Abstract
Background:
To compare the enamel protection efficacy of stannous-containing sodium fluoride and sodium monofluorophosphate (MFP)/triclosan dentifrices marketed in India in an in situ erosion model with acidic challenge.
Materials and Methods:
This randomised and controlled, in situ, supervised, double-blind clinical trial employed a two-treatment, four-period crossover design, wherein subjects wore an appliance fitted with human enamel samples 6 h/day during each 10 day treatment period and swished twice daily with their assigned dentifrice slurry: Oral-B® Pro-Health (maximum 1,000 ppm F as sodium fluoride with stannous chloride) or Colgate® Strong Teeth with Cavity Protection (maximum 1,000 F as sodium MFP and triclosan). Subjects swished with 250 ml of orange juice over a 10 min period after each treatment and twice daily for the acidic erosive challenge. Enamel samples were measured for tooth surface loss using contact profilometry at baseline and day 10.
Results:
A total of 34 subjects were randomised to treatment; 32 subjects completed the final visit. Baseline profilometry measurements of the specimen surfaces were near zero within ± 0.3 μm, and no statistically significant difference (P > 0.48) on average was observed between the two test dentifrices. At day 10, the stannous-containing dentifrice demonstrated 88% less erosion (P < 0.0001) relative to the MFP/triclosan dentifrice. Estimated medians (95% confidence intervals) were 0.21 μm (0.17, 0.25) for the stannous-containing dentifrice versus 1.66 μm (1.39, 1.99) for the MFP/triclosan dentifrice. Both dentifrices were well-tolerated.
Conclusions:
Compared with MFP/triclosan toothpaste, a stabilised stannous-containing sodium fluoride dentifrice gave statistically significantly greater protection against tooth enamel surface loss in situ following repeated acid erosive challenge.
Keywords: Dentifrice, erosion, sodium fluoride, stannous fluoride, triclosan
INTRODUCTION
Dental erosion results when the enamel surface is excessively challenged by significant acidic insult, shifting the local pH below 4.5, and rendering the inherent protective features of the dental pellicle insufficient to shield against attrition, abrasion, and potentially permanent erosive damage.[1,2,3,4] In contrast to dental caries, dental erosion is elicited by nonbacterial and lower-pH acids. Some dietary acids can dissolve fluoroapatite and lead to irreversible surface damage without intervention.[1,2,3,4] Dental erosion can be caused by intrinsic factors such as refluxed gastric acid, but is often precipitated by dietary sources.[5,6] In particular, the increasingly popular consumption by both adults and children of highly acidic beverages including fruit juices, soft drinks, energy drinks, and sports beverages is thought to be a significant contributor to the surprisingly high worldwide prevalence of dental erosion.[6,7,8,9,10] Surveys from disparate locales of children, adolescents, and young adults have found the prevalence of tooth erosion to range from approximately 25% to as high as 78%.[11,12,13,14,15,16,17,18,19,20] Nayak et al.[21] have reported that almost one-third of a population of 5-year-old children assessed in India had evidence of tooth erosion.
Tooth surface loss from erosion can bring about dentinal hypersensitivity and/or loss of function, potentially requiring costly and invasive professional restorative treatment.[22] Alternatively, preventive measures at the early stage of acid enamel dissolution are a superior strategy from both a patient comfort and cost standpoint. The extent and severity of erosion is influenced by factors such as the individual's oral hygiene practices, the length and regularity of acid exposures, and the adequacy of saliva levels to neutralize acid attacks;[23] these constitute potential preventive intervention points in the erosion process.
Through extensive ongoing research and innovation in recent years, stannous fluoride dentifrices-once hindered by formulation challenges-are now readily available in optimized aesthetically-pleasing, stabilised formulations proven in a myriad of investigations to provide numerous therapeutic benefits, including enamel erosion protection. For maximum global consumer accessibility, patented stannous-containing sodium fluoride formulations with multiple benefits have also been developed, utilizing stannous chloride and sodium fluoride to provide stabilisation and delivery of the stannous ion and fluoride ion, respectively. Recently, a stannous-containing sodium fluoride dentifrice (maximum 1000 ppm fluoride) was introduced in India, which has shown in vitro superiority for erosion protection when compared with various controls.[24,25,26,27]
This paper reports on a randomised and controlled, in situ clinical investigation undertaken to evaluate the comparative antierosion benefits of this new stannous-containing dentifrice compared with a marketed sodium monofluorophosphate (sodium MFP)/triclosan control dentifrice in an adult population.
