in plant extracts derived using hot and cold extraction methods
DISCUSSION
All the four plant extracts inhibited the growth of S. mutans, S. sanguis, and S. salivarius. A. nilotica, P. guajava, and E. hybrid extracts derived from hot extraction process exhibited a significantly higher zone of inhibition against all the three bacteria compared to M. koenigii L. Sprengel and chlorhexidine. The extracts of A. nilotica, P. guajava, and E. hybrid derived using cold infusion method also exhibited maximum efficacy against S. mutans compared to M. koenigii L. Sprengel and chlorhexidine. The efficacy of cold extract of A. nilotica against S sanguis was comparable to that produced by chlorhexidine while other extracts were found to be less effective in comparison with chlorhexidine. However, chlorhexidine exhibited a significantly higher mean inhibition zone against S. salivarius compared to all other plant extracts. These results suggest all the four plant extracts to be effective in inhibiting the growth of the three bacteria tested in the present study irrespective of the extraction method.
The qualitative assay of A. nilotica extract derived using cold extraction process revealed the presence of anthraquinones, terpenoids, saponins, flavonoids, tannins, and cardiac glycosides. However, the extract derived using hot extraction method failed to show the presence of saponins. Deshpande and Kadam et al.[12] found the ethanolic extracts of A. nilotica to be effective in inhibiting the growth of S. mutans (mean diameter of the inhibition zone was 31 mm ±0.7) and the antimicrobial activity was attributed to the presence of alkaloids, saponins, cardiac glycosides, tannins, flavonoids, and anthraquinones. Dabur et al.[19] found the extracts of A. nilotica (Babul) to possess antimicrobial and antifungal properties. The antimicrobial potential of A. nilotica found in the present study is consistent with these studies.
The qualitative assay of P. guajava extract derived using cold extraction showed the presence of alkaloids, anthraquinones, terpenoids, saponins, flavonoids, tannins, and cardiac glycosides. The extract derived from hot extraction failed to show the presence of alkaloids and saponins. The antimicrobial efficacy of P. guajava could be attributed to guajaverin, psidiolic acid, and other essential oil constituents such as monoterpenes, 1.8-cineol, ρ-cimen, and acetate of α-terpenil.[15] Hema et al.[16] found P. guajava to exert inhibitory effect against Pseudomonas lundensis, Aspergillus niger, and Aspergillus flavus. Our study demonstrated the antimicrobial potential of P. guajava similar to the findings of these studies and others.[20,21]
The Eucalyptus extract using both hot and cold extraction process possessed terpenoids, saponins, flavonoids, tannins, and cardiac glycosides with no difference in terms of phytochemical constituents. Nagata et al.[14] found Macrocarpals A, B, and C in Eucalyptus leaves to exhibit antibacterial activity against periodontopathic bacteria. The P. gingivalis displayed the greatest sensitivity. Takarada et al.[22] found Eucalyptus oil to exert an inhibitory effect on various oral bacteria that included Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans, Fusobacterium nucleatum, S. mutans, and Streptococcus sobrinus. Our results were similar to these studies.
The cold extracts of M. koenigii L. Sprengel found the presence of alkaloids, terpenoids, tannins, and cardiac glycosides. The extracts derived using hot extraction failed to show the presence of alkaloids and terpenoids. Ningappa et al.[17] found antioxidant protein isolated from curry leaves to inhibit various human pathogenic bacteria. Sunitha et al.[18] found the alcoholic extracts of curry leaves to inhibit dental caries microorganisms similar to the findings in the present study. Ramesh et al.[23] concluded that the use of curry leaf extracts as home remedies creates an oral environment which is unfavorable for microbes. This indirectly reduces the microbial colonization and modifies oral environment, thus preventing plaque accumulation, periodontal diseases, and dental caries. These studies and our results clearly demonstrate the usefulness of M. koenigii L. Sprengel in oral health care.