MATERIALS AND METHODS
Volunteers in good general health and at least 18 years old were recruited from Bristol University and the Bristol Dental School and Hospital. The study protocol and subject consent form had previously been reviewed and approved by the National Research Ethics Service Committee South West - Exeter (13/SW/0039), United Kingdom. Prospective subjects who provided written informed consent at the prestudy screening visit received clinical oral soft tissue evaluations, following which medical, demographic, and concomitant medication information was elicited. Those subjects who were deemed qualified for study enrolment met all entrance inclusion and exclusion criteria [Table 1].
Table 1.
Inclusion and exclusion criteria
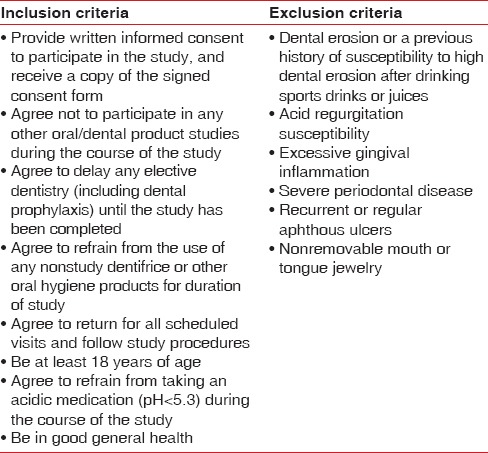
This randomised single centre, double-blind, supervised-usage clinical trial-conducted according to good clinical practice guidelines-employed a two-treatment, four-period crossover design and an in situ erosion model based on Hooper et al.[28] to compare the enamel protection effectiveness of two dentifrices with differing fluoride systems. Ten weekday treatment days were included in each of the four study periods, and each study period was approximately 2 weeks long. Following enrolment, palatal intraoral appliances fitted with two enamel samples were constructed for subjects. A computer-generated randomisation list was provided by the study sponsor and supplied to study site personnel who randomly assigned participants to one of four treatment sequences: AABB, BBAA, ABBA, and BAAB, where the letters correspond to the two-treatments. Accordingly, each subject used the two test dentifrices 2 times during the trial:
Maximum 1,000 ppm F as sodium fluoride and stannous chloride as a key excipient, marketed in India as Oral-B® Pro-Health dentifrice (The Procter and Gamble Company, Cincinnati, OH, USA)
Maximum 1,000 ppm F as sodium MFP with triclosan, marketed in India as Colgate® Strong Teeth with Cavity Protection (Colgate-Palmolive, New York, NY, USA).
Before presenting for their visit on every treatment day, subjects brushed with the supplied nontreatment dentifrice and toothbrush in their customary manner and according to oral and written instructions: 0.32% sodium fluoride (1450 ppm fluoride) marketed dentifrice (Crest® Decay Protection, The Procter and Gamble Company, Cincinnati, OH); and an Oral-B® 35 manual toothbrush (The Procter and Gamble Company, Cincinnati, OH). These products were to be used on nontreatment days, weekends, in the morning and evening before and after treatment day visits, and prior to and following wearing of the dental appliances during treatment phases.
Treatment day procedures followed the predefined order summarised in Figure 1. At the clinical site on treatment days, subjects first inserted their custom palatal intraoral appliance containing the enamel samples and wore them approximately 6 h, whereby the samples were exposed to the treatment dentifrice and successive erosive challenges. While supervised by site personnel, study participants swished for 60 s with their assigned treatment toothpaste in slurry form twice each treatment day (baseline and 3 h after baseline). Swishing was followed by a rinse with 10 ml of water. Following each slurry treatment as well as at 2 and 6 h postbaseline (i.e. 4 times daily), the enamel samples worn via the intraoral appliances were given an erosive challenge with the administration of orange juice (Sainsbury's Supermarkets Ltd, 33 Holborn, London, UK). Over one timed minute, subjects sipped the juice (25 ml), swished it thoroughly, and expectorated. This sequence was repeated 10 times, resulting in the exposure of the enamel samples to 250 ml of orange juice over 10 min during each replication. Ten minute acid challenges were used because this time period represents a reasonable time for consumption of a juice beverage or soft drink.[28,29] Subjects were prohibited from food and drink-other than small water sips-when wearing their appliance between slurry treatments and erosive challenges. The appliances were removed and stored in a moist container during a mid-day lunch break.
Figure 1.
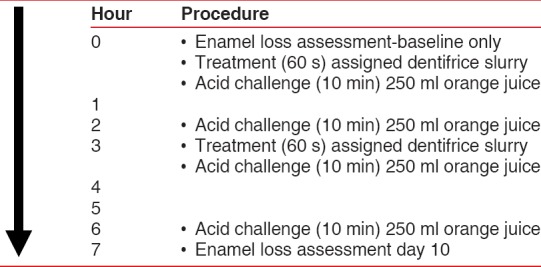
Study procedures – treatment days 1–10
Dentifrice slurries were prepared by clinical site personnel by mixing 3 g of dentifrice with 10 ml of water. To ensure study blinding, the identity of each assigned slurry was unknown to subjects, who were directed not to discuss the slurry physical properties with other subjects or clinical site personnel. Further, access to the product dispensing room was prohibited during treatment for the investigator and those involved in surface profilometry assessment and recording.
Fresh enamel samples were inserted in the intraoral appliance at the initiation of each new study period. A final clinical oral assessment and medical exit interview were conducted within 2 weeks of completion of the fourth treatment period.
Recently extracted, adult caries-free human third molars were obtained following ethical approval for use in preparation of the enamel samples at the clinical site according to institutional standard operating procedures.[28]
At the start and end of each treatment day, the palatal appliances containing the enamel samples were disinfected by dipping them for 3 min in Corsodyl® mouthrinse with 0.2% w/v chlorhexidine gluconate (GlaxoSmithKline, Brentford, Middlesex, UK) and rinsed with tap water. Samples were disinfected through soaking for 20 min in a mixture of 0.5% chlorhexidine and 70% aqueous ethanol before the profilometry measurements, after removal from the appliances, and again postmeasurement after being re-taped and replaced in the appliances. A “moist pot” (container with a cotton wool, water-moistened pad) was used for storage of the intraoral palatal appliances when absent from the mouth, to prevent dehydration.
A calibrated surface (contact) profilometer [Figure 2], was used to quantify enamel loss. A stainless steel jig held samples in place during the assessments. Operating in a controlled environment, the measuring head was fitted with a diamond stylus to follow the surface of the enamel under test, and transversed the specimen at a constant speed of 10 mm/min. The measuring heads’ signals were processed on an electrical control unit for display on a monitor screen.
Figure 2.

Contact Surface Profilomter
Two baseline measurements were secured for each enamel sample, where readings were taken across a demarcated 2-3 mm treatment area to be exposed to the test treatments and erosive challenges. Postexposure profilometry readings were measured in the same demarcated area at treatment day 10 of all treatment periods.
A clinical assessment of oral hard and soft tissues was utilized to assess safety.
Statistical analyses
Using 36 subjects, at least 80% power to detect a 2-sided 5% significant difference between the treatment dentifrices would be achieved provided the natural log scale effect size (mean difference divided by the error standard deviation) was 0.50 or greater for this crossover design.