The cold extraction method offered higher zone of inhibition compared to hot extraction method with A. nilotica and E. hybrid against S. sanguis. However, the extracts of P. guajava and M. koenigii L. Sprengel offered higher efficacy against S. sanguis and S. mutans, respectively, when extracted using hot extraction process. The relatively superior results using cold extraction method could be due to thermal decomposition of the target compounds in the plants as the Soxhlet extraction usually occurs at the boiling point of the solvent for a long time.[24] However, the higher yield in hot extraction process using Soxhlet apparatus makes this the method of choice for plant extraction in most situations. The variations in the antimicrobial efficacy between the hot and cold extraction methods using different plant extracts against the plaque bacteria highlight the need to employ both methods before choosing a particular extraction process for the plant.
Our results with respect to antimicrobial efficacy of the four plant extracts using the two methods of extraction against primary plaque colonizers could not be compared with previously published literature as this was the first of its kind. The extraction using solvents other than ethanol may yield different results which need to be evaluated. The study included only primary plaque colonizers, while the inclusion of other bacteria involved in dental caries (Lactobacillus acidophilus) as well as secondary and tertiary plaque colonizers would have made the results more conclusive. The antimicrobial efficacy of the individual plant extracts was assessed in the present study while their combinations could have offered synergistic benefits.
SUMMARY AND CONCLUSION
All the four plant extracts in the present study exhibited antimicrobial efficacy against S. mutans, S. sanguis, and S. salivarius irrespective of the extraction method. A. nilotica, P. guajava, and E. hybrid extracts showed higher mean inhibition zones compared to M. koenigii L. Sprengel. These plant extracts have the potential to be used as antiplaque agents and could be considered as herbal alternatives to chlorhexidine. Although significant benefits with regard to some plant extracts using either hot or cold extraction methods were noted against some bacteria, overall, it can be inferred that the cold extraction method retains more chemical constituents than hot extraction methods. However, considering the fact that even hot extraction method also yields antimicrobial efficacy which is either comparable to cold extraction method or sometimes superior with some plant extracts against certain bacteria and very high yield, we feel hot extraction method to be the choice when large quantity of the extract is to be produced. Moreover, the solvent used in the hot extraction process (Soxhlet extraction) can be recovered and reused which makes the procedure cost effective. The antimicrobial efficacy testing using the combination of these plant extracts on Lactobacillus acidophilus, secondary and tertiary plaque colonizers is the need of the hour. This may facilitate the evolution of a polyherbal formulation that can inhibit the most common dental caries and plaque bacteria simultaneously.
ACKNOWLEDGEMENTS
We sincerely thank the management of People's University, Bhopal Madhya Pradesh for their kind permission and co-operation in completing this research project.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Marsh PD. Dental plaque as a biofilm and a microbial community – Implications for health and disease. BMC Oral Health. 2006;6(Suppl 1):S14. doi: 10.1186/1472-6831-6-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroes I, Lepp PW, Relman DA. Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci U S A. 1999;96:14547–52. doi: 10.1073/pnas.96.25.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005;83:661–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. Am J Dent. 2009;22:3–8. [PubMed] [Google Scholar]
- 5.Shekar C, Cheluvaiah MB, Namile D. Prevalence of dental caries and dental fluorosis among 12 and 15 years old school children in relation to fluoride concentration in drinking water in an endemic fluoride belt of Andhra Pradesh. Indian J Public Health. 2012;56:122–8. doi: 10.4103/0019-557X.99902. [DOI] [PubMed] [Google Scholar]