The primary outcome measure of efficacy in this study was dental erosion, measured by profilometry at day 10. For each subject, treatment period, and visit, the average of four erosion measurements was calculated using two replicate measurements from each of two enamel sections. Since the day 10 enamel loss distribution was right-skewed, the data were transformed using the natural log function to make the distribution bell-shaped before performing between-treatment analysis that assumed normality. A general linear mixed model was used to compare treatments, and the final model included period and treatment as fixed effects and subject as a random effect. Neither the carryover effect nor the baseline covariate was statistically significant (P > 0.33) and each was removed from the statistical model. From the final statistical model, estimated means on the natural log scale were back-transformed by using the exponential function (emean) to obtain the estimated medians or 50th percentiles on the original scale (μm), and 95% confidence intervals (CI) were calculated. All statistical comparisons were two-sided with a significance level of 0.05.
RESULTS
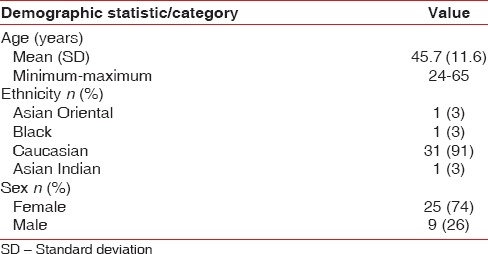
Thirty-four subjects were enrolled and randomised to a test product sequence; 32 (94%) completed the study. The study was conducted between April and July 2013. Two subjects discontinued after Period 3 due to nonproduct related factors. The study population had an average age of 45.7 years (standard deviation 11.60), and ranged in age from 24 to 65 years. Female volunteers constituted 74% of the enrolled volunteers, and 91% of subjects were Caucasian [Table 2].
Table 2.
Demographics summary
The baseline profilometry measurements of the specimen surfaces were near zero within ± 0.3 μm, and no statistically significant difference (P > 0.48) on average was observed between the two test dentifrices. As displayed in Table 3, after 10 days of dietary acid challenge, the treatment comparison of the profilometry surface levels demonstrated that the stannous-containing dentifrice produced statistically significantly (P < 0.0001) less erosion by 88% resulting in greater enamel protection compared to the MFP/triclosan dentifrice, with an estimated median of 0.21 μm and a 95% CI of (0.17, 0.25) for the stannous-containing dentifrice compared to an estimated erosion median of 1.66 μm and 95% CI of (1.39, 1.99) when subjects used the MFP/triclosan dentifrice. Using the natural log scale, estimated means from the statistical model were calculated for the stannous-containing and MFP/triclosan test dentifrices, with the aforementioned estimated medians (μm) calculated by application of the exponential function. In the enamel loss statistical model used to compare treatment dentifrices on the natural log scale, neither the carryover effect nor the baseline measurement covariate demonstrated evidence of being statistically significant (P > 0.33) at day 10, and so both effects were removed from the final statistical model.
Table 3.
Enamel loss (μm) treatment comparison at day 10

Table 4 summarises basic descriptive statistics. The observed median of the data was 0.19 μm for the stannous-containing dentifrice and 1.62 μm for the MFP/triclosan dentifrice. These simple medians of the data were consistent with estimates from the more complex statistical model that adjusted for period and subject to subject variability with estimated medians of 0.21 and 1.66 for the NaF/stannous and MFP/triclosan dentifrices, respectively. However, the means from Table 4 were inflated relative to the medians due to the right-skewed nature of the data distribution. Figures 3 and 4 display the individual subject and period level data as well as boxplot summaries of the distribution for both the original and natural logarithm scales, respectively. The right-skewed nature of the data was readily apparent, especially as erosion levels increased for the lesser effective MFP/triclosan dentifrice, which initiated the need to perform statistical modeling of the data on the natural log scale in order to comply with the normality assumptions.
Table 4.
Enamel loss (μm) summary statistics at day 10 by treatment
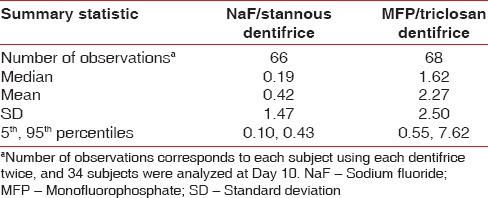
Figure 3.
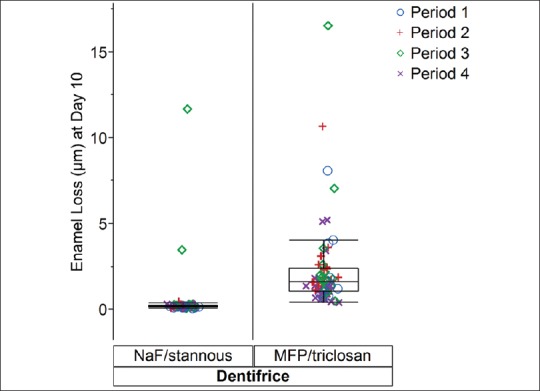
Enamel Loss (μm) at Day 10 versus Dentifrice displaying Period
Figure 4.
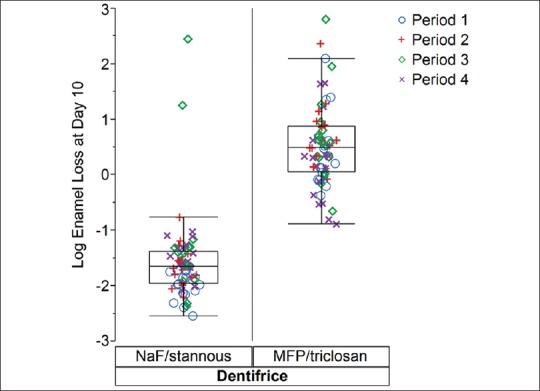
Log Enamel Loss at Day 10 versus Dentifrice displaying Period
Both dentifrices were well-tolerated. One adverse event, a mouth ulcer rated mild in severity, was reported in a subject who withdrew following period 1. Upon questioning and clinical examination, the event was deemed not related to study product use.
DISCUSSION
The brand name and packaging of certain fluoride dentifrices may convey a message that these products provide strong fortifying protection against all potential threats to dental enamel integrity. In the area of tooth erosion, however, the existing high erosion prevalence rates coupled with widespread dentifrice use suggests that not all fluoride systems provide clinically meaningful acid-induced tooth surface loss protection. Stannous-containing dentifrices have demonstrated erosion protection, with significantly greater benefits relative to other fluoride dentifrices substantiated in multiple published investigations.[24,25,26,27,28,30,31,32,33,34,35,36,37] Stannous fluoride has been shown to form insoluble tin-rich complexes that have the potential to enhance the resistance of the tooth surface against acid challenges.[38]
Faller and Eversole[30] compared the ability of various fluoride dentifrices to form a protective barrier layer to erosive acid in vitro; they found the SnF2 treatment was significantly more effective than sodium fluoride (NaF), sodium MFP, and amine fluoride. Similarly, in separate research by this investigator,[35] a stabilised stannous fluoride dentifrice significantly outperformed NaF, sodium MFP, NaF plus 5% potassium nitrate, and sodium MFP plus 8% arginine bicarbonate dentifrices in enamel protection of extracted human teeth from erosive acid damage, using a controlled series of tests involving four dietary acids. Furthermore, in a trial employing 24 cycles of citric acid challenge to bovine enamel samples by Paepegaey et al.,[36] the stabilised SnF2 dentifrice was significantly (P < 0.0001) more effective in preventing enamel erosion when contrasted with NaF and MFP dentifrices, and was 62% (P < 0.0001) better in enamel loss protection versus a water control. Similar findings have been demonstrated for the stannous-based dentifrice recently introduced in India. In two separate in vitro studies by Chen[24] and Su,[25] the stabilised stannous-containing dentifrice significantly (P < 0.05) outperformed MFP/triclosan dentifrice in erosion resistance following multiple acidic challenges. The results of the in situ clinical trial reported here are confirmatory, providing statistically significant clinical evidence of the markedly greater effectiveness (88% less erosion) for a newly introduced stabilised stannous-containing toothpaste in providing enamel protection to withstand repeated acid attacks when compared to an MFP/triclosan dentifrice marketed as a tooth-strengthener. One might argue the in situ benefit should be manifested in long-term human erosion clinical studies, however, those studies are impractical given the slow progression of this condition.