- 6.Albandar JM, Brown LJ, Löe H. Clinical features of early-onset periodontitis. J Am Dent Assoc. 1997;128:1393–9. doi: 10.14219/jada.archive.1997.0058. [DOI] [PubMed] [Google Scholar]
- 7.Peck MT, Africa CW, Stephen LX, Marnewick J, Majeed A. An in-vitro analysis of the antimicrobial efficacy of herbal toothpastes on selected primary plaque colonizers. Int J Clin Dent Sci. 2011;2:28–32. [Google Scholar]
- 8.Yee R, Sheiham A. The burden of restorative dental treatment for children in Third World countries. Int Dent J. 2002;52:1–9. [PubMed] [Google Scholar]
- 9.Mouradian WE, Wehr E, Crall JJ. Disparities in children's oral health and access to dental care. JAMA. 2000;284:2625–31. doi: 10.1001/jama.284.20.2625. [DOI] [PubMed] [Google Scholar]
- 10.Eley BM. Antibacterial agents in the control of supragingival plaque – A review. Br Dent J. 1999;186:286–96. doi: 10.1038/sj.bdj.4800090. [DOI] [PubMed] [Google Scholar]
- 11.Thakur R, Jain N, Pathak R, Sandhu SS. Practices in wound healing studies of plants. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1155/2011/438056. 438056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshpande SN, Kadam DG. Phytochemical analysis and antibacterial activity of Acacia nilotica against Streptococcus mutans. Int J Pharm Pharm Sci. 2013;5:236–8. [Google Scholar]
- 13.Pai MB, Prashant GM, Murlikrishna KS, Shivakumar KM, Chandu GN. Antifungal efficacy of Punica granatum, Acacia nilotica, Cuminum cyminum and Foeniculum vulgare on Candida albicans: an in vitro study. Indian J Dent Res. 2010;21:334–6. doi: 10.4103/0970-9290.70792. [DOI] [PubMed] [Google Scholar]
- 14.Nagata H, Inagaki Y, Yamamoto Y, Maeda K, Kataoka K, Osawa K, et al. Inhibitory effects of macrocarpals on the biological activity of Porphyromonas gingivalis and other periodontopathic bacteria. Oral Microbiol Immunol. 2006;21:159–63. doi: 10.1111/j.1399-302X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 15.Andrade-Neto M, Alencar JW, Silveira ER, Cunha AN. Volatile constituents of Psidium pohlianum Berg. and Psidium guyanensis Pers. J Essent Oils Res. 1994;6:299–300. [Google Scholar]
- 16.Hema R, Kumaravel S, Elanchezhiyan N. Antimicrobial activity of some of the South-Indian spices and Herbals against food pathogens. Global J Pharmacol. 2009;3:38–40. [Google Scholar]
- 17.Ningappa MB, Dhananjaya BL, Dinesha R, Harsha R, Srinivas L. Potent antibacterial property of APC protein from Curry leaves (Murraya koenigii L.) Food Chem. 2010;118:747–50. [Google Scholar]
- 18.Sunitha JD, Patel S, Madhusudan AS, Ravindra SV. An in vitro antimicrobial activity of few plant extracts on dental caries microorganisms. Int J A PS BMS. 2012;3:294–303. [Google Scholar]
- 19.Dabur R, Gupta A, Mandal TK, Singh DD, Bajpai V, Gurav AM, et al. Antimicrobial activity of some Indian medicinal plants. Afr J Tradit Complement Altern Med. 2007;4:313–8. doi: 10.4314/ajtcam.v4i3.31225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismail M, Minhas PS, Khanum F, Sahana VM, Sowmya C. Antibacterial activity of leaves extract of Guava (Psidium guajava) Int J Res Pharm Biomed Sci. 2012;3:1–3. [Google Scholar]
- 21.Gonçalves FA, Andrade Neto M, Bezerra JN, Macrae A, Sousa OV, Fonteles-Filho AA, et al. Antibacterial activity of GUAVA, Psidium guajava Linnaeus, leaf extracts on diarrhea-causing enteric bacteria isolated from Seabob shrimp, Xiphopenaeus kroyeri (Heller) Rev Inst Med Trop Sao Paulo. 2008;50:11–5. doi: 10.1590/s0036-46652008000100003. [DOI] [PubMed] [Google Scholar]
- 22.Takarada K, Kimizuka R, Takahashi N, Honma K, Okuda K, Kato T. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol Immunol. 2004;19:61–4. doi: 10.1046/j.0902-0055.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 23.Ramesh G, Nagarajappa R, Madhusudan AS, Sandesh N, Batra M, Sharma A, et al. Estimation of salivary and tongue coating pH on chewing household herbal leaves: A randomized controlled trial. Anc Sci Life. 2012;32:69–75. doi: 10.4103/0257-7941.118531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 2006;17:300–12. [Google Scholar]