Ideally, patients would adhere to their dental professionals’ recommendations to brush and floss regularly and thoroughly to remove dental plaque. In actuality, habits and practices surveys reveal that efficient toothbrushing and frequent flossing are not normative,[39,40] leading to unacceptably high levels of oral disease worldwide.[41] With education and patient motivation, changed behavior can be effected, but sustainable improvements to ingrained habits have been shown to be challenging.[42] While oral hygiene practices differ somewhat across the globe, most adults report that at a minimum they use a toothbrush and dentifrice at least once daily.[39,43] Incorporating a dentifrice with proven clinical antierosion protection, therefore, such as that afforded by the new Oral-B Pro-Health stabilised stannous-containing toothpaste, is a simple yet highly beneficial means of reducing the threat of tooth surface loss without the need for adding an extra step or product into the daily oral hygiene routine.
ACKNOWLEDGEMENT
The authors would like to thank Melanie Miner, Aneta Wilberg, Vicki Widmeyer, Uta Mesples, Lisa Sagel, and Shelly Campbell for their assistance with this research and/or publication.
Footnotes
Source of Support: The study was supported by the Procter and Gamble Company
Conflict of Interest: Drs. He and Barker are employees of the Procter and Gamble Company.
REFERENCES
- 1.Mahoney EK, Kilpatrick NM. Dental erosion: Part 1. Aetiology and prevalence of dental erosion. N Z Dent J. 2003;99:33–41. [PubMed] [Google Scholar]
- 2.Ganss C. Definition of erosion and links to tooth wear. In: Lussi A, editor. Dental Erosion. From Diagnosis to Therapy (Monographs in Oral Science) 1st ed. Basel: Karger; 2006. pp. 9–16. [DOI] [PubMed] [Google Scholar]
- 3.Addy M, Shellis RP. Interaction between attrition, abrasion and erosion in tooth wear. In: Lussi A, editor. Dental Erosion. From Diagnosis to Therapy (Monographs in Oral Science) 1st ed. Basel: Karger; 2006. pp. 17–31. [DOI] [PubMed] [Google Scholar]
- 4.Amaechi BT, Higham SM, Edgar WM, Milosevic A. Thickness of acquired salivary pellicle as a determinant of the sites of dental erosion. J Dent Res. 1999;78:1821–8. doi: 10.1177/00220345990780120901. [DOI] [PubMed] [Google Scholar]
- 5.Moazzez R, Bartlett D, Anggiansah A. Dental erosion, gastro-oesophageal reflux disease and saliva: How are they related? J Dent. 2004;32:489–94. doi: 10.1016/j.jdent.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Zero DT, Lussi A. Etiology of enamel erosion: Intrinsic and extrinsic factors. In: Addy M, Embery G, Edgar WM, Orschardon R, Dunitz M, editors. Tooth Wear and Sensitivity. London: Martin Dunitz Ltd; 2000. pp. 121–40. [Google Scholar]
- 7.von Fraunhofer JA, Rogers MM. Effects of sports drinks and other beverages on dental enamel. Gen Dent. 2005;53:28–31. [PubMed] [Google Scholar]
- 8.Jain P, Hall-May E, Golabek K, Agustin MZ. A comparison of sports and energy drinks – Physiochemical properties and enamel dissolution. Gen Dent. 2012;60:190–7. [PubMed] [Google Scholar]
- 9.Rirattanapong P, Vongsavan K, Surarit R. Effect of soft drinks on the release of calcium from enamel surfaces. Southeast Asian J Trop Med Public Health. 2013;44:927–30. [PubMed] [Google Scholar]
- 10.Jaeggi T, Lussi A. Prevalence, incidence and distribution of erosion. Monogr Oral Sci. 2006;20:44–65. doi: 10.1159/000093350. [DOI] [PubMed] [Google Scholar]
- 11.Al-Dlaigan YH, Shaw L, Smith A. Dental erosion in a group of British 14-year-old, school children. Part I: Prevalence and influence of differing socioeconomic backgrounds. Br Dent J. 2001;190:145–9. doi: 10.1038/sj.bdj.4800908. [DOI] [PubMed] [Google Scholar]
- 12.Deery C, Wagner ML, Longbottom C, Simon R, Nugent ZJ. The prevalence of dental erosion in a United States and a United Kingdom sample of adolescents. Pediatr Dent. 2000;22:505–10. [PubMed] [Google Scholar]
- 13.Nahás Pires Corrêa MS, Nahás Pires Corrêa F, Nahás Pires Corrêa JP, Murakami C, Mendes FM. Prevalence and associated factors of dental erosion in children and adolescents of a private dental practice. Int J Paediatr Dent. 2011;21:451–8. doi: 10.1111/j.1365-263X.2011.01150.x. [DOI] [PubMed] [Google Scholar]
- 14.Taji S, Seow WK. A literature review of dental erosion in children. Aust Dent J. 2010;55:358–67. doi: 10.1111/j.1834-7819.2010.01255.x. [DOI] [PubMed] [Google Scholar]
- 15.Kazoullis S, Seow WK, Holcombe T, Newman B, Ford D. Common dental conditions associated with dental erosion in schoolchildren in Australia. Pediatr Dent. 2007;29:33–9. [PubMed] [Google Scholar]
- 16.Wiegand A, Müller J, Werner C, Attin T. Prevalence of erosive tooth wear and associated risk factors in 2-7-year-old German kindergarten children. Oral Dis. 2006;12:117–24. doi: 10.1111/j.1601-0825.2005.01167.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Lin HC, Chen JH, Liang HY. The prevalence of dental erosion and associated risk factors in 12-13-year-old school children in Southern China. BMC Public Health. 2010;10:478. doi: 10.1186/1471-2458-10-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantonanaki M, Koletsi-Kounari H, Mamai-Homata E, Papaioannou W. Dental erosion prevalence and associated risk indicators among preschool children in Athens, Greece. Clin Oral Investig. 2013;17:585–93. doi: 10.1007/s00784-012-0730-4. [DOI] [PubMed] [Google Scholar]
- 19.Manaf ZA, Lee MT, Ali NH, Samynathan S, Jie YP, Ismail NH, et al. Relationship between food habits and tooth erosion occurrence in Malaysian University students. Malays J Med Sci. 2012;19:56–66. [PMC free article] [PubMed] [Google Scholar]
- 20.Nunn JH, Gordon PH, Morris AJ, Pine CM, Walker A. Dental erosion – changing prevalence? A review of British National childrens’ surveys. Int J Paediatr Dent. 2003;13:98–105. doi: 10.1046/j.1365-263x.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 21.Nayak SS, Ashokkumar BR, Ankola AV, Hebbal MI. Distribution and severity of erosion among 5-year-old children in a city in India. J Dent Child (Chic) 2010;77:152–7. [PubMed] [Google Scholar]
- 22.West NX, Sanz M, Lussi A, Bartlett D, Bouchard P, Bourgeois D. Prevalence of dentine hypersensitivity and study of associated factors: A European population-based cross-sectional study. J Dent. 2013;41:841–51. doi: 10.1016/j.jdent.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Lussi A, Jaeggi T. Erosion – Diagnosis and risk factors. Clin Oral Investig. 2008;12(Suppl 1):S5–13. doi: 10.1007/s00784-007-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H. Evaluation of toothpastes’ anti-erosion efficacy via novel in-vitro models. J Dent Res. 2013;92 Abstract 246. [Google Scholar]
- 25.Su Y. Effect of stabilised stannous-based fluoride toothpaste on dental erosion. J Dent Res. 2013;92 Abstract 188. [Google Scholar]
- 26.Faller RV, Eversole SL, Tzeghai GE. Enamel protection: A comparison of marketed dentifrice performance against dental erosion. Am J Dent. 2011;24:205–10. [PubMed] [Google Scholar]
- 27.Shi Y, Chen H, Sun L, Strand M, Zsiska M, White DJ. Evaluation of anti-sensitivity efficacy of stabilized stannous-based fluoride toothpaste. J Dent Res. 2013;92 Abstract 235. [Google Scholar]
- 28.Hooper SM, Newcombe RG, Faller R, Eversole S, Addy M, West NX. The protective effects of toothpaste against erosion by orange juice: Studies in situ and in vitro. J Dent. 2007;35:476–81. doi: 10.1016/j.jdent.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Hooper S, Hughes J, Parker D, Finke M, Newcombe RG, Addy M, et al. A clinical study in situ to assess the effect of a food approved polymer on the erosion potential of drinks. J Dent. 2007;35:541–6. doi: 10.1016/j.jdent.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Faller RV, Eversole SL. Ability of fluoride compounds to deposit a protective barrier layer onto enamel. Caries Res. 2009;43:224. [Google Scholar]
- 31.Khambe D, Eversole SL, Mills T, Faller RV. Enamel protection: Deposition and retention of SnF2 on enamel surfaces. J Dent Res. 2010;89 Abstract 526. [Google Scholar]
- 32.Yan J, Faller RV, Ji N. HAP dissolution study I: SnF 2 vs. NaF solution study. J Dent Res. 2009;88 Abstract 2112. [Google Scholar]
- 33.Khambe D, Eversole S, Faller R, Wagner M, Mills T. Surface protective mechanism of SnF2 against irreversible acid damage. J Dent Res. 2009;88 Abstract 3371. [Google Scholar]
- 34.Baig A, Lawless M, Faller R. HAP dissolution study II: SnF 2 vs. NaF dentifrice study. J Dent Res. 2009;88 Abstract 725. [Google Scholar]
- 35.Faller RV, Eversole SL. Enamel protection from acid challenge – benefits of marketed fluoride dentifrices. J Clin Dent. 2013;24:25–30. [PubMed] [Google Scholar]
- 36.Paepegaey AM, Day TN, Boulding A, Harris R, Barker ML, Bellamy PG. In vitro comparison of stannous fluoride, sodium fluoride, and sodium monofluorophosphate dentifrices in the prevention of enamel erosion. J Clin Dent. 2013;24:73–8. [PubMed] [Google Scholar]
- 37.Willumsen T, Ogaard B, Hansen BF, Rølla G. Effects from pretreatment of stannous fluoride versus sodium fluoride on enamel exposed to 0.1 M or 0.01 M hydrochloric acid. Acta Odontol Scand. 2004;62:278–81. doi: 10.1080/00016350410000174. [DOI] [PubMed] [Google Scholar]
- 38.Faller RV, Eversole SL. Protective effects of SnF2-Part III. Mechanism of barrier layer attachment. Int Dent J. 2014;64(Suppl 1):16–21. doi: 10.1111/idj.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inglehart M, Tedesco LA. Behavioral research related to oral hygiene practices: A new century model of oral health promotion. Periodontol 2000. 1995;8:15–23. doi: 10.1111/j.1600-0757.1995.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 40.Bakdash B. Current patterns of oral hygiene product use and practices. Periodontol 2000. 1995;8:11–4. doi: 10.1111/j.1600-0757.1995.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 41.Beaglehole R, Benzian H, Crail J, Mackay J, King J, Lacey C, editors. 1st ed. Brighton, UK: Dental Education Ltd and Myriad Editions; 2009. The Oral Health Atlas: Mapping A Neglected Global Health Issue; pp. 1–124. [Google Scholar]
- 42.Kumanyika SK, Van Horn L, Bowen D, Perri MG, Rolls BJ, Czajkowski SM, et al. Maintenance of dietary behavior change. Health Psychol. 2000;19:42–56. doi: 10.1037/0278-6133.19.suppl1.42. [DOI] [PubMed] [Google Scholar]
- 43.Maes L, Vereecken C, Vanobbergen J, Honkala S. Tooth brushing and social characteristics of families in 32 countries. Int Dent J. 2006;56:159–67. doi: 10.1111/j.1875-595x.2006.tb00089.x. [DOI] [PubMed] [Google Scholar]


